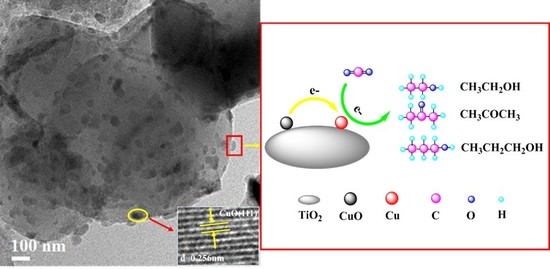
CuO Nanoparticles Supported on TiO2 with High Efficiency for CO2 Electrochemical Reduction to Ethanol
Abstract
:1. Introduction
2. Results and Discussion
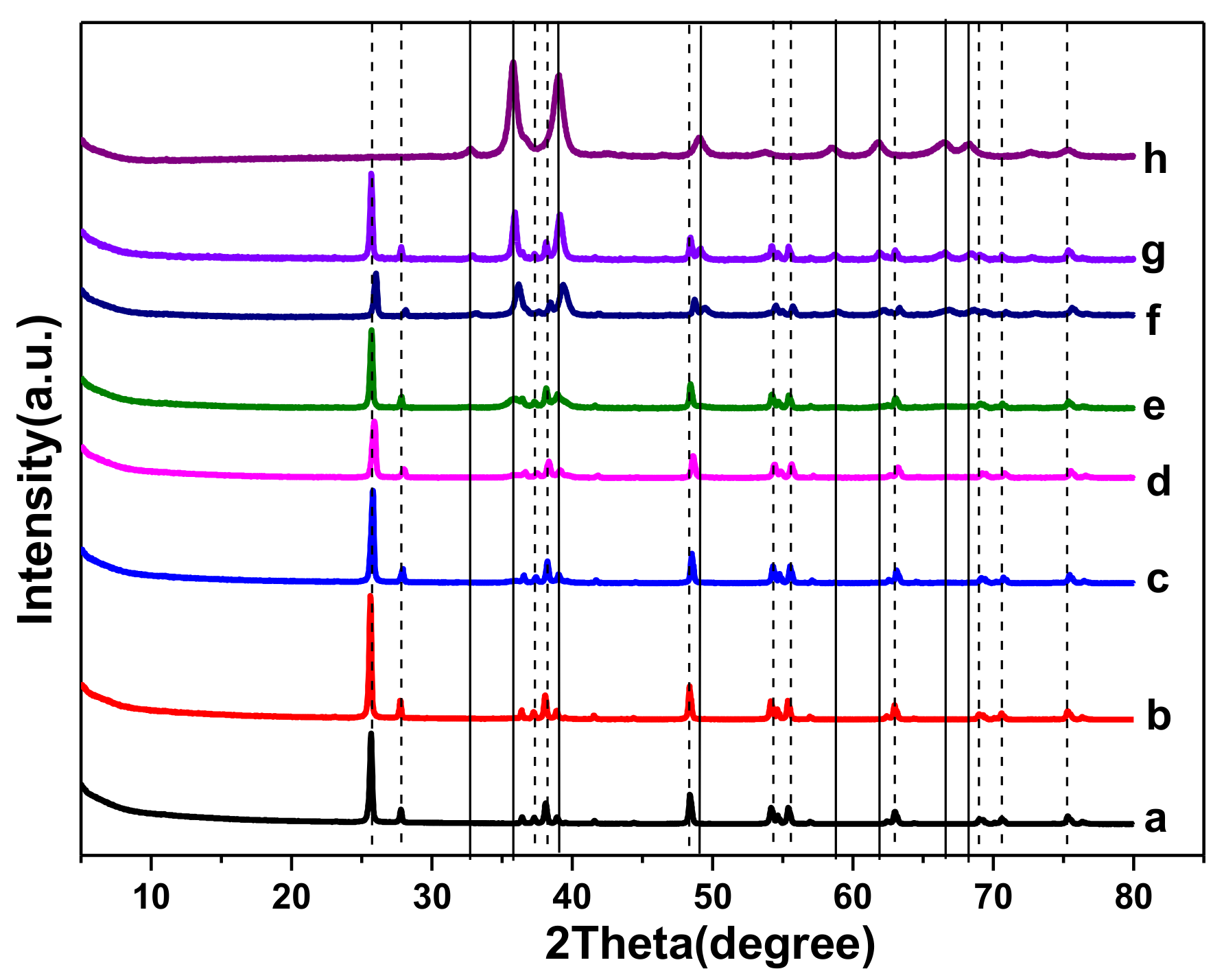
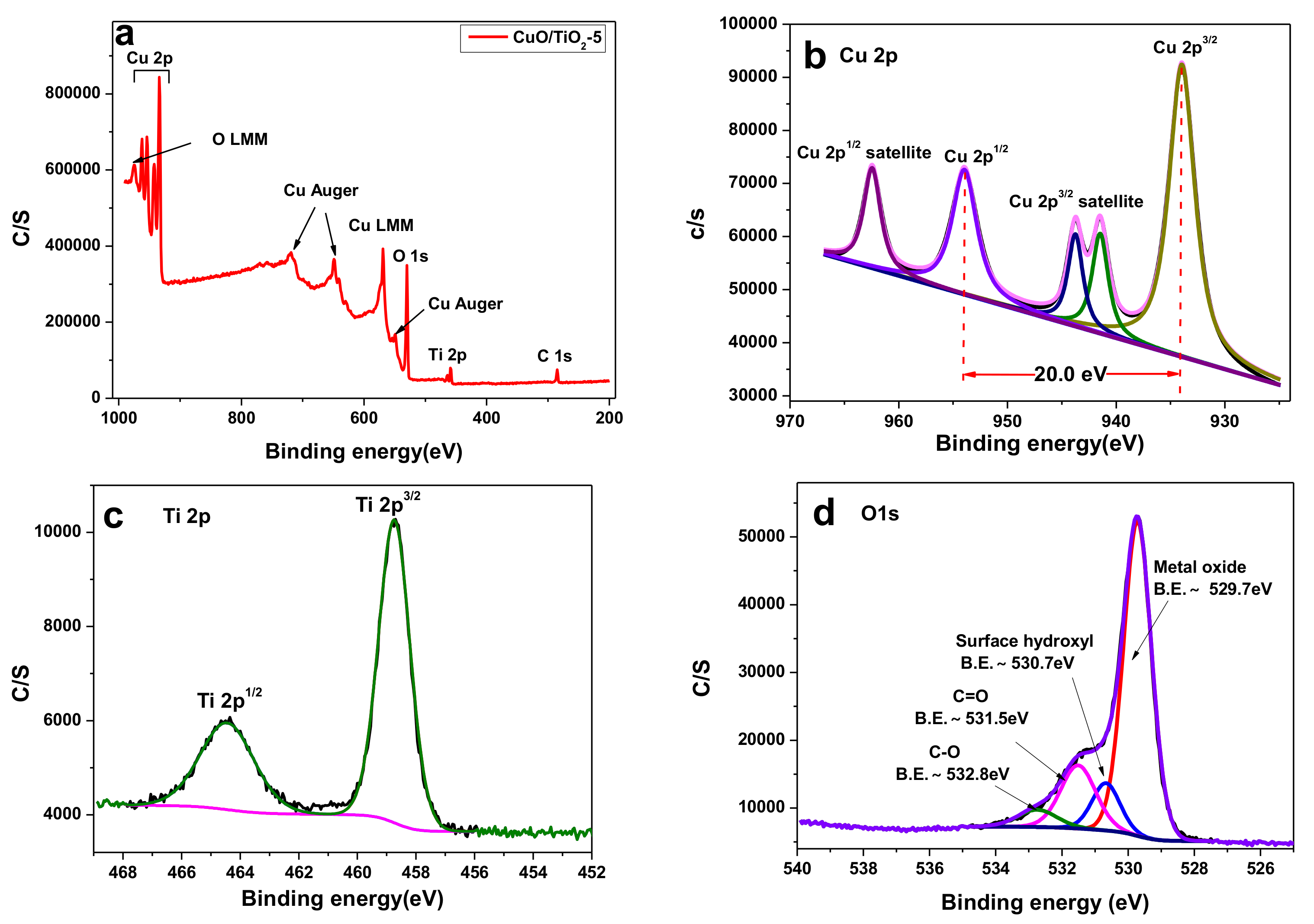
2.1. Catalyst Characterizations
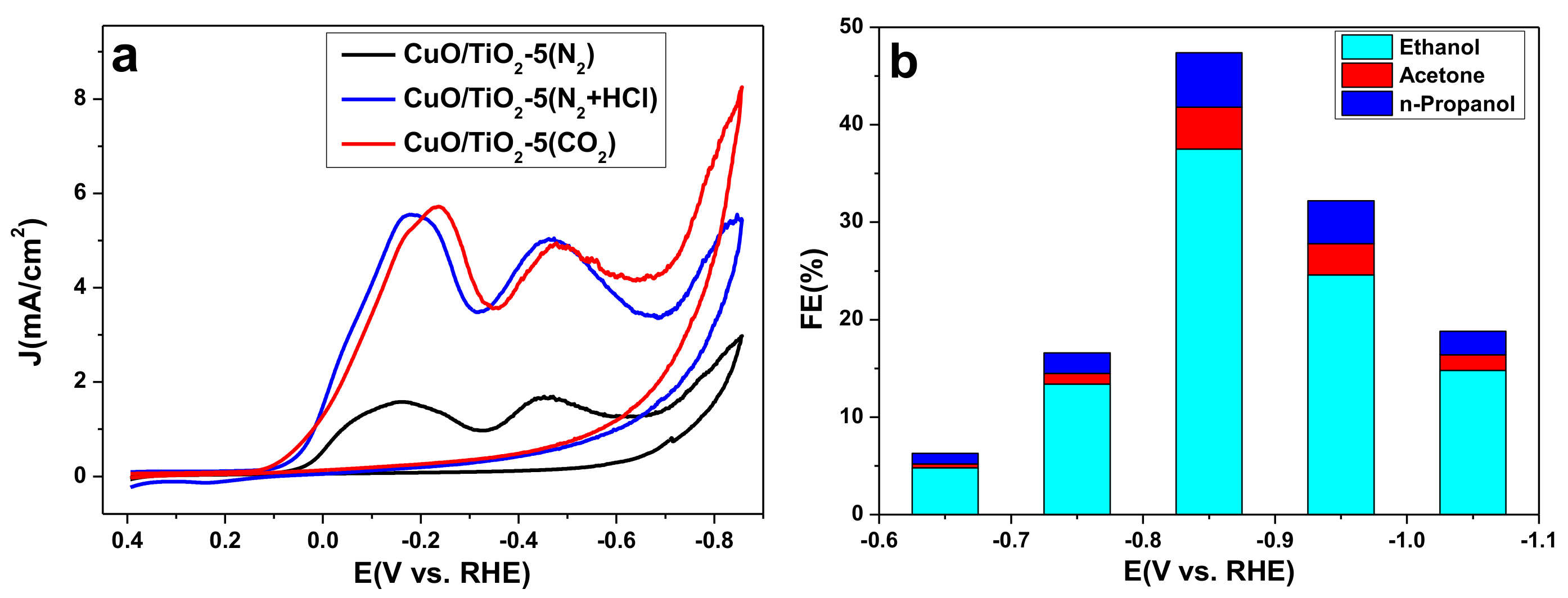
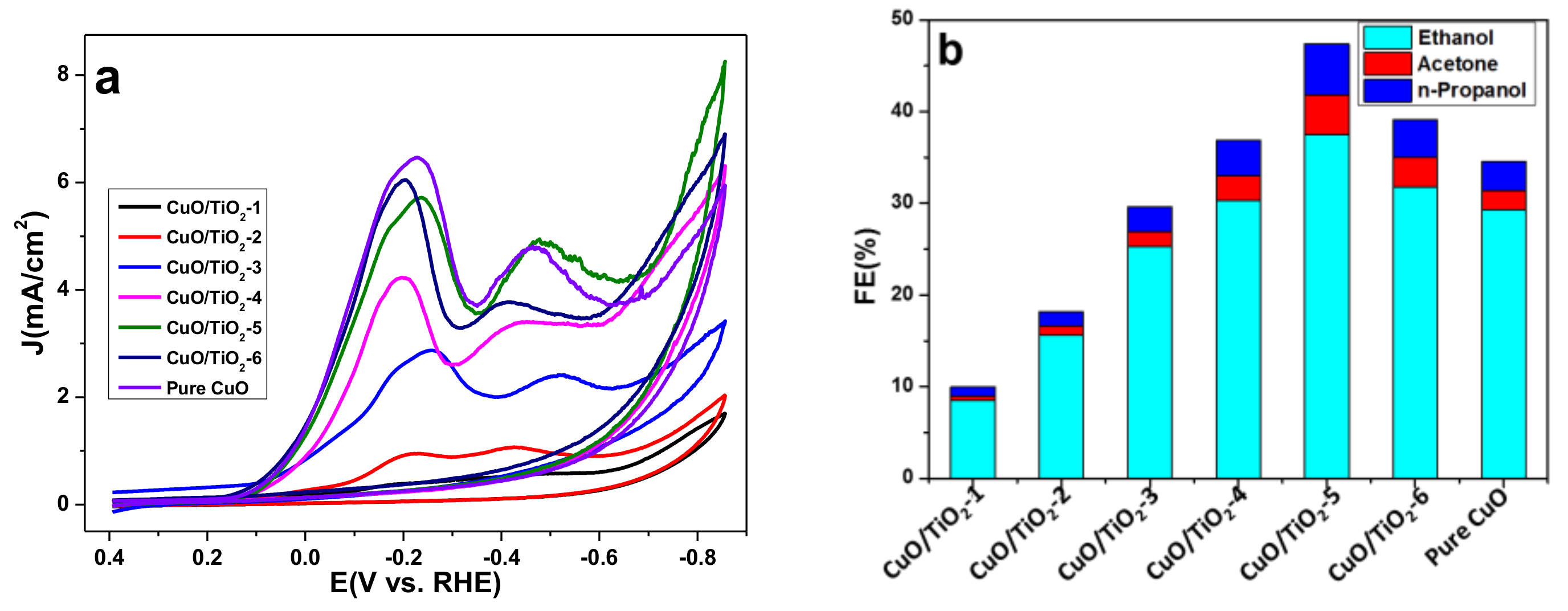
2.2. Catalyst Properties
3. Materials and Methods
3.1. Materials and Instruments
3.2. Materials Synthesis
3.3. Electrode Preparation and Electrochemical Test
3.4. Product Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kumar, B.; Brian, J.P.; Atla, V.; Kumari, S.; Bertram, K.A.; White, R.T.; Spurgeon, J.M. New trends in the development of heterogeneous catalysts for electrochemical CO2 reduction. Catal. Today 2016, 270, 19–30. [Google Scholar] [CrossRef]
- Qiao, J.L.; Liu, Y.Y.; Hong, F.; Zhang, J.J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef] [PubMed]
- Costentin, C.; Robert, M.; Saveant, J.M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 2013, 42, 2423–2436. [Google Scholar] [CrossRef] [PubMed]
- Jitaru, M.; Lowy, D.A.; Toma, M.; Toma, B.C.; Oniciu, L. Electrochemical reduction of carbon dioxide on flat metallic cathodes. J. Appl. Electrochem. 1997, 27, 875–889. [Google Scholar] [CrossRef]
- Kumar, B.; Brian, J.P.; Atla, V.; Kumari, S.; Bertram, K.A.; White, R.T.; Spurgeon, J.M. Controlling the Product Syngas H2:CO Ratio through Pulsed-Bias Electrochemical Reduction of CO2 on Copper. ACS Catal. 2016, 6, 4739–4745. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazabal, G.O.; Perez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef]
