Metal Oxide-Modified PES Membranes for Efficient Separation of Oil-in-Water Emulsions and Trace Organic Compounds
Abstract
1. Introduction
2. Results
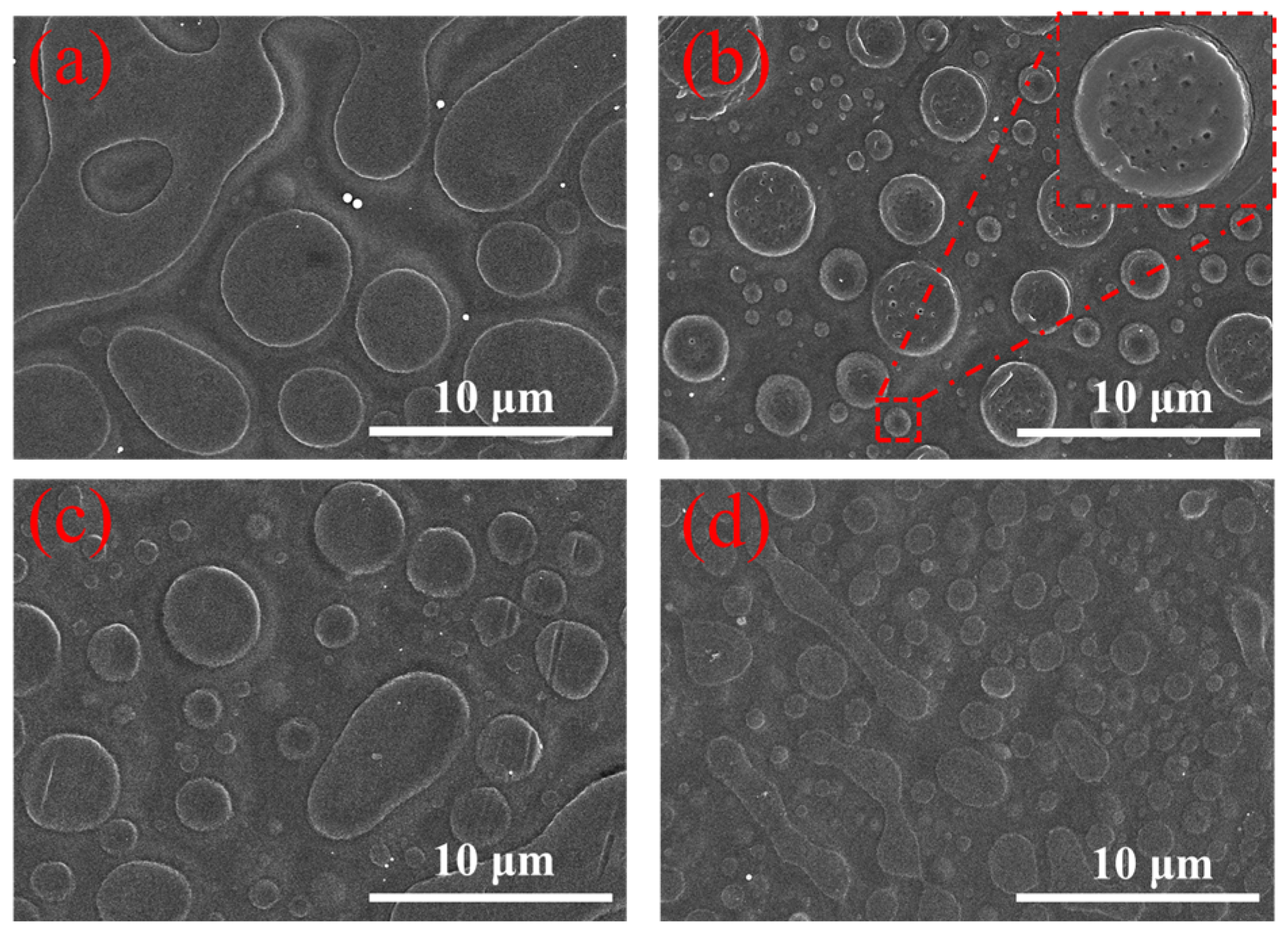
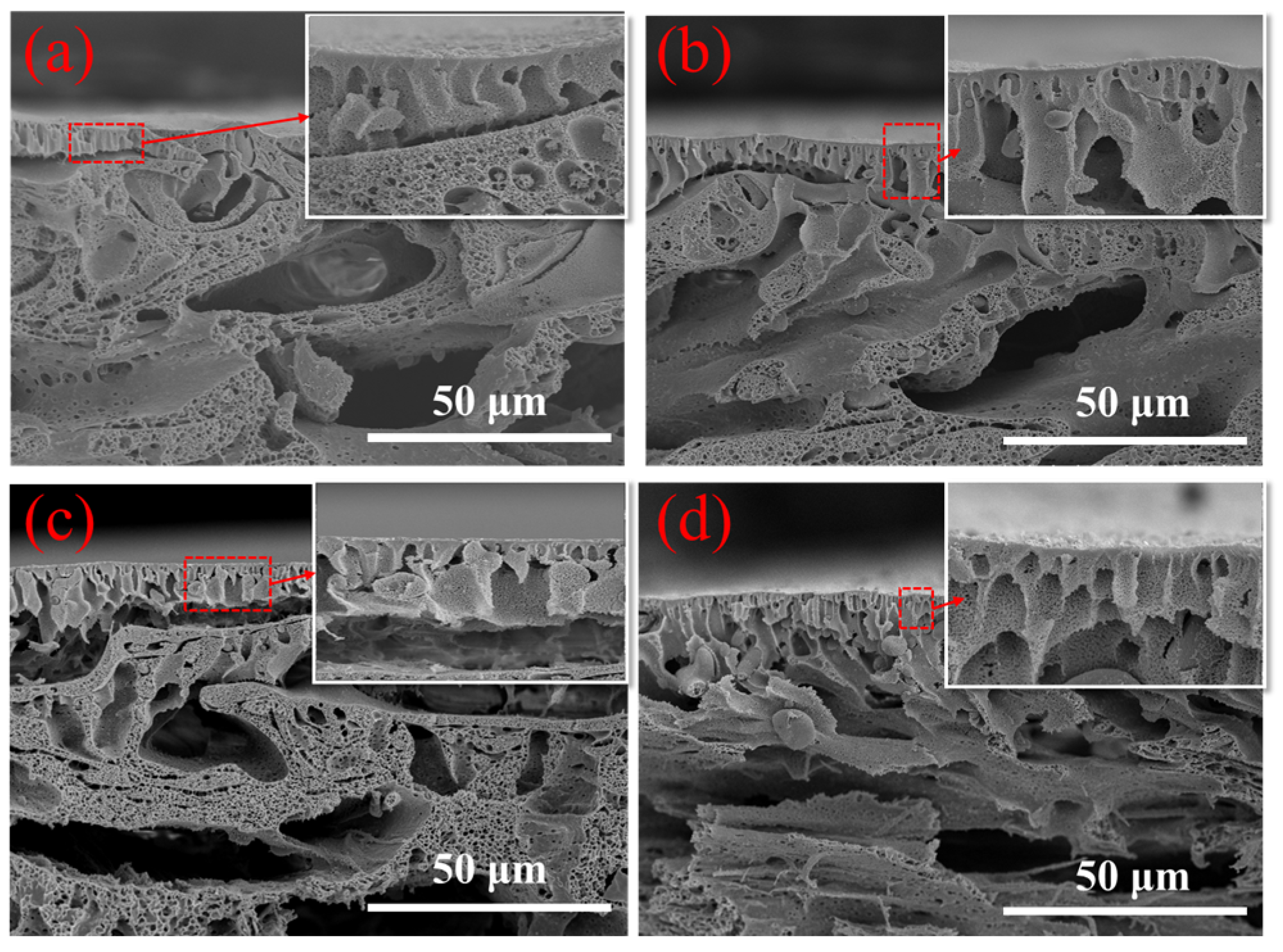
2.1. SEM Image Analysis of the Modified FO Membranes
2.2. FTIR Analysis of Modified FO Membranes
2.3. Roughness Analysis of Modified FO Membranes
2.4. Zeta Potential Analysis of Modified FO Membranes
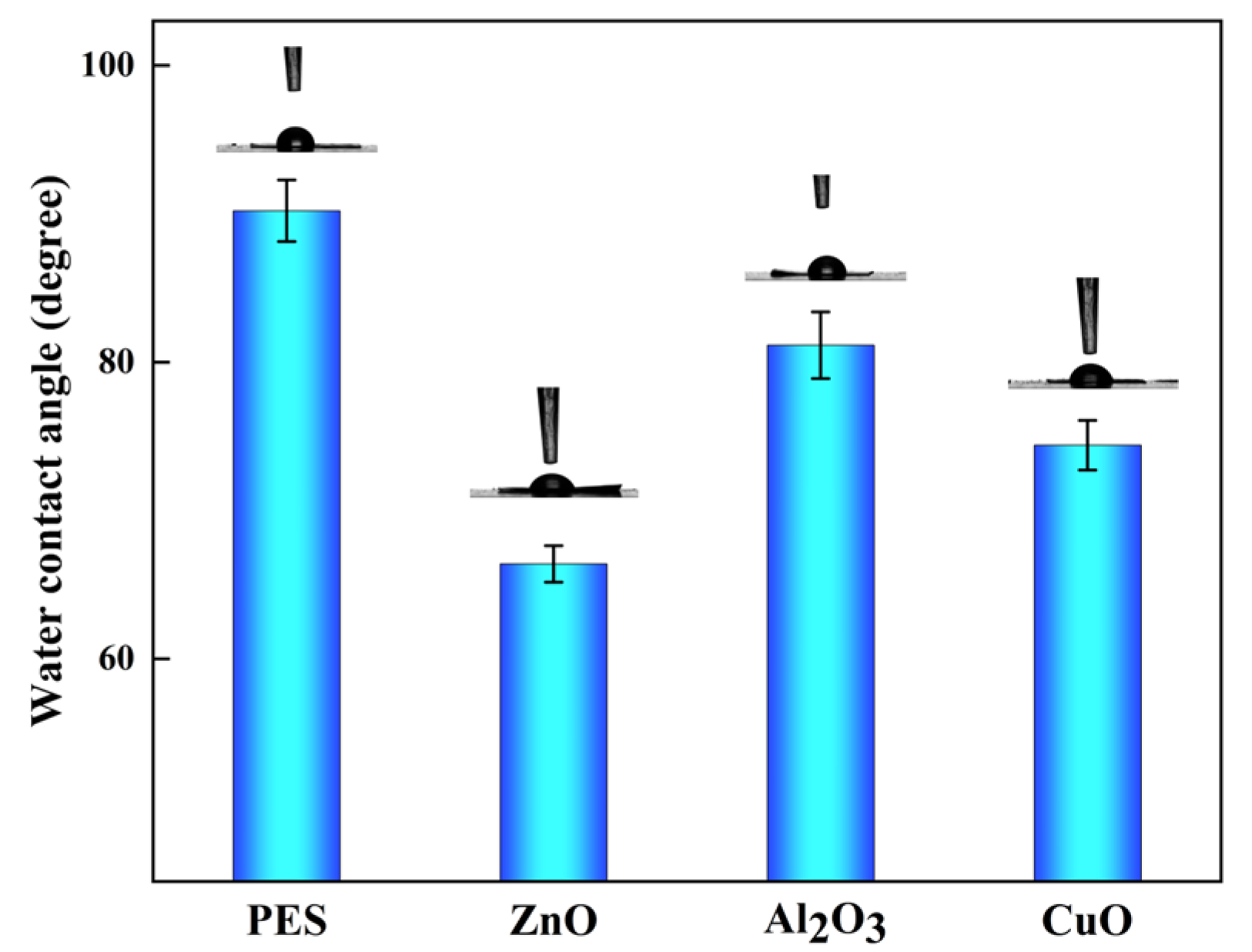
2.5. Wettability of Modified Membranes
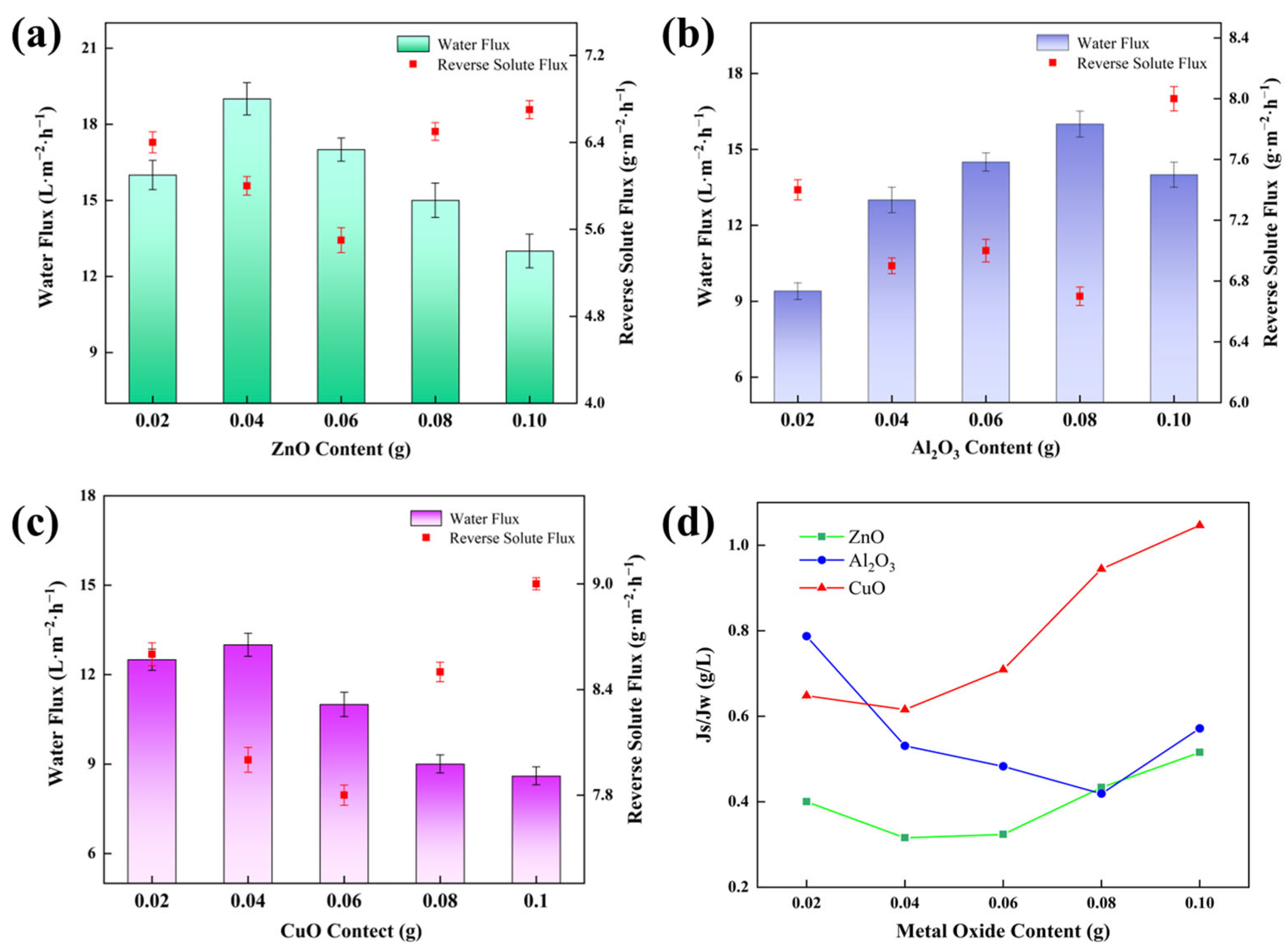
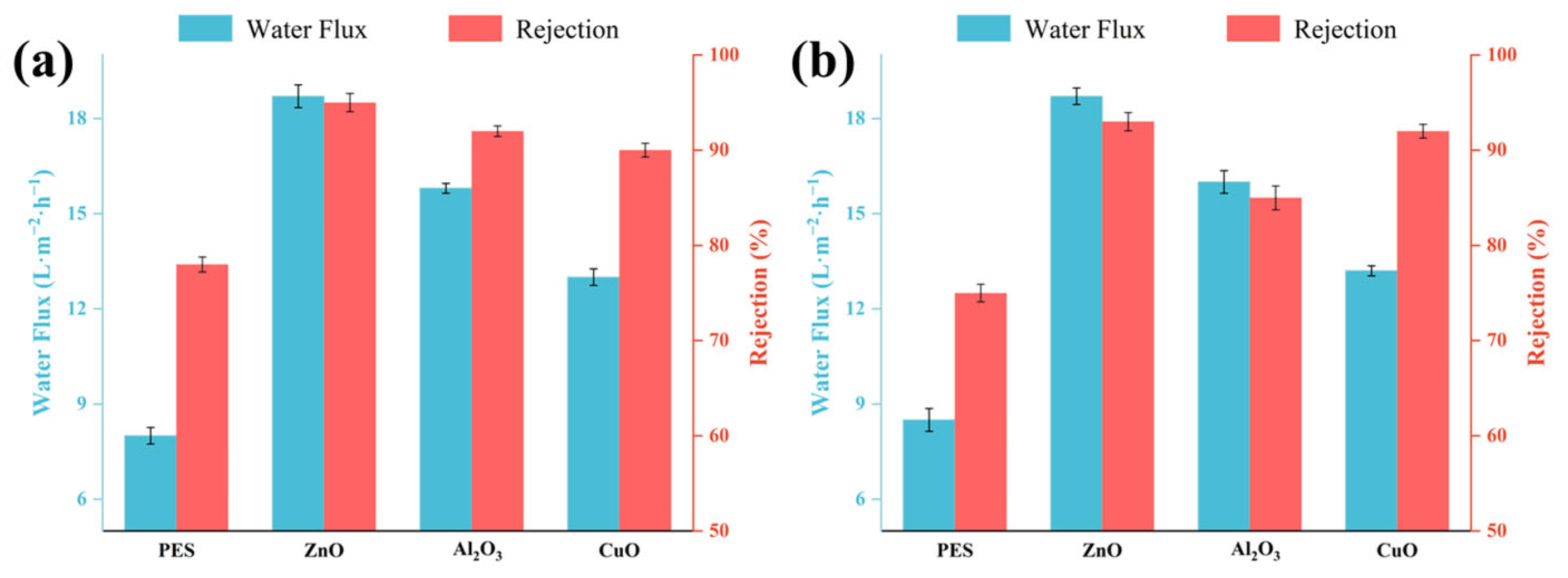
2.6. Effect of Metal Oxide Content on Membrane Separation Performance
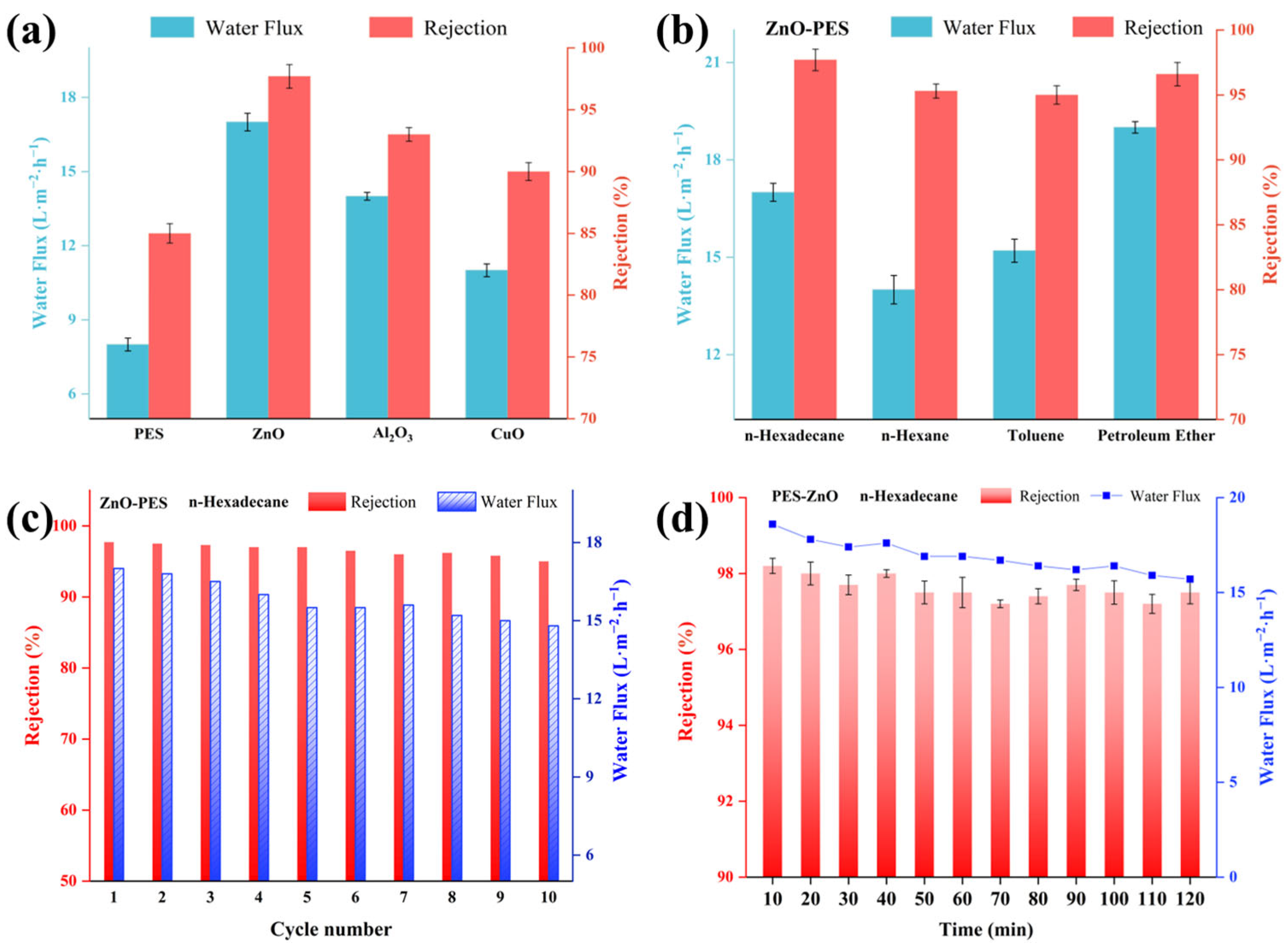
2.7. Rejection Performance of Modified Membranes for Trace Organic Compounds
2.8. Oil–Water Separation Performance and Antifouling Properties of Modified Membranes
3. Materials and Methods
3.1. Materials
3.2. Preparation of Modified Membranes
3.3. Preparation of Emulsified Oil
3.4. Characterization
3.5. Performance Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rout, D.R.; Jena, H.M.; Baigenzhenov, O.; Hosseini-Bandegharaei, A. Graphene-based materials for effective adsorption of organic and inorganic pollutants: A critical and comprehensive review. Sci. Total Environ. 2023, 863, 160871. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, X.; Gu, Z.; Tong, Y.; Meng, F.; Sun, L.; Liu, H.; Wang, Q. High-efficiency purification of alkali-surfactant-polymer flooding produced water by ultrasonication-ionic liquids combination: Performance and separation mechanism. Sep. Purif. Technol. 