The Development of Ni-Al Aerogel-Based Catalysts via Supercritical CO2 Drying for Photocatalytic CO2 Methanation
Abstract
1. Introduction
2. Results and Discussion
2.1. Textural Properties
2.2. X-Ray Diffraction
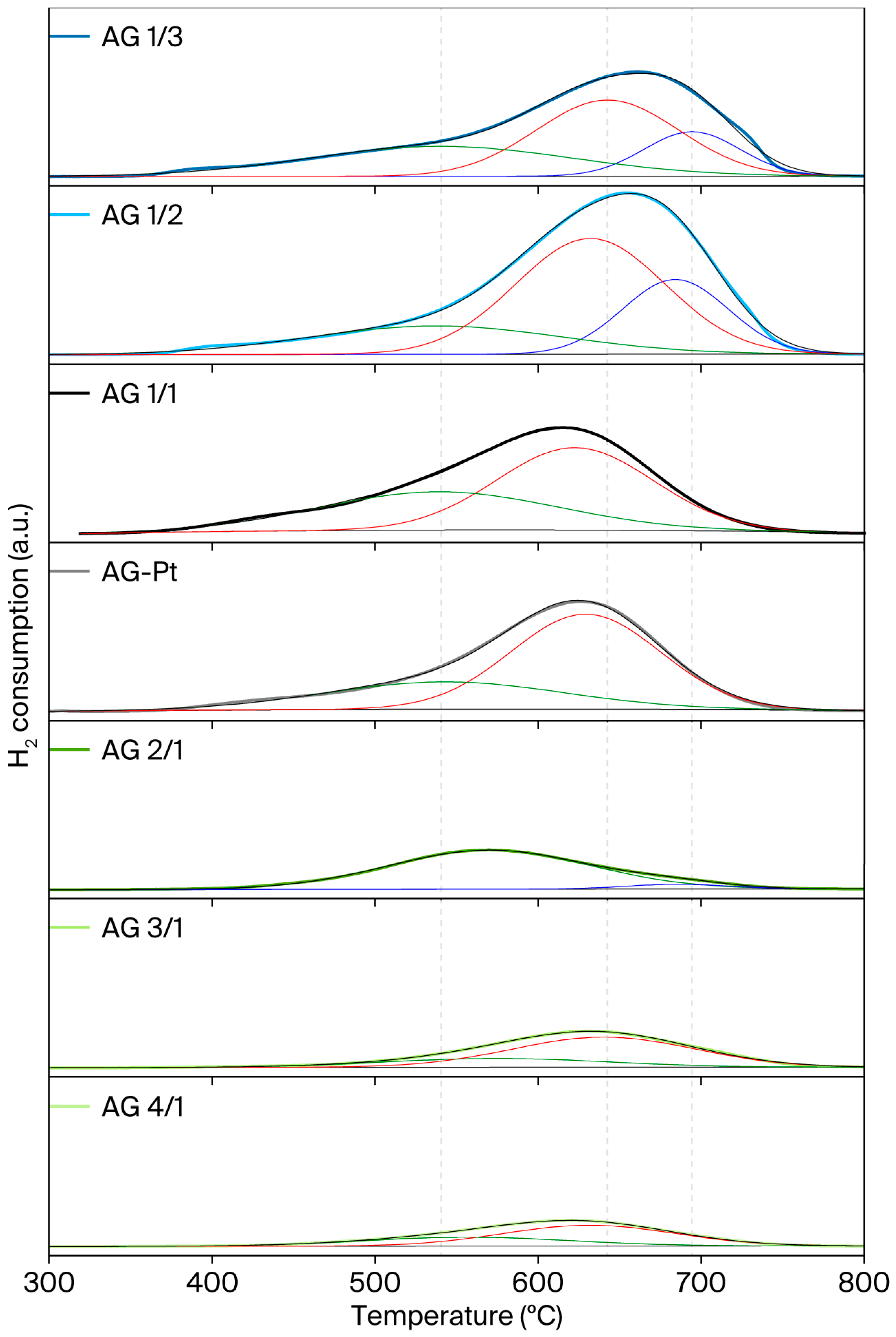
2.3. H2 Temperature-Programmed Reduction (H2-TPR)
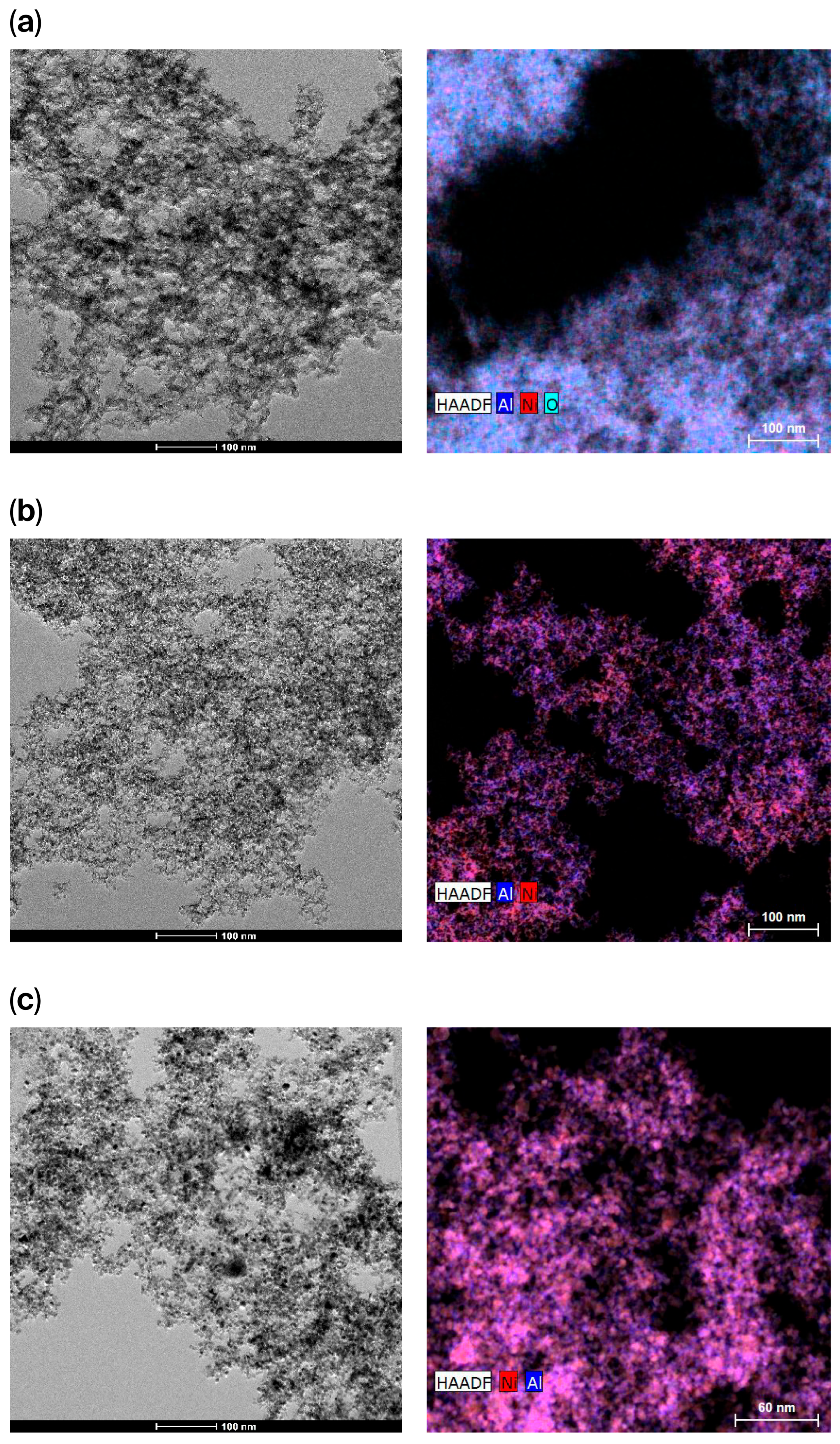
2.4. Transmission Electron Microscopy (TEM)
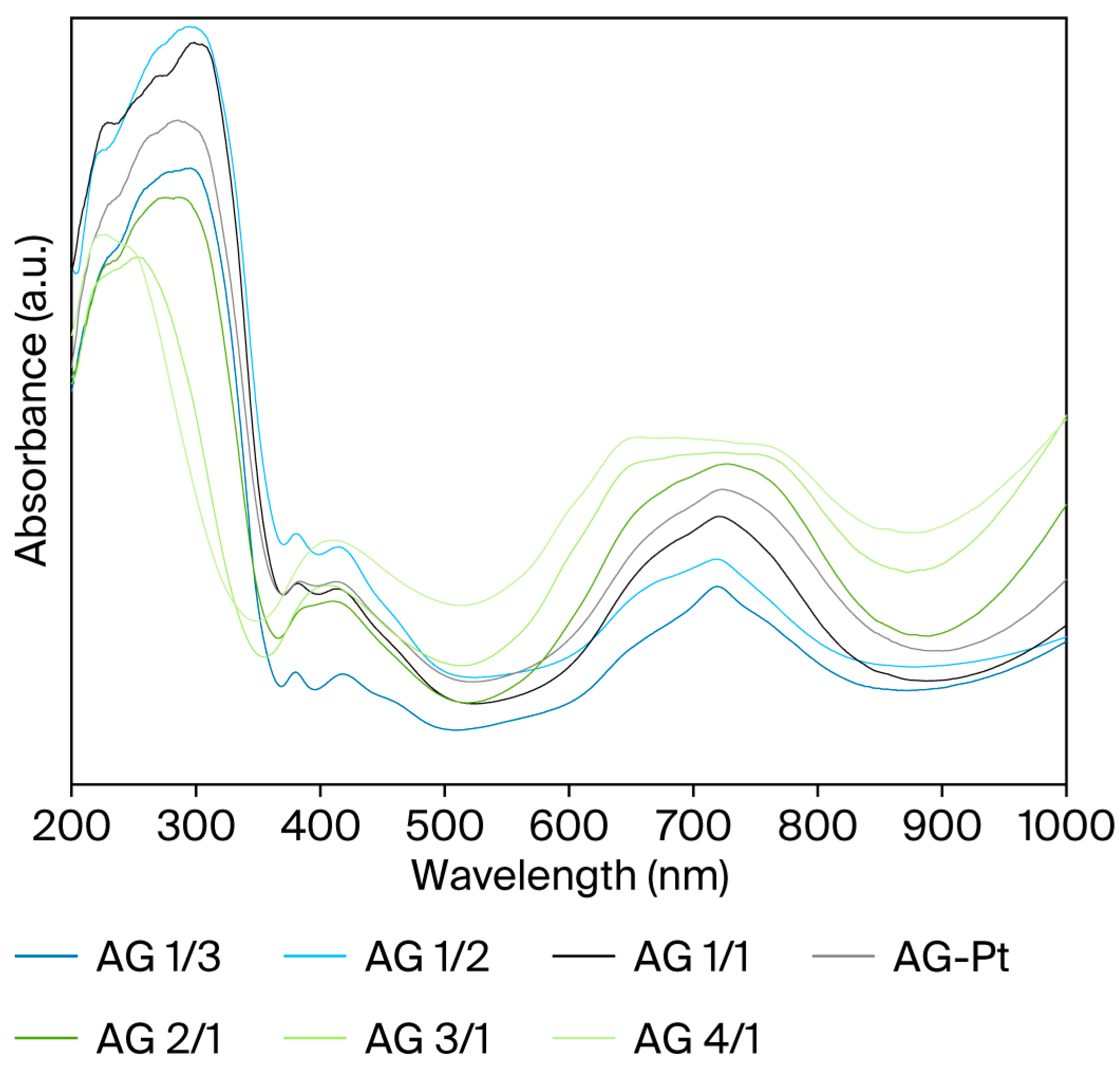
2.5. UV-Visible Diffuse Reflectance Spectroscopy (UV-Vis DRS)
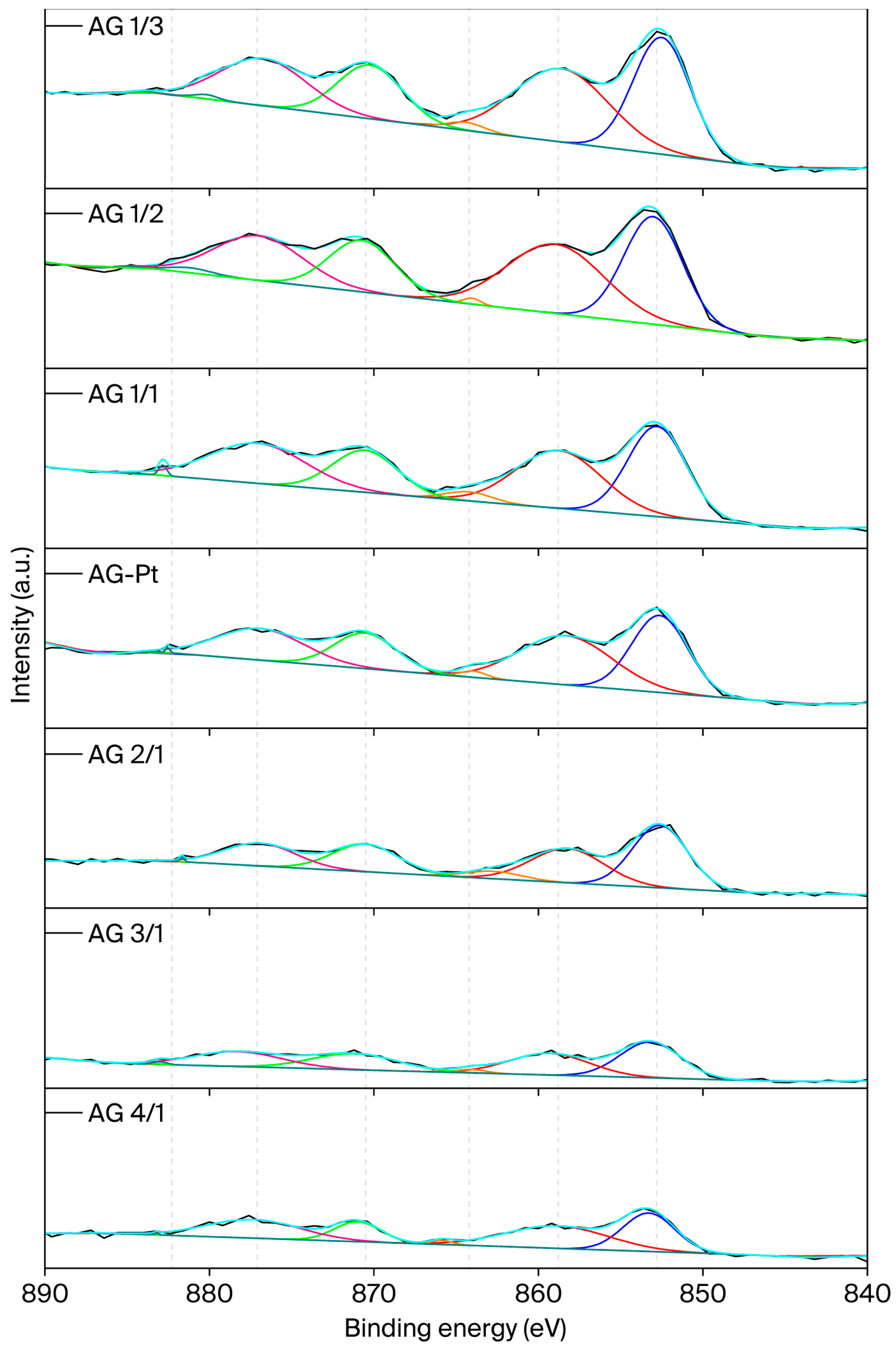
2.6. X-Ray Photoelectron Microscopy (XPS)
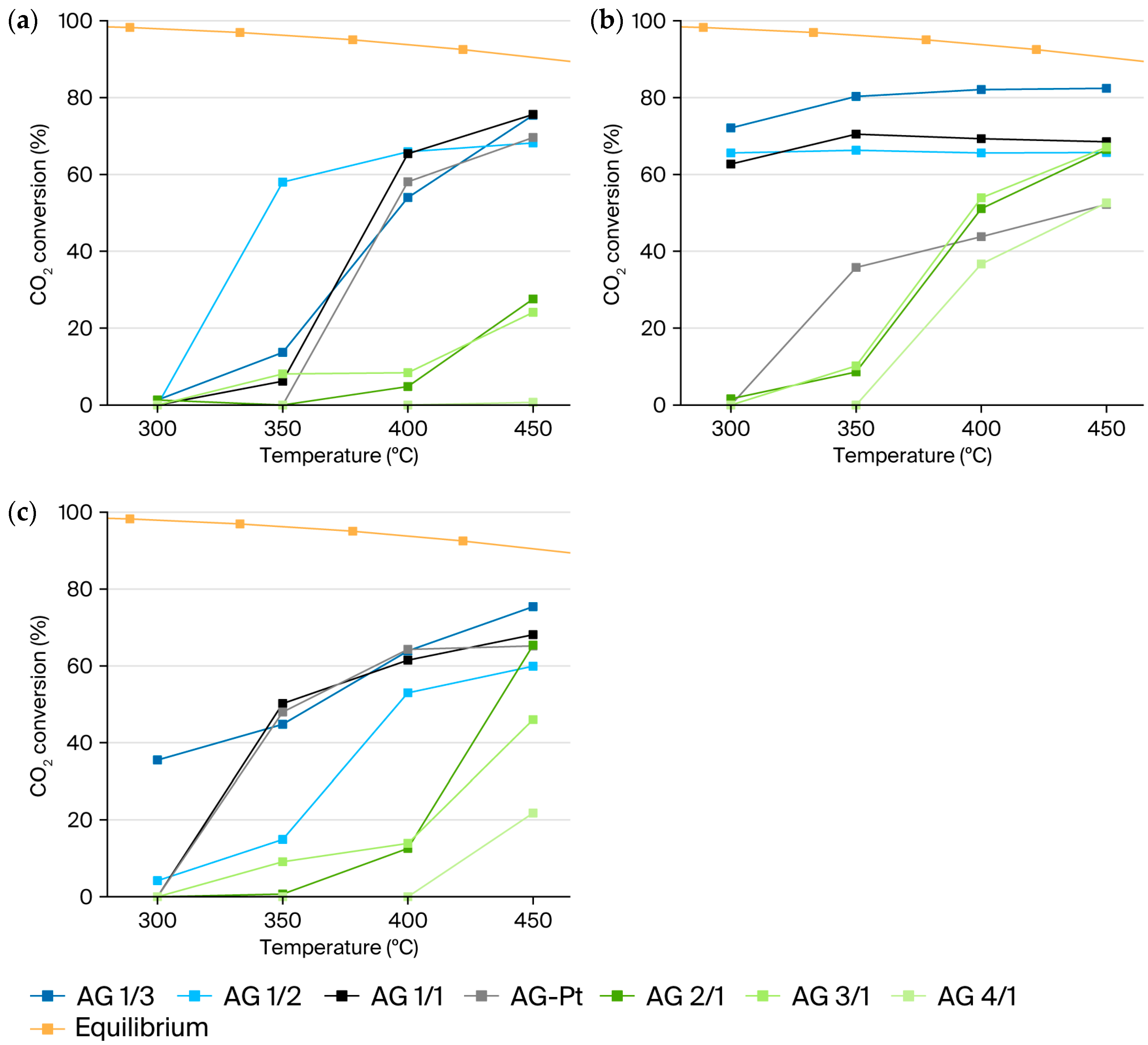
2.7. CO2 Methanation Activity Performance
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AG | Aerogel |
| lCO2 | Liquid CO2 |
| ICP-OES | Inductively Coupled Plasma–Optical Emission Spectroscopy |
| BET | Brunauer, Emmet, and Teller Theory |
| BJH | Barrett–Joyner–Halenda |
| XRD | X-Ray Diffraction |
| H2-TPR | H2 Temperature-Programmed Reduction |
| TEM | Transmission Electron Microscopy |
| HAADF | High-Angle Annular Dark Field |
| EDX | Energy-Dispersive X-Ray Spectroscopy |
| UV-Vis DRS | UV-Visible Diffuse Reflectance Spectroscopy |
| XPS | X-Ray Photoelectron Spectroscopy |
| TCD | Thermal Conductivity Detector |
| FID | Flame Ionization Detector |
References
- Liu, Z.; Deng, Z.; Davis, S.J.; Ciais, P. Global Carbon Emissions in 2023. Nat. Rev. Earth Environ. 2024, 5, 253–254. [Google Scholar] [CrossRef]
- Lewandowska-Bernat, A.; Desideri, U. Opportunities of power-to-gas technology in different energy systems architectures. Appl. Energy 2018, 228, 57–67. [Google Scholar] [CrossRef]
- Karakaya, C.; Kee, R.J. Progress in the direct catalytic conversion of methane to fuels and chemicals. Prog. Energy Combust. Sci. 2016, 55, 60–97. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Guan, N.; Li, L. Methane Activation and Utilization: Current Status and Future Challenges. Energy Technol. 2019, 8, 1900826. [Google Scholar] [CrossRef]
