1. Introduction
In the field of agriculture, pesticides play a vital role in growing more crops by killing pests and increasing production per acre [
1,
2]. Developing countries use agricultural pesticides to produce more crops, but due to their toxicity and bad effects on both the environment and human health, this causes a major problem [
2,
3,
4,
5,
6,
7]. Pesticides are used in agriculture in large quantities every year, which adversely affect the food chain, human health, and biodiversity [
8]. Due to a lack of education, knowledge, incorrect perception, regulation, and pesticide toxicity, agricultural workers and other populations result in death in many parts of the world [
9,
10,
11].
Pesticides are crucial in agriculture for enhancing crop yields by effectively controlling pests, weeds, and diseases. They have been utilized since the 1940s, significantly contributing to food security by increasing production per acre [
12]. However, the application of pesticides must be judicious; excessive use can harm beneficial organisms and lead to environmental degradation [
13,
14]. Key aspects include the fact that pesticides protect crops from harmful organisms, ensuring higher yields. They are essential for managing pests, weeds, and diseases, which can otherwise devastate crops [
14].
Overuse can lead to biodiversity loss and the contamination of soil and water [
12]. Integrated Pest Management (IPM) strategies can mitigate these risks by combining chemical and non-chemical methods [
12]. Biopesticides offer eco-friendly alternatives, reducing reliance on synthetic chemicals [
15]. Sustainable practices are necessary to balance productivity with environmental health. While pesticides are vital for crop production, their negative impacts on ecosystems and human health necessitate careful management and the exploration of sustainable alternatives.
Environmental concerns of each pesticide or class of pesticides are specific. Among pesticides, organophosphates and their degradation products are extremely hazardous to human health and ecology on the Earth and persist for a long time. Therefore, the presence of organophosphates and their degradation products in fruits, water, vegetables, and Earth needs more attention and control to ensure safe human health and ecology [
16,
17,
18,
19]. Organophosphates (OPs) are widely recognized for their hazardous effects on human health and the environment due to their persistence and bioaccumulation. These compounds, commonly used in agriculture, inhibit acetylcholinesterase, leading to severe neurological and physiological disorders in humans and wildlife [
20,
21]. Their degradation products can remain in ecosystems for extended periods, posing risks to biodiversity and food safety. The following points highlight the critical aspects of OPs and their impacts.
OPs are linked to acute and chronic health issues, including respiratory and reproductive problems [
20]. Neurotoxicity is a significant concern, with documented cases of poisoning from both occupational and environmental exposure [
21]. OPs are known for their long-lasting presence in soil and water, affecting non-target species and disrupting ecosystems [
22]. Studies show that certain OPs can remain detectable in food products and human tissues, raising alarm over food safety [
23]. Innovative bioremediation techniques using microbes and enzymes are being developed to detoxify OPs from contaminated environments [
24]. Research indicates that specific bacterial strains can effectively degrade OPs, reducing their ecological footprint [
22]. Despite the advancements in bioremediation, the widespread use of OPs continues to pose significant challenges, necessitating ongoing research and regulatory measures to mitigate their impact on health and the environment.
TiO
2 and ZnO are recognized as effective photocatalysts due to their large band gap energy, ease of synthesis, and chemical stability. Recent studies have demonstrated the photocatalytic degradation of chlorpyrifos, an organophosphate pesticide, using ZnO nanoparticles under UV irradiation. The efficiency of these photocatalysts can be significantly enhanced by incorporating metallic and non-metallic dopants, which improve their stability and reusability [
25,
26]. Moreover, the use of nanocomposites, such as those combining ZnO and TiO
2 with superparamagnetic iron oxide nanoparticles, has shown remarkable degradation rates of chlorpyrifos, achieving up to 95.6% removal efficiency [
27]. Factors such as pH, catalyst dosage, and light intensity also play crucial roles in optimizing photocatalytic performance [
28]. Despite the promising results, the environmental impact of using such photocatalysts in large-scale applications remains a concern, necessitating further research into their long-term effects and sustainability in real-world scenarios. Although both TiO
2 and ZnO have been extensively studied, there is a notable lack of research focusing on the ZnO-mediated photocatalytic degradation of specific organophosphates such as hexamethylphosphoramide (HMPA) and omethoate, especially under natural sunlight. Moreover, to the best of our knowledge, the application of sonochemically synthesized ZnO nanoparticles for this purpose has not yet been reported, indicating a significant research gap that this study aims to address.
Previous studies on both TiO
2 and ZnO indicate a large band gap [
29]. Both TiO
2 and ZnO are recognized for their large band gaps, making them suitable for various applications in nanotechnology and solar energy. TiO
2 typically exhibits a band gap of around 3.0 eV, while ZnO has a similar band gap, which can be tuned through doping and structural modifications. The following points highlight their characteristics and applications: TiO
2 has a wide band gap of approximately 3.0 eV, limiting its absorption to UV light [
30]. ZnO also possesses a large band gap, which is advantageous for UV photodetectors and solar cells [
31].
Doping TiO
2 with Zn can reduce its band gap, enhancing its photocatalytic activity and extending light absorption into the visible range [
32]. The multilayer TiZn oxide films show similar band gap values to TiO
2, indicating potential for solar cell applications [
33]. Both materials are utilized as photoanodes in solar cells, where their large band gaps facilitate efficient energy conversion [
34]. The combination of TiO
2 and ZnO in composite films enhances their electrodynamic properties, making them promising candidates for renewable energy technologies [
33]. While the large band gaps of TiO
2 and ZnO present challenges for visible light absorption, ongoing research into doping and composite structures aims to overcome these limitations, potentially broadening their application in energy-harvesting technologies. The catalytic studies of ZnO show greater performance than TiO
2 in both acidic and basic media for the degradation of organic compounds, and thus, their photocatalytic properties need to be explored further [
35,
36]. Also, ZnO absorbs a greater amount of light compared to TiO
2 [
37,
38].
Synthesis of ZnO nanoparticles has gained importance because of their specific shape and size due to their use in various fields of life. ZnO has a large band gap with 60 eV energy [
39,
40].
Zinc oxide (ZnO) can be synthesized through various methods, each offering unique advantages and applications. The hydrothermal method, for instance, allows for the production of nanoparticles with controlled size and morphology, as demonstrated by the synthesis of ZnO nanoparticles from zinc nitrate using sodium hydroxide at 120 °C, yielding the smallest particle size [
41]. The sol–gel method, often combined with hydrothermal processes, facilitates the growth of well-aligned ZnO nanorods, achieving a preferential orientation and specific crystallite sizes [
42]. Additionally, the polyol method has been compared with hydrothermal synthesis, revealing differences in particle characteristics influenced by temperature and synthesis conditions [
43]. These methods highlight the versatility in ZnO synthesis, catering to diverse applications in electronics and biomedicine. However, while these methods are effective, they may also present challenges such as scalability and cost, which can impact their practical applications in industry.
