Abstract
In this investigation, a hierarchical FeCo-layered double hydroxide (FeCo-LDH) electrochemical membrane material was prepared by a simple in situ hydrothermal method. The prepared material formed a 3D honeycomb-structured FeCo-LDH-modified carbon felt (FeCo-LDH/CF) catalytic layer with uniform open pores on a CF substrate with excellent catalytic activity and was served as the cathode in a flow-through electro-Fenton (FTEF) reactor. The electrocatalyst demonstrated excellent treatment performance (99%) in phenol simulated wastewater (30 mg L−1) under the optimized operating conditions (applied voltage = 3.5 V, pH = 6, influent flow rate = 15 mL min−1) of the FTEF system. The high removal rate could be attributed to (i) the excellent electrocatalytic oxidation performance and low interfacial charge transfer resistance of the FeCo-LDH/CF electrode as the cathode, (ii) the ability of the synthesized FeCo-LDH to effectively promote the conversion of H2O2 to •OH under certain conditions, and (iii) the flow-through system improving the mass transfer efficiency. In addition, the degradation process of pollutants within the FTEF system was additionally illustrated by the •OH dominant ROS pathway based on free radical burst experiments and electron paramagnetic resonance tests. This study may provide new insights to explore reaction mechanisms in FTEF systems.
1. Introduction
At present, phenol, exemplified by phenol, have garnered considerable attention on account of their deleterious effects on humans and the environment. Phenol undergoes selective hydrogenation to yield a range of valuable by-products, including hexanone and cyclohexanol. In addition to this, it is a crucial raw material particularly in the production of bisphenol A [,]. Estimates suggest that each year a significant volume of phenolic waste is released into the environment without undergoing treatment. Studies have demonstrated that prolonged human exposure to phenol at lethal levels can evoke a series of physiological effects, including breathing distress and injury to brain tissues [,,,,], in addition to impacts on water, soil, and air as a result of its solubility when released. Furthermore, the release of it into the environment can have detrimental effects on water, soil, and air due to its solubility. In recent years, current wastewater treatment technologies for phenol-containing wastewater have faced a number of challenges. These include low treatment efficiency, high energy consumption, and expensive operating costs. Balancing efficiency and cost become challenging when relying solely on one treatment technology. As a case in point, membrane separation technology boasts a number of key advantages, including low chemical use, high scalability, and a compact equipment footprint. However, it should be noted that the retention and adsorption of membrane modules can limit the removal of organic pollutants, and these modules are prone to fouling problems during operation; furthermore, the traditional Fenton has the problems of slow Fe2+ regeneration and low utilization rate of H2O2, and the utilization rate of H2O2 can reach 99.7% []. Electrochemical advanced oxidation processes (EAOPs) generate free radicals with strong oxidizing capacity, which can mineralize organic matter, thus exhibiting excellent pollutant removal ability. Batool et al. studied the MnO2-CC electrocatalytic system, and the mineralization rate of TCS was over 99% []. It must be emphasized, however, that EAOPs inherently demand significant energy input and are vulnerable to the formation of toxic by-products and other drawbacks. Consequently, it is imperative to select a treatment technology that is highly efficient in the removal of phenolics.
Electro-Fenton (EF), a subset of advanced oxidation processes (AOPs), relies on a functional cathode to produce H2O2 via a two-electron oxygen reduction reaction (2e−ORR) (Equations (1) and (2), where * represents the unoccupied active site while HOO* signifies the formation of the single adsorption intermediate) [,,]. Subsequently, H2O2 reacts with Fe2+ to produce •OH radicals (Equation (3)), which degrade organic pollutants. Meanwhile, the cathode continuously regenerates Fe2+ via reduction (Equation (4)). Within the EF system, pollutant removal relies on the non-selective reactivity of •OH radicals. Consequently, boosting •OH concentration and optimizing H2O2 conversion are imperative to elevate the degradation efficiency of phenol [,,]. Flow-through electro-Fenton (FTEF), an enhanced variant of the conventional Fenton process, operates by applying an external electric field. This architecture facilitates the cyclic regeneration of Fe2+ ions, consequently minimizing the formation of iron-based sludge and curbing the consumption of iron-based reagents []. Electrodes featuring a high specific surface area and serving as dual-function filtration media [], have demonstrated improved mass transfer efficiency for pollutants. These electrodes are widely regarded for their high efficiency. Notwithstanding its merits, the technology is hampered by two key drawbacks: the low generation efficiency of •OH, the dominant reactive agent in EF [], and the sluggish Fe3+ reduction to Fe2+, where Fe2+ depletion happens at a rate roughly three orders of magnitude faster than its renewal. Further enhancements are imperative to augment the permeate flux across electrodes and fabricate electrodes with elevated electroactive surface areas in FTEF systems [].
In recent decades, transition metal-based materials, such as Ni-, Fe- and Co-based compounds, have emerged as highly promising catalysts owing to their adjustable electronic structure and diverse nanoscale architectures. Recent studies highlight that Fe-Co-based and Co-containing base metal electrocatalysts have shown notable efficacy in key green reactions. Kuznetsova et al. and Zhou et al. both demonstrated such catalysts’ effectiveness in nitrate-related sustainable processes [,]. And extensive studies have confirmed that the CoFe-LDH cathode exhibits a wide range of anion tolerance and pH adaptability. Their layered structure exhibits stability in a variety of ionic environments due to selective oxygen anion adsorption and pH-buffered anion exchange, while surface hydroxyl groups (-OH) enable efficient H2O2 activation under acidic to alkaline conditions by acting as pH-dependent charge regulators [,,]. Among them, layered double hydroxide (LDHs) materials consisting of coplanar octahedral MO6 layers have drawn significant attention due to their large specific surface area (>100 m2 g−1) and fast anion transfer channels [], and moreover, the catalytic performance is significantly enhanced by the synergistic effect of the laminar metal elements, which have been verified as efficient for Oxygen Evolution Reaction (OER) [,,,]. It has been shown that Fe- and Co-based LDHs materials exhibit excellent pollutant mineralization in the electro-Fenton system, and the FeIIFeIII-LDH cathode degraded the antibiotic sulfamethoxazole with an efficiency of up to 98% (120 min) []. However, the inherent poor conductivity characteristic of LDHs restricts the enhancement of their electrocatalytic performance and application [,,], and in order to obtain higher electrocatalytic performance, the augmentation of active site quantity (specific surface area), regulation of electronic properties, and modification of surface properties are imperative for electrocatalysts. In the previous literature, the FeCo-LDH system demonstrated a dual reaction center mechanism, where the Co site was able to enhance the 2e−ORR selectivity to more than 85% by optimizing the *OOH adsorption free, while the Fe site significantly promoted H2O2 activation by accelerating the redox cycle (kobs = 0.67 s−1) (Equations (3) and (4)). The synergistic interaction between the two metals led to a 3.2-fold enhancement in the apparent rate constant for phenol degradation relative to the monometallic system []. Notably, Fe substantially boosted the catalytic activity of the Co-based material (Equations (5)–(7)) [].
In this study, FeCo-LDH/CF electrochemical membrane was prepared by a simple in situ hydrothermal method. In this experiment, this was used as the membrane electrode material and applied in an FTEF to investigate the operation mechanism with the goal of improving the degradation performance and exploring the mechanism. The morphology of the cathode membrane electrode was characterized using scanning electron microscopy (SEM), combined with X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), and Fourier-transform infrared spectrometry (FT-IR); the synthesized electrochemical membranes were characterized for their electrochemical performances using an electrochemical workstation (CHI660E), and the system was also studied through the stability analysis, versatility analysis, and energy consumption analysis performance studies. Quenching experiments, electron paramagnetic resonance (EPR), and free radical trapping assays were employed to identify Reactive Oxygen Species (ROS) and elucidate the phenol degradation mechanism in the Fenton system. The results indicated that hydroxyl radicals served as the dominant oxidizing radicals in the system. The system’s design integrates a flow-through architecture to enhance interfacial mass transfer rates, alongside electrochemical membranes with high active site loading to optimize filtration electrocatalytic oxidation and free radical conversion. To further boost degradation performance, the reaction mechanism from reactants, intermediates to products were comprehensively studied.
2. Results and Discussion
2.1. The Electrode Structure Enables Catalytic Activity
The morphology of the materials synthesized under various conditions was obtained by SEM, and the SEM photographs of the virgin CF, FeCo-LDH powder, and FeCo-LDH/CF composites synthesized at 70 °C, 90 °C, and 120 °C for 12 h are presented in Figure 1. The carbon fibers of the pristine CF have a cross-packed pore structure (Figure 1a,b). A large number of FeCo-LDH showing anisotropic growth appeared on the CF produced by solvothermal synthesis, and the FeCo-LDH/CF synthesized at different temperatures showed different morphological and structural characteristics. When the synthesis temperature was 70 °C, FeCo-LDH showed an agglomerated but interstitial particle morphology on the CF substrate (Figure 1c,f), which indicated that only the LDH particles nucleated at this temperature []. Upon raising the synthesis temperature to 90 °C, FeCo-LDH with a lamellar hexagonal morphology grew perpendicularly on CF (Figure 1d,g), forming a 3D honeycomb structure with uniformly open pores, which can provide abundant reaction sites for the catalytic reaction. While some studies have shown that FeCo-LDH can achieve higher density growth on CF substrates at 120 °C than at 70 °C and 90 °C [,,], but in this study, after the synthesis temperature was increased to 120 °C, the FeCo-LDH grown on CF was in the form of fragmented flakes or filaments, where the FeCo-LDH underwent intensive growth. After that, the honeycomb structure began to collapse. The structure of FeCo-LDH powder generated at 120 °C further illustrates the collapse of the honeycomb structure (Figure 1e). The data indicate that this temperature may compromise structural integrity. Crucially, XRD results (Figure 2a) provide complementary evidence that samples synthesized at 120 °C exhibit broadened diffraction peaks and reduced crystallinity, indicating lattice disorder and degraded crystal quality. This morphological deterioration correlates directly with diminished electrochemical performance. Thus, 90 °C is optimal for preserving the performance-critical honeycomb structure under these synthesis conditions. At this point, a portion of hexagonal flaky FeCo-LDH is still present, but its structure changes from a honeycomb to a rod or filamentary sea urchin structure []. The FeCo-LDH powder obtained by Imran Shakir et al. synthesized at 130 °C for 10 h has an intercalated structure of varying sizes []. The FeCo-LDH powder obtained by synthesizing at 90 °C for 7 h was a dense sea urchin-like structure, and the FeCo-LDH powder obtained by synthesizing at 90 °C for 21 h has the same structure, but a slight collapse was observed at 50 scale, and the degradation rate of the sea urchin-like structure dropped after the first cycle (11%), and the degradation rate exceeded 60% after the seventh cycle, which presents that the sea urchin-like structure is less effective than the sea urchin structure in the electrocatalytic field, which is not better than the honeycomb structure. Energy dispersive X-ray (EDX) spectral results (Figure 1i–l) showed the homogeneous distribution of O, C, Fe, and Co elements over FeCo-LDH/CF.