- Reske, R.; Duca, M.; Oezaslan, M.; Schouten, K.J.P.; Koper, M.T.M.; Strasser, P. Controlling Catalytic Selectivities during CO2 Electroreduction on Thin Cu Metal Overlayers. J. Phys. Chem. Lett. 2013, 4, 2410–2413. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, Y.M.; Quan, X.; Chen, S.; Yu, H.T. CO2 Electroreduction at Low Overpotential on Oxide-Derived Cu/Carbons Fabricated from Metal Organic Framework. ACS Appl. Mater. Interfaces 2017, 9, 5302–5311. [Google Scholar] [CrossRef] [PubMed]
- Ohya, S.; Kaneco, S.; Katsumata, H.; Suzuki, T.; Ohta, K. Electrochemical reduction of CO2 in methanol with aid of CuO and Cu2O. Catal. Today 2009, 148, 329–334. [Google Scholar] [CrossRef]
- Kaneco, S.; Iiba, K.; Katsumata, H.; Suzuki, T.; Ohta, K. Electrochemical reduction of high pressure carbon dioxide at a Cu electrode in cold methanol with CsOH supporting salt. Chem. Eng. J. 2007, 128, 47–50. [Google Scholar] [CrossRef]
- Whipple, D.T.; Kenis, P.J.A. Prospects of CO2 Utilization via Direct Heterogeneous Electrochemical Reduction. J. Phys. Chem. Lett. 2010, 1, 3451–3458. [Google Scholar] [CrossRef]
- Kaneco, S.; Iiba, K.; Katsumata, H.; Suzuki, T.; Ohta, K. Electrochemical reduction of high pressure CO2 at a Cu electrode in cold methanol. Electrochim. Acta. 2006, 51, 4880–4885. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Oh, Y.; Vrubel, H.; Guidoux, S.; Hu, X. Electrochemical reduction of CO2 in organic solvents catalyzed by MoO2. Chem. Commun. 2014, 50, 3878–3881. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kanan, M.W. Tin Oxide Dependence of the CO2 Reduction Efficiency on Tin Electrodes and Enhanced Activity for Tin/Tin Oxide Thin-Film Catalysts. J. Am. Chem. Soc. 2012, 134, 1986–1989. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Kanan, M.W. CO2 Reduction at Low Overpotential on Cu Electrodes Resulting from the Reduction of Thick Cu2O Films. J. Am. Chem. Soc. 2012, 134, 7231–7234. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.R.; Dubois, D.L. Development of Molecular Electrocatalysts for CO2 Reduction and H2 Production/Oxidation. Acc. Chem. Res. 2009, 42, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Diercks, C.S.; Zhang, Y.B.; Kornienko, N.; Nichols, E.M.; Zhao, Y.; Paris, A.R.; Kim, D.; Yang, P.; Yaghi, O.M. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 2015, 349, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.L.; Zhong, H.X.; Zhang, T.T.; Xu, W.B.; Li, X.F.; Zhang, H.M. Copper Electrode Fabricated via Pulse Electrodeposition: Toward High Methane Selectivity and Activity for CO2 Electroreduction. ACS Catal. 2017, 7, 6302–6310. [Google Scholar] [CrossRef]
- Hori, Y.; Takahashi, I.; Koga, O.; Hoshi, N. Electrochemical reduction of carbon dioxide at various series of copper single crystal electrodes. J. Mol. Catal. A Chem. 2003, 199, 39–47. [Google Scholar] [CrossRef]
- Nie, X.; Esopi, M.R.; Janik, M.J.; Asthagiri, A. Selectivity of CO2 Reduction on Copper Electrodes: The Role of the Kinetics of Elementary Steps. Angew. Chem. Int. Ed. 2013, 52, 2459–2462. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, S.; Esparza, A.T.G.; Jedidi, A.; Cavallo, L.; Takanabe, K. Cu–Sn Bimetallic Catalyst for Selective Aqueous Electroreduction of CO2 to CO. ACS Catal. 2016, 6, 2842–2851. [Google Scholar] [CrossRef]
