A Comparative Study of MFI Zeolite Derived from Different Silica Sources: Synthesis, Characterization and Catalytic Performance
Abstract
1. Introduction
2. Results and Discussion
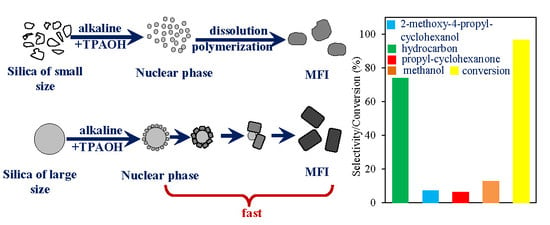
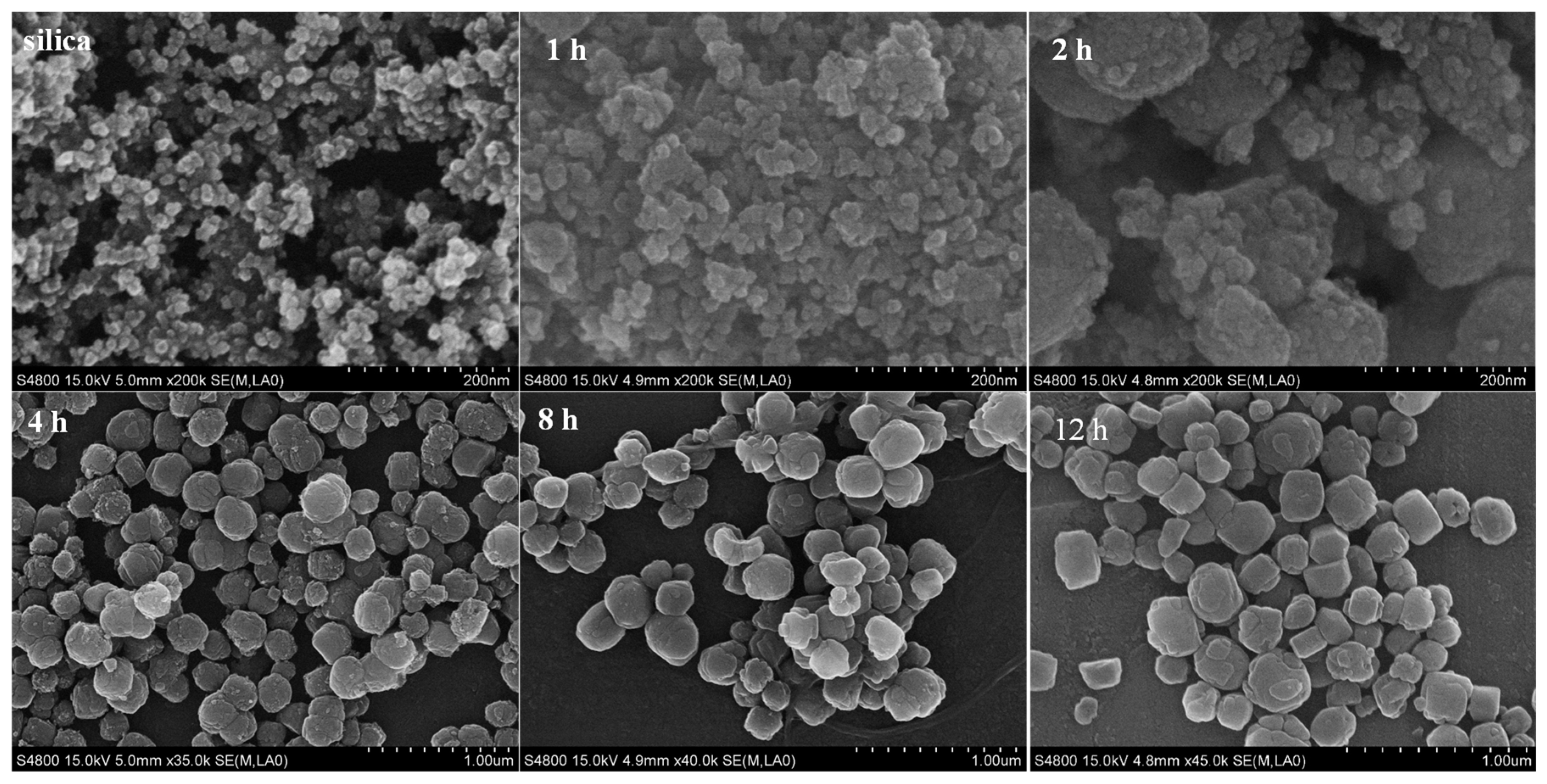
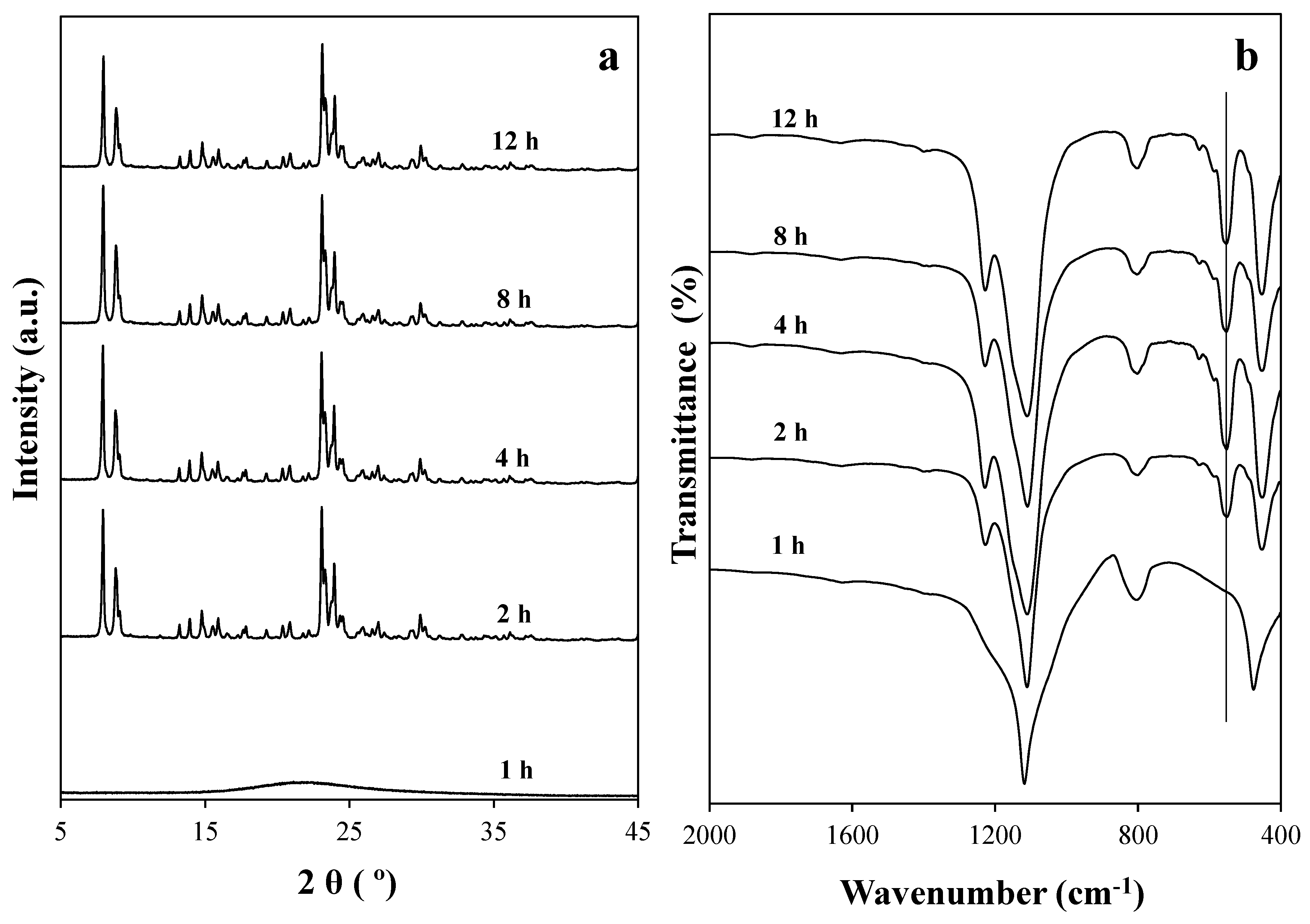
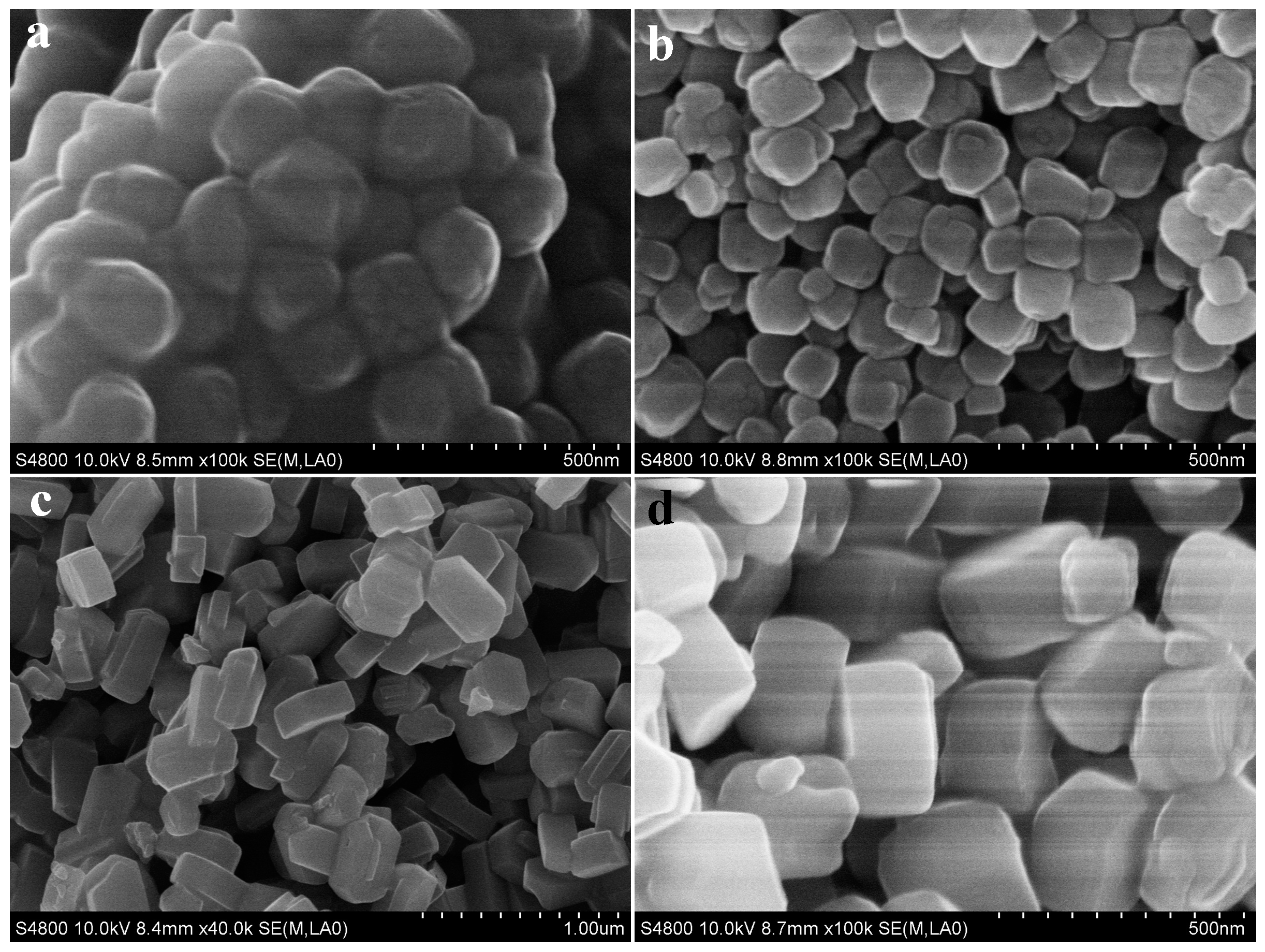
2.1. The Effect of Silica Sources and Synthesis Time on the Preparation of Silicalite-1
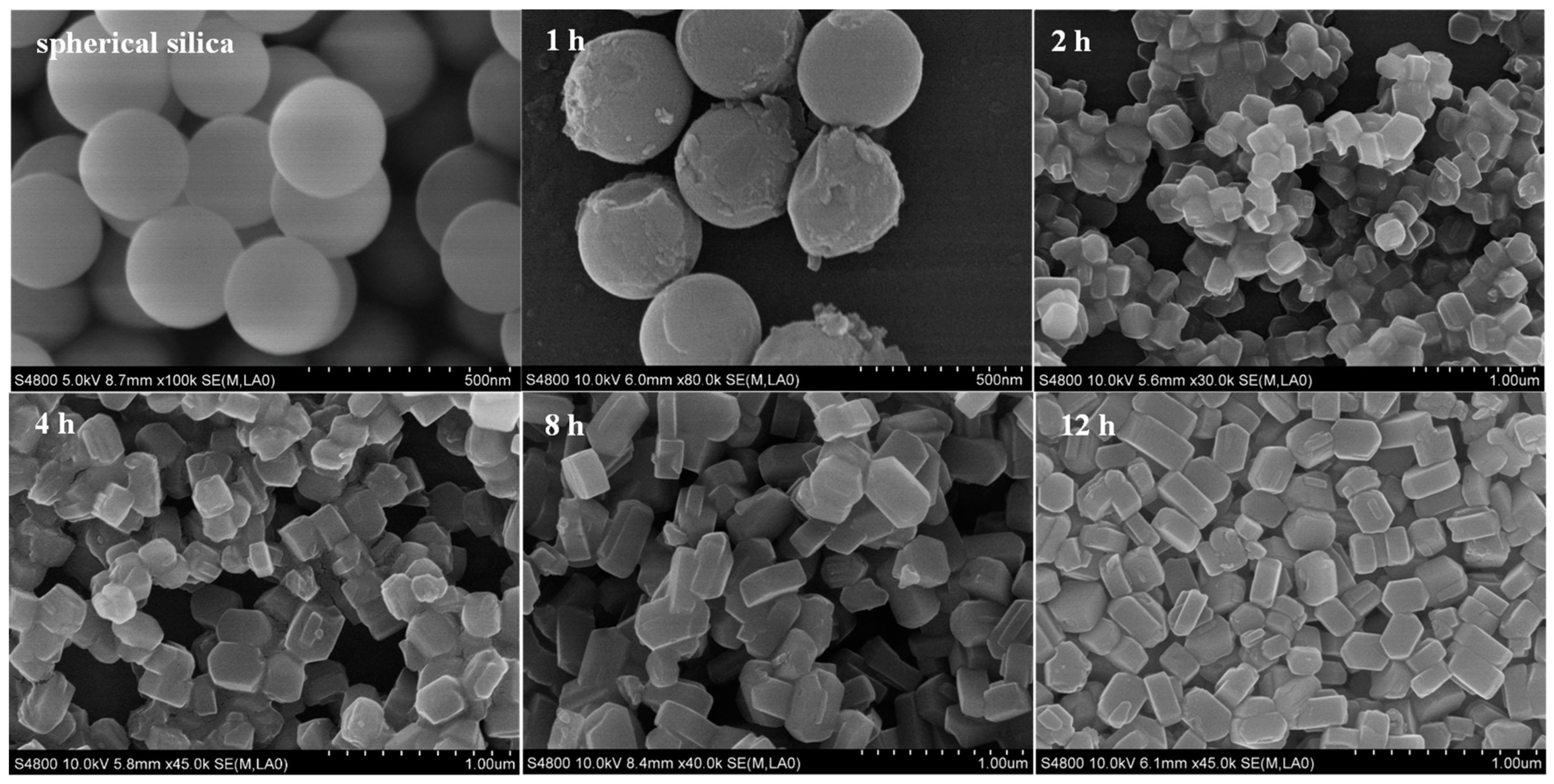
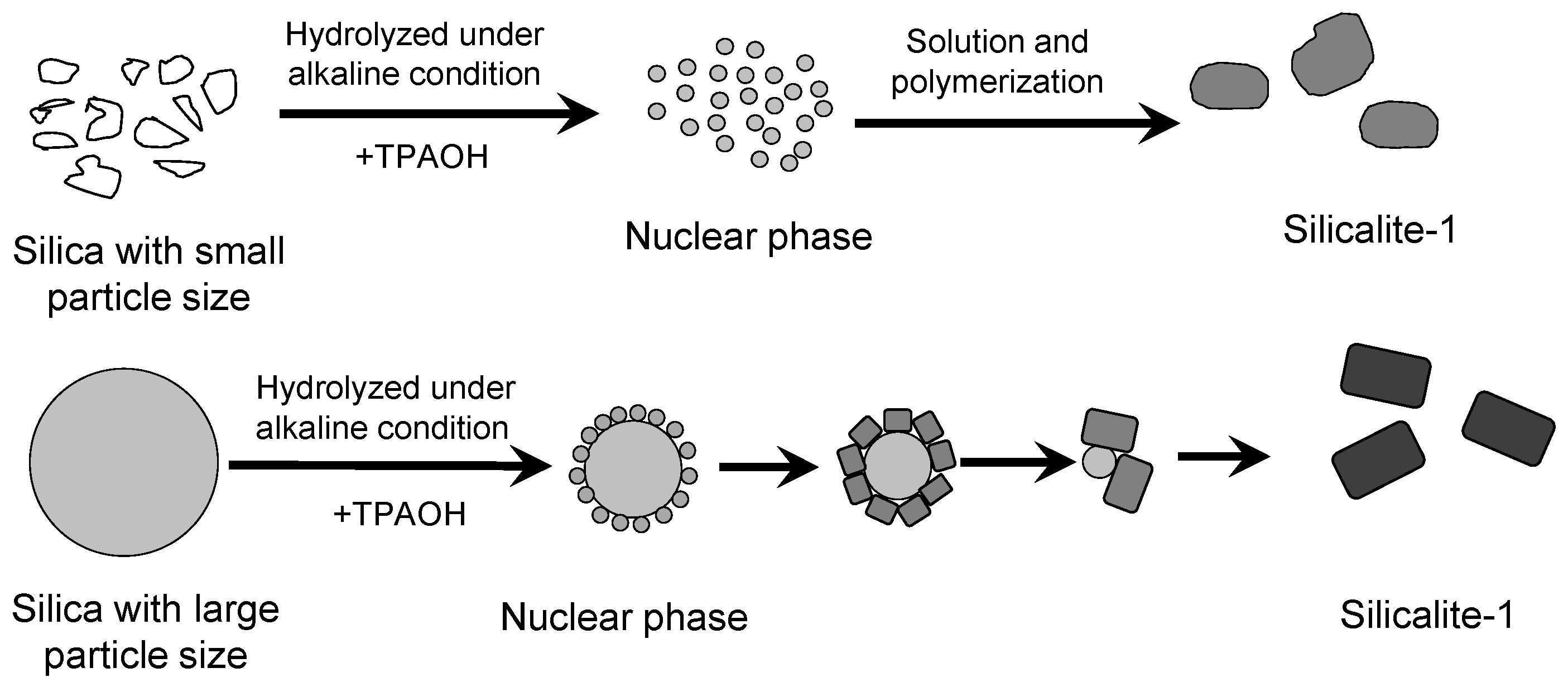
2.2. The Effect of Spherical Silica Particle Size on Synthesis of Silicalite-1
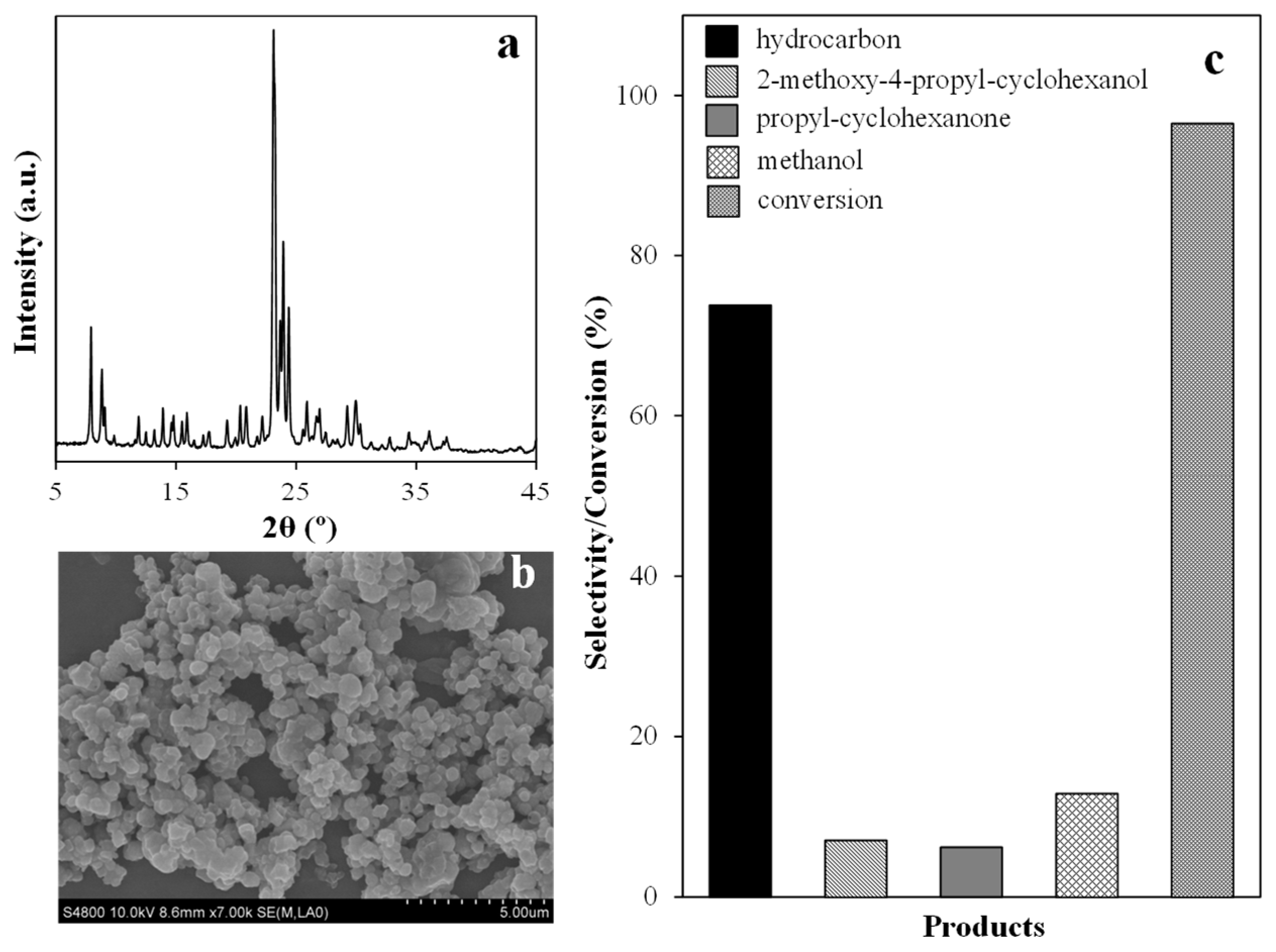
2.3. Synthesis of ZSM-5 and Catalytic Performance
3. Experimental
3.1. Chemicals
3.2. Synthesis Process
3.3. Characterization
3.4. HDO of Eugenol
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhou, W.; Zhang, S.Y.; Hao, X.Y.; Guo, H.; Zhang, C.; Zhang, Y.Q.; Liu, S.X. MFI-type boroaluminosilicate: A comparative study between the direct synthesis and the templating method. J. Solid State Chem. 2006, 179, 855–865. [Google Scholar] [CrossRef]
- Sarmah, B.; Satpati, B.; Srivastava, R. Highly efficient and recyclable basic mesoporous zeolite catalyzed condensation, hydroxylation, and cycloaddition reactions. J. Colloid. Interface Sci. 2017, 493, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Akhmetzyanova, U.; Opanasenko, M.; Horacek, J.; Montanari, E.; Cejka, J.; Kikhtyanin, O. Zeolite supported palladium catalysts for hydroalkylation of phenolic model compounds. Microporous Mesoporous Mater. 2017, 252, 116–124. [Google Scholar] [CrossRef]