ZnO can be prepared by different methods, such as sonochemical, sol–gel, solid state, microwave-assisted, precipitation, polyol, and hydrothermal. The primary objective of this study is to synthesize ZnO nanoparticles using a modified sonochemical method and assess their photocatalytic degradation performance against highly toxic organophosphates (HMPA and omethoate) under ambient sunlight conditions. The degradation products are characterized using GC/EIMS to ensure effective and safe breakdown. Technical-grade organophosphates, i.e., hexamethyl phosphoramide (HMPA) and omethoate, were taken as a model pesticide for the degradation studies. Organophosphates (HMPA and omethoate) were chemisorbed on the surface of nanocrystalline ZnO for a specific time, and the degradation products were then extracted in two different solvents, i.e., DCM (dichloromethane) and IPA (isopropyl alcohol), and analyzed on GC/EIMS for identification and confirmation. The novelty of this work lies in the use of natural sunlight as a sustainable energy source and the use of GC/EIMS analysis to confirm the formation of less toxic degradation products. A comparison between ZnO and TiO2 is included to highlight ZnO’s advantages, such as greater light absorption capacity, higher surface reactivity, and cost-effectiveness. ZnO also demonstrates enhanced photocatalytic activity in various pH conditions and exhibits better charge carrier mobility than TiO2, making it a promising alternative for environmental remediation.
4. Results and Discussion
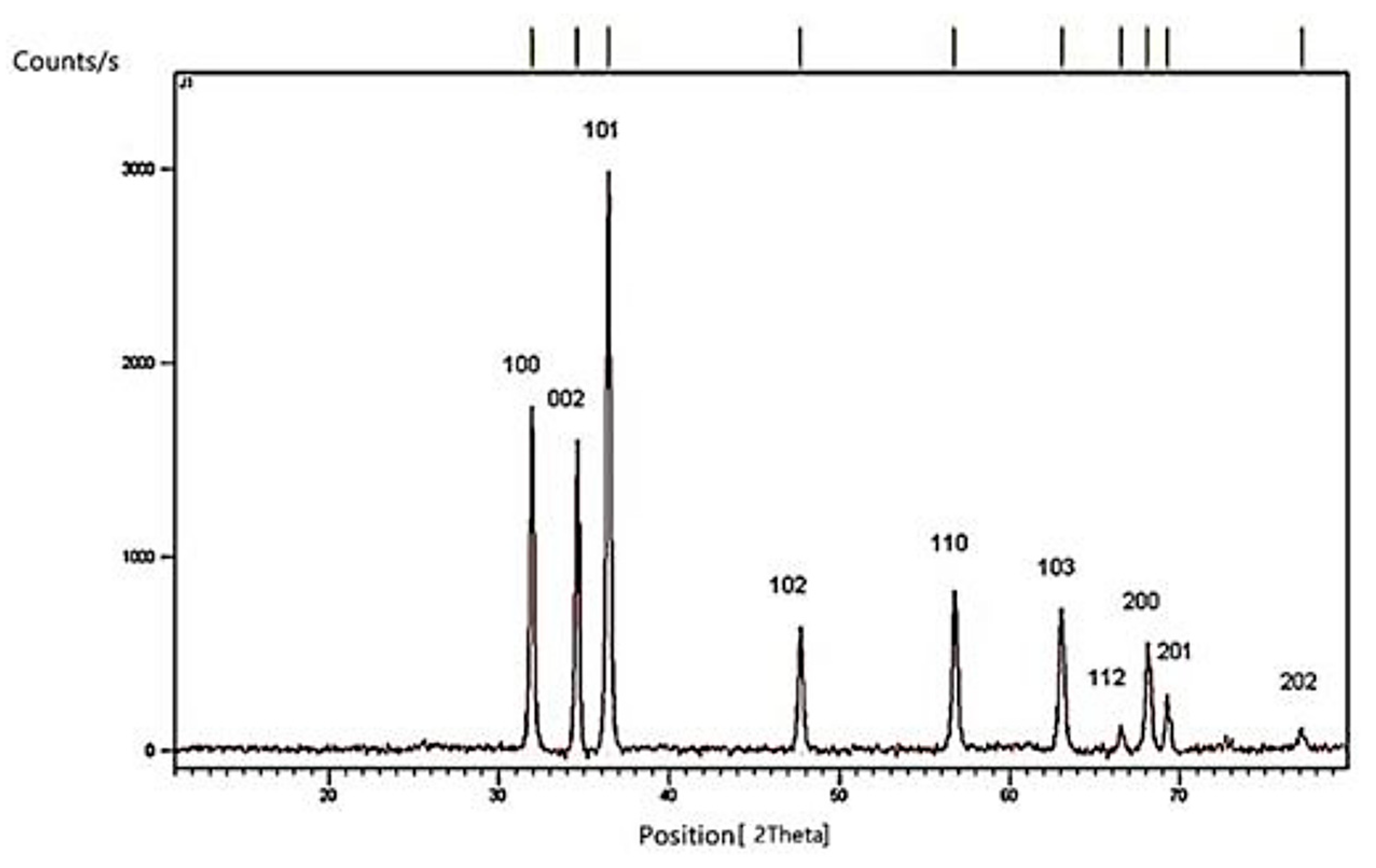
The XRD pattern of the synthesized material displayed (
Figure 1) diffraction peaks at 2θ
O values of 31.90°, 34.61°, 36.44°, 47.65°, 56.77°, 62.99°, 66.50°, 68.66°, 69.23°, and 77.13°, corresponding to the (100), (002), (101), (102), (110), (103), (112), (200), (201), and (202) Miller indices, respectively, confirming the formation of the zincite phase. The presence of sharp and intense peaks indicated a high degree of crystallinity. The average crystallite size was calculated using the Scherrer equation.
A size of 23 ± 1 nm was obtained. The obtained XRD data align well with the reference pattern 00-001-1136, indicating a hexagonal phase (space group P63_33mc) with lattice parameters a = 3.2420 Å, c = 5.1760 Å, and Z = 2, confirming the hexagonal structure of the ZnO nanoparticles.
