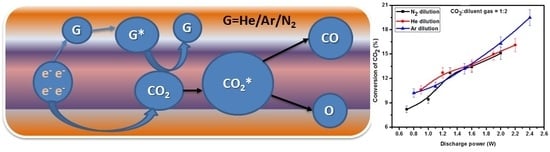
DBD Plasma Assisted CO2 Decomposition: Influence of Diluent Gases
Abstract
1. Introduction
2. Results and Discussion
2.1. Discharge Characterization
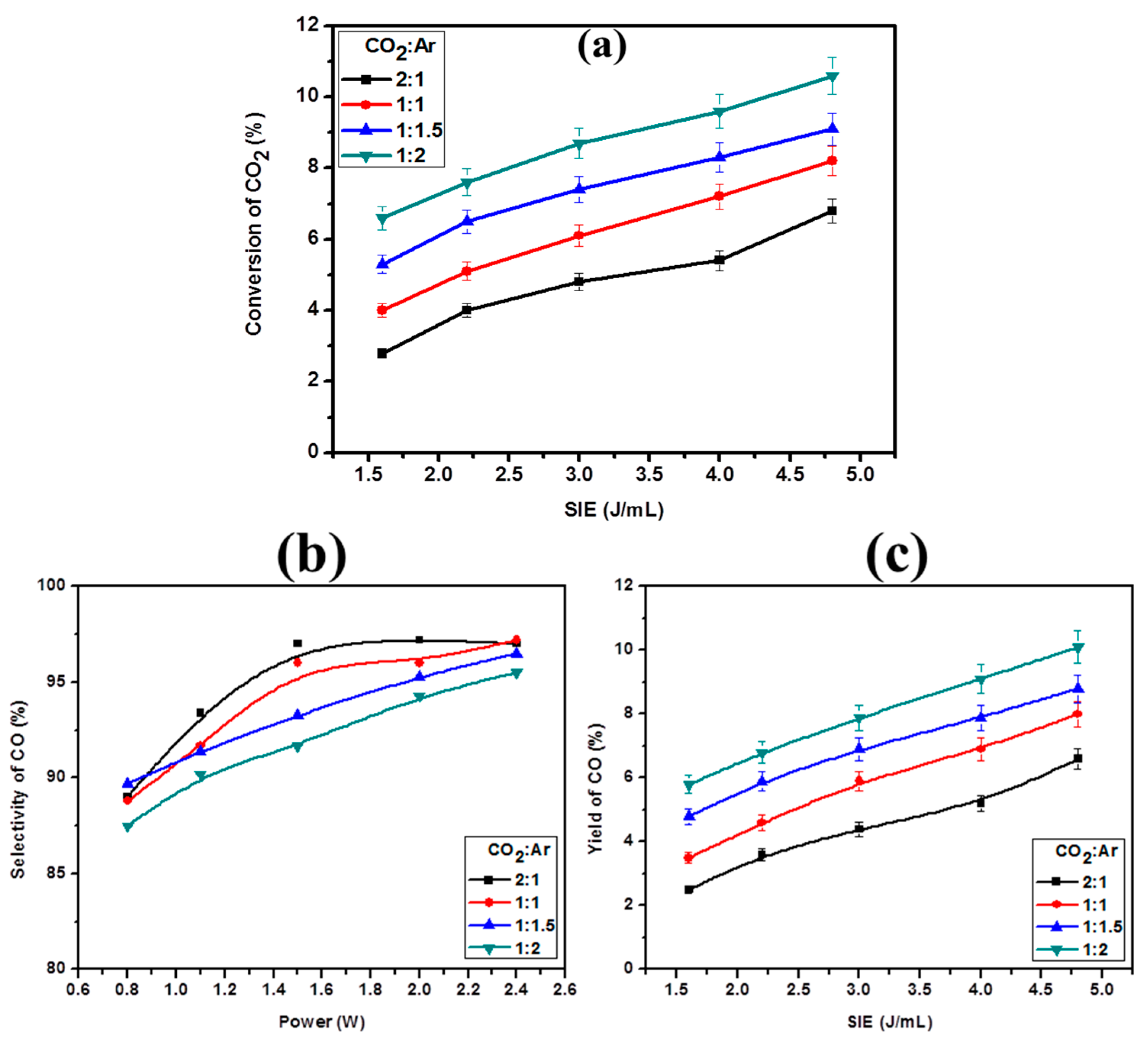
2.2. Effect of CO2:Ar Mole Ratio on CO2 Decomposition
2.3. Effect of Glass Beads on CO2 Decomposition
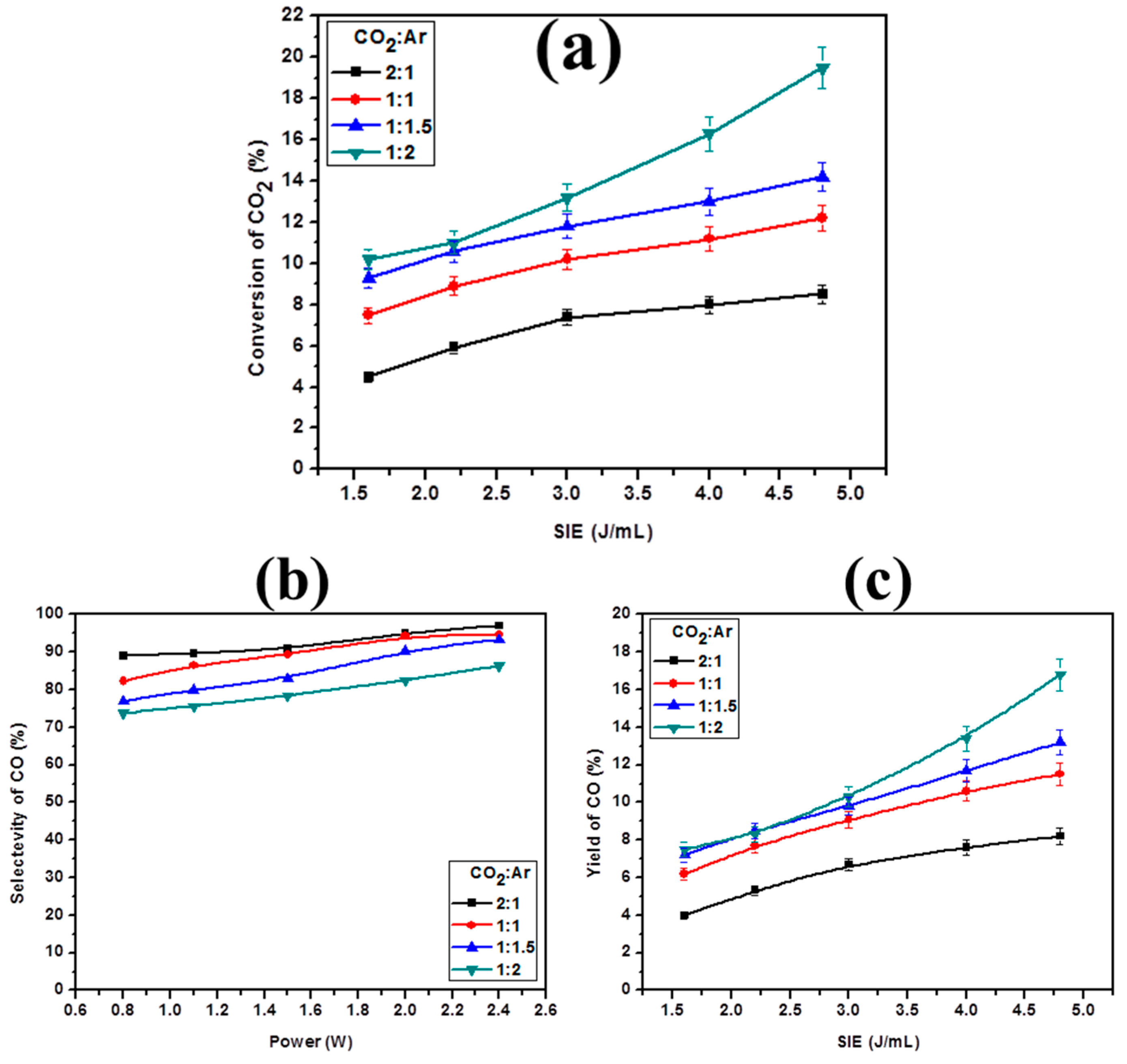
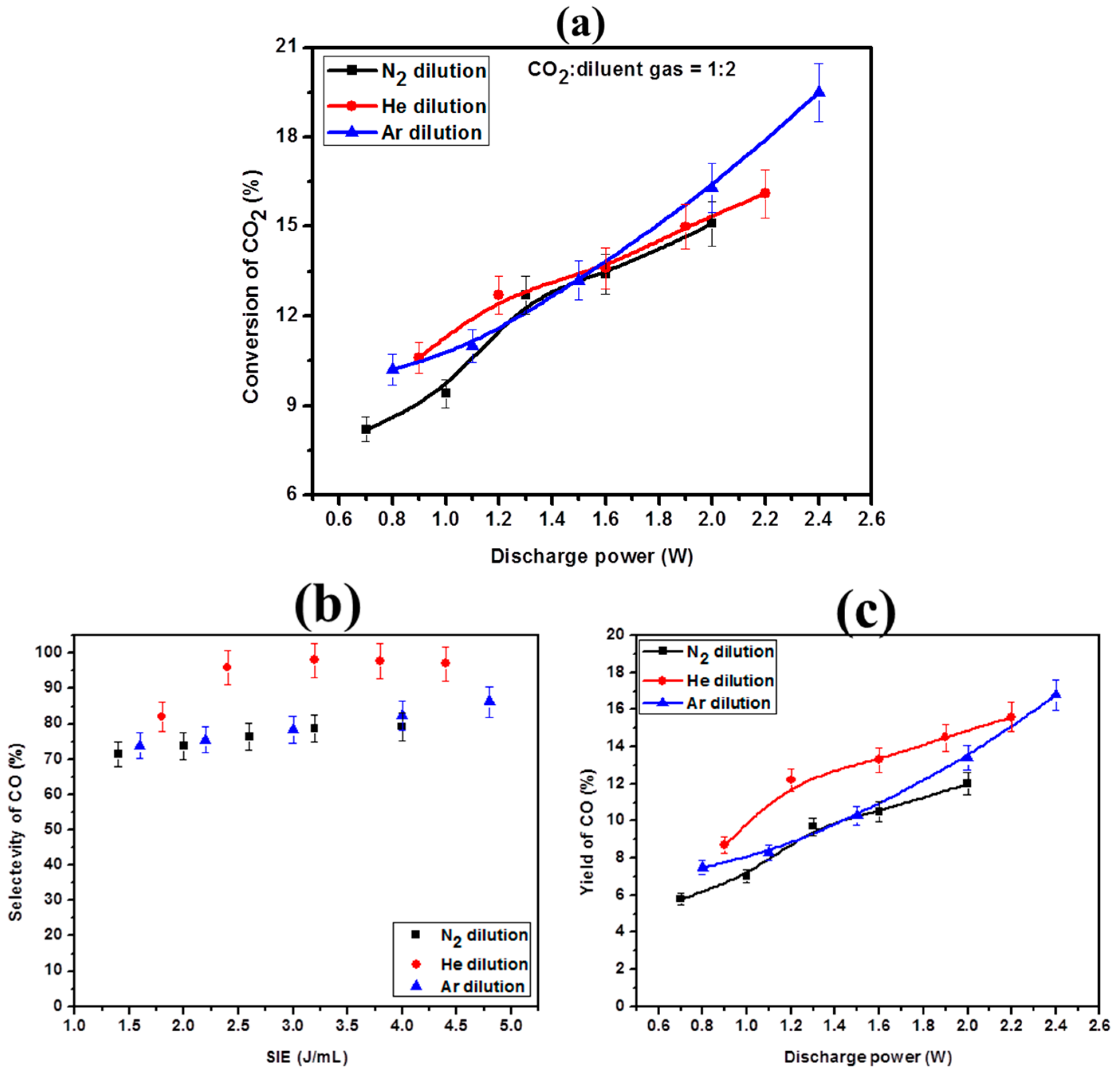
2.4. Effect of Diluent Gases with Packed Bed DBD
2.5. Effect of Diluent Gases on the Reaction Rate
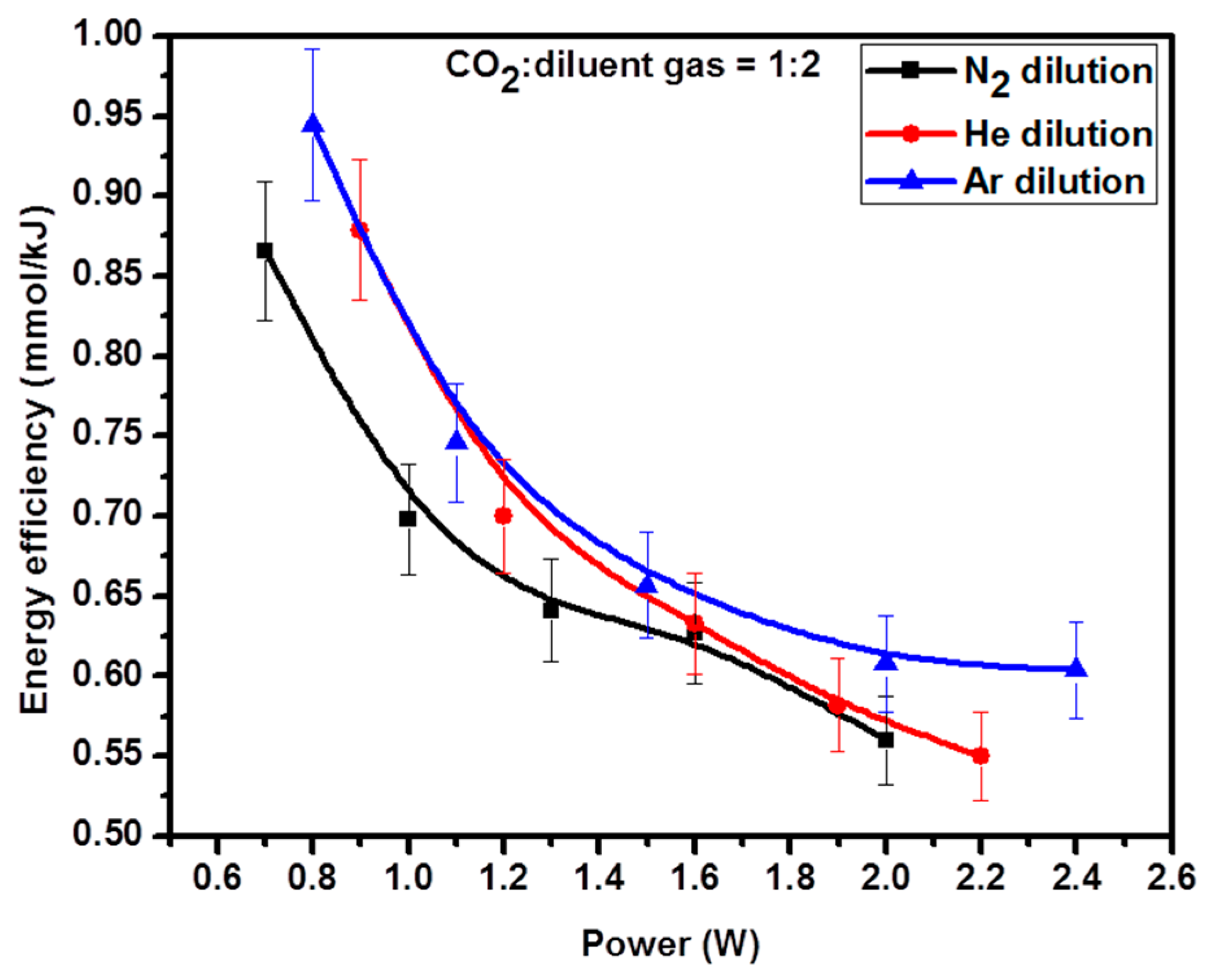
2.6. Energy Efficiency
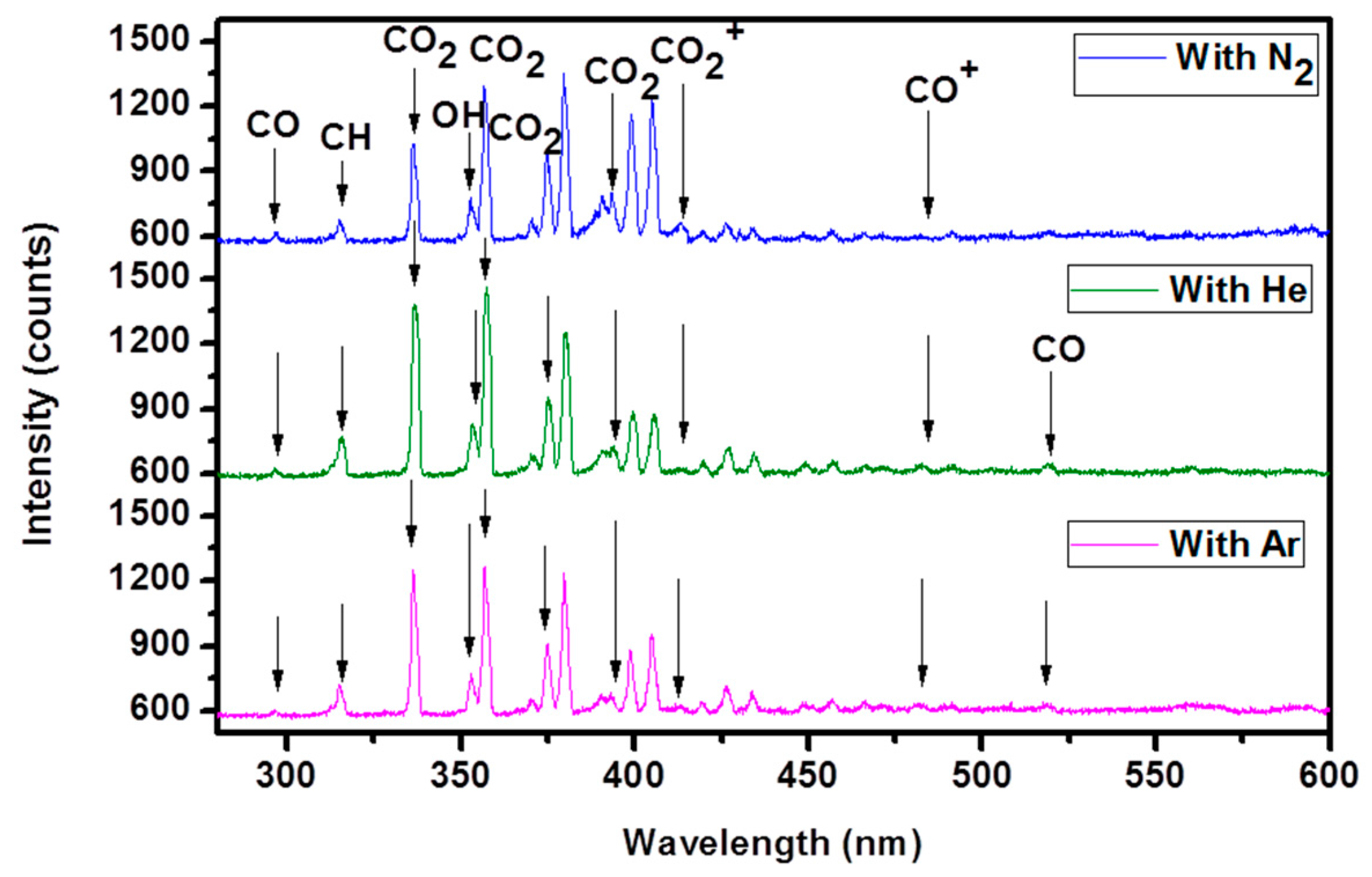
2.7. Optical Emission Spectra (OES)
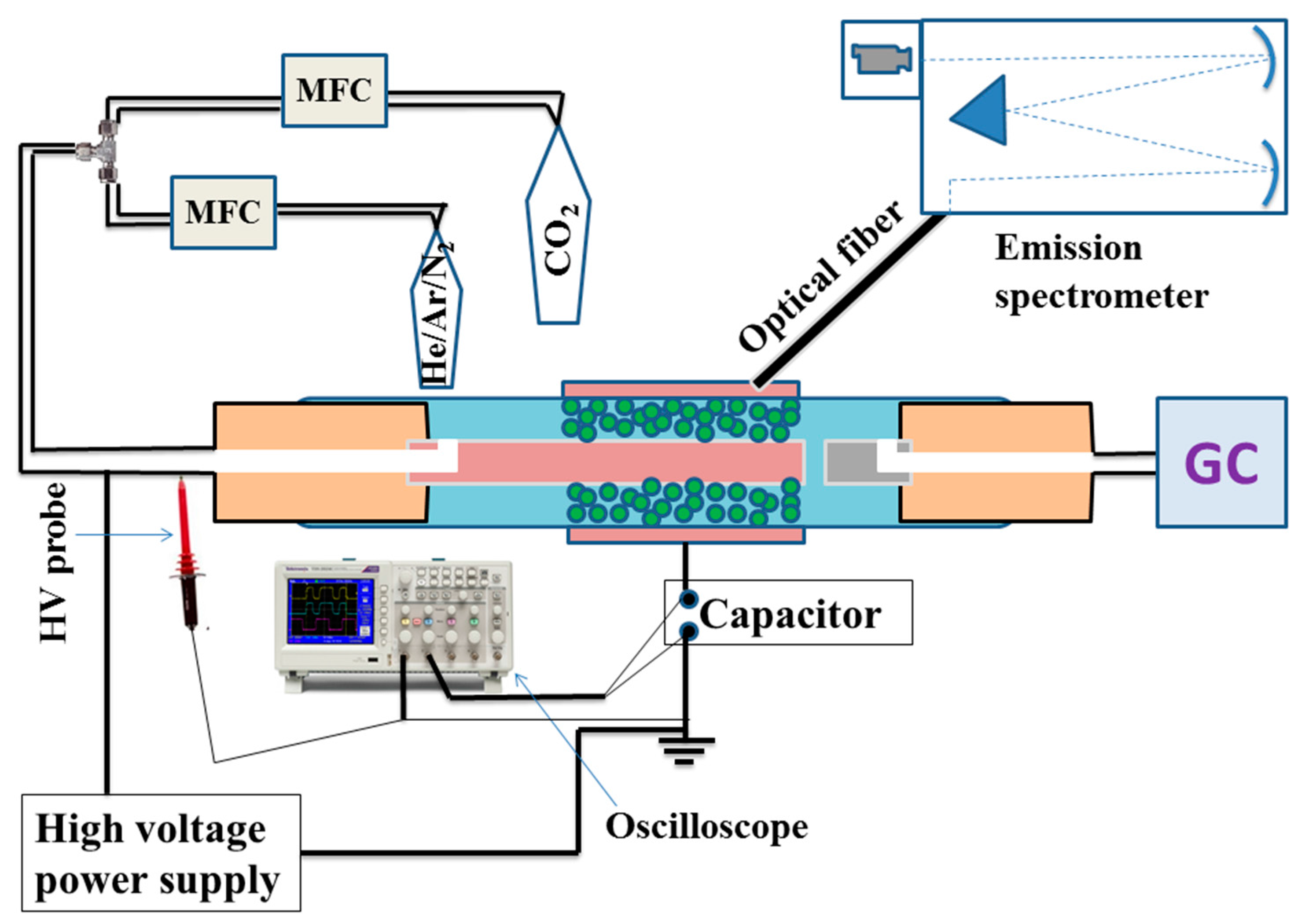
3. Experimental Set-Up
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zeng, Y.; Tu, X. Plasma-Catalytic CO2 Hydrogenation at Low Temperatures. IEEE Trans. Plasma Sci. 2016, 44, 405–411. [Google Scholar] [CrossRef]
- Mahammadunnisa, S.; Reddy, E.L.; Ray, D.; Subrahmanyam, C.; Whitehead, J.C. CO2 reduction to syngas and carbon nanofibres by plasma-assisted in situ decomposition of water. Int. J. Greenh. Gas Control 2013, 16, 361–363. [Google Scholar] [CrossRef]