- Silva, A.F.; Fernandes, A.; Antunes, M.M.; Neves, P.; Rocha, S.M.; Ribeiro, M.F.; Pillinger, M.; Ribeiro, J.; Silva, C.M.; Valente, A.A. TUD-1 type aluminosilicate acid catalysts for 1-butene oligomerisation. Fuel 2017, 209, 371–382. [Google Scholar] [CrossRef]
- Grenev, I.V.; Gavrilov, V.Y. Calculation of adsorption properties of aluminophosphate and aluminosilicate zeolites. Adsorption 2017, 23, 903–915. [Google Scholar] [CrossRef]
- Moreno-Recio, M.; Jimenez-Morales, I.; Arias, P.L.; Santamaria-Gonzalez, J.; Maireles-Torres, P. The Key Role of Textural Properties of Aluminosilicates in the Acid-Catalysed Dehydration of Glucose into 5-Hydroxymethylfurfural. Chemistryselect 2017, 2, 2444–2451. [Google Scholar] [CrossRef]
- Rillig, M.C.; Wagner, M.; Salem, M.; Antunes, P.M.; George, C.; Ramke, H.G.; Titirici, M.M.; Antonietti, M. Material derived from hydrothermal carbonization: Effects on plant growth and arbuscular mycorrhiza. Appl. Soil Ecolog. 2010, 45, 238–242. [Google Scholar] [CrossRef]
- Al-Jubouri, S.M.; Holmes, S.M. Hierarchically porous zeolite X composites for manganese ion-exchange and solidification: Equilibrium isotherms, kinetic and thermodynamic studies. Chem. Eng. J. 2017, 308, 476–491. [Google Scholar] [CrossRef]
- Losch, P.; Pinar, A.B.; Willinger, M.G.; Soukup, K.; Chavan, S.; Vincent, B.; Pale, P.; Louis, B. H-ZSM-5 zeolite model crystals: Structure-diffusion-activity relationship in methanol-to-olefins catalysis. J. Catal. 2017, 345, 11–23. [Google Scholar] [CrossRef]
- Hernandez-Tamargo, C.E.; Roldan, A.; de Leeuw, N.H. A density functional theory study of the structure of pure-silica and aluminium-substituted MFI nanosheets. J. Solid State Chem. 2016, 237, 192–203. [Google Scholar] [CrossRef]