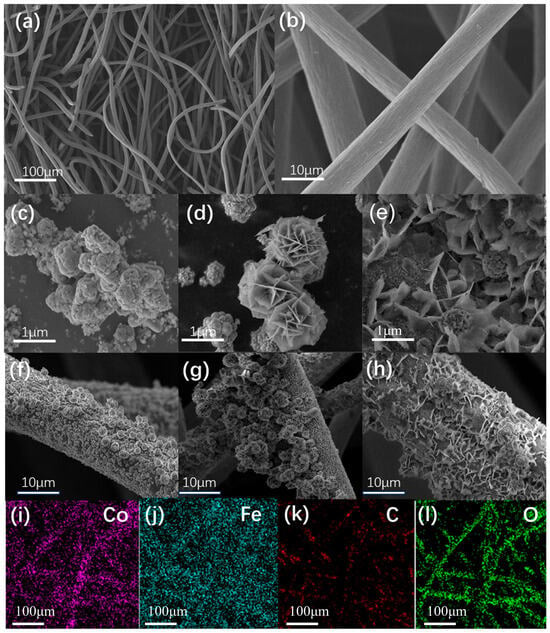
Figure 1.
SEM and EDX results of the materials: (a,b) are the SEM images of the pristine carbon mats; (c–e) are the SEM images of FeCo-LDH powders prepared by synthesizing the powders at 70 °C, 90 °C, and 120 °C for 12 h, respectively; (f–h) are the SEM images of FeCo-LDH/CF prepared by synthesizing the powders at 70 °C, 90 °C, and 120 °C for 12 h, respectively; (i–l) EDX analysis of FeCo-LDH/CF prepared by 12 h of synthesis at 90 °C, respectively.
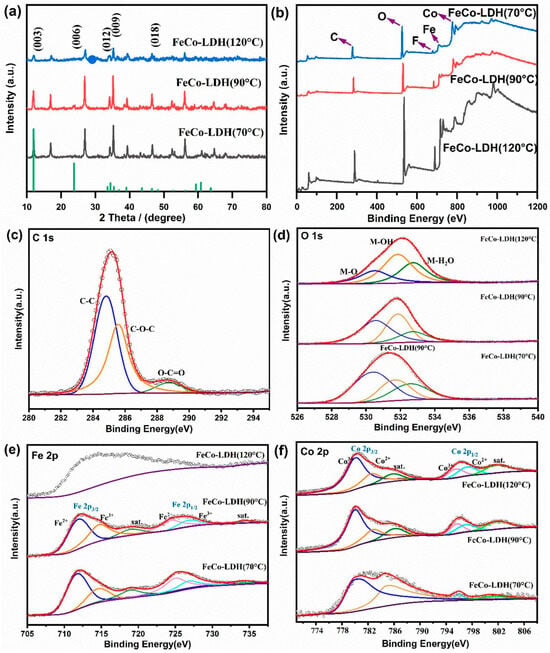
Figure 2.
XRD and XPS spectra of the synthesized materials under different conditions (a) XRD images of FeCo-LDH powders prepared by synthesizing at 70 °C, 90 °C, and 120 °C for 12 h (phase identification was performed using the ICDD PDF-2 database, with the matched PDF card number 50-0235). (b) XPS full spectrum of FeCo-LDH powders synthesized at 70 °C, 90 °C, and 120 °C. (c) C 1s high-resolution spectrum. (d) O 1s high-resolution spectrum. (e) Fe 2p high-resolution spectrum. (f) Co 2p high-resolution spectrum.
To further analyze the synthesis of FeCe-LDH as well as the crystalline grains, the XRD patterns of FeCo-LDH powders obtained by synthesizing them for 12 h at temperatures of 70 °C, 90 °C, and 120 °C, separately, were tested (Figure 2a), the XRD patterns were analyzed using MDI Jade software (9.0) with the ICDD PDF-2 database (MDI-500.csd internal index), and all the samples showed characteristic diffraction peaks at 11.9°, 23.7°, 34.3°, 35.4°, 46.5°, and 61.1°, which are in good agreement with the reported FeCo-LDH characteristic lattice planes (003), (006), (012), (009), (018), and (113) in good agreement [,], which indicates the successful synthesis of FeCo-LDH on CF substrate, and sharp (003)/(006) XRD peaks confirm highly ordered stacking of 90 °C-synthesized FeCo-LDH. Notably, peak positions across all samples remain largely unchanged, though their intensities exhibit temperature-dependent variations. Specifically, samples synthesized at 120 °C show diminished characteristic peak intensity compared to those prepared at lower temperatures. The intensity of characteristic peaks in the samples decreased, while the diffraction peaks originally located at 61.1° and 64.7° vanished completely, indicating that the temperature affects both the lattice parameters and crystallization of FeCo-LDH [,]. Additional crystalline phases were identified, while the LDH phase supported on the CF substrate showed typical purity. FTIR spectroscopy (Figure 3a) unveiled functional groups in FeCo-LDH synthesized at varying temperatures, with a characteristic hydroxyl stretching band observed between 2500 cm−1 and 3900 cm−1 in the typical LDH structure. The FTIR spectra of FeCo-LDH prepared at 70 °C and 90 °C exhibit subtle peak variations near 3500 cm−1, which stem from stretching and bending deformation of M•OH (M=Fe, Co). On the contrary, the absorption bands at 33,258.79 cm−1, 3321.70 cm−1, and 3498.64 cm−1 in FeCo-LDH are associated with stretching vibrations of hydrogen-bonded O-H groups, which arise from water molecules adsorbed on the surface or within interlayers. []. At 1640.92 cm−1 and 1644.71 cm−1, the observed absorption bands relate to H-O-H bending vibrations in water molecules held by hydrogen bonding []. A strong absorption band observed at 1500.84 cm−1 in the FTIR spectrum of FeCo-LDH synthesized at 120 °C is attributed to the N-O stretching vibration of nitrate anions adsorbed on the surface and in interlayer spaces, exposing more active sites. While conventional synthesis (120–150 °C) typically promotes nitrate intercalation, our XRD data reveal compromised crystallinity at 120 °C (broadened peaks in Figure 2a) and our catalytic results, strongly suggesting the predominant species is surface-bound organic nitro (-NO2) rather than intercalated nitrate []. These anions are derived from the nitrate salts (i.e., Co(NO3)2∙6H2O and Fe(NO3)3∙9H2O) incorporated during material preparation []. In addition, due to the adsorption of CO2 gas from the atmosphere or CO2 from the decomposition of urea added during the hydrothermal reaction in the interlayers of the LDH structure, stretching vibrations belonging to the CO32− at 1053.16 cm−1, 1382.80 cm−1, and 1384.18 cm−1, separately, appeared []. The absorption peaks detected in the lower wavenumber regions (699.67 cm−1 and 836.75 cm−1, 700.67 cm−1 and 809.26 cm−1, 693.04 cm−1, and 831.23 cm−1, respectively) can be assigned to the stretching vibrations of M-O or O-M-O bonds (M=Fe, Co) in FeCo-LDH [].
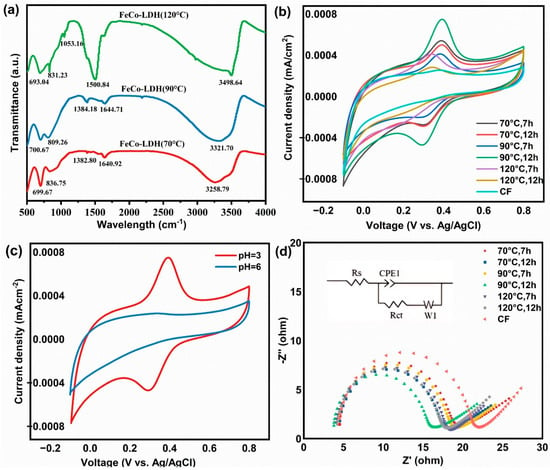
Figure 3.
FTIR profiles and electrochemical properties of materials synthesized under different conditions: (a) FTIR profiles of FeCo-LDH powders fabricated at 70 °C, 90 °C, and 120 °C for 12 h, individually. (b) CV tests of different FeCo-LDH/CF and CF films in O2-saturated 50 mM Na2SO4 electrolyte at pH = 3, with a scanning rate of 10 mV/s and a potential range of −0.1 to 0.8 V (vs. Ag/AgCl). (c) CV profiles of FeCo-LDH/CF (synthesized at 90 °C for 12 h) in O2-saturated 50 mM Na2SO4 electrolyte under pH 3 and pH 6 conditions, with a scanning rate of 10 mV/s and a potential range of −0.1 to 0.8 V (vs. Ag/AgCl). (d) EIS curves of different FeCo-LDH/CF and CF films in O2-saturated 50 mM Na2SO4 electrolyte, measured at open circuit potential with a frequency range of 0.01 to 1,000,000 Hz and an AC amplitude of 0.01 V.
