Synthesis of Thermal-Stable Aviation Fuel Additives with 4-Hydroxy-2-butanone and Cycloketones
Abstract
1. Introduction
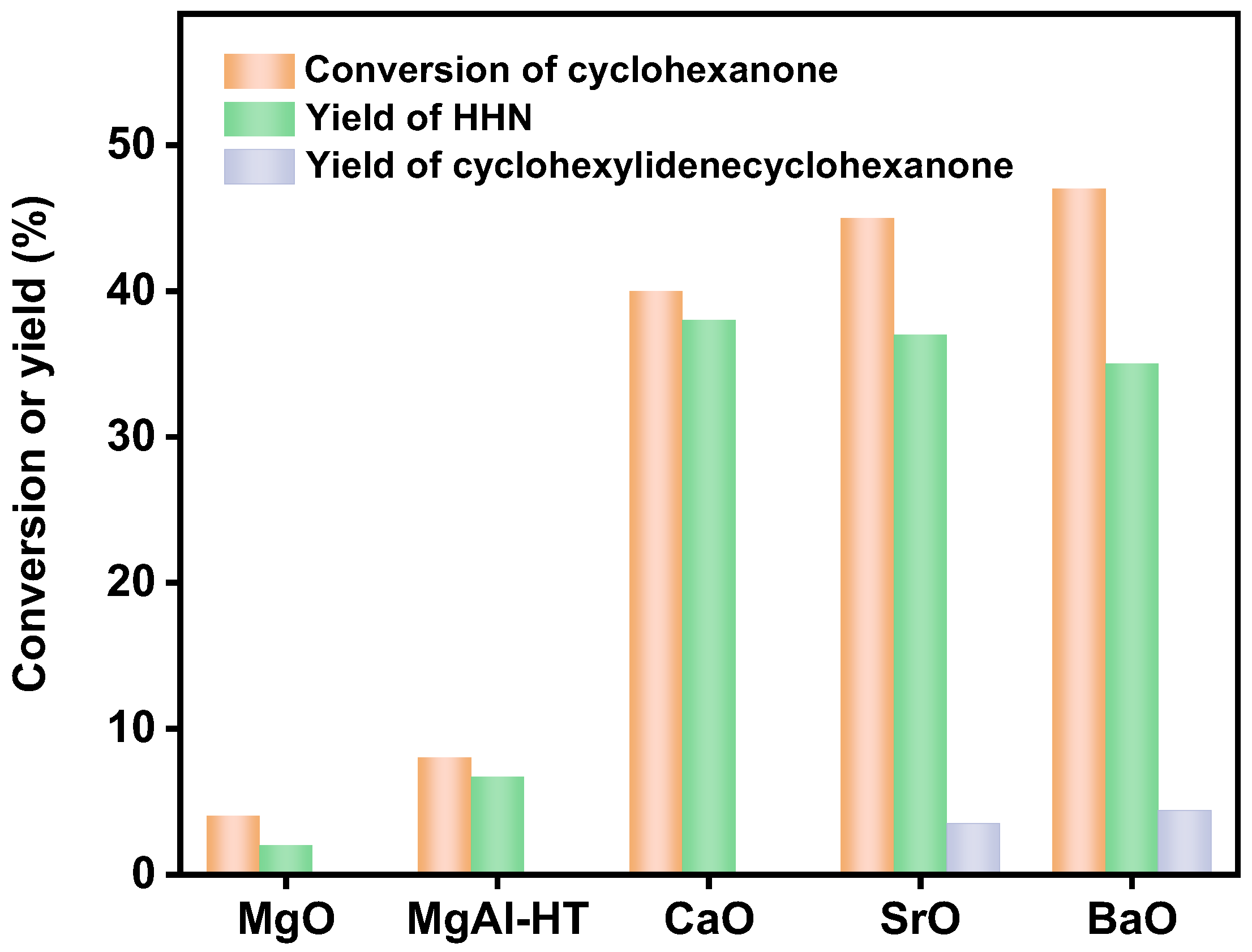
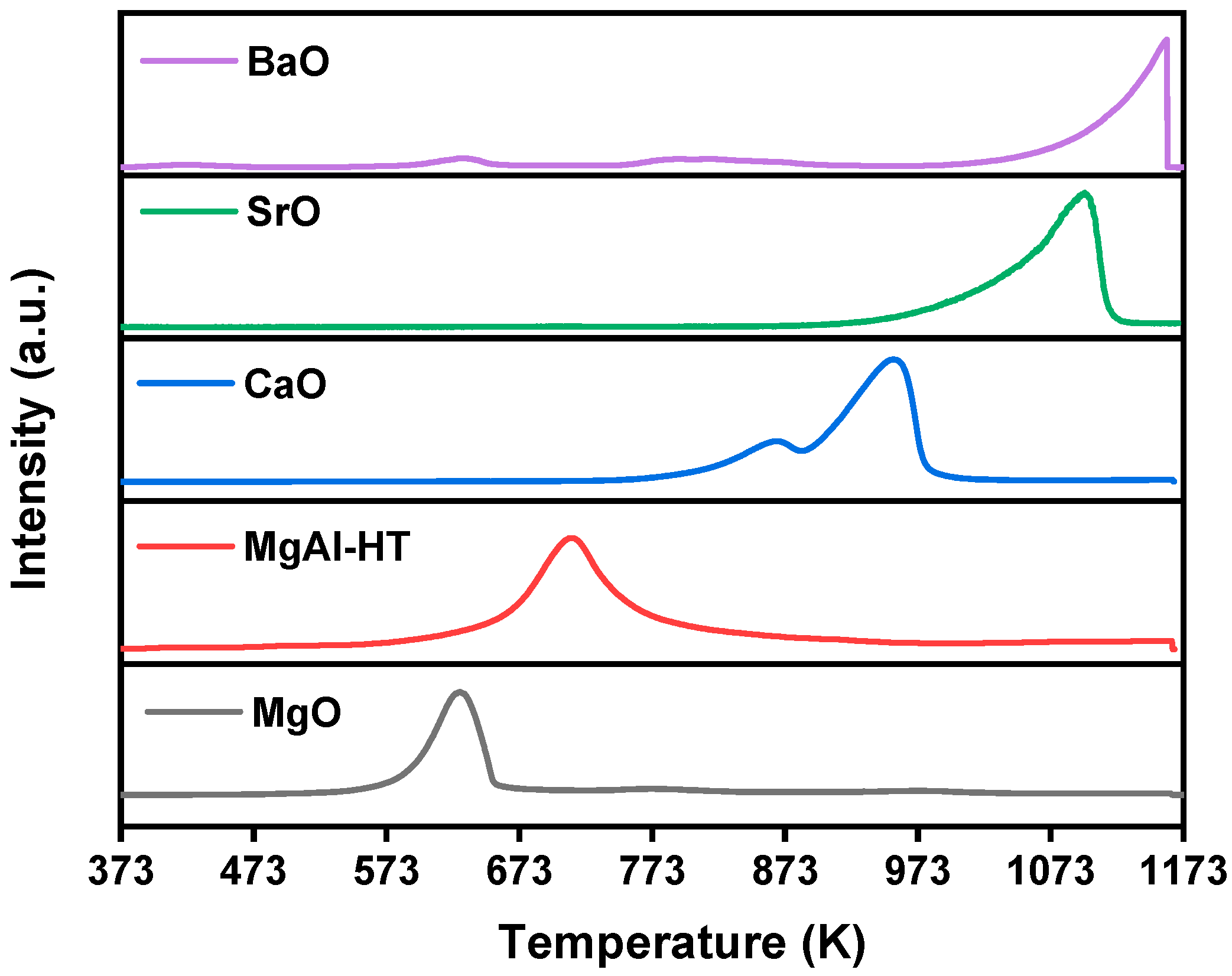
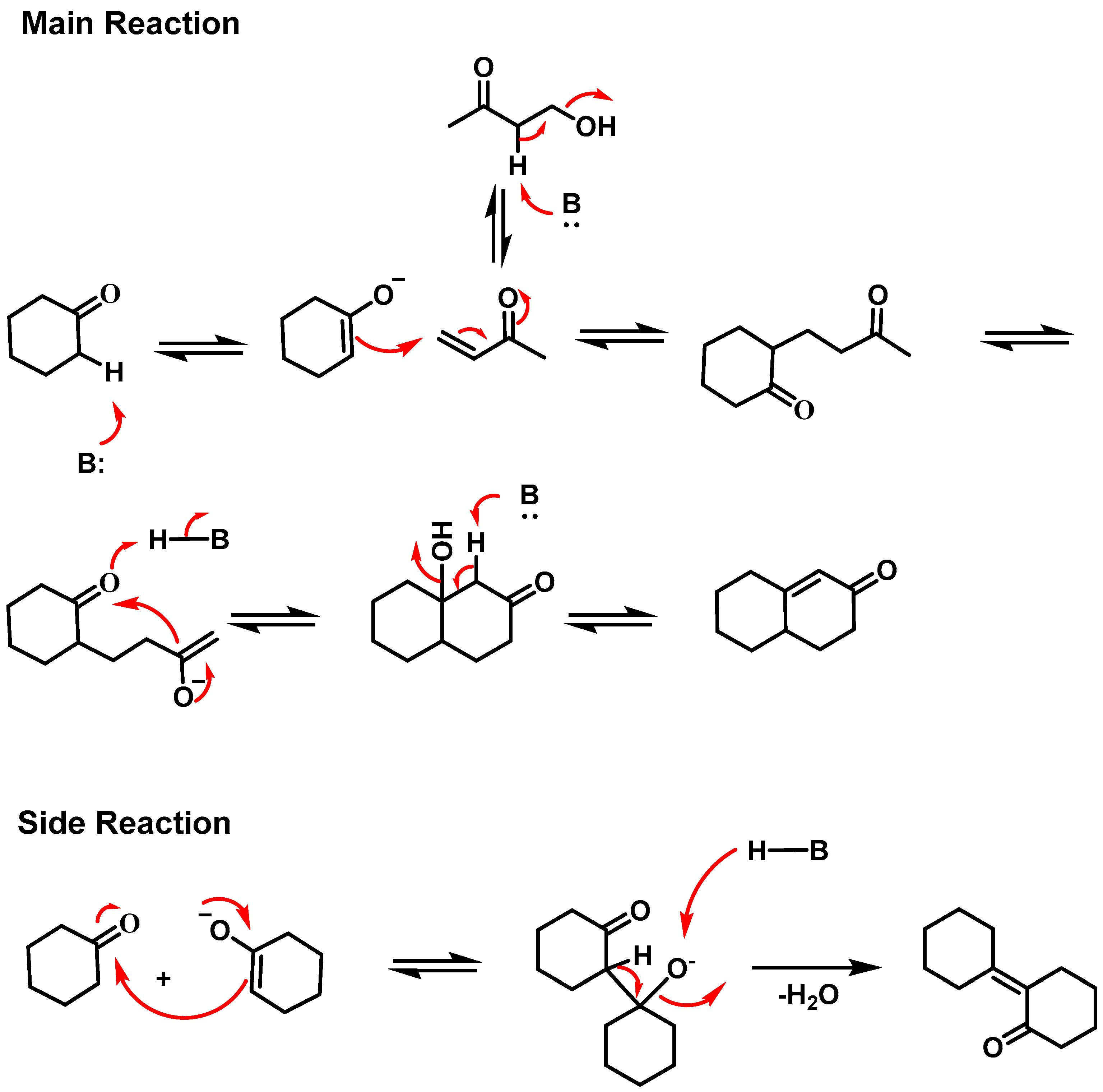
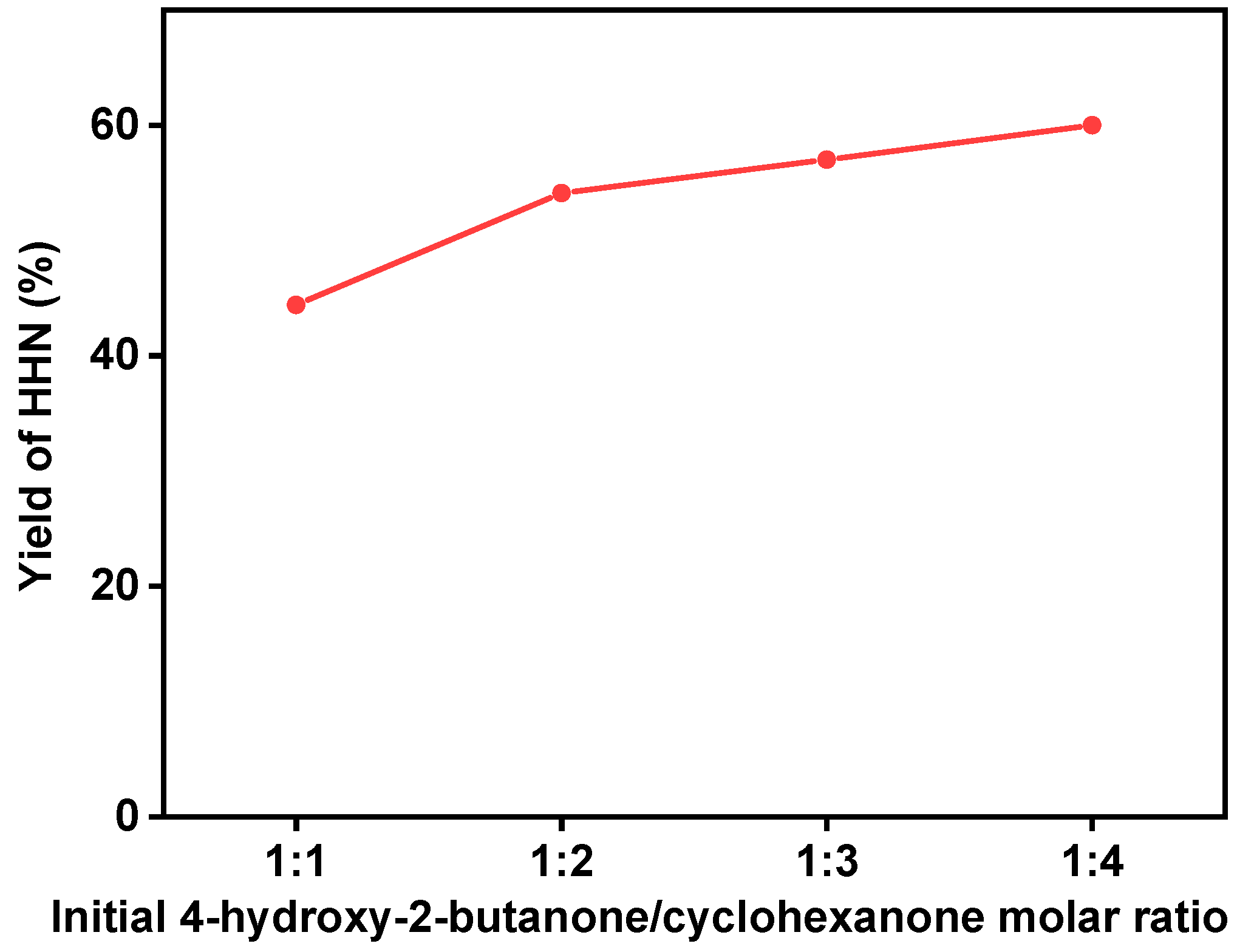
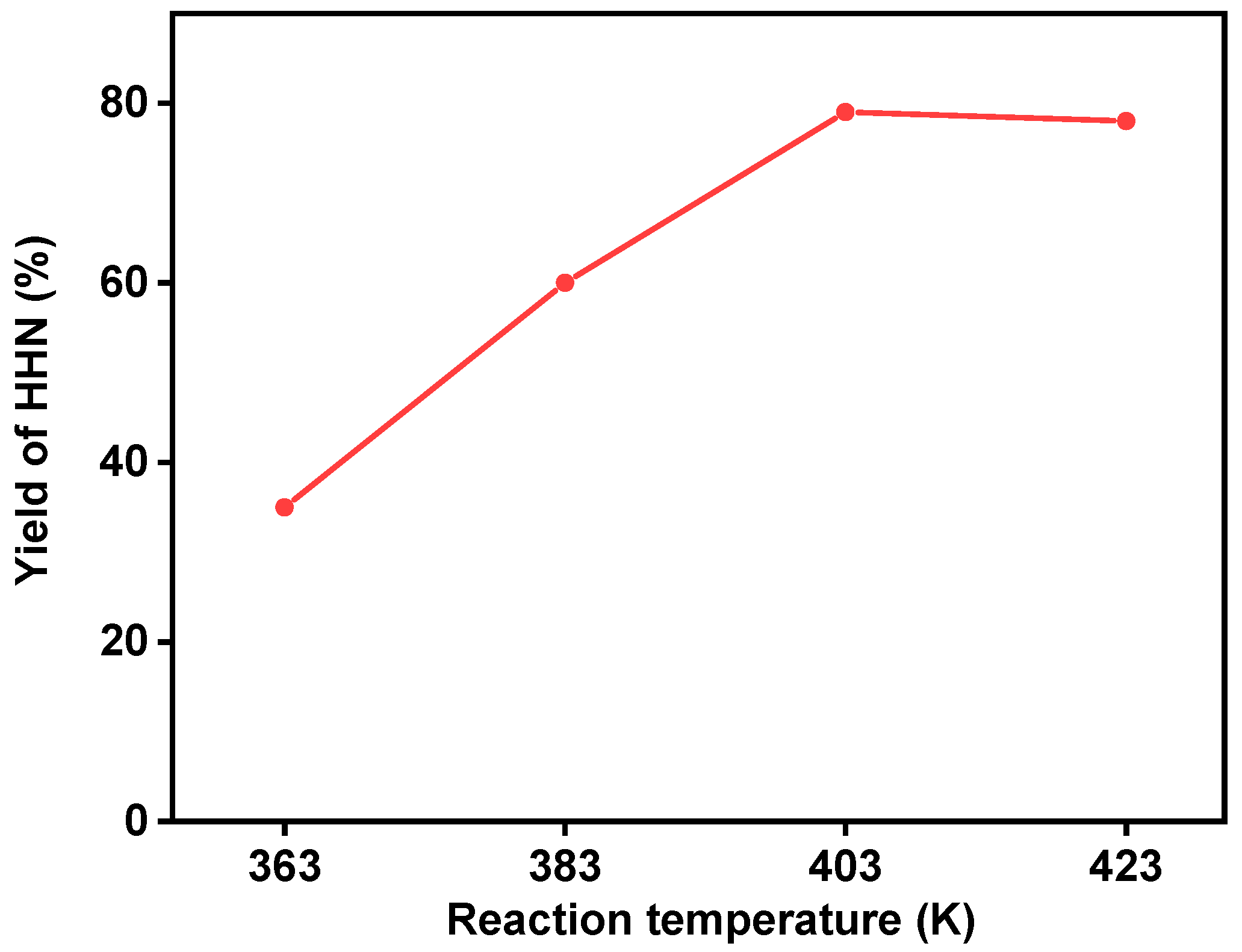
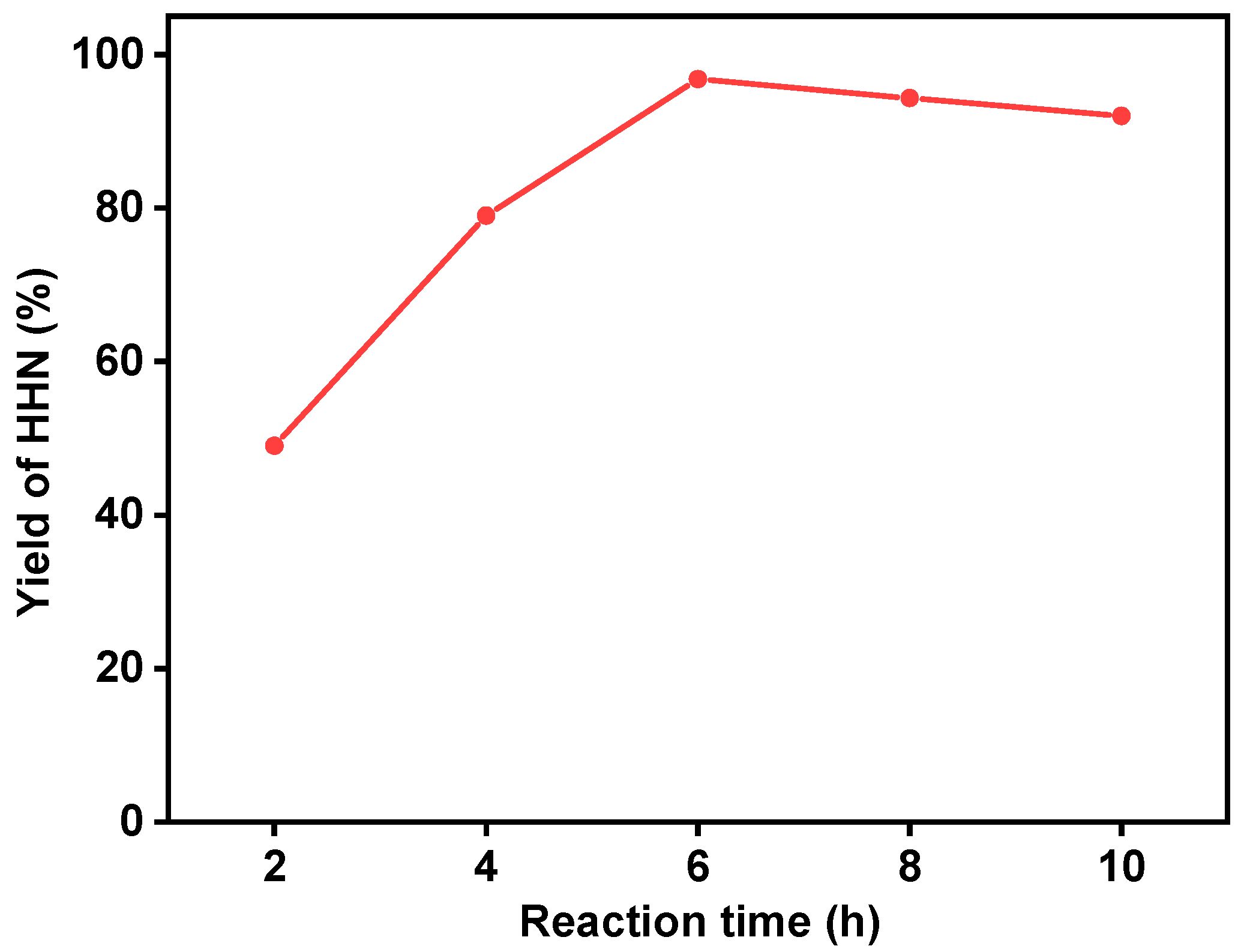
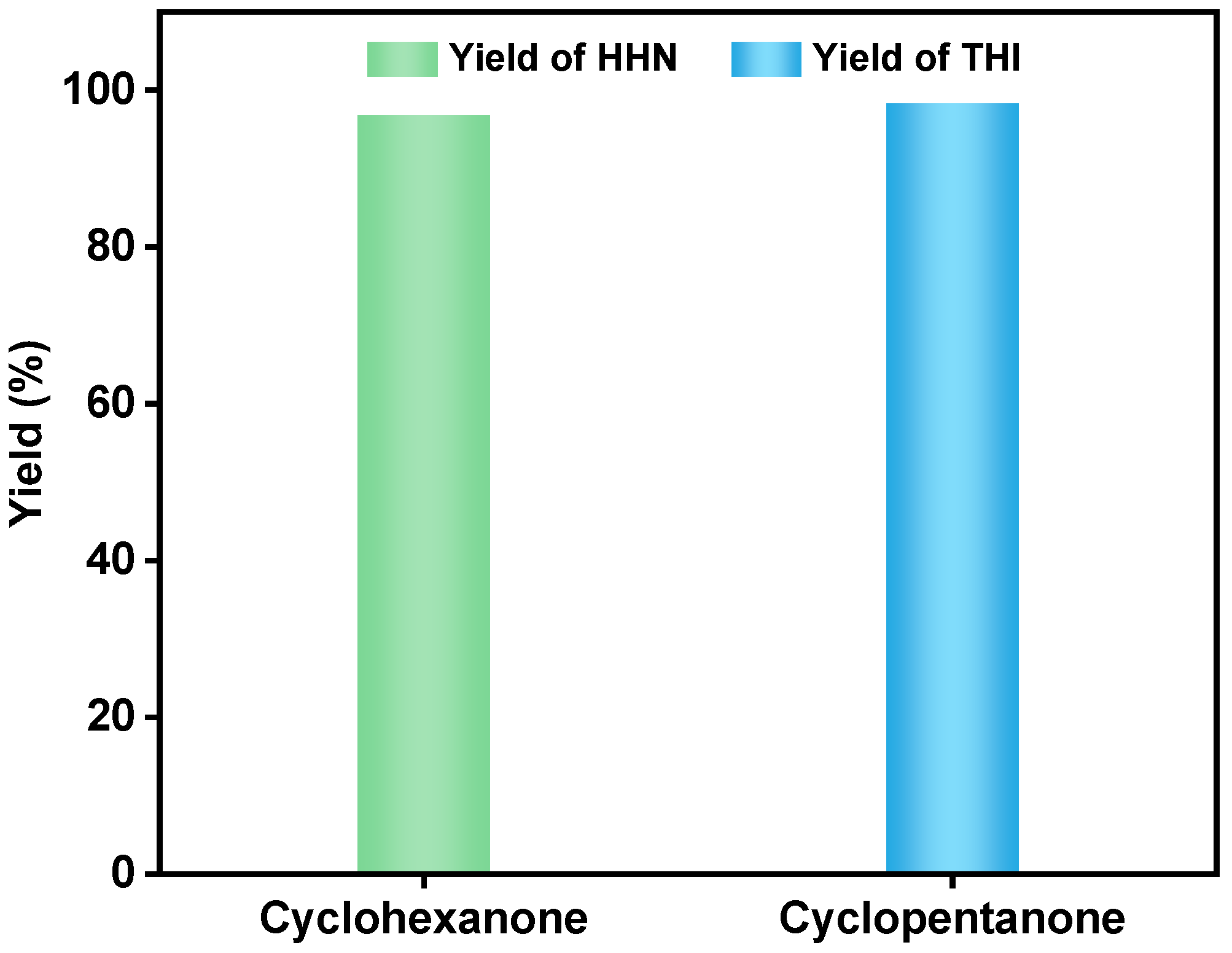
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Activity Tests
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huber, G.W.; Chheda, J.N.; Barrett, C.J.; Dumesic, J.A. Production of liquid alkanes by aqueous-phase processing of biomass-derived carbohydrates. Science 2005, 308, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.G.; Quintana, R.L. Synthesis of renewable jet and diesel fuels from 2-ethyl-1-hexene. Energy Environ. Sci. 2010, 3, 352–357. [Google Scholar] [CrossRef]
- Corma, A.; de la Torre, O.; Renz, M.; Villandier, N. Production of high-quality diesel from biomass waste products. Angew. Chem. Int. Ed. 2011, 50, 2375–2378. [Google Scholar] [CrossRef]
- Anbarasan, P.; Baer, Z.C.; Sreekumar, S.; Gross, E.; Binder, J.B.; Blanch, H.W.; Clark, D.S.; Toste, F.D. Integration of chemical catalysis with extractive fermentation to produce fuels. Nature 2012, 491, 235–239. [Google Scholar] [CrossRef]
- Corma, A.; de la Torre, O.; Renz, M. Production of high quality diesel from cellulose and hemicellulose by the sylvan process: Catalysts and process variables. Energy Environ. Sci. 2012, 5, 6328–6344. [Google Scholar] [CrossRef]
