Ag-Cu Synergism-Driven Oxygen Structure Modulation Promotes Low-Temperature NOx and CO Abatement
Abstract
1. Introduction
2. Results and Discussion
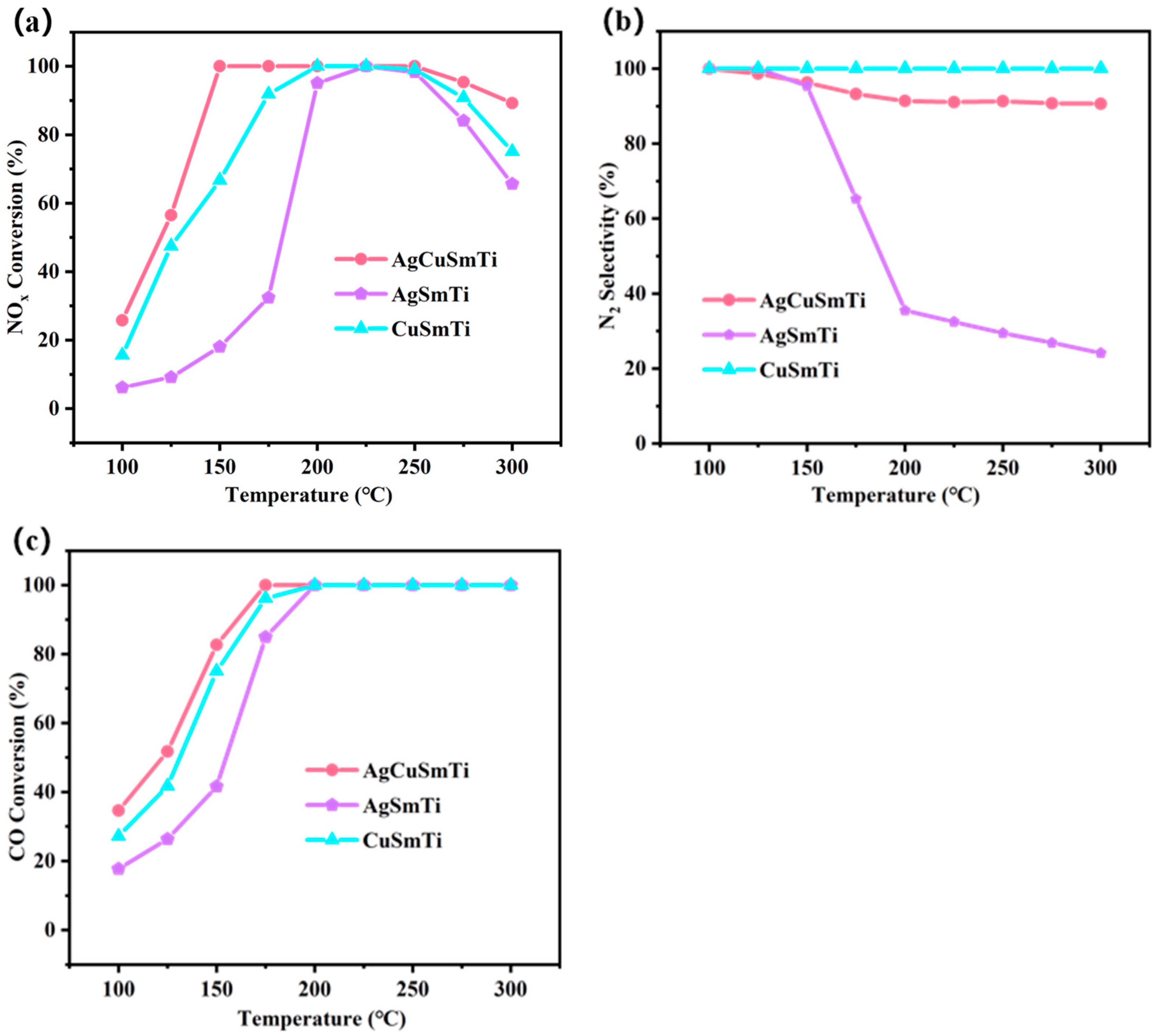
2.1. Evaluation of Catalytic Performance
2.2. Structural and Chemical Characterization of the Catalyst
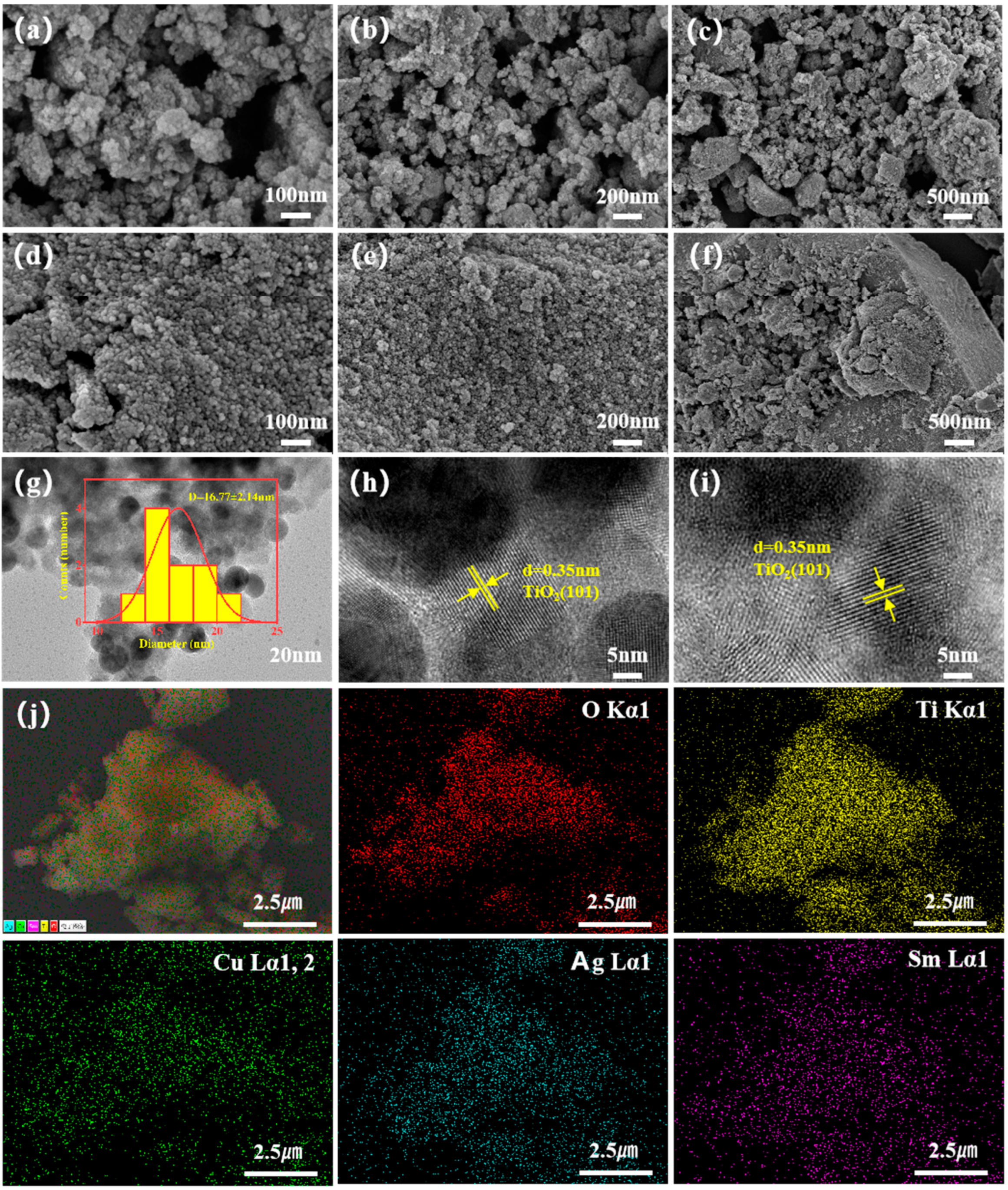
2.2.1. Morphological Characterization of the Catalysts
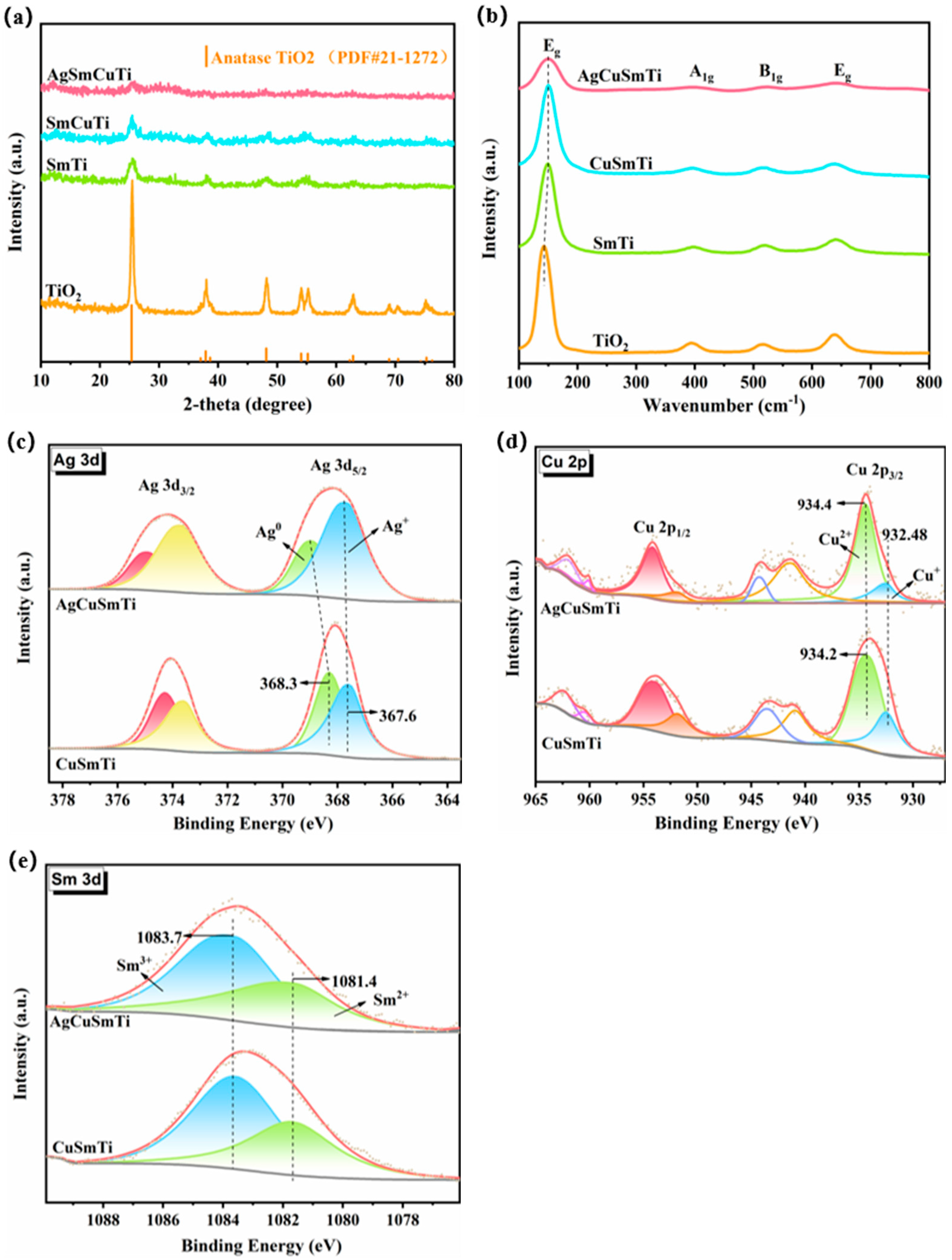
2.2.2. Evidence for Ag-Cu Interaction
2.2.3. Structural Regulation of Oxygen
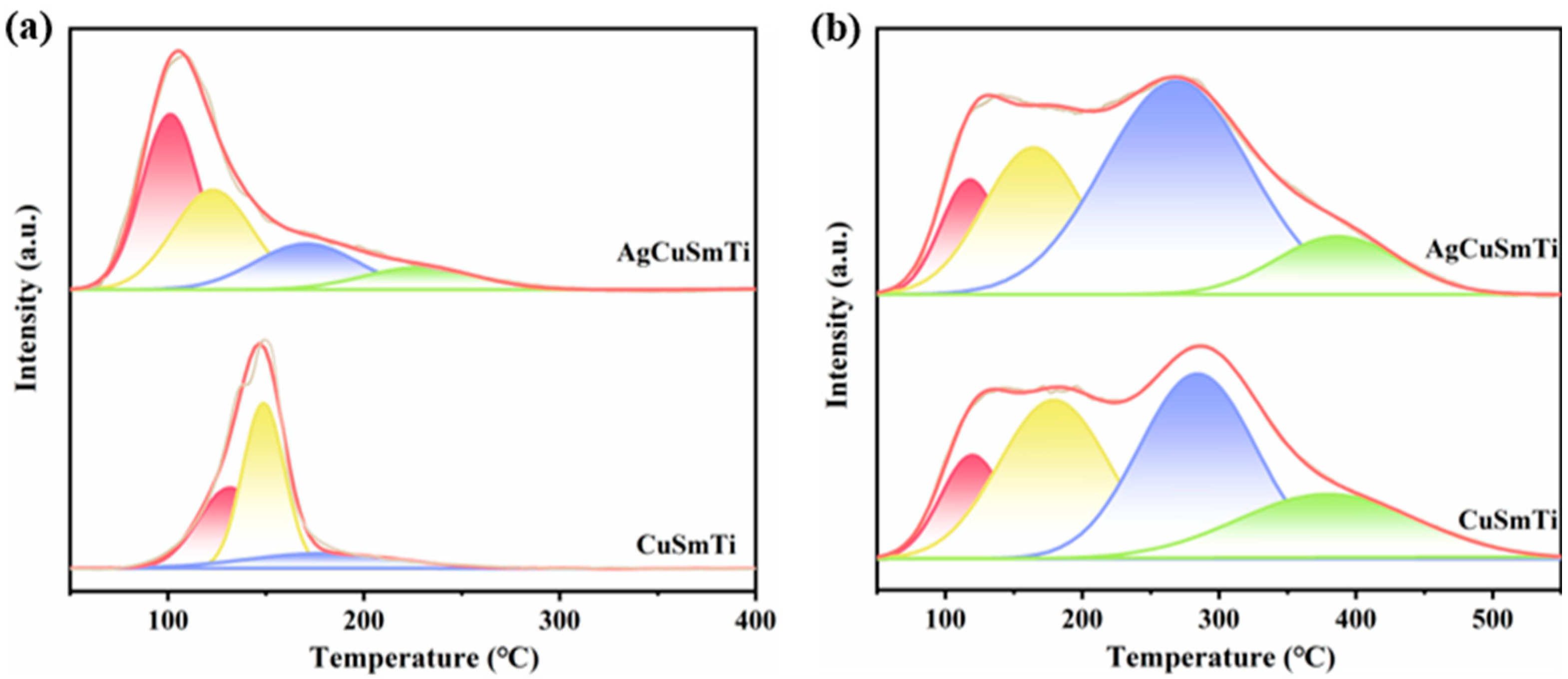
2.2.4. Redox Properties and Surface Acidity Characterization
2.3. Proposed Reaction Mechanism Investigation
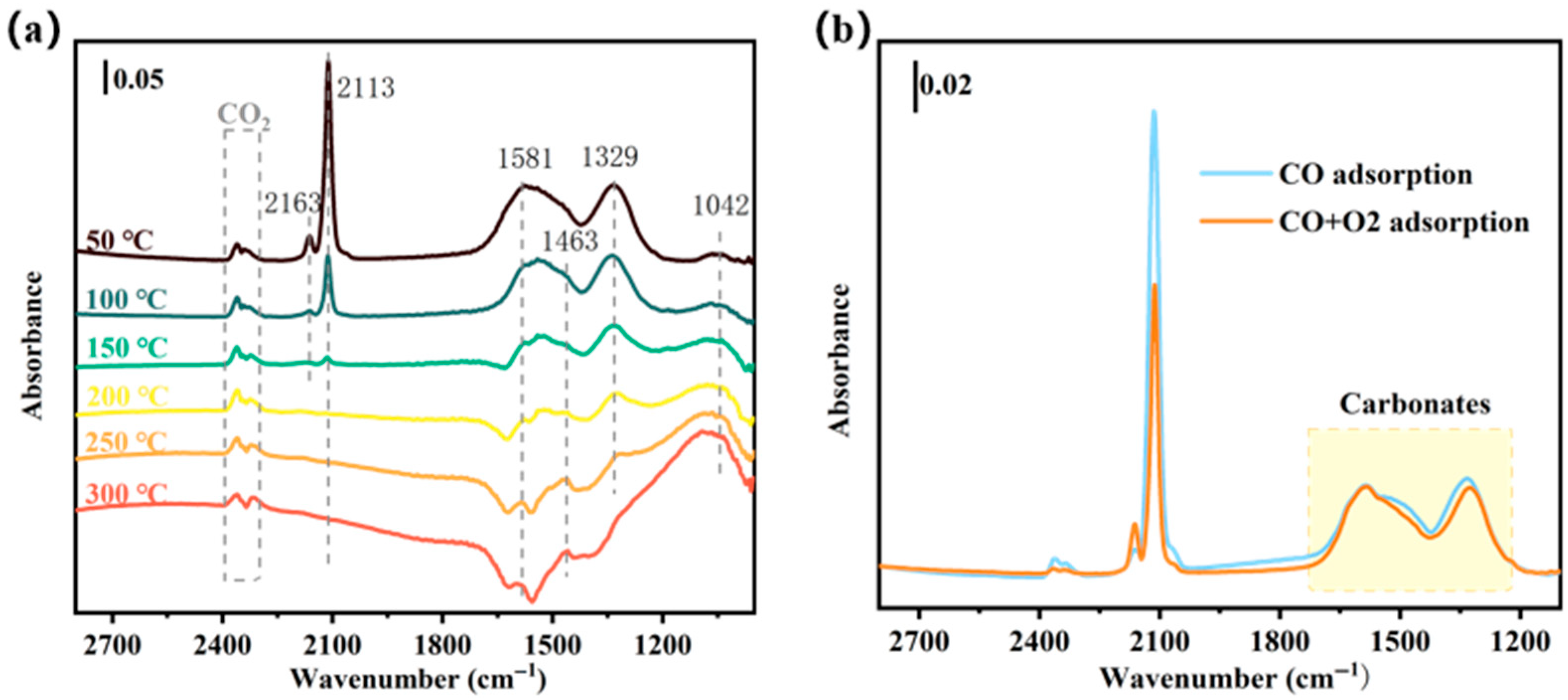
2.3.1. Proposed Reaction Mechanism of CO Oxidation
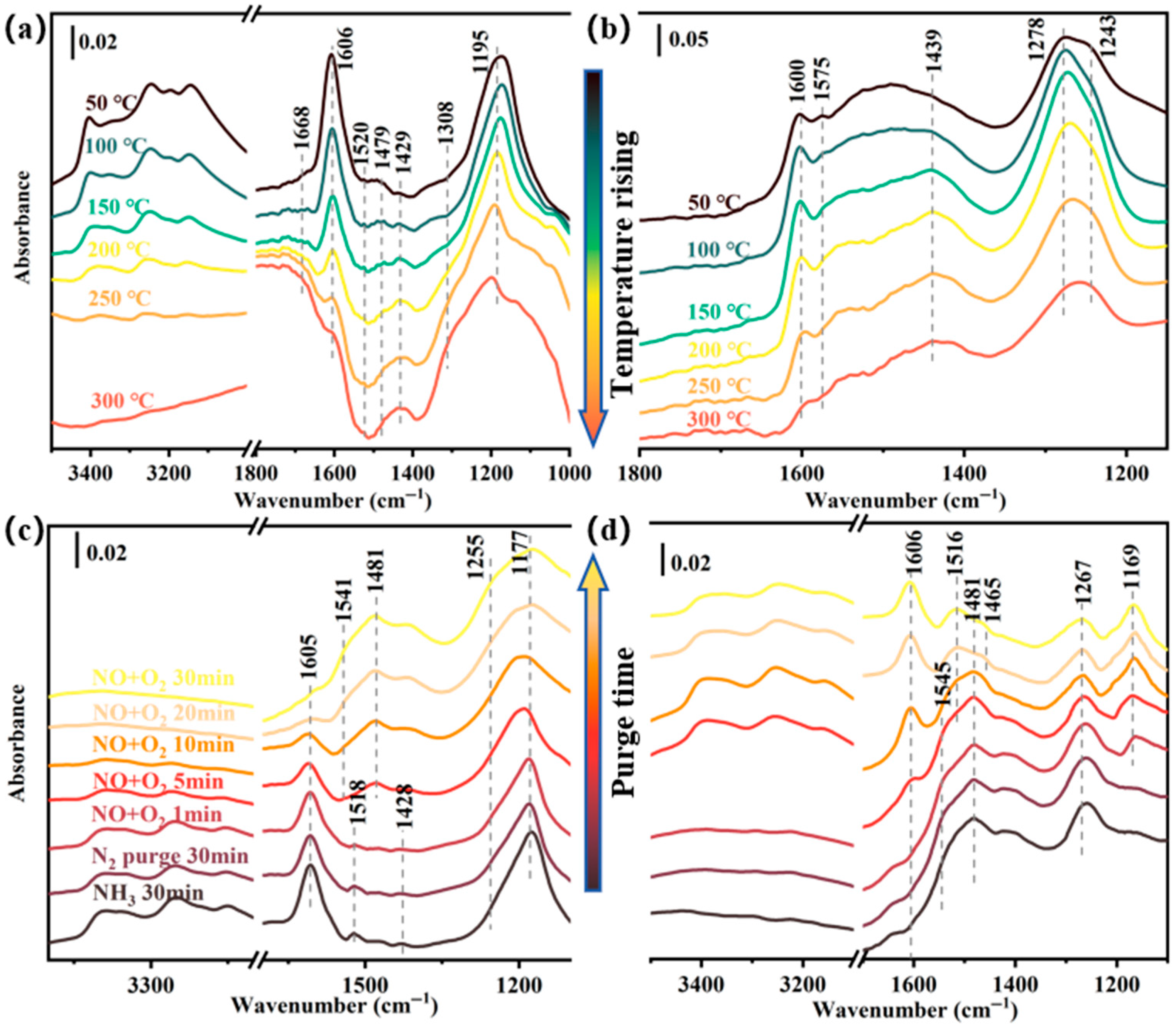
2.3.2. Proposed Reaction Mechanism of NH3-SCR
3. Experimental Section
3.1. Catalyst Preparation
3.2. Catalyst Activity Test
3.3. Reagents and Gases
3.4. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Han, Q.; Zhu, T.; Xu, W. Catalytic NO Reduction by CO over Ca–Fe Oxides in the Presence of O2 with Sintering Flue Gas Circulation. Ind. Eng. Chem. Res. 2020, 59, 20624–20629. [Google Scholar] [CrossRef]
- Gan, L.; Shi, W.; Li, K.; Chen, J.; Peng, Y.; Li, J. Synergistic Promotion Effect between NOx and Chlorobenzene Removal on MnOx–CeO2 Catalyst. ACS Appl. Mater. Interfaces 2018, 10, 30426–30432. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, Y.; Jiang, W.; Pu, Y.; Yang, L.; Dai, Z.; Yao, L. The promotion of NH3-SCR performance by AC addition on Mn-Fe/Z catalyst. Sep. Purif. Technol. 2023, 322, 124274. [Google Scholar] [CrossRef]
- Zhu, M.; Lai, J.-K.; Tumuluri, U.; Wu, Z.; Wachs, I.E. Nature of Active Sites and Surface Intermediates during SCR of NO with NH3 by Supported V2O5–WO3/TiO2 Catalysts. J. Am. Chem. Soc. 2017, 139, 15624–15627. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, J.-H.; Bak, Y.-C.; Amal, R.; Scott, J. Pt-V2O5-WO3/TiO2 catalysts supported on SiC filter for NO reduction at low temperature. Korean J. Chem. Eng. 2005, 22, 844–851. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Zhang, J.-L.; Liu, Z.-J.; Du, C.-B. Recent Advances and Research Status in Energy Conservation of Iron Ore Sintering in China. JOM 2017, 69, 2404–2411. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C.; Mohan, D.; Prasad, R. Advances in transition metal oxide catalysts for carbon monoxide oxidation: A review. Adv. Compos. Hybrid. Mater. 2019, 2, 626–656. [Google Scholar] [CrossRef]
