Abstract
In the race for industrialization and urbanization, the concentration of greenhouse gases like CO2 and CH4 is growing rapidly and ultimately resulting in global warming. An Ni-based catalyst over MgO support (Ni/MgO) offers a catalytic method for the conversion of these gases into hydrogen and carbon monoxide through the dry reforming of methane (DRM) reaction. In the current research work, 1–4 wt% strontium is investigated as a cheap promoter over a 5Ni/MgO catalyst to modify the reducibility and basicity for the goal of excelling the H2 yield and H2/CO ratio through the DRM reaction. The fine catalytic activities’ correlations with characterization results (like X-ray diffraction, surface area porosity, photoelectron–Raman–infrared spectroscopy, and temperature-programmed reduction/desorption (TPR/TPD)) are established. The 5Ni/MgO catalyst with a 3 wt.% Sr loading attained the highest concentration of stable active sites and the maximum population of very strong basic sites. 5Ni3Sr/MgO surpassed 53% H2 yield (H2/CO ~0.8) at 700 °C and 85% H2 yield (H2/CO ratio ~0.9) at 800 °C. These outcomes demonstrate the catalyst’s effectiveness and affordability. Higher Sr loading (>3 wt%) resulted in a weaker metal–support contact, the production of free NiO, and a lower level of catalytic activity for the DRM reaction. The practical and cheap 5Ni3Sr/MgO catalyst is scalable in industries to achieve hydrogen energy goals while mitigating greenhouse gas concentrations.
1. Introduction
Global warming has emerged as one of the most vital challenges of the 21st century, with increasing greenhouse gas (GHG) emissions significantly altering Earth’s climate systems [1]. CO2 and CH4 are the primary contributors to global warming, primarily generated by human activities such as industrial processes, fossil fuel combustion, and agricultural practices [2,3]. Mitigating the environmental impact of these gases requires innovative solutions to reduce their concentration in the atmosphere [4]. Among various strategies, converting greenhouse gases into valuable chemicals or fuels has gained considerable attention, presenting a sustainable approach to addressing climate change [5]. Dry reforming of methane (DRM) is a catalytic process that converts two potent greenhouse gases, CH4 and CO2, into valuable syngas, a mixture of H2 and CO [6,7]. Syngas is a critical feedstock for synthesizing valuable chemicals, such as methanol, and serves as a fuel precursor in the Fischer–Tropsch process. DRM is particularly attractive due to its environmental benefits and potential for industrial-scale application. However, its practical implementation is hindered by challenges such as catalyst deactivation caused by carbon deposition and sintering under high-temperature conditions [8,9]. Developing robust and efficient catalysts is crucial to overcoming these limitations and enabling the widespread adoption of DRM. The development of DRM catalysts has been extensively studied, with transition metals such as Ni emerging as a preferred choice due to their high activity and cost-effectiveness [9,10]. However, Ni-based catalysts often suffer from rapid deactivation due to coking and sintering during the reaction [11]. To address these issues, research has focused on modifying catalyst supports, incorporating promoters, and improving catalyst composition to overcome these problems. Kustov et al. observed worse catalytic activity with “10 wt% Ni-catalyst dispersed over Al2O3” than Ni dispersed over SiO2 or SiO2-Al2O3 [12]. As CO2 is an acid gas, it can interact with a basic material. Among various supports investigated in DRM, a basic support like MgO may be more advantageous than an acidic support like Al2O3 or a neutral support like SiO2 [13]. However, with Ni-based catalysts, MgO forms a solid MgO·NiO solution at high temperatures, and the low reducibility of such mixed oxides is always a limitation in the DRM reaction [14,15]. Usman et al. used a very high wt% of Ni (80 wt%) over MgO through the microemulsion method to ensure high catalytic activity due to the presence of more metallic Ni [16]. However, the use of such a high amount of Ni is again questionable.
By using basic promoters such as La and alkaline earth metals like Ca and Sr, the interaction with CO2 and stability of active sites over the catalyst surface can be further modified [17,18,19]. However, a La-promoted Ni/Al2O3 catalyst was prone to carbonate and coke formation [18]. In the same way, a calcium-promoted Ni/meso-SiO2 catalyst showed inferior catalytic activity towards DRM due to excessive coke deposition [19]. The formation of an Sr-Ni-O system (like SrNiO2.5, Sr5Ni4O11, and Sr9Ni7O21) by thermal decomposition of Sr-nitrate and Ni-nitrate solutions indicates the extent of interaction between Sr and Ni [20,21]. Sr addition may increase the basicity, structural stability, and stabilization of the NiO phase [22,23]. The presence of strontium was found to be responsible for the formation of strong basic sites over Ni/Al2O3 [24]. In the same way, strontium addition over Ni/La2O3 was found to induce basicity as well as surface oxygen species [25]. Over alumina–zirconia support, the promotional addition of Sr was found to optimize the size of Ni [26]. The presence of strontium was found to enhance the interaction of CO2 with the catalyst surface [27,28]. MgO support is known for its excellent thermal stability, basicity, and ability to disperse active metal sites. But MgO-supported Ni catalysts suffer from low reducibility, low concentration of active sites, and low activity. The addition of a small amount of strontium (as a promoter) may bring stabilization of active sites and may facilitate greater interaction of CO2 with the catalyst surface. This study investigates the catalytic performance of 5NixSr/MgO (x = 1, 2, 3, 4 wt%) in hydrogen production through the DRM process, focusing on its activity, stability, and resistance to carbon formation. By systematically evaluating the effects of varying Sr loadings, the study aims to provide insights into the synergistic interactions between Ni, Sr, and MgO in the DRM process. Through this research, we aim to address the persistent challenges of catalyst deactivation and contribute to the development of a sustainable DRM process. The findings will not only advance the understanding of Ni-based catalysts but also offer practical implications for the large-scale application of DRM in reducing greenhouse gas emissions and supporting the global transition toward a low-carbon economy.
2. Results and Discussion
2.1. Catalyst Activity
The catalytic performance of 5Ni/MgO and Sr-promoted 5Ni/MgO catalysts during the DRM reaction is illustrated in Figure 1. The H2 yield trends over time on stream (TOS) are presented in Figure 1A, showing that the unpromoted 5Ni/MgO catalyst exhibits the lowest hydrogen production, maintaining around 36% hydrogen yield throughout the reaction. With Sr promotion, hydrogen yield improves significantly, with 5Ni3Sr/MgO achieving the highest hydrogen yield (~56%) and demonstrating stable performance over 420 min of reaction. A similar trend is observed in the CO yield (Figure 1B), where 5Ni3Sr/MgO and 5Ni4Sr/MgO catalysts outperform the others, maintaining CO yields above 61%. The hydrogen-to-carbon monoxide ratio (Figure 1C) provides crucial insights into syngas composition. Ideally, in DRM, the H2/CO ratio should be close to 1. The Sr-promoted catalysts exhibit H2/CO ratios between 0.75 and 0.80, whereas the unpromoted 5Ni/MgO catalyst shows a lower ratio ~0.65, suggesting the prompt presence of H2-consuming reactions like reverse water–gas shift over unpromoted catalysts. Figure 1D highlights the effect of reaction temperature on the catalytic activity of 5Ni3Sr/MgO at 700 °C and 800 °C for 12 h time on stream. A distinct enhancement in both H2 and CO yields is observed when the temperature is increased. At 700 °C, the catalyst achieves a moderate H2 yield of 53%, CO yield of 64%, and H2/CO ratio of 0.89 (Figure 1D and Figure S1); however, at 800 °C, hydrogen yield stabilizes around 85%, CO yield approaches 88%, and H2/CO ratio reaches 0.95 (Figure S1). Additionally, the catalyst maintains stable performance over 12 h, indicating its stability under reaction conditions. Figure 1D also compares the experimental and equilibrium yields for H2 and CO over a 5Ni3Sr/MgO catalyst. It shows that at both 700 °C and 800 °C, the experimental yields are consistently below the corresponding equilibrium curves. This is expected as equilibrium represents the theoretical maximum conversion. The experimental data show the system approaching a steady-state yield but not reaching the equilibrium limit.
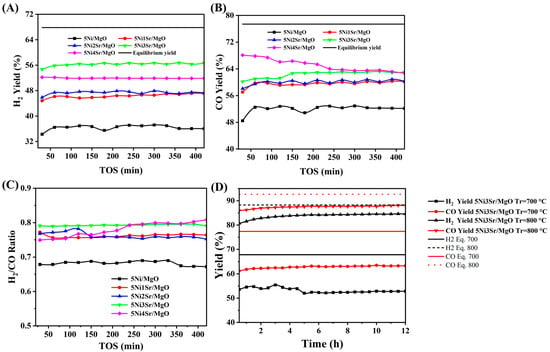
Figure 1.