- Xia, Q.-N.; Cuan, Q.; Liu, X.-H.; Gong, X.-Q.; Lu, G.-Z.; Wang, Y.-Q. Pd/NbOPO4 multifunctional catalyst for the direct production of liquid alkanes from aldol adducts of furans. Angew. Chem. Int. Ed. 2014, 53, 9755–9760. [Google Scholar] [CrossRef]
- Sacia, E.R.; Balakrishnan, M.; Deaner, M.H.; Goulas, K.A.; Toste, F.D.; Bell, A.T. Highly selective condensation of biomass-derived methyl ketones as a source of aviation fuel. ChemSusChem 2015, 8, 1726–1736. [Google Scholar] [CrossRef]
- Shao, Y.; Xia, Q.; Dong, L.; Liu, X.; Han, X.; Parker, S.F.; Cheng, Y.; Daemen, L.L.; Ramirez-Cuesta, A.J.; Yang, S.; et al. Selective production of arenes via direct lignin upgrading over a niobium-based catalyst. Nat. Commun. 2017, 8, 16104. [Google Scholar] [CrossRef]
- Cao, Z.; Dierks, M.; Clough, M.T.; Daltro de Castro, I.B.; Rinaldi, R. A Convergent Approach for a Deep Converting Lignin-First Biorefinery Rendering High-Energy-Density Drop-in Fuels. Joule 2018, 2, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, G.; Hu, Y.; Wang, A.; Lu, F.; Zou, J.-J.; Cong, Y.; Li, N.; Zhang, T. Integrated Conversion of Cellulose to High-Density Aviation Fuel. Joule 2019, 3, 1028–1036. [Google Scholar] [CrossRef]
- Lebedeva, D.; Schick, L.W.; Cracco, D.; Sangsuwan, W.; Castiella-Ona, G.; Silva, D.O.; Marson, A.; Svensson Grape, E.; Inge, A.K.; Rossi, L.M.; et al. Sustainable aviation fuel from prehydrolysis liquors. Green Chem. 2024, 26, 7258–7267. [Google Scholar] [CrossRef]