- Li, X.; Ren, S.; Jiang, Y.; Chen, Z.; Wang, L.; Liu, M.; Chen, T. In situ IR spectroscopy study of NO removal over CuCe catalyst for CO-SCR reaction at different temperature. Catal. Today 2023, 418, 114082. [Google Scholar] [CrossRef]
- Hamada, H.; Haneda, M. A review of selective catalytic reduction of nitrogen oxides with hydrogen and carbon monoxide. Appl. Catal. A Gen. 2012, 421–422, 1–13. [Google Scholar] [CrossRef]
- Zeng, Y.; Rong, W.; Zhang, S.; Wang, Y.; Zhong, Q. Promoting NH3-SCR denitration via CO oxidation over CuO promoted V2O5-WO3/TiO2 catalysts under oxygen-rich conditions. Fuel 2022, 323, 124357. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Liu, J.; Li, G.; Zhao, Y.; Wang, Y.; Lv, Y. Effects of defective structure originating from N incorporation-evaporation of Co-based biomass carbon catalysts on methane dry reforming. Fuel 2024, 357, 129752. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Han, F.; Zhao, Y. Effect of Fe addition on the structure and SCR reactivity of one-pot synthesized Cu-SSZ-13. J. Environ. Chem. Eng. 2022, 10, 107888. [Google Scholar] [CrossRef]
- Shen, Z.; Xing, X.; She, Y.; Wang, S.; Lv, M.; Li, J.; Li, H. Revealing of K and SO2 poisoning mechanism on CuCeOx catalyst for low-temperature CO oxidation. Chem. Eng. J. 2024, 485, 149373. [Google Scholar] [CrossRef]
- Liu, Q.; Mi, J.; Chen, X.; Wang, S.; Chen, J.; Li, J. Effects of phosphorus modification on the catalytic properties and performance of CuCeZr mixed metal catalyst for simultaneous removal of CO and NOx. Chem. Eng. J. 2021, 423, 130228. [Google Scholar] [CrossRef]
- Shen, Z.; Xing, X.; She, Y.; Guo, P.; Ren, S.; Niu, W.; Li, J.; Li, H.; Meng, H. Unveiling the promoting mechanism of Mo on the performance of CuCeOx catalyst for simultaneously NH3-SCR denitration and CO oxidation under oxygen-rich conditions. Sep. Purif. Technol. 2025, 355, 129561. [Google Scholar] [CrossRef]