- Shi, G.D.; Yu, L.; Ba, X.; Zhang, X.S.; Zhou, J.Q.; Yu, Y. Copper nanoparticle interspersed MoS2 nanoflowers with enhanced efficiency for CO2 electrochemical reduction to fuel. Dalton Trans. 2017, 46, 10569–10577. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fu, J.J.; Zhu, W.L.; Chen, Z.Z.; Shen, B.; Wu, L.H.; Xi, Z.; Wang, T.Y.; Lu, G.; Zhu, J.J.; et al. Tuning Sn-Catalysis for Electrochemical Reduction of CO2 to CO via the Core/Shell Cu/SnO2 Structure. J. Am. Chem. Soc. 2017, 139, 4290–4293. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sust. Energ. Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Yang, H.G.; Cheng, H.M.; Lu, G.Q. Titania-based photocatalysts—Crystal growth, doping and heterostructuring. J. Mater. Chem. 2010, 20, 831–843. [Google Scholar] [CrossRef]
- Ravichandran, C.; Kennady, C.J.; Chellammal, S.; Thangavelu, S.; Anantharaman, P.N. Indirect electroreduction of o-nitrophenol to o-aminophenol on titanium dioxide coated titanium electrodes. J. Appl. Electrochem. 1991, 21, 60–63. [Google Scholar] [CrossRef]
- Chu, D.; Qin, G.; Yuan, X.; Xu, M.; Zheng, P.; Lu, J. Fixation of CO2 by Electrocatalytic Reduction and Electropolymerization in Ionic Liquid-H2O Solution. ChemSusChem 2008, 1, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.L.; Diwald, O.; Yates, J.T. CO2 as a Probe for Monitoring the Surface Defects on TiO2 (110) Temperature-Programmed Desorption. J. Phys. Chem. B 2003, 107, 11700–11704. [Google Scholar] [CrossRef]
- Cueto, L.F.; Hirata, G.A.; Sanchez, E.M. Thin-film TiO2 electrode surface characterization upon CO2 reduction processes. J. Sol-Gel Sci. Technol. 2006, 37, 105–109. [Google Scholar] [CrossRef]