Catalytic activity results in 5Ni/MgO and 5NixSr/MgO (x = 1, 2, 3, 4 wt%) catalysts at 700 °C: (A) H2 yield vs. TOS, (B) CO yield vs. TOS, (C) H2/CO ratio vs. TOS. (D) Experimental and equilibrium yields for H2 and CO are compared during 12 h over a 5Ni3Sr-MgO catalyst at 700 °C and 800 °C.
2.2. Characterization Results
The X-ray diffraction (XRD) patterns of the synthesized catalysts are presented in Figure 2A–C. Ni supported over MgO (Ni/MgO) shows the diffraction peaks corresponding to cubic MgO at Bragg’s angles 36.8°, 42.8°, 62.3°, 74.5°, and 78.4° [JCPDS reference number 00-043-1022]. Additionally, minor peaks related to Mg (OH)2 are also observed at approximately 38.2° and 62.2° [JCPDS reference number 00-001-1169] [29]. The diffraction peaks for cubic NiO are also detected at 37°, 43°, and 62° Bragg’s angles (JCPDS reference number 96-432-0506). However, these peaks are merged with cubic MgO peaks. The diffraction pattern of 1 wt.% Sr-promoted 5Ni/MgO is similar as 5Ni/MgO (Figure S2). Figure 2B shows the XRD patterns of Ni/MgO and Sr-promoted Ni/MgO catalysts. The presence of an orthorhombic SrCO3 phase is confirmed by a diffraction peak at approximately 25.2° (JCPDS Card 00-001-0556) in Sr-promoted catalysts. As a general observation, upon Sr loading from 1 to 4 wt.%, the diffraction peak intensity for the orthorhombic SrCO3 phase is increased (Figure 2B, Table 1). The crystalline sizes of orthorhombic SrCO3 over 5Ni1Sr/MgO, 5Ni2Sr/MgO, 5Ni3Sr/MgO, and 5Ni4Sr/MgO catalysts are 8.4 nm, 8.8 nm, 10.5 nm, and 13.1 nm, respectively. Interestingly, upon higher Sr loading (2–3 wt.%) over 5Ni/MgO, shifts of the diffraction pattern are observed, which indicate the structural modifications upon higher loading. The diffraction patterns of 5Ni3Sr/MgO and 5Ni4Sr/MgO catalysts are shifted slightly to higher and lower Bragg’s angles, respectively. The shift to a higher Bragg angle indicates compression in the lattice [30]. The shift of the diffraction pattern towards a lower Bragg’s angle over 5Ni4Sr/MgO is due to the formation of MgO·NiO solid solution [31]. Overall, it can be said that the formation of MgO·NiO solid solution is prohibited up to 3 wt.% Sr loading over the 5Ni/MgO catalyst.
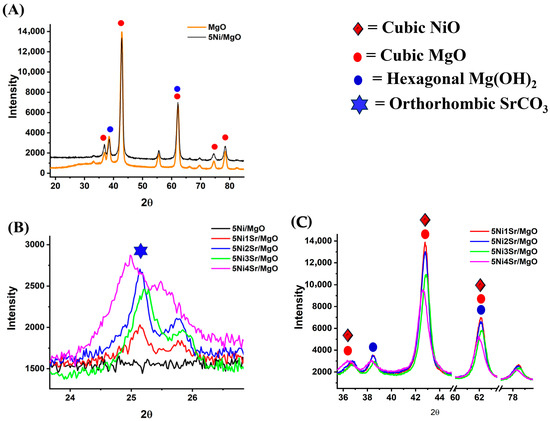
Figure 2.
XRD patterns of (A) MgO and Ni/MgO in the 2θ range of 20–80°, (B) X-ray diffraction pattern of 5NixSr/MgO (x = 0, 1, 2, 3, 4 wt%) in the 2θ range of 24–27°, and (C) 5NixSr/MgO (x = 1–4 wt%) in the 2θ range of 42–80°.

Table 1.
The principal characterization results and catalytic activity over different catalysts.
Figures S3 and S4 present the textural properties of the MgO, 5Ni/MgO, and Sr-promoted 5Ni/MgO catalysts. The N2 adsorption–desorption isotherms exhibit type IV behavior with H3 hysteresis loops, indicating pores with a wide distribution of sizes, including larger mesopores and possibly macrospores, and interconnected pores. The pore size distribution (dV/dlog(w) vs. pore width) plot (in Figure S3) indicates a multimodal distribution, with larger mesopores contributing to the overall porosity. In the MgO support, mesopores of a smaller size range are also exclusively present, whereas Ni/MgO and Sr-promoted MgO catalysts are populated with mesopores of a larger size range exclusively (Figure S4). MgO has a 45.7 m2/g surface area, 0.22 cm3/g pore volume, and 21 nm average pore diameter. Upon impregnation of Ni over MgO, the surface area, pore volume, and average pore diameter are improved greatly to 77.42 m2/g, 0.69 cm3/g, and 40.27 nm, respectively (Figure S3A, Table 1). It indicates the expansion of the MgO framework upon the incorporation of Ni into it. With the introduction of Sr (1–3 wt%), the surface area, pore volume, and pore diameter decrease progressively, suggesting partial blockage of mesopores by Sr species (Figure S3B–D). Upon further addition of Sr (4 wt%), all surface parameters are increased again (Figure S3E). In XRD, expansion of the crystalline lattice was noticed over the 5Ni4Sr/MgO catalyst. The expanded lattice aligns and results in enhanced surface parameters (68.55 m2/g surface area, 0.59 cm3/g pore volume, and 37.4 nm pore diameter). Figure S3F shows a relationship between average pore diameter and pore volume. The pore volume expansion results in the enlargement of the average pore diameter and vice versa.
The Raman spectra of 5Ni/MgO and Sr-promoted 5Ni/MgO catalysts are presented in Figure 3A. Raman is inactive for pure MgO [32]. For pure NiO, disorder-induced one-phonon Raman vibration (1P) at 560 cm−1 and two-phonon (2P) Raman vibrations at 740 cm−1 and 1100 cm−1 were reported [33]. Here, the 5Ni/MgO catalyst shows prominent peaks at about 562 cm−1 and 1097 cm−1, which are attributed to the 1P and 2P vibration bands for NiO. The FTIR spectra (Figure 3B) further confirm the presence of carbonate species and surface functional groups. The peaks at 862 cm−1 and 1458 cm−1 correspond to CO32− vibrations [34,35], whereas the broad peak at 3445 cm−1 and the peak at 3724 cm−1 are attributed to hydroxyl groups, indicating surface-adsorbed water and hydroxyl interactions [36,37]. It should be noted that the carbonated vibration peak is also observed over 5Ni/MgO and MgO (Figure S5). In XRD, the crystalline peak of SrCO3 is observed, but the diffraction pattern for MgCO3 is not obtained. The basic MgO can absorb CO2 (which is acidic) from the air and form surface carbonate species that may not be crystalline. Upon increasing the Sr loading, the peak intensity for the carbonate phase does not change much.
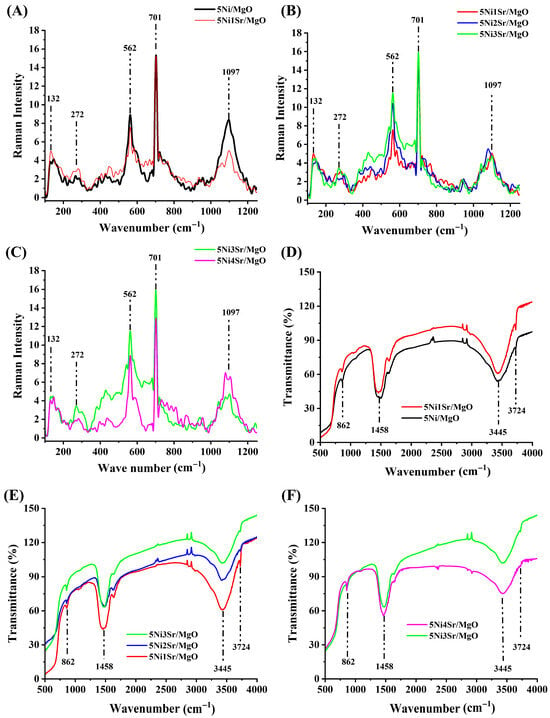
Figure 3.