- Barakov, R.; Shcherban, N.; Yaremov, P.; Solomakha, V.; Vyshnevskyy, A.; Ilyin, V. Low-temperature synthesis, structure, sorption properties and acidity of zeolite ZSM-5. J. Porous Mater. 2016, 23, 517–528. [Google Scholar] [CrossRef]
- Mi, S.; Wei, T.; Sun, J.; Liu, P.; Li, X.; Zheng, Q.; Gong, K.; Liu, X.; Gao, X.; Wang, B.; Zhao, H.; Liu, H.; Shen, B. Catalytic function of boron to creating interconnected mesoporosity in microporous Y zeolites and its high performance in hydrocarbon cracking. J. Catal. 2017, 347, 116–126. [Google Scholar] [CrossRef]
- Chung, K.H.; Park, H.; Jeon, K.J.; Park, Y.K.; Jung, S.C. Microporous Zeolites as Catalysts for the Preparation of Decyl Glucoside from Glucose with 1-Decanol by Direct Glucosidation. Catalysts 2016, 6, 216. [Google Scholar] [CrossRef]
- Menoufy, M.F.; Nadia, A.E.; Ahmed, H.S. Catalytic Dewaxing for Lube Oil Production. Petrol. Sci. Technol. 2009, 27, 568–574. [Google Scholar] [CrossRef]
- Xin, H.; Li, X.; Fang, Y.; Yi, X.; Hu, W.; Chu, Y.; Zhang, F.; Zheng, A.; Zhang, H.; Li, X. Catalytic dehydration of ethanol over post-treated ZSM-5 zeolites. J. Catal. 2014, 312, 204–215. [Google Scholar] [CrossRef]
- Li, X.; Xing, J.; Zhou, M.; Zhang, H.; Huang, H.; Zhang, C.; Song, L.; Li, X. Influence of crystal size of HZSM-5 on hydrodeoxygenation of eugenol in aqueous phase. Catal. Commun. 2014, 56, 123–127. [Google Scholar] [CrossRef]