- Balster, L.M.; Corporan, E.; DeWitt, M.J.; Edwards, J.T.; Ervin, J.S.; Graham, J.L.; Lee, S.-Y.; Pal, S.; Phelps, D.K.; Rudnick, L.R.; et al. Development of an advanced, thermally stable, coal-based jet fuel. Fuel Process. Technol. 2008, 89, 364–378. [Google Scholar] [CrossRef]
- Heyne, J.S.; Boehman, A.L.; Kirby, S. Autoignition studies of trans- and cis-decalin in an ignition quality tester (IQT) and the development of a high thermal stability unifuel/single battlefield fuel. Energy Fuels 2009, 23, 5879–5885. [Google Scholar] [CrossRef]
- Liu, Q.; Xue, K.; Jia, T.; Shen, Z.; Han, Z.; Pan, L.; Zou, J.-J.; Zhang, X. Effect of cis/trans molecular structures on pyrolysis performance and heat sink of decalin isomers. Front. Chem. Sci. Eng. 2023, 18, 5. [Google Scholar] [CrossRef]
- Chen, F.; Li, N.; Yang, X.; Li, L.; Li, G.; Li, S.; Wang, W.; Hu, Y.; Wang, A.; Cong, Y.; et al. Synthesis of high-density aviation fuel with cyclopentanol. ACS Sustain. Chem. Eng. 2016, 4, 6160–6166. [Google Scholar] [CrossRef]
- Wang, R.; Li, G.; Tang, H.; Wang, A.; Xu, G.; Cong, Y.; Wang, X.; Zhang, T.; Li, N. Synthesis of Decaline-Type Thermal-Stable Jet Fuel Additives with Cycloketones. ACS Sustain. Chem. Eng. 2019, 7, 17354–17361. [Google Scholar] [CrossRef]
- Nie, G.; Zhang, X.; Pan, L.; Wang, M.; Zou, J.-J. One-pot production of branched decalins as high-density jet fuel from monocyclic alkanes and alcohols. Chem. Eng. Sci. 2018, 180, 64–69. [Google Scholar] [CrossRef]