- Ma, J.; Zhu, C.; Mao, K.; Jiang, W.; Low, J.; Duan, D.; Ju, H.; Liu, D.; Wang, K.; Zang, Y.; et al. Sustainable methane utilization technology via photocatalytic halogenation with alkali halides. Nat. Commun. 2023, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- van Schyndel, J.; Goos, E.; Naumann, C.; Hardi, J.S.; Oschwald, M. Effects of Compounds in Liquefied Methane on Rocket Engine Operation. Aerospace 2022, 9, 698. [Google Scholar] [CrossRef]
- Vogt, C.; Monai, M.; Kramer, G.J.; Weckhuysen, B.M. The renaissance of the Sabatier reaction and its applications on Earth and in space. Nat. Catal. 2019, 2, 188–197. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Tian, J.; Liao, Y.; Zhai, J.; Fang, X.; Xu, X.; Wang, X. Tuning CO adsorption states via enhanced metal-support interaction for boosting CO2 methanation activity of Ru/rutile-TiO2. Fuel 2023, 360, 130600. [Google Scholar] [CrossRef]
- Rueda-Navarro, C.M.; González-Fernández, M.; Cabrero-Antonino, M.; Dhakshinamoorthy, A.; Ferrer, B.; Baldoví, H.G.; Navalón, S. Solar-Assisted CO2 Methanation via Photocatalytic Sabatier Reaction by Calcined Titanium-based Organic Framework Supported RuOx Nanoparticles. ChemCatChem 2024, 16, e202400991. [Google Scholar] [CrossRef]
- Yang, C.; Wang, W.; Zhuo, H.; Shen, Z.; Zhang, T.; Yang, X.; Huang, Y. Phase engineering of Ru-based nanocatalysts for enhanced activity toward CO2 methanation. Chin. J. Catal. 2024, 61, 226–236. [Google Scholar] [CrossRef]
- Sun, S.; Higham, M.D.; Zhang, X.; Catlow, C.R.A. Multiscale Investigation of the Mechanism and Selectivity of CO2 Hydrogenation over Rh(111). ACS Catal. 2024, 14, 5503–5519. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Flores, K.; Solís-García, A.; Lam, S.J.; Cervantes-Gaxiola, M.; Castillo-López, R.; Leyva, J.R.; Gómez, S.; Flores-Aquino, E.; Zepeda, T. Modulating selectivity in CO2 Methanation through Rhodium catalysts supported on Zirconia-Chemically grafted SBA-15. Mol. Catal. 2024, 558, 114035. [Google Scholar] [CrossRef]
- Dai, X.; Sun, Y. Mechanism of photocatalytic CO2 methanation on ultrafine Rh nanoparticles. Nanoscale Horizons 2024, 9, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Meloni, E.; Cafiero, L.; Renda, S.; Martino, M.; Pierro, M.; Palma, V. Ru- and Rh-Based Catalysts for CO2 Methanation Assisted by Non-Thermal Plasma. Catalysts 2023, 13, 488. [Google Scholar] [CrossRef]
