Selective Hydrogenation of Benzene to Cyclohexene over Monometallic Ru Catalysts: Investigation of ZnO and ZnSO4 as Reaction Additives as Well as Particle Size Effect
Abstract
1. Introduction
2. Results
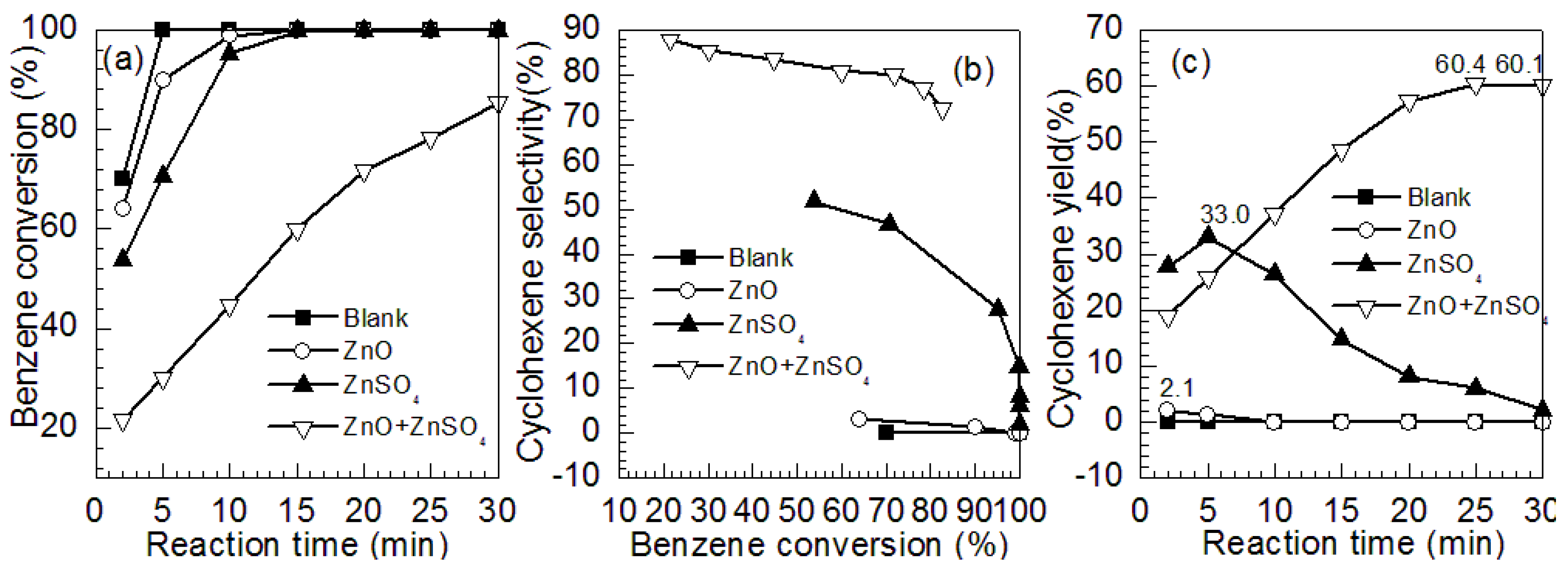
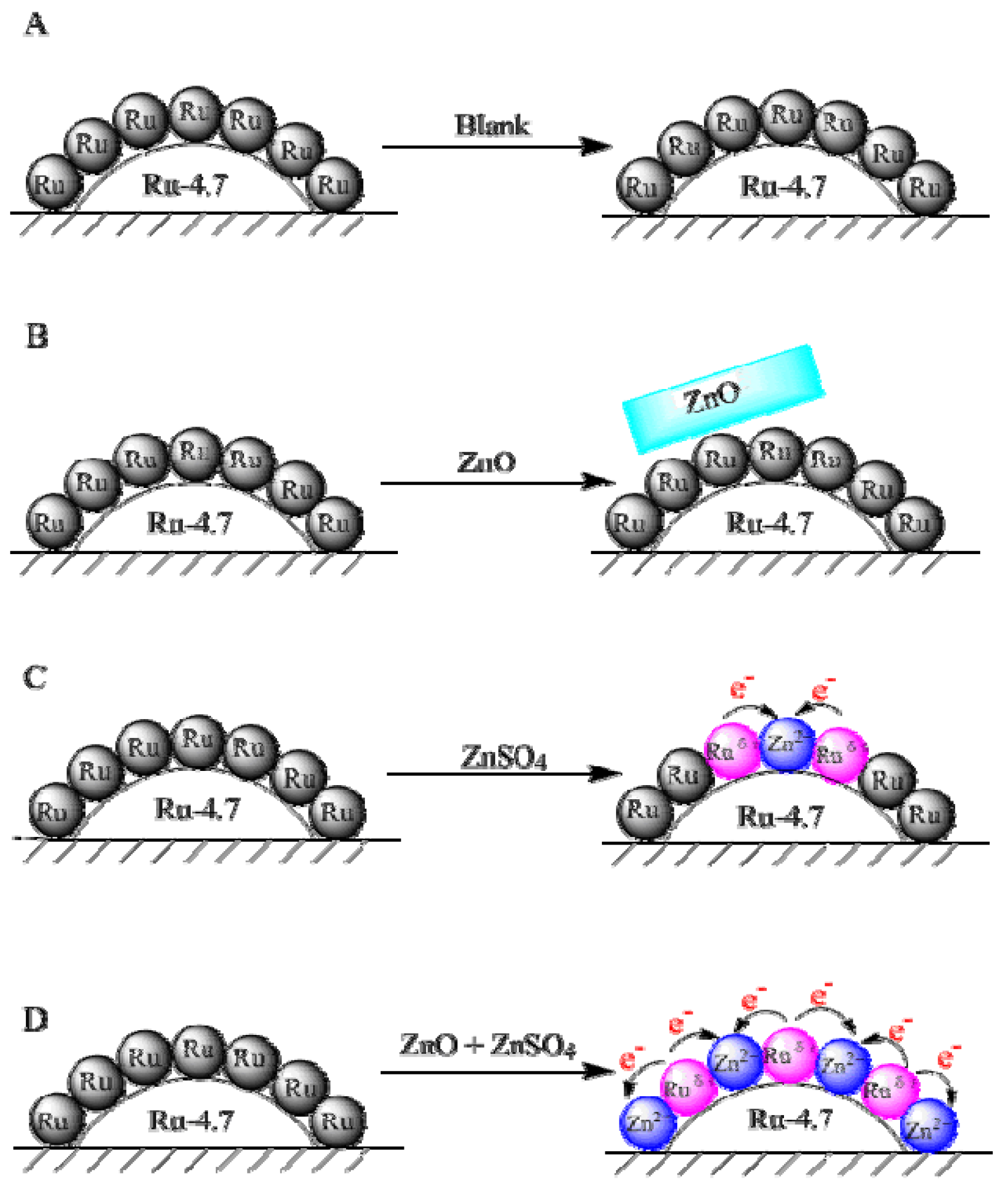
2.1. Effects of ZnO and ZnSO4
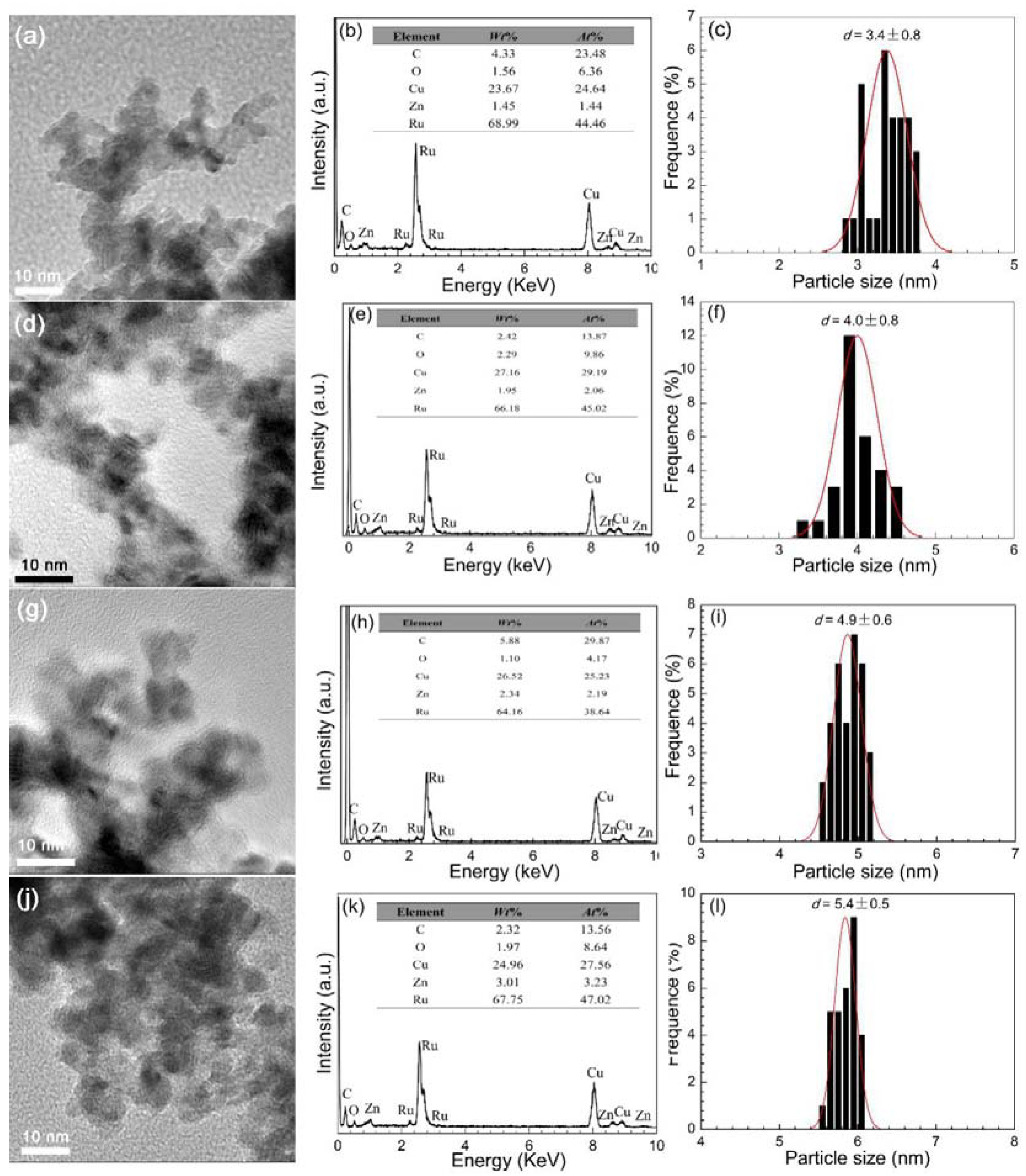
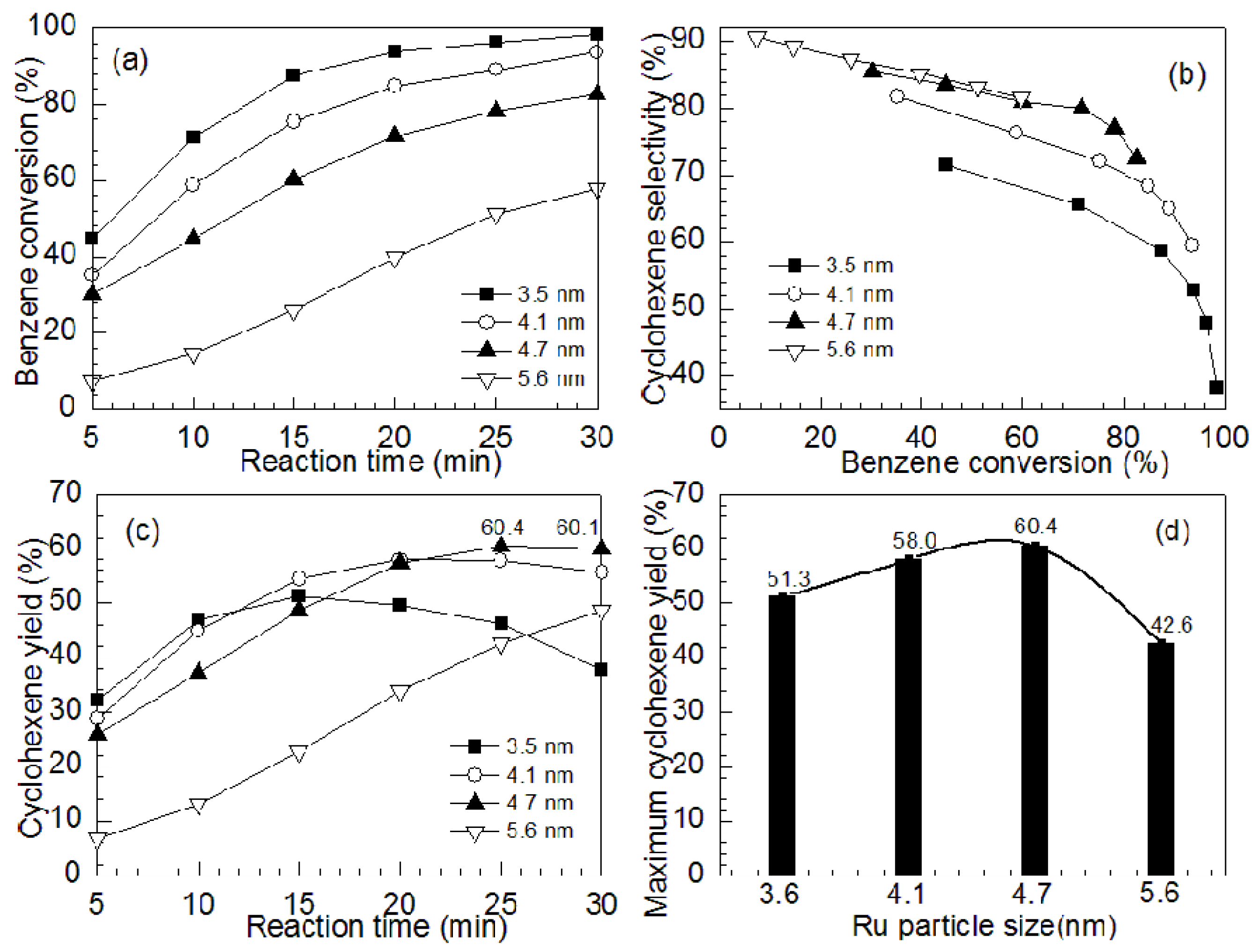
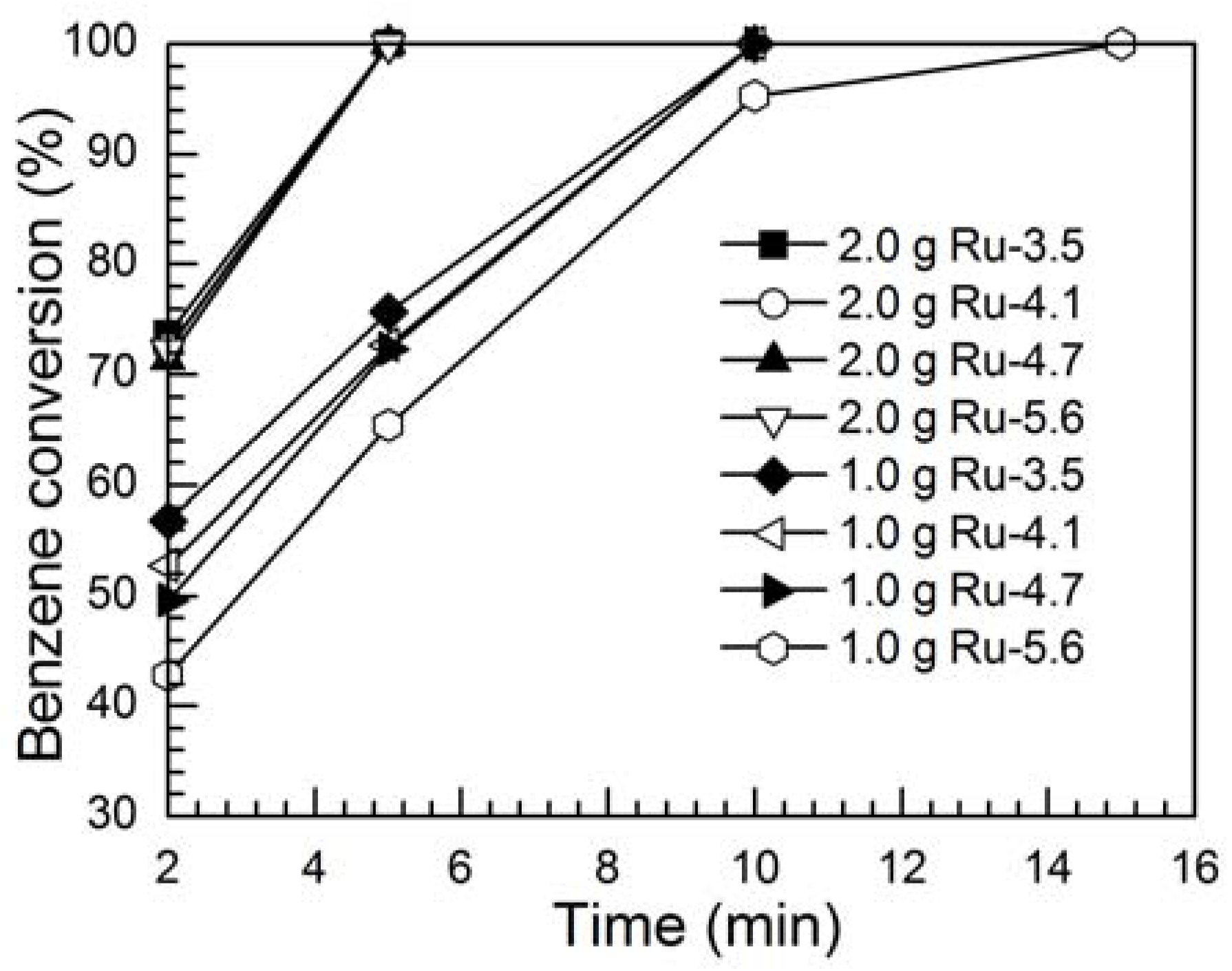
2.2. Effects of Particle Size of Ru Catalysts
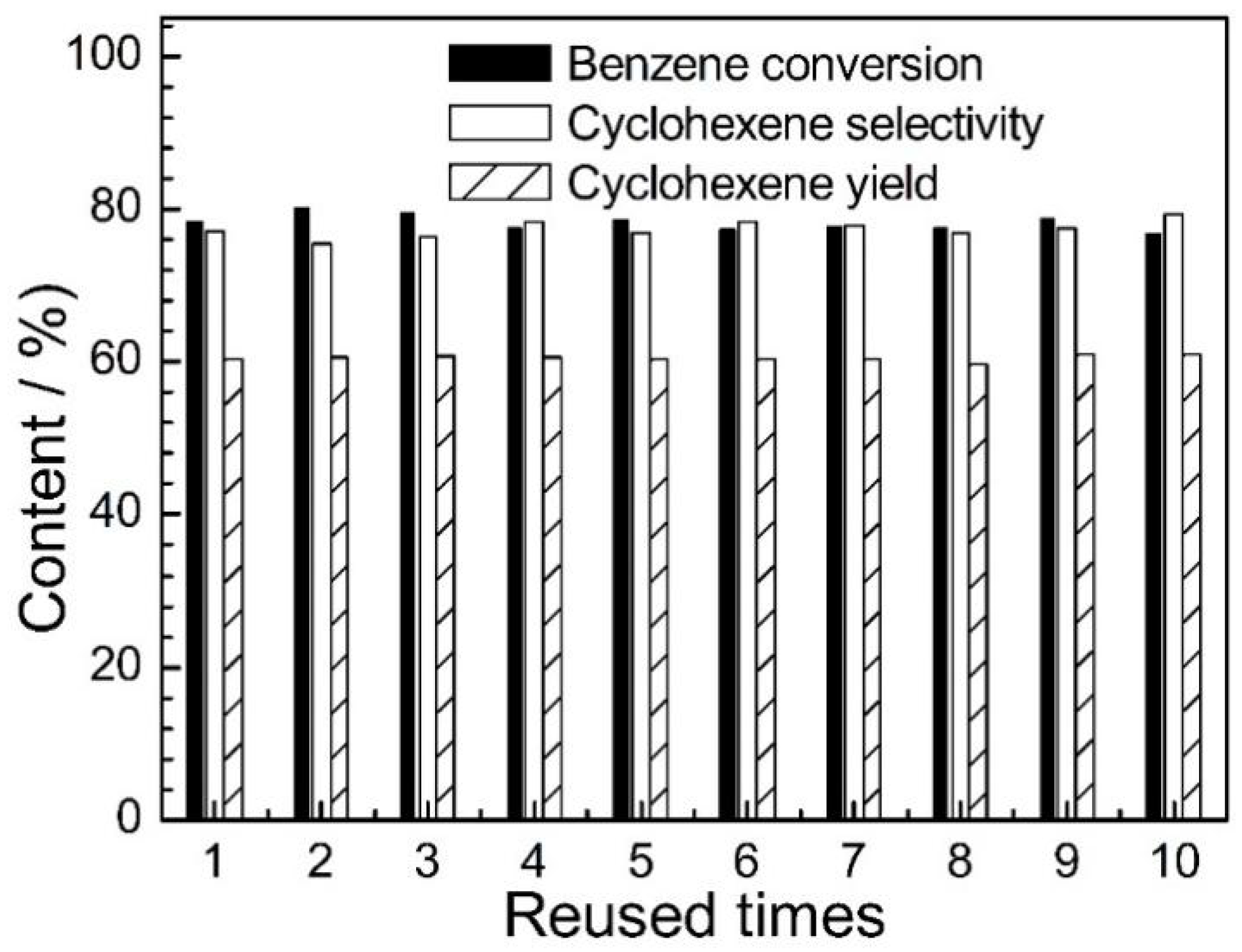
2.3. Reusability of Ru-4.7 Catalyst
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Catalysts
3.3. Catalytic Experimental Procedure
3.4. Catalysts Characterization
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yan, X.H.; Zhang, Q.; Zhu, M.Q.; Wang, Z.B. Selective hydrogenation of benzene to cyclohexene over Ru-Zn/ZrO2 catalysts prepared by a two step impreganation method. J. Mol. Catal. A Chem. 2016, 413, 85–93. [Google Scholar] [CrossRef]
- Zhou, G.B.; Pei, Y.; Jiang, Z.; Fan, K.N.; Qiao, M.H.; Sun, B.; Zong, B.N. Doping effects of B in ZrO2 on structural and catalytic properties of Ru/B-ZrO2 catalysts for benzene partial hydrogenation. J. Catal. 2014, 311, 393–403. [Google Scholar] [CrossRef]
- Sun, H.J.; Chen, Z.H.; Li, C.G.; Chen, L.X.; Li, Y.; Peng, Z.K.; Liu, Z.Y.; Liu, S.C. Selective hydrogenation of benzene to cyclohexene over monometallic Ru catalysts: Investigation of ZnO and ZnSO4 as reaction additives as well as particle size effect. Catalysts 2018, 8, 104. [Google Scholar] [CrossRef]
