Cathode Catalyst PdAgCo/C for Optimal Performance of the Alkaline Anion Exchange Membrane Direct Ammonia Fuel Cells
Abstract
1. Introduction
2. Results
2.1. Physicochemical Characterizations
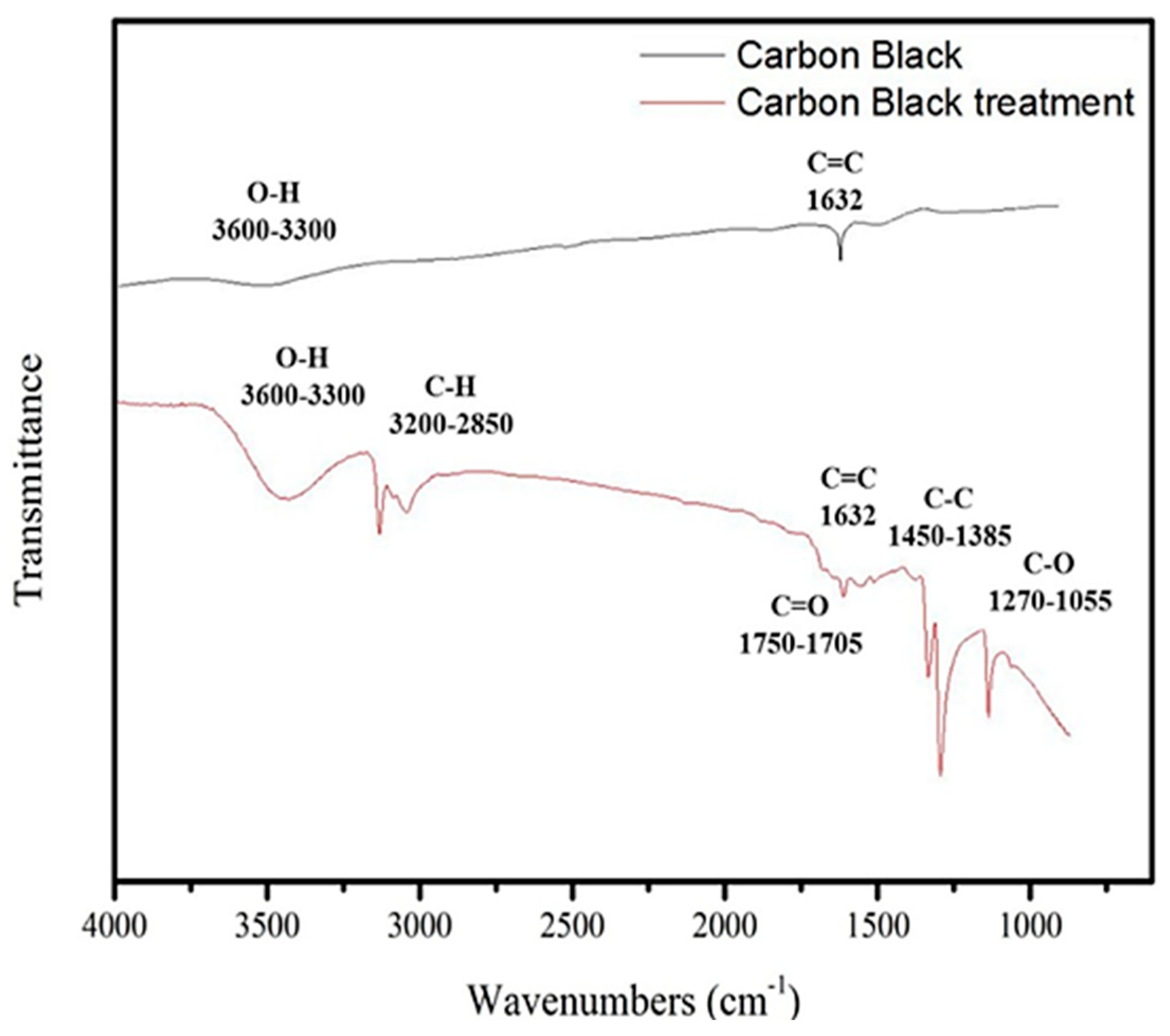
2.1.1. FTIR Analysis of Carbon Black
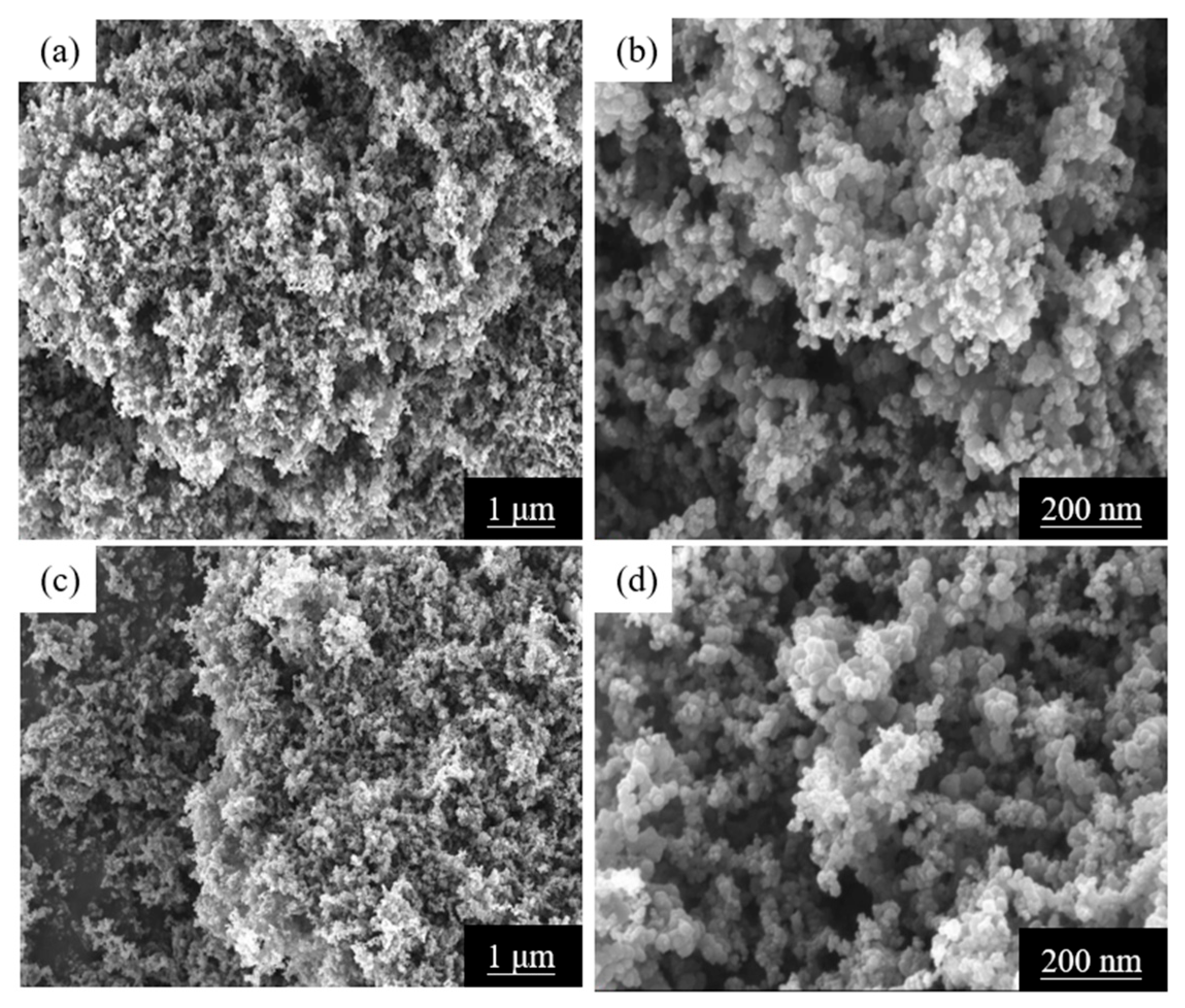
2.1.2. SEM and EDS Analysis of Ag/C
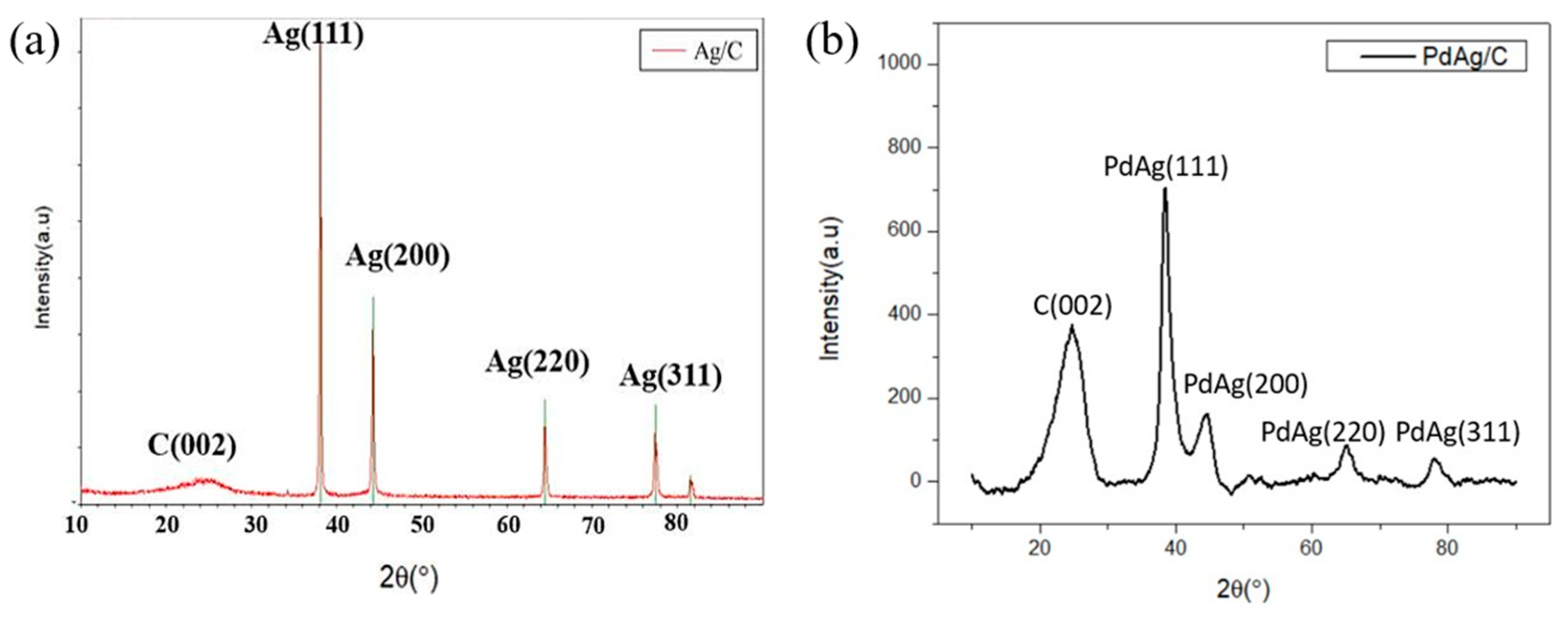
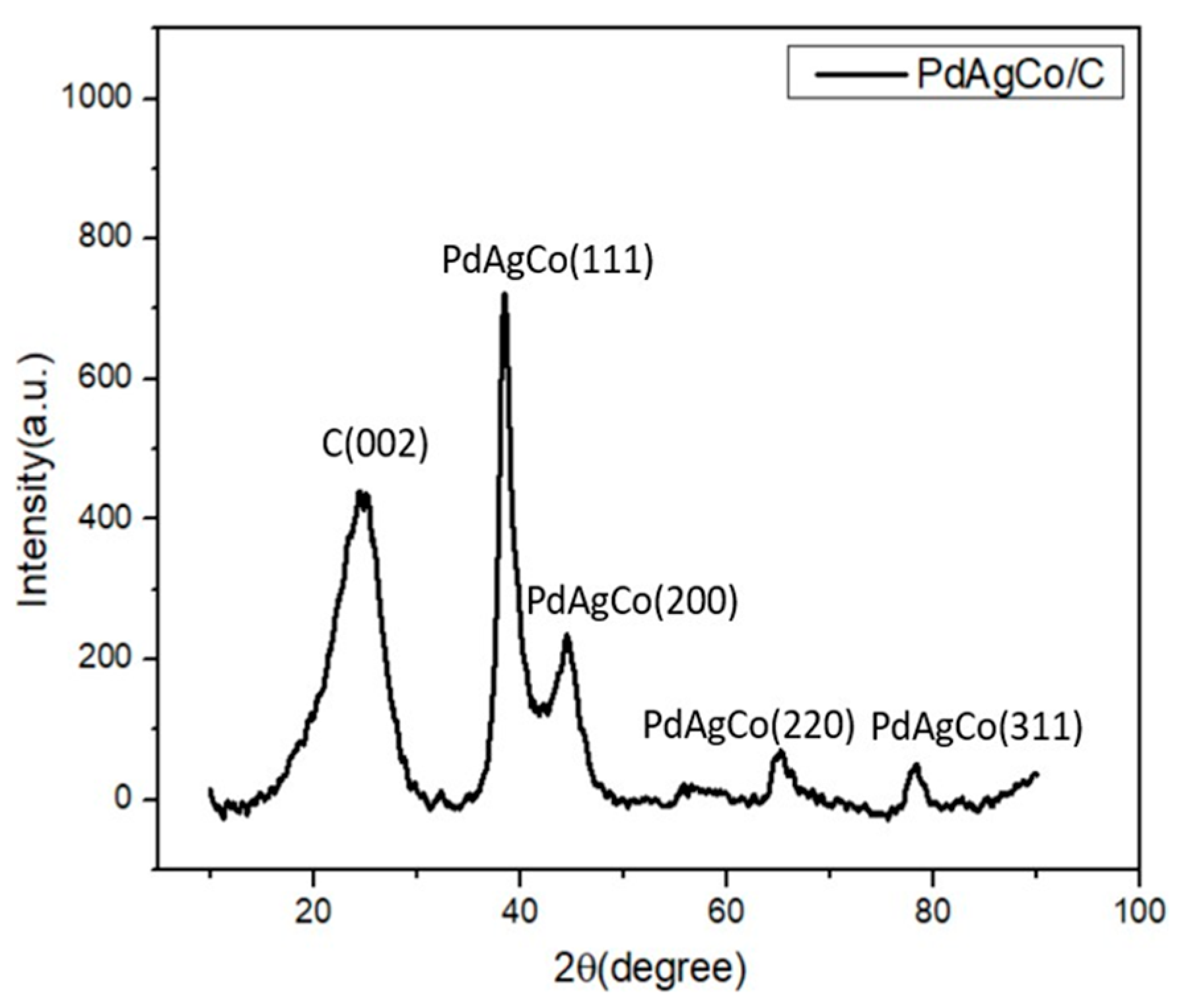
2.1.3. XRD Analysis of Ag/C, PdAg/C, and PdAgCo/C
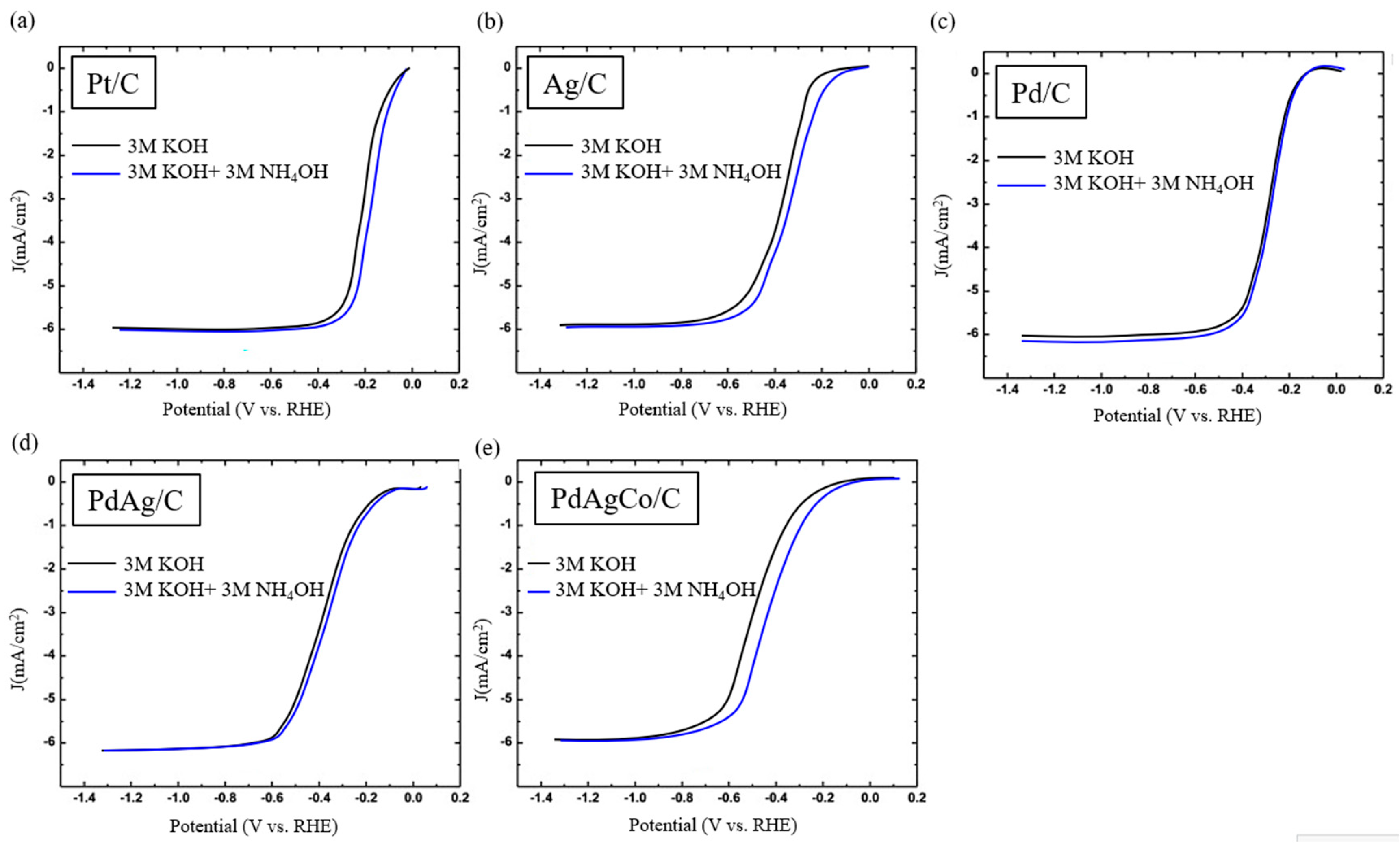
2.2. Electrochemical Characterization
2.3. Impact of Operating Parameters on the Power Density
2.3.1. Anode Fuel Concentration and Composition
2.3.2. Operating Temperature
2.3.3. Cathode Humidification Temperature
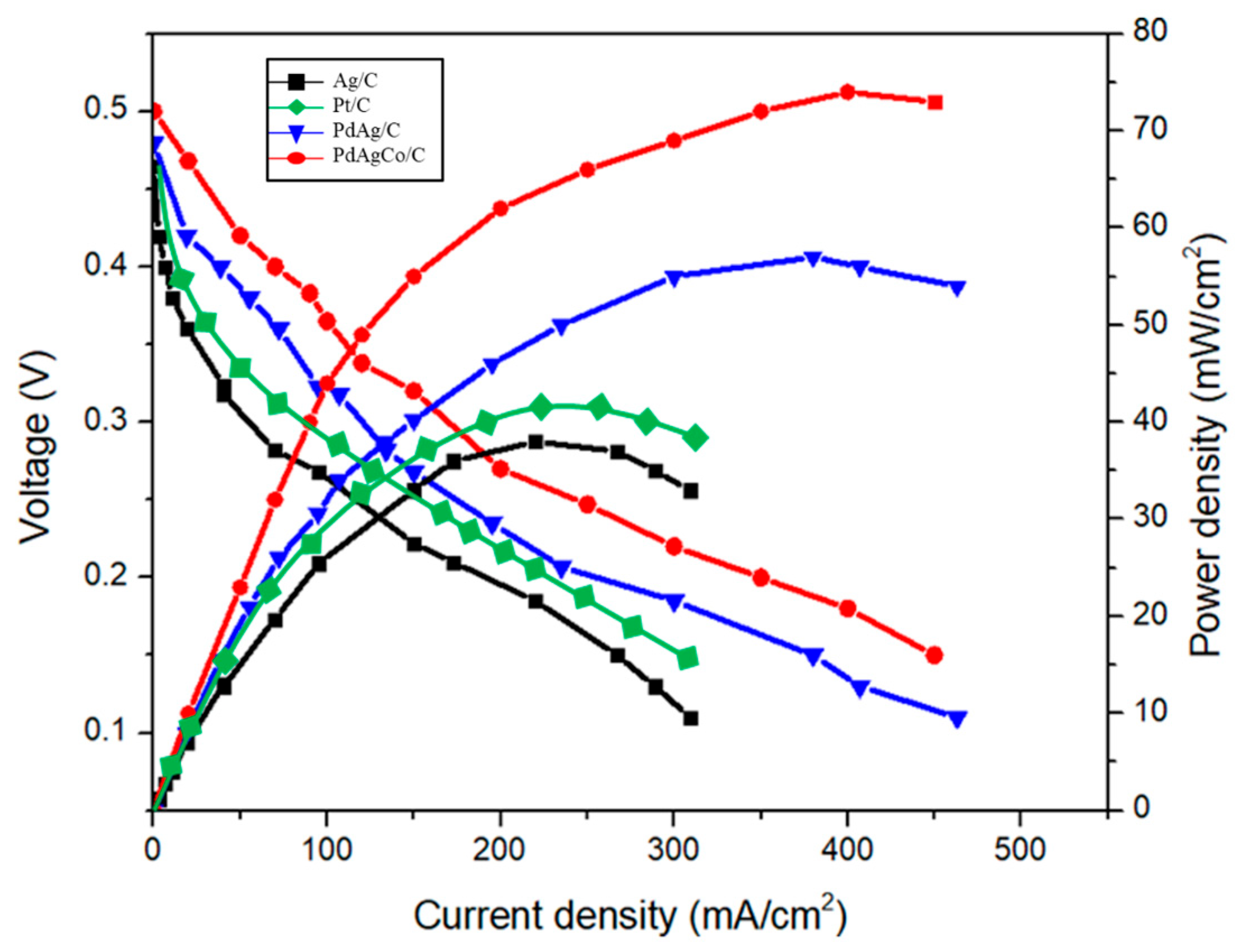
2.3.4. Performance Analysis of the Catalysts
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Catalysts
3.2.1. Anode Catalysts
3.2.2. Cathode Catalysts
3.3. Calibration of Working Parameters
3.3.1. Anode Fuel Concentration and Composition
3.3.2. Operating Temperature
3.3.3. Cathode Humidification Temperature
3.4. Optimal Load Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Osman, A.I.; Chen, L.; Yang, M.; Msigwa, G.; Farghali, M.; Fawzy, S.; Rooney, D.W.; Yap, P.-S. Cost, environmental impact, and resilience of renewable energy under a changing climate: A review. Environ. Chem. Lett. 2023, 21, 741–764. [Google Scholar] [CrossRef]
- Freitas, W.D.S.; D’Epifanio, A.; Ficca, V.C.A.; Placidi, E.; Arciprete, F.; Mecheri, B. Tailoring active sites of iron-nitrogen-carbon catalysts for oxygen reduction in alkaline environment: Effect of nitrogen-based organic precursor and pyrolysis atmosphere. Electrochim. Acta 2021, 391, 138899. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, Y.; Setzler, B.P.; Abbasi, R.; Gottesfeld, S.; Yan, Y. A high-performance 75 W direct ammonia fuel cell stack. Cell Rep. Phys. Sci. 2022, 3, 100829. [Google Scholar] [CrossRef]
