The Role of NiO in Reactive Adsorption Desulfurization Over NiO/ZnO-Al2O3-SiO2 Adsorbent
Abstract
1. Introduction
2. Results and Discussion
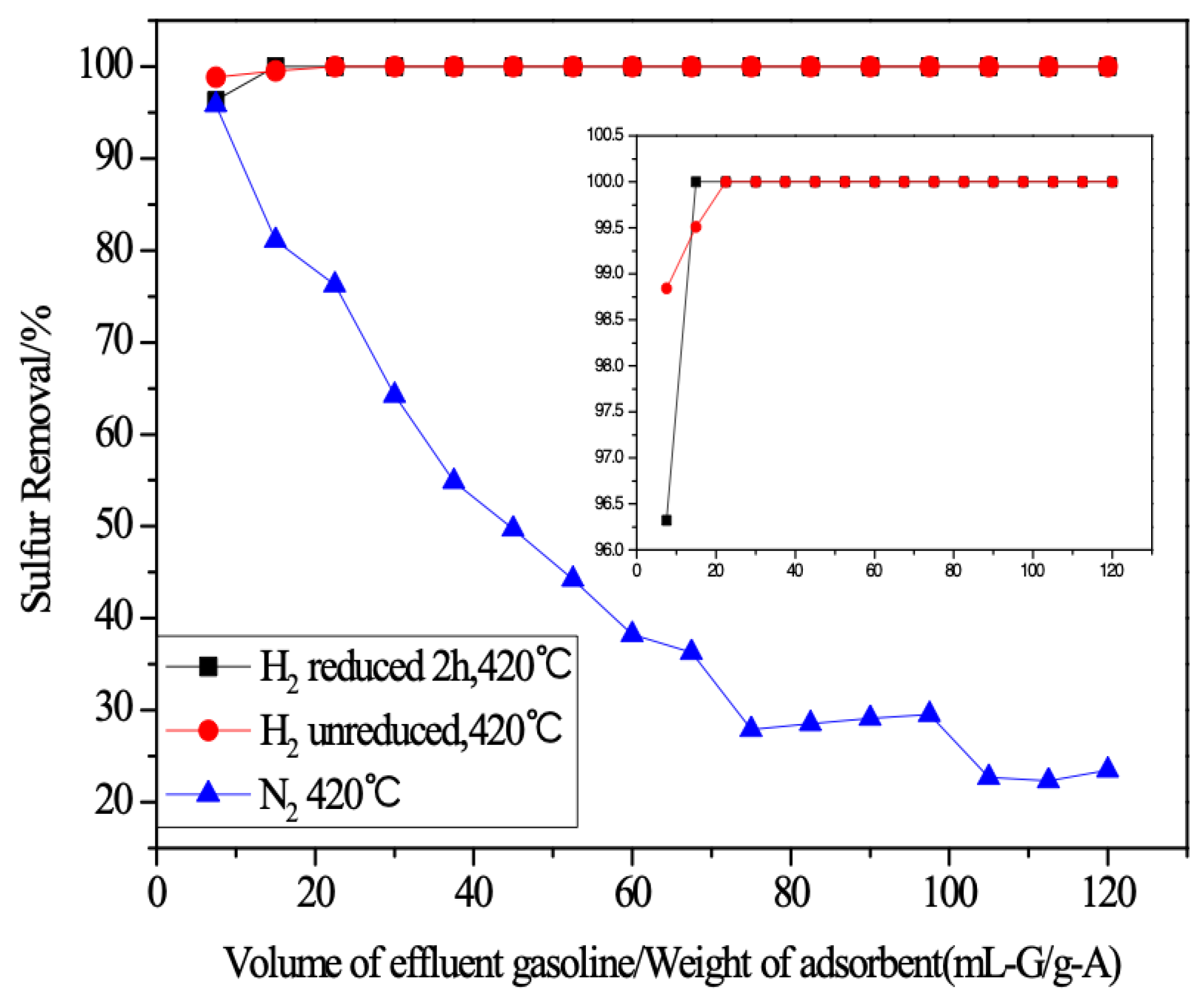
2.1. RADS Performance under Different Atmospheres
2.2. XRD Characterization
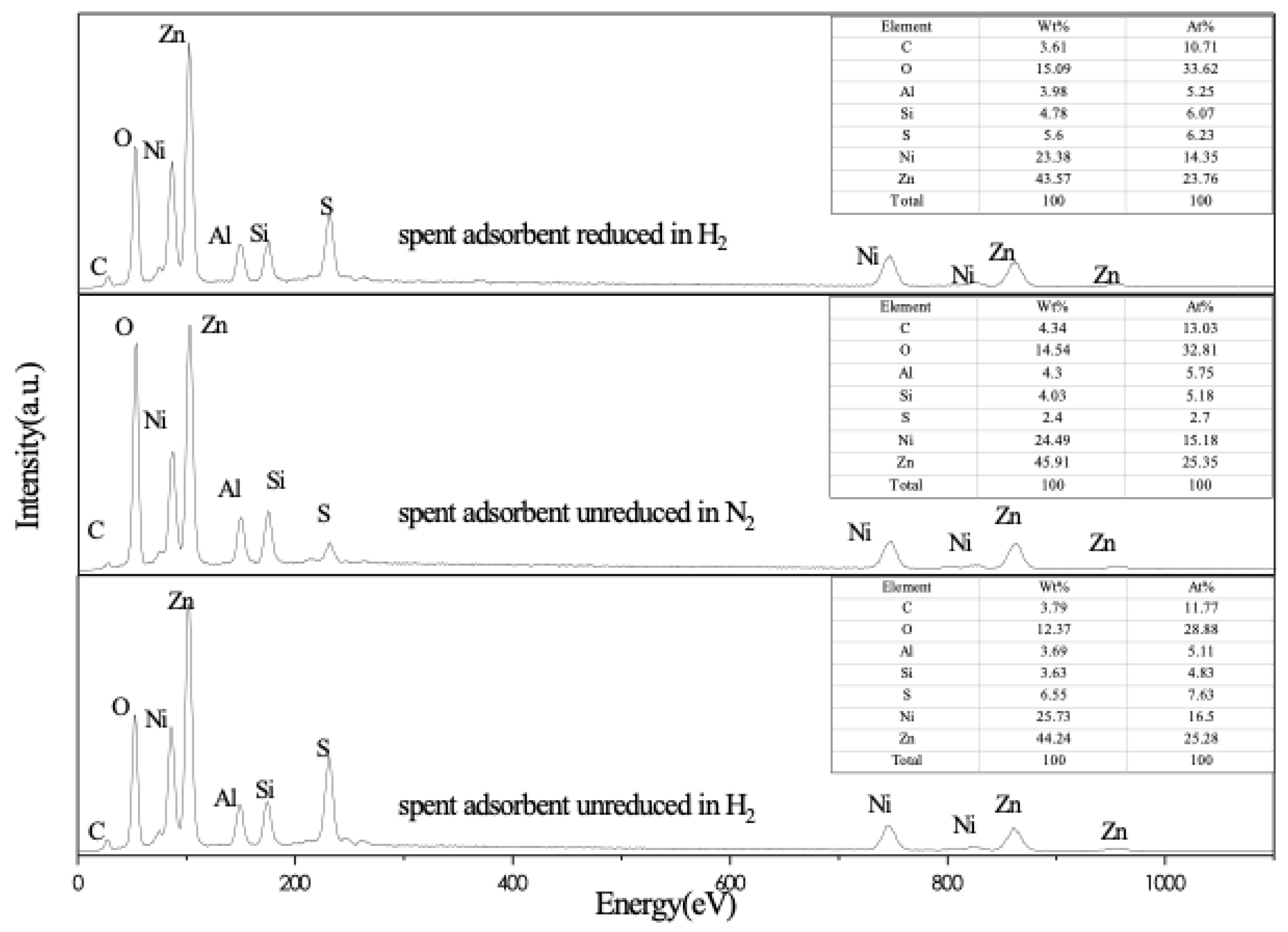
2.3. EDS Results
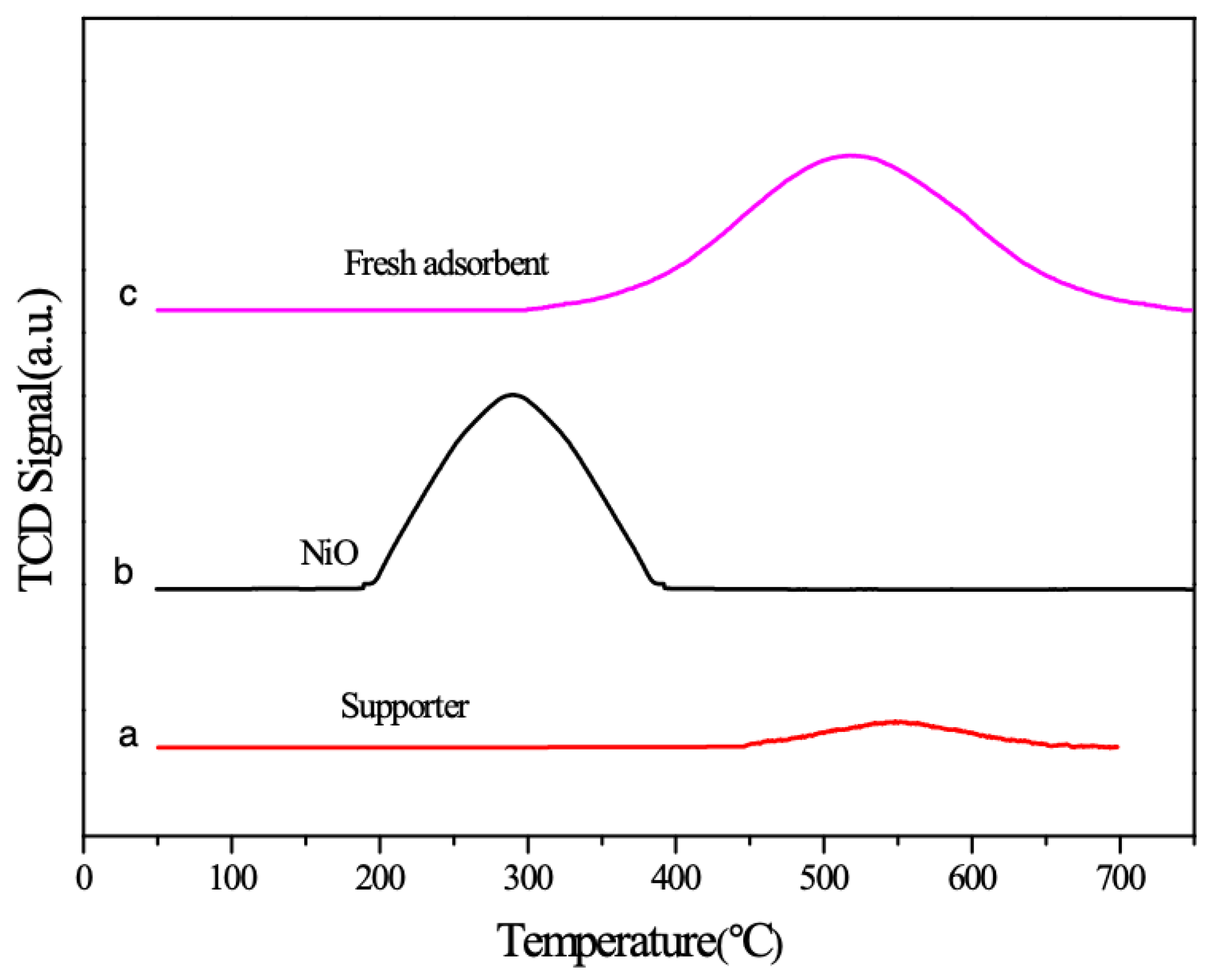
2.4. H2-Temperature Programmed Reduction (TPR) Characterization
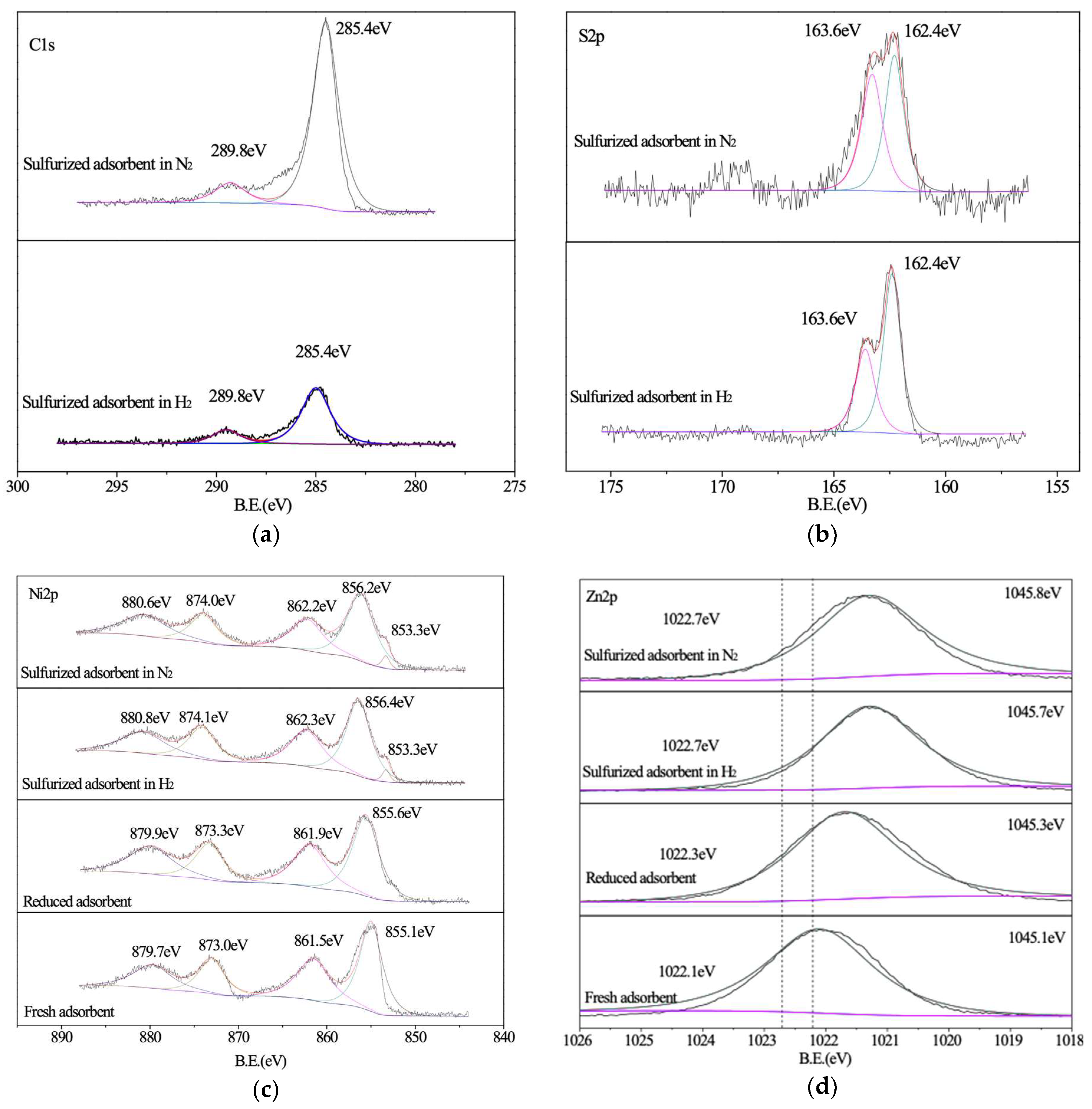
2.5. X-Ray Photoelectron Spectroscopy (XPS) Characterization
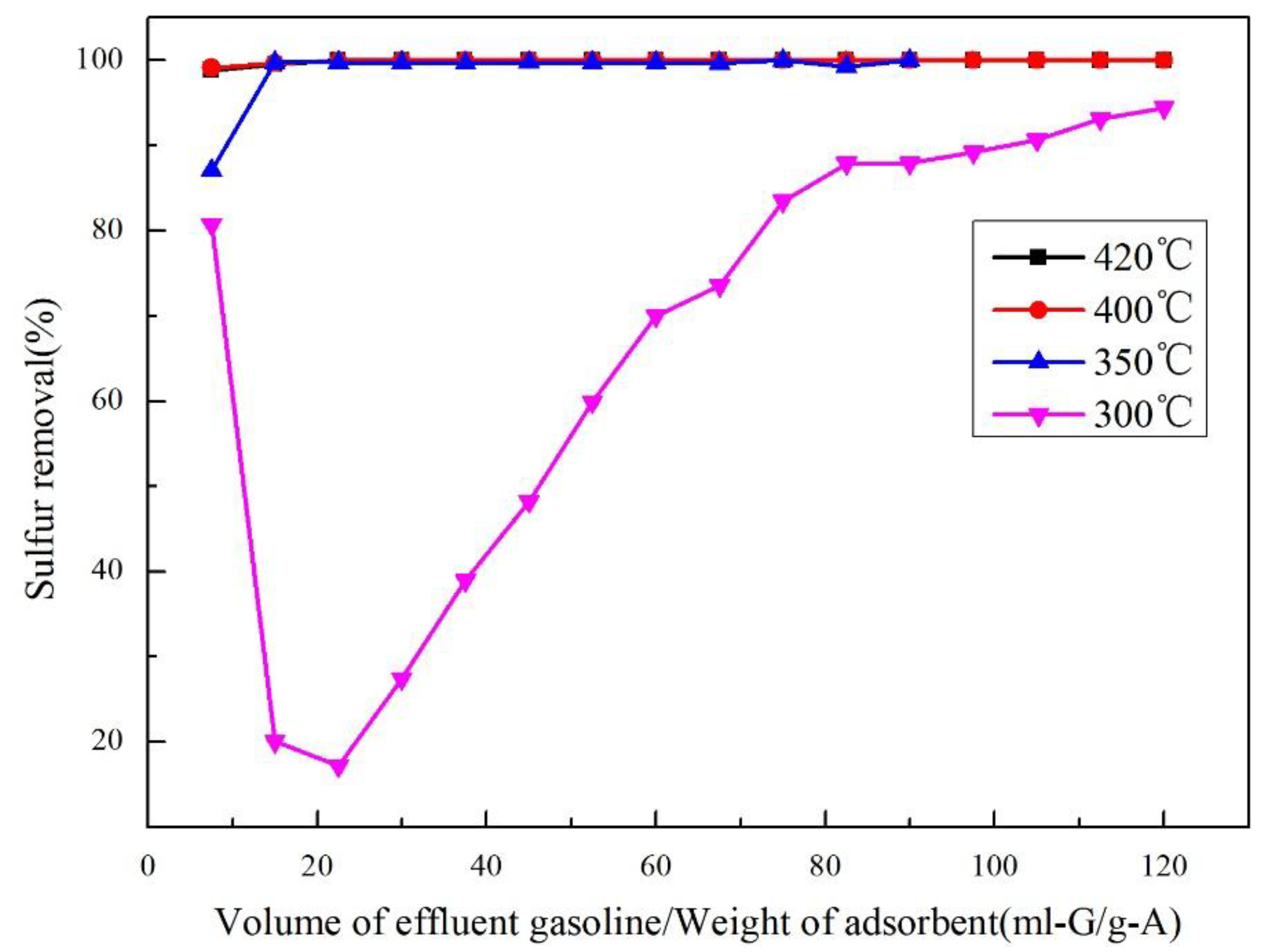
2.6. RADS Performance at Different Temperatures
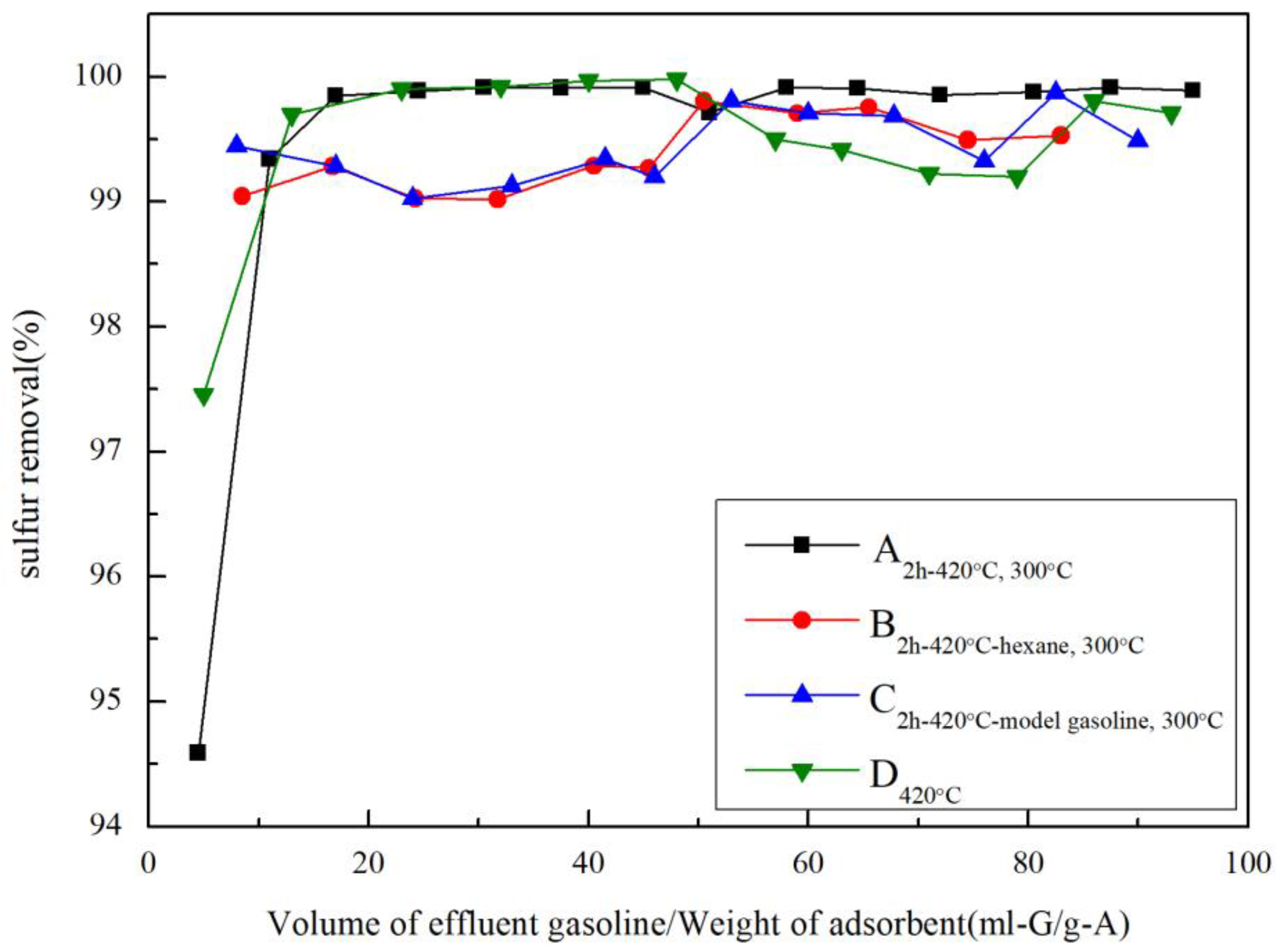
2.7. Effects of Pretreatments on RADS Performance
3. Materials and Methods
3.1. Adsorbent Preparation and Feedstock Properties
3.2. Characterization of Adsorbents
3.3. Sulfur Adsorption Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oyama, S.T.; Gott, T.; Zhao, H.; Lee, Y.K. Transition metal phosphide hydroprocessing catalysts: A review. Catal. Today 2009, 143, 94–107. [Google Scholar] [CrossRef]
