Abstract
The discharge of oily wastewater threatens the ecosystem and human health, and the efficient treatment of oily wastewater is confronted with problems of high mass transfer resistance at the oil-water-solid multiphase interface, significant light shielding effect, and easy deactivation of photocatalysts. Although traditional physical separation methods avoid secondary pollution by chemicals and can effectively separate floating oil and dispersed oil, they are ineffective in removing emulsified oil with small particle sizes. To address these complex challenges, photocatalytic technology and photocatalysis-based improved technologies have emerged, offering significant application prospects in degrading organic pollutants in oily wastewater as an environmentally friendly oxidation technology. In this paper, the degradation mechanism, kinetic mechanism, and limitations of conventional photocatalysis technology are briefly discussed. Subsequently, the surface interface modulation functions of metal doping and heterojunction energy band engineering, along with their applications in enhancing the light absorption range and carrier separation efficiency, are reviewed. Focus on typical studies on the separation and degradation of aqueous and oily phases using photocatalytic membrane technology, and illustrate the advantages and mechanisms of photocatalysts loaded on the membranes. Finally, other new approaches and converging technologies in the field are outlined, and the challenges and prospects for the future treatment of oily wastewater are presented.
1. Introduction
With the rapid development of the global economy, environmental pollution caused by oily wastewater has become increasingly prominent, mainly including oilfield produced water, refinery wastewater, food and catering oil wastewater, and ship oil pollution wastewater. The composition of oilfield wastewater is quite complex, mainly including drilling cuttings, oils, high concentration of salts, heavy metals and surfactants [1,2]. The surfactants inside make oilfield wastewater an oil-in-water emulsion that is extremely difficult to be removed [3,4]. Oilfield wastewater is also characterized by poor biodegradability, high COD value, richness in salt and heavy metals [5].
Therefore, oily wastewater has become one of the key areas of research due to its complex composition, high treatment difficulty, and toxic high-concentration persistent pollutants, which cause serious harm. In the face of the increasingly severe problem of the ecological environment of the waters, China has introduced and improved continuously the relevant emission control standards. Cai et al. conducted a comprehensive review of the water pollutant discharge standards of river basins in China and presented an overview of their development and current status [6]. The pollutants in oily wastewater mainly include floating oil, dispersed oil, emulsified oil and dissolved oil, each of which has different physicochemical properties [7,8]. Among them, emulsified and dissolved oils are small in particle size and high in chemical stability, which makes traditional treatment methods (such as gravity separation, chemical flocculation, biodegradation, etc.) less efficient in removing these pollutants, and may even lead to secondary pollution [9,10]. The discharge of untreated oily wastewater into the environment may trigger eutrophication of water bodies, decrease in dissolved oxygen, and cause long-term harm to aquatic ecosystems and human health. For example, petroleum hydrocarbons can enter the food chain through bioconcentration, leading to chronic toxicity, cumulative effects on organisms and even carcinogenic risks [11]. Therefore, the development of efficient and sustainable oily wastewater treatment technologies has become a current research hotspot in the field of environmental governance. Photocatalytic technology is regarded as a potential solution to crack the problem of oily wastewater treatment by virtue of its green, low energy consumption and deep mineralization advantages [12]. However, the complex interactions at the oil-water-solid multiphase interface present three major challenges to traditional photocatalytic systems: the light shielding effect, limitations in mass transfer kinetics, and irreversible deactivation of the photocatalyst.
In recent years, researchers have made significant progress in broadening the photo-response range and improving the carrier separation efficiency through metal doping [13], heterostructure construction [14] and other strategies. However, it is difficult to break through the interfacial mass transfer barrier of oil degradation by single material modification, and there is an urgent need to integrate the technology from micro-mechanism to macro-system. Only by crossing the gap between material design and technology integration, can the engineering breakthrough of photocatalytic technology be realized.
In this paper, we focus on the interfacial synergistic mechanism and innovative optimization of photocatalytic degradation of oily wastewater, and analyzing the cross-scale correlation between doping modification, heterojunction engineering and photocatalytic membrane coupling technology. It elucidates the microscopic mechanism of oily wastewater degradation, the principle of photocatalysis and the connotation of dynamics fitting, provides theoretical support and technical solutions to solve the problems of photocatalyst deactivation, high energy consumption and poor water quality fluctuation in the practical engineering, and finally summarizes some new methods and convergent technologies for the treatment of oily wastewater in anticipation of injecting a new momentum into the development of the technologies for oily wastewater degradation.
2. Challenges and Technologies for Degrading Oily Wastewater
2.1. Complexity of Oily Wastewater
As a typical multi-phase and multi-component dispersed system in the oily wastewater, the complexity stems from the physical mixing of petroleum hydrocarbons and their derivatives, as well as the dynamic chemical interactions and ecological synergistic effects between pollutants. From the molecular level, hydrophobic components such as polycyclic aromatic hydrocarbons (PAHs) and benzene, toluene, ethylbenzene, and xylene (BTEX) in the oil phase are embedded in the core of emulsified oil droplets through micellar solubilization, forming quasi-stable colloids with particle sizes ranging from 0.1–100 μm, and the surface charge is regulated by the ionic strength of seawater, which results in the ζ-potential inversion of the surface [15], leading to a sudden decrease in the efficiency of the microbubble adsorption in the traditional air flotation process. Moreover, the interfacial interaction significantly affects the material transfer and reaction efficiency. Due to the high viscosity and low diffusivity of the oil phase, mass transfer resistance in water is high, and a covering layer is easily formed on the surface of the photocatalyst, hindering the diffusion of active oxide species in the water phase to the oil molecules and their contact with the photocatalyst [16]. The problem is made worse by the imbalance between interfacial tension and wettability, where the hydrophobic photocatalyst surface is susceptible to be covered by the oil phase, forming a continuous oil film encapsulating the photocatalyst particles and restricting the diffusion of aqueous-phase reactants to the active sites [17]. In addition, the size mismatch between oil droplets and photocatalyst particles [18]. This leads to insufficient length of the three-phase contact line, limiting the effective area of the reaction interface. The absorption and scattering effects of oil-phase contaminants on incident light result in the light energy not reaching the photocatalyst surface effectively, severely weakening the driving force of the photocatalytic reaction [19]. These factors have a synergistic effect, severely restricting the material transfer and reaction efficiency of the multiphase system in oily wastewater.
Secondly, metal ions generated during the production process form lipophilic metal-organic complexes by chelating with thiol groups in oil additives. These substances can penetrate the oil-water interface film to accelerate the stabilization of the emulsion system. When wastewater is discharged into water, they can also accumulate in the hepatopancreas of marine organisms through the bioaccumulation effect, triggering the abnormal activation of the PAHs metabolic enzyme CYP1A and DNA adduct generation [20]. PAHs metabolism enzyme CYP1A abnormal activation and DNA adduct generation. More seriously, although international maritime regulations limit the bilge water oil concentration (≤15 ppm) [21], they do not consider adequately the ecological risk equivalent of dissolved PAHs in the oil phase: the equivalent concentration of benzo[a]pyrene detected continuously after the Deepwater Horizon accident confirms that emulsified oil droplets, even if compliant with the emission standards, can still carry genotoxic and highly cyclic PAHs carry genotoxic, highly cyclic PAHs (4–6 rings) in the water column for long periods of time [22]. This physicochemical-toxicological composite mechanism makes the existing treatment technology system based on oil droplet size distribution and total oil concentration control face fundamental challenges. There is an urgent need to develop a multistage coupled treatment process that can deconstruct the oil-water-solid three-phase interface and target degradation simultaneously of oily wastewater.
The multiphase oil forms of oily wastewater typically include high concentrations of dispersed oils, emulsified oils, suspended particles, etc. (Table 1). Among them, emulsified oils have high stability due to electrostatic repulsion and interfacial films that are difficult to coalescence. The chemical treatment and biodegradation of dissolved oil has a long cycle and high cost, the mixing of different oils requires multi-stage treatment, the difficulty of technical synergy is high, the coagulation technology may bring ecological hazards, and the traditional treatment technology has high energy consumption, which is not conducive to sustainable development.

Table 1.
Description of the main pollutants in marine oily wastewater.
2.2. Limitations of Traditional Techniques
Physical separation methods are usually used for the in-situ treatment of oily wastewater, including gravity separation, gravity-coagulation separation and filtration separation, etc. (Figure 1). It avoids the secondary pollution of chemical agents, and can separate floating oil and dispersed oil effectively, but cannot remove emulsified oil effectively that particle size less than 20 μm. Moreover, the widespread use of additives such as surfactants on ships means that oily water from ships is more likely to be emulsified. The adhesion of emulsified oil droplets is enhanced by hydrophobicity, which makes physical separation more difficult [23]. Therefore, the effective treatment of emulsified oil has become one of the limiting factors for the efficient treatment of oily sewage on ships. In addition, the high salinity content of oily sewage from ships is often mixed with seawater [24], which further increases the difficulty.
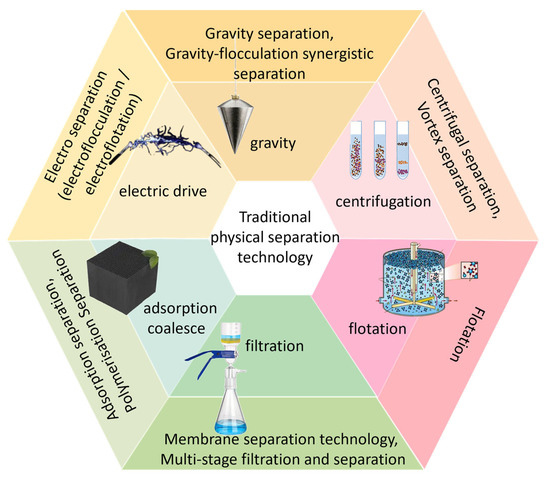
Figure 1.
Commonly used physical separation methods.
Gravity separation technology achieves natural stratification through oil-water density difference, simple structure, low maintenance cost, can separate small flow wastewater, but the separation effect is not very satisfactory, the efficiency is affected by the configuration of the internal components, the traditional API design must be optimized to adapt to small-scale scenarios [25]. Researchers have found that gravity separation technology can be combined with membrane separation [26,27,28]. For example, ions loaded onto stainless steel mesh can be used for gravity-driven separation of complex oily wastewater [29], and the oil removal efficiency can be further improved with technological improvements.
Gravity-flocculation synergistic separation technology [30] includes a tank and multiple zones arranged sequentially within the tank: the impurity separation zone intercepts particulate impurities in the influent, the flocculation zone mixes chemical agents, the water distribution zone ensures smooth water flow into the oil-water separation zone, and so on. The collected floating oil is recovered through a floating oil collection device, and the effluent meets discharge or reuse standards. Zhao et al. [31] proposed an axial separator using a multi-stage separation mode for oil-water separation. They verified the feasibility of multi-stage separation through an experimental platform. The simulation results of the mixed model and the Reynolds stress model were in good agreement with the experimental results, and ultimately achieved a separation efficiency of over 93%.
In addition, membrane separation technology uses ultrafiltration, which has good retention properties but is expensive [32], while reverse osmosis membranes need to solve the problem of long-term oil contamination [33], which achieves oil-water separation through size exclusion effect. Aggregation and separation had a wide range of applications, allowing for the combination of oversized particles, ultra-high concentrations, and surfactants [34]. Electrocoagulation (EC) process is designed to overcome the drawbacks of conventional wastewater treatment techniques and is very effective in removing organic pollutants including dye wastewater. It can reduce sludge generation and effectively separate heavy metal ions such as iron, nickel, copper and zinc [35]. Other centrifugal separation, Such as air flotation separation techniques, adsorption separation, and vortex separation, also have their own characteristics, but none of them are effective when used alone, so there is an urgent need to develop a degradation technology with good removal effect. The discussion below covers the topic of photocatalytic technology, which has received a great deal of attention in recent years.