- Abbasi, R.; Setzler, B.P.; Wang, J.; Zhao, Y.; Wang, T.; Gottesfeld, S.; Yan, Y. Low-temperature direct ammonia fuel cells: Recent developments and remaining challenges. Curr. Opin. Electrochem. 2020, 21, 335–344. [Google Scholar] [CrossRef]
- Dolan, R.H.; Anderson, J.E.; Wallington, T.J. Outlook for ammonia as a sustainable transportation fuel. Sustain. Energy Fuels 2021, 5, 4830–4841. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Z.; Esan, O.C.; Huo, X.; Shi, X.; An, L. Development and performance evaluation of a passive direct ammonia fuel cell. J. Power Sources 2023, 570, 233057. [Google Scholar] [CrossRef]
- Osmieri, L.; He, Y.; Chung, H.T.; McCool, G.; Zulevi, B.; Cullen, D.A.; Zelenay, P. La–Sr–Co oxide catalysts for oxygen evolution reaction in anion exchange membrane water electrolyzer: The role of electrode fabrication on performance and durability. J. Power Sources 2022, 556, 232484. (In English) [Google Scholar] [CrossRef]
- Cairns, E.J.; Simons, E.L.; Tevebaugh, A.D. Ammonia–Oxygen Fuel Cell. Nature 1968, 217, 780–781. [Google Scholar] [CrossRef]
- Abbasi, R.; Wang, H.; Lattimer, J.R.C.; Xu, H.; Wu, G.; Yan, Y. Effect of Ammonia on the Electrocatalysis of Oxygen Reduction Reaction in Base. J. Electrochem. Soc. 2020, 167, 164510. [Google Scholar] [CrossRef]
- Kapałka, A.; Cally, A.; Neodo, S.; Comninellis, C.; Wächter, M.; Udert, K.M. Electrochemical behavior of ammonia at Ni/Ni(OH)2 electrode. Electrochem. Commun. 2010, 12, 18–21. [Google Scholar] [CrossRef]
- Xu, W.; Lan, R.; Du, D.; Humphreys, J.; Walker, M.; Wu, Z.; Wang, H.; Tao, S. Directly growing hierarchical nickel-copper hydroxide nanowires on carbon fibre cloth for efficient electrooxidation of ammonia. Appl. Catal. B Environ. 2017, 218, 470–479. [Google Scholar] [CrossRef]
- Xu, W.; Du, D.; Lan, R.; Humphreys, J.; Miller, D.N.; Walker, M.; Wu, Z.; Irvine, J.T.S.; Tao, S. Electrodeposited NiCu bimetal on carbon paper as stable non-noble anode for efficient electrooxidation of ammonia. Appl. Catal. B Environ. 2018, 237, 1101–1109. [Google Scholar] [CrossRef]