2025, 363, 132255. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, C.; Bi, J.; Qu, X.; Zhang, R.; Liu, X.; Yang, Y. Improved emulsified oil removal approach for industrial coal pyrolysis wastewater by flocculation under pressurized CO2 atmosphere. J. Clean. Prod. 2023, 418, 138213. [Google Scholar] [CrossRef]
- Khan, A.H.; Aziz, H.A.; Khan, N.A.; Hasan, M.A.; Ahmed, S.; Farooqi, I.H.; Dhingra, A.; Vambol, V.; Changani, F.; Yousefi, M.; et al. Impact, disease outbreak and the eco-hazards associated with pharmaceutical residues: A Critical review. Int. J. Environ. Sci. Technol. 2022, 19, 677–688. [Google Scholar] [CrossRef]
- Perumal, M.K.K.; Gandhi, D.; Renuka, R.R.; Lakshminarayanan, A.; Thiyagarajulu, N.; Kamaraj, C. Advanced nano-based adsorbents for purification of pharmaceutical residue polluted water: A critical review. Process Saf. Environ. Prot. 2024, 186, 552–565. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y.; Zhou, Y.; Zhu, J.; Chen, G. Fe3+-modified polyamide membrane with a porous network structure: Rapid permeation and efficient interception of trace organic compounds. Sep. Purif. Technol. 2025, 353, 128294. [Google Scholar] [CrossRef]
- Ma, Z.; Shu, G.; Lu, X. Preparation of an antifouling and easy cleaning membrane based on amphiphobic fluorine island structure and chemical cleaning responsiveness. J. Membr. Sci. 2020, 611, 118403. [Google Scholar] [CrossRef]