To confirm the chemical environments of elements, XPS measurements were carried out on FeCo-LDH powders prepared via 12 h synthesis at 70 °C, 90 °C, and 120 °C. The recorded spectra included the full scan, along with high-resolution scans of C 1s, O 1s, Fe 2p, and Co 2p. In the samples the elements Co, Fe, C, O and F were detected (Figure 2b), respectively, and the element F appeared in comparison with the EDS test results, which was probably introduced by the ammonium fluoride used in the synthesis process and the possible role of F is to neutralize the excess positive charge in the LDH layer []. The highest peak of F was found in the sample synthesized at 90 °C, which may be a result of the better synthesis of LDH. Split-peak fitting of C1s showed three characteristic signal peaks (Figure 2c): the characteristic peaks of C-C at 284.80 eV, C-O at 285.55 eV, and C=O at 288.69 eV, respectively, with C-C having the highest relative abundance. The O1s spectra (Figure 2d) show three distinct peaks: those at 530.31 eV and 531.62 eV correspond to lattice oxygen and unsaturated oxygen (i.e., M-O and M•OH, M=Fe, Co), respectively, while the peak at 532.31 eV is assigned to physically and chemically adsorbed H2O in the FeCo-LDH nanostructure []. It is clear that the intensity of M•OH bonds increases significantly with increasing temperature, but the intensity of peaks associated with M-O decreases significantly at 120 °C, whereas the highest content of M-O and M•OH is found in the material obtained at 90 °C, suggesting that at this time, FeCo-LDH contains more oxygen-containing compounds associated with the catalytic reaction []. The Fe 2p profiles (Figure 2e) of FeCo-LDH obtained at both 70 °C and 90 °C showed two main peaks at 711.86 eV and 724.96 eV, corresponding to Fe 2p1/2 and Fe 2p3/2, respectively, whereas the peaks at 718.92 eV and 734.41 eV belonged to the satellite peaks, which indicated that a high spin was present in the LDH material. In summary, FeCo-LDH/CF synthesized at 90 °C exhibits structural advantages due to its honeycomb structure (Figure 1d,g), high crystallinity (XRD, Figure 2a), and bimetallic synergism (XPS, Figure 2e,f). In the following section, the catalytic mechanism and performance limits will be further revealed by combining electrochemical tests (CV, EIS) and the optimization of system operating parameters.
2.2. Optimization of System Performance
Building on the structural characterization in Section 3.1, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were employed to access the electrochemical properties of FeCo-LDH/CF. This aimed to elucidate how the material structure influences catalytic activity. The CV curves of FeCo-LDH/CF in 50 mM Na2SO4 obtained under different treatment conditions are shown in Figure 3b,c; for the CF film, no obvious reversible oxidation/reduction peaks were found. The FeCo-LDH/CF integral area was significantly larger than that of the other materials, indicating that the materials have more active surface sites. However, to further test the electrocatalytic performance of the material in different pH environments, a CV curve test was added for pH = 6 (Figure 3c), at which time there was no obvious redox peak. A short-term appearance of obvious reversible oxidation/reduction peaks at pH = 3 suggests that there was a leaching of Fe3+, and Co2+ into solution under acidic conditions, exposing more active sites and thus briefly increasing the electrochemical activity. Many studies have proved that initial leaching of transition metals can generate vacancies or defects, transiently enhancing activity by exposing underlying active sites []. Metal ion leaching may induce alterations in the microenvironment of the electrode interface, thereby promoting side reactions. Additionally, LDH exhibits lower stability under strongly acidic conditions. The metal leaching measurement further verified this analysis, while further analysis is needed to observe the trend of current density and CV peak shape with time, as well as to further validate the activation effect and side-reaction interference (excluding metal ion contribution). Jia et al. explored the level of cobalt leaching under neutral water conditions []. Ganiyu et al. observed that the peak current rose as the pH value decreased, and there was no significant redox peak at pH 5.83 due to the stability of the material []. The lack of distinct redox peaks at pH = 6 demonstrates the coating’s high stability [,]. The electrochemical impedance spectroscopy (EIS) technique was employed to assess the electrical conductivity of the prepared electrochemical membranes, as illustrated in Figure 3d. The obtained EIS data were fitted using an equivalent circuit model, in which RS represents the series resistance, primarily associated with the solution resistance; RCT denotes the interfacial charge transfer resistance; CPE1 serves as the non-ideal capacitive element applied in the fitting process; and W1 indicates the Warburg impedance. Notably, the diameter of the semicircle in the EIS plot corresponds to the actual magnitude of RCT. The RCT values of the pure CF material and the material obtained from the synthesis condition of 90 °C for 12 h are 21.6 Ω and 15.9 Ω, respectively. The FeCo-LDH coating enhances the conductivity of CF, and with the incorporation of electrode materials, the charge transfer resistance decreases, leading to enhanced charge transfer efficiency and an accelerated electrochemical reaction rate. This phenomenon indicates a high density of active sites within the material. The above electrochemical tests showed that FeCo-LDH/CF has excellent ORR activity and electron transfer efficiency. In order to further evaluate its potential for practical applications, this study conducted a systematic investigation into the impacts of operational parameters on the phenol degradation rate.
The FeCo-LDH/CF composite served as the cathode in an FTEF system, where phenol was employed as a model contaminant to evaluate phenol elimination efficiency and system-wide H2O2 generation. Experiments were conducted under conditions of 100 mM Na2SO4 electrolyte, a 3.5 V applied potential, and pH = 6 (Figures S3 and S4). It was shown that the average concentration of H2O2 generated by FeCo-LDH/CF at 90 °C for 12 h of synthesis conditions was lower than that of CF, which was only 65 μmol L−1, but 100% degradation rate could be achieved [,]. It stems from its ordered structural superiority and bimetallic center, which significantly promotes H2O2 transformation [,]. Within 20 min from the beginning of the reaction, the H2O2 yields of all the materials were low due to mass transfer limitations. After experimental observation, when the CF membrane was used as the electrode, the phenol removal was only 49% after 150 min of reaction, which could not be carried out due to the lack of catalyst Fenton reaction. At this time, the phenol removal was contributed by the anodic oxidation, and the average concentration of the generated H2O2 in the system was 96 μmol L−1. It is noteworthy that, after the addition of FeCo-LDH to the FTEF system, three cathodic membranes showed slightly lower removal efficiencies of phenol than the CF cathodic membrane in the first 30 min of the reaction, which may be attributed to the agglomeration phenomenon of the catalysts on the three cathodic membranes or the higher thickness of the loaded catalytic layer, which increased the mass-transfer resistance and resulted in a slower catalytic rate for the generation of •OH from H2O2. However, after 30 min, the degradation efficiency of all FeCo-LDH/CF membranes for phenol exceeded that of CF membranes, which could be ascribed to the abundance of electroactive sites in the FeCo-LDH catalytic layer. Of the average concentrations of H2O2 generated by FeCo-LDH/CF at 90 °C, 12 h, and 120 °C, 12 h synthesis conditions were lower than that of CF, especially FeCo-LDH/CF at 90 °C, 12 h, which was only 65 μmol L−1. This phenomenon can be ascribed to the mesoporous hydrotalcite structure of the material, which imparts a larger electrochemical surface area to it. Such a structure provides abundant active sites for heterogeneous catalytic reactions, aligning well with the SEM, CV, and EIS results. These characterizations revealed robust electrocatalytic performance, enabling rapid decomposition of pollutants by in situ generated H2O2 and efficient electron cycling starting from the 20th minute of the reaction. FeCo-LDH/CF membranes obtained at a synthesis temperature of 70 °C produced on average 207 μmolL−1 of hydrogen peroxide, but the phenol removal performance was poor, probably because at lower temperatures, FeCo-LDH showed a blocky agglomeration on the CF substrate and lacked sufficient catalytically active sites, which was consistent with the SEM results.
The stability and degradation activity of the FTEF system was determined across a broad pH ranging from 3 to 9 (Figure 4a). The present framework showed 91%, 100% and 78% phenol removal after 150 min of reaction at pH = 3, 6, and 9, respectively. It has been shown that the non-homogeneous Fenton reaction takes place on the surface of the FeCo-LDH catalyst. This mechanism extends the operational pH range of the Fenton course with no or very little Fe/Co sludge generation. At pH = 3, when there is Fe3+/Co2+ leaching, phenol removal is good, as shown in the CV curves of Figure 3b, and the ICP-MS analysis of the reaction effluent shows that the amount of leaching of Fe and Co is higher, 0.57 mg L−1 and 0.63 mg L−1, and that the EF reaction occurs in the native solution, respectively. Phenol degradation was attributed to the synergistic action of heterogeneous oxidation on the cathode surface and homogeneous oxidation in the bulk solution []. As the pH shifted toward alkalinity, the FeCo-LDH catalyst exhibited enhanced stability. Specifically, the leaching amount of Fe decreased to 0.28 mg L−1 and that of Co was 0.39 mg L−1 at pH = 6, while the amount of Fe leaching quantity was only 0.05 mg L−1 and that of Co was 0.05 mg L−1 at pH = 9. Concurrently, the phenol removal efficiency increased, suggesting that phenol degradation under alkaline conditions was primarily driven by the heterogeneous Fenton reaction occurring on the catalyst surface. The reason for the reduction in phenol elimination rate after the pH was elevated to the alkaline region was that the alkaline environment affected the oxidizing ability of the free radicals and the generation of the active species, for example, the generation of HO2− instead of H2O2 (Equation (8)) [,].
