Synergistic Sequestration and Hydroxyapatite-Based Recovery of Phosphorus by the Coupling Process of CaCl2/Modified Oyster Shell and Circulating Fluidized Bed Reactor
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Oyster Shell (OS) and Modified Oyster Shell (MOS)
2.2. Separation Profiles of Phosphorus by the OS-Based Materials
2.2.1. Effect of Modification Temperature
2.2.2. Effect of Particle Size
2.2.3. Effect of MOS-800 Dosage
2.3. Phosphorus Separation Profiles in Semicontinuous-Flow Testes of Coupling Processes
2.4. Effects of Coexisting Ions on Phosphate Sequestration in the Semicontinuous CaCl2/MOS-800/CFB Process
2.4.1. Effects of Coexisting Anions
2.4.2. Effects of Coexisting Cations
2.5. Phosphorus Recovery Profiles in Continuous Flow CFB Processes
2.6. Characterization of Reclaimed Products
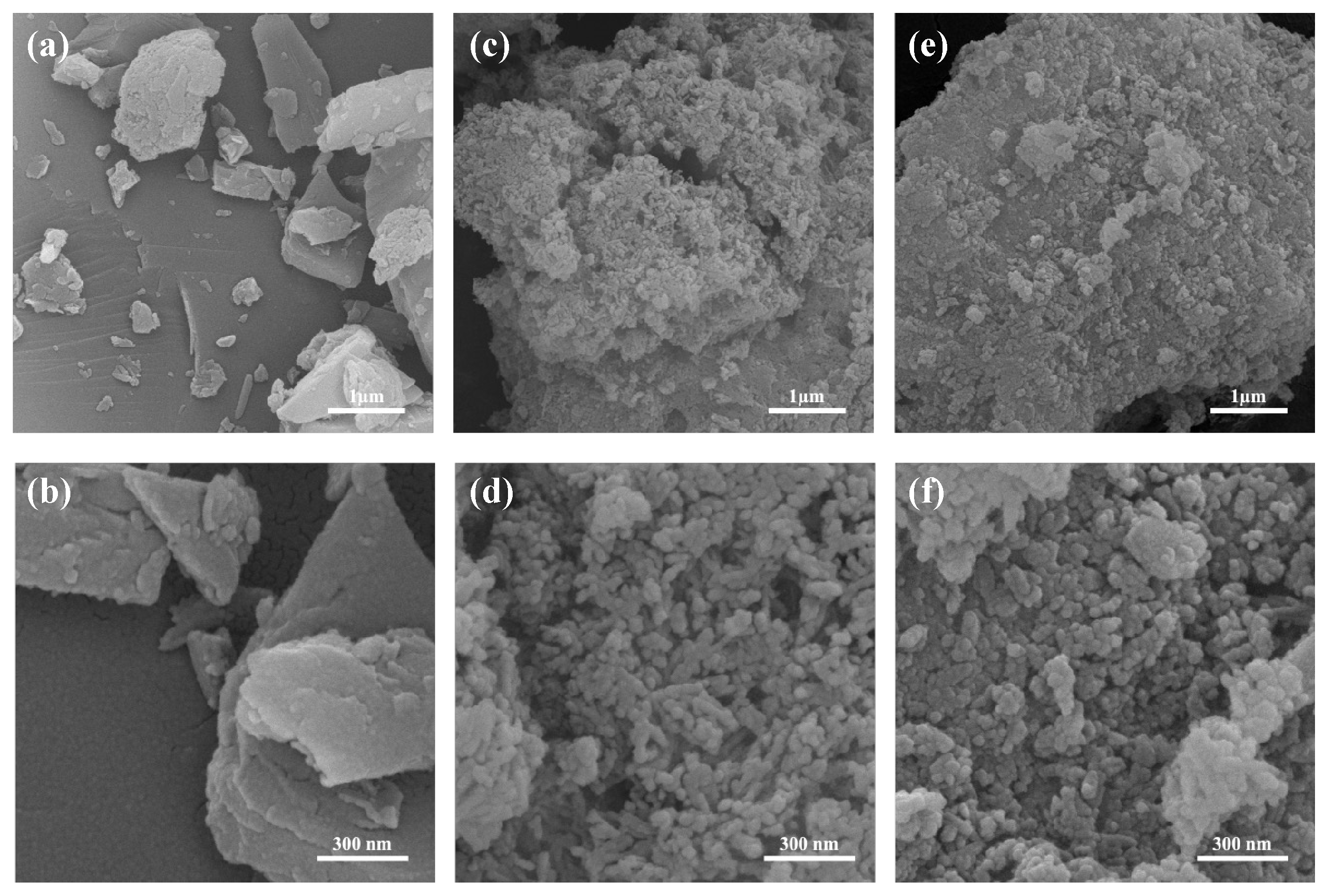
2.6.1. SEM
2.6.2. XRD, Raman, and FT-IR
2.6.3. XPS
2.7. Leaching Properties of MOS-800 and Supersaturation Index
2.7.1. Leaching Characterization of MOS-800
2.7.2. Supersaturation Index of Possible Ca-P Complexes
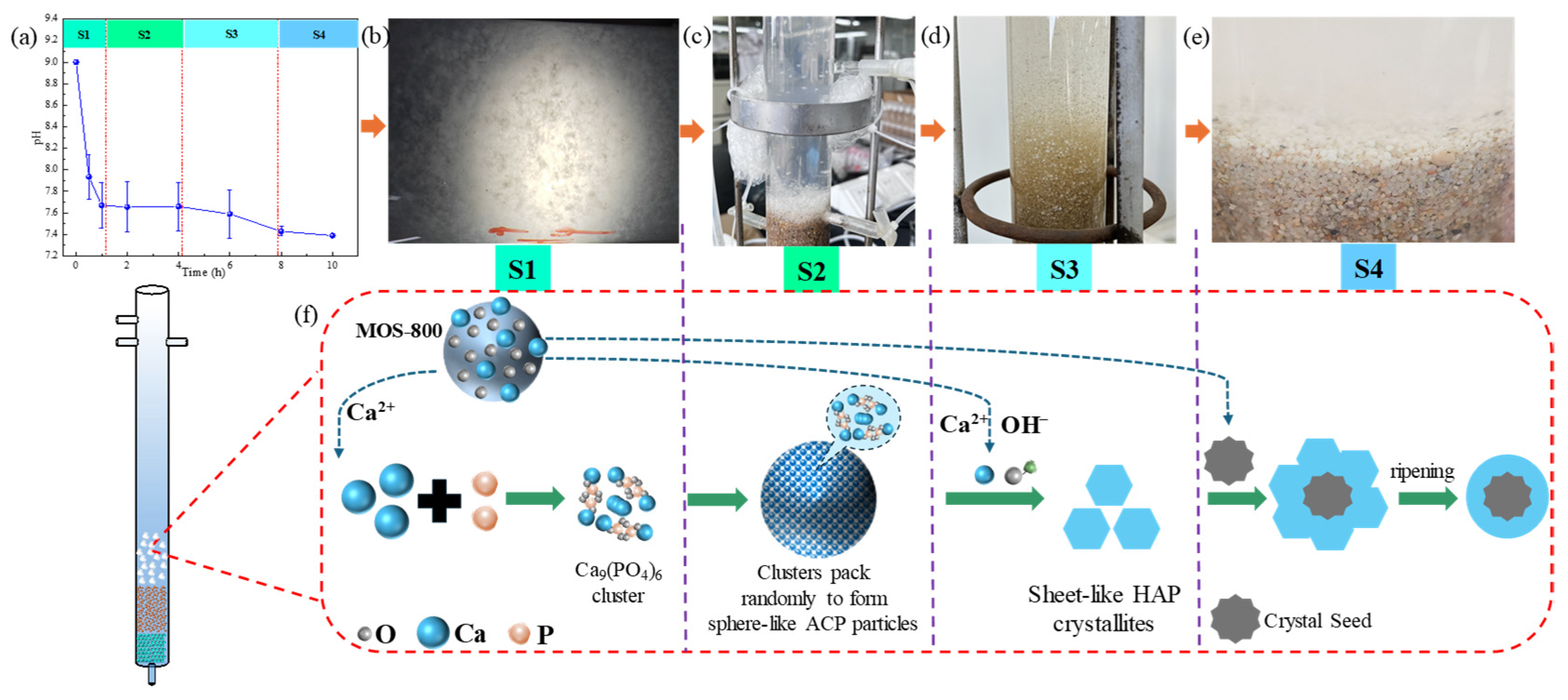
2.8. Heterogeneous Crystallization Mechanisms in the CaCl2/MOS-800/CFB Process
3. Materials and Methods
3.1. Reagents and Materials
3.2. Preparation of Modified Oyster Shell (MOS)
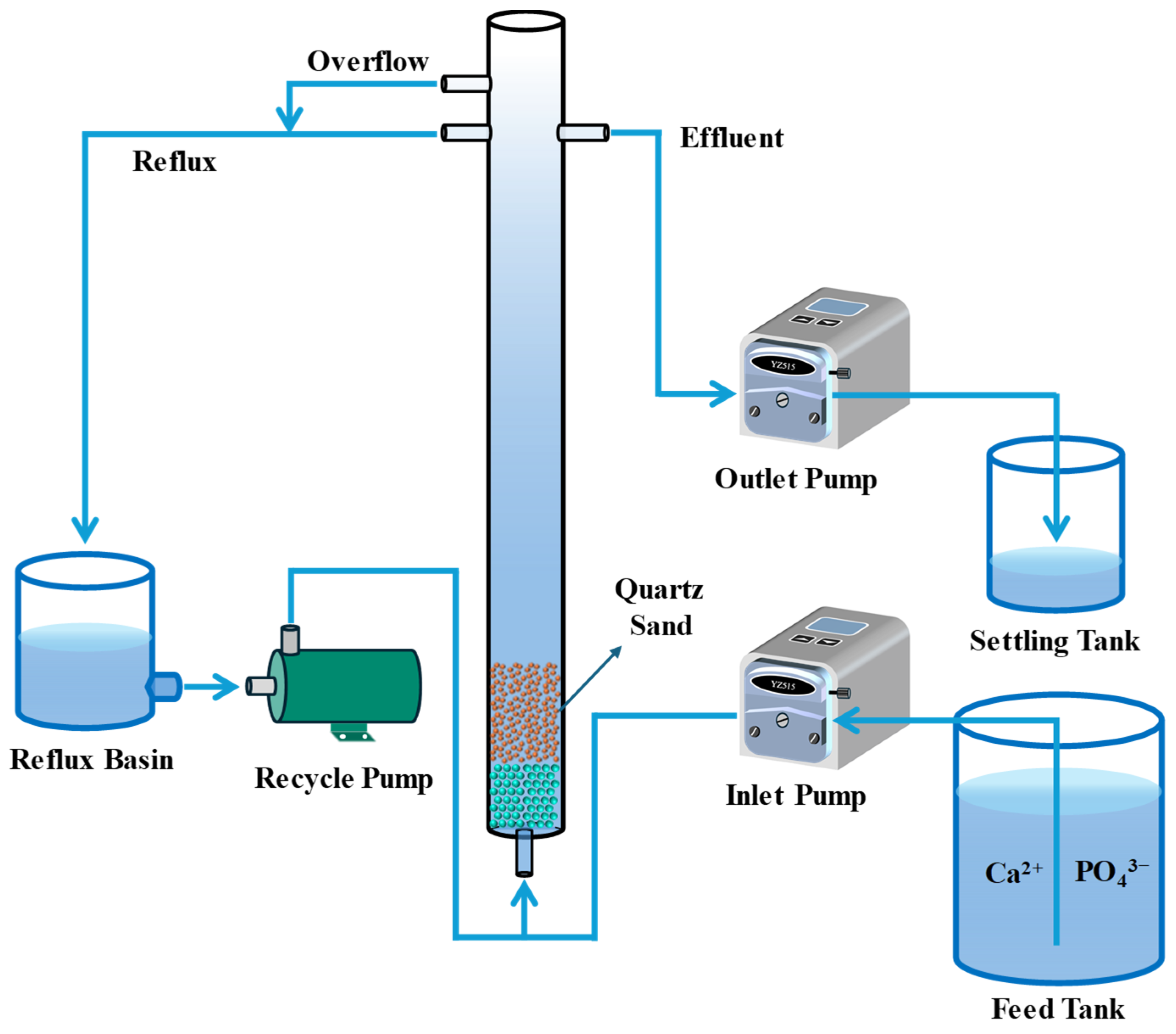
3.3. Circulating Fluidized Bed System
3.4. Experimental Setup and Design
3.5. Analysis and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xing, C.; Shi, J.; Cui, F.; Shen, J.; Li, H. Fe2+/H2O2-Strengite method with the enhanced settlement for phosphorus removal and recovery from pharmaceutical effluents. Chemosphere 2021, 277, 130343. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Qiang, X.; Gu, W.; Ma, Z.; Wang, G. An overview of biological mechanisms and strategies for treating wastewater from printing and dyeing processes. J. Water Process Eng. 2023, 55, 104242. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Li, L.; Ai, J.; He, H.; Yu, J.; Li, P.; Zhang, W. Environmental impact and optimization suggestions of pig manure and wastewater treatment systems from a life cycle perspective. Sci. Total Environ. 2023, 905, 167262. [Google Scholar] [CrossRef]
- Jupp, A.R.; Beijer, S.; Narain, G.C.; Schipper, W.; Slootweg, J.C. Phosphorus recovery and recycling—Closing the loop. Chem. Soc. Rev. 2021, 50, 87–101. [Google Scholar] [CrossRef]