- Wu, Y.; Bhalothia, D.; Chou, J.-P.; Lee, G.-W.; Hu, A.; Chen, T.-Y. Electronic metal–support interaction in Pd/CoNi-hydroxides with enhanced CO adsorption for boosting CO2 methanation. Sustain. Energy Fuels 2024, 9, 847–854. [Google Scholar] [CrossRef]
- Yang, T.; Beniwal, A.; Bhalothia, D.; Yan, C.; Wang, C.-H.; Chen, T.-Y. Oxygen vacancies coupled with surface silicide facilitate CO2 activation at near-room temperature for efficient methane productivity on Ni-oxide supported Pd nanoparticles. Sustain. Energy Fuels 2024, 8, 3399–3411. [Google Scholar] [CrossRef]
- Danielis, M.; Jiménez, J.D.; Rui, N.; Moncada, J.; Betancourt, L.E.; Trovarelli, A.; Rodriguez, J.A.; Senanayake, S.D.; Colussi, S. Tuning the hydrogenation of CO2 to CH4 over mechano-chemically prepared palladium supported on ceria. Appl. Catal. A Gen. 2023, 660, 119185. [Google Scholar] [CrossRef]
- Memon, M.A.; Jiang, Y.; Hassan, M.A.; Ajmal, M.; Wang, H.; Liu, Y. Heterogeneous Catalysts for Carbon Dioxide Methanation: A View on Catalytic Performance. Catalysts 2023, 13, 1514. [Google Scholar] [CrossRef]
- Garbarino, G.; Bellotti, D.; Riani, P.; Magistri, L.; Busca, G. Methanation of carbon dioxide on Ru/Al2O3 and Ni/Al2O3 catalysts at atmospheric pressure: Catalysts activation, behaviour and stability. Int. J. Hydrogen Energy 2015, 40, 9171–9182. [Google Scholar] [CrossRef]
- Spataru, D.; Carvalho, G.; Quindimil, A.; Lopes, J.M.; Henriques, C.; Bacariza, C. CO2 methanation under more realistic conditions: Influence of O2 and H2O on Ni-based catalysts’ performance. Chem. Eng. J. 2024, 496, 153709. [Google Scholar] [CrossRef]
- Ma, L.; Ye, R.; Huang, Y.; Reina, T.R.; Wang, X.; Li, C.; Zhang, X.L.; Fan, M.; Zhang, R.; Liu, J. Enhanced low-temperature CO2 methanation performance of Ni/ZrO2 catalysts via a phase engineering strategy. Chem. Eng. J. 2022, 446, 137031. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Z.; Feng, K.; Yan, B. Eu3+ doping-promoted Ni-CeO2 interaction for efficient low-temperature CO2 methanation. Appl. Catal. B Environ. 2022, 317, 121800. [Google Scholar] [CrossRef]