- Lv, D.; Liu, J.; Zhang, G.; Wang, Y.; Zhao, Y.; Li, G. Novel insights for simultaneous NOx and CO Removal: Cu+-Sm3+-Ov-Ti4+ asymmetric active site promoting NH3-SCR coupled with CO oxidation reaction. Chem. Eng. J. 2024, 481, 148534. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Zhan, J.; Zhang, X.; Lian, H.-Y.; Zhou, H.; Yi, X.; Yang, H.-H.; Shan, J. Enhancing water resistance of α-MnO2 catalyst for efficient airborne benzene oxidation at mild temperatures via facile Ag incorporation. J. Environ. Chem. Eng. 2023, 11, 110725. [Google Scholar] [CrossRef]
- Rao, R.; Liang, H.; Hu, C.; Dong, H.; Dong, X.; Tang, Y.; Fang, S.; Ling, Q. A melamine-assisted pyrolytic synthesis of Ag-CeO2 nanoassemblys for CO oxidation: Activation of Ag-CeO2 interfacial lattice oxygen. Appl. Surf. Sci. 2022, 571, 151283. [Google Scholar] [CrossRef]
- Md Saad, S.K.; Ali Umar, A.; Ali Umar, M.I.; Tomitori, M.; Abd Rahman, M.Y.; Mat Salleh, M.; Oyama, M. Two-Dimensional, Hierarchical Ag-Doped TiO2 Nanocatalysts: Effect of the Metal Oxidation State on the Photocatalytic Properties. ACS Omega 2018, 3, 2579–2587. [Google Scholar] [CrossRef]
- Ren, S.; Li, X.; He, C.; Chen, L.; Wang, L.; Li, F. Surface tailoring on bifunctional CuOx/MnO2 catalyst to promote the selective catalytic reduction of NO with NH3 and oxidation of CO with O2. Sep. Purif. Technol. 2024, 346, 127471. [Google Scholar] [CrossRef]
- Li, X.; Lai, C.; Zhang, Y.; Wang, S.; Ding, S. Bifunctional catalysts V-Cu/TiO2 for selective catalytic reduction of NOx and CO oxidation under oxygen-rich conditions. Mol. Catal. 2024, 569, 114574. [Google Scholar] [CrossRef]
- Gui, R.; Xiao, J.; Gao, Y.; Li, Y.; Zhu, T.; Wang, Q. Simultaneously achieving selective catalytic reduction of NOx with NH3 and catalytic oxidation of CO with O2 over one finely optimized bifunctional catalyst Mn2Cu1Al1Ox at low temperatures. Appl. Catal. B Environ. 2022, 306, 121104. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, H.; Zhang, X.; Ji, M.; Wang, S.; Wang, S. CuW/CeZr Catalysts: A Dual-Function Catalyst for Selective Catalytic Reduction of NO and CO Oxidation Under Oxygen-Rich Conditions. Catal. Lett. 2021, 151, 3361–3371. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Guo, Y.; Zheng, Y.; Zhu, T. Interactions between CO oxidation and selective catalytic reduction of NO with NH3 over Mn-based catalysts. Catal. Sci. Technol. 2022, 12, 4776–4788. [Google Scholar] [CrossRef]