- Cakmak, E.K.; Hartl, M.; Kisser, J.; Cetecioglu, Z. Phosphorus mining from eutrophic marine environment towards a blue economy: The role of bio-based applications. Water Res. 2022, 219, 118505. [Google Scholar] [CrossRef] [PubMed]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. WIREs Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Barbieri, P.; MacDonald, G.K.; Bernard De Raymond, A.; Nesme, T. Food system resilience to phosphorus shortages on a telecoupled planet. Nat. Sustain. 2021, 5, 114–122. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, S.; Wang, S.; Yan, X.; Qian, J.; Pan, B. Phosphorus in water: A review on the speciation analysis and species specific removal strategies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 435–456. [Google Scholar] [CrossRef]
- Izadi, P.; Izadi, P.; Eldyasti, A. Design, operation and technology configurations for enhanced biological phosphorus removal (EBPR) process: A review. Rev. Environ. Sci. Bio/Technol. 2020, 19, 561–593. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Y.; Wang, Z.; Lv, M.; Tang, A.; Yu, Y.; Qu, X.; Chen, Z.; Wen, Q.; Li, A. Insight into the synthesis and adsorption mechanism of adsorbents for efficient phosphate removal: Exploration from synthesis to modification. Chem. Eng. J. 2022, 442, 136147. [Google Scholar] [CrossRef]
- Deng, L.; Dhar, B.R. Phosphorus recovery from wastewater via calcium phosphate precipitation: A critical review of methods, progress, and insights. Chemosphere 2023, 330, 138685. [Google Scholar] [CrossRef]
- Cordell, D.; Rosemarin, A.; Schröder, J.J.; Smit, A.L. Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere 2011, 84, 747–758. [Google Scholar] [CrossRef]
- Riewklang, K.; Kaan Dereli, R.; Nakason, K.; Jin, G.; Panyapinyopol, B. Assessing phosphorus recovery from anaerobic digestion effluent of tapioca starch processing in a pilot—Scale fluidized—Bed homogeneous crystallizer: Effects of operation modes. Chem. Eng. J. 2024, 488, 150825. [Google Scholar] [CrossRef]
- Wilfert, P.; Dugulan, A.I.; Goubitz, K.; Korving, L.; Witkamp, G.J.; Van Loosdrecht, M.C.M. Vivianite as the main phosphate mineral in digested sewage sludge and its role for phosphate recovery. Water Res. 2018, 144, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, C.; Zhang, X.; Deng, S.; Cheng, X.; Waite, T.D. Phosphate Recovery from Aqueous Solutions via Vivianite Crystallization: Interference of FeII Oxidation at Different DO Concentrations and pHs. Environ. Sci. Technol. 2023, 57, 2105–2117. [Google Scholar] [CrossRef] [PubMed]
