Reusable Three-Dimensional TiO2@MoS2 Core–Shell Photoreduction Material: Designed for High-Performance Seawater Uranium Extraction
Abstract
1. Introduction
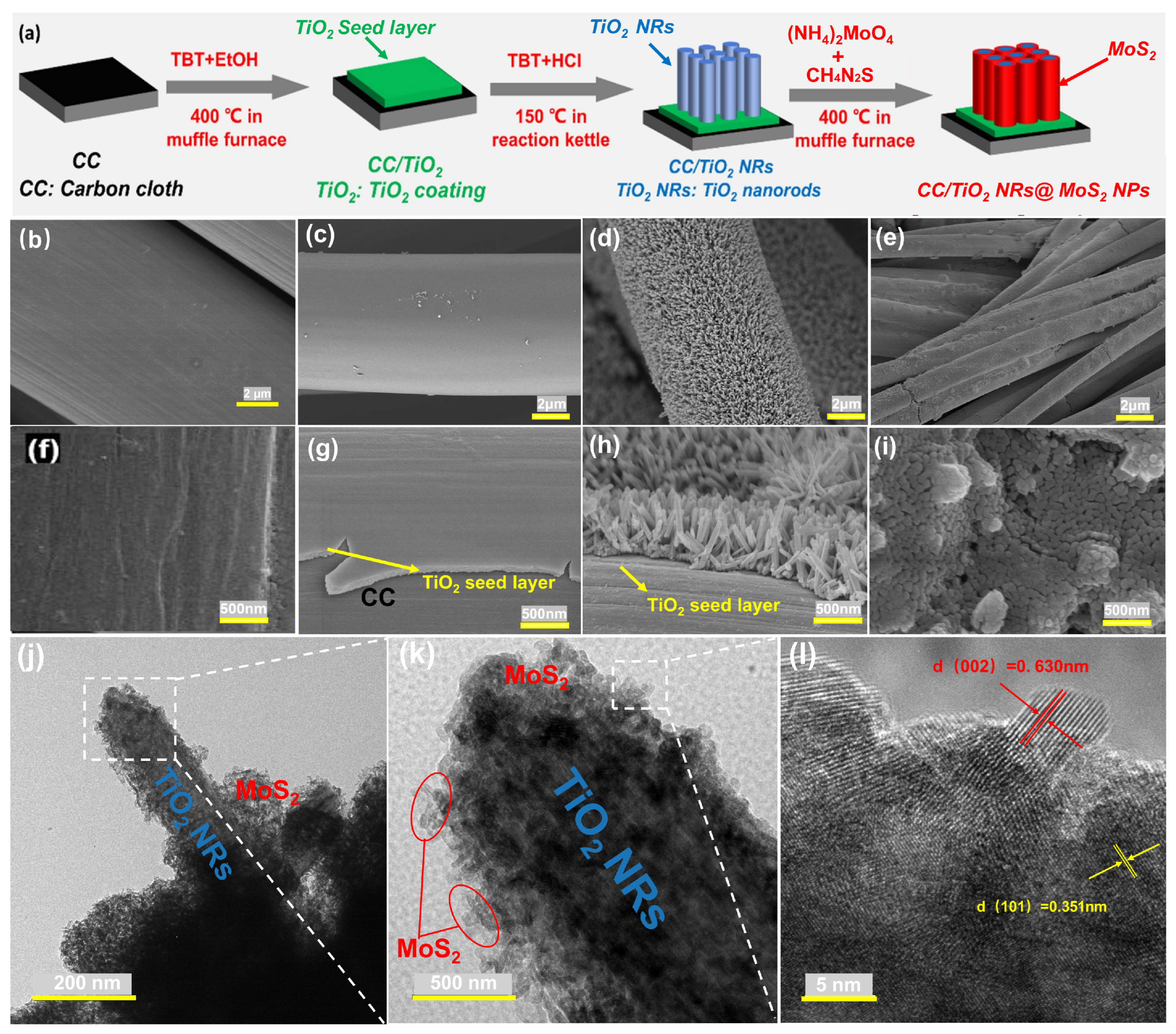
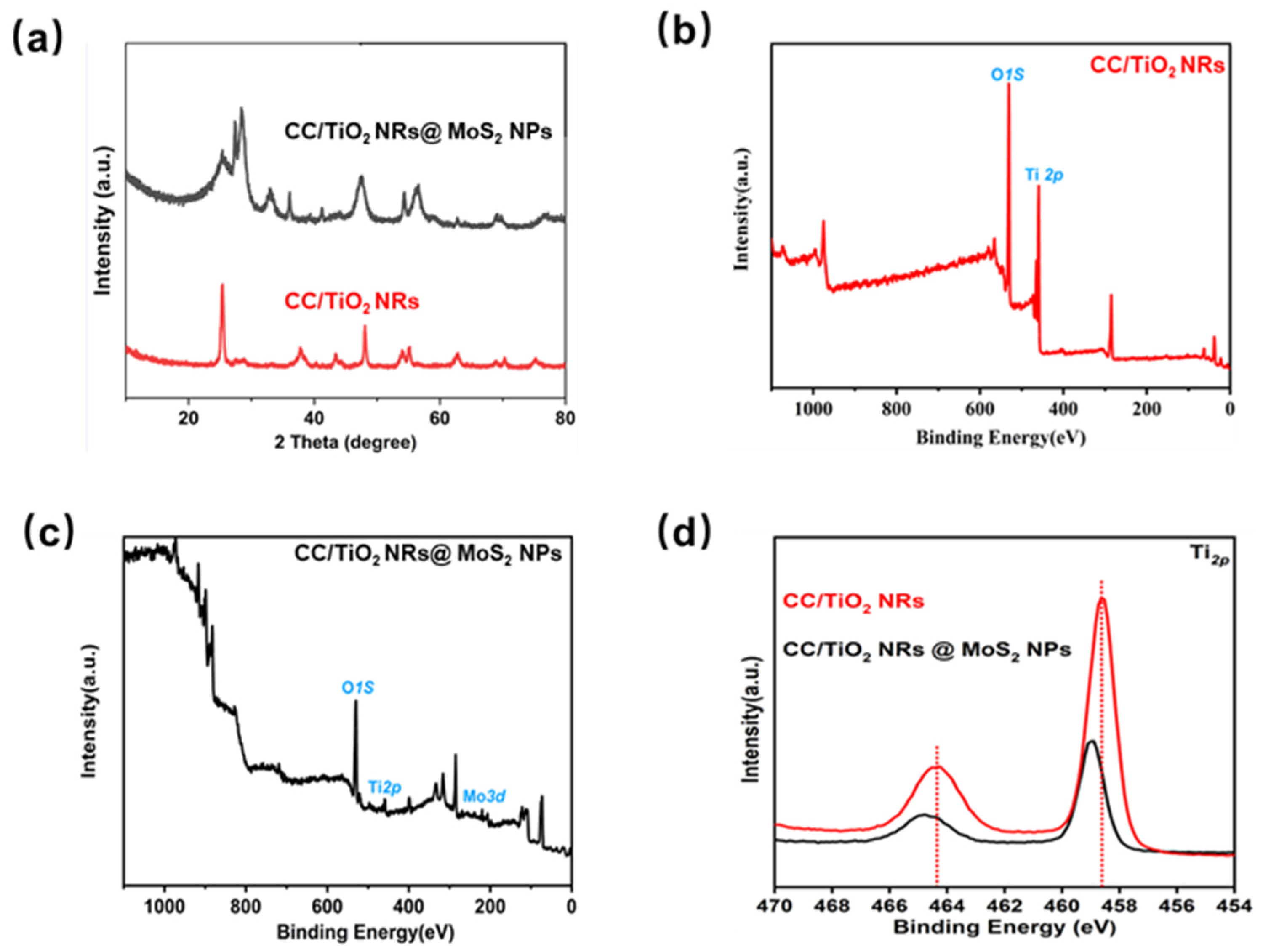
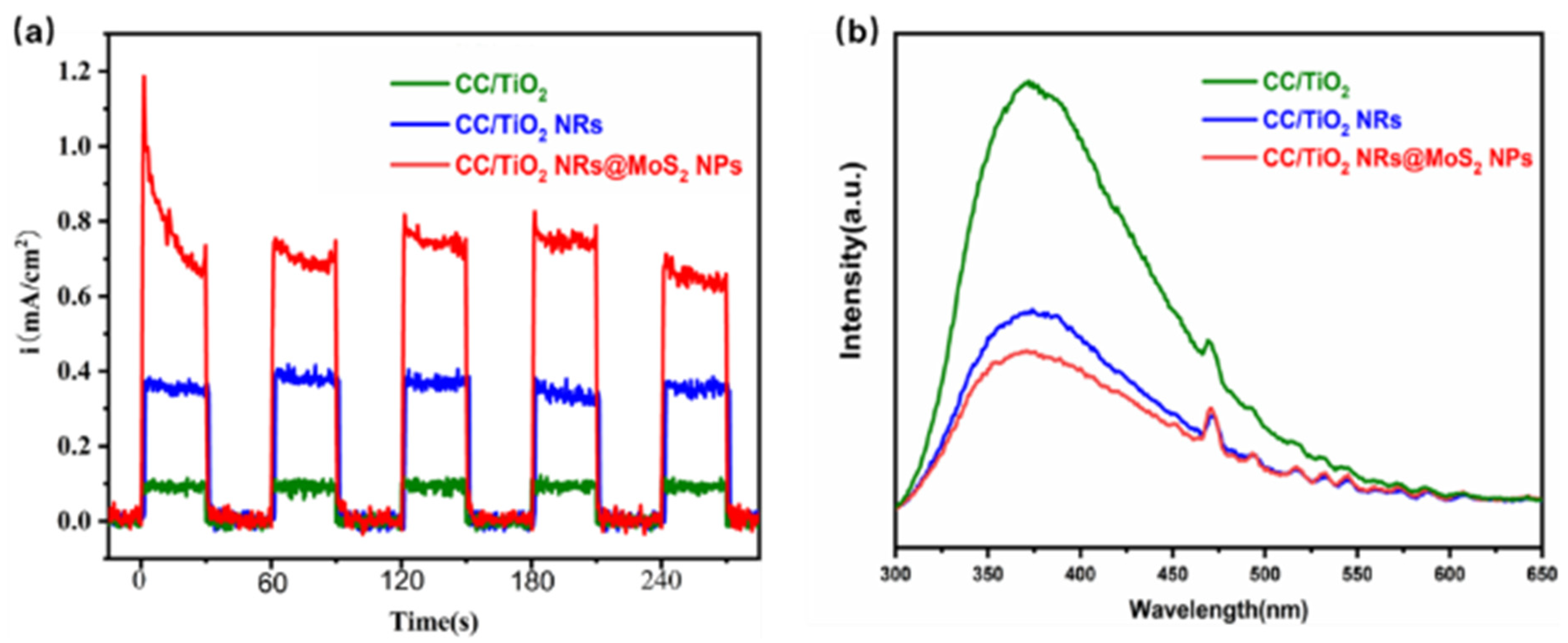
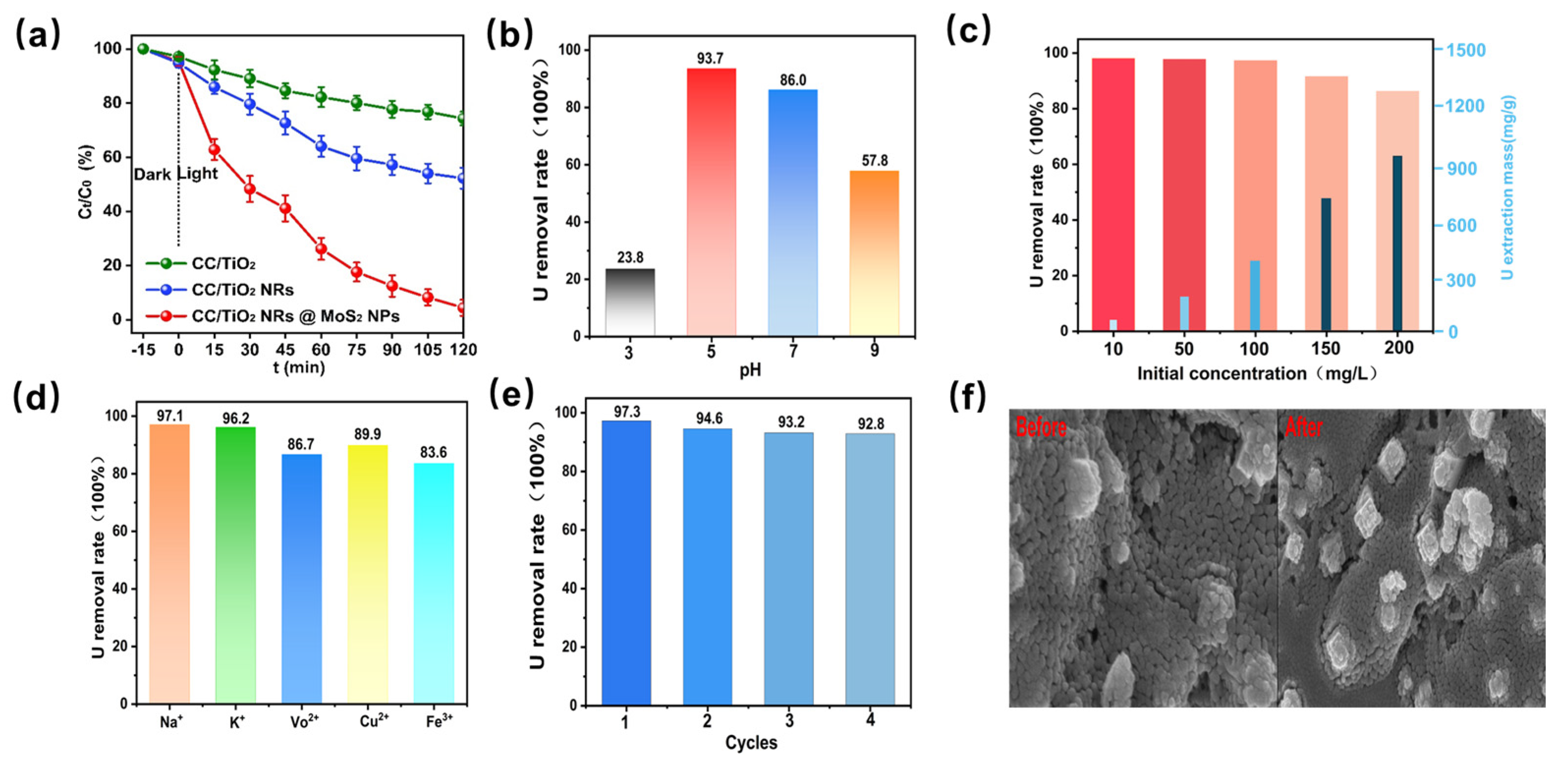
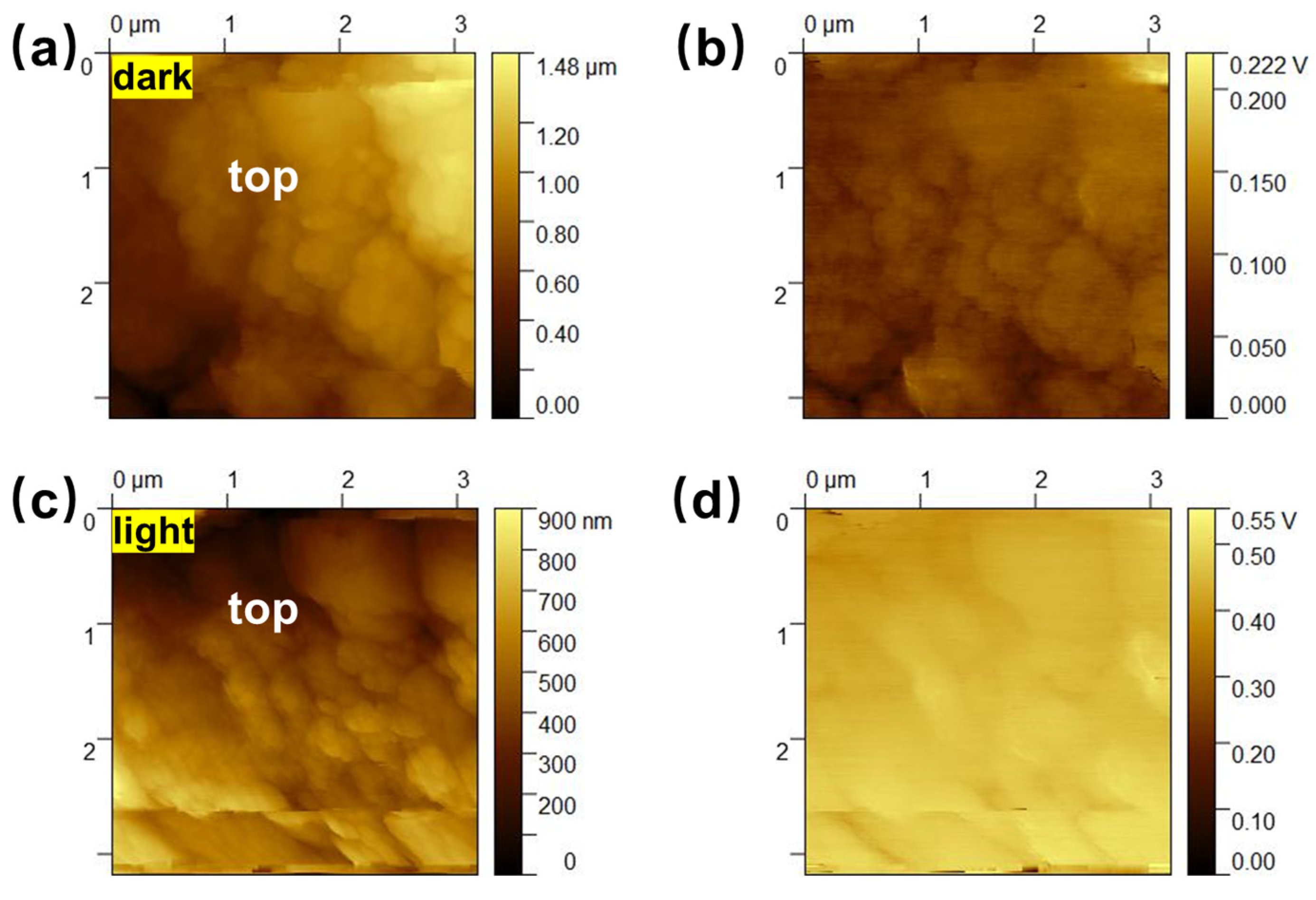
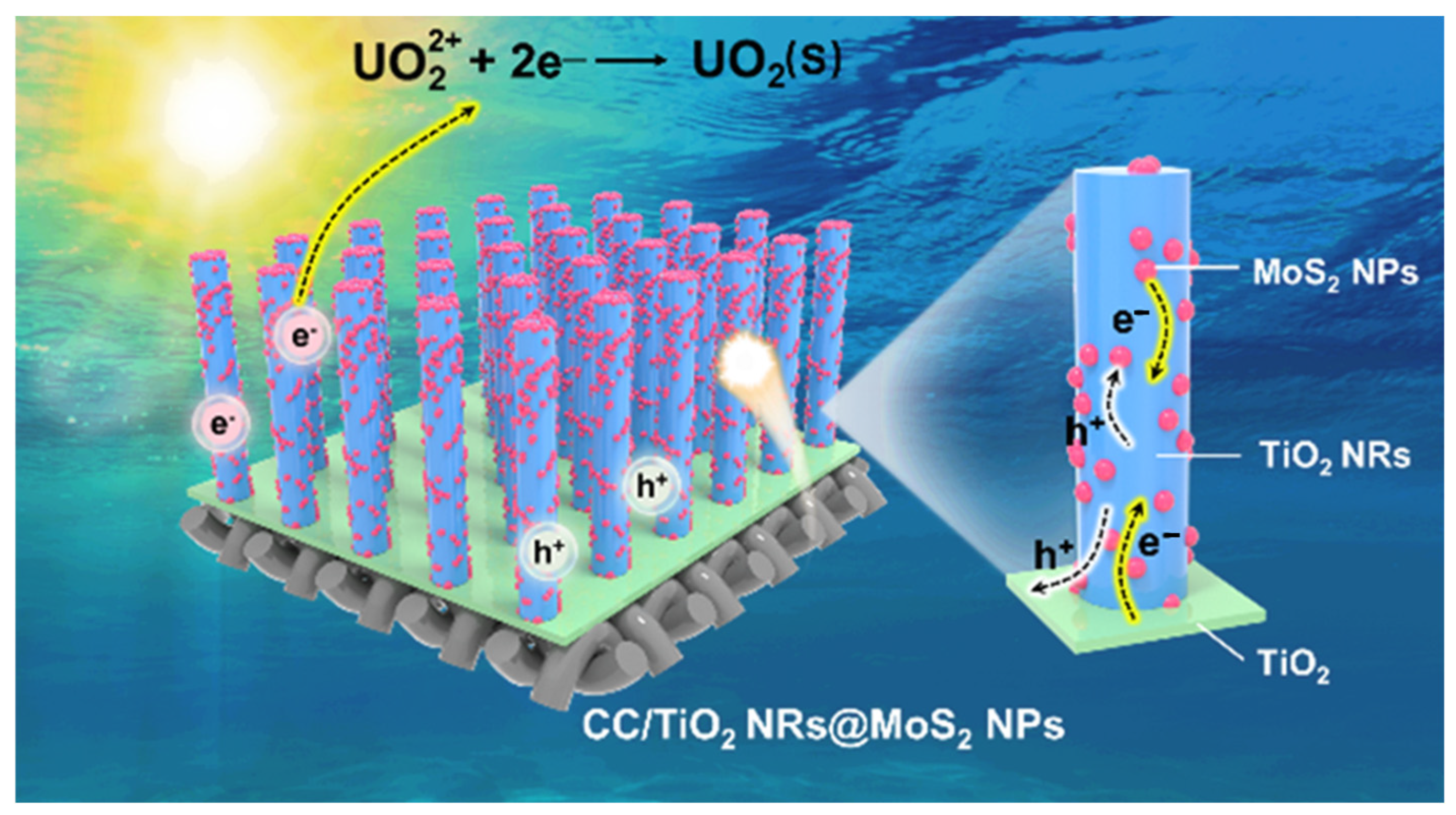
2. Results and Discussions
3. Experimental
3.1. Materials and Reagents
3.2. Preparation of Photocatalysts
3.2.1. Preparation of CC/TiO2 Seeds (Carbon Fiber-Supported TiO2 Seed Layer)
3.2.2. Preparation of CC/TiO2 NRs (Carbon Fiber-Supported TiO2 Nanorod Arrays)
3.2.3. Preparation of CC/TiO2NRs@MoS2 NPs (Carbon Fiber-Supported 3D Core–Shell Structure)
3.3. Characterization
3.4. Uranium Extraction Performance Evaluation
3.4.1. Preparation of Uranium-Containing Simulated Seawater
3.4.2. Uranium Concentration Detection Method
3.4.3. Photocatalytic Uranium Extraction Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gralla, F.; Abson, D.J.; Møller, A.P.; Lang, D.J.; Von Wehrden, H. Energy Transitions and National Development Indicators: A Global Review of Nuclear Energy Production. Renew. Sustain. Energy Rev. 2017, 70, 1251–1265. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling Lithium-Ion Batteries from Electric Vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Xu, Z.; Xiao, J.; Qin, Y. Recycling Hazardous and Valuable Electrolyte in Spent Lithium-Ion Batteries: Urgency, Progress, Challenge, and Viable Approach. Chem. Rev. 2023, 123, 8718–8735. [Google Scholar] [CrossRef]