- Wang, J.; Xing, Y.; Su, W.; Li, K.; Zhang, W. Bifunctional Mn-Cu-CeOx/γ-Al2O3 catalysts for low-temperature simultaneous removal of NOx and CO. Fuel 2022, 321, 124050. [Google Scholar] [CrossRef]
- Resitoglu, I.A.; Keskin, A. Hydrogen applications in selective catalytic reduction of NOx emissions from diesel engines. Int. J. Hydrog. Energy 2017, 42, 23389–23394. [Google Scholar] [CrossRef]
- Gao, C.; Shi, J.-W.; Fan, Z.; Wang, B.; Wang, Y.; He, C.; Wang, X.; Li, J.; Niu, C. “Fast SCR” reaction over Sm-modified MnOx-TiO2 for promoting reduction of NOx with NH3. Appl. Catal. A Gen. 2018, 564, 102–112. [Google Scholar] [CrossRef]
- Wang, B.; Wang, M.; Han, L.; Hou, Y.; Bao, W.; Zhang, C.; Feng, G.; Chang, L.; Huang, Z.; Wang, J. Improved Activity and SO2 Resistance by Sm-Modulated Redox of MnCeSmTiOx Mesoporous Amorphous Oxides for Low-Temperature NH3-SCR of NO. ACS Catal. 2020, 10, 9034–9045. [Google Scholar] [CrossRef]
- Sun, H.; Wang, H.; Qu, Z. Construction of CuO/CeO2 Catalysts via the Ceria Shape Effect for Selective Catalytic Oxidation of Ammonia. ACS Catal. 2023, 13, 1077–1088. [Google Scholar] [CrossRef]
- Djaoued, Y.; Badilescu, S.; Ashrit, P.V.; Bersani, D.; Lottici, P.P.; Robichaud, J. Study of Anatase to Rutile Phase Transition in Nanocrystalline Titania Films. J. Sol-Gel Sci. Technol. 2002, 24, 255–264. [Google Scholar] [CrossRef]
- Alhomoudi, I.A.; Newaz, G. Residual stresses and Raman shift relation in anatase TiO2 thin film. Thin Solid. Film. 2009, 517, 4372–4378. [Google Scholar] [CrossRef]
- Choudhury, B.; Dey, M.; Choudhury, A. Defect generation, d-d transition, and band gap reduction in Cu-doped TiO2 nanoparticles. Int. Nano Lett. 2013, 3, 25. [Google Scholar] [CrossRef]
- Liu, H.; Fan, Z.; Sun, C.; Yu, S.; Feng, S.; Chen, W.; Chen, D.; Tang, C.; Gao, F.; Dong, L. Improved activity and significant SO2 tolerance of samarium modified CeO2-TiO2 catalyst for NO selective catalytic reduction with NH3. Appl. Catal. B Environ. 2019, 244, 671–683. [Google Scholar] [CrossRef]