- Cañón-Alvarado, M.; Blanco, C.; Daza, C. Exploring dolomite as a promising support for Ni catalysts in CO2 methanation. J. Environ. Chem. Eng. 2024, 12, 112224. [Google Scholar] [CrossRef]
- Liu, F.; Park, Y.S.; Diercks, D.; Kazempoor, P.; Duan, C. Enhanced CO2 Methanation Activity of Sm0.25Ce0.75O2-δ–Ni by Modulating the Chelating Agents-to-Metal Cation Ratio and Tuning Metal–Support Interactions. ACS Appl. Mater. Interfaces 2022, 14, 13295–13304. [Google Scholar] [CrossRef] [PubMed]
- Kristiani, A.; Takeishi, K. CO2 methanation over nickel-based catalyst supported on yttria-stabilized zirconia. Catal. Commun. 2022, 165, 106435. [Google Scholar] [CrossRef]
- Song, W.; Wang, K.; Wang, X.; Ma, Q.; Zhao, T.-S.; Bae, J.W.; Gao, X.; Zhang, J. Boosting low temperature CO2 methanation by tailoring Co species of CoAlO catalysts. Chem. Eng. Sci. 2024, 298, 120405. [Google Scholar] [CrossRef]
- Fu, W.; Liu, S.; He, Y.; Chen, J.; Ren, J.; Chen, H.; Sun, R.; Tang, Z.; Mebrahtu, C.; Zeng, F. CO2 hydrogenation over CoAl based catalysts: Effects of cobalt-metal oxide interaction. Appl. Catal. A Gen. 2024, 678, 119720. [Google Scholar] [CrossRef]
- O’cOnnell, G.E.; Tan, T.H.; Yuwono, J.A.; Wang, Y.; Kheradmand, A.; Jiang, Y.; Kumar, P.V.; Amal, R.; Scott, J.; Lovell, E.C. Seeing the light: The role of cobalt in light-assisted CO2 methanation. Appl. Catal. B Environ. 2023, 343, 123507. [Google Scholar] [CrossRef]
- Mehrabi, M.; Ashjari, M.; Meshkani, F. Cobalt supported on silica–alumina nanocomposite for use in CO2 methanation process: Effects of Si/Al molar ratio and Co loading on catalytic activity. Res. Chem. Intermed. 2023, 50, 219–238. [Google Scholar] [CrossRef]
- Prabhakar, J.K.; Apte, P.A.; Deo, G. Exploring optimal total metal loading of Ni3Fe/Al2O3 catalyst for CO2 methanation and its kinetic model. Fuel 2024, 367, 131447. [Google Scholar] [CrossRef]
- Li, J.; Xu, Q.; Han, Y.; Guo, Z.; Zhao, L.; Cheng, K.; Zhang, Q.; Wang, Y. Efficient photothermal CO2 methanation over NiFe alloy nanoparticles with enhanced localized surface plasmon resonance effect. Sci. China Chem. 2023, 66, 3518–3524. [Google Scholar] [CrossRef]