- Hu, Z.; Guan, D.; Sun, Z.; Zhang, Z.; Shan, Y.; Wu, Y.; Gong, C.; Ren, X. Osmotic cleaning of typical inorganic and organic foulants on reverse osmosis membrane for textile printing and dyeing wastewater treatment. Chemosphere 2023, 336, 139162. [Google Scholar] [CrossRef]
- Bogler, A.; Lin, S.; Bar-Zeev, E. Biofouling of membrane distillation, forward osmosis and pressure retarded osmosis: Principles, impacts and future directions. J. Membr. Sci. 2017, 542, 378–398. [Google Scholar] [CrossRef]
- Ong, C.S.; Al-anzi, B.; Lau, W.J.; Goh, P.S.; Lai, G.S.; Ismail, A.F.; Ong, Y.S. Anti-Fouling Double-Skinned Forward Osmosis Membrane with Zwitterionic Brush for Oily Wastewater Treatment. Sci. Rep. 2017, 7, 6904. [Google Scholar] [CrossRef]
- Almoalimi, K.; Liu, Y.-Q. Fouling and cleaning of thin film composite forward osmosis membrane treating municipal wastewater for resource recovery. Chemosphere 2022, 288, 132507. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-T.; Fu, P.; Li, W.-L.; Yu, Y.-H.; Zhang, Z.-L.; Fan, H.-Y.; Huang, X.-J.; Xu, Z.-K.; Wan, L.-S. Cross-linked g-C3N4 nanofibers enable thermal stable composite membranes for high-performance loose nanofiltration. Chem. Eng. J. 2024, 494, 153197. [Google Scholar] [CrossRef]
- Mohammed, S.; Aburabie, J.; Hashaikeh, R. A review on the potential of cellulose nanomaterials for the development of thin film composite polyamide membranes for water treatment. Chemosphere 2024, 363, 142927. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, K.; Arthanareeswaran, G.; Bose, A.C.; Kumar, P.S. Hydrophilic hierarchical carbon with TiO2 nanofiber membrane for high separation efficiency of dye and oil-water emulsion. Sep. Purif. Technol. 2020, 241, 116709. [Google Scholar] [CrossRef]