- Guo, X.; Lin, F.; Gao, M.; Dai, Q.; Zhan, W.; Wang, L.; Guo, Y.; Wang, A.; Guo, Y. Enhanced catalytic performance and N2 yield of Ag/CeO2 catalyst by Cu modification for NVOCs removal. Chem. Eng. J. 2024, 488, 151117. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Y.; Liu, S.; Chen, J.; Jia, W. SCR catalyst doped with copper for synergistic removal of slip ammonia and elemental mercury. Fuel Process. Technol. 2018, 181, 268–278. [Google Scholar] [CrossRef]
- Wang, M.; Ren, S.; Jiang, Y.; Su, B.; Chen, Z.; Liu, W.; Yang, J.; Chen, L. Insights into co-doping effect of Sm and Fe on anti-Pb poisoning of Mn-Ce/AC catalyst for low-temperature SCR of NO with NH3. Fuel 2022, 319, 123763. [Google Scholar] [CrossRef]
- Zang, P.; Liu, J.; He, Y.; Zhang, G.; Li, G.; Wang, Y.; Lv, Y. LDH-derived preparation of CuMgFe layered double oxides for NH3-SCR and CO oxidation reactions: Performance study and synergistic mechanism. Chem. Eng. J. 2022, 446, 137414. [Google Scholar] [CrossRef]
- Liu, S.; Xue, W.; Ji, Y.; Xu, W.; Chen, W.; Jia, L.; Zhu, T.; Zhong, Z.; Xu, G.; Mei, D.; et al. Interfacial oxygen vacancies at Co3O4-CeO2 heterointerfaces boost the catalytic reduction of NO by CO in the presence of O2. Appl. Catal. B: Environ. 2023, 323, 122151. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, L.; Li, L.; Liu, L.; Cao, Y.; Dong, X.; Gao, F.; Deng, Y.; Tang, C.; Chen, Z.; et al. Investigation of the structure, acidity, and catalytic performance of CuO/Ti0.95Ce0.05O2 catalyst for the selective catalytic reduction of NO by NH3 at low temperature. Appl. Catal. B Environ. 2014, 150–151, 315–329. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, Z.; Quan, X.; Wang, H.; Shu, Y. Selective catalytic oxidation of ammonia to N2 over wire–mesh honeycomb catalyst in simulated synthetic ammonia stream. Chem. Eng. J. 2013, 233, 233–241. [Google Scholar] [CrossRef]
- Wang, X.; Qin, M.; Xu, Y.; Li, Q. Improvement of Cu-SAPO-34 hydrothermal stability by tuning P/Al ratio for selective catalytic reduction of NO by NH3. J. Colloid. Interface Sci. 2023, 638, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, Z.; Huang, Q.; Chen, M.; Xu, L.; Shen, Y.; Zhu, S. Synergetic removal of elemental mercury and NO over TiCe0.25Sn0.25Ox catalysts from flue gas: Performance and mechanism study. Chem. Eng. J. 2019, 360, 990–1002. [Google Scholar] [CrossRef]