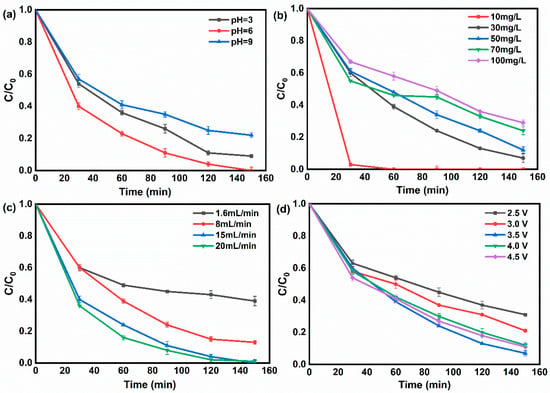
Figure 4.
(a) Effect of pH on phenol removal rate with reaction time under the conditions of phenol concentration 30 mg L−1, applied voltage 3.5 V, electrolyte concentration 100 mM, and influent flow rate 15 mL min−1. (b) Effect of feed concentration with reaction time on phenol removal rate under the conditions of applied voltage 3.5 V, electrolyte concentration 100 mM, pH = 6, and influent flow rate 15 mL min−1. (c) Effect of influent flow rate on phenol removal rate under the conditions of phenol concentration 30 mg L−1, electrolyte concentration 100 mM, pH = 6, and applied voltage 3.5 V. (d) Effect of applied voltage on phenol removal rate with reaction time under the conditions of phenol concentration 30 mg L−1, electrolyte concentration 100 mM, pH = 6, and influent flow rate 15 mL min−1.
The feed concentration affects the loading of the system and the mass transfer resistance of the solution. The influence of feed concentration on phenol removal from water by the FTEF system was investigated under an applied voltage of 3.5 V, an electrolyte concentration of 100 mM, pH = 6, and an influent flow rate of 15 mL min−1 as depicted in Figure 4b. Phenol removal efficiency declined as the feed concentration increased. At a feed concentration of 10 mg L−1, complete phenol removal was attained exclusively within the initial 30 min of the reaction. When the feed concentration was increased to 30 mg L−1 and 50 mg L−1, the phenol removal rates were 93% and 88%, respectively. When the feed concentration was further elevated, the phenol removal rate dropped sharply to about 70%, at which time the high concentration of phenol molecules increased the mass transfer resistance, affected the electron transport rate, and even caused the blockage of membrane pores []. In addition, the increase in phenol concentration also means the increase in degradation by-products, which increase the consumption of •OH []. The influence of influent flow rate on phenol removal from water by the FTEF system was also investigated by conducting several trials under a phenol concentration of 30 mg L−1, an electrolyte concentration of 100 mM, pH = 6, and an applied voltage of 3.5 V. The outcomes are presented in Figure 4c. As the flow rate was elevated from 1.6 mL min−1 to 20 mL min−1, the phenol removal efficiency rose from 61% to 99% following 150 min of reaction because the open voids of the honeycomb structure can reduce the mass transfer resistance and support efficient reaction at high flow rates. Compared to conventional electrochemical filters with limited contact area between membrane electrodes and feed solution, which need to be operated at a lower flow rate to ensure pollutant removal [,,], a recirculation reaction mode was adopted in this study, and the increased fluid velocities not only imply an increase in the number of circulations of simulated wastewater through the FTEF system, but also enhance the mass transfer process. In addition, the flow rate directly determines the contact time between the oxidant (e.g., •OH, SO4−•) and the target molecules, and increasing the flow rate accelerates the process of solute molecules reaching the active sites present on the surface of the electrochemical membrane and increases the number of contacts between solute molecules and the surface-active sites of the membrane [,].
Figure 4d shows the exploration of how applied voltage affects the system’s treatment of organic pollutants. The system was operated at a concentration of 30 mg L−1 of phenol. The effect of the magnitude of the applied voltage on the phenol removal by the FTEF system was investigated with a phenol concentration of 30 mg L−1, an electrolyte concentration of 100 mM, pH = 6, and an influent flow rate of 15 mL min−1 (Figure 4d). The phenol removal by the system increased with increasing the applied voltage from 2.5 V to 3.5 V, 69% and 79%, respectively, and the highest phenol removal (93%) was achieved at 3.5 V. The system was able to remove phenol from the water with the increase in applied voltage from 2.5 V to 3.5 V, respectively. This is attributed to the fact that increasing the voltage over a range increases the yield of H2O2. However, when the voltage was further increased, the phenol removal rate decreased instead, while during the experiment, a large number of bubbles were found to appear near the cathode electrode, which was the occurrence of the side reaction 4e−ORR (Equation (9)), and as the concentration of H2O2 buildup decreased, the decomposition reaction of H2O2 was generated on the electrode surface (Equations (11) and (12)), and hydrogen precipitation reaction (Equations (10) and (11)), which leads to a decrease in pH in the vicinity of the electrode, thus disrupting the Fenton reaction in the system []. Similarly, in the study it was found that the hydrogen peroxide accumulation decreases after increasing with increasing current []. On one hand, the production of H2O2 was reduced, and on the other hand, the conversion of H2O2 to •OH [,] was affected, which consequently influenced phenol degradation in the FTEF system. Based on the optimization of the operating parameters, the stability of FeCo-LDH/CF in long-term operation was further investigated to verify its engineering practicality.
Owing to their inherent stability, the materials enabled the system to undergo six consecutive contaminant removal cycles. Evaluation of the membrane electrodes’ reusability was conducted under operational conditions: 30 mg L−1 phenol concentration, 3.5 V applied voltage, 100 mM electrolyte, pH = 6, and 15 mL min−1 influent flow rate, with each cycle lasting 150 min. Following each experiment, the cathode was carefully rinsed and refreshed with fresh phenol solution. Phenol removal efficiencies of more than 90% were achieved in the first four cycles (Figure 5a), but with a slight decrease: from 99% in the first cycle to 93% in cycle4, and 89% and 85% at the end of the fifth cycle and the sixth cycle, respectively. Despite prolonged continuous operation inducing pore blockage and partial deactivation of membrane electrode active sites, the FTEF system maintained effective pollutant removal during the initial 750 min (i.e., up to the fifth cycle). By the end of six cycles, the system retained a stable TOC removal rate of approximately 70%, as shown in Figure 5b. SEM characterization of the FeCo-LDH/CF was carried out after 900 min of operation (Figure 5c). After a long period of operation, the catalyst on the membrane electrodes showed slight detachment phenomenon, but basically no fouling phenomenon, and the typical honeycomb structure was maintained, which indicated that the FeCo-LDH/CF electrochemical membrane had good mechanical and chemical stability. The ICP-MS results were analyzed for the effluent after each cycle (Table S6), and there was no significant increase in metal leaching from the cathode membrane after 900 min of continuous operation. The system’s long-term operational viability is supported by its structural stability and minimal leaching.
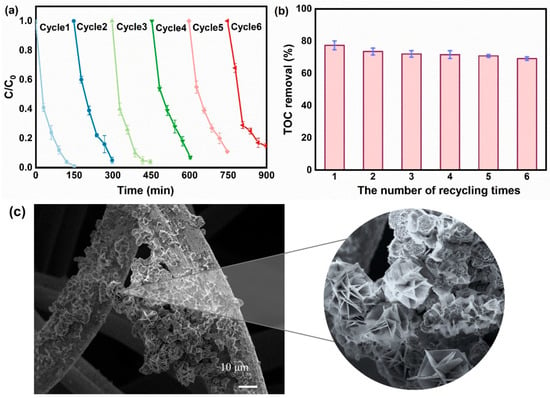
Figure 5.
(a) Phenol removal rate of 6 cycles of continuous operation of the flow-through electric Fenton system; (b) TOC removal rate of the flow-through electro-Fenton system after 6 cycles of continuous operation and (c) SEM images of the FeCO-LDH/CF electrode after 900 min of continuous operation. The experiments were conducted under the conditions of phenol concentration 30 mg L−1, applied voltage 3.5 V, electrolyte concentration 100 mM, pH = 6, influent flow rate 15 mL min−1; each experimental cycle was 150 min.
2.3. Multi-Scale Analysis of Degradation Mechanisms and Pathways
Phenol degradation experiments were performed in filtration mode, conventional intermittent mode, and flow-through electrolysis mode (Figure 6a) to assess the impact of mass transfer patterns on organic matter removal. In the filtration-only mode, the phenol removals of CF and FeCo-LDH/CF membranes were very low, only 12% and 16%, when the adsorption interception of the membranes was playing a role in the system. Adding an electric field, the phenol removal of CF and FeCo-LDH/CF membranes were 46% and 61%, respectively, providing electrochemical oxidation to promote phenol degradation, in which the electric field was able to act synergistically with the membranes: the FeCo-LDH/CF membranes adsorbed the pollutant molecules and enhanced the interaction of phenol molecules with the oxidizing free radicals formed on the electrodes surface; as a consequence, the removal efficiency was increased []. In the flow-through electrolysis mode, the phenol removal rates of CF and FeCo-LDH/CF membranes were as high as 67% and 99%, which may be ascribed to the enhanced interfacial mass transfer efficiency of the flow-through configuration, which increased the contact opportunities between the contaminants and the strong oxidants, and even the uncatalyst-modified CF electrode achieved higher phenol removal rates than the FeCo-LDH/CF electrode in the conventional intermittent mode. In addition, the continuous passage of influent water through the membrane allowed the contaminants to reach not only the membrane surface or nearby degradation, but also the membrane surface or membrane pores, between which the deposited FeCo-LDH had a 3D honeycomb structure, which could provide abundant reactive active sites, thus improving the phenol removal rate. The phenol degradation efficiency was only 28% by using a Ti mesh cathode instead of FeCo-LDH/CF membrane cathode (Figure 6b). In addition, the improvement of the mass transfer process in the circulation model was further analyzed by studying the phenol degradation kinetics and CA curves in different models. The fitting results of zero-, one-, and two-stage kinetic models (Table S4) showed that the phenol degradation process in both intermittent mode and circulation electrolysis mode conformed to quasi-one-stage kinetics. In contrast to conventional intermittent operation, flow-through electrolysis mode exhibited a marked enhancement in phenol removal efficiency, with the reaction rate constant (k) approximately 5-fold higher than that of the intermittent mode (Figure 6c) [].