- Snoeckx, R.; Heijkers, S.; Wesenbeeck, K.V.; Lenaerts, S.; Bogaerts, A. CO2 conversion in a dielectric barrier discharge plasma: N2 in the mix as helping hand or problematic impurity? Energy Environ. Sci. 2016, 9, 999–1011. [Google Scholar] [CrossRef]
- Chandana, L.; Subrahmanyam, C. Degradation and Mineralization of Aqueous Phenol by an Atmospheric Pressure Catalytic Plasma Reactor. J. Environ. Chem. Eng. 2016. [Google Scholar] [CrossRef]
- Chandana, L.; Lakshminarayana, B.; Subrahmanyam, C. Influence of hydrogen peroxide on the simultaneous removal of Cr(VI) and methylene blue from aqueous medium under atmospheric pressure plasma jet. J. Environ. Chem. Eng. 2015, 3, 2760–2767. [Google Scholar] [CrossRef]
- Baravian, G.; Chaleix, D.; Choquet, P.; Nauche, P.L.; Puech, V.; Rozoy, M. Oil removal from iron surfaces by atmospheric-pressure barrier discharges. Surf. Coat. Technol. 1999, 115, 66–69. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Renken, A.; Kiwi-Minsker, L. Catalytic abatement of volatile organic compounds assisted by non-thermal plasma, Part II. Optimized catalytic electrode and operating conditions. Appl. Catal. B Environ. 2006, 65, 157–162. [Google Scholar] [CrossRef]