- Spod, H.; Lucas, M.; Claus, P. Performance of Ru/La2O3–ZnO Catalyst for the Selective Hydrogenation of Benzene to Cyclohexene. Catalysts 2015, 5, 1756–1769. [Google Scholar] [CrossRef]
- Sun, H.J.; Chen, J.J.; Huang, Z.X.; Liu, Z.Y.; Liu, S.C. Selective hydrogenation of benzene to cyclohexene over the nano-sized Ru-Zn catalyst modified by Rrabic gum. Chin. J. Inorg. Chem. 2016, 32, 202–210. [Google Scholar]
- Liao, H.G.; Ouyang, D.H.; Zhang, J.; Xiao, Y.J.; Liu, P.L.; Hao, F.; You, K.Y.; Luo, H.A. Benzene hydrogenation over oxide-modified MCM-41 supported ruthenium-lanthanum catalyst: The influence of zirconia crystal form and surface hydrophilicity. Chem. Eng. J. 2014, 243, 207–216. [Google Scholar] [CrossRef]
- Martins, L.M.D.; Carabineiro, S.A.C.; Wang, J.; Rocha, B.G.M.; Maldonado-Hódar, F.J.; Pombeiro, A.J.L.D.O. Supported Gold Nanoparticles as Reusable Catalysts for Oxidation Reactions of Industrial Significance. ChemCatChem 2017, 9, 1211–1221. [Google Scholar] [CrossRef]
- Martins, L.M.; de Almeida, M.P.; Carabineiro, S.A.; Figueiredo, J.L.; Pombeiro, A.J. Heterogenisation of a C-Scorpionate FeII Complex on Carbon Materials for Cyclohexane Oxidation with Hydrogen Peroxide. ChemCatChem 2013, 5, 3847–3856. [Google Scholar] [CrossRef]
- De Almeida, M.P.; Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Lauterbach, T.; Rominger, F.; Hashmi, A.S.K.; Pombeiro, A.J.L.; Figueiredo, J.L. Homogeneous and heterogenised new gold C-scorpionate complexes as catalysts for cyclohexane oxidation. Catal. Sci. Technol. 