- Yan, P.; Peng, H.; Zhang, X.; Rabiee, H.; Ahmed, M.; Weng, Y.; Rozhkovskaya, A.; Vogrin, J.; Konarova, M.; Zhu, Z. Unlocking the role of Ni-Fe species in CO2 methanation. Fuel 2024, 374, 132373. [Google Scholar] [CrossRef]
- Aziz, M.A.; Jalil, A.A.; Hassan, N.S.; Bin Bahari, M.; Hatta, A.H.; Abdullah, T.A.T.; Jusoh, N.W.C.; Setiabudi, H.D.; Saravanan, R. Kinetic exploration of CO2 methanation over nickel loaded on fibrous mesoporous silica nanoparticles (CHE-SM). Process. Saf. Environ. Prot. 2024, 186, 1229–1241. [Google Scholar] [CrossRef]
- Ilsemann, J.; Murshed, M.M.; Gesing, T.M.; Kopyscinski, J.; Bäumer, M. On the support dependency of the CO2 methanation–decoupling size and support effects. Catal. Sci. Technol. 2021, 11, 4098–4114. [Google Scholar] [CrossRef]
- Zhou, L.; Guo, X.; Hu, X.; Zhang, Y.; Cheng, J.; Guo, Q. CO2 methanation reaction over La-modified NiAl catalysts derived from hydrotalcite-like precursors. Fuel 2024, 362, 130888. [Google Scholar] [CrossRef]
- Abahussain, A.A.M.; Al-Fatesh, A.S.; Rajput, Y.B.; Osman, A.I.; Alreshaidan, S.B.; Ahmed, H.; Fakeeha, A.H.; Al-Awadi, A.S.; El-Salamony, R.A.; Kumar, R. Impact of Sr Addition on Zirconia–Alumina-Supported Ni Catalyst for COx-Free CH4 Production via CO2 Methanation. ACS Omega 2024, 9, 9309–9320. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Traitangwong, A.; Hu, M.; Zuo, C.; Meeyoo, V.; Peng, Z.; Li, C. Carbon Dioxide Methanation over Nickel-Based Catalysts Supported on Various Mesoporous Material. Energy Fuels 2018, 32, 3681–3689. [Google Scholar] [CrossRef]
- Bacariza, M.; Graça, I.; Lopes, J.; Henriques, C. Enhanced activity of CO2 hydrogenation to CH4 over Ni based zeolites through the optimization of the Si/Al ratio. Microporous Mesoporous Mater. 2018, 267, 9–19. [Google Scholar] [CrossRef]
- Quindimil, A.; De-La-Torre, U.; Pereda-Ayo, B.; González-Marcos, J.A.; González-Velasco, J.R. Ni catalysts with La as promoter supported over Y- and BETA- zeolites for CO2 methanation. Appl. Catal. B Environ. 2018, 238, 393–403. [Google Scholar] [CrossRef]
- Gao, B.; Wang, I.-W.; Ren, L.; Haines, T.; Hu, J. Catalytic Performance and Reproducibility of Ni/Al2O3 and Co/Al2O3 Mesoporous Aerogel Catalysts for Methane Decomposition. Ind. Eng. Chem. Res. 2018, 58, 798–807. [Google Scholar] [CrossRef]