Raman spectra of (A) 5Ni/MgO and 5Ni1Sr/MgO, (B) 5NixSr/MgO (x = 1, 2, 3 wt%), and (C) 5NixSr/MgO (x = 3, 4 wt%). FT-IR of (D) 5Ni/MgO and 5Ni1Sr/MgO, (E) 5NixSr/MgO (x = 1, 2, 3 wt%), and (F) 5NixSr/MgO (x = 3, 4 wt%).
The DRM catalysts are reduced under hydrogen with the goal of preparing active sites of Ni for the reaction. However, when the reaction is carried out, CO2 gas (one of the constituents of the feed gas in DRM) can oxidize the metallic Ni into NiO again [38]. H2 gas is generated during DRM, which further helps to attain the metallic state of Ni again. Overall, during this reduction–oxidation–reduction treatment, the active site distribution is further modified. To understand this phenomenon, a reduction–oxidation–reduction cyclic experiment is designed by H2TPR-CO2TPD-H2TPR. In this cyclic experiment, the first H2-TPR shows the reduction profile over fresh catalyst, whereas the last H2-TPR shows the reduction profile after “sequential reduction–oxidation treatment” (Figure S6). It is speculated that the reduction profile of the fresh catalyst system (in the first H2-TPR) is centered at about 750 °C, whereas, in the last H2-TPR, the reduction profile is observable before 700 °C. That means the sequential oxidation–reduction–oxidation treatment (by H2TPR-CO2TPD-H2TPR) enhances the edge of reducibility and creates all active sites within the reaction temperature (700 °C).
The last H2-TPR expresses the reduction profile, which persists during the DRM reaction, and it is presented in Figure 4A–C. The consumption of H2 during the first H2-TPR and last H2-TPR (in the H2TPR-CO2TPD-H2TPR cyclic experiment) is shown in Table 2. It is noticed that H2 consumption in the last H2 TPR is higher in non-promoted catalysts and 1–3 wt% Sr-promoted catalysts. This means that after reduction–oxidation–reduction treatment of the catalysts, the number of active sites is increased over these catalysts. Upon the highest Sr loading (4 wt%), the hydrogen consumption is lower in the last H2-TPR than in the first H2-TPR. This means that the total number of active sites present during the first H2-TPR (over fresh catalyst) is not recovered after reduction–oxidation–reduction treatment. The reduction temperature for the pure NiO was reported to be about 340 °C [39]. Here, the reduction profile of 5Ni/MgO is composed of two reduction peaks centered at about 475 °C and 650 °C, which are attributed to “NiO under moderate interaction” and “NiO under strong attraction”, respectively [40]. It should be noted that “NiO under strong interaction” can generate more stable active sites than “NiO under moderate interaction”. However, the H2 consumption over 5Ni/MgO is found to be the lowest (11.7 cm3/g), which indicates the lowest concentration of active sites over the non-promoted catalyst. Upon loading of 1–2 wt.% Sr, the intensity of reduction peaks is increased. 5Ni2Sr/MgO and 5Ni3Sr/MgO consume 12.9 cm3/g and 15.6 cm3/g H2, respectively. This indicates the growing population of active sites upon the addition of 1–2 wt.% Sr. Upon increasing the Sr loading to 3 wt.%, the intensity of the reduction peak at a higher temperature reaches the maximum. That means the 5Ni3Sr/MgO catalyst has the highest concentration of stable active sites. Upon a higher loading of Sr (4 wt.%) over 5Ni/MgO, the concentration of NiO under weak interaction is decreased, and the reduction profile is extended to lower temperatures (up to 340 °C). This temperature range was reported for the reduction of bulk NiO [39]. The result indicates a deterioration of the 5Ni4Sr/MgO catalyst’s metal–support interaction. A section of the NiO in this catalyst seems to be less affected by the support, suggesting a weaker connection.
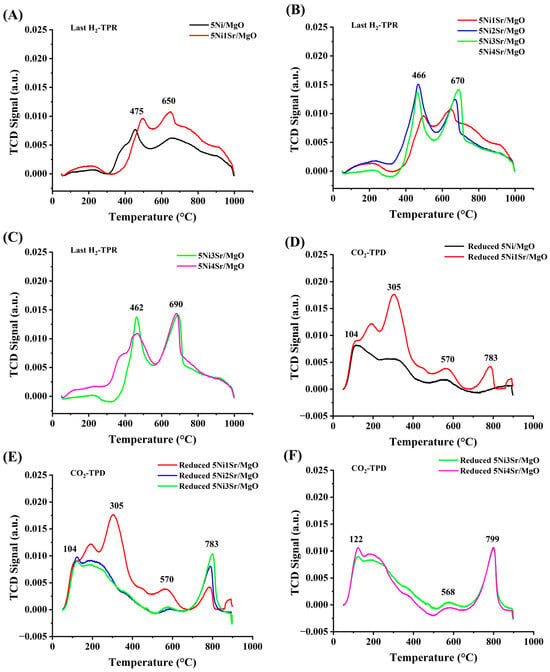
Figure 4.
The last H2-TPR of the cyclic H2TPR-O2TPO-H2TPR experiment of (A) 5Ni/MgO and 5Ni1Sr/MgO, (B) 5NixSr/MgO (x = 1, 2, 3 wt%), and (C) 5NixSr/MgO (x = 3, 4 wt%). CO2-TPD of reduced catalysts (D) 5Ni/MgO and 5Ni1Sr/MgO, (E) 5NixSr/MgO (x = 1, 2, 3 wt%), and (F) 5NixSr/MgO (x = 3, 4 wt%).

Table 2.
The amount of H2 desorbed at different temperature ranges.
The basic profile of the reduced catalyst system is studied by CO2-TPD and presented in Figure 4D–F. The MgO support is basic and it can also interact with acidic gas like CO2, and the introduction of Sr-O, like basic oxide, increases the interaction between the CO2 and the catalyst’s surface. The desorption peaks centered at about 100–305 °C are denoted as weak to moderate [41], whereas the desorption peak at about 550 °C is related to strong basic sites. Weak basic and strong basic sites are attributed to surface hydroxyl and surface oxide ions [27,42]. The desorption peak at about 775 °C is related to the dissociation of bonded carbonate species [43,44], which can be termed as very strong basic sites. Upon increasing Sr loading over 5Ni/MgO, the desorption peak at about 775 °C is intensified. Additionally, 3 wt.% Sr-loaded 5Ni/MgO catalyst has the highest concentration of very strong basic sites. The higher concentration of Sr+2 binds CO2 more efficiently [27,28]. Ghorbaei et al. reported effective CO2 diffusivity by SrO-bonded CO2 (as SrCO3 species) [43] at high temperatures. Overall, these bonded CO2 species are available over the catalyst surface for the DRM reaction. The CO2-TPD of 5Ni1Sr/MgO is noticeable because of the desorption of the highest amount of CO2 (Table 1). This indicates the presence of the highest concentration of weak to strong basic sites over 1 wt.% Sr-promoted 5Ni/MgO catalyst.
The XPS spectra of the Mg(1s), Ni (2p), and Sr (3d) are presented in Figure 5A–C. The binding energies for Mg (1s) spectra at 1305.5 eV and Sr (3d) spectra at 134.4 eV show the presence of Mg2+ and Sr2+ oxidation states, respectively [27,45]. The Ni 2p3/2 spectra at 857 eV and Ni 2p1/2 spectra at 875 eV correspond to the Ni2+ oxidation state [46]. As a general observation, the peak intensity of Ni2+ and Mg2+ is decreased, whereas Sr2+ is increased upon loading of Sr over the 5Ni/MgO. It indicates the surface enrichment by Sr2+ after promoter addition. Generally, at higher Sr loading, the peak intensity for Mg (1s) decreases, but in the case of 5Ni3Sr/MgO, it is observed that the peak intensity is higher (than the rest of the Sr-promoted catalysts), and it is shifted towards lower binding energy. From these findings, two points can be concluded. The 5Ni3Sr/MgO catalyst shows greater surface enrichment of Mg2+ and Sr2+ compared to the other Sr-promoted catalysts. Secondly, the 5Ni3Sr/MgO catalyst displays an increased negative charge density around Mg2+. This is attributed to the enrichment of SrO, which creates a higher concentration of oxide ions in the vicinity of the Mg2+. A high concentration of SrO over 5Ni3Sr/MgO modifies the electronic environment around Mg2+ and results in oxide enrichment about Mg2+. Raman spectra of spent 5Ni/MgO and spent Sr-promoted catalysts are presented in Figure 5D. The spectra exhibit three distinct peaks at 1347 cm−1, 1590 cm−1, and 2667 cm−1, corresponding to the D-band (defect carbon), G-band (graphitic carbon), and 2D-band (overtone of the D-band), respectively [47,48]. A lower ID/IG ratio of carbon indicates a higher degree of graphitization and vice versa. The graphitic carbon intensity is found to be maximum with the addition of 2–3 wt.% Sr over the 5Ni/MgO catalyst, whereas the 5Ni3Sr/MgO catalyst has the highest degree of graphitization (lowest ID/IG ratio).