2013, 3, 3056–3069. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Martins, L.M.D.R.S.; Avalos-Borja, M.; Buijnsters, J.G.; Pombeiro, A.J.L.; Figueiredo, J.L. Gold nanoparticles supported on carbon materials for cyclohexane oxidation with hydrogen peroxide. Appl. Catal. A Gen. 2013, 467, 279–290. [Google Scholar] [CrossRef]
- Sun, H.J.; Li, S.H.; Zhang, Y.X.; Jiang, H.B.; Qu, L.L.; Liu, Z.Y. Selective hydrogenation of benzene to cyclohexene in continuous reaction device with two reaction reactors in serie over Ru-Co-B/ZrO2 catalysts. Chin. J. Catal. 2013, 34, 1482–1488. [Google Scholar] [CrossRef]
- Sun, H.J.; Jiang, H.B.; Li, S.H.; Wang, H.X.; Pan, Y.J.; Dong, Y.Y.; Liu, S.C.; Liu, Z.Y. Selective hydrogenation of benzene to cyclohexene over nanocomposite Ru-Mn/ZrO2 catalyst. Chin. J. Catal. 2013, 34, 684–694. [Google Scholar] [CrossRef]
- Wang, Z.B.; Zhang, Q.; Lu, X.F.; Chen, S.J.; Liu, C.J. Ru-Zn catalysts for selective hydrogenation of benzene using coprecipitation in low alkalinity. Chin. J. Catal. 2015, 36, 400–407. [Google Scholar] [CrossRef]
- Sun, H.J.; Li, Y.Y.; Li, S.H.; Zhang, Y.X.; Liu, S.C.; Liu, Z.Y.; Ren, B.Z. ZnSO4 and La2O3 as Co-Modifier of the Monoclinic Ru Catalyst for Selective Hydrogenation of Benzene to Cyclohexene. Acta. Phys. Chim. Sin. 2014, 30, 1332–1340. [Google Scholar]
- Canpbell, P.S.; Santini, C.C.; Bayard, F.; Chauvin, Y.; Collière, V.; Podgoršek, A.; Gomes, M.F.C.; Sá, J. Olefin hydrogenation by ruthenium nanoparticles in ionic liquid media: Does size matter? J. Catal. 2010, 275, 99–107. [Google Scholar] [CrossRef]