- Xie, J.; Zhuang, W.; Wei, Z.; Ning, Y.; Yu, Z.; Ju, W. Construction of Acid-Base Synergetic Sites on Mg-bearing BEA Zeolites Triggers the Unexpected Low-Temperature Alkylation of Phenol. Chemcatchem 2017, 9, 1076–1083. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Wang, S. Hydrodeoxygenation of bio-oil over Pt-based supported catalysts: Importance of mesopores and acidity of the support to compounds with different oxygen contents. Rsc Adv. 2013, 3, 12635–12640. [Google Scholar] [CrossRef]
- Beiragh, H.H.; Omidkhah, M.; Abedini, R.; Khosravi, T.; Pakseresht, S. Synthesis and characterization of poly (ether-block-amide) mixed matrix membranes incorporated by nanoporous ZSM-5 particles for CO2/CH4 separation. Asia-Pac. J. Chem. Eng. 2016, 11, 522–532. [Google Scholar] [CrossRef]
- Lakhane, M.; Khairnar, R.; Mahabole, M. Metal oxide blended ZSM-5 nanocomposites as ethanol sensors. Bull. Mater. Sci. 2016, 39, 1483–1492. [Google Scholar] [CrossRef]
- Pande, H.B.; Parikh, P.A. Novel Application of ZSM-5 Zeolite: Corrosion-Resistant Coating in Chemical Process Industry. J. Mater. Eng. Perform. 2013, 22, 190–199. [Google Scholar] [CrossRef]
- McDonnell, A.M.P.; Beving, D.; Wang, A.J.; Chen, W.; Yan, Y.S. Hydrophilic and antimicrobial zeolite coatings for gravity-independent water separation. Adv. Funct. Mater. 2005, 15, 336–340. [Google Scholar] [CrossRef]
- Jiao, K.; Xu, X.; Lv, Z.; Song, J.; He, M.; Gies, H. Synthesis of nanosized Silicalite-1 in F- media. Microporous Mesoporous Mater. 2016, 225, 98–104. [Google Scholar] [CrossRef]
- Mohammed, M.A.A.; Salmiaton, A.; Azlina, W.A.K.G.W.; Amran, M.S.M.; Fakhru’l-Razi, A.; Taufiq-Yap, Y.H. Hydrogen rich gas from oil palm biomass as a potential source of renewable energy in Malaysia. Renew. Sustain. Energy Rev. 2011, 15, 1258–1270. [Google Scholar] [CrossRef]