- Cichy, B.; Kużdżał, E.; Krztoń, H. Phosphorus recovery from acidic wastewater by hydroxyapatite precipitation. J. Environ. Manag. 2019, 232, 421–427. [Google Scholar]
- Eggers, E.; Dirkzwager, A.H.; Van Der Honing, H. Full-Scale Experiences with Phosphate Crystallization in a Crystalactor®. Water Sci. Technol. 1991, 23, 819–824. [Google Scholar] [CrossRef]
- Guan, Q.; Zeng, G.; Song, J.; Li, Y.; Yang, L.; Wang, Z.; Liu, C. Highly efficient phosphorus and potassium recovery from urine via crystallization process in a fluidized bed reactor system. J. Environ. Chem. Eng. 2021, 9, 105623. [Google Scholar] [CrossRef]
- Riewklang, K.; Polprasert, C.; Nakason, K.; Polprasert, S.; Kwonpongsagoon, S.; Mahasandana, S.; Panyapinyopol, B. Enhancing chemical phosphorus precipitation from tapioca starch anaerobic digestion effluent in a modified pilot-scale fluidized bed reactor. Environ. Res. 2023, 231, 116277. [Google Scholar] [CrossRef]
- Cruz-Hernández, M.; Velázquez-Herrera, F.D.; Fetter, G. Synthetic hydroxyapatites as high-performance fertilizers for lettuce plant growth. Rhizosphere 2023, 26, 100718. [Google Scholar] [CrossRef]
- Lee, J.-I.; Kang, J.-K.; Oh, J.-S.; Yoo, S.-C.; Lee, C.-G.; Jho, E.H.; Park, S.-J. New insight to the use of oyster shell for removing phosphorus from aqueous solutions and fertilizing rice growth. J. Clean. Prod. 2021, 328, 129536. [Google Scholar] [CrossRef]
- Jang, H.; Kang, S.-H. Phosphorus removal using cow bone in hydroxyapatite crystallization. Water Res. 2002, 36, 1324–1330. [Google Scholar] [PubMed]
- Kim, E.; Yim, S.; Jung, H.; Lee, E. Hydroxyapatite crystallization from a highly concentrated phosphate solution using powdered converter slag as a seed material. J. Hazard. Mater. 2006, 136, 690–697. [Google Scholar] [CrossRef]
- Santos, A.F.; Lopes, D.V.; Alvarenga, P.; Gando-Ferreira, L.M.; Quina, M.J. Phosphorus removal from urban wastewater through adsorption using biogenic calcium carbonate. J. Environ. Manag. 2024, 351, 119875. [Google Scholar] [CrossRef]
- Su, P.; Huo, Q.; Zhang, J.; Zhao, G.; Quan, B.; Zhang, C. Recovery of phosphorus from public toilet press filtrate using Ca-rich fly ash through the formation of hydroxyapatite (HAP). Resour. Conserv. Recycl. Adv. 2023, 17, 200138. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Thenepalli, T.; Han, C.; Ahn, J.-W. Synthesis of aragonite-precipitated calcium carbonate from oyster shell waste via a carbonation process and its applications. Korean J. Chem. Eng. 2017, 34, 225–230. [Google Scholar] [CrossRef]