- Tian, E.; Wang, X.; Zhao, Y.; Ren, Y. Middle support layer formation and structure in relation to performance of three-tier thin film composite forward osmosis membrane. Desalination 2017, 421, 190–201. [Google Scholar] [CrossRef]
- Aniagor, C.O.; Igwegbe, C.A.; Iwuozor, K.O.; Iwuchukwu, F.U.; Eshiemogie, S.; Menkiti, M.C.; Ighalo, J.O. CuO nanoparticles as modifiers for membranes: A review of performance for water treatment. Mater. Today Commun. 2022, 32, 103896. [Google Scholar] [CrossRef]
- Chen, H.; Huang, M.; Wang, Z.; Gao, P.; Cai, T.; Song, J.; Zhang, Y.; Meng, L. Enhancing rejection performance of tetracycline resistance genes by a TiO2/AgNPs-modified nanofiber forward osmosis membrane. Chem. Eng. J. 2020, 382, 123052. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, L.; Zhang, J.; Wen, H.; Zhu, Z.; Wang, P.; Liu, Y.; Liu, C. Template-etched sodium alginate hydrogel as the sublayer to improve the FO performance with double barriers for high metal ion rejection. Chem. Eng. J. 2021, 413, 127425. [Google Scholar] [CrossRef]
- Soleymani Lashkenari, A.; Hamed Mosavian, M.T.; Peyravi, M.; Jahanshahi, M. Biofouling mitigation of bilayer polysulfone membrane assisted by zinc oxide-polyrhodanine couple nanoparticle. Prog. Org. Coat. 2019, 129, 147–158. [Google Scholar] [CrossRef]
- Huang, A.; Chen, L.-H.; Kan, C.-C.; Hsu, T.-Y.; Wu, S.-E.; Jana, K.K.; Tung, K.-L. Fabrication of zinc oxide nanostructure coated membranes for efficient oil/water separation. J. Membr. Sci. 2018, 566, 249–257. [Google Scholar] [CrossRef]