- Kang, R.; Wei, X.; Bin, F.; Wang, Z.; Hao, Q.; Dou, B. Reaction mechanism and kinetics of CO oxidation over a CuO/Ce0.75Zr0.25O2-δ catalyst. Appl. Catal. A Gen. 2018, 565, 46–58. [Google Scholar] [CrossRef]
- Qi, L.; Yu, Q.; Dai, Y.; Tang, C.; Liu, L.; Zhang, H.; Gao, F.; Dong, L.; Chen, Y. Influence of cerium precursors on the structure and reducibility of mesoporous CuO-CeO2 catalysts for CO oxidation. Appl. Catal. B Environ. 2012, 119–120, 308–320. [Google Scholar] [CrossRef]
- Davó-Quiñonero, A.; Bailón-García, E.; López-Rodríguez, S.; Juan-Juan, J.; Lozano-Castelló, D.; García-Melchor, M.; Herrera, F.C.; Pellegrin, E.; Escudero, C.; Bueno-López, A. Insights into the Oxygen Vacancy Filling Mechanism in CuO/CeO2 Catalysts: A Key Step Toward High Selectivity in Preferential CO Oxidation. ACS Catal. 2020, 10, 6532–6545. [Google Scholar] [CrossRef]
- Lu, J.; Wang, J.; Zou, Q.; He, D.; Zhang, L.; Xu, Z.; He, S.; Luo, Y. Unravelling the Nature of the Active Species as well as the Doping Effect over Cu/Ce-Based Catalyst for Carbon Monoxide Preferential Oxidation. ACS Catal. 2019, 9, 2177–2195. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Liang, P.; Min, X.; Sun, T. SnO2-modified CuMnOx catalysts for humid CO oxidation at low temperature. J. Ind. Eng. Chem. 2024, 138, 300–310. [Google Scholar] [CrossRef]
- Sasmaz, E.; Wang, C.; Lance, M.J.; Lauterbach, J. In situ spectroscopic investigation of a Pd local structure over Pd/CeO2 and Pd/MnOx–CeO2 during CO oxidation. J. Mater. Chem. A 2017, 5, 12998–13008. [Google Scholar] [CrossRef]
- Dann, E.K.; Gibson, E.K.; Blackmore, R.H.; Catlow, C.R.A.; Collier, P.; Chutia, A.; Erden, T.E.; Hardacre, C.; Kroner, A.; Nachtegaal, M.; et al. Structural selectivity of supported Pd nanoparticles for catalytic NH3 oxidation resolved using combined operando spectroscopy. Nat. Catal. 2019, 2, 157–163. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, H.; Teng, W.; Li, J.; Liu, W.; Ren, S.; Yang, J.; Liu, Q. Comparative study on N2O formation pathways over bulk MoO3 and MoO3-x nanosheets decorated Fe2O3-containing solid waste NH3-SCR catalysts. Fuel 2023, 337, 127210. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, R.; Wang, M.; Li, Y.; Tong, Y.; Yang, P.; Zhu, Y. Two steps synthesis of CeTiOx oxides nanotube catalyst: Enhanced activity, resistance of SO2 and H2O for low temperature NH3-SCR of NOx. Appl. Catal. B Environ. 2021, 282, 119542. [Google Scholar] [CrossRef]