- Lee, K.; Kim, M.; Kim, H. Catalytic nanoparticles being facet-controlled. J. Mater. Chem. 2010, 20, 3791–3798. [Google Scholar] [CrossRef]
- Pushkarev, V.V.; An, K.; Alayoglu, S.; Beaumont, S.K.; Somorjai, G.A. Hydrogenation of benzene and toluene over size controlled Pt/SBA-15 catalysts: Elucidation of the Pt particle size effect on reaction kinetics. J. Catal. 2012, 292, 64–72. [Google Scholar] [CrossRef]
- Nagahara, H.; Ono, M.; Konishi, M.; Fukuoka, Y. Partial hydrogenation of benzene to cyclohexene. Appl. Surf. Sci. 1997, 121, 448–451. [Google Scholar] [CrossRef]
- Milone, C.; Neri, G.; Donato, A.; Musolino, M.G.; Mercadante, L. Selective Hydrogenation of Benzene to Cyclohexene on Ru/γ-Al2O3. J. Catal. 1996, 159, 253–258. [Google Scholar] [CrossRef]
- Bu, J.; Liu, J.L.; Chen, X.Y.; Zhuang, J.H.; Yan, S.R.; Qiao, M.H.; He, H.Y.; Fan, K.N. Ru/SBA-15 catalysts for partial hydrogenation of benzene to cyclohexene: Tuning the Ru crystallite size by Ba. Catal. Commun. 2008, 9, 2612–2615. [Google Scholar] [CrossRef]
- Sun, H.J.; Zhang, X.D.; Chen, Z.H.; Zhou, X.L.; Guo, W.; Liu, Z.Y.; Liu, S.C. Monolayer dispersed Ru-Zn Catalyst and its performance in the Selective Hydrogenation of Benzene to Cyclohexene. Chin. J. Catal. 2011, 32, 224–230. [Google Scholar] [CrossRef]
- Sun, H.J.; Chen, X.L.; Huang, Z.X.; Liu, Z.Y.; Liu, S.C. Effect of NaOH concentration on performance of Ru-Zn catalyst for selective hydrogenation of benzene to cyclohexene. CIESC J. 2016, 67, 1324–1332. [Google Scholar]