- da Silva, A.F.P.; Araújo, E.M.; de Lucena Lira, H.; da Ferreira, R.S.B.; da Nóbrega Medeiros, V.; Oliveira, S.S.L. Synthesis of Polysulfone/Alumina hollow fiber membranes for water treatment in the presence of indigo blue dye. Res. Soc. Dev. 2021, 10, e18610110863. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Karami, F.; Farahani, S.K.; Bandehali, S.; Shen, J.; Bagheripour, E.; Seidypoor, A. Tailoring the separation performance and antifouling property of polyethersulfone based NF membrane by incorporating hydrophilic CuO nanoparticles. Korean J. Chem. Eng. 2020, 37, 866–874. [Google Scholar] [CrossRef]
- Khezraqa, H.; Samiei, A.; Etemadi, H.; Salami-Kalajahi, M. Effect of Copper Oxide Nanoparticles on the Performance of Polyvinyl Chloride Membranes. Chem. Eng. Technol. 2023, 46, 2412–2422. [Google Scholar] [CrossRef]
- Kujawa, J.; Pianka, K.; Al-Gharabli, S.; Jankowski, W.; Flanc, Z.; Kujawski, W. Rare earth metal oxides smart modifiers in 3D re-entrant surface architecture for efficient membrane separation. Desalination 2024, 586, 117788. [Google Scholar] [CrossRef]
- Sevinchan, Y.; Hopkinson, P.E.; Bakulin, A.A.; Herz, J.; Motzkus, M.; Vaynzof, Y. Improving Charge Separation across a Hybrid Oxide/Polymer Interface by Cs Doping of the Metal Oxide. Adv. Mater. Interfaces 2016, 3, 1500616. [Google Scholar] [CrossRef]