- Kim, J.H.; Ma, X.; Zhou, A.; Song, C. Ultra-deep desulfurization and denitrogenation of diesel fuel by selective adsorption over three different adsorbents: A study on adsorptive selectivity and mechanism. Catal. Today 2006, 111, 74–83. [Google Scholar] [CrossRef]
- Jeon, H.J.; Chang, H.K.; Kim, S.H.; Kim, J.N. Removal of Refractory Sulfur Compounds in Diesel Using Activated Carbon with Controlled Porosity. Energy Fuels 2009, 23, 2537–2543. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Z.; Liu, B.; Xia, Q.; Yu, M. Adsorption of Benzothiophene and Dibenzothiophene on Ion-Impregnated Activated Carbons and Ion-Exchanged Y Zeolites. Energy Fuels 2008, 22, 3858–3863. [Google Scholar] [CrossRef]
- Khare, G.P. Desulfurization Process and Novel Bimetallic Sorbent Systems for Same. U.S. Patent 6,531,053, 11 March 2003. [Google Scholar]
- Khare, G.P. Process for the Production of a Sulfur Sorbent. U.S. Patent 6,184,176, 6 February 2001. [Google Scholar]
- Turaga, U.T.; Gislason, J.J. Desulfurization and Novel Compositions for Same. U.S. Patent 7,201,839, 10 April 2007. [Google Scholar]