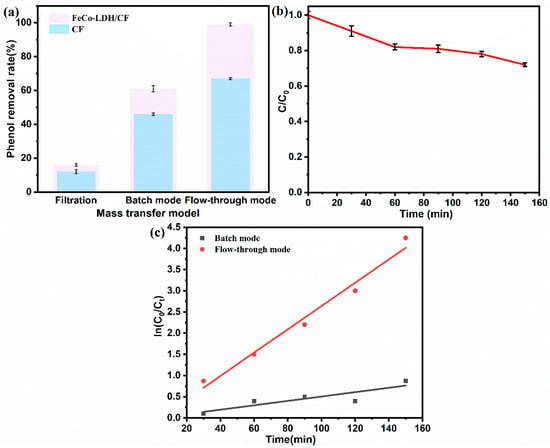
Figure 6.
(a) Phenol removal from CF and FeCo-LDH/CF membranes in different operation modes. (b) Phenol removal from Ti mesh as cathode. (c) First-stage kinetic fitting for phenol removal process.
The previous section provides a cursory assessment of the reasons for the increased efficiency of the system in treating pollutants, and further elaboration of the mechanism of action of the system, i.e., the substances that produce the action, is needed. To explore the types of ROS produced in the FeCo-LDH/CF electro-filtration system, free radical quenching experiments were conducted by adding different bursting agents (tert-butanol, methanol, and furfuryl alcohol) to the system. As illustrated in Figure 7a, the introduction of an adequate quantity of furfuryl alcohol exerted negligible influence on organic matter removal, while the addition of methanol reduced the phenol removal to 61%, and the addition of sufficient amount of tertiary butanol severely limited the removal of phenol in this system to 37%. Bursting experiments preliminarily indicated that •OH was the dominant ROS in the FeCo-LDH/CF electro-filtration system, while SO4−• also played a role, with •OH contributing about 63% of the phenol degradation rate. EPR analysis with DMPO as the •OH trapping agent was employed to further characterize ROS generated during phenol oxidation. As depicted in Figure 7b, the FeCo-LDH/CF system exhibited four characteristic peaks with an intensity ratio of 1:2:2:1, which matched the signature of the DMPO•OH adduct. In addition, the characteristic peaks of DMPO•OH disappeared after the addition of a sufficient amount of tert-butanol, and these phenomena further confirmed the presence of •OH. The pathway and mechanism of phenol degradation by the FeCo-LDH/CF cathodic electro-Fenton system are shown in Figure S6. In addition to the initial addition of a small amount of hydrogen peroxide initiating reactant, hydrogen peroxide was also generated during the process, and the source of •OH was guessed to be oxygen, for which experiments were carried out to investigate the contribution of cathodic fluxed O2 in phenol removal. The experimental findings are depicted (Figure 7c). The phenol degradation rate is lower than 60% when oxygen is not passed through the FTEF system; at this time, the system can only utilize the dissolved oxygen inherent in the solution as well as the dissolved oxygen entering from the air, and the adsorption energy of the cathode material for oxygen is still limited, and the adsorption of oxygen has been improved way of the study of Yu et al. []. After constant aeration to the influent water with the air passed through the cathode material, the system obtains sufficient O2 for H2O2 production, and a phenol removal rate of 99% was obtained, which shows that the FeCo-LDH/CF electrode has limited efficiency in utilizing the oxygen inherent in the solution, and that oxygen has a decisive role in the production of •OH. As a functional positive electrode, the CF component in FeCo-LDH/CF serves as a highly active and stable 2e−ORR catalyst, critically influencing •OH generation. In contrast, FeCo-LDH alone exhibits a significantly high energy barrier for oxygen adsorption, leading to minimal H2O2 production due to its intrinsic properties []. This experiment did not verify the relationship between the amount of oxygen passed through and the amount of H2O2 produced, and the amount of oxygen dissolved is yet to be investigated, with the electrocatalytic cathode sufficiently adsorbing oxygen. Following the confirmation of •OH presence, the SA method was employed to indirectly quantify the •OH yield in the FeCo-LDH/CF electro-filtration system [], where SA was able to capture •OH to produce stable 2,3-DHBA and 2,5-DHBA, and the sum of the contents of the two products was roughly equal to the content of •OH. The variation in •OH content in this system is shown in Figure 7d, and the content of OH increases gradually with the reaction time, from the initial 0.013 mmol to 0.50 mmol after 150 min.
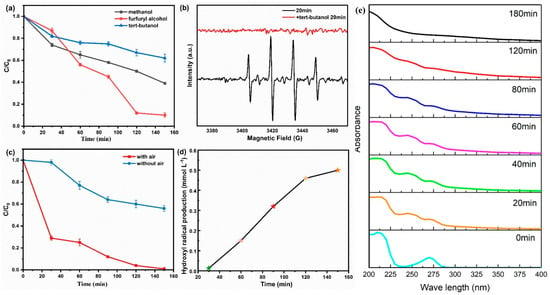
Figure 7.
(a) Phenol removal after addition of different bursting agents. (b) EPR spectra of DMPO•OH without and with the addition of tert-butanol. (c) Effect of O2 content on phenol removal. (d) •OH yield of the FTEF system. (e) UV spectral absorption spectra of phenol after different reaction durations.
Next, the pathway of phenol degradation and the pathway of ROS generation action were analyzed, and the material changes during phenol degradation were analyzed by UV-Vis’s absorption spectroscopy (Figure 7e). The characteristic absorption peaks of phenol were concentrated at 210–215 nm and 270–275 nm, and the intensities of the peaks at these two locations gradually decreased until disappeared with the increase in degradation time. Thirty minutes after reaction initiation, a new characteristic peak was observed at 245–250 nm, assignable to the formation of benzoquinone with an absorption wavelength of λmax = 248 nm. By 120 min, the characteristic peaks at 210–215 nm and 245–50 nm were weakened, while the characteristic peaks at 270–275 nm disappeared, which might be due to the further degradation of the intermediates, catechol and hydroquinone. Until 180 min later, all the characteristic peaks disappeared and no new peaks appeared, indicating that phenol might be completely degraded to CO2 and H2O at this time, which is in accordance with Figure 6a results. It was also observed that the color of the effluent solution of the phenol-simulated wastewater underwent a process of changing from dark brown to light yellow and finally was colorless as the reaction proceeded. Although the flow-through electrochemical system in this study achieved more than 90% phenol removal, the TOC removal of the effluent was less than 80%, suggesting that other organic intermediates were generated during the phenol removal process. With a view to analyze the degradation products of phenol, the effluent water from the FTEF system was tested by HPLC at different time intervals. By comparing the results with the retention time of standard samples, the degradation products of phenol were hydroquinone, catechol, maleic acid, and glycolic acid (Table S5). After 20 min, hydroquinone began to accumulate; after 40 min, catechol appeared; after 60 min, cisbutylene dioic acid began to accumulate; as the reaction proceeded, the peak areas of hydroquinone and catechol decreased dramatically in the second half of the reaction; after 150 min, phenol showed a very low peak area, and cisbutylene dioic acid disappeared. The formation of ethanedioic acid, however, was detected. This suggests that hydroxylation of reactive phenol occurs as a rapid step, whereas the ring-opening reaction (maleic acid → ethanedioic acid) serves as the rate-determining step. It can be speculated that the appearance of hydroquinone and catechol during phenol degradation indicates hydroxylation of phenol under the attack of OH []; these intermediates open the conjugated structure of the benzene ring under the strong oxidation of •OH and SO4−• to produce maleic acid; as the oxidation reaction proceeds, maleic acid was decomposed into the simpler structure of ethanedioic acid, which was eventually converted to CO2 and H2O. The degradation process of the system is shown in Figure 8.
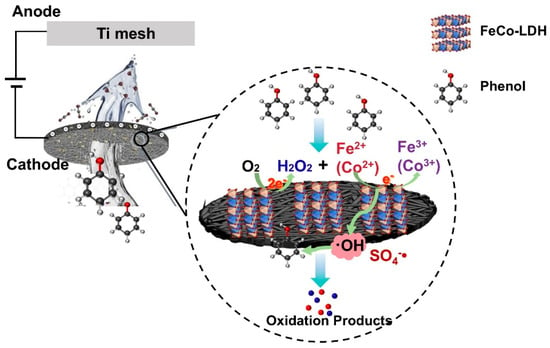
Figure 8.
Schematic diagram of the degradation of phenol by a flow-through electrochemical system with FeCo-LDH/CF electrochemical membrane as cathode.
3. Materials and Methods
3.1. Reagents and Materials
Phenol(), methylene blue (), cobalt nitrate hexahydrate (), ferric nitrate nonahydrate (), urea (), ammonium fluoride (), tert-butyl alcohol, ethanol, furfuryl alcohol, methyl orange, ammonia, potassium ferrocyanide,4-aminoantipyrine, anhydrous sodium sulfate, 5,5-dimethyl-1-pyrrolidinium-N-oxide, 2,3-dihydroxybenzoic acid, and 2,5-dihydroxybenzoic acid used are analytically pure and can be used without further purification, acetonitrile is chromatographically pure, and can be used without further purification, and acetonitrile is color spectroscopically pure. The chemicals and materials used in the experiments were counted (Tables S1 and S2), and the determination of organic substances during the experiments (Text S1 and Figure S2).