- Dudek, G.; Gnus, M.; Strzelewicz, A.; Turczyn, R.; Krasowska, M. The influence of metal oxides on the separation properties of hybrid alginate membranes. Sep. Sci. Technol. 2018, 53, 1178–1190. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Amini, S.H.; Khodabakhshi, A.R.; Bagheripour, E.; Van der Bruggen, B. Activated carbon nanoparticles entrapped mixed matrix polyethersulfone based nanofiltration membrane for sulfate and copper removal from water. J. Taiwan Inst. Chem. Eng. 2018, 82, 169–178. [Google Scholar] [CrossRef]
- Chou, P.-I.; Ghim, D.; Gupta, P.; Singamaneni, S.; Lee, B.; Jun, Y.-S. Surface Functional Groups Affect Iron (Hydr)oxide Heterogeneous Nucleation: Implications for Membrane Scaling. Environ. Sci. Technol. 2023, 57, 11056–11066. [Google Scholar] [CrossRef]
- Hosakun, Y.; Halász, K.; Horváth, M.; Csóka, L.; Djoković, V. ATR-FTIR study of the interaction of CO2 with bacterial cellulose-based membranes. Chem. Eng. J. 2017, 324, 83–92. [Google Scholar] [CrossRef]
- Choi, M.J.; Park, C.K.; Lee, Y.M. Chelate membrane from poly(vinyl alcohol)/poly(n-salicylidene allyl amine) blend. II. Effect of co(II) content on oxygen/nitrogen separation. J. Appl. Polym. Sci. 1995, 58, 2373–2379. [Google Scholar] [CrossRef]
- Alsohaimi, I.H. Comparative study of PS and PES and their sulfonated forms in antifouling behavior and rejection efficiency. J. King Saud Univ. Sci. 2024, 36, 103576. [Google Scholar] [CrossRef]
- Thejaswini, H.C.; Agasanapura, B.; Hopwood, J. Deposition and characterization of ZnO films using microplasma at atmospheric pressure. Thin Solid Film. 2016, 603, 328–333. [Google Scholar] [CrossRef]