- Hu, F.; Chen, X.; Tu, Z.; Lu, Z.-H.; Feng, G.; Zhang, R. Graphene Aerogel Supported Ni for CO2 Hydrogenation to Methane. Ind. Eng. Chem. Res. 2021, 60, 12235–12243. [Google Scholar] [CrossRef]
- Canales, R.; Barrio, V.L. Photo- and Thermocatalytic CO2 Methanation: A Comparison of Ni/Al2O3 and Ni–Ce Hydrotalcite-Derived Materials under UV and Visible Light. Materials 2023, 16, 5907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yao, P.; Li, F.; Pan, L.; Xiong, W.; Zhang, Y.; Li, X. Catalytic Pyrolysis of Waste Textiles for Hydrogen-Rich Syngas Production over NiO/Al2O3 Catalyst. Processes 2024, 13, 15. [Google Scholar] [CrossRef]
- Oliveira, G.V.; de Macedo, V.; Urquieta-González, E.A.; Magriotis, Z.M.; Pereira, C.A. Fe2O3/γ-Al2O3 and NiO/γ-Al2O3 catalysts for the selective catalytic oxidation of ammonia. Catal. Today 2024, 444, 114991. [Google Scholar] [CrossRef]
- Tian, T.; Zhao, Z.-M.; Zhang, Y.; Jing, J.; Li, W.-Y. Insight into the Nature of Nickel Active Sites on the NiAl2O4 Catalyst for Phenanthrene Hydrogenation Saturation. ACS Omega 2025, 10, 8303–8313. [Google Scholar] [CrossRef] [PubMed]
- Valecillos, J.; Iglesias-Vázquez, S.; Landa, L.; Remiro, A.; Bilbao, J.; Gayubo, A.G. Insights into the Reaction Routes for H2 Formation in the Ethanol Steam Reforming on a Catalyst Derived from NiAl2O4 Spinel. Energy Fuels 2021, 35, 17197–17211. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, G.; Chitsazan, S.; Phung, T.K.; Riani, P.; Busca, G. Preparation of supported catalysts: A study of the effect of small amounts of silica on Ni/Al2O3 catalysts. Appl. Catal. A Gen. 2015, 505, 86–97. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Chen, C.; Zou, X.; Shang, X.; Ding, W.; Lu, X. Investigation of mesoporous NiAl2O4/MOx (M = La, Ce, Ca, Mg)–γ-Al2O3 nanocomposites for dry reforming of methane. RSC Adv. 2017, 7, 33143–33154. [Google Scholar] [CrossRef]
- Garbarino, G.; Campodonico, S.; Perez, A.R.; Carnasciali, M.M.; Riani, P.; Finocchio, E.; Busca, G. Spectroscopic characterization of Ni/Al2O3 catalytic materials for the steam reforming of renewables. Appl. Catal. A Gen. 2013, 452, 163–173. [Google Scholar] [CrossRef]