- Okanishi, T.; Katayama, Y.; Muroyama, H.; Matsui, T.; Eguchi, K. SnO2-modified Pt electrocatalysts for ammonia–fueled anion exchange membrane fuel cells. Electrochim. Acta 2015, 173, 364–369. [Google Scholar] [CrossRef]
- Habibzadeh, F.; Miller, S.L.; Hamann, T.W.; Smith, M.R., III. Homogeneous electrocatalytic oxidation of ammonia to N(2) under mild conditions. Proc. Natl. Acad. Sci. USA 2019, 116, 2849–2853. [Google Scholar] [CrossRef]
- Lyu, Z.-H.; Fu, J.; Tang, T.; Zhang, J.; Hu, J.-S. Design of ammonia oxidation electrocatalysts for efficient direct ammonia fuel cells. EnergyChem 2023, 5, 100093. [Google Scholar] [CrossRef]
- Chen, R.; Zheng, S.; Yao, Y.; Lin, Z.; Ouyang, W.; Zhuo, L.; Wang, Z. Performance of direct ammonia fuel cell with PtIr/C, PtRu/C, and Pt/C as anode electrocatalysts under mild conditions. Int. J. Hydrogen Energy 2021, 46, 27749–27757. [Google Scholar] [CrossRef]
- De Vooys, A.C.A.; Koper, M.T.M.; Van Santen, R.A.; Van Veen, J.A.R. The role of adsorbates in the electrochemical oxidation of ammonia on noble and transition metal electrodes. J. Electroanal. Chem. 2001, 506, 127–137. [Google Scholar] [CrossRef]
- Garcia, A.C.; Paganin, V.A.; Ticianelli, E.A. CO tolerance of PdPt/C and PdPtRu/C anodes for PEMFC. Electrochim. Acta 2008, 53, 4309–4315. [Google Scholar] [CrossRef]
- Shao, M.H.; Sasaki, K.; Adzic, R.R. Pd-Fe nanoparticles as electrocatalysts for oxygen reduction. J. Am. Chem. Soc. 2006, 128, 3526–3527. [Google Scholar] [CrossRef]
- Sheng, W.; Gasteiger, H.A.; Shao-Horn, Y. Hydrogen Oxidation and Evolution Reaction Kinetics on Platinum: Acid vs Alkaline Electrolytes. J. Electrochem. Soc. 2010, 157, B1529. [Google Scholar] [CrossRef]
- Yu, P.-S.; Liu, C.-T.; Feng, B.; Wan, J.-F.; Li, L.; Du, C.-Y. Highly efficient anode catalyst with a Ni@PdPt core–shell nanostructure for methanol electrooxidation in alkaline media. Int. J. Miner. Metall. Mater. 2015, 22, 1101–1107. [Google Scholar] [CrossRef]
- Småbråten, D.R.; Strømsheim, M.D.; Peters, T.A. Hydrogen and Ammonia Co-Adsorption on M(1 1 1) and Pd3M(1 1 1) (M = Pd, Ru, Ag, Au, Cu) Surfaces. Membranes 2025, 15, 135. [Google Scholar] [CrossRef]