- Cimenler, U.; Joseph, B.; Kuhn, J.N. Hydrocarbon steam reforming using Silicalite-1 zeolite encapsulated Ni-based catalyst. Aiche J. 2017, 63, 200–207. [Google Scholar] [CrossRef]
- Ge, C.; Li, Z.; Chen, G.; Qin, Z.; Li, X.; Dou, T.; Dong, M.; Chen, J.; Wang, J.; Fan, W. Kinetic study of vapor-phase Beckmann rearrangement of cyclohexanone oxime over silicalite-1. Chem. Eng. Sci. 2016, 153, 246–254. [Google Scholar] [CrossRef]
- Kabalan, I.; Rioland, G.; Nouali, H.; Lebeau, B.; Rigolet, S.; Fadlallah, M.B.; Toufaily, J.; Hamiyeh, T.; Daou, T.J. Synthesis of purely silica MFI-type nanosheets for molecular decontamination. Rsc Adv. 2014, 4, 37353–37358. [Google Scholar] [CrossRef]
- Sanhoob, M.A.; Muraza, O. Synthesis of silicalite-1 using fluoride media under microwave irradiation. Microporous Mesoporous Mater. 2016, 233, 140–147. [Google Scholar] [CrossRef]
- Han, S.W.; Kim, J.; Ryoo, R. Dry-gel synthesis of mesoporous MFI zeolite nanosponges using a structure-directing surfactant. Microporous Mesoporous Mater. 2017, 240, 123–129. [Google Scholar] [CrossRef]
- Meng, X.; Xiao, F.S. Green Routes for Synthesis of Zeolites. Chem. Rev. 2014, 114, 1521–1543. [Google Scholar] [CrossRef]
- Mintova, S.; Valtchev, V. Effect of the silica source on the formation of nanosized silicalite-1: An in situ dynamic light scattering study. Microporous Mesoporous Mater. 2002, 55, 171–179. [Google Scholar] [CrossRef]
- Pan, F.; Lu, X.; Zhu, Q.; Zhang, Z.; Yan, Y.; Wang, T.; Chen, S. Direct synthesis of HZSM-5 from natural clay. J. Mater. Chem. A 2015, 3, 4058–4066. [Google Scholar] [CrossRef]