3. Mechanism of Degradation of Oily Wastewater by Photocatalysts
The complex components and stable emulsion structure of oily wastewater make it difficult to effectively remove the oil pollutants in traditional physical, chemical and biological treatment methods. In recent years, photocatalytic technology has become a research hotspot for the deep treatment of oily wastewater due to these characteristics of high efficiency, sustainable and environmentally friendly [36]. The multiphase catalytic reaction involves several steps, such as diffusion of reactants from the bulk phase to the photocatalyst surface, adsorption of reactants, catalytic reaction, product resolution and product diffusion [37,38]. Photocatalysts can generate active species with high oxidative capacity by absorbing the light energy and stimulating the electron-hole pairs (e−/h+), and then generate active species with high oxidative capacity (such as •OH and )), which can degrade difficult-to-degrade organic pollutants effectively in oily wastewater and achieve complete mineralization of pollutants. However, the industrial application of photocatalysts still faces challenges due to their low quantum efficiency, narrow light response range, and susceptibility to photogenerated carrier complexation. Therefore, an in-depth study of the basic mechanisms and kinetic characteristics of the photocatalytic degradation of oily wastewater, as well as optimizing the performance of photocatalysts, is of great theoretical and engineering value.
The core mechanism of photocatalytic degradation of oily wastewater relies on the excitation and redox reaction of photogenerated electron-hole (e−/h+) pairs in semiconductor materials (TiO2, WO3, Cu2O, ZnO, SnO2, g-C3N4, etc.) [39,40,41,42,43,44]. This process involves several key steps, including photoexcitation, carrier migration, complexation, and redox reactions, which are interrelated and together determine the efficiency of photocatalytic degradation and mineralization of the final effect. The reaction principles and formulas of some photocatalysts are listed, as shown in Figure 2.
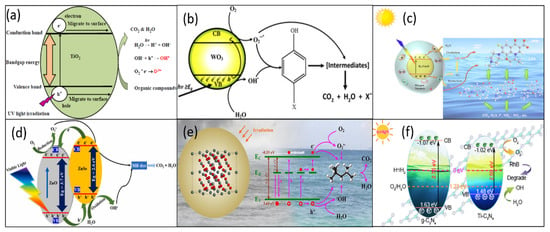
Figure 2.
(a) TiO2 degradation of organic matter [39]. (b) WO3 degradation of phenolic actives [40]. (c) K2-Cu2O sample on levofloxacin [41]. (d) ZnO/ZnSe photocatalysts for degradation of methylene blue dyes [42]. (e) Anoxic SnO2 degradation of oil pollutants [43]. (f) Degradation of RhB by Ti doped g-C3N4 [44].
3.1. Photocatalytic Degradation Process
- (1)
- Photoexcitation
The basic working principle of photocatalysts is based on the absorption of light by semiconductor materials. When a semiconductor photocatalyst absorbs photons with energies greater than its forbidden bandwidth, the electrons (e−) in the valence band (VB) are excited into the conduction band (CB). Equation (1) shows the basic reaction that forms electron-hole pairs (e−/h+).
In Equation (1), hv represents the energy of the incident photon, e− is the photogenerated electron, and h+ is the photogenerated hole, and the band gap of the photocatalyst determines its range of response to light. Table 2 lists the effect of the band gap energy of photocatalysts on the degradation of oily wastewater.

Table 2.
Effect of the band gap energy of photocatalysts on the degradation of oily wastewater.
- (2)
- Carrier migration and complexation
After the formation of photogenerated electron-hole pairs, they undergo two possible pathways: electrons (e−) and holes (h+) migrate to the surface of the catalyst and undergo redox reactions with adsorbed O2 or H2O (Equations (2) and (3)), respectively, to produce reactive oxygen species. The recombination of electron-hole pairs causes energy loss, thereby reducing photocatalytic efficiency.
Since carrier complexation significantly reduces the catalytic efficiency, the optimization of the electronic structure of the photocatalysts to extend the carrier lifetime is the key to improve the photocatalytic degradation efficiency. Common optimal strategies include the formation of Schottky barriers by noble metal nanoparticles to promote the directional transfer of electrons, electron-hole separation through band gap modulation, and the enhancement of carrier mobility by means of oxygen vacancies, metal ion doping, and so on.
- (3)
- Redox reactions
Oil compounds primarily consist of complex macromolecules such as long-chain alkanes, alkenes, aromatic hydrocarbons, esters, and others. Photocatalytic degradation is a stepwise oxidation process. Initially, the process targets the terminal ends or weak bond sites of molecular chains, generating oxygen-containing intermediates such as alcohols, aldehydes, and ketones [51]. Then, the intermediates undergo further oxidation and cleavage, forming short-chain carboxylic acids (such as formic acid, acetic acid, oxalic acid, etc.) and small-molecule aldehydes and ketones. Finally, hydroxyl radicals () and superoxide radicals () can non-selectively attack short-chain organic compounds in oily wastewater, causing their oxidative degradation and ultimately converting them into CO2 and H2O. The overall reaction process can be summarized as Equation (4).
Wang et al. [51] explored the degradation of road oil stains using a photocatalyst (nano-TiO2), finding that after 24 h of UV irradiation, crude oil degradation exceeded 90%. They also demonstrated that the reaction is light-driven rather than heat-driven, and that beyond a light intensity of 30 W/m2, degradation efficiency no longer changes due to increased electron-hole recombination. Nano-TiO2 tends to agglomerate, but its specific surface area can be increased through modification, thereby enhancing the number of active sites for the reaction. King et al. [46] found that large-molecule polycyclic aromatic hydrocarbons (PAHs) in the oil from the Deepwater Horizon spill degraded faster, as their larger specific surface area absorbed more UV light, generating more reactive oxygen species. Hydrophobic-treated TiO2 significantly enhanced the degradation of small-molecule PAHs while inhibiting the degradation of large-molecule PAHs, as the enhanced effect of TiO2 photocatalysts is size-dependent and subject to a light-shielding effect. Additionally, it was demonstrated that modifying the surface of photocatalysts can enhance their adsorption capacity for oil molecules (e.g., hydrophobic modification, surfactant-assisted modification, loading onto porous carriers, etc.), and close adsorption contact can effectively enhance hole oxidation and radical attack. Furthermore, Mohd et al. [52] focused on the specific environmental issue of diesel pollution in the Malacca Strait and systematically and quantitatively analyzed the key parameters influencing the efficiency of photocatalytic diesel degradation. They first clearly proposed the priority order of parameter influences (calcination temperature > pH > initial concentration > dosage) and provided an in-depth explanation of the core role of calcination temperature. Higher calcination temperatures (e.g., 700 °C and 900 °C) significantly increase crystallinity, thereby enhancing light absorption capacity and promoting the generation and separation of photo-generated electron-hole pairs. This ultimately leads to higher diesel degradation efficiency (60–80%). The Sapawe team is an active researcher and key promoter of green synthesis of nano-photocatalysts for environmental remediation, particularly in oil-contaminated wastewater treatment. They have successfully synthesized various metal oxide nanoparticles (such as ZnO [53], NiO [54], SiO2 [55], and ZrO2 [56]) and their composites using natural plant extracts as reducing agents and stable agents. Recently, they investigated the effects of microwave irradiation time [57] and microwave power [58] on ZnO particles, discovering that these factors can enhance the photocatalytic degradation activity of phenol. Diesel fuel has a complex composition (containing various saturated hydrocarbons, aromatic hydrocarbons, etc.), and its degradation is typically more challenging than that of a single dye, requiring a stepwise approach. The team’s technology is targeted in terms of application scope and addressing real-world environmental issues, continuously driving the development of photocatalytic technology in the field of oil-contaminated wastewater treatment.
The catalytic degradation efficiency of oil-contaminated wastewater is highly dependent on the form of the oil contamination. For emulsified oil, the active free radicals generated during the photocatalytic process not only degrade oil molecules but also attack emulsifier molecules, disrupting the hydrophilic-hydrophobic balance of oil droplets, making them easier to coalesce and be adsorbed and degraded by the photocatalyst. If the photocatalyst cannot penetrate the core of the oil droplets, interfacial oxidation is required, where larger oil droplets gradually split into smaller droplets, increasing the specific surface area and accelerating degradation. When combined with membrane technology, membrane physical retention followed by concentration of the oil phase and subsequent catalytic degradation can enhance efficiency. Dissolved oil can be directly mineralized. However, free oil/oil-solid mixtures first require addressing mass transfer limitations. Strongly oxidative systems can break organic-inorganic bonds, but due to large oil layers obstructing photocatalyst diffusion, auxiliary degradation techniques such as adsorption, ultrasonication, or mechanical stirring are needed.
3.2. Kinetic Mechanism
The photocatalytic degradation of oily wastewater involves complex physicochemical reactions, and its kinetic mechanism is affected by a variety of factors, such as pollutant species, photocatalyst performance, light intensity and solution environment. In order to quantitatively describe the photocatalytic degradation process, quasi-primary kinetic model, pseudo-primary kinetic model, Langmuir-Hinshelwood (L-H) kinetic model, and pseudo-secondary kinetic model are usually used for the analysis.
In conventional suspended-phase photocatalytic systems (e.g., TiO2 particle suspensions), the reaction kinetics usually follow the L-H model [59,60] with the Equation (5):
where, r denotes the degradation constant, k denotes the reaction rate constant, Kc denotes the adsorption equilibrium constant, and C0 denotes the concentration of the oily water. Under low concentration conditions (KC ≪ 1), the equation can be simplified as r ≈ , which exhibits quasi-level kinetics, implying that the reaction rate is proportional to the pollutant concentration. Under high concentration conditions (KC ≫ 1): the equation is simplified as r ≈ k, which exhibits zero-level kinetics, implying that the reaction rate of the concentration is independent and is limited by the active sites on the surface of the photocatalyst or the rate of the photogenerated carrier production. Reaction rate kinetics determine the rate of pollutant degradation and reveal the dependence of reaction rate on reactant concentration [61]. The apparent reaction kinetics of photocatalysts are influenced by various factors, such as membrane module filling density, temperature, photocatalyst dosage, and light source type. The data in Table 3 can prove this.

Table 3.
Photocatalyst kinetic reaction table.
4. Photocatalytic Materials Surface Interface Modulation
The surface interface modulation of photocatalytic materials is the core strategy to enhance their light absorption, charge separation efficiency and surface reaction activity, which involves the comprehensive enhancement of surface chemistry, physical structure and interfacial band engineering. The main strategies include metal doping, non-metal doping, heterogeneous structure building, interfacial energy band engineering, morphology and microstructure design and surface hydrophobicity modulation. The breakthrough of the efficiency bottleneck of traditional materials is of great significance in promoting the practical application of photocatalytic technology in energy and environment fields. The development of photocatalytic coupled nanomaterials has greatly contributed to the efficient degradation of oily wastewater. Japanese scientists Kenichi Honda and Akira Fujishima [64] first discovered that TiO2 could decompose water under UV irradiation in 1972, which initiated the research boom of modern photocatalytic technology. In 2002, Ziolli et al. [65] reported that nano TiO2 photocatalytic degradation performance of marine oily wastewater, which laid the foundation for the application of TiO2-based photocatalysts in oily wastewater treatment.