- Ma, S.C.; Lan, Y.C.; Perez, G.M.J.; Moniri, S.; Kenis, P.J.A. Silver Supported on Titania as an Active Catalyst for Electrochemical Carbon Dioxide Reduction. ChemSusChem 2014, 7, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, L.; Guo, R.R.; Zeng, S.; Wang, H.; Lu, J.X. Electroreduction of CO2 into Ethanol over an Active Catalyst: Copper Supported on Titania. Catalysts 2017, 7, 220. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, M.P.; Hu, Q.L.; Li, S.M.; Wang, H.; Lu, J.X. Cu/TiO2 nanoparticles modified nitrogen-doped graphene as a highly efficient catalyst for the selective electroreduction of CO2 to different alcohols. J. CO2 Util. 2018, 24, 334–340. [Google Scholar] [CrossRef]
- Fang, B.Z.; Xing, Y.L.; Bonakdarpour, A.; Zhang, S.C.; Wilkinson, D.P. Hierarchical CuO-TiO2 Hollow Microspheres for Highly Efficient Photodriven Reduction of CO2 to CH4. ACS Sustain. Chem. Eng. 2015, 3, 2381–2388. [Google Scholar] [CrossRef]
- Choi, H.J.; Kang, M. Hydrogen production from methanol/water decomposition in a liquid photosystem using the anatase structure of Cu loaded TiO2. Int. J. Hydrog. Energy 2007, 32, 3841–3848. [Google Scholar] [CrossRef]
- Chi, D.H.; Yang, H.P.; Du, Y.F.; Lv, T.; Sui, G.J.; Wang, H.; Lu, J.X. Morphology-controlled CuO nanoparticles for electroreduction of CO2 to ethanol. RSC Adv. 2014, 4, 37329–37332. [Google Scholar] [CrossRef]
- Kulkarni, P.; Mahamuni, S.; Chandrachood, M.; Mulla, I.S.; Sinha, A.P.B.; Nigavekar, A.S.; Kulkarni, S.K. Photoelectron spectroscopic studies on a silicon interface with Bi2Sr2CaCu2BO8+δ high Tc superconductor. J. Appl. Phys. 1990, 67, 3438–3442. [Google Scholar] [CrossRef]