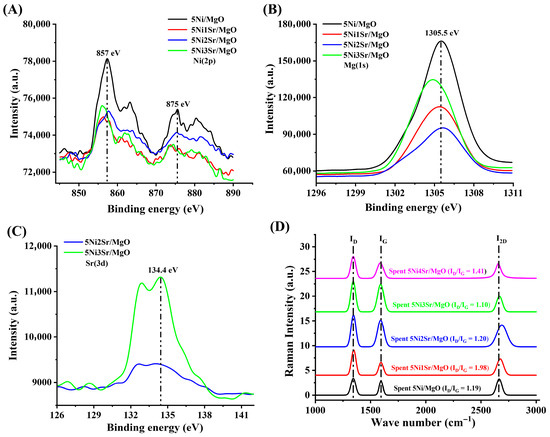
Figure 5.
The XPS spectra of 5Ni/MgO and 5Ni3Sr/MgO catalysts: (A) Mg(1s), (B) Ni(2p), and (C) Sr(3d). (D) Raman spectra of spent 5Ni/MgO and 5NixSr/MgO (x = 1, 2, 3, 4 wt%).
The thermogravimetric analysis (TGA) of the spent catalysts is presented in Figure 6. The weight loss profiles of spent 5Ni3Sr/MgO catalysts used in the DRM reaction at 700 °C and 800 °C were analyzed. The initial weight loss at lower temperatures can be attributed to the evaporation of adsorbed moisture. The weight gain at about 400 °C is due to oxidation of metallic Ni into NiO [38]. The further steady loss with increasing temperature is due to the oxidation of carbon deposits over the catalyst surface. At elevated temperatures (>800 °C), a more significant weight loss is observed, which may be associated with the decomposition of SrCO3, as previously studied [49]. Notably, the catalyst that is investigated at 700 °C has an additional weight gain at about 400 °C. Due to this weight gain, this catalyst has less weight loss (6.59%) than the catalyst investigated at 800 °C (weight loss 7.8%).
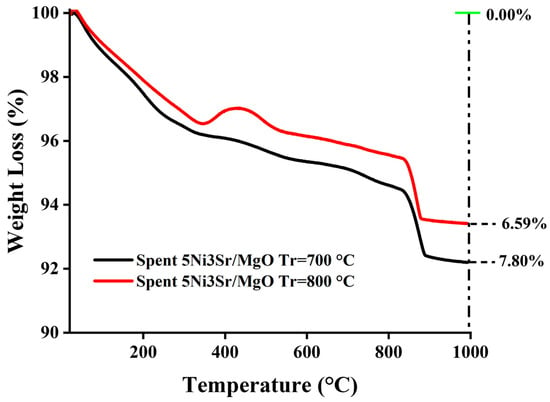
Figure 6.
Thermogravimetric analysis of 5Ni3Sr/MgO at 700 °C and 800 °C.
The transmission electron microscopy (TEM) analysis of fresh and spent 5Ni/Sr/MgO catalysts is presented in Figure 7. In the fresh 5Ni3Sr/MgO catalyst, the Ni nanoparticles are well dispersed over the MgO support, as observed in the TEM images (A–C). The particle size distribution (D) of fresh 5Ni3Sr/MgO catalyst indicates an average Ni particle size of 7.71 nm. However, after the DRM reaction, the TEM images of the spent 5Ni3Sr/MgO catalyst (E–G) reveal slight agglomeration of Ni particles. The particle size distribution (H) of the spent 5Ni3Sr/MgO shows an increase in average Ni particle size to 12.72 nm, suggesting moderate particle growth during the reaction. Despite this increase, the growth in Ni particle size remains controlled, indicating the structural stability of the catalyst under DRM reactions.
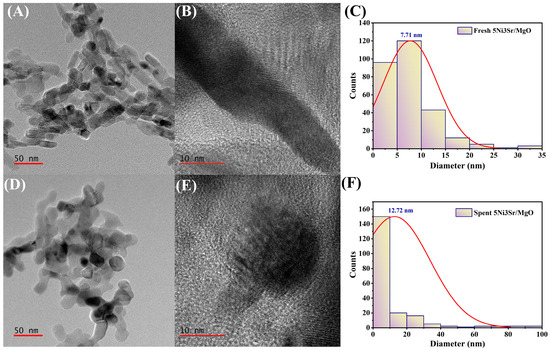
Figure 7.
TEM image of fresh 5Ni3Sr/MgO (A) at 50 nm scale and (B) at 10 nm scale. (C) Particle size distribution of 5Ni3Sr/MgO. TEM image of spent 5Ni3Sr/MgO (D) at 50 nm scale and (E) at 10 nm scale. (F) Particle size distribution of spent 5Ni3Sr/MgO.
3. Discussion
In the DRM reaction, CH4 and CO2 are passed over the catalyst and from syngas, H2 and CO, where the stoichiometric ratio of H2 and CO should be 1. But over 5Ni/MgO catalyst, the H2/CO ratio is lower than 0.67. It indicates that the produced H2 (from syngas) is further consumed over the catalyst surface, which brings down the H2/CO ratio. Among these gases (CO2, H2, CO), various H2-consuming reactions may take place, such as CO + H2 → H2O + C, CO2 + 2H2 → 2H2O + C and CO2 + H2 → H2O + CO (reverse water gas shift reaction; RWGS) [50]. However, at ≥700 °C reaction temperature, only RWGS remains thermodynamically feasible. This means it can be said that the low H2/CO ratio over 5Ni/MgO is due to the prominent presence of the RWGS reaction. The 5Ni/MgO catalyst shows high crystallinity and possesses the largest surface area, pore volume, and pore diameter among the tested materials. The fresh catalyst has the highest concentration of Ni2+ species (Figure 5A), but under the reduction–oxidation–reduction conditions (during DRM reaction), the lowest concentration of active sites remains there (Figure 4A). This catalyst lacks very strong basic sites (Figure 4D). With the presence of a minimum concentration of active sites, lack of very strong basic sites, and prominent significance of the RWGS reaction, the catalytic activity of 5Ni/MgO is the lowest and it attains ~36% H2 yield over 420 min.