- Martins, M.C.; Santos, E.B.H.; Marques, C.R. First study on oyster-shell-based phosphorous removal in saltwater—A proxy to effluent bioremediation of marine aquaculture. Sci. Total Environ. 2017, 574, 605–615. [Google Scholar]
- Inthapanya, X.; Wu, S.; Han, Z.; Zeng, G.; Wu, M.; Yang, C. Adsorptive removal of anionic dye using calcined oyster shells: Isotherms, kinetics, and thermodynamics. Environ. Sci. Pollut. Res. 2019, 26, 5944–5954. [Google Scholar] [CrossRef]
- Currie, J.A.; Harrison, N.R.; Wang, L.; Jones, M.I.; Brooks, M.S. A preliminary study of processing seafood shells for eutrophication control. Asia-Pac. J. Chem. Eng. 2007, 2, 460–467. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, X.; Bai, J.; Li, X.; Wang, C.; Cai, J.; Li, Y.; Liang, W.; Chang, S.; Jiang, Y. Utilization of oyster shell nano-hydroxyapatite modified red-brick waste as an environmentally friendly composite filler for Cd(II) and Cr(VI) adsorption: Preparation, property and mechanism. J. Water Process Eng. 2024, 59, 104955. [Google Scholar] [CrossRef]
- Legodi, M.A.; De Waal, D.; Potgieter, J.H.; Potgieter, S.S. Rapid determination of CaCO3 in mixtures utilising FT—IR spectroscopy. Miner. Eng. 2001, 14, 1107–1111. [Google Scholar] [CrossRef]
- Park, K.; Sadeghi, K.; Thanakkasaranee, S.; Park, Y.I.; Park, J.; Nam, K.H.; Han, H.; Seo, J. Effects of calcination temperature on morphological and crystallographic properties of oyster shell as biocidal agent. Int. J. Appl. Ceram. Technol. 2021, 18, 302–311. [Google Scholar] [CrossRef]
- Khiri, M.Z.A.; Matori, K.A.; Zainuddin, N.; Abdullah, C.A.C.; Alassan, Z.N.; Baharuddin, N.F.; Zaid, M.H.M. The usability of ark clam shell (Anadara granosa) as calcium precursor to produce hydroxyapatite nanoparticle via wet chemical precipitate method in various sintering temperature. SpringerPlus 2016, 5, 1206. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.H.J.M.D.; Pontin, D.; Ponzi, G.G.D.; Stepanha, A.S.D.G.E.; Martel, R.B.; Schütz, M.K.; Einloft, S.M.O.; Dalla Vecchia, F. Application of Fourier Transform infrared spectroscopy (FTIR) coupled with multivariate regression for calcium carbonate (CaCO3) quantification in cement. Constr. Build. Mater. 2021, 313, 125413. [Google Scholar] [CrossRef]
- Chang, L.; Feng, Y.; Wang, B.; Huang, X.; Yang, D.-P.; Lu, Y. Dual functional oyster shell-derived Ag/ZnO/CaCO3 nanocomposites with enhanced catalytic and antibacterial activities for water purification. RSC Adv. 2019, 9, 41336–41344. [Google Scholar] [CrossRef]