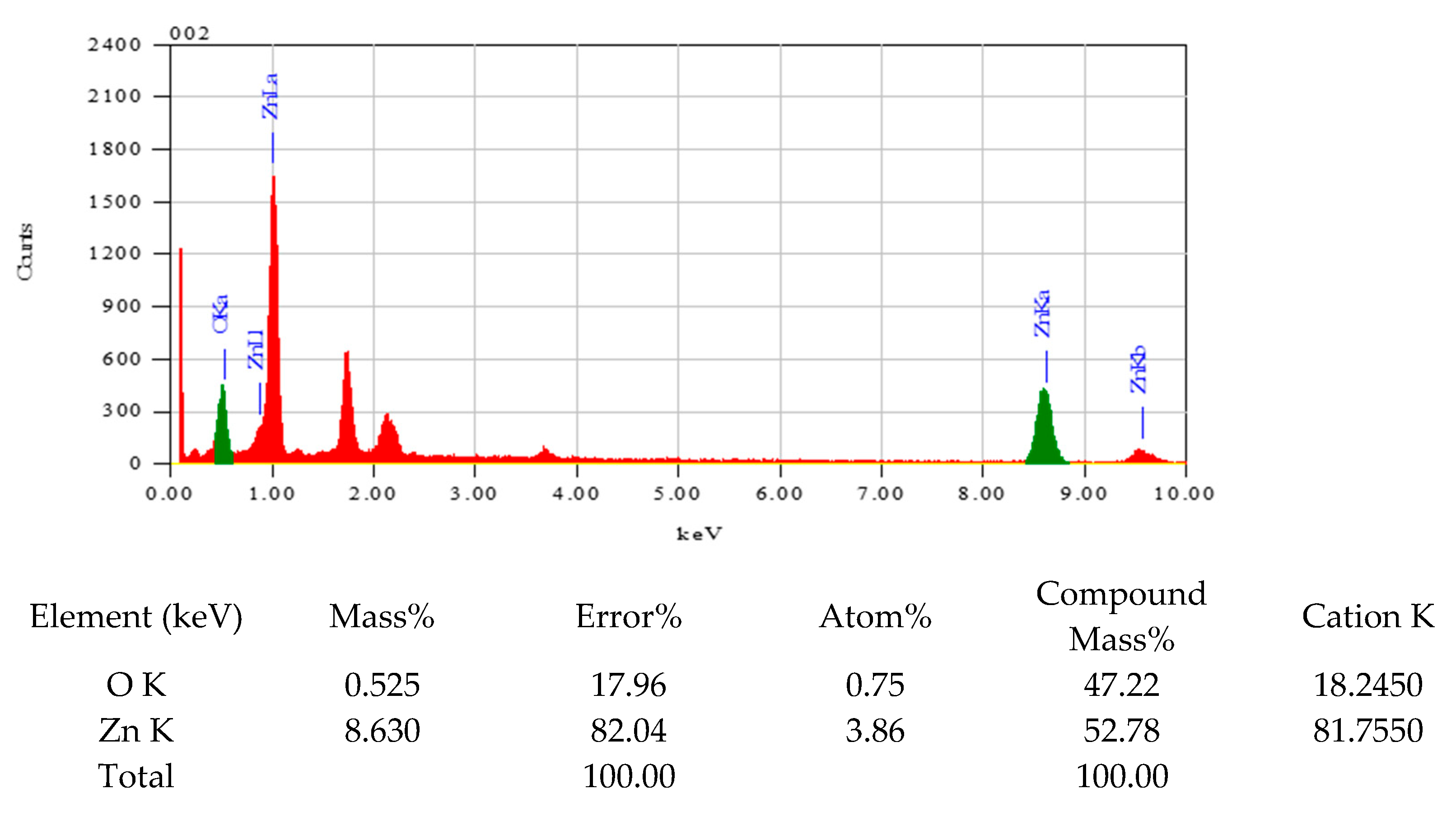
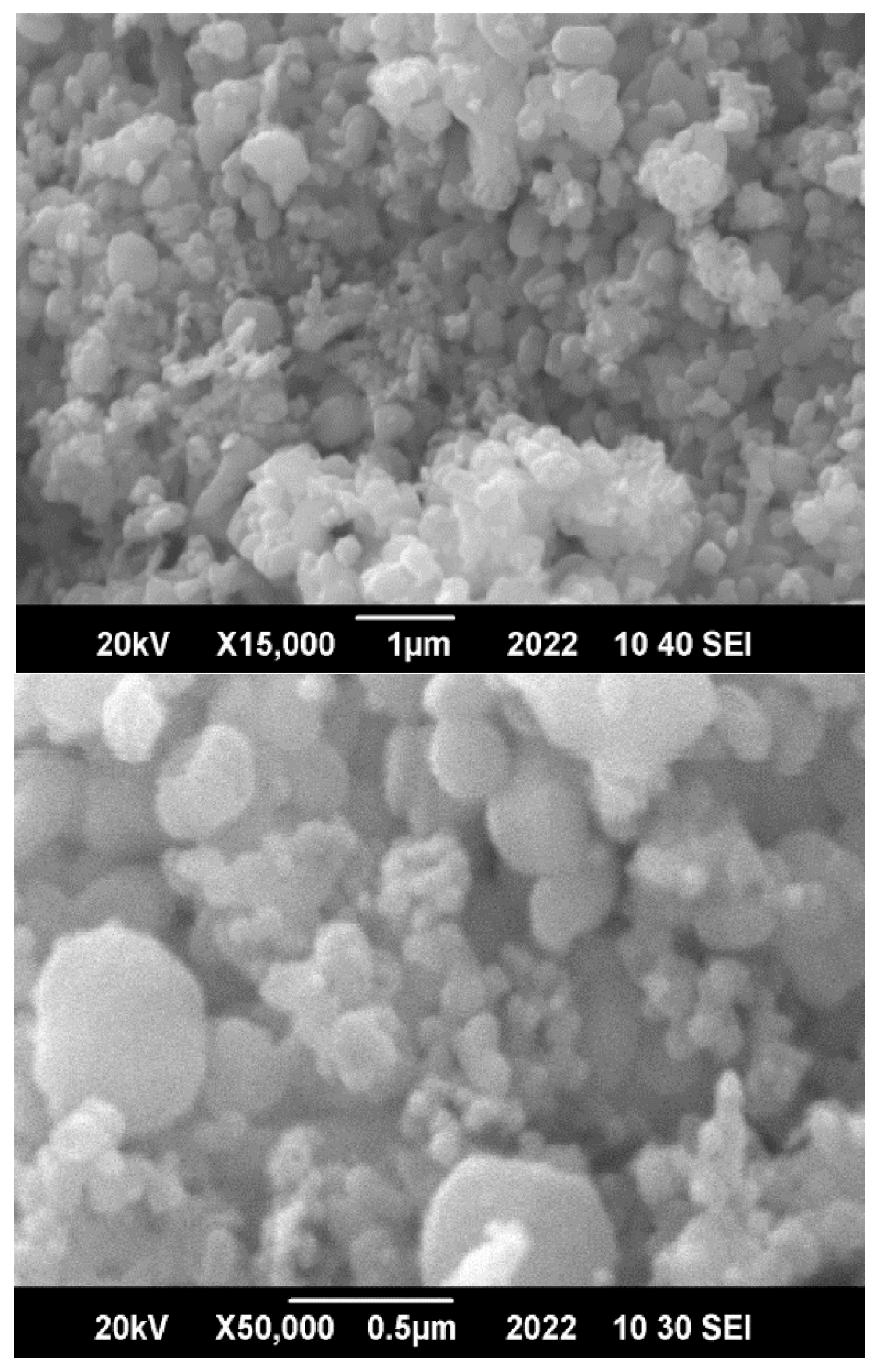
To determine the morphology, particle size, shape, and elemental composition of the synthesized ZnO nanoparticles, including the metal-to-oxygen ratio, scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) analyses were conducted. The SEM micrograph (
Figure 2) reveals that the ZnO nanoparticles exhibit a predominantly spherical shape with a uniform size distribution, indicating the successful synthesis of nanostructured material.