Although TiO2 photocatalytic activity is high, stable and eco-friendly, its application is still limited by the large forbidden bandwidth (Eg ≈ 3.2 eV, which only responds to ultraviolet light), the high rate of electron-hole pair complexation, the tendency of the catalysts to agglomerate, and the photocatalytic degradation efficiency limited by the light conditions. Other photocatalysts have similar shortcomings. To overcome these shortcomings, researchers have developed a series of methods, including nanometal doping and heterostructure building, which can enhance the stability of photocatalysis effectively and lay the foundation for the application of photocatalysts in oily wastewater treatment.
4.1. Doping of Nanometals
Nanometals (Au [66], Ag [67], Fe [47], Pt [48], etc.) have been widely used in photocatalyst modification studies due to their excellent electron transport ability, surface plasmon resonance effect, and excellent catalytic activity. The introduction of noble or transition metals can not only expand the spectral response range of photocatalysts, but also inhibit the complexation of photogenerated electron-hole pairs effectively and improve the quantum efficiency of the photocatalysts, thus enhancing the photocatalytic degradation of organic pollutants [66]. In the study of photocatalytic degradation of oily wastewater, nano-metal doping technology has been shown to improve the stability of the photocatalysts, reduce the agglomeration phenomenon, and enhance the degradation of difficult-to-degrade oil pollutants.
Hu et al. [67] prepared 1 at% Ag (Figure 3a) and 3 at% Li (Figure 3b) doped ZnO photocatalysts by co-precipitation, sonochemistry and systematically investigated their catalytic performance in degrading diesel. The experiments showed that both Li-ZnO and Ag-ZnO exhibited higher photocatalytic activities compared with undoped ZnO, among which Li-ZnO showed better degradation efficiency under sunlight irradiation, and finally the floating 3 at% Li/ZnO/polypropylene multifaceted spherical photocatalysts were successfully prepared (A in Figure 3c), and degradation rates of the diesel fuel and crude oil are 65% and 58% respectively in 8 h. The photocatalytic performance and degradation rate of 3% Li/ZnO/polypropylene floating-type polyhedral spherical photocatalysts were also studied. This was attributed to the fact that Li doping led to the lattice distortion of ZnO and introduced additional electronic states within the forbidden band, which improved the photogenerated electron migration rate, reduced the chances of electron-hole pair complexation, resulted in a lower bandgap and enhanced visible light absorption. Liu et al. [68] prepared Ag-TiO2 nanofiber membranes with silver on the surface, primarily utilizing the surface plasmon resonance (SPR) effect. In contrast, Merdoud et al. [69] used a solid-state method to synthetic Ag-TiO2 with silver incorporated into the crystal lattice, reducing the bandgap by forming defect levels. The surface deposition of silver may be more suitable for oily wastewater, as its large specific surface area nanofiber structure facilitates the adsorption of oil molecules. However, the stability of silver in the lattice-doped scheme may be an issue, especially in complex water quality environments. These studies indicate that alkali metal doping can effectively optimize the electronic structure of photocatalysts, enhancing their performance in the photocatalytic degradation of oil pollutants.

Figure 3.
(a) 1 at% Ag/ZnO, TEM image after 700° calcination, (b) 3 at% Li/ZnO, TEM image after 700° calcination, (c) A is the loaded photocatalyst obtained by coupling agent hair preparation, and B is the unloaded polypropylene multifaceted sphere [67].
On the other hand, Ghasemi et al. [47] conducted a study involving dual modification. First, iron atoms were incorporated into the ZSM-5 zeolite framework via hydrothermal synthesis (forming the Fe-ZSM-5 carrier), followed by loading nano-TiO2 onto it using the sol-gel method (Figure 4a). This structure is not a simple physical mixture. XRD and FT-IR data confirm that iron has entered the zeolite lattice (no free iron oxide peaks), and TiO2 has formed Ti-O-Si/Ti-O-Fe chemical bonds with the carrier. It was found that the photocatalyst could remove up to 80% of chemical oxygen demand (COD) from refinery wastewater after 3 h of UV irradiation. As shown in Figure 4b, the peaks disappeared significantly after photocatalytic treatment under ultraviolet light and sunlight, indicating that the majority of pollutants were degraded. Such an effect stems from the fact that Fe exists mainly in the form of Fe3+ and introduces oxygen vacancies within the TiO2 lattice, which improves the carrier migration efficiency, and the Fe doping significantly reduces the charge transfer impedance of TiO2, which enhances the catalytic activity and long-term stability. The carrier features an ingenious design in which the Fe-ZSM-5 exhibits photocatalytic activity, forming a synergistic effect with the TiO2. Characterization reveals that TiO2 loading results in the formation of a mesoporous structure with a specific surface area of 304.6 m2/g. This structure retains microporous adsorption capacity while facilitating mass transfer. This demonstrates the catalyst’s highly efficient sunlight-driven degradation capabilities, and its ease of recovery makes it a practical solution for the treatment of industrial oily wastewater.
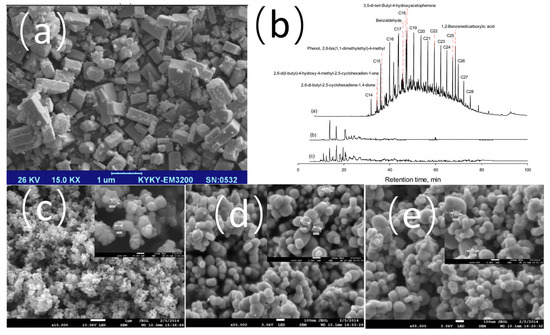
Figure 4.
(a) TEM image of Fe-ZSM-5 endo-photocatalyst, (b) GC/MS chromatograms of wastewater before photocatalytic treatment under UV light, after treatment and under sunlight at optimal conditions [47]. (c–e) 0.25 wt%,0.5 wt% Pt/TiO2 and 1.0 wt% Pt/TiO2 [48].
Cheng et al. [48] investigated the photocatalytic degradation performance of Pt nanoparticles loaded with TiO2 (Figure 4c,e) with different mass fractions (0.25 wt%, 0.5 wt%, and 1.0 wt%) on palm oil effluent (POME). Research has shown that when the Pt mass fraction is 0.5 wt% and the photocatalyst loading is 1.0 g/L, the photocatalytic degradation rate reaches a maximum of 90% within 8 h. Although Pt only slightly reduces the bandgap (3.20 eV vs. 3.16 eV), the visible light activity is significantly enhanced (11% vs. 2%). This differs from the mechanism of other metal dopants, which significantly alter the bandgap, suggesting that Pt’s primary role may be to promote charge separation rather than broaden the spectral response. Recent studies have found that exposure to high-energy crystal planes (such as TiO2 (101)) can improve the dispersion and stability of the active component Pt [70]. The mechanism lies in the fact that the (101) crystal face has a higher surface energy, which anchors Pt (OH)2 via covalent bonds, directly leading to a 7.55-fold increase in Pt dispersion (1.82 atoms/nm2 vs. 0.24 atoms/nm2), thereby improving toluene degradation efficiency by 60%. Bi et al. [71] used defective TiO2 as a carrier to prepare Pt-TiO2 catalysts. By introducing oxygen vacancies into TiO2 via high-energy electron beam irradiation, the catalyst’s bandgap was reduced from 2.67 eV to 2.47 eV, resulting in a threefold increase in photocurrent and promoting the •OH generation pathway. Crystal plane regulation optimizes the dispersion of active sites, defect engineering enhances oxidation capacity, and photothermal synergy significantly improves the degradation efficiency of oily wastewater. Future research could focus on the design of bifunctional catalysts (e.g., Pt-TiO2 (101)/Ov) to simultaneously address adsorption, photoresponse, and deep mineralization issues.
In addition to monometallic doping, bimetallic modifications have received extensive attention in photocatalytic studies. Cybula et al. [66] prepared gold-doped titanium dioxide (Figure 5a), palladium-doped titanium dioxide, and titanium dioxide doped with both metals (Figure 5b,c). Figure 5d illustrates that the 0.5 Pd_1.25 Au/TiO2 prepared by calcination at 350 °C exhibits excellent degradation effects on aqueous phenol and gaseous toluene after 60 min of visible light irradiation. In this study, under UV irradiation, the degradation efficiency of Au-Pd/TiO2 even reached 100% after 20 min, which was significantly higher than that of the monometallic-doped Au-TiO2 (89.2%) and Pd-TiO2 (55.6%) (Figure 5e). This study is the first systematic investigation of the effect of calcination temperature on gold-palladium bimetallic-modified rutile-phase TiO2, and innovatively uses a 415 nm LED light source for the degradation of toluene in the gas phase. Visible light LEDs consume significantly less energy than traditional xenon lamps, making them more suitable for practical applications. The selection of toluene and phenol as model pollutants is also highly representative, with the former being a volatile organic compound (VOC) and the latter a water-phase difficult-to-degrade organic compound, thereby covering the two typical scenarios of oily wastewater. They found that the activity of the bimetallic catalyst was highest at low-temperature calcination (350 °C). Contrary to the traditional view that high temperatures enhance crystallinity, this study demonstrated that high temperatures cause palladium to segregate to the surface, forming a PdO shell layer, which instead hinders the synergistic effect between gold and palladium. This conclusion aligns with the Sapawe team’s emphasis on the importance of calcination temperature. However, it provides a more refined mechanism of action, suggesting that temperature should be adjusted based on the metal combination to maintain the alloy structure, rather than simply pursuing high or low temperatures. However, the catalyst loading in the study was only 0.5–1.25 mol%, and activity decreased after repeated use, indicating that both economic viability and stability still have room for improvement.
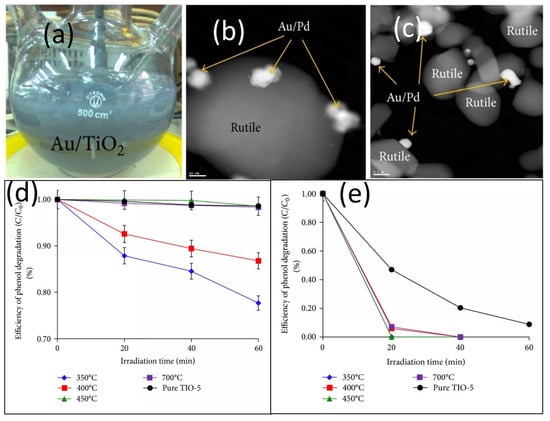
Figure 5.
(a) Au-modified TiO2, (b,c) High-resolution HAADF images of Au/Pd nanoparticles deposited on rutile surface examples calcined at 350 °C and 700 °C, Phenol degradation efficiencies of 0.5 Pd_1.25 Au/TiO2 samples calcined at different temperatures: (d) in the visible light and (e) under UV-Vis. light [66].
Zielińska-Jurek et al. [72] further prepared Ag-TiO2, Au-TiO2 and Ag/Au-TiO2 precious metal-based composite photocatalysts (Figure 6b,d) by microemulsion method and investigated their photocatalytic activity systematically in phenol degradation. It was found that the sample containing 1.5 mol% Ag-0.5% Au showed the best photocatalytic activity, with about 65% of phenol degraded after 3 h of irradiation under visible light (Figure 6a). And the photodegradation of phenol under visible light was increased by 80% for the sample containing 4.5 mol% Ag-1.5 mol%Au_5 (Figure 6c). The Ag/Au-TiO2 composite catalysts showed significantly higher photocatalytic activity than monometallic Ag-modified or Au-modified TiO2. As shown in Figure 6, this is a schematic diagram of the degradation mechanism of phenol on the surface of Ag/Au-TiO2 nanoparticles. The catalytic activity was superior when the Ag content was higher than that of Au. This result indicates that bimetallic doping can effectively enhance the catalytic activity of the photocatalysts in the visible light region, and reveals the key role of Ag in improving the photocatalytic performance of the composites.