- Ray, D.; Reddy, P.M.K.; Subrahmanyam, C. Glass Beads Packed DBD-Plasma Assisted Dry Reforming of Methane. Top. Catal. 2017. [Google Scholar] [CrossRef]
- Ray, D.; Reddy, P.M.K.; Subrahmanyam, C. Ni-Mn/γ-Al2O3 assisted plasma dry reforming of methane. Catal. Today 2017. [Google Scholar] [CrossRef]
- Horvath, G.; Skalný, J.D.; Mason, N.J. FTIR study of decomposition of carbon dioxide in dc corona discharges. J. Phys. D Appl. Phys. 2008, 41, 225207. [Google Scholar] [CrossRef]
- Mikoviny, T.; Kocan, M.; Matejcik, S.; Mason, N.J.; Skalny, J.D. Experimental study of negative corona discharge in pure carbon dioxide and its mixtures with oxygen. J. Phys. D Appl. Phys. 2004, 37, 64. [Google Scholar] [CrossRef]
- Wen, Y.; Jiang, X. Decomposition of CO2 using pulsed corona discharges combined with catalyst. Plasma Chem. Plasma Process. 2001, 21, 665–678. [Google Scholar] [CrossRef]
- Kozák, T.; Bogaerts, A. Splitting of CO2 by vibrational excitation in non-equilibrium plasmas: A reaction kinetics model. Plasma Sources Sci. Technol. 2014, 23, 045004. [Google Scholar] [CrossRef]
- Wang, Y.F.; Tsai, G.H.; Shih, M.; Hsieh, L.T.; Chang, W.C. Direct Conversion of Methane into Methanol and Formaldehyde in an RF Plasma Environment II: Effects of Experimental Parameters. Aerosol Air Qual. Res. 2005, 5, 211–224. [Google Scholar] [CrossRef]
- Indarto, A.; Yang, D.R.; Choi, J.W.; Lee, H.; Song, H.K. Gliding arc plasma processing of CO2 conversion. J. Hazard. Mater. 2007, 146, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, T.; Okazaki, K. Non-thermal plasma catalysis of methane: Principles, energy efficiency, and applications. Catal. Today 2013, 211, 29–38. [Google Scholar] [CrossRef]
- Mei, D.; Tu, X. Conversion of CO2 in a cylindrical dielectric barrier discharge reactor: Effects of plasma processing parameters and reactor design. J. CO2 Util. 2017, 19, 68–78. [Google Scholar] [CrossRef]
- Mei, D.; He, Y.L.; Liu, S.; Yan, J.; Tu, X. Optimization of CO2 Conversion in a Cylindrical Dielectric Barrier Discharge Reactor Using Design of Experiments. Plasma Process. Polym. 2016, 13, 544–556. [Google Scholar] [CrossRef]