3.2. Synthesis of Electrodes
FeCo-LDH-modified CF electrodes were fabricated via an in situ hydrothermal method. Specifically, NH4F (0.125 M),, CO(NH2)2 (0.5 M), and were dissolved in deionized water, where Co: Fe = 2:1 (n/n). The mixture was transferred to a stainless-steel autoclave equipped with a Teflon liner after being stirred vigorously for 30 min to ensure homogeneity. The original CF was first treated with concentrated HNO3 and then successively washed in an ultrasonic bath for 15 min using deionized water, acetone, ethanol, and deionized water in sequence. The clean CF was placed into the autoclave with the growth solution and hydrothermally treated at temperatures of 70 °C, 90 °C, and 120 °C for 7 h and 12 h cycles, respectively. Reactions during the hydrothermal process mainly involved decomposition reactions of NH4F and CO(NH2)2 (Equations (13) and (14)). The alkaline solution generated by Equation (15) triggers homogeneous nucleation, crystallization, and the growth of metal hydroxides directly on CF substrates. During immersion in the growth solution, CFs serve as nucleation sites that facilitate the formation and growth of LDH structures []. Following the reaction, the LDH-coated substrates were rinsed exhaustively with deionized water. The solid LDH was then isolated from the suspension via filtration. Both the coated substrates and the filtered precipitate were dried in an oven at 45 °C, completing the synthesis of FeCo-LDH.
3.3. Evaluation of Electrode Morphology and Performance
To characterize the structural and morphological features of the electrodes, as well as their electrochemical properties, they were evaluated using the following methods. Structural, morphological, and particle size analyses of the samples were performed with a scanning electron microscope (SEM, TESCAN MIRA LMS, Brno, Czech Republic). Elemental composition characterization of the membrane electrodes was performed via X-ray photoelectron spectroscopy (XPS, Thermo Scientific ESCALAB 250Xi, Waltham, MA, USA) and X-ray diffraction (XRD, Rigaku Miniflex 600, Tokyo, Japan). Fourier transform infrared spectroscopy (FTIR, Thermo Scientific Nicolet iS2, Waltham, MA, USA) was utilized to assess the morphology of membrane materials, while an electrochemical workstation (CHI660E, Chenhua Instruments, Shanghai, China) was adopted to appraise the electrochemical properties of the synthesized electrochemical membranes. The electrode materials were evaluated via comprehensive photoelectrochemical measurements, including cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). CV scans were conducted at a rate of 10 mV s−1 and a scan voltage of −0.1–0.8 V (vs. Ag/AgCl), while EIS spectra were recorded at an open-circuit potential with an AC amplitude of 0.01 V in the frequency range of 0.01–106 Hz (Text S3).
3.4. Experiments on Phenol Degradation by FeCo-LDH/CF FTEF System
A 150 mL glass vessel was used to construct an FTEF system (Text S2 and Figure S1) with the prepared modified FeCo-LDH/CF electrochemical membrane as the cathode and titanium mesh as the anode. For the purpose of investigating the FeCo-LDH/CF cathode-based FTEF system’s capability in degrading pollutants, phenol was chosen as the target compound, Na2SO4 was used as the electrolyte, and the system was operated by maintaining an air flux of 300 mL min−1, plus the voltage provided by a DC power supply, and the degradation efficiency of the system on phenol was measured. For a deeper analysis of individual factor impacts, the execution of the FTEF system under changing operating scenarios was systematically compared and analyzed, such as feed concentration (10–100 mg L−1), applied voltage (2.5–4.5 V), solution pH (3.0, 6.0, and 9.0), and influent flow rate (1.6 mL min−1, 8.0 mL min−1, 15.0 mL min−1, and 20.0 mL min−1); and the performance of the FTEF system was investigated through the analysis of stability, versatility, and energy consumption. The system performance was investigated by stability analysis, versatility analysis, and energy consumption analysis (Texts S4 and S5 and Table S3); and the treatment mechanism of phenol in the Fenton system was explored through quenching experiments, EPR tests, and ROS identification via free radical trapping experiments.
4. Conclusions
This study utilized FeCo-LDH/CF electrochemical membranes and a novel FTEF system to overcome the limitations of the traditional EF technology, which effectively improved the problems of the traditional EF technology in terms of low yield of electroactive substances, poor mass transfer efficiency, narrow reaction pH range, and large production of iron sludge, etc. In this study, the FeCo-LDH/CF electrochemical membranes with abundant catalytic active sites were prepared and used as the cathode to construct an FTEF system. In this study, a FeCo-LDH/CF electrochemical membrane with abundant catalytic sites was prepared and used as the cathode to construct an FTEF system, and the performance of the system in the removal of organic pollutants was investigated in depth. The FeCo-LDH/CF electrochemical membrane is a 3D honeycomb structure, which can effectively promote the conversion of H2O2 to •OH, while the flow-through design of the system allows convective transfer of influent water, which increases the reaction rate by 5 times compared to the intermittent mode on the basis of higher steady state current, and is capable of achieving 40% higher phenol removal rate. The FeCo-LDH/CF system was able to achieve 78–100% phenol removal over a wide operating pH range, maintain 85% phenol removal and 70% TOC removal after six cycles of use, thus FeCo-LDH has great potential for practical wastewater treatment. The present study demonstrates the bimetallic synergy assisted by positively charged LDH for in situ generation and stabilization of radical ROS, which provides a new strategy for maximizing the performance of a non-homogeneous Fenton-like system with low chemical input and high mineralization efficiency, and provides a basis for further optimization of the conventional EF. Although this study has successfully developed an efficient FTEF system, there are still shortcomings. For example, the oxidation reaction may be kinetically limited at higher water fluxes. In order to optimize the system performance for practical applications, in-depth explorations on practical wastewater treatment evaluation, system stability and effluent safety are still needed. However, this experiment can still provide a reference for further enhancing the EF degradation efficiency by modifying the cathode with membrane filtration.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15070685/s1. Text S1: Methods for the determination of organic matter; Text S2: Assembly of the system; Text S3: Specific operational conditions for the characterization of materials; Text S4: Calculation of the removal rate of organic matter and analysis of energy consumption; Text S5: Energy consumption analysis; Text S6: Identification of ROS; Figure S1: Schematic diagram of the structure of the FTEF device and its working flow diagram; Figure S2: (a) Absorbance standard curve of 2,3-DHBA; (b) Absorbance standard curve of 2,5-DHBA; Figure S3: H2O2 concentrations with different FeCo-LDH/CF and CF electrode stacks (reaction conditions: 100 mM Na2SO4 electrolyte, 3.5 V applied voltage, pH = 6); Figure S4: Degradation of phenol by different FeCo-LDH/CF and CF as cathodes of FTEF systems (reaction conditions: phenol initial concentration of 30 mg L−1, electrolyte of 100 mM Na2SO4, applied voltage of 3.5 V, pH = 6, inlet flow rate of 15 mL min−1); Figure S5: FTEF system for (a) phenol removal and (b) TOC removal in different water matrices; (c) removal rates of different types of pollutants; (d) removal rate of mixed contaminants and TOC; Table S1: Main materials and instruments required in the catalyst synthesis process; Table S2: Main reagents required in the catalyst synthesis process; Table S3: Comparison of the performance of different electrochemical filtration systems; Table S4: Simulation results of phenol degradation process kinetics under different modes; Table S5: Detection results of phenol degradation products. Table S6: The flow-through electric Fenton system operates continuously for 6 cycles of Fe and Co leaching. References [,,,,,,].
Author Contributions
Conceptualization, F.L. and T.H.; methodology, F.L., T.H., H.D. and Y.Q.; software, H.D.; validation, H.D. and Y.Q.; formal analysis, F.L., T.H., H.D., Y.Q., Z.Y., Y.F. and W.S.; investigation, H.D. and Y.Q.; resources, F.L. and T.H.; data curation, H.D. and Y.Q.; writing—original draft preparation, Y.Q.; writing—review and editing, H.D.; visualization, H.D. and Y.Q.; project administration, F.L. and T.H.; funding acquisition, F.L. and T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32171615).