Upon the addition of 1 wt.% Sr over 5Ni/MgO, the surface area and pore volume decline, but the concentration of active sites as well as basic sites of a wide range (from weak to very strong strength) grows (Figure 4A,D). Raman’s results show that the carbon deposit over 5Ni1Sr/MgO has the lowest degree of graphitization and is easily oxidizable. Overall, with its enriched reducibility–basicity profile and deposit of oxidizable carbon, 5Ni1Sr/MgO shows enhanced catalytic activity (46–47% H2 yield with a 0.76 H2/CO ratio over 420 min) towards DRM. The improved H2/CO ratio shows the inhibition of RWGS up to an extent. The drop in the surface area, the rise in concentration of active sites, and the rise in concentration of very strong basic sites continue over 2 wt.% Sr loading also. 5Ni2Sr/MgO has comparable catalytic activity to that of the 5Ni1Sr/MgO catalyst. Over 5Ni3Sr/MgO, the crystallinity of the catalyst drops (Figure 2C), the surface area remains comparable (to that of 5Ni2Sr/MgO), the concentration of stable active sites is the highest, and the optimum concentration of very strong basic sites is attained (Figure 4B–D). The catalyst’s surface is enriched with Mg2+ and Sr2+ (more than the rest of the Sr-promoted catalysts), and the Mg2+–Sr2+ ion pair leads to oxide enrichment about Mg2+ (Figure 5B,C). All these surface phenomena make 5Ni3Sr/MgO the best DRM catalyst, and it achieves 55–57% H2 yield with a ~0.8 H2/CO ratio up to 420 min. With higher Sr loading (4 wt.%) over 5Ni/MgO, the crystallinity of the support is the lowest and the optimum concentration of very strong basic sites is also obtained, but the formation of bulk NiO species (weakly interacted NiO) limits the catalytic activity to ~52% H2 yield up to 420 min. This means 5Ni3Sr/MgO is the best catalyst in this series, and it was tested in a long-time study (12 h), where it showed a constant 53% H2 yield with a constant 0.8 H2/CO ratio at 700 °C. Upon increasing the reaction temperature to 800 °C over the 5Ni3Sr/MgO, the H2 yield went up to 85% and the H2/CO ratio to 0.95 for 12 h due to the endothermic nature of the reaction. After a thorough study, it was found that the active site and basic site functionalities over the catalyst surface catalyze the DRM reaction over 5Ni/MgO and 5NixSr/MgO (x = 1, 2, 3, 4 wt%) catalysts. The Ni NiO interconversion continues in the presence of oxidizing and reducing gases, and the concentration of metallic Ni in the presence of CO2 and H2 is the real active site for reaction. The salient reaction mechanism of DRM and characteristic features of the current catalyst system for regulating catalytic activity are presented in Figure 8. The “Ni” active site catalyzes CH4 decomposition into CHx (x = 1, 2, 3). In a CH4−D2 exchange experiment and DFT study, Zeng et al. showed that CH4 is adsorbed over metallic Ni and sequentially dehydrogenates over it [51]. CO2 is adsorbed, activated, and then dissociated over the catalyst surface. Sr+2 and MgO serve as sorbents of CO2. The trapped CO2 (by Sr+2 and MgO) is further activated at close proximity to Ni (at the Ni–support interface or Ni–promoter interface), and finally, activated CO2 is dissociated [52,53]. Overall, CO2 is dissociated into CO and O* over an oxygen-deficient lattice, and CO2 can be assisted by H* to form COOH* species which further decompose into CO and OH* [54,55,56,57]. The surface-active O* and OH* species oxidize CHx selectively into CHxO* and CHxOH*, respectively, which are finally decomposed into a mixture of CO and H2 (syngas). The adsorbed methane fragments may also be polymerized into a coke deposit over the catalyst surface. The stable active sites (shown by the H2-TPR reduction peak about 560 °C in Figure 4) and very strong basic sites (shown by the CO2 desorption peak about 780 °C) over the catalyst are found to be the most crucial factor in determining catalytic activity towards DRM. The minimum activity over 5Ni/MgO is due to the minimum concentration of active sites (including the minimum concentration of stable active sites) and the lack of very strong basic sites. The excellent activity over 3 wt% Sr-promoted 5Ni/MgO is due to the presence of the highest concentration of stable active sites and very strong basic sites.
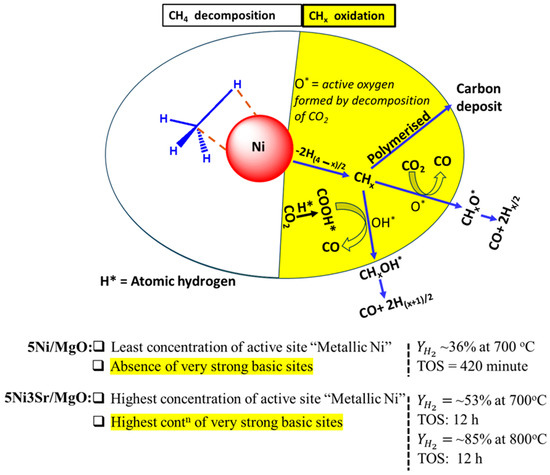
Figure 8.
The salient reaction mechanism of the DRM reaction and characteristic features of the current catalyst system for regulating catalytic activity. Note: Contn: concentration.
Figure 9 and Table S1 compare [58,59,60,61,62,63,64,65,66,67] the hydrogen yield of various catalyst systems. The current catalyst system outperforms others in terms of H2 yield [68,69,70,71,72]. The H2 yield over Ni catalyst dispersed over lanthana-zirconia was found to be comparable, but in terms of catalyst cost, again, the current catalyst system (5Ni3Sr/MgO) is cheaper. Overall, the current catalyst system attracts attention due to its high H2 yield at a lower cost.
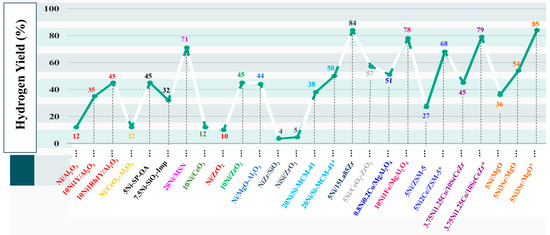
Figure 9.
Comparison of the hydrogen yield for DRM over a closely related catalyst. * = reaction at 800 °C. OA = oleic acid. Imp = impregnation method. MSN = mesoporous silica nanoparticles [58,59,60,61,62,63,64,65,66,67,68,69,70,71,72].
4. Experiment
4.1. Materials
Ni (NO3)2·6H2O (nickel nitrate hexahydrate; 98%, Alfa Aesar, Lancashire, UK), Sr (NO3)2·6H2O (strontium nitrate hexahydrate; Fisher, Frankfurt, Germany), MgO (Magnesium Oxide; 99.5%, BDH Chemicals, Dubai, United Arab Emirates), and distilled water are used to prepare catalysts.
4.2. Catalyst Preparation
The active metal nickel (Ni) and Sr promoter (x = 0, 1, 2, 3 wt.%) are incorporated on an MgO support. The MgO-based catalyst was synthesized with an Ni loading of 5 wt.% using nickel nitrate hexahydrate (Ni (NO3)3·6H2O, Alfa Aesar) by using the wet impregnation (WI) method. Ni and Sr metal precursors were prepared as an aqueous solution which was slowly added to the samples at 80 °C under constantly agitated surroundings until a slurry was formed. Then this slurry was dried overnight at 120 °C and calcined at 600 °C for 3 h. The catalyst was finely powdered prior to the process of catalytic application. The resulting catalysts were identified as 5NixSr/MgO (where x = 0, 1, 2, 3, 4 Sr wt.%).
4.3. Catalytic Performance Evaluation
The catalytic activity of the DRM was tested using a tubular stainless-steel reactor (Micro Activity Reference Catalytic Reaction, PID Eng. and Tech., Madrid, Spain) with a diameter of 9.1 mm and a height of 300 mm, operating at atmospheric pressure. The temperature was monitored by inserting a K-type thermocouple in the center. Hydrogen was passed to a 0.1 g catalyst sample at a rate of 30 mL/min for 1 h at 800 °C before starting the reaction. The remaining hydrogen was then removed by adding nitrogen, and the temperature was maintained at 800 °C. A total gas hourly space velocity (GHSV) of 42,000 mL/g.h. was achieved by conducting the DRM reaction at 1 atm and 800 °C with a reactant gas consisting of CH4:CO2:N2 at 30:30:10 mL/minute. Additional testing was performed on the best-performing catalyst at a lower GHSV of 15,000 mL/g.h. Nitrogen gas was used as both a carrier gas and an internal standard. Since DRM is an endothermic reaction requiring high temperatures, nitrogen facilitates heat dissipation to maintain a stable reaction environment. In addition to lowering carbon deposition and increasing catalyst durability, it helped control the reactor’s gas flow rate and pressure. A TCD-equipped online gas chromatograph was used to examine the gaseous product composition. The H2/CO molar ratio and hydrogen and carbon monoxide yields were determined using previously established formulae.
5. Conclusions
Ni dispersed over MgO (Ni/MgO) is highly crystalline; it occupies the highest surface area compared to the other promoted catalyst systems. However, it exhibits the lowest activity due to its low concentration of active sites and lack of very strong basic sites. Sr addition of 1 wt.% over Ni/MgO cultivated the highest concentration of basic sites and enhanced the population of active sites. These physico-chemical changes over 5Ni1Sr/MgO catalyze the DRM and yield 47% H2 with a 0.76 H2/CO ratio. The 5Ni3Sr/MgO catalyst displays the highest surface concentration of Mg2+ and Sr2+ together, along with the highest oxide enrichment surrounding Mg2+, compared to the other Sr-promoted catalysts. The high concentration of Mg2+ and Sr2+ enhances the formation of a high concentration of very strong basic sites and makes it easier for the optimal growth of stable active sites. The 5Ni3Sr/MgO catalyst achieves 55–53% H2 yield with ~0.8 H2/CO at 700 °C and 85% H2 yield with 0.96 H2/CO at 800 °C for 12 h. The higher loading of Sr (4 wt.%) over 5Ni/MgO weakens the metal–support interaction, thus generating free NiO species, which limits the catalytic activity further. The development of a cheap 5Ni3Sr/MgO catalyst for achieving high H2 yield (~85%) through DRM is economical for the catalyst community and industries.