- Kumar, S.; Ojha, A.K. Ni, Co and Ni–Co codoping induced modification in shape, optical band gap and enhanced photocatalytic activity of CeO2 nanostructures for photodegradation of methylene blue dye under visible light irradiation. RSC Adv. 2015, 6, 8651–8660. [Google Scholar] [CrossRef]
- Castillo-Saenz, J.; Nedev, N.; Valdez-Salas, B.; Curiel-Alvarez, M.; Mendivil-Palma, M.I.; Hernandez-Como, N.; Martinez-Puente, M.; Mateos, D.; Perez-Landeros, O.; Martinez-Guerra, E. Properties of Al2O3 Thin Films Grown by PE-ALD at Low Temperature Using H2O and O2 Plasma Oxidants. Coatings 2021, 11, 1266. [Google Scholar] [CrossRef]
- Roškarič, M.; Žerjav, G.; Zavašnik, J.; Pintar, A. The influence of synthesis conditions on the visible-light triggered photocatalytic activity of g-C3N4/TiO2 composites used in AOPs. J. Environ. Chem. Eng. 2022, 10, 107656. [Google Scholar] [CrossRef]
- Canales, R.; Gil-Calvo, M.; Barrio, V.L. UV- and visible-light photocatalysis using Ni–Co bimetallic and monometallic hydrotalcite-like materials for enhanced CO2 methanation in sabatier reaction. Heliyon 2023, 9, e18456. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ma, C.; Luo, J.; Kong, X.; Xu, Y.; Ma, G.; Wang, J.; Zhang, C.; Li, Z.; Ding, M. Preparation of Ni based mesoporous Al2O3 catalyst with enhanced CO2 methanation performance. RSC Adv. 2019, 9, 8684–8694. [Google Scholar] [CrossRef] [PubMed]
- Italiano, C.; Llorca, J.; Pino, L.; Ferraro, M.; Antonucci, V.; Vita, A. CO and CO2 methanation over Ni catalysts supported on CeO2, Al2O3 and Y2O3 oxides. Appl. Catal. B Environ. 2020, 264, 118494. [Google Scholar] [CrossRef]
- Gash, A.E.; Satcher, J.H.; Simpson, R.L. Monolithic nickel(II)-based aerogels using an organic epoxide: The importance of the counterion. J. Non-Crystalline Solids 2004, 350, 145–151. [Google Scholar] [CrossRef]
- Gil Seo, J.; Youn, M.H.; Bang, Y.; Song, I.K. Effect of Ni/Al atomic ratio of mesoporous Ni–Al2O3 aerogel catalysts on their catalytic activity for hydrogen production by steam reforming of liquefied natural gas (LNG). Int. J. Hydrogen Energy 2010, 35, 12174–12181. [Google Scholar] [CrossRef]