- Rafi, M.; Anwar, U.; Alnasir, M.H.; Ramzan, A.; Noor, N.A.; Mumtaz, S. Rietveld refined structural, optical and temperature dependent impedance spectroscopy of NiO–ZnO heterostructure composite: Synthesized through solid state method for high-frequency devices. Ceram. Int. 2024, 50, 38600–38609. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Wu, B.; Meng, J. A nano-Al2O3 modified polypropylene hollow fiber membrane with enhanced biofilm formation in membrane aerated biofilm reactor application. J. Environ. Chem. Eng. 2024, 12, 112524. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Aber, S.; Vatanpour, V.; Mahmoodi, N.M. Development of hydrophilic microporous PES ultrafiltration membrane containing CuO nanoparticles with improved antifouling and separation performance. Mater. Chem. Phys. 2019, 222, 338–350. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Song, Q.-W.; Mo, C.-R.; Yu, W.-W.; Hu, C.-Y. Effect of neutralization treatment on properties of chitosan/bamboo leaf flavonoids/nano-metal oxide composite films and application of films in antioxidation of rapeseed oil. Int. J. Biol. Macromol. 2023, 242, 124951. [Google Scholar] [CrossRef]

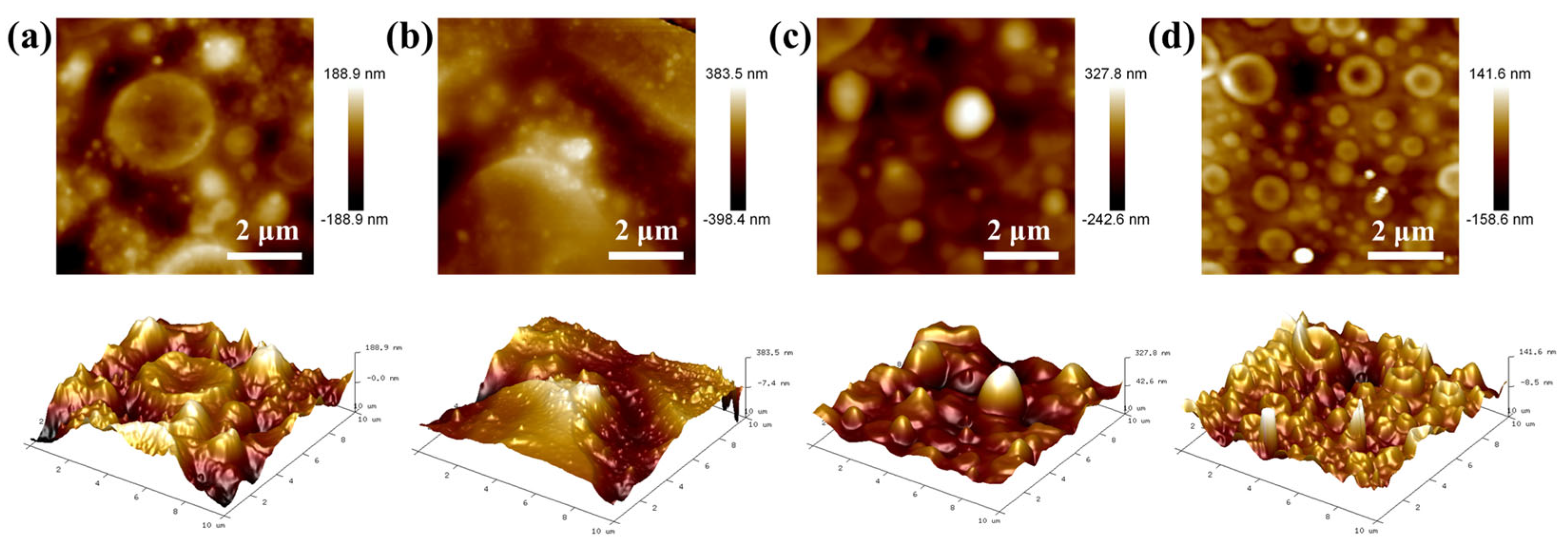

| Membrane Material | Ra (±SD)/nm | Rg (±SD)/nm | Rz (±SD)/nm | Surface Morphology |
|---|---|---|---|---|
| PES | 43.6 () | 56.2 () | 346 () | Ridge-valley Type |
| ZnO-PES | 83.7 () | 106 () | 733 () | Ridge-valley Type |
| Al2O3-PES | 52.7 () | 71 () | 532 () | Ridge-valley Type |
| CuO-PES | 28.0 () | 37.8 () | 426 () | Ridge-valley Type |
| Membrane Type | Zeta Potentials (±SD)/mV |
|---|---|
| PES-based Membrane | −12.9 () |