- Hu, S.C.; Chen, Y.W. Partial Hydrogenation of Benzene to Cyclohexene on Ruthenium Catalysts Supported on La2O3−ZnO Binary Oxides. Ind. Eng. Chem. Res. 1997, 36, 5153–5159. [Google Scholar] [CrossRef]
- Sun, H.J.; Wang, H.X.; Jiang, H.B.; Li, S.H.; Liu, S.C.; Liu, Z.Y.; Yuan, X.M.; Yang, K.J. Eeffect of (Zn(OH)2)3(ZnSO4)(H2O)5 on the performance of Ru-Zn catalyst for benzene selective hydrogenation to cyclohexene. Appl. Catal. A Gen. 2013, 450, 160–168. [Google Scholar] [CrossRef]
- Fuggle, J.C.; Madey, T.E.; Steinkilberg, M.; Menzel, D. Photoelectron spectroscopic studies of adsorption of CO and oxygen on Ru(001). Surf. Sci. 1975, 52, 521–541. [Google Scholar] [CrossRef]
- Börje, F. ESCA studies on the charge distribution in some dinitrogen complexes of Rhenium, Iridium, Ruthenium, and Osmium. Acta Chem. Scand. 1973, 27, 287–302. [Google Scholar]
- Wehner, P.S.; Mercer, P.N. Interaction of H2 and CO with Rh4(CO)12 supported on ZnO. J. Catal. 1983, 84, 244–247. [Google Scholar] [CrossRef]
- Dake, L.S.; Baer, D.R.; Zachara, J.M. Auger parameter measurements of zinc compounds relevant to zinc transport in the environment. Surf. Interface Anal. 1989, 14, 71–75. [Google Scholar] [CrossRef]
- Deroubaix, G.; Marcus, P. X-ray photoelectron spectroscopy analysis of copper and zinc oxides and sulphides. Surf. Interface Anal. 1992, 18, 39–46. [Google Scholar] [CrossRef]
- Strohmeier, B.R.; Hercules, D.M. Surface spectroscopic characterization of the interaction between zinc ions and γ-alumina. J. Catal. 1984, 86, 266–279. [Google Scholar] [CrossRef]