- Ethiraj, A.S.; Kang, D.J. Synthesis and characterization of CuO nanowires by a simple wet chemical method. Nanoscale Res. Lett. 2012, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.Y.; Liang, R.M.; Wu, P.W.; Chen, J.Y.; Hsieh, Y.C. Electrochemical reduction of CO2 by Cu2O-catalyzed carbon clothes. Mater. Lett. 2009, 63, 1001–1003. [Google Scholar] [CrossRef]
- Gupta, K.; Bersani, M.; Darr, J.A. Highly efficient electro-reduction of CO2 to formic acid by nano-copper. J. Mater. Chem. A 2016, 4, 13786–13794. [Google Scholar] [CrossRef]
- Qiao, J.L.; Fan, M.Y.; Fu, Y.S.; Bai, Z.Y.; Ma, C.Y.; Liu, Y.Y.; Zhou, X.D. Highly-active copper oxide/copper electrocatalysts induced from hierarchical copper oxide nanospheres for carbon dioxide reduction reaction. Electrochim. Acta. 2015, 153, 559–565. [Google Scholar] [CrossRef]
- Ghijsen, J.; Tjeng, L.H.; Van-Elp, J.; Esks, H.; Westerink, J.; Sawatzky, C.A.; Czyzyk, M.T. Electronic structure of Cu2O and CuO. Phys. Rev. B 1988, 38, 11322–11330. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Aljaber, A.S.; AlQaradawi, S.Y.; Allam, N.K. TiO2 nanotubes with ultrathin walls for enhanced water splitting. Chem. Commun. 2015, 51, 12617–12620. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Qiao, S.; Fang, Y.; Tian, J.; Mcleod, J.; Song, Y.; Huang, H.; Liu, Y.; Kang, Z. Third-order nonlinear optical properties of carboxyl group dominant carbon nanodots. J. Mater. Chem. C 2016, 4, 8490–8495. [Google Scholar] [CrossRef]
- Yu, B.Y.; Kwak, S.Y. Carbon quantum dots embedded with mesoporous hematite nanospheres as efficient visible light-active photocatalysts. J. Mater. Chem. 2012, 22, 8345–8353. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; MacFarlane, D.R. Carbon Quantum Dots/Cu2O Heterostructures for Solar-Light-Driven Conversion of CO2 to Methanol. Adv. Energy Mater. 2015, 5, 1401077. [Google Scholar] [CrossRef]