- Liu, C.; Hu, Y.; Li, G.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T.; Li, N. Synthesis of renewable alkylated decalins with p-quinone and 2-methyl-2,4-pentanediol. Sustain. Energy Fuels 2022, 6, 834–840. [Google Scholar] [CrossRef]
- Tanner, R.; Gill, P.; Wells, R.; Bailie, J.E.; Kelly, G.; Jackson, S.D.; Hutchings, G.J. Aldol condensation reactions of acetone and formaldehyde over vanadium phosphate catalysts: Comments on the acid–base properties. Phys. Chem. Chem. Phys. 2002, 4, 688–695. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, Y.; Yin, H.; Yuan, S. Reactivity of Formaldehyde during 4-Hydroxy-2-butanone Synthesis in Supercritical State. ACS Omega 2022, 7, 43450–43461. [Google Scholar] [CrossRef] [PubMed]
- Hronec, M.; Fulajtarova, K. Selective transformation of furfural to cyclopentanone. Catal. Commun. 2012, 24, 100–104. [Google Scholar] [CrossRef]
- Yang, Y.; Du, Z.; Huang, Y.; Lu, F.; Wang, F.; Gao, J.; Xu, J. Conversion of furfural into cyclopentanone over Ni-Cu bimetallic catalysts. Green Chem. 2013, 15, 1932–1940. [Google Scholar] [CrossRef]
- Fang, R.; Liu, H.; Luque, R.; Li, Y. Efficient and selective hydrogenation of biomass-derived furfural to cyclopentanone using Ru catalysts. Green Chem. 2015, 17, 4183–4188. [Google Scholar] [CrossRef]
- Li, X.-L.; Deng, J.; Shi, J.; Pan, T.; Yu, C.-G.; Xu, H.-J.; Fu, Y. Selective conversion of furfural to cyclopentanone or cyclopentanol using different preparation methods of Cu-Co catalysts. Green Chem. 2015, 17, 1038–1046. [Google Scholar] [CrossRef]