| ZnO-PES Membrane | −15.7 () |
| Al2O3-PES Membrane | −10.1 () |
| CuO-PES Membrane | −14.9 () |
| Membrane Type | PES (g) | PVP (g) | NMP (g) | ZnO (g) | Al2O3 (g) | CuO (g) |
|---|---|---|---|---|---|---|
| PES | 18 | 4 | 78 | |||
| ZnO-PES | 18 | 4 | 78 | 0.02 | ||
| 0.04 | ||||||
| 0.06 | ||||||
| 0.08 | ||||||
| 0.1 | ||||||
| Al2O3-PES | 18 | 4 | 78 | 0.02 | ||
| 0.04 | ||||||
| 0.06 | ||||||
| 0.08 | ||||||
| 0.1 | ||||||
| CuO-PES | 18 | 4 | 78 | 0.02 | ||
| 0.04 | ||||||
| 0.06 | ||||||
| 0.08 | ||||||
| 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Yang, W.; Xu, Y.; Sun, C.; Zhu, Y.; Chen, G. Metal Oxide-Modified PES Membranes for Efficient Separation of Oil-in-Water Emulsions and Trace Organic Compounds. Catalysts 2025, 15, 604. https://doi.org/10.3390/catal15060604
Li J, Yang W, Xu Y, Sun C, Zhu Y, Chen G. Metal Oxide-Modified PES Membranes for Efficient Separation of Oil-in-Water Emulsions and Trace Organic Compounds. Catalysts. 2025; 15(6):604. https://doi.org/10.3390/catal15060604
Chicago/Turabian StyleLi, Jinze, Wensheng Yang, Yang Xu, Chengfeng Sun, Yingying Zhu, and Geng Chen. 2025. "Metal Oxide-Modified PES Membranes for Efficient Separation of Oil-in-Water Emulsions and Trace Organic Compounds" Catalysts 15, no. 6: 604. https://doi.org/10.3390/catal15060604
APA StyleLi, J., Yang, W., Xu, Y., Sun, C., Zhu, Y., & Chen, G. (2025). Metal Oxide-Modified PES Membranes for Efficient Separation of Oil-in-Water Emulsions and Trace Organic Compounds. Catalysts, 15(6), 604. https://doi.org/10.3390/catal15060604