- Mei, D.; Zhu, X.; Wu, C.; Ashford, B.; Williams, T.P.; Tu, X. Plasma-photocatalytic conversion of CO2 at low temperatures: Understanding the synergistic effect of plasma-catalysis. Appl. Catal. B Environ. 2016, 182, 525–532. [Google Scholar] [CrossRef]
- Zeng, Y.; Tu, X. Plasma-catalytic hydrogenation of CO2 for the cogeneration of CO and CH4 in a dielectric barrier discharge reactor: Effect of argon addition. J. Phys. D Appl. Phys. 2017, 50, 184004. [Google Scholar] [CrossRef]
- Ramakers, M.; Michielsen, I.; Aerts, R.; Meynen, V.; Bogaerts, A. Effect of Argon or Helium on the CO2 Conversion in a Dielectric Barrier Discharge. Plasma Process. Polym. 2015, 12, 755–763. [Google Scholar] [CrossRef]
- Wang, T.; Liu, H.; Xiong, X.; Feng, X. Conversion of carbon dioxide to carbon monoxide by pulse dielectric barrier discharge plasma. IOP Conf. Ser. Earth Environ. Sci. 2017, 52, 012100. [Google Scholar] [CrossRef]
- Yap, D.; Tatibouët, J.-M.; Batiot-Dupeyrat, C. Carbon dioxide dissociation to carbon monoxide by non-thermal plasma. J. CO2 Util. 2015, 12, 54–61. [Google Scholar] [CrossRef]