Data Availability Statement
The generated data are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xue, G.; Yin, L.; Shao, S.; Li, G. Recent Progress on Selective Hydrogenation of Phenol toward Cyclohexanone or Cyclohexanol. Nanotechnology 2022, 33, 072003. [Google Scholar] [CrossRef]
- Chen, H.; Sun, J. Selective Hydrogenation of Phenol for Cyclohexanone: A Review. J. Ind. Eng. Chem. 2021, 94, 78–91. [Google Scholar] [CrossRef]
- Raza, W.; Lee, J.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J. Removal of Phenolic Compounds from Industrial Waste Water Based on Membrane-Based Technologies. J. Ind. Eng. Chem. 2019, 71, 1–18. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Rajan, P.S.; Arun, J.; Brindhadevi, K.; Le, Q.H.; Pugazhendhi, A. A Review on Recent Advancements in Extraction, Removal and Recovery of Phenols from Phenolic Wastewater: Challenges and Future Outlook. Environ. Res. 2023, 237, 117005. [Google Scholar] [CrossRef] [PubMed]
- Enguita, F.J.; Leitão, A.L. Hydroquinone: Environmental Pollution, Toxicity, and Microbial Answers. BioMed Res. Int. 2013, 2013, 542168. [Google Scholar] [CrossRef]
- Chouhan, S.; Yadav, S.K.; Prakash, J.; Swati; Singh, S.P. Effect of Bisphenol A on Human Health and Its Degradation by Microorganisms: A Review. Ann. Microbiol. 2014, 64, 13–21. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Y.; Li, X.; Liu, Y.; Chen, Y.; Cheng, J.; Zhu, X.; Wang, G.; Xie, J. Unlocking the Power of Manganese-Doped MoS2 Hollow Nanotubes: A Game-Changing Fenton Co-Catalyst for Ciprofloxacin Degradation. Chem. Eng. J. 2025, 511, 162009. Available online: https://www.sciencedirect.com/science/article/pii/S1385894725028359 (accessed on 12 July 2025). [CrossRef]
- Batool, A.; Shao, S.; Majhi, K.C.; Mushtaq, A.; Jiang, Y.; Ho, W.; Tsang, Y.F.; He, Y.; Yee Leung, K.M.; Lam, J.C. MnO2-Catalyzed Electrocatalytic Mineralization of Triclosan in Chlorinated Wastewater. Environ. Sci. Ecotechnol. 2025, 25, 100559. Available online: https://www.sciencedirect.com/science/article/pii/S2666498425000377 (accessed on 12 July 2025). [CrossRef]
- Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W.; et al. Enabling Direct H2O2 Production through Rational Electrocatalyst Design. Nat. Mater. 2013, 12, 1137–1143. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Viswanathan, V.; Hansen, H.A.; Rossmeisl, J.; Nørskov, J.K. Unifying the 2e− and 4e− Reduction of Oxygen on Metal Surfaces. J. Phys. Chem. Lett. 2012, 3, 2948–2951. [Google Scholar] [CrossRef]
- Yan, B.; Shi, Z.; Lin, J.; Zhang, L.; Han, L.; Shi, X.; Yang, Q. Boosting Heterogeneous Fenton Reactions for Degrading Organic Dyes via the Photothermal Effect under Neutral Conditions. Environ. Sci. Nano 2022, 9, 532–541. [Google Scholar] [CrossRef]
- Jain, B.; Singh, A.K.; Kim, H.; Lichtfouse, E.; Sharma, V.K. Treatment of Organic Pollutants by Homogeneous and Heterogeneous Fenton Reaction Processes. Environ. Chem. Lett. 2018, 16, 947–967. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Zhang, J.; Luo, L.; Yang, Y.; Huang, H.; Peng, H.; Tang, L.; Mu, Y. Insight into Electro-Fenton and Photo-Fenton for the Degradation of Antibiotics: Mechanism Study and Research Gaps. Chem. Eng. J. 2018, 347, 379–397. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, G.; Ji, Q.; Liu, H.; Hua, X.; Xia, H.; Sillanpää, M.; Qu, J. pH-Independent Production of Hydroxyl Radical from Atomic H*-Mediated Electrocatalytic H2O2 Reduction: A Green Fenton Process without Byproducts. Environ. Sci. Technol. 2020, 54, 14725–14731. [Google Scholar] [CrossRef] [PubMed]
- Ly, Q.V.; He, K.; Maqbool, T.; Sun, M.; Zhang, Z. Exploring the Potential Application of Hybrid Permonosulfate/Reactive Electrochemical Ceramic Membrane on Treating Humic Acid-Dominant Wastewater. Sep. Purif. Technol. 2022, 286, 120513. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, M.; Hedtke, T.; Deshmukh, A.; Zhou, X.; Weon, S.; Elimelech, M.; Kim, J.-H. Mechanism of Heterogeneous Fenton Reaction Kinetics Enhancement under Nanoscale Spatial Confinement. Environ. Sci. Technol. 2020, 54, 10868–10875. [Google Scholar] [CrossRef]
- Hakizimana, I.; Zhao, X.; Wang, C.; Mutabazi, E.; Zhang, C. Insights into the Flow-through Electrochemical System in Water and Wastewater Treatment: Influential Factors, Advantages, Challenges, and Perspective. Sep. Purif. Technol. 2024, 331, 125533. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, M.; Zhang, L.; Li, N.; Qian, T.; Yan, C.; Lu, J. Quaternary Medium-Entropy Alloy Metallene with Strong Charge Polarization for Highly Selective Urea Electrosynthesis from Carbon Dioxide and Nitrate. ACS Nano 2025, 19, 7273–7282. [Google Scholar] [CrossRef]
- Kuznetsova, I.; Kultin, D.; Lebedeva, O.; Nesterenko, S.; Murashova, E.; Kustov, L. Intermetallic Compound and Solid Solutions of Co75Me25 (Me: Si, Fe, Cr) as Catalysts for the Electrochemical Reaction of Nitrate Conversion to Ammonia. Int. J. Mol. Sci. 2025, 26, 1650. [Google Scholar] [CrossRef]
- Yin, Q.; Rao, D.; Zhang, G.; Zhao, Y.; Han, J.; Lin, K.; Zheng, L.; Zhang, J.; Zhou, J.; Wei, M. CoFe–Cl Layered Double Hydroxide: A New Cathode Material for High-Performance Chloride Ion Batteries. Adv. Funct. Mater. 2019, 29, 1900983. Available online: https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/adfm.201900983 (accessed on 11 July 2025). [CrossRef]
- Liu, P.F.; Yang, S.; Zhang, B.; Yang, H.G. Defect-Rich Ultrathin Cobalt-Iron Layered Double Hydroxide for Electrochemical Overall Water Splitting. ACS Appl. Mater. Interfaces 2016, 8, 34474–34481. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.M.; Song, J.; Kim, Y.K.; Oh, J.; Kim, K.Y.; Noh, W.Y.; Byun, W.J.; Lee, J.U.; Yang, C.; Jang, J.W.; et al. High-Performance Electrochemical and Photoelectrochemical Water Splitting at Neutral pH by Ir Nanocluster-Anchored CoFe-Layered Double Hydroxide Nanosheets. Nano Lett. 2023, 23, 5092–5100. Available online: https://pubs.acs.org/doi/10.1021/acs.nanolett.3c01024?ref=PDF (accessed on 11 July 2025). [CrossRef] [PubMed]
- Wang, L.; Xu, S.-M.; Yang, X.; He, S.; Guan, S.; Waterhouse, G.I.N.; Zhou, S. Exploiting Co Defects in CoFe-Layered Double Hydroxide (CoFe-LDH) Derivatives for Highly Efficient Photothermal Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 54916–54926. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.; Zhong, Y.; Cui, L.; Yang, W.; Razal, J.M.; Barrow, C.J.; Liu, J. Facile Construction of MgCo2O4@CoFe Layered Double Hydroxide Core-Shell Nanocomposites on Nickel Foam for High-Performance Asymmetric Supercapacitors. J. Power Sources 2021, 484, 229288. [Google Scholar] [CrossRef]
- Paramanik, L.; Mohapatra, L.; Choi, D.Y.; Yoo, S.H. Enhanced Photocatalytic Performance of MXene-Modified Cation-Exchanged CoFe-LDH/CoFeCrO4 Heterostructure for Antibiotic Degradation and Hydrogen Production through Synergistic Charge Dynamics. Compos. Part B Eng. 2024, 278, 111416. [Google Scholar] [CrossRef]
- Yu, D.; He, J.; Wang, Z.; Pang, H.; Li, L.; Zheng, Y.; Chen, Y.; Zhang, J. Mineralization of Norfloxacin in a CoFe–LDH/CF Cathode-Based Heterogeneous Electro-Fenton System: Preparation Parameter Optimization of the Cathode and Conversion Mechanisms of H2O2 to OH. Chem. Eng. J. 2021, 417, 129240. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Liu, D.; Zou, Y.; Wang, S. Water-Plasma-Enabled Exfoliation of Ultrathin Layered Double Hydroxide Nanosheets with Multivacancies for Water Oxidation. Adv. Mater. 2017, 29, 1701546. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Thi, X.H.L.; Bechelany, M.; Oturan, N.; Papirio, S.; Esposito, G.; van Hullebusch, E.; Cretin, M.; Oturan, M.A. Electrochemical Mineralization of Sulfamethoxazole over Wide pH Range Using FeIIFeIII LDH Modified Carbon Felt Cathode: Degradation Pathway, Toxicity and Reusability of the Modified Cathode. Chem. Eng. J. 2018, 350, 844–855. [Google Scholar] [CrossRef]
- Yuan, F.; Zhang, E.; Liu, Z.; Yang, K.; Zha, Q.; Ni, Y. Hollow CoSx Nanoparticles Grown on FeCo-LDH Microtubes for Enhanced Electrocatalytic Performances for the Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2021, 4, 12211–12223. [Google Scholar] [CrossRef]
- Sohrabi, H.; Khataee, A.; Ghasemzadeh, S.; Majidi, M.R.; Orooji, Y. Layer Double Hydroxides (LDHs)-Based Electrochemical and Optical Sensing Assessments for Quantification and Identification of Heavy Metals in Water and Environment Samples: A Review of Status and Prospects. Trends Environ. Anal. Chem. 2021, 31, e00139. [Google Scholar] [CrossRef]
- Hameed, A.; Batool, M.; Liu, Z.; Nadeem, M.A.; Jin, R. Layered Double Hydroxide-Derived Nanomaterials for Efficient Electrocatalytic Water Splitting: Recent Progress and Future Perspective. ACS Energy Lett. 2022, 7, 3311–3328. [Google Scholar] [CrossRef]
- Yang, F.; Sliozberg, K.; Sinev, I.; Antoni, H.; Bähr, A.; Ollegott, K.; Xia, W.; Masa, J.; Grünert, W.; Cuenya, B.R.; et al. Synergistic Effect of Cobalt and Iron in Layered Double Hydroxide Catalysts for the Oxygen Evolution Reaction. ChemSusChem 2017, 10, 156–165. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Huong Le, T.X.; Bechelany, M.; Esposito, G.; Van Hullebusch, E.D.; Oturan, M.A.; Cretin, M. A Hierarchical CoFe-Layered Double Hydroxide Modified Carbon-Felt Cathode for Heterogeneous Electro-Fenton Process. J. Mater. Chem. A 2017, 5, 3655–3666. [Google Scholar] [CrossRef]
- Yang, L.; Xu, D.; Yang, H.; Luo, X.; Liang, H. Structurally-Controlled FeNi LDH/CNTs Electro-Fenton Membrane for In-Situ Electro-Generation and Activation of Hydroxyl Radicals toward Organic Micropollutant Treatment. Chem. Eng. J. 2022, 432, 134436. [Google Scholar] [CrossRef]
- Shakir, I.; Almutairi, Z.; Shar, S.S. Fabrication of Nanostructured Iron-Cobalt Layered Double Hydroxide: An Innovative Approach for the Facile Synthesis. J. Saudi Chem. Soc. 2022, 26, 101500. [Google Scholar] [CrossRef]
- Li, R.; Zhang, D.; Chen, H.; Wang, S.; Ling, Y.; Fang, C. Morphological and Electronic Regulation of Zn,S-Incorporated FeCo–LDH Nanosheets for Boosting the Bi-Electrocatalytic Oxygen Evolution and Urea Oxidation Reactions. ACS Sustain. Chem. Eng. 2023, 11, 16098–16107. [Google Scholar] [CrossRef]
- Abedi, H.; Mehrpooya, M. Synthesis of Three-Metal Layered Double Hydroxide and Dual Doped Graphene Oxide Composite as a Novel Electrocatalyst for Oxygen Reduction Reaction. J. Alloys Compd. 2021, 875, 160047. [Google Scholar] [CrossRef]
- Understanding Catalyst Poisoning in Precious Metal Catalysts: Causes, Problems, and Solutions. Available online: https://www.samaterials.com/content/catalyst-poisoning-in-precious-metal-catalysts-causes-problems-and-solutions.html (accessed on 12 July 2025).