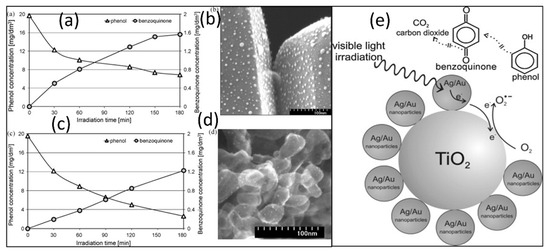
Figure 6.
(a) Efficiency of phenol degradation and benzoquinone formation under visible light in the presence of Ag/Au-TiO2-4, (b) STEM image of Ag/Au nanoparticles deposited on the surface of TiO2-5 (sample Ag/Au-TiO2-4), (c) efficiency of phenol degradation and benzoquinone formation under visible light in the presence of Ag/Au-TiO2-5, (d) STEM images of Ag/Au nanoparticles deposited on the TiO2-6 surface (sample Ag/Au-TiO2-5), (e) mechanism of phenol degradation on the surface of Ag/Au-TiO2 nanoparticles [72].
Additionally, Rahman et al. [73] found that when K-TiO2 is prepared using the electrochemical doping method, the entry of K+ into the TiO2 lattice triggers multiple effects, including a reduction in the bandgap, the reduction of Ti4+ to Ti3+/Ti2+ to form electron traps, an increase in surface hydroxyl groups, and enhanced adsorption efficiency due to the preferential orientation of the crystal along the (004) plane. Hajri et al. [74] used the initial wet impregnation method to prepare Sr-TiO2, where strontium doping enhances performance through different mechanisms. For example, at 0.8 wt% Sr, the formation of Sr-TiO2 heterojunctions promotes charge separation, increases oxygen vacancy concentration, and expands the unit cell volume to provide more active sites. Ultimately, the formic acid degradation rate increased by 3.36 times. Beltrán et al. [75] replaced Ti4+ with Cd2+ to generate oxygen vacancies, while the amino/hydroxyl groups of chitosan improved dispersion through chemical bonding (C-O-Ti). Interestingly, despite a 7.7-fold decrease in specific surface area, catalytic efficiency increased by 45%, indicating that surface chemical properties have a greater impact than physical structure. Additionally, the team used food-grade TiO2 and shrimp shell-derived chitosan, a low-cost raw material choice that holds significant potential for the engineering of oil-containing wastewater treatment.
Overall, research on metal-doped photocatalysts in environmental remediation has primarily focused on the degradation of single organic pollutants (e.g., phenols, dyes), with advantages including a simple system, clear reaction mechanisms, and easily quantifiable degradation kinetics. However, research on photocatalytic treatment of actual oily wastewater remains lagging, primarily constrained by three core challenges: first, the coexistence of oils, emulsifiers, and suspended solids forms a multi-phase system that easily obscures catalyst active sites while hindering photon penetration, significantly reducing light utilization efficiency. Second, traditional kinetic evaluation metrics based on the degradation of single pollutants (such as pseudo-first-order rate constants) are difficult to adapt to the comprehensive water quality parameters (such as COD and BOD) that need to be considered in the treatment of oily wastewater. Third, oil contamination on the catalyst surface leads to hydrophobic modification, further hindering the diffusion of pollutants and the contact of active species, causing rapid catalyst deactivation. Through a three-pronged innovative approach—enhancing anti-pollution capabilities via material modification, achieving stepwise treatment through process integration, and innovating standards to quantify comprehensive performance—the gap between single-pollutant degradation mechanisms and the complex requirements of oil-contaminated wastewater treatment can be effectively bridged, providing critical theoretical support and technical solutions for the industrial application of photocatalytic technology.
4.2. Heterostructure Construction
The photocatalytic performance of single semiconductor photocatalysts can be improved effectively by constructing heterojunctions, as they often suffer from high photogenerated electron-hole complexation rates, insufficient visible light responsiveness and limited catalytic activity. This makes them more efficient and stable in oily wastewater treatment.
Joodi et al. [76] investigated the formation of ZnO-C3N4/r-PET by loading ZnO with g-C3N4 on recycled beverage bottles made from poly ethylene terephthalate (PET), and the wider individual peaks in the UV-Vis DRS indicated that heterojunctions were formed between these components (Figure 7a). As shown in Figure 7b, it has been demonstrated that the degradation efficiency increases with increasing ZnO content. This phenomenon can be attributed to the fact that ZnO is small in size and can be uniformly attached to the surface of the samples. This is in contrast to samples with a high specific surface area and a large pore volume, which results in improved photocatalytic activity. This indicates that the construction of heterojunction can effectively enhance its degradation of benzene pollutants and improve the photocatalytic degradation efficiency of the photocatalyst.
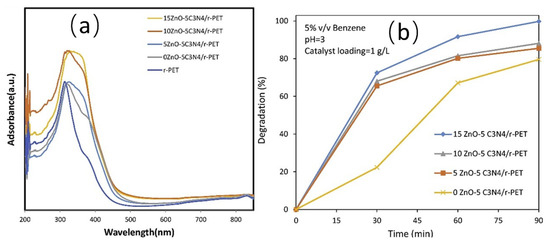
Figure 7.
(a) UV-Vis DRS analysis results of prepared samples, (b) photodegradation of benzene using ZnO-C3N4/r-PET photocatalysts made with different levels of ZnO [76].
The subsequent researchers explored the effects of salinity [77], wind [78], and type of light source [79] on pollutant degradation. The removal of salt-reduced benzene was simulated in seawater, the salts were ironized and their negative charge was adsorbed onto the photocatalyst, thus preventing hydroxyl groups from adsorbing onto it. The removal process of low-salt benzene was simulated in a model solution of seawater. The salt was ironized, resulting in the adsorption of its negative charge onto the photocatalyst. This process prevented the adsorption of hydroxyl groups onto the photocatalyst effectively. It has been established that the negative charge of the salts may also adsorb onto the positive benzene ions, thereby preventing them from depositing on the photocatalyst. In conditions of elevated wind speeds, the interaction between the photocatalyst and pollutants is augmented, resulting in an accelerated degradation rate. Moreover, the removal of benzene is more effective under sunlight than under simulated sunlight. It is important to note that sunlight is a combination of visible light and ultraviolet light, which is difficult to replicate with laboratory xenon lamps.
WO3 displays pronounced absorption characteristics in the visible light range, attributable to its diminutive bandgap. In addition to this, it exhibits anti-photocorrosion properties, excellent optical properties, superior physicochemical properties, and stable physicochemical properties. However, the weak photogenerated electron mobility of the material is a limiting factor in its photocatalytic performance. Samuel et al. [80] combined WO3 with TiO2 and further doped it with graphene oxide (GO), a ternary composite photocatalyst of TiO2-WO3@GO. Experiments were conducted to study the degradation of the photocatalyst, with the objective of determining the effect of energy storage in WO3 under conditions of darkness. The results demonstrated that the energy storage effect of WO3 can maintain partial catalytic activity under these conditions. The composite photocatalyst has been demonstrated to optimize the photogenerated electron migration pathways, thereby significantly reducing the electron-hole recombination rate and enhancing the photocatalytic degradation of oil pollution. As shown in Table 4, the total organic carbon (TOC) removal rates and electron-hole recombination phenomena are presented at varying levels of WO3 doping. As demonstrated in the tabular data, at a WO3 doping level of 10 wt%, the photocatalyst attains the maximum TOC removal rate. However, beyond this doping level, there is a decline in catalytic activity. This phenomenon can be ascribed to the agglomeration effect of WO3 on the photocatalyst surface, resulting in a reduction in specific surface area and consequent impairment of photocatalytic efficiency.

Table 4.
Effect of different WO3 doping on photocatalytic degradation performance (4 h of light) [80].
In the study by Yaacob et al. [50], ZrO2-TiO2 heterojunction photocatalysts (Figure 8a) were prepared using the sol-gel method. The performance of these photocatalysts in the degradation of oily wastewater was then investigated. The findings demonstrated that the degradation rate of 100 ppm oily wastewater attained 95% within a span of 5 h, which is considerably higher than that observed for pure TiO2. The incorporation of ZrO2 has been demonstrated to augment the catalyst’s specific surface area, thereby enhancing its capacity for oil pollutant adsorption. This, in turn, facilitates the adhesion of oil to the catalyst surface, while concurrently optimizing the migration pathways of photo-generated electrons. As demonstrated in Figure 8b, the photodegradation process of oily wastewater exhibits a strong correlation with the first-order kinetic equation. The interaction between ZrO2 and TiO2, and the formation of heterojunctions, significantly enhance photocatalytic activity. However, excessive ZrO2 loading has been shown to reduce the generation rate of electron-hole pairs and deteriorate the degradation efficiency of oily wastewater due to a decrease in active sites (Figure 8c).
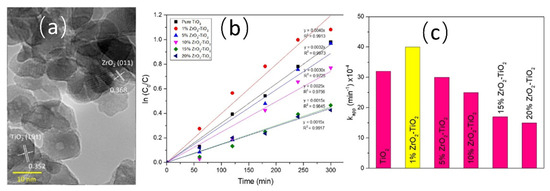
Figure 8.
(a) TEM images of ZrO2-TiO2 heterojunction photocatalysts, (b) photodegradation curves of pure TiO2 and various ZrO2-TiO2 heterojunction photocatalysts with different molar ratios under UV irradiation, (c) degradation rate constants of different photocatalysts for oily wastewater [50].
Zhang et al. [81] prepared ZnO/SnO2 composite nanoproduct photocatalysts with a heterojunction structure utilizing the chemical precipitation method. Orthogonal experimental methods were employed to determine the optimal reaction conditions for the degradation of marine diesel fuel, and the extent of influence of various factors on the degradation efficiency was thoroughly investigated. In optimal conditions, the oil removal rate in seawater reached as high as 91.54%. The influence of various factors on the degradation efficiency was ranked as follows: catalyst doping ratio > initial diesel concentration > solution pH value > UV irradiation time > catalyst dosage and hydrogen peroxide concentration. This study provides significant directions for the optimization of degradation efficiency in the context of oil pollutants. He et al. [49] synthesized TiO2 using the sol-gel method, MoS2 using the hydrothermal method, and then prepared TiO2/MoS2 composite materials using the composite hydrothermal method. SEM and EDAX results showed that the two materials were physically composite. Band structure analysis revealed that the composite material had a narrower bandgap (2.80 eV vs. 3.20 eV) and extended light absorption into the visible light region (400–800 nm), forming a type II heterojunction to promote charge separation. After the heterojunction is formed, the layered structure of MoS2 provides more active sites for adsorbing pollutants, while the combination with the spherical structure of TiO2 increases the specific surface area (51.48 m2/g), facilitating the enrichment and degradation of pollutants on the catalyst surface and enhancing catalytic efficiency. Specifically, under illumination, the TiO2/MoS2 composite material generates photo-generated electron-hole pairs, where photo-generated electrons migrate from the conduction band of TiO2 to the conduction band of MoS2, while holes migrate in the opposite direction, significantly suppressing electron-hole recombination. Additionally, electrons migrating to MoS2 react with O2 to form , while holes in the valence band of TiO2 oxidize H2O to form ). These strong oxidants can degrade organic compounds (such as alkanes and aromatics) and suspended solids. Optimizing experimental conditions is also critical, with the best results achieved in an acidic environment at pH = 3–4. Acidic conditions may enhance the interaction between the catalyst surface charge and pollutants, improving adsorption efficiency. Additionally, appropriate catalyst dosage and reaction time are also important.