- Ray, D.; Subrahmanyam, C. CO2 decomposition in a packed DBD plasma reactor: Influence of packing materials. RSC Adv. 2016, 6, 39492–39499. [Google Scholar] [CrossRef]
- Dunn, F.A. The dielectric constants of argon, carbon dioxide, nitrogen, and oxygen determined at an audio frequency. Can. J. Phys. 1961, 12, 1480–1498. [Google Scholar] [CrossRef]
- Linga Reddy, E.; Biju, V.M.; Subrahmanyam, C. Production of hydrogen from hydrogen sulfide assisted by dielectric barrier discharge. Int. J. Hydrog. Energy 2012, 37, 2204–2209. [Google Scholar] [CrossRef]
- Mei, D.; Zhu, X.; He, Y.L.; Yan, D.J.; Tu, X. Plasma-assisted conversion of CO2 in a dielectric barrier discharge reactor: Understanding the effect of packing materials. Plasma Sources Sci. Technol. 2015, 24, 015011. [Google Scholar] [CrossRef]
- Garcia-Cosio, G.; Calixto-Rodriguez, M.; Martinez, H. Low-pressure plasma discharge of Ar/N2/CO2 ternary mixture. Topic number B6200954. In Proceedings of the 29th International Conference on Phenomena in Ionized Gases (ICPIG 2009), Cancun, Mexico, 12–17 July 2009. [Google Scholar]