The EDX analysis (
Figure A1,
Appendix A) confirms the purity of the synthesized ZnO nanoparticles, showing no detectable impurities. The elemental composition, particularly the zinc-to-oxygen ratio, was determined to be 52:47, which is close to the theoretical stoichiometric ratio, indicating minimal oxygen vacancies or excess zinc in the material. This suggests that the synthesis method employed is effective at producing high-purity ZnO nanoparticles, with a well-defined morphology and elemental composition.
The synthesized ZnO nanoparticles exhibit a strong absorption edge at around 375 nm, as observed through UV–Vis spectroscopy. Using the Tauc plot method and assuming a direct band gap transition, the calculated optical band gap of the nanoparticles was approximately 3.2 eV. This value aligns well with the reported band gaps of ZnO nanostructures in previous studies, confirming the material’s photocatalytic potential under UV and solar irradiation.
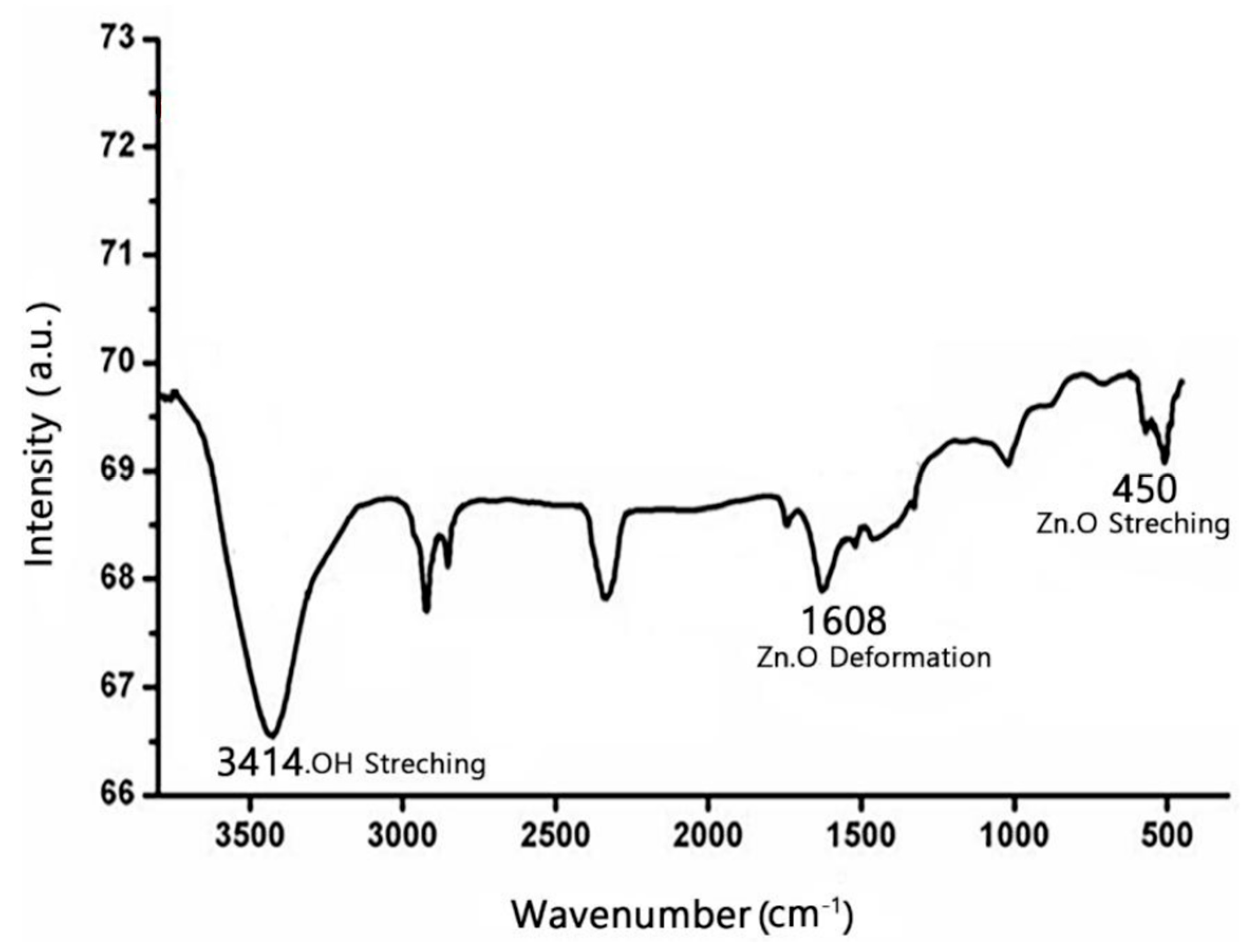
The FTIR spectra of the synthesized nanocrystalline ZnO material exhibited an absorption band at 457 cm
−1, which corresponds to the characteristic stretching vibration mode of Zn–O bonds in ZnO nanoparticles, confirming the formation of ZnO. Additionally, broad peaks at 3465 cm
−1 and 3789 cm
−1 are likely attributed to the presence of surface hydroxyl groups, which are common in nanoparticles due to moisture adsorption or incomplete surface passivation (
Figure 3). These hydroxyl groups can enhance the reactivity of the ZnO nanoparticles, making them more suitable for applications such as photocatalysis and adsorption (
Figure 3).
The FTIR spectrum of the synthesized ZnO nanoparticles aligns closely with the standard reference spectra of ZnO nanoparticles, as cataloged under I-NO:77947, in the Atlas of Polymer and Plastics Analysis, Volume 3. This match further validates the successful synthesis and confirms the structural integrity of the nanoparticles.