- Zhang, G.S.; Zhu, M.M.; Zhang, Q.; Liu, Y.M.; He, H.Y.; Cao, Y. Towards quantitative and scalable transformation of furfural to cyclopentanone with supported gold catalysts. Green Chem. 2016, 18, 2155–2164. [Google Scholar] [CrossRef]
- Deng, Q.; Gao, R.; Li, X.; Wang, J.; Zeng, Z.; Zou, J.-J.; Deng, S. Hydrogenative Ring-Rearrangement of Biobased Furanic Aldehydes to Cyclopentanone Compounds over Pd/Pyrochlore by Introducing Oxygen Vacancies. ACS Catal. 2020, 10, 7355–7366. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, H.; Sun, Y.; Liu, X.; Zhang, M.; Luo, Y.; Gao, J.; Xu, J. Selective tandem hydrogenation and rearrangement of furfural to cyclopentanone over CuNi bimetallic catalyst in water. Chin. J. Catal. 2021, 42, 2216–2224. [Google Scholar] [CrossRef]
- Lin, C.-J.; Huang, S.-H.; Lai, N.-C.; Yang, C.-M. Efficient Room-Temperature Aqueous-Phase Hydrogenation of Phenol to Cyclohexanone Catalyzed by Pd Nanoparticles Supported on Mesoporous MMT-1 Silica with Unevenly Distributed Functionalities. ACS Catal. 2015, 5, 4121–4129. [Google Scholar] [CrossRef]
- Liu, S.; Han, J.; Wu, Q.; Bian, B.; Li, L.; Yu, S.; Song, J.; Zhang, C.; Ragauskas, A.J. Hydrogenation of Phenol to Cyclohexanone over Bifunctional Pd/C-Heteropoly Acid Catalyst in the Liquid Phase. Catal. Lett. 2019, 149, 2383–2389. [Google Scholar] [CrossRef]