- Ma, Q.; Nengzi, L.; Li, B.; Wang, Z.; Liu, L.; Cheng, X. Heterogeneously Catalyzed Persulfate with Activated Carbon Coated with CoFe Layered Double Hydroxide (AC@CoFe-LDH) for the Degradation of Lomefloxacin. Sep. Purif. Technol. 2020, 235, 116204. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, W.; Li, J.-G.; Li, Z.; Ao, X.; Xue, K.-H.; Ostrikov, K.K.; Tang, J.; Wang, C. Rh-Engineered Ultrathin NiFe-LDH Nanosheets Enable Highly-Efficient Overall Water Splitting and Urea Electrolysis. Appl. Catal. B Environ. 2021, 284, 119740. [Google Scholar] [CrossRef]
- Jia, W.; Li, Y.; Chen, C.; Wu, Y.; Liang, Y.; Du, J.; Feng, X.; Wang, H.; Wu, Q.; Guo, W.-Q. Unveiling the Fate of Metal Leaching in Bimetal-Catalyzed Fenton-like Systems: Pivotal Role of Aqueous Matrices and Machine Learning Prediction. J. Hazard. Mater. 2024, 477, 135291. [Google Scholar] [CrossRef]
- Feng, C.; Wang, F.; Liu, Z.; Nakabayashi, M.; Xiao, Y.; Zeng, Q.; Fu, J.; Wu, Q.; Cui, C.; Han, Y.; et al. A Self-Healing Catalyst for Electrocatalytic and Photoelectrochemical Oxygen Evolution in Highly Alkaline Conditions. Nat. Commun. 2021, 12, 5980. Available online: https://www.nature.com/articles/s41467-021-26281-0 (accessed on 12 July 2025). [CrossRef] [PubMed]
- Yang, W.; Zhou, M.; Oturan, N.; Bechelany, M.; Cretin, M.; Oturan, M.A. Highly Efficient and Stable FeIIFeIII LDH Carbon Felt Cathode for Removal of Pharmaceutical Ofloxacin at Neutral pH. J. Hazard. Mater. 2020, 393, 122513. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, S.; Wang, H.; Ding, J.; Liu, S.; Huang, Z.; Sun, W.; Liu, G.; Wang, L.; Xu, W. Construction of High-Performance Asymmetric Supercapacitor Based on FeCo-LDH@C3N4 Composite Electrode Material with Penetrating Structure. J. Energy Storage 2022, 56, 106034. [Google Scholar] [CrossRef]
- Lin, S.; Zhou, Z.; Wu, H.; Yin, S.; Wang, Y. Electrochemical Oxidation of Aniline Using a High-Flux CNT Filter. J. Water Process Eng. 2022, 46, 102536. [Google Scholar] [CrossRef]
- Shi, C.; Yu, S.; Li, C. Fabrication of Aligned Carbon Nanofiber Doped with SnO2-Sb for Efficient Electrochemical Removal of Tetracycline. Chem. Eng. J. 2022, 441, 136052. [Google Scholar] [CrossRef]
- Guo, D.; Liu, Y.; Ji, H.; Wang, C.-C.; Chen, B.; Shen, C.; Li, F.; Wang, Y.; Lu, P.; Liu, W. Silicate-Enhanced Heterogeneous Flow-Through Electro-Fenton System Using Iron Oxides under Nanoconfinement. Environ. Sci. Technol. 2021, 55, 4045–4053. [Google Scholar] [CrossRef]
- Li, Z.; Shen, C.; Liu, Y.; Ma, C.; Li, F.; Yang, B.; Huang, M.; Wang, Z.; Dong, L.; Wolfgang, S. Carbon Nanotube Filter Functionalized with Iron Oxychloride for Flow-through Electro-Fenton. Appl. Catal. B Environ. 2020, 260, 118204. [Google Scholar] [CrossRef]
- Kong, L.; Guo, J.; Li, Y.; Shao, J.; Ren, L.; Jia, J.; Ying, D. Using Flow-through Reactor to Enhance Ferric Ions Electrochemical Regeneration in Electro-Fenton for Wastewater Treatment. Process Saf. Environ. Prot. 2024, 191, 907–916. [Google Scholar] [CrossRef]
- Ghasemi, M.; Khataee, A.; Gholami, P.; Cheshmeh Soltani, R.D. Template-Free Microspheres Decorated with Cu-Fe-NLDH for Catalytic Removal of Gentamicin in Heterogeneous Electro-Fenton Process. J. Environ. Manag. 2019, 248, 109236. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, F.; Hou, X.; Wang, L.; Wu, W.; Wang, L.; Li, Y.; Xie, H. Fe@Fe2O3/Etched Carbon Felt as a Cathode for Efficient Bisphenol a Removal in a Flow-through Electro-Fenton System: Electron Transfer Pathway and Underlying Mechanism. Sep. Purif. Technol. 2024, 334, 125982. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, S.; Xu, A.; Wei, K.; Han, W.; Li, J.; Sun, X.; Shen, J.; Liu, X.; Wang, L. A Multi-Walled Carbon Nanotube Electrode Based on Porous Graphite-RuO2 in Electrochemical Filter for Pyrrole Degradation. Chem. Eng. J. 2017, 330, 956–964. [Google Scholar] [CrossRef]
- Gui, L.; Chen, Z.; Chen, B.; Song, Y.; Yu, Q.; Zhu, W.; Hu, Q.; Liu, Y.; Zheng, Z.; Ze, L.; et al. Preparation and Characterization of ZnO/PEG-Co(II)-PbO2 Nanocomposite Electrode and an Investigation of the Electrocatalytic Degradation of Phenol. J. Hazard. Mater. 2020, 399, 123018. [Google Scholar] [CrossRef]
- Ma, J.; Li, H.; Yang, Y.; Li, X. Influence of Water Matrix Species on Persulfate Oxidation of Phenol: Reaction Kinetics and Formation of Undesired Degradation Byproducts. Water Sci. Technol. 2018, 2017, 340–350. [Google Scholar] [CrossRef]
- Fu, X.; Han, Y.; Xu, H.; Su, Z.; Liu, L. Electrochemical Study of a Novel High-Efficiency PbO2 Anode Based on a Cerium-Graphene Oxide Co-Doping Strategy: Electrodeposition Mechanism, Parameter Optimization, and Degradation Pathways. J. Hazard. Mater. 2022, 422, 126890. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, M.; Wang, X.; Wang, C.; Lu, D.; Ma, W.; Kube, S.A.; Ma, J.; Elimelech, M. Janus Electrocatalytic Flow-through Membrane Enables Highly Selective Singlet Oxygen Production. Nat. Commun. 2020, 11, 6228. [Google Scholar] [CrossRef]
- Guo, D.; Wang, Y.; Lu, P.; Liu, J.; Liu, Y. Flow-through Electro-Fenton Using Nanoconfined Fe-Mn Bimetallic Oxides: Ionization Potential-Dependent Micropollutants Degradation Mechanism. Appl. Catal. B Environ. Energy 2023, 328, 122538. [Google Scholar] [CrossRef]
- Jia, X.; Cao, P.; Qin, X.; Chen, S.; Yu, H.; Quan, X. High-Efficiency Electrochemical Activation of H2O2 into OH Enabled by Flow-through FeOCl-Modified Carbon Electrode for Organic Pollutants Degradation. Sep. Purif. Technol. 2022, 295, 121279. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Yao, Q.; Wang, Y.; Fang, X.; Shen, C.; Li, F.; Huang, M.; Wang, Z.; Sand, W.; et al. Supported Atomically-Precise Gold Nanoclusters for Enhanced Flow-through Electro-Fenton. Environ. Sci. Technol. 2020, 54, 5913–5921. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Q.; Jing, J.; Song, G.; Zhou, M. Biomass Derived S, N Self-Doped Catalytic Janus Cathode for Flow-through Metal-Free Electrochemical Advanced Oxidation Process: Better Removal Efficiency and Lower Energy Consumption under Neutral Conditions. Chem. Eng. J. 2023, 466, 143283. [Google Scholar] [CrossRef]
- Zheng, J.; Ma, J.; Wang, Z.; Xu, S.; Waite, T.D.; Wu, Z. Contaminant Removal from Source Waters Using Cathodic Electrochemical Membrane Filtration: Mechanisms and Implications. Environ. Sci. Technol. 2017, 51, 2757–2765. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).