- Shao, M.H.; Huang, T.; Liu, P.; Zhang, J.; Sasaki, K.; Vukmirovic, M.B.; Adzic, R.R. Palladium monolayer and palladium alloy electrocatalysts for oxygen reduction. Langmuir 2006, 22, 10409–10415. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Yerramilli, A.S.; D’Oliveira, A.; Alford, T.L.; Boffito, D.C.; Patience, G.S. Experimental methods in chemical engineering: X-ray diffraction spectroscopy—XRD. Can. J. Chem. Eng. 2020, 98, 1255–1266. [Google Scholar] [CrossRef]
- Achrai, B.; Zhao, Y.; Wang, T.; Tamir, G.; Abbasi, R.; Setzler, B.P.; Page, M.; Yan, Y.; Gottesfeld, S. A Direct Ammonia Fuel Cell with a KOH-Free Anode Feed Generating 180 mW cm−2 at 120 °C. J. Electrochem. Society 2020, 167, 134518. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Luo, M.; Shi, X.; Pan, Z.; Chen, R.; An, L. Monitoring ammonia and water transport through anion exchange membranes in direct ammonia fuel cells. J. Power Sources 2025, 628, 235900. [Google Scholar] [CrossRef]
- Yang, Y.; Peltier, C.R.; Zeng, R.; Schimmenti, R.; Li, Q.; Huang, X.; Yan, Z.; Potsi, G.; Selhorst, R.; Lu, X.; et al. Electrocatalysis in Alkaline Media and Alkaline Membrane-Based Energy Technologies. Chem. Rev. 2022, 122, 6117–6321. [Google Scholar] [CrossRef]
- Kim, K.H.; Yu, J.-K.; Lee, H.S.; Choi, J.H.; Noh, S.Y.; Yoon, S.K.; Lee, C.-S.; Hwang, T.-S.; Rhee, Y.W. Preparation of Pt-Pd catalysts for direct formic acid fuel cell and their characteristics. Korean J. Chem. Eng. 2007, 24, 518–521. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Cullen, D.A.; Hwang, S.; Wang, M.; Li, B.; Liu, K.; Karakalos, S.; Lucero, M.; Zhang, H.; et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 2018, 1, 935–945. [Google Scholar] [CrossRef]