- Inoue, Y.; Yasumori, I. Catalysis by Alkaline Earth Metal Oxides. III. X-Ray Photoelectron Spectroscopic Study of Catalytically Active MgO, CaO, and BaO Surfaces. Bull. Chem. Soc. Jpn. 1981, 54, 1505–1510. [Google Scholar] [CrossRef]
- Sugama, T.; Kukacka, L.E.; Carciello, N.; Hocker, N.J. Study of interactions at water-soluble polymer/Ca(OH)2 or gibbsite interfaces by XPS. Cem. Concr. Res. 1989, 19, 857–867. [Google Scholar] [CrossRef]
- Supamathanon, N.; Boonserm, K.; Lisnund, S.; Chanlek, N.; Rungtaweevoranit, B.; Khemthong, P.; Wittayakun, J.; Osakoo, N. Development of CaO supported on modified geopolymer catalyst for transesterification of soybean oil to biodiesel. Mater. Today Commun. 2021, 29, 102822. [Google Scholar] [CrossRef]
- Tran, T.-T.; Tran, N.-N.T.; Sugiyama, S.; Liu, J.-C. Enhanced phosphate removal by thermally pretreated waste oyster shells. J. Mater. Cycles Waste Manag. 2021, 23, 177–185. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, X.; Dong, Y.; Ma, Y. Removal of high-concentration phosphate by calcite: Effect of sulfate and pH. Desalination 2012, 289, 66–71. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Carbonate and Magnesium Interactive Effect on Calcium Phosphate Precipitation. Environ. Sci. Technol. 2008, 42, 436–442. [Google Scholar] [CrossRef]
- Dai, J.; Yang, H.; Yan, H.; Shangguan, Y.; Zheng, Q.; Cheng, R. Phosphate adsorption from aqueous solutions by disused adsorbents: Chitosan hydrogel beads after the removal of copper(II). Chem. Eng. J. 2011, 166, 970–977. [Google Scholar] [CrossRef]
- Lei, Y.; Zhan, Z.; Saakes, M.; Van Der Weijden, R.D.; Buisman, C.J.N. Electrochemical Recovery of Phosphorus from Acidic Cheese Wastewater: Feasibility, Quality of Products, and Comparison with Chemical Precipitation. ACS EST Water 2021, 1, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Pan, H.; Xu, X.; Tang, R. Toward a Detailed Understanding of Magnesium Ions on Hydroxyapatite Crystallization Inhibition. Cryst. Growth Des. 2014, 14, 763–769. [Google Scholar] [CrossRef]
- Ha, T.-H.; Mahasti, N.N.N.; Lin, C.-S.; Lu, M.-C.; Huang, Y.-H. Enhanced struvite (MgNH4PO4·6HXO) granulation and separation from synthetic wastewater using fluidized-bed crystallization (FBC) technology. J. Water Process Eng. 2023, 53, 103855. [Google Scholar] [CrossRef]