The 5Ni3Sr/MgO catalyst (investigated in this study) produces excellent H2 yield (85%) with a 0.95 H2/CO ratio. The cheap catalyst system, practical catalyst preparation procedure, and high catalytic activity make the 5Ni3Sr/MgO catalyst scalable for syngas production. Syngas is a known synthetic feedstock for high-value-added chemicals, including gasoline and diesel [73]. H2 production from syngas can be further scaled up by using the water gas shift reaction coupled with a membrane reactor (WGS-MR) [74]. The DRM-allied WGS-MR process may offer the opportunity of on-site hydrogen production from methane for fuel cells and industrial hydrogen production for reduction processes in refineries and ammonia synthesis.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15090853/s1. Catalyst characterization S1. Figure S1. (D) H2/CO ratio vs. TOS (12 h) for 5Ni3Sr/MgO catalyst at 700 and 800 °C. Figure S2A. The X-ray diffraction pattern for 5Ni/MgO and 5Ni1Sr/MgO catalysts. Figure S2B. The X-ray diffraction pattern for 5Ni/MgO and 5NixSr/MgO (x = 1, 2, 3, 4 wt%) catalysts. Figure S3. N2 adsorption–desorption isotherms of 5Ni/MgO and 5NixSr/MgO (x = 1, 2, 3, 4 wt%). Figure S4. N2 adsorption–desorption isotherms of MgO. Figure S5. FTIR of MgO and 5Ni/MgO. Figure S6. Cyclic H2TPR-O2TPO-H2TPR profile of (A) 5Ni/MgO, (B) 5Ni1Sr/MgO, (C) 5Ni2Sr/MgO, (D) 5Ni3Sr/MgO, (E) 5Ni4Sr/MgO. Table S1. Comparative catalyst activity for dry reforming of methane over a closely related catalyst system.
Author Contributions
A.S.B.: methodology, conceptualization, writing. A.B.: software, methodology, validation. F.A.A.A. and A.A.M.A.: formal analysis, data curation, methodology. K.J.C.: formal analysis, data curation, writing. M.O.B. and A.B.J.: data curation, validation, software, formal analysis. A.M.M.S.: writing—review and editing, R.K.: review, editing and writing. A.S.A.-F.: methodology, funding acquisition, project administration, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Ongoing Research Funding program (ORF-2025-612), King Saud University, Riyadh, Saudi Arabia., and Researchers Supporting Project number PNURSP2025R320, Princess Nourah bint Abdulrahman University.
Data Availability Statement
Data is contained within the article and Supplementary Material.
Acknowledgments
The authors would like to extend their sincere appreciation to the Ongoing Research Funding program (ORF-2025-612), King Saud University, Riyadh, Saudi Arabia. Also, the authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R230), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Peter, S.C. Reduction of CO2 to chemicals and fuels: A solution to global warming and energy crisis. ACS Energy Lett. 2018, 3, 1557–1561. [Google Scholar] [CrossRef]
- Pongratz, J.; Le Quéré, C. National contributions to climate change due to historical emissions of carbon dioxide, methane, and nitrous oxide since 1850. Sci. Data 2023, 10, 155. [Google Scholar] [CrossRef]
- Al-Ghussain, L. Global warming: Review on driving forces and mitigation. Environ. Prog. Sustain. Energy 2019, 38, 13–21. [Google Scholar] [CrossRef]
- Mac Kinnon, M.A.; Brouwer, J.; Samuelsen, S. The role of natural gas and its infrastructure in mitigating greenhouse gas emissions, improving regional air quality, and renewable resource integration. Prog. Energy Combust. Sci. 2018, 64, 62–92. [Google Scholar] [CrossRef]
- Jeffry, L.; Ong, M.Y.; Nomanbhay, S.; Mofijur, M.; Mubashir, M.; Show, P.L. Greenhouse gases utilization: A review. Fuel 2021, 301, 121017. [Google Scholar] [CrossRef]
- Shamsuddin, M.R.; Asikin-Mijan, N.; Marliza, T.S.; Miyamoto, M.; Uemiya, S.; Yarmo, M.A.; Taufiq-Yap, Y.H. Promoting dry reforming of methane via bifunctional NiO/dolomite catalysts for production of hydrogen-rich syngas. RSC Adv. 2021, 11, 6667–6681. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, A.; Singh, S.A.; Reddy, B.M.; Roy, S. Integrated CO2 capture and dry reforming of CH4 to syngas: A review. Langmuir 2024, 40, 14766–14778. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Tran, A.V.; Vo, D.-V.N.; Nguyen, H.T.; Rajamohan, N.; Trinh, T.H.; Nguyen, T.L.; Le, Q.V.; Nguyen, T.M. Methane dry reforming: A catalyst challenge awaits. J. Ind. Eng. Chem. 2024, 140, 169–189. [Google Scholar] [CrossRef]
- Huang, L.; Li, D.; Tian, D.; Jiang, L.; Li, Z.; Wang, H.; Li, K. Optimization of Ni-based catalysts for dry reforming of methane via alloy design: A review. Energy Fuels 2022, 36, 5102–5151. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Zeng, C.; Sun, W.; Fan, H.; Ma, Q.; Zhao, T.-S. Recent research progress of methane dry reforming to syngas. Fuel 2025, 398, 135535. [Google Scholar] [CrossRef]
- Chen, C.; Wei, J.; Lu, Y.; Duyar, M.S.; Huang, Y.; Lin, L.; Ye, R. Confinement effects over Ni-based catalysts for methane dry reforming. Catal. Sci. Technol. 2023, 13, 6089–6101. [Google Scholar] [CrossRef]
- Kustov, A.L.; Aymaletdinov, T.R.; Shesterkina, A.A.; Kalmykov, K.B.; Pribytkov, P.V.; Mishin, I.V.; Dunaev, S.F.; Kustov, L.M. Methane dry reforming: Influence of the SiO2 and Al2O3 supports on the catalytic properties of Ni catalysts. Mendeleev Commun. 2024, 34, 221–223. [Google Scholar] [CrossRef]
- Chaudhary, K.J.; Banabdwin, K.M.; Abahussain, A.A.M.; Fakeeha, A.H.; Wazeer, I.; Abu-Dahrieh, J.K.; Hasnain Bakhtiar, S.U.; Kumar, R.; Al-Fatesh, A.S. The Role of Pore Architect, Reducibility and Silica-Alumina Ratio over Ni-Containing Molecular Sieves for Methane Partial Oxidation. Catal. Lett. 2025, 155, 93. [Google Scholar] [CrossRef]
- Jafarbegloo, M.; Tarlani, A.; Mesbah, A.W.; Muzart, J.; Sahebdelfar, S. NiO–MgO solid solution prepared by sol–gel method as precursor for Ni/MgO methane dry reforming catalyst: Effect of calcination temperature on catalytic performance. Catal. Lett. 2016, 146, 238–248. [Google Scholar] [CrossRef]
- Martra, G.; Arena, F.; Baricco, M.; Coluccia, S.; Marchese, L.; Parmaliana, A. High loading Ni/MgO catalysts. Surface characterization by IR spectra of adsorbed CO. Catal. today 1993, 17, 449–458. [Google Scholar] [CrossRef]
- Usman, M.; Daud, W.M.A.W. An investigation on the influence of catalyst composition, calcination and reduction temperatures on Ni/MgO catalyst for dry reforming of methane. RSC Adv. 2016, 6, 91603–91616. [Google Scholar] [CrossRef]
- Maina, S.C.P.; Ballarini, A.D.; Vilella, J.I.; de Miguel, S.R. Study of the performance and stability in the dry reforming of methane of doped alumina supported iridium catalysts. Catal. Today 2020, 344, 129–142. [Google Scholar] [CrossRef]
- Li, K.; Pei, C.; Li, X.; Chen, S.; Zhang, X.; Liu, R.; Gong, J. Dry reforming of methane over La2O2CO3-modified Ni/Al2O3 catalysts with moderate metal support interaction. Appl. Catal. B Environ. 2020, 264, 118448. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, G.; Liu, J.; Xu, Y.; Lv, Y. Production of syngas via CO2 methane reforming process: Effect of cerium and calcium promoters on the performance of Ni-MSC catalysts. Int. J. Hydrogen Energy 2020, 45, 640–649. [Google Scholar] [CrossRef]