- Schutyser, W.; Van den Bosch, S.; Dijkmans, J.; Turner, S.; Meledina, M.; Van Tendeloo, G.; Debecker, D.P.; Sels, B.F. Selective nickel-catalyzed conversion of model and lignin-derived phenolic compounds to cyclohexanone-based polymer building blocks. ChemSusChem 2015, 8, 1805–1818. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Wang, C.; Wang, W. Highly Selective Hydrogenation of Phenols to Cyclohexanone Derivatives Using a Palladium@N-Doped Carbon/SiO2 Catalyst. Org. Process Res. Dev. 2021, 25, 2425–2431. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, T.; Han, B.; Liang, S.; Zhou, Y. Selective Phenol Hydrogenation to Cyclohexanone Over a Dual Supported Pd–Lewis Acid Catalyst. Science 2009, 326, 1250–1252. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, Q.; Wang, A.; Zhang, T. Direct Catalytic Hydrogenolysis of Kraft Lignin to Phenols in Choline-Derived Ionic Liquids. ACS Sustain. Chem. Eng. 2016, 4, 3850–3856. [Google Scholar] [CrossRef]
- Liao, Y.; Koelewijn, S.-F.; Van den Bossche, G.; Van Aelst, J.; Van den Bosch, S.; Renders, T.; Navare, K.; Nicolaï, T.; Van Aelst, K.; Maesen, M.; et al. A sustainable wood biorefinery for low–carbon footprint chemicals production. Science 2020, 367, 1385. [Google Scholar] [CrossRef]
- Yu, Z.; Zou, Z.; Wang, R.; Li, G.; Wang, A.; Cong, Y.; Zhang, T.; Li, N. Synthesis of Cyclopentadiene and Methylcyclopentadiene with Xylose or Extracted Hemicellulose. Angew. Chem. Int. Ed. 2023, 62, e202300008. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; Liu, Y.; Yao, Y.; Han, Y.; Wang, W.; Li, G.; Wang, A.; Cong, Y.; Zhang, T.; et al. Renewable indanone and thermal-stable aviation fuel from cellulose. Cell Rep. Sustain. 2024, 1, 100156. [Google Scholar] [CrossRef]