- Nefedov, V.I. A comparison of results of an ESCA study of nonconducting solids using spectrometers of different constructions. J. Electron. Spectrosc. Relat. Phenom. 1982, 25, 29–47. [Google Scholar] [CrossRef]
- Struijk, J.; Moene, R.; Kamp, T.V.D.; Scholten, J.J.F. Partial liquid phase hydrogenation of benzene to cyclohexene over ruthenium catalysts in the presence of an aqueous salt solution: II. Influence of various salts on the performance of the catalyst. Appl. Catal. A Gen. 1992, 89, 77–102. [Google Scholar] [CrossRef]
- Sun, H.J.; Dong, Y.Y.; Li, S.H.; Jiang, H.B.; Zhang, Y.X.; Liu, Z.Y.; Liu, S.C. The role of La in improving the selectivity to cyclohexene of Ru catalyst for hydrogenation of benzene. J. Mol. Catal. A Chem. 2013, 368–369, 119–124. [Google Scholar] [CrossRef]
- Sun, H.J.; Jiang, H.B.; Dong, Y.Y.; Wang, H.X.; Pan, Y.J.; Liu, S.C.; Tang, M.S.; Liu, Z.Y. Effect of alcohols as additives on the performance of a nano-sized Ru-Zn (2.8%) catalyst for selective hydrogenation of benzene to cyclohexene. Chem. Eng. J. 2013, 218, 415–424. [Google Scholar] [CrossRef]
- Sun, H.J.; Pan, Y.J.; Jiang, H.B.; Li, S.H.; Zhang, Y.X.; Liu, S.C.; Liu, Z.Y. Effect of transition metals (Cr, Mn, Fe, Co, Ni, Cu and Zn) on the hydrogenation properties of benzene over Ru-based catalyst. Appl. Catal. A Gen. 2013, 464–465, 1–9. [Google Scholar] [CrossRef]