- Cho, E.H.; Park, Y.-K.; Park, K.Y.; Song, D.; Koo, K.Y.; Jung, U.; Yoon, W.R.; Ko, C.H. Simultaneous impregnation of Ni and an additive via one-step melt-infiltration: Effect of alkaline-earth metal (Ca, Mg, Sr, and Ba) addition on Ni/γ-Al2O3 for CO2 methanation. Chem. Eng. J. 2022, 428, 131393. [Google Scholar] [CrossRef]
- Zinkevich, M. Constitution of the Sr–Ni–O system. J. Solid State Chem. 2005, 178, 2818–2824. [Google Scholar] [CrossRef]
- Taherian, Z.; Gharahshiran, V.S.; Wei, X.; Khataee, A.; Yoon, Y.; Orooji, Y. Revisiting the mitigation of coke formation: Synergism between support & promoters’ role toward robust yield in the CO2 reformation of methane. Nano Mater. Sci. 2024, 6, 536–547. [Google Scholar] [CrossRef]
- Chaudhary, M.L.; Kumar, R. Doped Ceria Catalyst System: Catalyzing Carbon Monoxide Transformation (A-Review). Orient. J. Chem. 2021, 37, 1262. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Naeem, M.A.; Fakeeha, A.H.; Abasaeed, A.E. CO2 reforming of methane to produce syngas over γ-Al2O3-supported NiSr catalysts. Bull. Chem. Soc. Jpn. 2013, 86, 742–748. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Kawi, S. Promotional effect of alkaline earth over Ni-La2O3 catalyst for CO2 reforming of CH4: Role of surface oxygen species on H2 production and carbon suppression. Int. J. Hydrogen Energy 2011, 36, 14435–14446. [Google Scholar] [CrossRef]
- Song, J.H.; Han, S.J.; Yoo, J.; Park, S.; Kim, D.H.; Song, I.K. Hydrogen production by steam reforming of ethanol over Ni–X/Al2O3–ZrO2 (X = Mg, Ca, Sr, and Ba) xerogel catalysts: Effect of alkaline earth metal addition. J. Mol. Catal. A Chem. 2016, 415, 151–159. [Google Scholar] [CrossRef]
- Abahussain, A.A.M.; Al-Fatesh, A.S.; Rajput, Y.B.; Osman, A.I.; Alreshaidan, S.B.; Ahmed, H.; Fakeeha, A.H.; Al-Awadi, A.S.; El-Salamony, R.A.; Kumar, R. Impact of Sr Addition on Zirconia–Alumina-Supported Ni Catalyst for CO x-Free CH4 Production via CO2 Methanation. ACS Omega 2024, 9, 9309–9320. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Zhou, M. Infrared Spectroscopy of [M(CO2)n] + (M = Ca, Sr, and Ba; n = 1–4) in the Gas Phase: Solvation-Induced Electron Transfer and Activation of CO2. J. Phys. Chem. A 2024, 128, 618–625. [Google Scholar] [CrossRef]
- El-Molla, S.A.; Hammed, M.N.; El-Shobaky, G.A. Catalytic conversion of isopropanol over NiO/MgO system doped with Li2O. Mater. Lett. 2004, 58, 1003–1011. [Google Scholar] [CrossRef]
- Santra, C.; Rahman, S.; Bojja, S.; James, O.O.; Sen, D.; Maity, S.; Mohanty, A.K.; Mazumder, S.; Chowdhury, B. Barium, calcium and magnesium doped mesoporous ceria supported gold nanoparticle for benzyl alcohol oxidation using molecular O2. Catal. Sci. Technol. 2013, 3, 360–370. [Google Scholar] [CrossRef]
- Titus, J.; Roussière, T.; Wasserschaff, G.; Schunk, S.; Milanov, A.; Schwab, E.; Wagner, G.; Oeckler, O.; Gläser, R. Dry reforming of methane with carbon dioxide over NiO–MgO–ZrO2. Catal. Today 2016, 270, 68–75. [Google Scholar] [CrossRef]
- Cazzanelli, E.; Kuzmin, A.; Mariotto, G.; Mironova-Ulmane, N. Study of vibrational and magnetic excitations in NicMg1−cO solid solutions by Raman spectroscopy. J. Phys. Condens. Matter 2003, 15, 2045. [Google Scholar] [CrossRef]
- Meng, K.; Qi, Y.; Wen, L.U.; Zheyong, F.A.N.; Jinhua, F.E.I.; Zheng, X.; Wheelock, T.D. Effect of calcination temperature on characteristics and performance of Ni/MgO catalyst for CO2 reforming of toluene. Chin. J. Catal. 2012, 33, 1508–1516. [Google Scholar] [CrossRef]
- Bishop, J.L.; King, S.J.; Lane, M.D.; Brown, A.J.; Lafuente, B.; Hiroi, T.; Roberts, R.; Swayze, G.A.; Lin, J.; Sánchez Román, M. Spectral properties of anhydrous carbonates and nitrates. Earth Sp. Sci. 2021, 8, e2021EA001844. [Google Scholar] [CrossRef]
- Przekop, R.E.; Marciniak, P.; Sztorch, B.; Czapik, A.; Stodolny, M.; Martyła, A. One-pot synthesis of Al2O3-La2O2CO3 systems obtained from the metallic precursor by the sol-gel method. J. Non-Cryst. Solids 2018, 479, 105–112. [Google Scholar] [CrossRef]
- Dai, F.; Zhuang, Q.; Huang, G.; Deng, H.; Zhang, X. Infrared spectrum characteristics and quantification of OH groups in coal. ACS Omega 2023, 8, 17064–17076. [Google Scholar] [CrossRef]
- Joshi, M.S.; Mohan Prabhu, K. Synthesis and characterization of ZSM-8-type zeolite crystals. Cryst. Res. Technol. 1988, 23, 561–566. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Li, G.; Zhao, Q.; Wu, X.S.; Wang, Y.; Sun, Y.; Hu, C. Synergy of oxygen vacancies and Ni0 species to promote the stability of a Ni/ZrO2 catalyst for dry reforming of methane at low temperatures. ACS Catal. 2023, 13, 6486–6496. [Google Scholar] [CrossRef]
- Jiang, Z.; Su, J.; Jones, M.O.; Shi, H.; Xiao, T.; Edwards, P.P. Catalytic Partial Oxidation of Methane over Ni-Based Catalysts Derived from Ni−Mg/Al Ternary Hydrotalcites. Energy Fuels 2009, 23, 1634–1639. [Google Scholar] [CrossRef]
- Zafarnak, S.; Rahimpour, M.R. Dry reforming of methane for CO2 conversion using sintering-resistant halloysite-supported Ni catalysts enhanced by alkaline-earth metal oxide promoters. Fuel 2025, 395, 135168. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Rajput, Y.B.; Bayazed, M.O.; Alrashed, M.M.; Abu-Dahrieh, J.K.; Elnour, A.Y.; Ibrahim, A.A.; Fakeeha, A.H.; Abasaeed, A.E.; Kumar, R. Impact of gallium loading and process conditions on H2 production from dry reforming of methane over Ni/ZrO2-Al2O3 catalysts. Appl. Catal. A Gen. 2024, 681, 119794. [Google Scholar] [CrossRef]
- Li, T.; Liang, Z.; Liu, J.; Zhang, Y.; Zhang, X.; Zhang, G. The role of cerium in CoLa bimetallic catalysts: Enhancing activation of CH4 and CO2 for improved DRM reaction. Int. J. Hydrogen Energy 2024, 61, 611–622. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, L.; Zhang, X.; Tu, B.; Ou, D.; Cheng, M. Carbonates formed during BSCF preparation and their effects on performance of SOFCs with BSCF cathode. Int. J. Hydrogen Energy 2012, 37, 19036–19044. [Google Scholar] [CrossRef]
- Nisa, K.S.; Suendo, V.; Sophiana, I.C.; Susanto, H.; Kusumaatmaja, A.; Nishiyama, N.; Budhi, Y.W. Effect of base promoter on activity of MCM-41-supported nickel catalyst for hydrogen production via dry reforming of methane. Int. J. Hydrogen Energy 2022, 47, 23201–23212. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, S.; Long, M.; Chen, D.; Duan, H.; Li, Y. Methane Chemical Looping Partial Oxidation Over NiO/Ce2 (SO4) 3-MgO Oxygen Carrier to Produce High Purity Syngas. In Proceedings of the TMS Annual Meeting & Exhibition, Orlando, FL, USA, 3–7 March 2024; Springer: Berlin/Heidelberg, Germany, 2024; pp. 420–434. [Google Scholar]
- Tao, X.; Yang, C.; Huang, L.; Xu, D. DBD plasma combined with catalysts derived from NiMgAlCe hydrotalcite for CO2 reforming of CH4. Mater. Chem. Phys. 2020, 250, 123118. [Google Scholar] [CrossRef]