- Jesudoss, S.K.; Vijaya, J.J.; Kaviyarasu, K.; Rajan, P.I.; Narayanan, S.; Kennedy, L.J. In vitro anti-cancer activity of organic template-free hierarchical M (Cu, Ni)-modified ZSM-5 zeolites synthesized using silica source waste material. J. Photochem. Photobiol. B. 2018, 186, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Khoshbin, R.; Oruji, S.; Karimzadeh, R. Catalytic cracking of light naphtha over hierarchical ZSM-5 using rice husk ash as silica source in presence of ultrasound energy: Effect of carbon nanotube content. Adv. Powder Technol. 2018, 29, 2176–2187. [Google Scholar] [CrossRef]
- Saleh, N.J.; Al-Zaidi, B.Y.S.; Sabbar, Z.M. A Comparative Study of Y Zeolite Catalysts Derived from Natural and Commercial Silica: Synthesis, Characterization, and Catalytic Performance. Arab. J. Sci. Eng. 2018, 43, 5819–5836. [Google Scholar] [CrossRef]
- Bai, P.; Tsapatsis, M.; Siepmann, J.I. Multicomponent Adsorption of Alcohols onto Silicalite-1 from Aqueous Solution: Isotherms, Structural Analysis, and Assessment of Ideal Adsorbed Solution Theory. Langmuir 2012, 28, 15566–15576. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhao, T.; Xu, X.; Li, F.; Sun, G. Hydrothermal synthesis of size-controlled silicalite-1 crystals. J. Porous Mater. 2011, 18, 509–515. [Google Scholar] [CrossRef]
- Sanchez-Flores, N.A.; Solache, M.; Olguin, M.T.; Legaspe, J.; Pacheco-Malagon, G.; Saniger, J.M.; Martinez, E.; Bulbulian, S.; Fripiat, J.J. Silicalite-1, an adsorbent for 2-, 3-, and 4-chlorophenols. Water Sci. Technol. 2012, 66, 247–253. [Google Scholar] [CrossRef]
- Shi, L.; Song, X.; Liu, G.; Guo, H. Effect of Catalyst Preparation on Hydroisomerization of n-Heptane over Pt/Silicalite-1. Catal. Lett. 2017, 147, 2549–2557. [Google Scholar] [CrossRef]