- Mazzieri, V.A.; L’Argentire, P.C.; Coloma-Pascual, F.; Fgoli, N.S. Effect of Chlorine on the Properties of Ru/Al2O3. Ind. Eng. Chem. Res. 2003, 42, 2269–2272. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhu, Y.; Liu, J.; Pei, Y.; Li, Z.H.; Li, H.; Li, H.X.; Qiao, M.H.; Fan, K.N. Discrimination of the roles of CdSO4 and ZnSO4 in liquid phase hydrogenation of benzene to cyclohexene. J. Catal. 2009, 268, 100–105. [Google Scholar] [CrossRef]
- Sun, H.J.; Chen, L.X.; Huang, Z.X.; Liu, S.C.; Liu, Z.Y. Particle Size Effect of Ru-Zn Catalysts on Selective Hydrogenation of Benzene to Cyclohexene. Chem. J. Chin. Univ. 2015, 36, 1969–1976. [Google Scholar]
- Liu, S.C.; Luo, G.; Han, M.L.; Li, Z.J. Characterization of the catalyst prepared by impregnation for selective hydrogenation of benzene to cyclohexene. Chin. J. Catal. 2001, 22, 559–562. [Google Scholar]
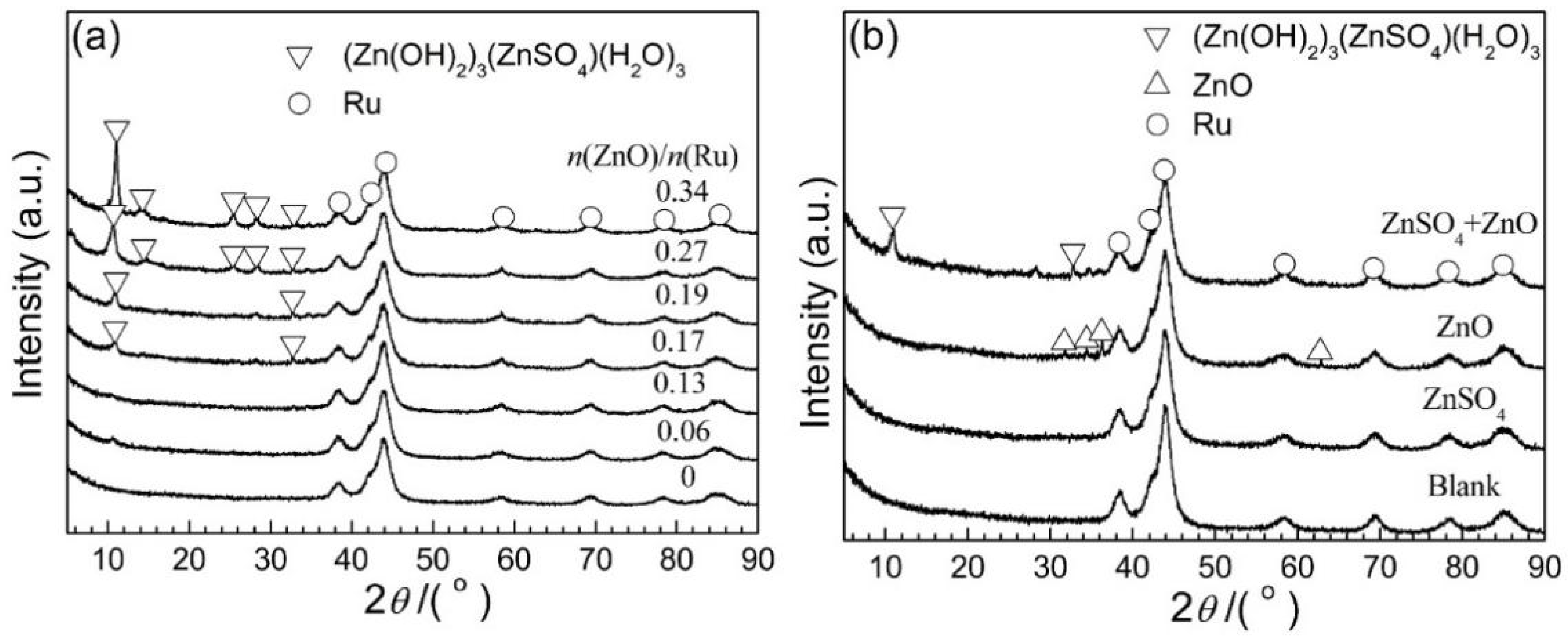

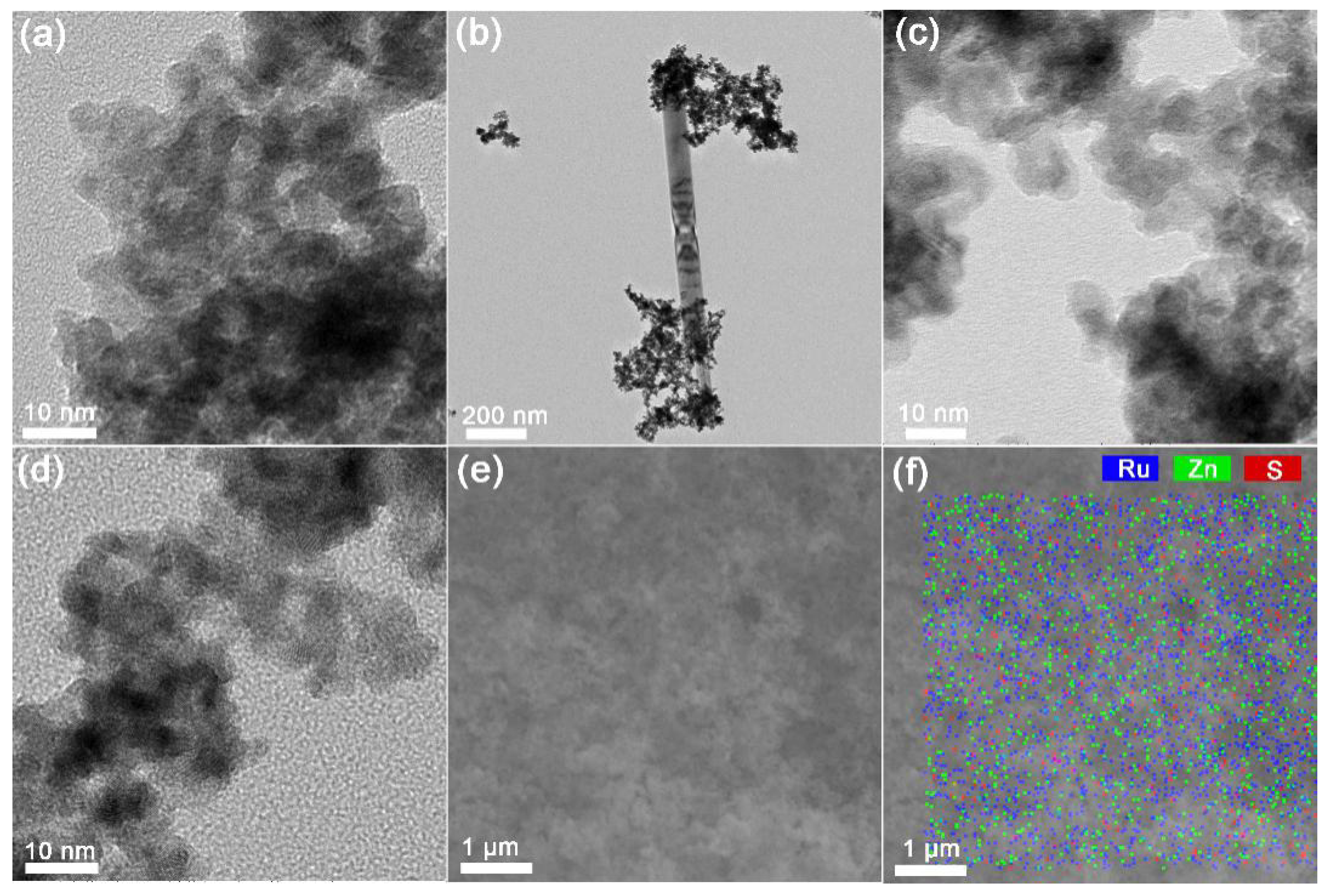
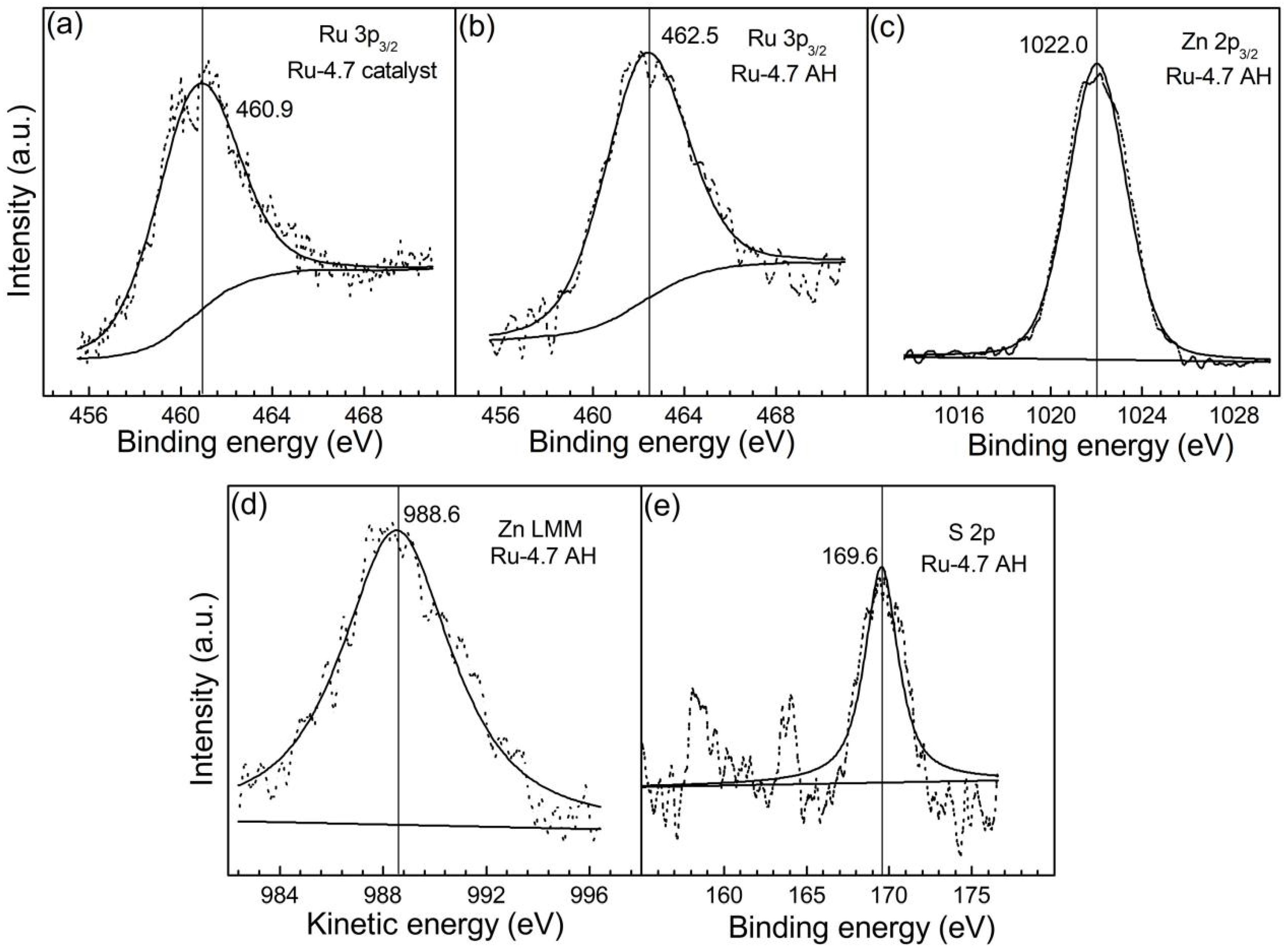



| n(ZnO)/n(Ru) | cZnSO4/mol·L‒1 | n(Zn)/n(Ru)AH 1 | n(S)/n(Ru) AH 1 | Particle Size 2/nm | pH Value 3 |
|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 4.6 | 6.97 |
| 0 | 0.62 | 0.0313 | 0.0026 | 4.7 | 5.53 |
| 0.06 | 0.62 | 0.1456 | 0.0067 | 4.7 | 5.78 |
| 0.13 | 0.62 | 0.1711 | 0.0111 | 4.6 | 5.89 |
| 0.17 | 0.62 | 0.1886 | 0.0156 | 4.8 | 5.92 |
| 0.19 | 0.62 | 0.2803 | 0.0336 | 4.7 | 6.01 |
| 0.27 | 0.62 | 0.3194 | 0.0381 | 4.8 | 6.13 |
| 0.34 | 0.62 | 0.4226 | 0.0436 | 4.6 | 6.25 |
| 0.19 | 0 | 0.1792 | 0 | 4.2 | 7.10 |
| Catalyst | SBET/m2 g−1 | Vp/cm3 g−1 | dp/nm |
|---|---|---|---|
| Ru-3.5 | 78 | 0.17 | 5.48 |