Duan et al. [82] further investigated that introducing single metal atoms into heterojunctions can further enhance the synergistic activity of photocatalysts. The researchers doped Co single metal atoms into the Z-type heterojunction g-C3N4/Bi2WO6, which demonstrated outstanding performance (96% COD removal rate and 92.8% TOC removal rate) when treating actual oily wastewater in a microbial fuel cell system. Under illumination, during the formation of the Z-type heterojunction between g-C3N4 and Bi2WO6, the conduction band electrons of g-C3N4 recombine with the valence band holes of Bi2WO6, retaining the strongly oxidizing holes (h+) in the valence band of g-C3N4 and the highly reducing electrons in the conduction band of Bi2WO6, thereby suppressing the e−−h+ recombination rate. Additionally, the Co3+/Co2+ redox pair acts as an electron mediator, accelerating charge transfer at the interface. The article highlights a triple synergistic effect. Cobalt doping optimizes the band structure of g-C3N4, shifting its visible light absorption to around 550 nm. Secondly, the Z-type heterojunction formed with Bi2WO6 significantly enhances carrier separation efficiency. Combining photocatalysis with a microbial fuel cell successfully enhances the biocatalytic system using photo-generated electrons. From the oil degradation pathway perspective, long-chain alkanes are first attacked by to form alcohol/ketone intermediates, then oxidized by Co3+ to break the chain into carboxylic acids, and finally mineralized by microorganisms. However, the system requires a UV-visible composite light source and specific microbial strains, resulting in relatively high operational costs.
Additionally, Pandey et al. [83] developed an innovative approach by constructing a type II heterojunction to selectively regulate the reductive degradation pathway, preparing SnS2/TiO2. The key innovation lies in utilizing the negative conduction band position (−0.52 V) of SnS2 to facilitate electron transfer, which may be highly effective for treating recalcitrant halogenated organic compounds in oily wastewater. However, the 20% TiO2 loading resulted in a specific surface area of only 46 m2/g, suggesting a trade-off between visible light absorption and the number of active sites. Natali Sora et al. [84] prepared TiO2/LaFeO3 using a simple ball-milling method, explicitly discussing the possibility of a p-n heterojunction. However, band structure calculations revealed that an effective heterojunction was not formed, with charge separation primarily facilitated by physical mixing. This conclusion is valuable, as it demonstrates that not all composite systems can form ideal heterojunctions. The breakthrough achieved by Sasikala et al. [85] in the preparation of MoS2/TiO2 lies in the use of single-crystal few-layer MoS2 substrates, which solves the problem of traditional MoS2 being prone to stacking and constructs a typical type II heterojunction. Efficient electron transfer is achieved through the close interface between single-crystal few-layer MoS2 and TiO2. Dai et al. [86] prepared a ZnO/TiO2 n-n-type heterojunction, which enables electron transfer from ZnO to TiO2 through bandgap matching, thereby reducing recombination probability. They innovatively applied a low-power light source (only a 36W UV lamp) and achieved a 98.6% degradation rate of the dye through component optimization. Sun et al. [87] constructed a ternary heterojunction via ball milling, achieving a 97.7% degradation rate of toluene at 220 °C and 80 mW/cm2 illumination. Most notably, their photothermal synergistic mechanism does not simply combine photocatalysis and thermal catalysis but reduces the activation energy of the reaction by 45% through oxygen vacancy-mediated charge transfer pathways. This has significant implications for oil-containing wastewater treatment, as lipid degradation typically requires high temperatures, while photothermal synergy can significantly reduce energy consumption.
In conclusion, heterostructure photocatalysts are primarily applied to the degradation of water-soluble organic pollutants (such as dyes, antibiotics, phenols, etc.). The main reason is that oily wastewater is a complex system, and heterojunctions rely on pollutant adsorption and radical attack, while the hydrophobicity and macromolecular structure of oil droplets hinder these two processes. For example, the degradation of methyl orange by ZnO/TiO2 requires molecular-level contact, but emulsified oil droplets can encapsulate the catalyst. Future improvements require breakthroughs from multiple angles. Material-wise, one can draw inspiration from the hydrophobic modification approach of MoS2/TiO2, but the more critical issue is addressing oil film coverage. Process-wise, combining pretreatment methods like flotation might be necessary, similar to the synergistic strategy of adding H2O2 to LaFeO3/TiO2.
5. Photocatalytic Coupled Membrane Technology
Membrane separation and photocatalytic degradation are two significant technologies for the purification of wastewater. Nevertheless, membrane fouling in membrane separation and low photocatalytic efficiency act as significant barriers to the practical implementation of these systems [49]. The integration of photocatalysis and membrane technology has the potential to capitalize on the respective strengths of both disciplines. Photocatalytic membranes are composed of three distinct layers: a photocatalytic layer, a support layer, and a separation layer [88]. Furthermore, functionalized layers may be incorporated with a view to enhancing membrane performance, whilst protective layers are also employed to extend the lifespan of the membrane. In the case of photocatalytic membranes, a comprehensive evaluation of performance is required, which must incorporate the performance of the photocatalyst in conjunction with that of the membrane. Factors influencing photocatalysts and optimization strategies have been discussed in previous sections. This section will explore their application in the degradation of oily wastewater from the perspectives of organic and inorganic membranes.
The support material of the photocatalytic membrane plays a key role in the photocatalytic membrane system, which not only provides mechanical support for the photocatalytic layer, but also needs to have certain chemical stability and permeability. Table 5 statistically illustrates advantages and applicable scenarios of popular photocatalytic membrane support materials, for example, PVDF is suitable for most water treatment scenarios, while ceramic materials and stainless steel mesh are suitable for high-temperature or strong corrosive environments.

Table 5.
Advantages and applicable scenarios of popular photocatalytic membrane support materials.
5.1. Organic Photocatalytic Membrane
Ding et al. [89] employed electrospinning to embed ZnO nanoparticles into piezoelectric PVDF fibers, preparing ZnO/PVDF core-shell structured membranes (CSMs), a method based on the research of Hemalatha [90]. Figure 9a–c show SEM images of commercial ZnO powder, PVDF, and ZnO/PVDF nanofibers, respectively. As shown in the figures, the fiber morphology changed significantly after embedding. Figure 9d,e show that the ZnO/PVDF kinetic curves exhibit high compatibility and significant degradation efficiency. A uniform water-in-oil emulsion was prepared using hexane and deionized water as the main components. After 60 min of xenon lamp irradiation, the RhB catalytic degradation efficiency reached a maximum of 88.32% (Figure 9f). This is due to the protective effect of PVDF, which keeps the ZnO material stable in solution, and the catalytic degradation function of ZnO. This enables the film to exhibit excellent cyclic stability and acid-alkali tolerance in oil-water separation processes. These findings provide new insights into the loading of semiconductor photocatalysts onto membranes and open up new possibilities for oil and wastewater treatment. Loana et al. [91] aim to overcome the limitations of traditional oily wastewater treatment methods have several limitations, including low efficiency, high cost and difficulty in treating stable emulsified wastewater. To overcome these limitations, Loana innovatively prepared polymer photocatalytic membranes, which demonstrated high efficiency in oil-water separation and pollutant degradation in oily wastewater treatment. The PVDF membrane containing TiO2 and graphene achieved 98.46% oil removal efficiency under UV light.
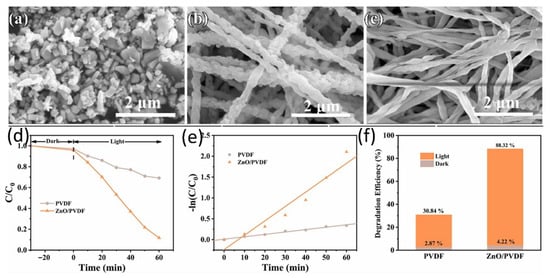
Figure 9.
(a–c) SEM images of commercial ZnO powders, PVDF and ZnO/PVDF nano-fibers, (d,e) kinetic curves and degradation efficiencies, (f) photodegradation efficiencies of PVDF EFM and ZnO/PVDF CSM under xenon irradiation [89].
Xiao et al. [92] combined SEP@MnO2/RGO composite membranes with in situ microbubble technology. using sepiolite (SEP) as a carrier to load MnO2, which was then composite with reduced graphene oxide (RGO) on a PVDF-based membrane. During treatment, a small amount of H2O2 was added to the oily wastewater, reacting with the MnO2 on the membrane surface to generate microbubbles, thereby simultaneously achieving oil removal, desalination, and heavy metal removal. This method demonstrates a triple synergistic effect. First, the membrane’s physical sieving: the composite membrane has a pore size of approximately 13 nm, smaller than the diameter of emulsified oil droplets (423 nm), enabling oil-water separation through size exclusion. Second, chemical adsorption: SEP and MnO2 can capture heavy metal ions (e.g., Cr6+, Ni2+), while RGO layers carry a negative charge, repelling salt ions via the Donnan effect. Finally, Equation (6) reflects the anti-fouling mechanism when H2O2 reacts with MnO2 on the surface of the membrane.
The oxygen microbubbles produced (as shown in Figure 10 of the paper) continuously scrub the membrane surface, and the bubbles carry away oil droplets during their ascent, preventing oil film formation. This explains why the contact angle suddenly increases from 28° to 117° (hydrophobic bubbles cover the surface) and why the flux decreases by only 30% after 12 h of continuous operation. This method is tolerant to high salt concentrations (1 mol/L NaCl) and strong acid-base environments (pH = 2–14), and only requires the addition of a small amount of H2O2 (20 μL), making it more economical than traditional chemical cleaning. However, it is important to note that H2O2 concentrations exceeding 30 mL can damage the membrane structure.
Figure 10.
Separation mechanism of the membrane, separation and anti-fouling mechanism of the in-situ bubble method [92].
Wang et al. [93] designed a pH-responsive fiber membrane that forms an integrated, efficient oil-containing wastewater treatment system combining separation, degradation, and self-cleaning. The rapid wettability switching of the pH-responsive membrane relies on the protonation/deprotonation of the carboxyl group of 12-amino dodecanoic acid. Under acidic conditions, the -COOH group forms a hydrophobic surface for heavy oil separation, while under alkaline conditions, the -COO- group forms a superhydrophilic surface for light oil separation. This bidirectional separation capability addresses the issue that traditional membranes cannot simultaneously handle both light and heavy oils. In terms of photocatalysis, TiO2 generates free radicals such as and under UV light, which can degrade oil and dyes adsorbed on the membrane surface (e.g., MB degradation rate > 90% in 6 min). Dynamic filtration experiments also demonstrate simultaneous water purification, and subsequent free radical capture experiments confirm that and e− play a dominant role. Electrospun fibers form a micron-nano porous network, combined with the roughness of the polydopamine (PDA) intermediate layer, creating a gradient pore size that enhances oil-water retention capacity, particularly for emulsified oil droplets, achieving a heavy oil separation flux of 6800 L·m−2·h−1 and a light oil separation flux exceeding 10,000 L·m−2·h−1. This effectively avoids the flux decay caused by oil fouling in traditional membranes, with a flux retention rate of over 95% after 50 cycles.
Zhang et al. [94] incorporated GO nanosheets into PAN nanofiber membranes prepared via ultrasonic-assisted deposition and biomimetic fixation methods. The SPAN@GO/M88A membrane demonstrated separation efficiencies exceeding 99% for various oil-in-water emulsions, attributed to its excellent mechanical stability, hydrophilicity, reasonable pore size distribution, superior photocatalytic performance, significant self-cleaning capability, and flux recovery rate. This addresses the issues of membrane fouling and flux decline encountered in traditional oil-water separation membranes when treating oily wastewater. Future research could further explore process optimization, cost reduction, and yield improvement. Figure 11 illustrates the membrane morphology and pore size distribution.