- Malko, D.; Kucernak, A.; Lopes, T. In situ electrochemical quantification of active sites in Fe–N/C non-precious metal catalysts. Nat. Commun. 2016, 7, 13285. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, G.; Wen, Y.; Chen, N.; Chen, W.; Regier, T.; Dynes, J.; Zheng, Y.; Sun, S. Atomically Dispersed Fe-Co Bimetallic Catalysts for the Promoted Electroreduction of Carbon Dioxide. Nano-Micro Lett. 2021, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Sekol, R.C.; Li, X.; Cohen, P.; Doubek, G.; Carmo, M.; Taylor, A.D. Silver palladium core–shell electrocatalyst supported on MWNTs for ORR in alkaline media. Appl. Catal. B Environ. 2013, 138, 285–293. [Google Scholar] [CrossRef]
- Chen, M.; Wang, L.; Yang, H.; Zhao, S.; Xu, H.; Wu, G. Nanocarbon/oxide composite catalysts for bifunctional oxygen reduction and evolution in reversible alkaline fuel cells: A mini review. J. Power Sources 2018, 375, 277–290. [Google Scholar] [CrossRef]
- Su, F.-C.; Yu Chu, G.; Yang, H. Silver nano-particles modification used as cathode catalysts to enhance anion exchange membrane fuel cells. Electrochim. Acta 2025, 534, 146544. [Google Scholar] [CrossRef]
- Su, F.-C.; Lu, Y.-H.; Prapainainar, P.; Yang, H. A Long-Term Stability Study of Co-Pc-Modified Nanosilver for Anion Exchange Membrane Fuel Cells. Catalysts 2025, 15, 25. [Google Scholar] [CrossRef]
- Bao, Y.; Zha, M.; Sun, P.; Hu, G.; Feng, L. PdNi/N-doped graphene aerogel with over wide potential activity for formic acid electrooxidation. J. Energy Chem. 2021, 59, 748–754. [Google Scholar] [CrossRef]
- Jeerh, G.; Zou, P.; Zhang, M.; Tao, S. Optimization of a Perovskite Oxide-Based Cathode Catalyst Layer on Performance of Direct Ammonia Fuel Cells. ACS Appl. Mater. Interfaces 2023, 15, 1029–1041. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, D.; Zhang, C.; Ming, P.; Xiao, Q. Optimization of the Cathode Catalyst Layer for Boosting Direct Ammonia Fuel Cell Performance by Orthogonal Tests. Energy Fuels 2023, 37, 8600–8613. [Google Scholar] [CrossRef]
- Sun, X.; Ma, B.; Hao, J.; Wu, Z.; Li, Y.; Liu, H.; Yang, Y.; Ren, X. Insight into Mass and Charge Transports in Alkaline Direct Ammonia Fuel Cell with Anti-Ammonia Poisoning Fe-N-C Cathode. 2025. Available online: https://ssrn.com/abstract=5194266 (accessed on 28 August 2025).