- Amiri, H.; Charkhi, A.; Moosavian, M.A.; Ahmadi, S.J.; Nourian, H. Performance improvement of PDMS/PES membrane by adding silicalite-1 nanoparticles: Separation of xenon and krypton. Chem. Pap. 2017, 71, 1587–1596. [Google Scholar] [CrossRef]
- Ding, X.; Chen, F.; Ju, Y.; Lu, S. Simulation and Thermodynamic Analysis of the Adsorption of Mixed CH4 and N-2 on Silicalite-1 Molecular Sieve. J. Nanosci. Nanotechnol. 2017, 17, 6732–6737. [Google Scholar] [CrossRef]
- Moreira, R.; Ochoa, E.; Pinilla, J.L.; Portugal, A.; Suelves, I. Liquid-Phase Hydrodeoxygenation of Guaiacol over Mo2C Supported on Commercial CNF. Effects of Operating Conditions on Conversion and Product Selectivity. Catalysts 2018, 8, 127. [Google Scholar] [CrossRef]
- Barot, S.; Nawab, M.; Bandyopadhyay, R. Alkali metal modified nano-silicalite-1: An efficient catalyst for transesterification of triacetin. J. Porous Mater. 2016, 23, 1197–1205. [Google Scholar] [CrossRef]
- Xue, T.; Wang, Y.M.; He, M.Y. Facile synthesis of nano-sized NH4-ZSM-5 zeolites. Microporous Mesoporous Mater. 2012, 156, 29–35. [Google Scholar] [CrossRef]
- Xin, H.; Koekkoek, A.; Yang, Q.; van Santen, R.; Li, C.; Hensen, E.J.M. A hierarchical Fe/ZSM-5 zeolite with superior catalytic performance for benzene hydroxylation to phenol. Chem. Commun. 2009, 7590–7592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xing, J.; Song, L.; Xin, H.; Lin, S.; Xing, L.; Li, X. Aqueous-phase hydrodeoxygenation of lignin monomer eugenol: Influence of Si/Al ratio of HZSM-5 on catalytic performances. Catal. Today 2014, 234, 145–152. [Google Scholar] [CrossRef]