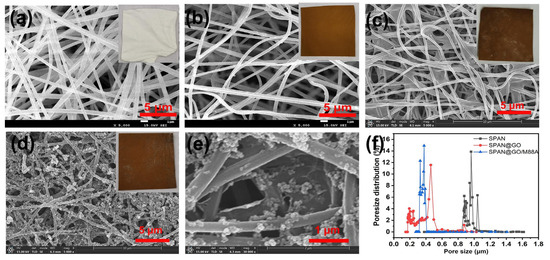
Figure 11.
SEM images and photographs of surface morphology for (a) PAN, (b) SPAN, (c) SPAN@GO0.5, (d) SPAN@GO0.5/M88A-4NFMS, (e) FM HD magnified image of SPAN@GO0.5/M88A-4N, (f) pore size distribution of the membrane [94].
Tian et al. [95] similarly proposed a multifunctional membrane based on electrospinning technology by combining carbon nanotubes (CNTs) and TiO2 into polyacrylonitrile (PAN) nanofibrous membranes to prepare a carbonized nanofibrous membrane (CPEN-CNTs/TiO2) with two self-cleaning functions (Figure 12d). These membranes exhibited efficient separation performance and self-cleaning by photocatalytic and pyrolysis methods for different types of oil-water emulsions including surfactant free emulsions, surfactant stability emulsions, dye/oil emulsions, and crude oil emulsions (Figure 12a–c), which allowed for regeneration of the membranes and long-term use. New ideas are provided to develop membranes with efficient self-cleaning performance. Qu et al. [96] achieved triple innovation by forming a heterojunction between self-assembled supramolecular PDINH and Fe-MOF, which was then composite with PAN nanofibers. The membrane exhibits switchable superhydrophobicity, photo-Fenton self-cleaning (degrading viscous crude oil contamination), and antibacterial properties. Notably, the flux of 1920 L·m−2·h−1 and separation efficiency of 99% under gravity-driven conditions are particularly outstanding, and the multiple reactive oxygen species generation mechanism was confirmed via ESR. These studies provide new insights for developing membranes with highly efficient self-cleaning performance.
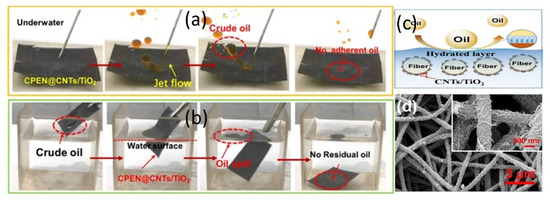
Figure 12.
(a) Photograph of sprayed crude oil, (b) separation process of dripping crude oil, (c) schematic membrane of the mechanism to prevent CPEN-CNTs/TiO2 from adhering to oil, (d) CPEN-CNTs/TiO2 [95].
Chaithra et al. [97] developed a material capable of simultaneously treating multiple pollutants, addressing the issue of low efficiency in treating complex wastewater. ZnO and Ag-ZnO were combined with cellulose acetate (CA) and polyvinylpyrrolidone (PVP) using electrospinning technology to prepare composite membranes with superhydrophilic and underwater superoleophobic properties for efficient oil-water separation. As shown in Figure 13a,b, which are SEM images and size distribution histograms of Ag-ZnO/DCA/PVP, the comparison of images before and after separation from an oil/water dispersion (Figure 13c,d) demonstrates rapid and efficient separation. However, while the experimental conditions are satisfactory, the degradation of actual wastewater still needs to be optimized.
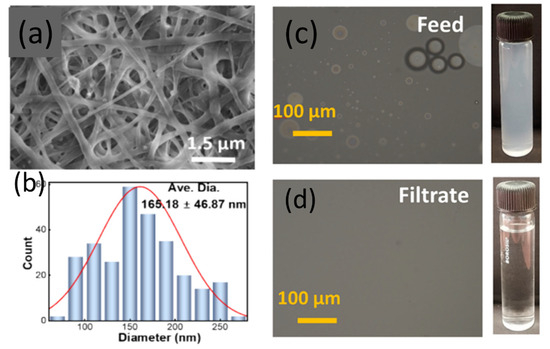
Figure 13.
(a,b) SEM images and size distribution histograms of Ag-ZnO/DCA/PVP, (c,d) microscopic images of feed and filtrate after oil/water emulsion separation [97].
A cellulose-based photocatalytic electrospun nanofiber membrane was proposed by Lu et al. [98]. The membrane was prepared using electrospinning technology and features a core-shell structure. It exhibits an exceptionally high MIL-100(Fe) loading capacity (78 wt%) and a large specific surface area (1105 m2/g). The membrane demonstrates excellent performance in oil-water emulsion separation, dye degradation, and Cr reduction, while exhibits good cycling performance. The core-shell structure not only enhances the membrane’s hydrophilicity and adsorption capacity from a material structural perspective but also broadens the light response range, enhances photocatalytic performance, and optimizes mass transfer and reaction kinetics through efficient charge separation and suppression of electron-hole pair recombination. Such a structure is also one of the future research directions.
5.2. Inorganic Photocatalytic Membrane
Gunatilake et al. [99] developed a novel, superhydrophobic, oil-repellent underwater membrane by coating stainless steel mesh with titanium dioxide nanofiber (Figure 14a). They then compared the coated membrane with uncoated samples to investigate its oil-water separation performance. The results showed that the membrane achieved separation efficiencies of approximately 90% and 99% for low- viscosity and high-viscosity oils, respectively, demonstrating its excellent oil-water separation capabilities. This high efficiency is primarily due to the rough micro-nano structure formed by the nanofibers. This structure creates a stable water film, imparting excellent underwater superhydrophobicity to the material. Simultaneously, the stainless steel mesh substrate provides robust mechanical support, enhancing the membrane’s stability and durability. The higher separation efficiency for high-viscosity oils is attributed to their greater viscosity, which makes them more easily blocked by the membrane structure.
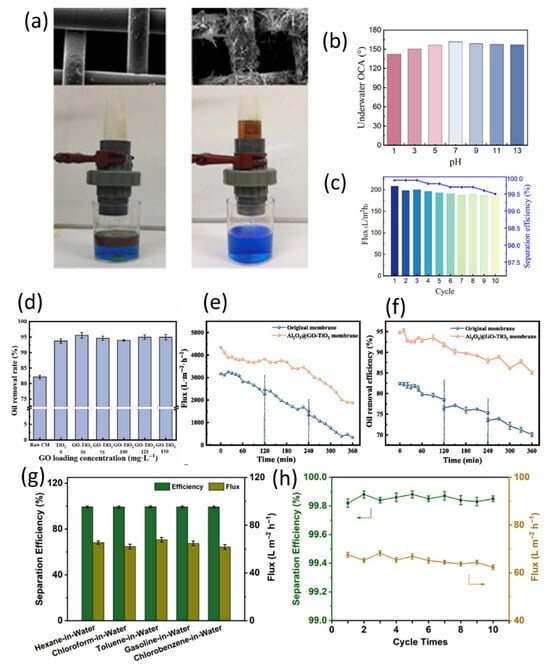
Figure 14.
(a) Uncoated (left) and TiO2 nanofiber-coated mesh (right) water-oil separation devices for separating oil-water from the corresponding filters [99]. (b) UOCA values of TiO2@SSM immersed in solutions of different pH values, (c) flux and separation efficiency of TiO2@SSM over 10 separation cycles [100]. (d) Oil removal rate at different GO loading levels, (e) flux and (f) oil removal efficiency of the original membrane after UV cleaning [102]. (g) Separation efficiency and flux for different types of multiphase oil-in-water emulsions, (h) separation efficiency over 10 consecutive separation cycles [105].
Yang et al. [100] used an electrochemical deposition method to uniformly load TiO2 nanoparticles onto stainless steel mesh (PSSM) for efficient oil-water emulsion separation. The TiO2@SSM membrane achieved a separation efficiency of up to 99.8% for oil-in-water emulsions and a rapid water flux of up to 208.0 L·m−2·h−1. Additionally, TiO2@SSM exhibits minimal performance degradation under strong acidic and alkaline high-salt conditions and demonstrates excellent corrosion resistance (Figure 14b). After 10 cycles, the water flux and separation efficiency remained above 185.0 L·m−2·h−1 and 99.5%, respectively (Figure 14c), indicating that the O/W emulsion separation and photocatalytic degradation performance were maintained, and the membrane could be rapidly and effectively repaired through re-electrodeposition, verifying the membrane’s photostability and recyclability. Xu et al. [101] focused more on structural design. They first constructed a TiO2 coating on the steel mesh surface to separate dispersed oil (with a flux as high as 3.6 × 104 L·m−2·h−1), and then used the hydrothermal method to grow TiO2 nanotube arrays to achieve emulsion breaking. After stacking the two membranes, the emulsified oil separation efficiency increased from 45% to 95%, which is a very practical improvement. The mechanical strength of the steel mesh substrate is a significant advantage, making this design more suitable for industrial applications.
Ceramic membranes with good stability and recyclability are commonly used for oil-water separation. Wang et al. [102] successfully prepared Al2O3@GO-TiO2 composite photocatalytic membranes using a two-step dip-coating method. This composite membrane successfully separated diesel-water emulsions, achieving a permeability of 4347.17 L·m−2·h−1 and an oil removal rate of 94.98% (Figure 14d), while maintaining excellent oil resistance, stability, and durability after 25 cycles and separation tests. Additionally, the composite modification with GO-TiO2 enhances the photocatalytic activity of the composite membrane compared to pure TiO2, and it demonstrates good flux and oil removal efficiency recovery when separating oilfield produced water (OPW) (Figure 14e,f). This is attributed to the fact that ultraviolet light can decompose and disperse surface fouling on the membrane, thereby effectively preventing pore blockage. Fu et al. [103] developed a novel photocatalytic self-cleaning ceramic composite membrane (M/S-TiNTs). Sulfur-doped titanium dioxide nanotubes (S-TiNTs) were loaded onto a whisker mullite hollow fiber substrate. The sulfur doping created oxygen vacancies, reducing the bandgap from 3.26 eV to 2.43 eV, enabling the material to utilize visible light and broadening its photoresponsive range, while the hollow fiber structure increased the contact area. The holes (h+), superoxide radicals (), and hydroxyl radicals () generated during the photocatalytic process can degrade pollutants, with holes playing a dominant role. The membrane combines physical retention performance, anti-fouling properties of a superhydrophilic surface (contact angle of 5°), and photocatalytic degradation of pollutants. The membrane maintains stable flux and retention efficiency (>98%) under acidic, alkaline, and saline conditions and at high temperatures. The flux recovery rate after 12 dynamic-static coupling cycles remains at 93.58%, indicating its potential for practical engineering applications. Akrami et al. [104] prepared Bi2WO6-based visible-light-responsive photocatalysts and combined them with zirconia/alumina ultrafiltration membranes in a slurry photocatalytic ceramic membrane reactor. When Bi2WO6 is exposed to visible light, electrons in its valence band are excited to the conduction band, forming electron-hole pairs. The holes possess strong oxidizing properties, enabling them to directly oxide organic compounds or react with water to generate hydroxyl radicals (). Meanwhile, the electrons can react with oxygen to form superoxide radicals (), and these active radicals further degrade organic pollutants. Additionally, the mechanism of action of composite catalysts such as NH2-MIL-125(Ti)/Bi2WO6 involves the formation of heterojunctions, which promote charge separation, reduce electron-hole recombination, and thereby enhance photocatalytic efficiency. NH2-MIL-125(Ti) is a metal-organic framework whose amino groups may enhance visible light absorption and provide additional active sites. Experimental parameters such as transmembrane pressure, flow rate, and light intensity influence system performance. Optimizing these parameters can improve TOC removal efficiency, with optimal removal rates reaching up to 92%. This demonstrates that operating conditions significantly impact the synergistic effect of photocatalysis-membrane separation.