- Ramakers, M.; Trenchev, G.; Heijkers, S.; Wang, W.; Bogaerts, A. Gliding Arc Plasmatron: Providing an Alternative Method for Carbon Dioxide Conversion. ChemSusChem 2017, 10, 2642–2652. [Google Scholar] [CrossRef] [PubMed]
- Bogaerts, A.; Berthelot, A.; Heijkers, S.; Kolev, S.; Snoeckx, R.; Sun, S.; Trenchev, G.; Laer, K.V.; Wang, W. CO2 conversion by plasma technology: Insights from modeling the plasma chemistry and plasma reactor design. Plasma Sources Sci. Technol. 2017, 26, 063001. [Google Scholar] [CrossRef]
- Kameshima, S.; Tamura, K.; Ishibashi, Y.; Nozaki, T. Pulsed dry methane reforming in plasma-enhanced catalytic reaction. Catal. Today 2015, 256, 67–75. [Google Scholar] [CrossRef]
- Kraus, M.; Egli, W.; Haffner, K.; Eliasson, B.; Kogelschatz, U.; Wokaun, A. Investigation of mechanistic aspects of the catalytic CO2 reforming of methane in a dielectric barrier discharge using optical emission spectroscopy and kinetic modeling. Phys. Chem. Chem. Phys. 2002, 4, 668–675. [Google Scholar] [CrossRef]