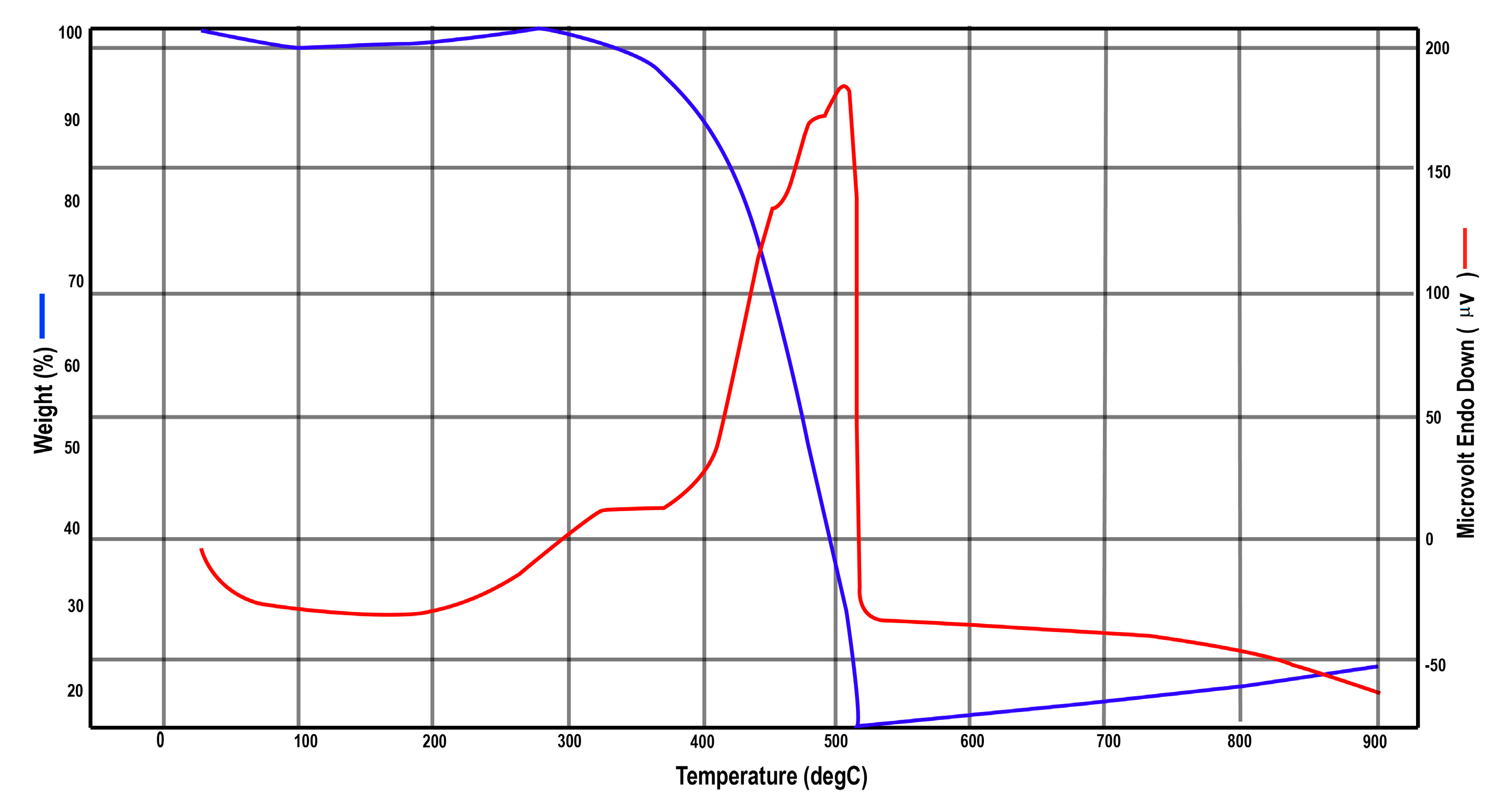
The thermogravimetric analysis (TGA) and differential thermal analysis (DTA) of the synthesized material were performed at a heating rate of 10 °C/min to investigate its thermal stability and decomposition profile. The TGA curve reveals that no significant weight loss occurs up to 300 °C, indicating the absence of volatile impurities or significant moisture loss at lower temperatures. Beyond 300 °C, a gradual weight loss begins and continues steadily up to 500 °C, after which the weight stabilizes, signifying the complete conversion of the material into nanocrystalline ZnO. Other researchers also observed similar results for the synthesized nanoparticles [
5,
6,
7,
8,
9,
10].
The weight loss observed between 300 °C and 500 °C is primarily attributed to the elimination of residual moisture, organic ligands, and low-melting-point additives present in the sample. This transition confirms that the calcination process at 500 °C results in the formation of phase-pure, stable nanocrystalline ZnO.
The DTA curve complements the TGA data, displaying an endothermic event beginning at 300 °C and continuing up to 500 °C, which corresponds to the decomposition of organics and the subsequent formation of ZnO. The combined TGA/DTA (
Figure A2,
Appendix A) provides critical insight into the thermal behavior of the material, confirming the optimal calcination temperature for achieving crystalline ZnO nanoparticles without further mass loss beyond 500 °C. Other researchers also observe similar results for ZnO nanoparticles [
1,
2,
3,
4,
5].
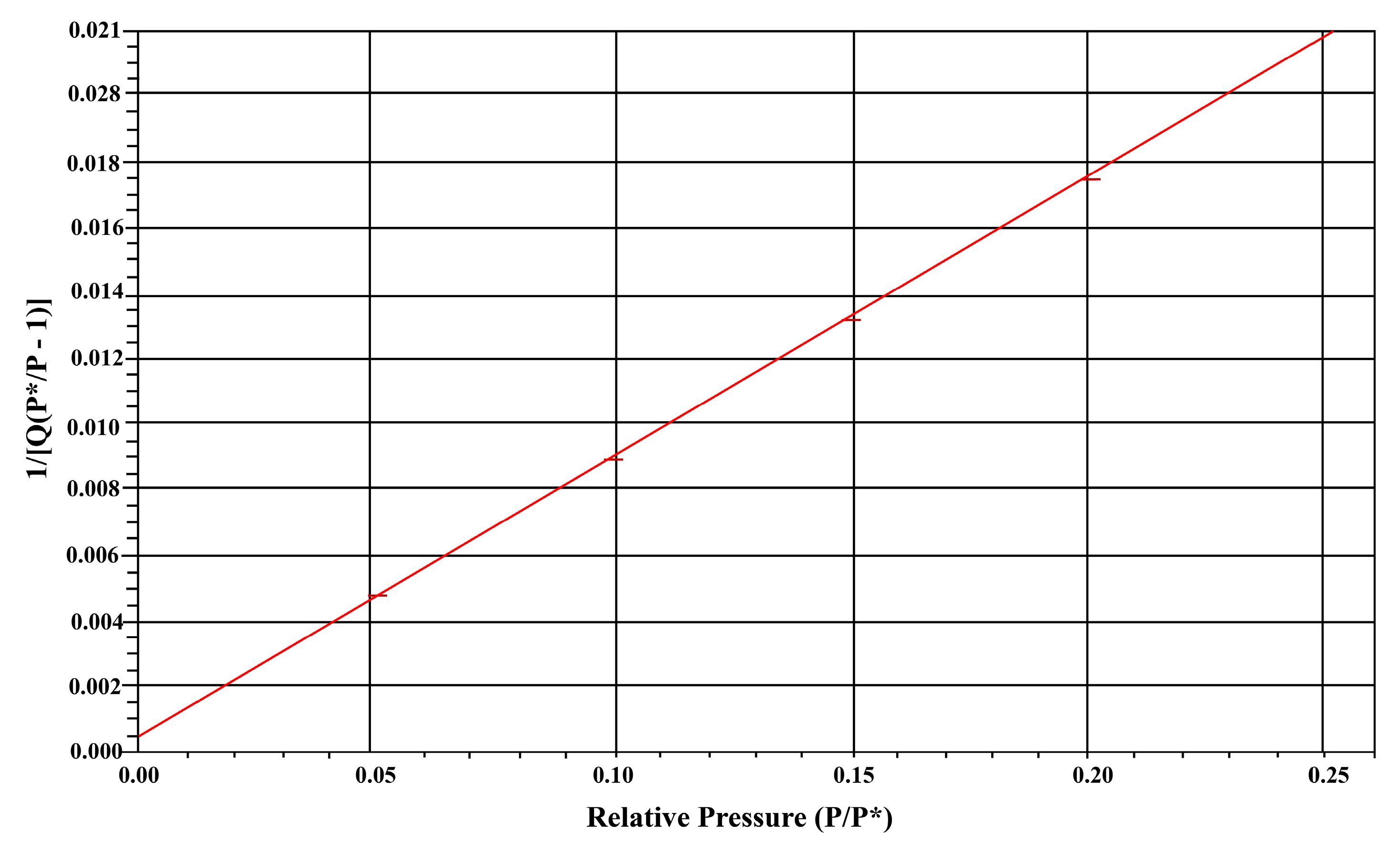
Nitrogen adsorption–desorption isotherms were measured using the N2-BET (Brunauer–Emmett–Teller) method to determine the specific surface area, pore volume, and pore size of the synthesized material. The analysis was conducted with a Micromeritics Gemini 2390 T system, using a sample density of 1.000 g/cm3 and a static pressure of 760 mm Hg to ensure accurate measurements.