- Li, W.; Liu, Y.; Zhang, Z.; Pan, Z.; Chen, R.; An, L. Performance of a hybrid direct ammonia fuel cell with hydrogen peroxide reduction. J. Power Sources 2024, 593, 233985. [Google Scholar] [CrossRef]
- Su, F.-C.; Yu, H.-H.; Yang, H. Anion-Exchange Membranes’ Characteristics and Catalysts for Alkaline Anion-Exchange Membrane Fuel Cells. Membranes 2024, 14, 246. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Rojas-Carbonell, S.; Yan, Y.; Kusoglu, A. Structure-transport relationships of poly(aryl piperidinium) anion-exchange membranes: Eeffect of anions and hydration. J. Membr. Sci. 2020, 598, 117680. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, H.; Li, C.; Sang, Q.; Xie, Y.; Zhang, X.; Liu, Y. Progress in the design and performance evaluation of catalysts for low-temperature direct ammonia fuel cells. Green Chem. Eng. 2025, 6, 54–67. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, G.; Yang, Y.; Song, W.; Yu, H.; Hao, J.; Shao, Z. PtIr/CNT as anode catalyst with high reversal tolerance in PEMFC. Int. J. Hydrogen Energy 2023, 48, 36500–36511. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, S.; Pastor, E.; Lázaro, M.J. Electrochemical behavior of the carbon black Vulcan XC-72R: Influence of the surface chemistry. Int. J. Hydrogen Energy 2018, 43, 7911–7922. [Google Scholar] [CrossRef]



| Elemental | Weight % |
|---|---|
| C | 55.98 |
| O | 3.9 |
| Ag | 40.12 |
| Total | 100.0 |
| Elemental | Weight % |
|---|---|
| C | 82.18 |
| O | 2.24 |
| Pd | 7.21 |
| Ag | 7.79 |
| Total | 100 |
| Elemental | Weight % |
|---|---|
| C | 82.18 |
| O | 3.02 |
| Pd | 6.01 |
| Ag | 6.3 |
| Co | 3.25 |
| Total | 100 |
| Parameters | Concentration Profile of Ammonia Water | Concentration Profile of KOH | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Anode fuel composition | 1 M NH4OH + 3 M KOH | 3 M NH4OH + 3 M KOH | 5 M NH4OH + 3 M KOH | 7 M NH4OH + 3 M KOH | 3 M NH4OH + 1 M KOH | 3 M NH4OH + 3 M KOH | 3 M NH4OH + 5 M KOH | 3 M NH4OH + 7 M KOH |
| Cathode oxidant | O2 | |||||||
| Flow rate of anode fuel (mL/min) | 32 | |||||||