| Ru-4.1 | 75 | 0.15 | 7.54 |
| Ru-4.7 | 69 | 0.13 | 8.60 |
| Ru-5.6 | 68 | 0.11 | 9.48 |
| Ru-3.5 AH | 70 | 0.15 | 6.16 |
| Ru-4.1 AH | 68 | 0.13 | 8.26 |
| Ru-4.7 AH | 65 | 0.11 | 8.88 |
| Ru-5.6 AH | 62 | 0.10 | 10.08 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Chen, Z.; Li, C.; Chen, L.; Li, Y.; Peng, Z.; Liu, Z.; Liu, S. Selective Hydrogenation of Benzene to Cyclohexene over Monometallic Ru Catalysts: Investigation of ZnO and ZnSO4 as Reaction Additives as Well as Particle Size Effect. Catalysts 2018, 8, 172. https://doi.org/10.3390/catal8050172
Sun H, Chen Z, Li C, Chen L, Li Y, Peng Z, Liu Z, Liu S. Selective Hydrogenation of Benzene to Cyclohexene over Monometallic Ru Catalysts: Investigation of ZnO and ZnSO4 as Reaction Additives as Well as Particle Size Effect. Catalysts. 2018; 8(5):172. https://doi.org/10.3390/catal8050172
Chicago/Turabian StyleSun, Haijie, Zhihao Chen, Chenggang Li, Lingxia Chen, Yan Li, Zhikun Peng, Zhongyi Liu, and Shouchang Liu. 2018. "Selective Hydrogenation of Benzene to Cyclohexene over Monometallic Ru Catalysts: Investigation of ZnO and ZnSO4 as Reaction Additives as Well as Particle Size Effect" Catalysts 8, no. 5: 172. https://doi.org/10.3390/catal8050172
APA StyleSun, H., Chen, Z., Li, C., Chen, L., Li, Y., Peng, Z., Liu, Z., & Liu, S. (2018). Selective Hydrogenation of Benzene to Cyclohexene over Monometallic Ru Catalysts: Investigation of ZnO and ZnSO4 as Reaction Additives as Well as Particle Size Effect. Catalysts, 8(5), 172. https://doi.org/10.3390/catal8050172