- Yang, T.; Luo, D.; Zhang, X.; Gao, S.; Gao, R.; Ma, Q.; Park, H.W.; Or, T.; Zhang, Y.; Chen, Z. Sustainable Regeneration of Spent Cathodes for Lithium-Ion and Post-Lithium-Ion Batteries. Nat. Sustain. 2024, 7, 776–785. [Google Scholar] [CrossRef]
- Parsons, J.; Buongiorno, J.; Corradini, M.; Petti, D. A Fresh Look at Nuclear Energy. Science 2019, 363, 105. [Google Scholar] [CrossRef]
- Chen, T.; Yu, K.; Dong, C.; Yuan, X.; Gong, X.; Lian, J.; Cao, X.; Li, M.; Zhou, L.; Hu, B.; et al. Advanced Photocatalysts for Uranium Extraction: Elaborate Design and Future Perspectives. Coord. Chem. Rev. 2022, 467, 214615. [Google Scholar] [CrossRef]
- Hu, Y.; Tang, D.; Shen, Z.; Yao, L.; Zhao, G.; Wang, X. Photochemically Triggered Self-Extraction of Uranium from Aqueous Solution under Ambient Conditions. Appl. Catal. B Environ. 2023, 322, 122092. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Wang, Y.; Liang, J.; Pan, D.; Qiang, S.; Fan, Q. An Overview and Recent Progress in the Heterogeneous Photocatalytic Reduction of U(VI). J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100320. [Google Scholar] [CrossRef]