- Shafiei, M.; Spizzirri, P.G.; Arsat, R.; Yu, J.; Du Plessis, J.; Dubin, S.; Kaner, R.B.; Kalantar-Zadeh, K.; Wlodarski, W. Platinum/graphene nanosheet/SiC contacts and their application for hydrogen gas sensing. J. Phys. Chem. C 2010, 114, 13796–13801. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Liu, Z.; Wang, L.; Han, P.; Xu, H.; Zhang, K.; Dong, S.; Yao, J.; Cui, G. Nitrogen-doped graphene nanosheets with excellent lithium storage properties. J. Mater. Chem. 2011, 21, 5430–5434. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Evaluation and performances comparison of calcium, strontium and barium carbonates during calcination/carbonation reactions for solar thermochemical energy storage. J. Energy Storage 2017, 13, 193–205. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Kasim, S.O.; Ibrahim, A.A.; Abasaeed, A.E.; Fakeeha, A.H. In situ auto-gasification of coke deposits over a novel Ni-Ce/W-Zr catalyst by sequential generation of oxygen vacancies for remarkably stable syngas production via CO2-reforming of methane. Appl. Catal. B Environ. 2021, 280, 119445. [Google Scholar] [CrossRef]
- Zeng, J.; Tarazkar, M.; Palmer, C.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Initial Steps in CH4 Pyrolysis on Cu and Ni. J. Phys. Chem. C 2021, 125, 18665–18672. [Google Scholar] [CrossRef]
- Wu, P.; Tao, Y.; Ling, H.; Chen, Z.; Ding, J.; Zeng, X.; Liao, X.; Stampfl, C.; Huang, J. Cooperation of Ni and CaO at interface for CO2 reforming of CH4: A combined theoretical and experimental study. Acs Catal. 2019, 9, 10060–10069. [Google Scholar] [CrossRef]
- Wang, H.; Li, R.; Wang, E.; Zhu, Z.; Zhang, J. CO2 capture and dissociation on novel Ni/CaO bifunctional materials: A theoretical study. Greenh. Gases Sci. Technol. 2024, 14, 411–426. [Google Scholar] [CrossRef]
- Zhu, Y.-A.; Chen, D.; Zhou, X.-G.; Yuan, W.-K. DFT studies of dry reforming of methane on Ni catalyst. Catal. Today 2009, 148, 260–267. [Google Scholar] [CrossRef]
- Wei, J.; Iglesia, E. Isotopic and kinetic assessment of the mechanism of reactions of CH4 with CO2 or H2O to form synthesis gas and carbon on nickel catalysts. J. Catal. 2004, 224, 370–383. [Google Scholar] [CrossRef]
- Ríos-Escudero, Á.; Costamagna, J.; Cárdenas-Jirón, G.I. Fukui Indexes Applied to the Reduced and Nonreduced Species of the Nickel(II) Tetraazadinaphtho[14]annulene Complex and Its Protonated Derivative. J. Phys. Chem. A 2004, 108, 7253–7260. [Google Scholar] [CrossRef]
- Akpan, E.; Sun, Y.; Kumar, P.; Ibrahim, H.; Aboudheir, A.; Idem, R. Kinetics, experimental and reactor modeling studies of the carbon dioxide reforming of methane (CDRM) over a new Ni/CeO2–ZrO2 catalyst in a packed bed tubular reactor. Chem. Eng. Sci. 2007, 62, 4012–4024. [Google Scholar] [CrossRef]
- Damyanova, S.; Shtereva, I.; Pawelec, B.; Mihaylov, L.; Fierro, J.L.G. Characterization of none and yttrium-modified Ni-based catalysts for dry reforming of methane. Appl. Catal. B Environ. 2020, 278, 119335. [Google Scholar] [CrossRef]
- Chein, R.Y.; Fung, W.Y. Syngas production via dry reforming of methane over CeO2 modified Ni/Al2O3 catalysts. Int. J. Hydrogen Energy 2019, 44, 14303–14315. [Google Scholar] [CrossRef]
- Pan, C.; Guo, Z.; Dai, H.; Ren, R.; Chu, W. Anti-Sintering Mesoporous Ni–Pd Bimetallic Catalysts for Hydrogen Production via Dry Reforming of Methane; Elsevier: Amsterdam, The Netherlands, 2020; Volume 45, ISBN 0360319920. [Google Scholar]
- Mourhly, A.; Kacimi, M.; Halim, M.; Arsalane, S. New low cost mesoporous silica (MSN) as a promising support of Ni-catalysts for high-hydrogen generation via dry reforming of methane (DRM). Int. J. Hydrogen Energy 2020, 45, 11449–11459. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Sebastian, V.; Monzon, A.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Yentekakis, I.V.; Goula, M.A. An in depth investigation of deactivation through carbon formation during the biogas dry reforming reaction for Ni supported on modified with CeO2 and La2O3 zirconia catalysts. Int. J. Hydrogen Energy 2018, 43, 18955–18976. [Google Scholar] [CrossRef]
- Hu, X.; Jia, X.; Zhang, X.; Liu, Y.; Liu, C.J. Improvement in the activity of Ni/ZrO2 by cold plasma decomposition for dry reforming of methane. Catal. Commun. 2019, 128, 105720. [Google Scholar] [CrossRef]
- Aghamohammadi, S.; Haghighi, M.; Karimipour, S. A comparative synthesis and physicochemical characterizations of Ni/Al2O3–MgO nanocatalyst via sequential impregnation and sol–gel methods used for CO2 reforming of methane. J. Nanosci. Nanotechnol. 2013, 13, 4872–4882. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, Y.; Wang, S.; Zhao, Q.; Mao, D.; Hu, C. Low-temperature catalytic CO2 dry reforming of methane on Ni-Si/ZrO2 catalyst. ACS Catal. 2018, 8, 6495–6506. [Google Scholar] [CrossRef]
- Aguiar, M.; Cazula, B.B.; Colpini, L.M.S.; Borba, C.E.; da Silva, F.A.; Noronha, F.B.; Alves, H.J. Si-MCM-41 obtained from different sources of silica and its application as support for nickel catalysts used in dry reforming of methane. Int. J. Hydrogen Energy 2019, 44, 32003–32018. [Google Scholar] [CrossRef]
- Abasaeed, A.; Kasim, S.; Khan, W.; Sofiu, M.; Ibrahim, A.; Fakeeha, A.; Al-Fatesh, A. Hydrogen yield from CO2 reforming of methane: Impact of La2O3 doping on supported Ni catalysts. Energies 2021, 14, 2412. [Google Scholar] [CrossRef]
- Kumar, P.; Sun, Y.; Idem, R.O. Comparative study of Ni-based mixed oxide catalyst for carbon dioxide reforming of methane. Energy Fuels 2008, 22, 3575–3582. [Google Scholar] [CrossRef]
- Aghaali, M.H.; Firoozi, S. Enhancing the catalytic performance of Co substituted NiAl2O4 spinel by ultrasonic spray pyrolysis method for steam and dry reforming of methane. Int. J. Hydrogen Energy 2021, 46, 357–373. [Google Scholar] [CrossRef]
- de Melo, M.A.F.; de Medeiros, R.L.B.A.; de Macedo, H.P.; de Melo, V.R.M.; de Oliveira, Â.A.S.; de Barros, J.M.F.; de Melo, D.M.A. Ni supported on Fe-doped MgAl2O4 for dry reforming of methane: Use of factorial design to optimize H2 yield. Int. J. Hydrogen Energy 2016, 41, 14047–14057. [Google Scholar]
- Chaudhary, K.J.; Al-Fatesh, A.S.; Ibrahim, A.A.; Osman, A.I.; Fakeeha, A.H.; Alhoshan, M.; Alarifi, N.; Ala’a, H.; Kumar, R. Enhanced hydrogen production through methane dry reforming: Evaluating the effects of promoter-induced variations in reducibility, basicity, and crystallinity on Ni/ZSM-5 catalyst performance. Energy Convers. Manag. X 2024, 23, 100631. [Google Scholar] [CrossRef]
- Rajput, Y.B.; Al-Fatesh, A.S.; Osman, A.I.; Bayazed, M.O.; Ibrahim, A.A.; Fakeeha, A.H.; Abasaeed, A.E.; Almubaddel, F.S.; Alothman, O.; Kumar, R. Enhancing hydrogen production via dry reforming of methane: Optimization of Co and Ni on scandia-ceria-zirconia supports for catalytic efficiency and economic feasibility. Fuel 2024, 378, 132843. [Google Scholar] [CrossRef]
- An, Y.; Lin, T.; Yu, F.; Yang, Y.; Zhong, L.; Wu, M.; Sun, Y. Advances in direct production of value-added chemicals via syngas conversion. Sci. China Chem. 2017, 60, 887–903. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, C.-Y. Water gas shift reaction for hydrogen production and carbon dioxide capture: A review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).