- Hao, Z.; Zhu, Q.; Lei, Z.; Li, H. CH4–CO2 reforming over Ni/Al2O3 aerogel catalysts in a fluidized bed reactor. Powder Technol. 2007, 182, 474–479. [Google Scholar] [CrossRef]

| Sample | Nominal Al/Ni Molar Ratio | Al/Ni Molar Ratio | Al/Pt Molar Ratio (wt %) | SBET (m2/g) | Vt, pore (cm3/g) | DBJH (nm) |
|---|---|---|---|---|---|---|
| AG 1/3 | 0.33 | 0.30 | --- | 156 ± 13 | 0.56 | 7.5 |
| AG 1/2 | 0.50 | 0.41 | --- | 207 ± 18 | 0.98 | 17.8 |
| AG 1/1 | 1.00 | 0.98 | --- | 215 ± 19 | 2.28 | 24.6 |
| AG-Pt | 1.00 | 0.97 | 1863 (0.1%) | 209 ± 19 | 0.84 | 6.8 |
| AG 2/1 | 2.00 | 1.93 | --- | 270 ± 25 | 3.26 | 66.6 |
| AG 3/1 | 3.00 | 2.94 | --- | 492 ± 50 | 5.50 | 67.0 |
| AG 4/1 | 4.00 | 3.79 | --- | 358 ± 35 | 3.63 | 11.5 |
| Sample | Band Gap Energy (eV) |
|---|---|
| AG 1/3 | 3.38 |
| AG 1/2 | 3.36 |
| AG 1/1 | 3.40 |
| AG-Pt | 3.39 |
| AG 2/1 | 3.46 |
| AG 3/1 | 3.52 |
| AG 4/1 | 3.87 |
| Catalyst | Al/Ni Atomic Ratio | Al/Pt Atomic Ratio |
|---|---|---|
| AG 1/3 | 1.00 | --- |
| AG 1/2 | 1.07 | --- |
| AG 1/1 | 1.67 | --- |
| AG-Pt | 1.63 | 2068 |
| AG 2/1 | 2.04 | --- |
| AG 3/1 | 3.17 | --- |
| AG 4/1 | 3.93 | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estevez, D.; Etxeberria, H.; Barrio, V.L. The Development of Ni-Al Aerogel-Based Catalysts via Supercritical CO2 Drying for Photocatalytic CO2 Methanation. Catalysts 2025, 15, 686. https://doi.org/10.3390/catal15070686
Estevez D, Etxeberria H, Barrio VL. The Development of Ni-Al Aerogel-Based Catalysts via Supercritical CO2 Drying for Photocatalytic CO2 Methanation. Catalysts. 2025; 15(7):686. https://doi.org/10.3390/catal15070686
Chicago/Turabian StyleEstevez, Daniel, Haritz Etxeberria, and Victoria Laura Barrio. 2025. "The Development of Ni-Al Aerogel-Based Catalysts via Supercritical CO2 Drying for Photocatalytic CO2 Methanation" Catalysts 15, no. 7: 686. https://doi.org/10.3390/catal15070686
APA StyleEstevez, D., Etxeberria, H., & Barrio, V. L. (2025). The Development of Ni-Al Aerogel-Based Catalysts via Supercritical CO2 Drying for Photocatalytic CO2 Methanation. Catalysts, 15(7), 686. https://doi.org/10.3390/catal15070686