- Xing, J.; Song, L.; Zhang, C.; Zhou, M.; Yue, L.; Li, X. Effect of acidity and porosity of alkali-treated ZSM-5 zeolite on eugenol hydrodeoxygenation. Catal. Today 2015, 258, 90–95. [Google Scholar] [CrossRef]
- Koekkoek, A.J.J.; Xin, H.; Yang, Q.; Li, C.; Hensen, E.J.M. Hierarchically structured Fe/ZSM-5 as catalysts for the oxidation of benzene to phenol. Microporous Mesoporous Mater. 2011, 145, 172–181. [Google Scholar] [CrossRef]



| Time | 200 m2/g SiO2 | 380 m2/g SiO2 | 300 nm SiO2 Spherical Silica | |||
|---|---|---|---|---|---|---|
| IR | XRD | IR | XRD | IR | XRD | |
| 1 h | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2 h | 7 | 16 | 47 | 17 | 71 | 100 |
| 4 h | 83 | 100 | 90 | 74 | 73 | 99 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, X.; Liu, J.; Wang, C. A Comparative Study of MFI Zeolite Derived from Different Silica Sources: Synthesis, Characterization and Catalytic Performance. Catalysts 2019, 9, 13. https://doi.org/10.3390/catal9010013
Zhang J, Li X, Liu J, Wang C. A Comparative Study of MFI Zeolite Derived from Different Silica Sources: Synthesis, Characterization and Catalytic Performance. Catalysts. 2019; 9(1):13. https://doi.org/10.3390/catal9010013
Chicago/Turabian StyleZhang, Jianguang, Xiangping Li, Juping Liu, and Chuanbin Wang. 2019. "A Comparative Study of MFI Zeolite Derived from Different Silica Sources: Synthesis, Characterization and Catalytic Performance" Catalysts 9, no. 1: 13. https://doi.org/10.3390/catal9010013
APA StyleZhang, J., Li, X., Liu, J., & Wang, C. (2019). A Comparative Study of MFI Zeolite Derived from Different Silica Sources: Synthesis, Characterization and Catalytic Performance. Catalysts, 9(1), 13. https://doi.org/10.3390/catal9010013