- Le, V.-G.; Vu, C.-T.; Shih, Y.-J.; Bui, X.-T.; Liao, C.-H.; Huang, Y.-H. Phosphorus and potassium recovery from human urine using a fluidized bed homogeneous crystallization (FBHC) process. Chem. Eng. J. 2020, 384, 123282. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, S.; Wu, D. Phosphorus harvesting from fresh human urine: A strategy of precisely recovering high-purity calcium phosphate and insights into the precipitation conversion mechanism. Water Res. 2022, 227, 119325. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Renard, F. Nucleation of Brushite and Hydroxyapatite from Amorphous Calcium Phosphate Phases Revealed by Dynamic In Situ Raman Spectroscopy. J. Phys. Chem. C 2020, 124, 15302–15311. [Google Scholar] [CrossRef]
- Ostroumov, M.; Faulques, E.; Lounejeva, E. Raman spectroscopy of natural silica in Chicxulub impactite, Mexico. Comptes Rendus. Géoscience 2002, 334, 21–26. [Google Scholar] [CrossRef]
- Kazanci, M.; Fratzl, P.; Klaushofer, K.; Paschalis, E.P. Complementary Information on In Vitro Conversion of Amorphous (Precursor) Calcium Phosphate to Hydroxyapatite from Raman Microspectroscopy and Wide-Angle X-Ray Scattering. Calcif. Tissue Int. 2006, 79, 354–359. [Google Scholar] [CrossRef]
- McMillan, P.; Wolf, G.; Lambert, P. A Raman spectroscopic study of shocked single crystalline quartz. Phys. Chem. Miner. 1992, 19, 71–79. [Google Scholar] [CrossRef]
- Mir, M.; Leite, F.L.; Herrmann Junior, P.S.D.P.; Pissetti, F.L.; Rossi, A.M.; Moreira, E.L.; Mascarenhas, Y.P. XRD, AFM, IR and TGA study of nanostructured hydroxyapatite. Mater. Res. 2012, 15, 622–627. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, Y.; Gu, L.; Zhu, M.; Yang, P.; Gu, C.; Liu, Z.; Feng, X.; Tan, W.; Huang, Q.; et al. Elucidating Phosphate and Cadmium Cosorption Mechanisms on Mineral Surfaces with Direct Spectroscopic and Modeling Evidence. Environ. Sci. Technol. 2024, 58, 20211–20223. [Google Scholar] [CrossRef]
- Choi, D.; Marra, K.G.; Kumta, P.N. Chemical synthesis of hydroxyapatite/poly(ε-caprolactone) composites. Mater. Res. Bull. 2004, 39, 417–432. [Google Scholar] [CrossRef]
- Gibson, I.R.; Bonfield, W. Novel synthesis and characterization of an AB-type carbonate-substituted hydroxyapatite. J. Biomed. Mater. Res. 2002, 59, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Paul, S.; Choudhury, A.R.; Balla, V.K.; Das, M.; Sinha, A. Synthesis of hydroxyapatite from Lates calcarifer fish bone for biomedical applications. Mater. Lett. 2017, 203, 89–92. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, W.; Wang, S.; Xu, R.; Hao, L.; Sun, W. Effects of calcium on phosphorus recovery from wastewater by vivianite crystallization: Interaction and mechanism analysis. J. Environ. Chem. Eng. 2023, 11, 110506. [Google Scholar] [CrossRef]