- Zheng, A.L.T.; Sinin, A.E.; Jin, W.T.; Feng, K.L.; Boonyuen, S.; Chung, E.L.T.; Lease, J.; Andou, Y. Rare Earth Elements for Enhancing Photocatalysis in Pollutant Degradation and Water Treatment. Int. J. Environ. Sci. Technol. 2025, 22, 7247–7270. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, V.; Kim, K.-H.; Kwon, E.E.; Younis, S.A. Metal-Organic Frameworks for Photocatalytic Detoxification of Chromium and Uranium in Water. Coord. Chem. Rev. 2021, 447, 214148. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, Z.; Geng, Y.; Li, H.; Wang, N.; Song, Y.; Wang, X.; Chen, J.; Wang, J.; Ma, S.; et al. Uranium Extraction from Seawater: Material Design, Emerging Technologies and Marine Engineering. Chem. Soc. Rev. 2023, 52, 97–162. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, C.; Ren, X.; Wang, X.; Wang, H.; Wang, X. Emerging Natural and Tailored Materials for Uranium-Contaminated Water Treatment and Environmental Remediation. Prog. Mater. Sci. 2019, 103, 180–234. [Google Scholar] [CrossRef]
- Huang, T.; Lu, J.; Xiao, R.; Wu, Q.; Yang, W. Enhanced Photocatalytic Properties of Hierarchical Three-Dimensional TiO2 Grown on Femtosecond Laser Structured Titanium Substrate. Appl. Surf. Sci. 2017, 403, 584–589. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Hahn, C.; Liu, B.; Yang, P. Photoelectrochemical Properties of TiO2 Nanowire Arrays: A Study of the Dependence on Length and Atomic Layer Deposition Coating. ACS Nano 2012, 6, 5060–5069. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, W.; Chen, X.; Wang, J.; Zhu, Y. Photocatalytic Activity Enhancement of Core-Shell Structure g-C3N4@TiO2 via Controlled Ultrathin g-C3N4 Layer. Appl. Catal. B Environ. 2018, 220, 337–347. [Google Scholar] [CrossRef]
- Wang, Z.; Tong, Y.; Dang, L.; Gao, F.; Lu, Q. Templated Synthesis of Titanium Dioxide Tube-in-Tube Superstructures with Enhanced Photocatalytic and Lithium Storage Performance. Chem. Eng. J. 2019, 370, 1434–1439. [Google Scholar] [CrossRef]
- Wei, N.; Liu, Y.; Feng, M.; Li, Z.; Chen, S.; Zheng, Y.; Wang, D. Controllable TiO2 Core-Shell Phase Heterojunction for Efficient Photoelectrochemical Water Splitting under Solar Light. Appl. Catal. B Environ. 2019, 244, 519–528. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, Y.; Zhang, B.; Zhou, J.; He, S. Gradient-Concentration RuCo Electrocatalyst for Efficient and Stable Electroreduction of Nitrate into Ammonia. Nat. Commun. 2024, 15, 6278. [Google Scholar] [CrossRef]
- Kim, K.-H.; Lee, H.; Huang, X.; Choi, J.H.; Chen, C.; Kang, J.K.; O’Hare, D. Energy-Efficient Electrochemical Ammonia Production from Dilute Nitrate Solution. Energy Environ. Sci. 2023, 16, 663–672. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Wang, M.; Zhang, L.; Jiang, Y.; Qian, T.; Xiong, J.; Yang, C.; Yan, C. In Situ Construction of Metal–Organic Frameworks as Smart Channels for the Effective Electrocatalytic Reduction of Nitrate at Ultralow Concentrations to Ammonia. ACS Catal. 