| Flow rate of cathode oxidant (sLmin) | 1 | |||||||
| Operating temperature (°C) | 110 | |||||||
| Anode and cathode catalyst | Pt/C | |||||||
| Power density (mW/cm2) | 33 | 40 | 39 | 38 | 32 | 40 | 35 | 31 |
| Anode fuel composition | 3 M NH4OH + 3 M KOH | ||
| Cathode Fuel | O2 | ||
| Flow rate of anode fuel (mL/min) | 32 | ||
| Flow rate of cathode oxidant (sL/min) | 1 | ||
| Cathode humidification temperature (°C) | 80 | 95 | 110 |
| Anode and cathode catalyst | Pt/C | ||
| Power density (mW/cm2) | 10 | 26 | 40 |
| Anode fuel composition | 3 M NH4OH + 3 M KOH | ||
| Cathode oxidant | O2 | ||
| Flow rate of anode fuel (mL/min) | 32 | ||
| Flow rate of cathode oxidant (sL/min) | 1 | ||
| Cathode humidification temperature (°C) | 70 | 80 | 90 |
| Anode and cathode catalyst | Pt/C | ||
| Power density (mW/cm2) | 30 | 36 | 40 |
| Anode Fuel | Cathode Oxidant | Anode Catalyst | Cathode Catalyst | Anode Catalyst Loading (mg/cm2) | Cathode Catalyst Loading (mg/cm2) | Power Density (mW/cm2) |
|---|---|---|---|---|---|---|
| 3 M NH4OH + 3 M KOH | O2 | Pt/C | Ag/C | 1 | 0.5 | 38 |
| Pt/C | 40 | |||||
| PdAgCo/C | 74 | |||||
| PdAg/C | 57 |
| Anode Fuel | Cathode Oxidant | Anode Catalyst | Cathode Catalyst | Anode Catalyst Loading (mg/cm2) | Cathode Catalyst Loading (mg/cm2) | Power Density (mW/cm2) |
|---|---|---|---|---|---|---|
| 3 M NH4OH + 3 M KOH | O2 | Pt/C | PdAgCo/C | 1 | 0.5 | 74 |
| PtIr/C | 172 |
| Anode Fuel | Cathode Oxidant | Anode Catalyst | Cathode Catalyst | Anode Catalyst Loading (mg/cm2) | Cathode Catalyst Loading (mg/cm2) | Power Density (mW/cm2) |
|---|---|---|---|---|---|---|
| 3 M NH4OH + 3 M KOH | O2 | PtIr/C | PdAgCo/C | 1 | 0.5 | 172 |
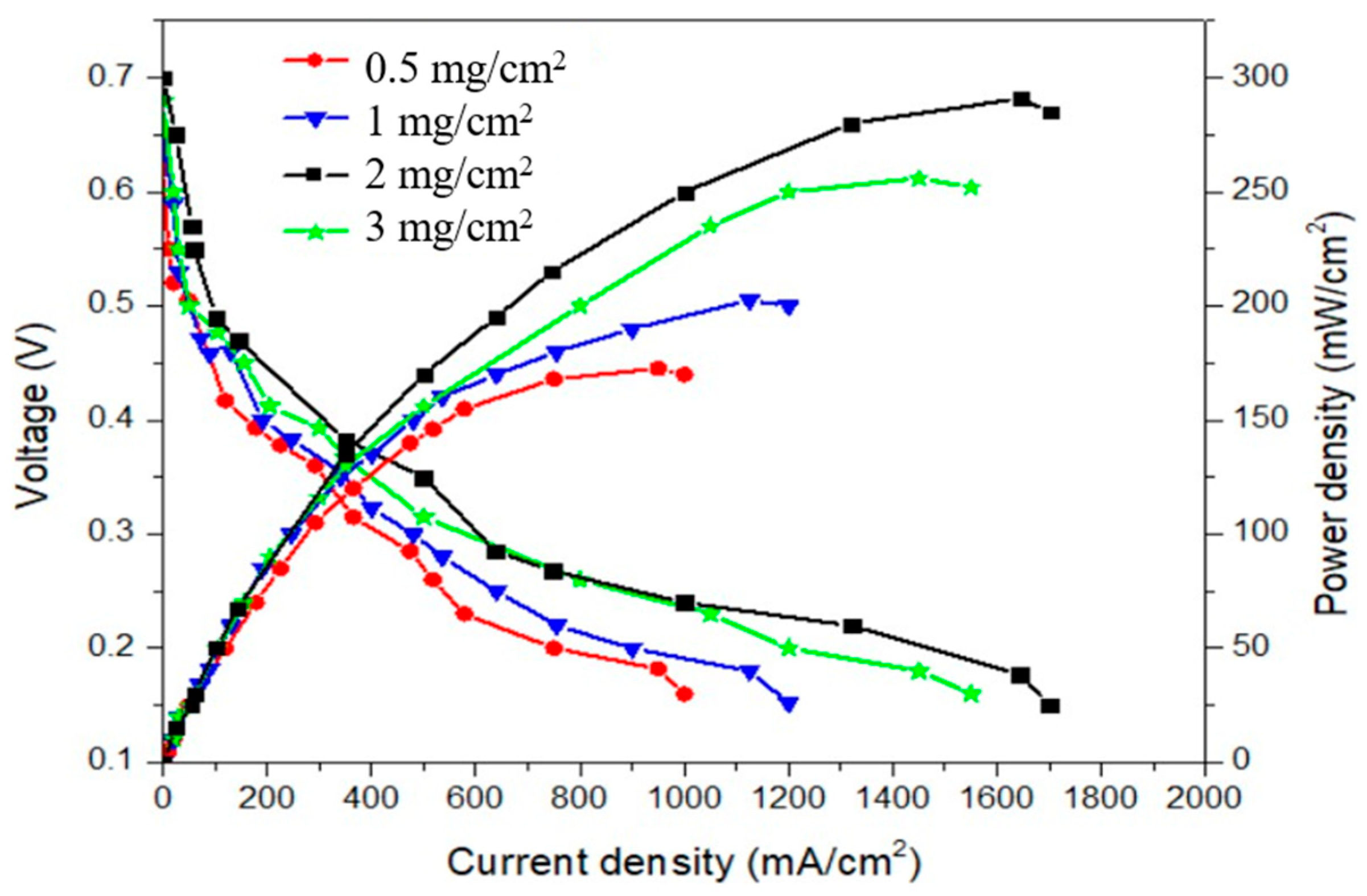
| 1 | 202 | |||||
| 2 | 291 | |||||
| 3 | 256 |
| Anode Fuel | Cathode Oxidant | Anode Catalyst | Cathode Catalyst | Anode Catalyst Loading (mg/cm2) | Cathode Catalyst Loading (mg/cm2) | Power Density (mW/cm2) |
|---|---|---|---|---|---|---|
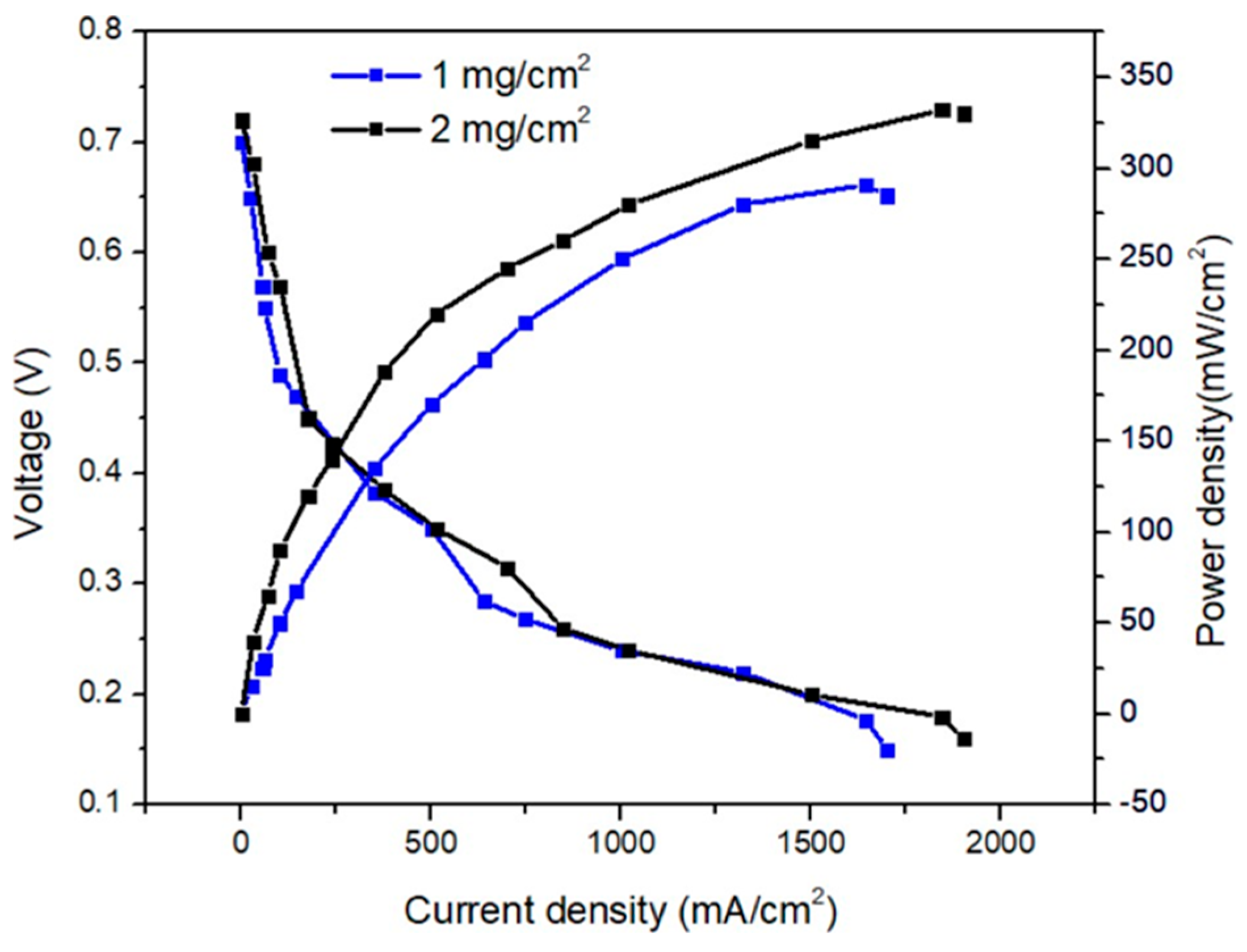
| 3 M NH4OH + 3 M KOH | O2 | PtIr/C | PdAgCo/C | 1 | 2 | 291 |
| 2 | 332 |