- Tu, X.; Whitehead, J.C. Plasma-catalytic dry reforming of methane in an atmospheric dielectric barrier discharge: Understanding the synergistic effect at low temperature. Appl. Catal. B Environ. 2012, 125, 439–448. [Google Scholar] [CrossRef]


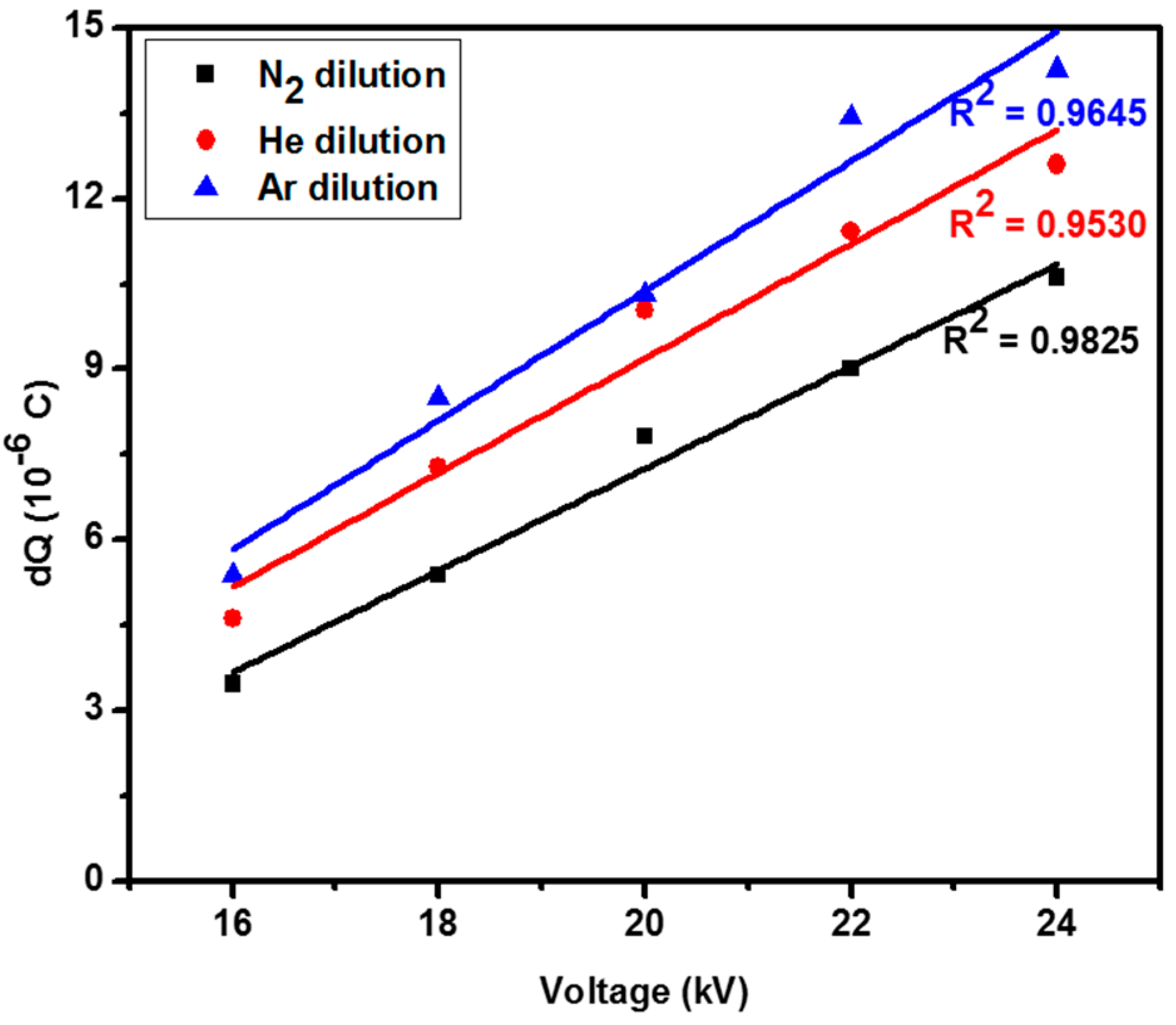

| Diluent Gas | Applied Voltage (kV) | Discharge Power (W) | Charge Transfer per Half Cycle (μC) |
|---|---|---|---|
| N2 | 24 | 2.0 | 10.61 |
| He | 24 | 2.2 | 12.60 |
| Ar | 24 | 2.4 | 14.28 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ray, D.; Saha, R.; Ch., S. DBD Plasma Assisted CO2 Decomposition: Influence of Diluent Gases. Catalysts 2017, 7, 244. https://doi.org/10.3390/catal7090244
Ray D, Saha R, Ch. S. DBD Plasma Assisted CO2 Decomposition: Influence of Diluent Gases. Catalysts. 2017; 7(9):244. https://doi.org/10.3390/catal7090244
Chicago/Turabian StyleRay, Debjyoti, Rajdeep Saha, and Subrahmanyam Ch. 2017. "DBD Plasma Assisted CO2 Decomposition: Influence of Diluent Gases" Catalysts 7, no. 9: 244. https://doi.org/10.3390/catal7090244
APA StyleRay, D., Saha, R., & Ch., S. (2017). DBD Plasma Assisted CO2 Decomposition: Influence of Diluent Gases. Catalysts, 7(9), 244. https://doi.org/10.3390/catal7090244