2023, 13, 9125–9135. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, Y.; Zhan, J.; Liu, G.; Liu, G.; Li, F.; Yu, F. Dopant-Induced Electronic States Regulation Boosting Electroreduction of Dilute Nitrate to Ammonium. Angew. Chem. Int. Ed. 2023, 62, e202303483. [Google Scholar] [CrossRef]
- Wang, M.; Ioccozia, J.; Sun, L.; Lin, C.; Lin, Z. Inorganic-Modified Semiconductor TiO2 Nanotube Arrays for Photocatalysis. Energy Environ. Sci. 2014, 7, 2182–2202. [Google Scholar] [CrossRef]
- Chand, H.; Liu, Z.; Wei, Q.; Gaballah, M.S.; Li, W.; Osmolovskaya, O.; Podurets, A.; Voznesenskiy, M.; Pismenskaya, N.; Padhye, L.P.; et al. Performance and Mechanism of Chromium Removal Using Flow Electrode Capacitive Deionization (FCDI): Validation and Optimization. Sep. Purif. Technol. 2024, 340, 126696. [Google Scholar] [CrossRef]
- Guo, H.; Wei, Q.; Wu, Y.; Qiu, W.; Li, H.; Zhang, C. Enhanced Nitrate Removal from Groundwater Using a Conductive Spacer in Flow-Electrode Capacitive Deionization. Chin. Chem. Lett. 2024, 35, 109325. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Mu, L.; Ma, P.-C. Three-Dimensional Titanium Dioxide/Graphene Hybrids with Improved Performance for Photocatalysis and Energy Storage. J. Colloid Interface Sci. 2018, 512, 647–656. [Google Scholar] [CrossRef]