Bao et al. [105] used a solvothermal synthesis method followed by vacuum deposition to prepare TiO2/Co3O4/GO (TCG) composite materials, which exhibited excellent superhydrophobicity and outstanding flexibility. Various oil-in-water emulsions, including hexane, chloroform, and gasoline, were tested, and the results showed that the TCG membrane achieved separation efficiencies exceeding 99% for all oil-in-water emulsions (Figure 14g), and maintained this efficiency after 10 experimental cycles (Figure 14h). Additionally, the linear relationship between ln(C/C0) and the exposure time (T) under light for 10 min indicates that the photodegradation of dyes by the TCG membrane follows pseudo-first-order kinetics. The membrane remains stable under both cold and hot conditions, offering potential for treating oily wastewater under harsh conditions.
In summary, past photocatalytic research has indeed gone through three stages. In the early stage, there was a frenzy to pursue catalyst activity, with various precious metal doping and heterostructure construction, but in actual wastewater treatment, there were problems such as difficulty in recovery and easy deactivation. In the middle stage, attempts were made at immobilization (such as loading on glass beads or ceramics), but mass transfer efficiency was poor. Currently, the mainstream has shifted towards membrane-assisted systems, as this technology offers advantages in terms of catalyst loss prevention (through carrier immobilization), spatiotemporal coupling of separation and degradation (direct mineralization of pollutants on the membrane surface), and engineering feasibility. However, current membrane-assisted systems still have significant shortcomings, such as PAN membranes, which although functionally rich, have weak mechanical strength, and TiO2-SSM membranes, which have high flux but limited functionality. Future breakthroughs may require interdisciplinary integration: for example, combining the mechanical strength of TiO2-SSM’s steel mesh substrate with the multifunctional photocatalytic layer of PAN membranes, or developing adaptive wettability coatings to address complex oil phases.
6. Other Technologies for Degrading Oily Wastewater
In the field of oily wastewater treatment, in addition to the elemental doping, heterogeneous construction and photocatalytic membrane technology mentioned in the appeal, there are a variety of innovative technologies, such as electrochemical oxidation technology, bio-enhanced treatment, air flotation coupled with photocatalysis and membrane separation upgrading, etc. These technologies demonstrate not only a single-point breakthrough in innovation, but also strong vitality and potential in technological integration. They provide more abundant and efficient solutions for oily wastewater treatment.
From the perspective of basic technological innovation, a series of targeted research results have opened up new paths for oily wastewater treatment, and Medeiros et al. [106] have focused on the study of bacterial cellulose filtration system, which has explored the performance of the system in removing oily pollutants. The experimental results showed that the bacterial cellulose filtration system showed excellent ability in many aspects when treating oily wastewater, which provided new ideas and directions for the application of filtration technology in oily wastewater treatment. Zhu et al. [107] devoted themselves to the preparation of alkylated chitosan sponges. Aiming at the difficult problem of emulsified oil removal, the sponge, with its excellent separation performance, provides a practical technical means to solve the problem of emulsified oil pollution and enriches the choice of emulsified oil removal technology. Bande et al. [108] achieved efficient removal of crude oil by using electroflotation technique to make electrodes of uniformly perforated aluminium plates.
In terms of technology integration and innovation, researchers have made great progress in oily wastewater treatment technology through cross-disciplinary technology integration. Ben et al. [109] combined electroflotation with coagulation to almost completely degrade oil-water emulsions of mediterranean marine crudes, and the researchers found that degradation was more effective in the presence of salt. This is because salt enhances the efficiency of coagulation, destabilisation and electroflotation synergistically by compressing the bilayer of oil droplets, thereby increasing the conductivity of the system and the salting-out effect. This significantly improves the degradation of oil-water emulsions in high-salt environments. Mostefa et al. [110] combined electroflotation with flocculation and similarly found that ions promoted the degradation of cutting oils. Su et al. [111] innovated the combination of photocatalytic technology and Pickering emulsion for the application of emulsion interface photocatalytic process. This fusion of technologies gives full play to the advantages of the two technologies, significantly improves the degradation rate of actual oily wastewater, and provides a new technological pathway for the deep treatment of oily wastewater. Wang et al. [112] prepared special electrodes elaborately for the special treatment needs of oily wastewater from ships, and applied them to the treatment of oily wastewater from ships. After practical application verification, the oil content of the treated water can meet the relevant standard requirements, which provides a reliable technical guarantee for the effective treatment of oily wastewater on board ships. Lu et al. [113] developed a core-shell microbial reactor to realize the integration of functional innovation, which has the dual functions of oil-water separation and degradation of organic pollutants, simplifying the process of oily wastewater treatment greatly, and improving the efficiency of the treatment. Rache et al. [114] combined MXene and photocatalyst into an electrostatically spun membrane, and this unique material composite not only improved the oil/water separation effect, but also endowed the membrane material with the characteristic of resistance to oil contamination, which further enhanced the effect and stability of oily wastewater treatment.
In addition, the photocatalytic in-situ Fenton oxidation system developed by Zhang et al. [115] combines the advantages of photocatalysis and Fenton oxidation, and is able to achieve effective treatment of diesel wastewater under complex environmental conditions. While Mokhbi et al. [116] further enhanced the removal rate of oily wastewater and achieved efficient degradation of real oily wastewater. Song et al. [117] integrated sulfite and novel electro Fenton technology, which showed excellent performance in the degradation of carbamazepine (CBZ), and provided a new approach and method for solving the problem of treating complex oily wastewater in real engineering. Table 6 lists and summarizes some new methods of oily sewage treatment.

Table 6.
Table of oily wastewater treated by the new methods.
In summary, these research efforts have provided diversified technology options for oily wastewater treatment, forming a progressive research pattern from basic technology innovation to technology integration innovation. However, the field of oily wastewater treatment still faces many challenges, and in the future we should further explore other effective degradation technologies in depth, strengthen the research on the synergistic effect between different technologies, and continuously improve and optimize the technical system of oily wastewater treatment, so as to better cope with the increasingly serious problem of oily wastewater pollution, and to achieve sustainable use of water resources and protection of the ecological environment.
7. Conclusions and Outlook
This paper briefly describes research advances in photocatalysis and photocatalysis-based improvements, and discusses the challenges faced by these technologies in practical oily wastewater treatment applications. Many researchers have used photocatalysis for surface interface modulation of materials or in combination with different technologies to overcome these problems. By comparing traditional oily wastewater treatment technologies (gravity separation, gravity-coalescence separation, and filtration separation, etc.), exploring the intrinsic advantages of nano-metal doping and heterogeneous structure building, and related applications, coupled methods for degradation of organic pollutants in oily wastewater are summarized and discussed (photocatalytic membranes, electro-flotation, microbial fuel cells, Fenton reaction, Pickering emulsion, etc.). These photocatalytic fusion techniques can significantly improve the removal efficiency of organic pollutants in oily wastewater. However, there are still many challenges for technology integration and innovation. Future research efforts should focus on the following challenges and directions:
- (1)
- To address the issues of uneven distribution, poor stability, and carrier recombination in metal-doped photocatalysts, it is necessary to develop atomic-level precise doping technology, combined with theoretical calculations to guide the design of multi-component composite structures, thereby enhancing the light response range and cycling stability. For heterojunction materials, interface engineering should be employed to optimize charge transfer efficiency, develop novel composite systems with broad-spectrum response, and explore low-temperature scalable fabrication processes to overcome interface compatibility and long-term performance limitations.
- (2)
- The central objective should be to enhance the catalytic efficiency and anti-pollution capabilities of membrane materials in a synergistic manner. Nano-structural optimization (e.g., three-dimensional porous frameworks and hydrophilic modifications) has been demonstrated to enhance mass transfer efficiency, while coupling with new energy sources has been shown to broaden spectral utilization. The development of self-cleaning membrane surfaces and intelligent response mechanisms is imperative, with process simulation being utilized to optimize membrane component design. This will promote the practical application of photocatalytic membrane technology in environmental governance and energy conversion.
- (3)
- Due to the limitations of existing technologies that focus on single-pollutant treatment, it is necessary to design catalysts that are stable, efficient and adaptable to multiple scenarios. This involves enhancing interference resistance to complex matrices through molecular-level material modification (e.g., defect engineering), and establishing a dynamic evaluation system that encompasses actual water quality parameters.
- (4)
- In order to surmount the obstacles inherent in technical collaboration, it is imperative to establish a multi-functional coupling system. The system’s design should be stable through energy matching and the development of innovative materials. The utilization of dynamic optimization algorithms, founded upon machine learning principles, can be contemplated in conjunction with in-situ monitoring and feedback control modules, thereby culminating in the formation of a closed-loop processing system for the purpose of resource recycling. The promotion of cooperation between industry, academia and research entities is imperative to expedite the transition of photocatalytic technology from its current state of laboratory development to its application in engineering contexts. This transition can be facilitated through the establishment of standardized processes and the optimization of costs.