- Xu, W.; Xiong, C.; Zhou, G.; Zhou, H. Removal of sulfur from FCC gasoline by using Ni/ZnO as adsorbent. Acta Pet. Sin. 2008, 24, 739–743. [Google Scholar] [CrossRef]
- Wang, T.; Wang, X.; Gao, Y.; Su, Y.; Miao, Z.; Wang, C.; Lu, L.; Chou, L.; Gao, X. Reactive adsorption desulfurization coupling aromatization on Ni/ZnO-Zn6Al2O9 prepared by ZnxAly(OH)2(CO3)z·xH2O precursor for FCC gasoline. J. Energy Chem. Res. 2015, 24, 503–511. [Google Scholar] [CrossRef]
- Fan, J.; Gang, W.; Yu, S.; Xu, C.; Zhou, H.; Zhou, G.; Gao, J. Research on Reactive Adsorption Desulfurization over Ni/ZnO-SiO2-Al2O3 Adsorbent in a Fixed-Fluidized Bed Reactor. Ind. Eng. Chem. Res. 2010, 49, 8450–8460. [Google Scholar] [CrossRef]
- Li, H.; Dong, L.; Zhao, L.; Xu, C. Enhanced Adsorption Desulfurization Performance over Mesoporous ZSM-5 by Alkali Treatment. Ind. Eng. Chem. Res. 2017, 56, 3813–3821. [Google Scholar] [CrossRef]
- Timko, M.T.; Wang, J.A.; Burgess, J.; Kracke, P.; Gonzalez, L.; Jayee, C.; Fischere, D.A. Roles of surface chemistry and structural defects of activated carbons in the oxidative desulfurization of benzothiophenes. Fuel 2016, 163, 223–231. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Zhang, Y.; Tang, M. Strong metal-support interactions between Ni and ZnO particles and their effect on the methanation performance of Ni/ZnO. Catal. Sci. Technol 2017, 7, 4413–4421. [Google Scholar] [CrossRef]
- Babich, I.V.; Moulijn, J.A. Science and technology of novel processes for deep desulfurization of oil refinery streams: a review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Huang, L.; Wang, G.; Qin, Z.; Dong, M.; Du, M.; Ge, H.; Li, X.; Zhao, Y.; Zhang, J.; Hu, T.; et al. In situ XAS study on the mechanism of reactive adsorption desulfurization of oil product over Ni/ZnO. Appl. Catal. B 2011, 106, 26–38. [Google Scholar] [CrossRef]
- Bezverkhyy, I.; Safonova, O.V.; Afanasiev, P.; Bellat, J.P. Reaction between Thiophene and Ni Nanoparticles Supported on SiO2 or ZnO: In Situ Synchrotron X-ray Diffraction Study. J. Phys. Chem. C 2009, 113. [Google Scholar] [CrossRef]
- Okamoto, H.J. Phase Equilib. Diffusion 2009, 30, 123. [Google Scholar]
- Ryzhikov, A.; Bezverkhyy, I.; Bellat, J.P. Reactive adsorption of thiophene on Ni/ZnO: Role of hydrogen pretreatment and nature of the rate determining step. Appl. Catal. B 2008, 84, 766–772. [Google Scholar] [CrossRef]
- Bezverkhyy, I.; Ryzhikov, A.; Gadacz, G.; Bellat, J.P. Kinetics of thiophene reactive adsorption on Ni/SiO2 and Ni/ZnO. Catal. Today 2008, 130, 199–205. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, X.; Cheng, Z. A comparative study on the ex situ and in situ presulfurization of hydrotreating catalysts. Catal. Today 2010, 158, 496–503. [Google Scholar] [CrossRef]
- Jin, F.Y.; Long, H.Y.; Song, W.C.; Xiong, G.; Guo, X.W.; Wang, X.S. Active Phase of a NiSO4 Catalyst Supported on γ-Al2O3 during in Situ Self-Sulfidation for Selective Hydrodesulfurization. Energy Fuels 2013, 27, 3394–3399. [Google Scholar] [CrossRef]
- Ju, F.; Liu, C.; Meng, C.; Gao, S.; Ling, H. Reactive Adsorption Desulfurization of Hydrotreated Diesel over a Ni/ZnO-Al2O3-SiO2 Adsorbent. Energy Fuels 2015, 29, 6057–6067. [Google Scholar] [CrossRef]