The N
2-BET results (
Figure A3 Appendix A) indicate that the synthesized material possesses a high specific surface area of 250 m
2/g, which is desirable for applications such as catalysis, adsorption, and sensing. Additionally, the pore volume was measured to be 0.023534 cm
3/g, suggesting a mesoporous structure, while the average pore size was found to be 18.58 Å (1.858 nm), further confirming the material’s mesoporosity. Similar results were observed by the other researchers [
10,
11,
12,
13,
14,
15].
The relatively high surface area and pore volume suggest that the synthesized material has abundant active sites, making it suitable for applications where enhanced surface interactions are critical, such as photocatalytic degradation, gas sensing, and adsorption of pollutants. The narrow-pore size distribution also implies a well-defined nanostructure, which can further enhance the material’s functional properties.
Nanocrystalline ZnO was synthesized using a modified sonochemical process, which facilitates the formation of well-defined nanostructures through ultrasonic irradiation. The catalytic activity of the synthesized ZnO nanoparticles was evaluated against organophosphate compounds, specifically hexamethyl phosphoramide (HMPA) and omethoate. Gas chromatography (GC) coupled with mass spectrometry (MS) was employed for analysis, using an Agilent Technologies GC 6850 system paired with an MS 5973i detector to ensure precise identification and quantification of degradation products.
All samples were analyzed with a CD-50 capillary column (30 m × 0.25 mm × 0.25 µm) optimized for organophosphate detection. The oven temperature was set to 270 °C, while the injector temperature was maintained at 280 °C to ensure efficient vaporization and transfer of the analytes. These conditions were carefully selected to optimize chromatographic resolution and peak symmetry.
The use of nanocrystalline ZnO as a catalyst offers the potential to enhance the degradation of toxic organophosphates through photocatalysis or adsorption-based processes, thereby reducing environmental pollutants. The precise analytical setup and temperature control during GC/MS analysis ensured reliable detection of degradation products, confirming the efficacy of the ZnO catalyst in breaking down these hazardous compounds.
The auxiliary temperature of the GC/MS system was maintained at 280 °C to ensure optimal transfer of analytes through the interface without condensation or degradation. A solvent delay of 3 min was applied to prevent solvent peaks from interfering with the analysis and to allow the system to focus on relevant components in the sample.
The oven and inlet temperatures were carefully ramped according to a predefined temperature program to facilitate efficient separation and proper elution of all analytes from the column. This temperature ramping ensured that even compounds with varying volatilities eluted with well-resolved peaks, enhancing the accuracy of the chromatographic analysis.
To maintain the precision and reliability of the results, the instrument was calibrated before each run using certified calibration standards. This step ensured that the system’s sensitivity and peak identification remained consistent across all analyses. The rigorous calibration process helped minimize potential errors in quantification and ensured high reproducibility of the results throughout the experimental study.
In the present study, the degradation products of hexamethylphosphoramide (HMPA) and omethoate were analyzed using gas chromatography–electron impact mass spectrometry (GC/EIMS) under two different conditions: at room temperature and under sunlight at varying time intervals. The extraction of these compounds was carried out using two different solvents, both sourced from E. MERCK Company, ensuring high-quality reagents for the experiments. Helium was employed as the carrier gas due to its inertness and ability to maintain stable flow, ensuring accurate and reliable separation of analytes (
Figure 4).
To evaluate the effectiveness of ZnO prepared by the modified sonochemical method, control experiments were conducted under identical conditions. Photolysis in the absence of a catalyst resulted in negligible degradation (~5%), while commercially available ZnO showed moderate activity (~45%). ZnO prepared via conventional precipitation showed ~52% efficiency. In contrast, our synthesized ZnO achieved ~89% degradation efficiency, clearly indicating enhanced performance due to an improved morphology and surface area resulting from the modified method.
The analytical procedure began with the injection of isopropyl alcohol (IPA) onto the GC/EIMS system to confirm the purity of the solvent. As expected, the chromatogram showed no extraneous peaks, indicating that only IPA was present. Following this, the purity of HMPA was assessed by running it through the same analytical method. The chromatogram confirmed the absence of any impurities, with only the HMPA peak appearing in the spectrum, validating the integrity of the reagent for subsequent experiments.
These preliminary purity checks were essential to ensure that the solvents and reactants did not introduce contaminants that could interfere with the detection of degradation products. This step also established a baseline for peak identification, enhancing the reliability of subsequent analyses. The comparison of degradation profiles under sunlight and room temperature provided insights into the photostability and environmental degradation behavior of both HMPA and omethoate.
Similarly, a controlled blank sample consisting of isopropyl alcohol (IPA) and ZnO powder was analyzed to confirm whether any interaction occurred between ZnO and IPA under the set experimental conditions. The chromatogram for the blank sample showed no additional peaks beyond those corresponding to IPA, confirming that the ZnO powder did not react with or degrade the IPA. This ensured that the ZnO catalyst was chemically inert toward the solvent, validating its suitability for further catalytic experiments.