- Hao, Z.; Jiao, Y.; Shi, Q.; Zhang, H.; Zhan, S. Improvement of NH3-SCR performance and SO2 resistance over Sn modified CeMoOx electrospun fibers at low temperature. Catal. Today 2019, 327, 37–46. [Google Scholar] [CrossRef]
- Wang, X.; Peng, J.; Wang, Y.; Guo, N.; Li, T.; Li, H.; Ren, D.; Gui, K. Poisoning mechanism of different Cd precursors on Fe-Ce/TiO2 catalyst for selective catalytic reduction of NOx with NH3. J. Environ. Chem. Eng. 2023, 11, 109625. [Google Scholar] [CrossRef]
- Jia, B.; Liu, J.; Kang, J.; Zhang, G.; Lv, D.; Wang, Y. Investigating NH3-SCR coupled with CO oxidation reaction mechanisms on titanium nanotube-loaded CuMnFe composite metal catalysts. Appl. Surf. Sci. 2024, 652, 159299. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, T.; Weng, X.; Wang, Y.; Wu, Z.; Wang, H. DRIFT Studies on the Selectivity Promotion Mechanism of Ca-Modified Ce-Mn/TiO2 Catalysts for Low-Temperature NO Reduction with NH3. J. Phys. Chem. C 2012, 116, 16582–16592. [Google Scholar] [CrossRef]
- Yao, W.; Wang, X.; Liu, Y.; Wu, Z. CeOP material supported CeO2 catalysts: A novel catalyst for selective catalytic reduction of NO with NH3 at low temperature. Appl. Surf. Sci. 2019, 467–468, 439–445. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Zhang, C.; Shan, W.; Shi, X. Mechanism of the selective catalytic reduction of NOx with NH3 over environmental-friendly iron titanate catalyst. Catal. Today 2011, 175, 18–25. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, B.; Liu, Y.; Wang, H.; Jin, R. DRIFT Study of Manganese/Titania-Based Catalysts for Low-Temperature Selective Catalytic Reduction of NO with NH3. Environ. Sci. Technol. 2007, 41, 5812–5817. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ge, M. DRIFT Study on Cerium−Tungsten/Titiania Catalyst for Selective Catalytic Reduction of NOx with NH3. Environ. Sci. Technol. 2010, 44, 9590–9596. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, S.; Wang, M.; Yang, J.; Chen, L.; Liu, W.; Liu, Q.; Su, B. Insights into samarium doping effects on catalytic activity and SO2 tolerance of MnFeOx catalyst for low-temperature NH3-SCR reaction. Fuel 2022, 321, 124113. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective Catalytic Reduction of NOx with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Liu, Y.; Nikiforov, A.; Ji, J.; Zhao, B.; Wang, J. The research on CO oxidation over Ce–Mn oxides: The preparation method effects and oxidation mechanism. Chemosphere 2023, 336, 139130. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ren, S.; Chen, Z.; Chen, L.; Wang, M.; Wang, L.; Wang, A. Promoting mechanism of CuO on catalytic performance of CeFe catalyst for simultaneous removal of NOx and CO. Fuel 2023, 347, 128435. [Google Scholar] [CrossRef]


| Sample | Surface Atomic Concentration (%) | Relative Concentration Ratio (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Ag | Cu | Sm | Ti | O | Cu2+/ (Cu+ + Cu2+) | Sm3+/ (Sm2+ + Sm3+) | Oβ/ (Oα + Oβ) | |
| AgCuSmTi | 8.79 | 2.61 | 5.50 | 24.63 | 58.46 | 82.51 | 60.98 | 77.77 |
| CuSmTi | - | 2.90 | 4.99 | 27.67 | 64.44 | 68.35 | 61.05 | 64.77 |
| Sample | α | β | γ |
|---|---|---|---|
| AgCuSmTi | 11.25% | 76.86% | 11.89% |
| CuSmTi | 11.5% | 68.15% | 20.35% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Wei, J.; Jia, B.; Liu, J.; Liu, X.; Wang, Y.; Zhao, Y.; Li, G.; Zhang, G. Ag-Cu Synergism-Driven Oxygen Structure Modulation Promotes Low-Temperature NOx and CO Abatement. Catalysts 2025, 15, 674. https://doi.org/10.3390/catal15070674
Li R, Wei J, Jia B, Liu J, Liu X, Wang Y, Zhao Y, Li G, Zhang G. Ag-Cu Synergism-Driven Oxygen Structure Modulation Promotes Low-Temperature NOx and CO Abatement. Catalysts. 2025; 15(7):674. https://doi.org/10.3390/catal15070674
Chicago/Turabian StyleLi, Ruoxin, Jiuhong Wei, Bin Jia, Jun Liu, Xiaoqing Liu, Ying Wang, Yuqiong Zhao, Guoqiang Li, and Guojie Zhang. 2025. "Ag-Cu Synergism-Driven Oxygen Structure Modulation Promotes Low-Temperature NOx and CO Abatement" Catalysts 15, no. 7: 674. https://doi.org/10.3390/catal15070674
APA StyleLi, R., Wei, J., Jia, B., Liu, J., Liu, X., Wang, Y., Zhao, Y., Li, G., & Zhang, G. (2025). Ag-Cu Synergism-Driven Oxygen Structure Modulation Promotes Low-Temperature NOx and CO Abatement. Catalysts, 15(7), 674. https://doi.org/10.3390/catal15070674