- Feng, J.; Graf, M.; Liu, K.; Ovchinnikov, D.; Dumcenco, D.; Heiranian, M.; Nandigana, V.; Aluru, N.R.; Kis, A.; Radenovic, A. Single-Layer MoS2 Nanopores as Nanopower Generators. Nature 2016, 536, 197–200. [Google Scholar] [CrossRef]
- Pham, V.P.; Yeom, G.Y. Recent Advances in Doping of Molybdenum Disulfide: Industrial Applications and Future Prospects. Adv. Mater. 2016, 28, 9024–9059. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.B.; Rafique, M.; Rafique, M.S.; Fatima, N.; Israr, Z. Metal oxide-and metal sulfide-based nanomaterials as photocatalysts. In Nanotechnology and Photocatalysis for Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 77–96. [Google Scholar]
- Mamiyev, Z.; Balayeva, N.O. PbS nanostructures: A review of recent advances. Mater. Today Sustain. 2023, 21, 100305. [Google Scholar] [CrossRef]
- Pan, M.; Cui, C.; Tang, W.; Guo, Z.; Zhang, D.; Xu, X.; Li, J. Carbon Cloth as an Important Electrode Support for the High Selective Electrosorption of Uranium from Acidic Uranium Mine Wastewater. Sep. Purif. Technol. 2022, 281, 119843. [Google Scholar] [CrossRef]
- Zhang, B.; Shan, X.; Yu, J.; Zhang, H.; Tawfik Alali, K.; Liu, Q.; Zhu, J.; Yu, J.; Liu, J.; Li, R.; et al. Facile Synthesis of TiO2-PAN Photocatalytic Membrane with Excellent Photocatalytic Performance for Uranium Extraction from Seawater. Sep. Purif. Technol. 2024, 328, 125026. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, H.; Liu, Q.; Liu, J.; Chen, R.; Yu, J.; Zhu, J.; Li, R.; Wang, J. Surface Hybridization of π-Conjugate Structure Cyclized Polyacrylonitrile and Radial Microsphere Shaped TiO2 for Reducing U(VI) to U(IV). J. Hazard. Mater. 2021, 416, 125812. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, R.; Liu, T.; Chen, Q.; Li, N.; Zhou, L.; Yu, K.; Liu, H.; Gong, X.; He, R.; et al. Integrating Surface Functional Modification and Energy-Level Adapted Coupling of Photocatalyst with Ultrafast Carrier Separation for Uranium Extraction. Sep. Purif. Technol. 2023, 309, 123121. [Google Scholar] [CrossRef]
- Wang, C.; Jiao, H.; Wu, Y.; Na, P. Amidoxime-Functionalized TiO2 Nanotube Arrays Plate Photocatalyst for Efficient Extracting and Recovering Uranium from Salt Lakes: Bench-Scale Experiments and Theoretical Calculation. Sep. Purif. Technol. 2024, 339, 126556. [Google Scholar] [CrossRef]
- Ma, X.; Meihaus, K.R.; Yang, Y.; Zheng, Y.; Cui, F.; Li, J.; Zhao, Y.; Jiang, B.; Yuan, Y.; Long, J.R.; et al. Photocatalytic Extraction of Uranium from Seawater Using Covalent Organic Framework Nanowires. J. Am. Chem. Soc. 2024, 146, 23566–23573. [Google Scholar] [CrossRef]
- Pan, J.; Xiao, B.; Zhu, W.; Yang, Y.; Huang, H.; Lian, Z.; Zhang, T.; Qiu, F.; Xue, S.; Pang, H. Photocatalytic Uranium Extraction Boosted by Dual Effective Active Sites of Porphyrin Metal-Organic Frameworks. Nano Res. 2024, 17, 6713–6720. [Google Scholar] [CrossRef]