| Cathode Catalyst | Cathode Loading (mgmetal + carbon/cm2) | Fuel (Anode/Cathode) | Fuel Cell Type | Operating Conditions Temperature (°C) | Power Density (mW/cm2) | Reference |
|---|---|---|---|---|---|---|
| Pt/C | 0.8 | H2/O | AEMFC | 70 | 912 | [25] |
| Fe-N-C | 4 | H2/O | AEMFC | 80 | 1300 | [29] |
| Mn-N-C | 2 | H2/O | AEMFC | 60 | 480 | [30] |
| Co@Co3O4/@N-C | 2.5 | H2/O | AEMFC | 70 | 550 | [31] |
| Ag/C | 2 | H2/O | AEMFC | 50 | 490 | [32] |
| PdAg/C (3:1) | 1 | H2/O | AEMFC | 60 | 680 | [33] |
| Ag/C | 1 | H2/O | AEMFC | 70 | 623 | [34] |
| Ag-Co/CNT | 1 | H2/O | AEMFC | 70 | 431.1 | [35] |
| PdNi/N-rGO | 1 (Pd only) | H2/O | Direct formate AEMFC | 60 | 180 | [36] |
| LaCr0.25Fe0.25Co0.5O3-δ (LCFCO) | 1.85 | 7 M NH3·H2O + 1 M KOH/CO2-free air | DAFC | 100 | 30.1 | [37] |
| Pt/C | 2 | 3 M NH3·H2O + 3 M KOH/Pure-O2 | DAFC | 80 | 158.6 | [38] |
| Fe-N-C | 2 | 1 M NH3·H2O + 3 M KOH/Pure-O2 | DAFC | 80 | 107 | [39] |
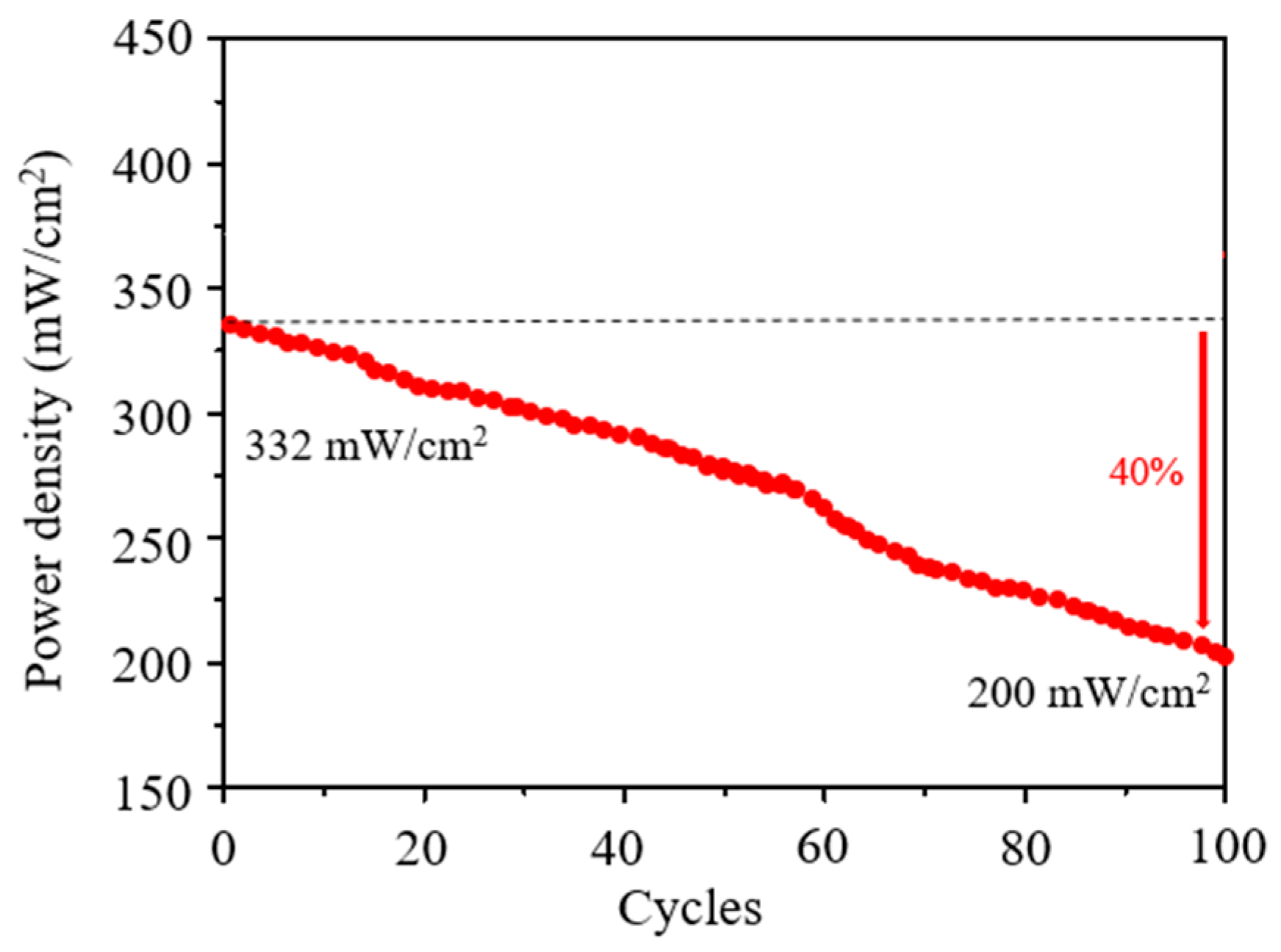
| PdAgCo/C | 2 | 3 M NH3·H2O + 3 M KOH/Pure-O2 | DAFC | 90 | 332 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vengatasalapathy, P.; Su, F.-C.; Su, Z.-J.; Lim, K.L.; Yang, H. Cathode Catalyst PdAgCo/C for Optimal Performance of the Alkaline Anion Exchange Membrane Direct Ammonia Fuel Cells. Catalysts 2025, 15, 825. https://doi.org/10.3390/catal15090825
Vengatasalapathy P, Su F-C, Su Z-J, Lim KL, Yang H. Cathode Catalyst PdAgCo/C for Optimal Performance of the Alkaline Anion Exchange Membrane Direct Ammonia Fuel Cells. Catalysts. 2025; 15(9):825. https://doi.org/10.3390/catal15090825
Chicago/Turabian StyleVengatasalapathy, Prithiv, Fa-Cheng Su, Zi-Jie Su, Kean Long Lim, and Hsiharng Yang. 2025. "Cathode Catalyst PdAgCo/C for Optimal Performance of the Alkaline Anion Exchange Membrane Direct Ammonia Fuel Cells" Catalysts 15, no. 9: 825. https://doi.org/10.3390/catal15090825
APA StyleVengatasalapathy, P., Su, F.-C., Su, Z.-J., Lim, K. L., & Yang, H. (2025). Cathode Catalyst PdAgCo/C for Optimal Performance of the Alkaline Anion Exchange Membrane Direct Ammonia Fuel Cells. Catalysts, 15(9), 825. https://doi.org/10.3390/catal15090825