- Ju, F.; Liu, C.; Li, K.; Meng, C.; Gao, S.; Ling, H. Reactive adsorption desulfurization of FCC gasoline over a Ca-Doped Ni-ZnO/Al2O3-SiO2 adsorbent. Energy Fuels 2016, 30, 6688–6697. [Google Scholar] [CrossRef]
- Ju, F.; Wang, M.; Luan, H.; Du, P.; Tang, Z.; Ling, H. Reactive adsorption desulfurization of NiO and Ni0 over NiO/ZnO-Al2O3-SiO2 adsorbents: role of hydrogen pretreatment. RSC Adv. 2018, 8, 33354–33360. [Google Scholar] [CrossRef]
- Rahbar, S.F.; Meshkani, F.; Rezaei, M. Ultrasound assisted co-precipitation synthesis and catalytic performance of mesoporous nanocrystalline NiO-Al2O3 powders. Ultrason. Sonochem. 2017, 34, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, J. Morphology of nickel/alumina catalysts. J. Catal. 1982, 76, 157–163. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. Reliable determination of chemical state in x-ray photoelectron spectroscopy based on sample-work-function referencing to adventitious carbon: Resolving the myth of apparent constant binding energy of the C 1s peak. Appl. Surf. Sci. 2018, 451, 99–103. [Google Scholar] [CrossRef]