- Tang, H.; Chen, F.; Li, G.; Yang, X.; Hu, Y.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T.; Li, N. Synthesis of jet fuel additive with cyclopentanone. J. Energy Chem. 2019, 29, 23–30. [Google Scholar] [CrossRef]
- Olsen, R.S.; Fataftah, Z.A.; Rathke, M.W. A Convenient Procedure for Carboxylation—Robinson Annulations of Ketones Using Triethylamine Base in the Presence of Magnesium Chloride. Synth. Commun. 1986, 16, 1133–1139. [Google Scholar] [CrossRef]
- Okano, T.; Satou, Y.; Tamura, M.; Kiji, J. Aldol Reaction and Robinson-Type Annelation Catalyzed by Lanthanoid Triisopropoxides. Bull. Chem. Soc. Jpn. 2006, 70, 1879–1885. [Google Scholar] [CrossRef]
- Li, S.; Li, N.; Li, G.; Li, L.; Wang, A.; Cong, Y.; Wang, X.; Xu, G.; Zhang, T. Protonated titanate nanotubes as a highly active catalyst for the synthesis of renewable diesel and jet fuel range alkanes. Appl. Catal. B 2015, 170–171, 124–134. [Google Scholar] [CrossRef]
- Ueno, K.; Zhao, Z.; Watanabe, M.; Angell, C.A. Protic Ionic Liquids Based on Decahydroisoquinoline: Lost Superfragility and Ionicity-Fragility Correlation. J. Phys. Chem. B 2012, 116, 63–70. [Google Scholar] [CrossRef]
- Dauben, W.G.; Hiskey, C.F.; Markhart, A.H., Jr. The Hydrogenation of Hydroxynaphthoic Acids1. J. Am. Chem. Soc. 1951, 73, 1393–1400. [Google Scholar] [CrossRef]