- Navío, J.A.; Macías, M.; Colón, G.; Sánchez-Soto, P.J.; Augugliaro, V.; Palmisano, L. Combined use of XPS, IR and EDAX techniques for the characterization of ZrO2-SiO2 powders prepared by a sol-gel process. Appl. Surf. Sci. 1994, 81, 325–329. [Google Scholar] [CrossRef]
- Kačiulis, S.; Mattogno, G.; Pandolfi, L.; Cavalli, M.; Gnappi, G.; Montenero, A. XPS study of apatite-based coatings prepared by sol–gel technique. Appl. Surf. Sci. 1999, 151, 1–5. [Google Scholar] [CrossRef]
- Mucalo, M.R.; Toriyama, M.; Yokogawa, Y.; Suzuki, T.; Kawamoto, Y.; Nagata, F.; Nishizawa, K. Growth of calcium phosphate on ion-exchange resins pre-saturated with calcium or hydrogenphosphate ions: An SEM/EDX and XPS study. J. Mater. Sci. Mater. Med. 1995, 6, 409–419. [Google Scholar] [CrossRef]
- Chaudhary, B.; Kshetri, Y.K.; Dhakal, D.R.; Lee, S.W.; Kim, T.-H. Synthesis and characterization of red-emitting Yb/Ho-CaSiO3 upconversion phosphors. Prog. Nat. Sci. Mater. Int. 2022, 32, 594–601. [Google Scholar] [CrossRef]
- Landis, W.J.; Martin, J.R. X-ray photoelectron spectroscopy applied to gold-decorated mineral standards of biological interest. J. Vac. Sci. Technol. Vac. Surf. Film. 1984, 2, 1108–1111. [Google Scholar] [CrossRef]
- Luo, J.; Peng, J.; Zhong, Z.; Long, X.; Yang, J.; Li, R.; Wan, J. A novel calcium peroxide/attapulgite-Fe(II) process for high concentration phosphate removal and recovery: Efficiency and mechanism. J. Environ. Manag. 2023, 343, 118166. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Lu, X.; Peng, Y.; Yang, Z.; Zhsssu, H. Effects of supersaturation control strategies on hydroxyapatite (HAP) crystallization for phosphorus recovery from wastewater. Environ. Sci. Pollut. Res. 2017, 24, 5791–5799. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Amorphous calcium (ortho)phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef]
- Yun, J.; Holmes, B.; Fok, A.; Wang, Y. A Kinetic Model for Hydroxyapatite Precipitation in Mineralizing Solutions. Cryst. Growth Des. 2018, 18, 2717–2725. [Google Scholar] [CrossRef]
- Liu, X.; Yang, S.; Liu, S.; Yang, Y. Performance and mechanism of phosphorus removal by slag ceramsite filler. Process Saf. Environ. Prot. 2021, 148, 858–866. [Google Scholar] [CrossRef]