- Herron, S.M.; Lawal, Q.O.; Bent, S.F. Polysulfide ligand exchange on zinc sulfide nanocrystal surfaces for improved film formation. Appl. Surf. Sci. 2015, 359, 106–113. [Google Scholar] [CrossRef]
- Smart, R.S.C.; Skinner, W.M.; Gerson, A.R. Xps of sulphide mineral surfaces: metal-deficient, polysulphides, defects and elemental sulphur. Surf. Interface Anal. 1999, 28, 101–105. [Google Scholar] [CrossRef]
- Ertl, G.; Hierl, R.; Knözinger, H.; Thiele, N.; Urbach, H.P. XPS study of copper aluminate catalysts. Appl. Surf. Sci. 1980, 5, 49–64. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Legrand, D.; Bancroft, G.M. Interpretation of Ni2p XPS spectra of Ni conductors and Ni insulators. Phys. Chem. Miner. 2000, 27, 357–366. [Google Scholar] [CrossRef]
- Seader, J.D.; Henley, E.J. Separation Process Principles; Wiley: New York, NY, USA, 1998. [Google Scholar]


| Reduction | Temp. /°C | 440 | |
| Hydrogen pressure/MPa | 2.0 | ||
| Reduction time/h | 2 | ||
| Adsoption desulfurization | In H2 | Temp. /°C | 300, 350, 400, 420 |
| Hydrogen pressure/MPa | 2.9 | ||