- Rao, C.S.; Banerjee, D.K. A stereospecific synthesis of trans-1β-hydroxy-7-methoxy-11-methyl-1,2,3,4,9,10,11,12-octahydrophenanthrene. Tetrahedron 1963, 19, 1611–1616. [Google Scholar] [CrossRef]
- Huckel, W.; Schluter, R.; Doll, W.; Reimer, F. Stereochemistry of bicyclic ring systems. XII. Stereoisomerism of hydrindane and its derivatives. 4. Hydrindanes substituted in the 6-membered ring. Justus Liebigs Ann. Chem. 1937, 530, 166–183. [Google Scholar] [CrossRef]



| Catalyst | SBET (m2 g−1) 1 | Amount of Base Sites (μmol g−1) 2 |
|---|---|---|
| MgO | 44.4 | 4.8 |
| MgAl-HT | 202.0 | 6.5 |
| CaO | 8.9 | 13.7 |
| SrO | 5.1 | 5.3 |
| BaO | 5.6 | 74.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, A.; Zou, Z.; Cong, Y.; Yin, Y.; Li, N. Synthesis of Thermal-Stable Aviation Fuel Additives with 4-Hydroxy-2-butanone and Cycloketones. Catalysts 2025, 15, 826. https://doi.org/10.3390/catal15090826
Zhu A, Zou Z, Cong Y, Yin Y, Li N. Synthesis of Thermal-Stable Aviation Fuel Additives with 4-Hydroxy-2-butanone and Cycloketones. Catalysts. 2025; 15(9):826. https://doi.org/10.3390/catal15090826
Chicago/Turabian StyleZhu, Anran, Zhufan Zou, Yu Cong, Yinghua Yin, and Ning Li. 2025. "Synthesis of Thermal-Stable Aviation Fuel Additives with 4-Hydroxy-2-butanone and Cycloketones" Catalysts 15, no. 9: 826. https://doi.org/10.3390/catal15090826
APA StyleZhu, A., Zou, Z., Cong, Y., Yin, Y., & Li, N. (2025). Synthesis of Thermal-Stable Aviation Fuel Additives with 4-Hydroxy-2-butanone and Cycloketones. Catalysts, 15(9), 826. https://doi.org/10.3390/catal15090826