- Xu, M.; Yu, F.; Liu, Y.; Li, W.; Li, C.; Song, F.; Wu, G.; Xu, Z.; Qiu, J. Integrating Uranyl-Affinity “Hooks” into Conjugated Polymers Achieving Giant Built-in Electric Field for Boosting Photocatalytic Uranium Extraction from Seawater. Macromolecules 2024, 57, 5679–5690. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, C.; Zhao, T.; Zhou, F.; Zhao, B. Reusable Three-Dimensional TiO2@MoS2 Core–Shell Photoreduction Material: Designed for High-Performance Seawater Uranium Extraction. Catalysts 2025, 15, 769. https://doi.org/10.3390/catal15080769
Xie C, Zhao T, Zhou F, Zhao B. Reusable Three-Dimensional TiO2@MoS2 Core–Shell Photoreduction Material: Designed for High-Performance Seawater Uranium Extraction. Catalysts. 2025; 15(8):769. https://doi.org/10.3390/catal15080769
Chicago/Turabian StyleXie, Chen, Tianyi Zhao, Feng Zhou, and Bohao Zhao. 2025. "Reusable Three-Dimensional TiO2@MoS2 Core–Shell Photoreduction Material: Designed for High-Performance Seawater Uranium Extraction" Catalysts 15, no. 8: 769. https://doi.org/10.3390/catal15080769
APA StyleXie, C., Zhao, T., Zhou, F., & Zhao, B. (2025). Reusable Three-Dimensional TiO2@MoS2 Core–Shell Photoreduction Material: Designed for High-Performance Seawater Uranium Extraction. Catalysts, 15(8), 769. https://doi.org/10.3390/catal15080769