- Gholami, M.; O’Sullivan, A.D.; Mackey, H.R. Calcinated sea urchin shell waste for rapid phosphate removal from greywater for application to nature-based systems. Process Saf. Environ. Prot. 2025, 194, 955–966. [Google Scholar] [CrossRef]
- Yang, J.; Long, X.; Feng, X.; Wan, J. Simultaneous sorption of orthophosphate and phosphonate from RO concentrate by kaolin/lanthanum carbonate composites: Experimental investigation and multi-objective artificial neural network modeling. J. Environ. Chem. Eng. 2023, 11, 109776. [Google Scholar] [CrossRef]
- Guo, S.; Liu, Y.; Li, J. Nanocatalysts for the Degradation of Refractory Pollutants. Catalysts 2024, 14, 444. [Google Scholar] [CrossRef]
- Guo, S.; Chen, M.; Wei, Y.; You, L.; Cai, C.; Wei, Q.; Zhou, K. Designing hierarchically porous zero-valent iron via 3D printing to degrade organic pollutants by activating peroxymonosulfate using high-valent iron-oxo species. Chem. Eng. J. 2023, 476, 146523. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Visual MINTEQ 3.0 User Guide; KTH, Department of Land and Water Recources: Stockholm, Sweden, 2011. [Google Scholar]














Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, X.; Yang, N.; Wang, H.; Fang, J.; Wang, R.; Zhong, Z.; Yu, P.; Xu, X.; Huang, H.; Wan, J.; et al. Synergistic Sequestration and Hydroxyapatite-Based Recovery of Phosphorus by the Coupling Process of CaCl2/Modified Oyster Shell and Circulating Fluidized Bed Reactor. Catalysts 2025, 15, 706. https://doi.org/10.3390/catal15080706
Long X, Yang N, Wang H, Fang J, Wang R, Zhong Z, Yu P, Xu X, Huang H, Wan J, et al. Synergistic Sequestration and Hydroxyapatite-Based Recovery of Phosphorus by the Coupling Process of CaCl2/Modified Oyster Shell and Circulating Fluidized Bed Reactor. Catalysts. 2025; 15(8):706. https://doi.org/10.3390/catal15080706
Chicago/Turabian StyleLong, Xuejun, Nanshan Yang, Huiqi Wang, Jun Fang, Rui Wang, Zhenxing Zhong, Peng Yu, Xuelian Xu, Hao Huang, Jun Wan, and et al. 2025. "Synergistic Sequestration and Hydroxyapatite-Based Recovery of Phosphorus by the Coupling Process of CaCl2/Modified Oyster Shell and Circulating Fluidized Bed Reactor" Catalysts 15, no. 8: 706. https://doi.org/10.3390/catal15080706
APA StyleLong, X., Yang, N., Wang, H., Fang, J., Wang, R., Zhong, Z., Yu, P., Xu, X., Huang, H., Wan, J., Lu, X., & Wu, X. (2025). Synergistic Sequestration and Hydroxyapatite-Based Recovery of Phosphorus by the Coupling Process of CaCl2/Modified Oyster Shell and Circulating Fluidized Bed Reactor. Catalysts, 15(8), 706. https://doi.org/10.3390/catal15080706