- Li, C.W.; Ciston, J.; Kanan, M.W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 2014, 508, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhang, Y.; Wang, X.; Wang, Q. Electrochemical formation and reduction of copper oxide nanostructures in alkaline media. Electrochem. Commun. 2013, 36, 99–102. [Google Scholar] [CrossRef]
- Gao, S.; Lin, Y.; Jiao, X.C.; Sun, Y.F.; Luo, Q.Q.; Zhang, W.H.; Li, D.Q.; Yang, J.L.; Xie, Y. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 2016, 529, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T.M. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Zhang, J.-J.; Yang, M.-P.; Meng, W.-J.; Wang, H.; Lu, J.-X. CuO Nanoparticles Supported on TiO2 with High Efficiency for CO2 Electrochemical Reduction to Ethanol. Catalysts 2018, 8, 171. https://doi.org/10.3390/catal8040171
Yuan J, Zhang J-J, Yang M-P, Meng W-J, Wang H, Lu J-X. CuO Nanoparticles Supported on TiO2 with High Efficiency for CO2 Electrochemical Reduction to Ethanol. Catalysts. 2018; 8(4):171. https://doi.org/10.3390/catal8040171
Chicago/Turabian StyleYuan, Jing, Jing-Jie Zhang, Man-Ping Yang, Wang-Jun Meng, Huan Wang, and Jia-Xing Lu. 2018. "CuO Nanoparticles Supported on TiO2 with High Efficiency for CO2 Electrochemical Reduction to Ethanol" Catalysts 8, no. 4: 171. https://doi.org/10.3390/catal8040171
APA StyleYuan, J., Zhang, J.-J., Yang, M.-P., Meng, W.-J., Wang, H., & Lu, J.-X. (2018). CuO Nanoparticles Supported on TiO2 with High Efficiency for CO2 Electrochemical Reduction to Ethanol. Catalysts, 8(4), 171. https://doi.org/10.3390/catal8040171