All analyses were performed at room temperature, and no special measures were taken to control ambient humidity or light exposure, reflecting typical environmental conditions. Additionally, all sample preparations were carried out at room temperature to maintain consistency throughout the study. While no effort was made to exclude ambient light, the exposure was kept uniform for all samples to ensure a fair comparison of results, particularly for the degradation studies conducted under sunlight and room temperature conditions.
These controlled conditions ensured that any observed changes in the experimental samples could be attributed to the degradation behavior of the organophosphates rather than unintended environmental influences or interactions between ZnO and the solvent.
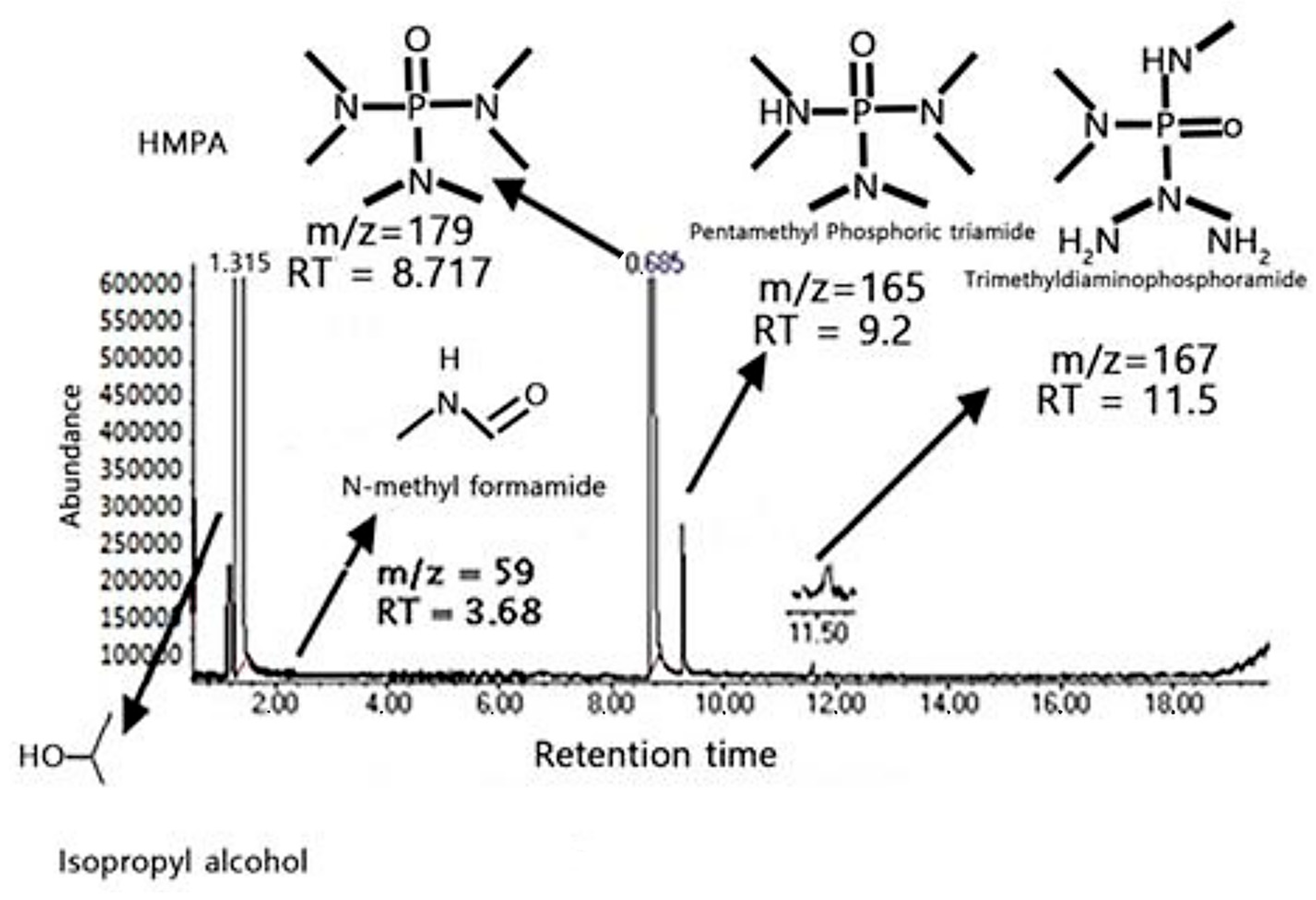
The GC/EIMS analysis in
Figure 4 provides insights into the degradation behavior of hexamethyl phosphoramide (HMPA), confirming the formation of specific byproducts under the experimental conditions, as shown in the total ion chromatogram (TIC), which highlights the retention times (RTs) and mass-to-charge ratios (m/z) of the parent compound and its degradation products. The primary peak at RT = 8.717 min with m/z = 179 corresponds to the parent compound HMPA, indicating its initial presence in the sample, while the peak at RT = 3.68 min with m/z = 59 represents N-methyl formamide, which is a non-toxic degradation product formed through partial hydrolysis involving the cleavage of phosphoramidic bonds.
Additional peaks observed at RT = 9.2 min (m/z = 165) and RT = 11.5 min (m/z = 167) correspond to pentamethyl phosphoric triamide and trimethyl amino phosphorimidate, respectively, suggesting that the degradation of HMPA proceeds via sequential dealkylation and phosphoramidate intermediates before complete breakdown. The presence of a solvent peak for isopropyl alcohol (IPA) in the chromatogram confirms the purity of the solvent, ensuring that no additional peaks or byproducts interfere with the analysis.
The degradation behavior observed in the TIC demonstrates that HMPA undergoes partial breakdown, yielding N-methylformamide, pentamethyl phosphoric triamide, and trimethyl amino phosphorimidate through complex pathways involving hydrolysis, dealkylation, and phosphoramidic bond cleavage. The formation of these non-toxic intermediates highlights the catalytic efficiency of ZnO nanoparticles under mild conditions, suggesting their potential for environmental remediation by transforming hazardous organophosphates into safer products.
The TIC spectrum confirms that HMPA, a persistent organophosphate, is partially degraded into non-toxic intermediates through catalytic reactions facilitated by ZnO nanoparticles, demonstrating the efficacy of ZnO as a photocatalyst for environmental detoxification. This finding reinforces the potential of ZnO-based catalytic systems for the sustainable degradation of hazardous organophosphates, offering a practical approach for developing efficient remediation strategies.
As demonstrated in
Table 1, hexamethylphosphoramide (HMPA) exhibited stability both at room temperature and under direct sunlight, showing no signs of degradation in the absence of a catalyst. However, when HMPA was exposed to sunlight for 6 h in the presence of ZnO as a catalyst, it underwent significant degradation, yielding non-toxic byproducts. The primary degradation products identified through their total ion chromatograms (TICs) were N-methylformamide and pentamethylphosphoric tri-imide, among others, indicating successful breakdown of the parent organophosphate compound.