| Weight hourly space velocity (WHSV)/h−1 | 9.9 | ||
| H2 volume/Gasoline weight (mL/g) | 70 | ||
| In N2 | Temp. /°C | 420 | |
| Nitrogen pressure/MPa | 2.9 | ||
| Weight hourly space velocity (WHSV)/h−1 | 9.9 | ||
| N2 volume/Gasoline weight (mL/g) | 70 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, F.; Wang, M.; Wu, T.; Ling, H. The Role of NiO in Reactive Adsorption Desulfurization Over NiO/ZnO-Al2O3-SiO2 Adsorbent. Catalysts 2019, 9, 79. https://doi.org/10.3390/catal9010079
Ju F, Wang M, Wu T, Ling H. The Role of NiO in Reactive Adsorption Desulfurization Over NiO/ZnO-Al2O3-SiO2 Adsorbent. Catalysts. 2019; 9(1):79. https://doi.org/10.3390/catal9010079
Chicago/Turabian StyleJu, Feng, Miao Wang, Tian Wu, and Hao Ling. 2019. "The Role of NiO in Reactive Adsorption Desulfurization Over NiO/ZnO-Al2O3-SiO2 Adsorbent" Catalysts 9, no. 1: 79. https://doi.org/10.3390/catal9010079
APA StyleJu, F., Wang, M., Wu, T., & Ling, H. (2019). The Role of NiO in Reactive Adsorption Desulfurization Over NiO/ZnO-Al2O3-SiO2 Adsorbent. Catalysts, 9(1), 79. https://doi.org/10.3390/catal9010079