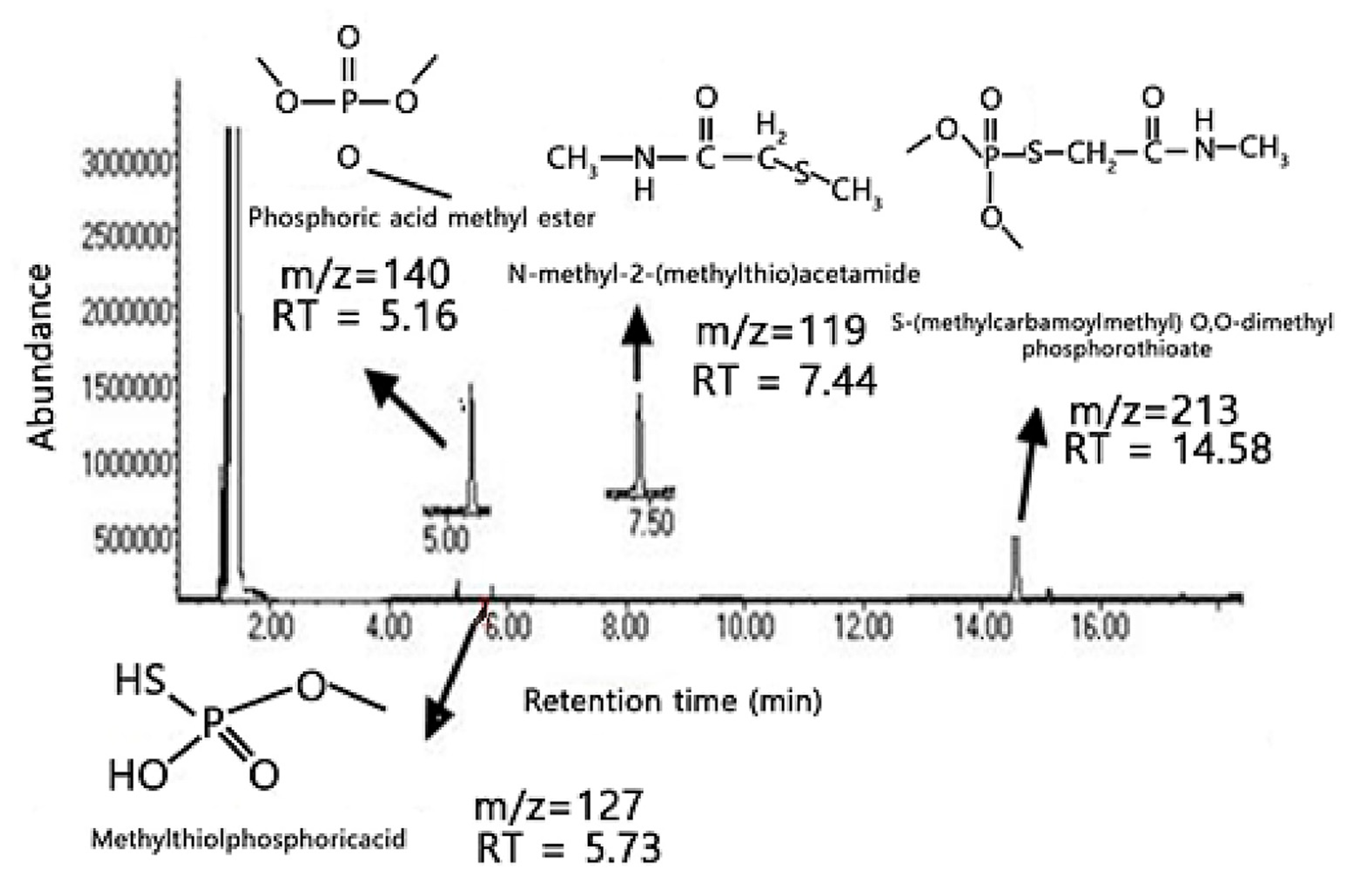
The total ion chromatogram (TIC) presented in
Figure 5 provides detailed insights into the degradation products of an organophosphate compound, likely omethoate, based on the identified byproducts. Each peak in the chromatogram is associated with specific retention times (RTs) and mass-to-charge ratios (m/z), reflecting the stepwise breakdown of the parent compound under the experimental conditions. The peak at RT = 5.16 min with m/z = 140 corresponds to phosphoric acid trimethyl ester, indicating that the degradation process involves the formation of phosphoric esters, which suggests hydrolysis of the phosphoric backbone: a common step in the breakdown of organophosphates. At RT = 5.73 min, the peak with m/z = 127 represents methylthiophosphoric acid, a sulfur-containing byproduct, suggesting that the degradation pathway includes demethylation and sulfur release, likely involving cleavage of
p = S bonds.
Further along, the peak at RT = 7.44 min with m/z = 119 corresponds to N-methyl-2-(methylthio)acetamide, reflecting the formation of amide derivatives with sulfur-containing groups, confirming that the degradation pathway involves sequential demethylation and amide formation. A more complex byproduct, S-methylkarbamoylmethyl O, O-dimethyl phosphorothioate, appears at RT = 14.58 min with m/z = 213, indicating that the degradation retains some of the original phosphorothioate structure, though in a modified form, suggesting that while the process is not entirely complete, it leads to less toxic intermediates.
The TIC in
Figure 5 highlights the formation of several key degradation products, suggesting that the breakdown of the organophosphate proceeds via hydrolysis, demethylation, and sulfur release. The presence of the phosphoric acid trimethyl ester and methylthiophosphoric acid confirms that the organophosphate structure undergoes gradual decomposition, forming smaller, less toxic intermediates. The detection of N-methyl-2-(methylthio)acetamide and S-methylkarbamoylmethyl O, O-dimethyl phosphorothioate further supports the hypothesis that the degradation pathway involves complex modifications of both the phosphorus and sulfur components. These degradation products demonstrate that the organophosphate compound can be broken down into intermediates with reduced toxicity, indicating that the catalytic system—potentially facilitated by ZnO nanoparticles—is effective. The observed byproducts provide insights into the underlying reaction pHs, including hydrolysis, demethylation, and amide formation, showcasing the versatility of the catalytic system in promoting multiple degradation pathways.
This catalytic degradation process highlights the effectiveness of ZnO under photochemical conditions, suggesting that the material facilitates photo-induced reactions that break down HMPA into environmentally benign components. The formation of these specific byproducts provides insight into the degradation pathway of HMPA, wherein cleavage of phosphoramidic bonds and demethylation processes occur.
The ability of ZnO to degrade HMPA only under sunlight emphasizes the role of photoactivation in driving the catalytic process, underscoring its potential for environmental remediation applications. These findings demonstrate that ZnO can serve as a viable photocatalyst for transforming persistent organophosphates into safer chemical species under mild and sustainable conditions.
Scheme 1 shows the proposed mechanism of degradation in the current investigation.