Author Contributions
Investigation, Y.Z. (Yulin Zhao), C.Y., Y.X., Y.F., G.C. and Y.Z. (Yingying Zhu); Data curation, Y.Z. (Yulin Zhao), C.Y., Y.X. and Y.F.; Writing—original draft, Y.Z. (Yulin Zhao) and Y.Z. (Yingying Zhu); Validation, Y.Z. (Yulin Zhao) and C.Y.; Investigation, Y.Z. (Yulin Zhao), C.Y., Y.X. and Y.F.; Methodology, Y.Z. (Yingying Zhu); Supervision, Y.Z. (Yingying Zhu); Conceptualization, Y.Z. (Yingying Zhu). All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the Ningbo Natural Science Foundation, China (grant No.: 2022J109) and Zhejiang provincial Key R&D programme (2025C02257(SD2)).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hedayatipour, M.; Jaafarzadeh, N.; Ahmadmoazzam, M. Removal optimization of heavy metals from effluent of sludge dewatering process in oil and gas well drilling by nanofiltration. J. Environ. Manag. 2017, 203 Pt 1, 151–156. [Google Scholar] [CrossRef]
- Caliani, I.; De Marco, G.; Cappello, T.; Giannetto, A.; Mancini, G.; Ancora, S.; Maisano, M.; Parrino, V.; Cappello, S.; Bianchi, N.; et al. Assessment of the effectiveness of a novel BioFilm-Membrane BioReactor oil-polluted wastewater treatment technology by applying biomarkers in the mussel Mytilus galloprovincialis. Aquat. Toxicol. 2022, 243, 106059. [Google Scholar] [CrossRef]
- Cao, M.; Xiao, F.; Yang, Z.J.; Chen, Y.B.; Lin, L.G. Purification of oil-containing emulsified wastewater via PAN nanofiber membrane loading PVP-UiO-66-NH2. Sep. Purif. Technol. 2022, 297, 121514. [Google Scholar] [CrossRef]
- Meng, N.; Priestley, R.C.E.; Zhang, Y.Q.; Wang, H.Y.; Zhang, X.W. The effect of reduction degree of GO nanosheets on microstructure and performance of PVDF/GO hybrid membranes. J. Membr. Sci. 2016, 501, 169–178. [Google Scholar] [CrossRef]
- Li, P.; Zhao, D.X.; Zhang, Y.G.; Sun, L.Q.; Zhang, H.M.; Lian, M.R.; Li, B.A. Oil-field wastewater treatment by hybrid membrane-aerated biofilm reactor (MABR) system. Chem. Eng. J. 2015, 264, 595–602. [Google Scholar] [CrossRef]
- Cai, Y. The research on present situation and formulation of water pollutant discharge standards of river basins in China. IOP Conf. Ser. 2019, 237, 022044. [Google Scholar]
- Sun, Y.J.; Zhu, C.Y.; Zheng, H.L.; Sun, W.Q.; Xu, Y.H.; Xiao, X.F.; You, Z.Y.; Liu, C.Y. Characterization and coagulation behavior of polymeric aluminum ferric silicate for high-concentration oily wastewater treatment. Chem. Eng. Res. Des. 2017, 119, 23–32. [Google Scholar] [CrossRef]
- Jamaly, S.; Giwa, A.; Hasan, S.W. Recent improvements in oily wastewater treatment: Progress, challenges, and future opportunities. J. Environ. Sci. 2015, 27, 15–30. [Google Scholar] [CrossRef]
- Huang, L.; Peng, F.; Ohuchi, F.S. “In situ” XPS study of band structures at Cu2O/TiO2 heterojunctions interface. Surf. Sci. 2009, 603, 2825–2834. [Google Scholar] [CrossRef]
- Mahmood, A.; Wang, X.; Xie, X.F.; Sun, J. Degradation behavior of mixed and isolated aromatic ring containing VOCs: Langmuir-Hinshelwood kinetics, photodegradation, in-situ FTIR and DFT studies. J. Environ. Chem. Eng. 2021, 9, 105069. [Google Scholar] [CrossRef]
- Chen, B.K.; Zheng, C.H.; Bi, J.C.; Qu, X.; Zhang, R.; Liu, X.B.; Yang, Y.Q. Improved emulsified oil removal approach for industrial coal pyrolysis wastewater by flocculation under pressurized CO2 atmosphere. J. Clean. Prod. 2023, 418, 138213. [Google Scholar] [CrossRef]
- Ahuja, T.; Brighu, U.; Saxena, K. Recent advances in photocatalytic materials and their applications for treatment of wastewater: A review. J. Water Process Eng. 2023, 53, 103759. [Google Scholar] [CrossRef]
- Ou, Y.J.; Shi, J.Q.; Zhao, D.; Zheng, Y. Research progress of metal ion doped TiO2 photocatalyst and its characterization technology. J. Funct. Mater. 2021, 52, 2018–2024. [Google Scholar]
- Wang, S.S.; Liao, W.Y.; Su, H.; Pang, S.Q.; Yang, C.; Fu, Y.N.; Zhang, Y. Review on the application of semiconductor heterostructures in photocatalytic hydrogen evolution: State-of-the-art and outlook. Energy Fuels 2023, 37, 1633–1656. [Google Scholar] [CrossRef]
- Li, Y.F.; Wang, M.X.; Sun, D.J.; Li, Y.J.; Wu, T. Effective removal of emulsified oil from oily wastewater using surfactant-modified sepiolite. Appl. Clay Sci. 2018, 157, 227–236. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Zhang, Y.H.; He, Y.F.; Xu, X.L.; Zhao, X. Oil density and viscosity affect emulsion stability and destabilization mechanism. J. Food Eng. 2024, 366, 111864. [Google Scholar] [CrossRef]
- Yekeen, N.; Padmanabhan, E.; Syed, A.H.; Sevoo, T.; Kanesen, K. Synergistic influence of nanoparticles and surfactants on interfacial tension reduction, wettability alteration and stabilization of oil-in-water emulsion. J. Pet. Sci. Eng. 2020, 186, 106779. [Google Scholar] [CrossRef]
- Liu, L.C.; Corma, A. Metal catalysts for heterogeneous catalysis: From single atoms to nanoclusters and nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Zhao, L.; Yao, S.; Chen, W.Y.; Cao, Y.Q.; Qian, G.; Zhou, X.G.; Duan, X.Z. Structure optimization and selection of catalyst particle for highly efficient gas oil hydrodenitrogenation. Chem. Eng. Sci. 2023, 277, 118871. [Google Scholar] [CrossRef]
- Olteanu, M.; Baraitaru, A.; Panait, A.M.; Dumitru, D.; Boboc, M.; Deák, G. Advanced SiO2 composite materials for heavy metal removal from wastewater. Water Air Soil Pollut. 2019, 230, 179. [Google Scholar] [CrossRef]
- Church, J.; Lundin, J.D.; Mercado, D.; Willner, M.R.; Lee, W.H.; Paynter, D.M. Identification and characterization of bilgewater emulsions. Sci. Total Environ. 2019, 691, 981–995. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, N.W. A county-level risk assessment of the deep water horizon oil spill in the gulf of Mexico. Geogr. Rev. 2016, 106, 360–380. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, K.; Xu, Z.K.; Xiong, Y.J.; Liu, J.; Cai, C.; Huang, X.F. A comprehensive review on the behavior and evolution of oil droplets during oil/water separation by membranes. Adv. Colloid Interface Sci. 2023, 319, 102971. [Google Scholar] [CrossRef] [PubMed]
- Li, G.T.; Zhang, C.G.; Wang, L.L. Research progress on treatment technology of oily sewage from ships. J. Waterw. Harb. 2024, 45, 256–263. [Google Scholar]
- López-Vazquez, C.M.; Fall, C. Improvement of a gravity oil separator using a designed experiment. Water Air Soil Pollut. 2004, 157, 33–52. [Google Scholar] [CrossRef]
- Han, L.; Shen, L.G.; Lin, H.J.; Cheng, T.H.; Wen, J.M.; Zeng, Q.Q.; Xu, Y.C.; Li, R.J.; Zhang, M.J.; Hong, H.C.; et al. Three dimension-printed membrane for ultrafast oil/water separation as driven by gravitation. Nano Energy 2023, 111, 108351. [Google Scholar] [CrossRef]
- Cao, M.; Fan, X.; Yang, Z.J.; Chen, Y.B.; Lin, L.G. Construction of polytetrafluoroethylene nanofiber membrane via continuous electrospinning/electrospraying strategy for oil-water separation and demulsification. Sep. Purif. Technol. 2022, 287, 120575. [Google Scholar] [CrossRef]
- Gao, L.; Gu, H.H.; Wang, C.X.; Wu, H.L.; Ye, C. Superhydrophilic and oil-resistant SiO2/PU fiber membrane for oil-in-water emulsion separation under gravity. Fibers Polym. 2024, 25, 1623–1634. [Google Scholar] [CrossRef]
- Zhang, R.N.; Xu, Y.C.; Shen, L.G.; Li, R.J.; Lin, H.G. Preparation of nickel@polyvinyl alcohol (PVA) conductive membranes to couple a novel electrocoagulation-membrane separation system for efficient oil-water separation. J. Membr. Sci. 2022, 653, 120541. [Google Scholar] [CrossRef]
- Yin, X.Y.; Ma, X.Y.; He, Y.; Li, H.J.; Fan, Y.; He, T.; Guo, X.; Chen, L. Robust self-cleaning urchin-like Ni/Co LDH stainless steel mesh for gravity-driven oil/water emulsion separation and catalytic degradation of aromatic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127186. [Google Scholar] [CrossRef]
- Zhao, L.; Zeng, X.B.; Zhao, W.G.; Zhu, F.Q.; Hou, M.W.; Fan, G.M. Structural optimization for an axial oil-water separator with multi-stage separation. Heat Mass Transf. 2021, 57, 1949–1963. [Google Scholar] [CrossRef]
- Geng, S.; Chen, D.; Guo, Z.; Li, Q.; Wen, M.; Wang, J.; Guo, K.; Wang, J.; Wang, Y.; Yu, L.; et al. Halloysite-nanotube-mediated high-flux γ-Al2O3 ultrafiltration membranes for semiconductor wastewater treatment. Membranes. 2025, 15, 130. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Yang, X.B.; Yan, L.L.; Bai, Y.P.; Li, S.W.; Sorokin, P.; Shao, L. Biomimetic nanoparticle-engineered superwettable membranes for efficient oil/water separation. J. Membr. Sci. 2021, 618, 118525. [Google Scholar] [CrossRef]
- Abhyankar, I.; Hirlekar, S.; Prabhune, A.; Nisal, A. Bridging the gap: An investigation of biosurfactants-polymer systems. Curr. Opin. Colloid Interface Sci. 2024, 72, 101806. [Google Scholar] [CrossRef]
- Ammar, M.; Yousef, E.; Mahmoud, M.A.; Ashraf, S.; Baltrusaitis, J. A comprehensive review of the developments in electrocoagulation for the removal of contaminants from wastewater. Separations 2023, 10, 337. [Google Scholar] [CrossRef]
- Li, H.F.; Cao, Y.J.; Liu, P.X.; Li, Y.Z.; Zhou, A.J.; Ye, F.; Xue, S.; Yue, X.P. Ammonia-nitrogen removal from water with g-C3N4-rGO-TiO2 Z-scheme system via photocatalytic nitrification-denitrification process. Environ. Res. 2022, 205, 112434. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Ji, S.F.; Chen, C.; Peng, Q.; Wang, D.S.; Li, Y.D. Single-atom catalysts: Synthetic strategies and electrochemical applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.W.; Wang, H.T. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Aslam, M.; Ismail, I.M.I.; Chandrasekaran, S.; Hameed, A.; Chandrasekaran, A. Hameed, Morphology controlled bulk synthesis of disc-shaped WO3 powder and evaluation of its photocatalytic activity for the degradation of phenols. J. Hazard. Mater. 2014, 276, 120–128. [Google Scholar] [CrossRef]
- Nie, J.K.; Yu, X.J.; Liu, Z.B.; Wei, Y.C.; Zhang, J.; Zhao, N.N.; Yu, Z.; Yao, B.H. Boosting principles for the photocatalytic performance of Cr-doped Cu2O crystallites and mechanisms of photocatalytic oxidation for levofloxacin. Appl. Surf. Sci. 2022, 576, 151842. [Google Scholar] [CrossRef]
- Gupta, T.; Chauhan, R.P. Optical, dielectric and photocatalytic properties of ZnO, ZnSe, and ZnO/ZnSe photocatalyst. Opt. Mater. 2023, 142, 114045. [Google Scholar] [CrossRef]
- Liu, J.Q.; Zhang, Q.R.; Tian, X.Y.; Hong, Y.; Nie, Y.C.; Su, N.N.; Jin, G.H.; Zhai, Z.X.; Fu, C. Highly efficient photocatalytic degradation of oil pollutants by oxygen deficient SnO2 quantum dots for water remediation. Chem. Eng. J. 2021, 404, 127146. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Nius, Y.; Zhang, X.C.; Jiang, Z.Y.; Zheng, J.M.; Guo, C.F. Combination of experimental and theoretical investigation on Ti-doped g-C3N4 with improved photo-catalytic activity. Appl. Surf. Sci. 2019, 489, 427–434. [Google Scholar] [CrossRef]
- Shi, Z.X.; Li, Y.M.; Dong, L.M.; Guan, Y.H.; Bao, M.T. Deep remediation of oil spill based on the dispersion and photocatalytic degradation of biosurfactant-modified TiO2. Chemosphere 2021, 281, 130744. [Google Scholar] [CrossRef] [PubMed]
- King, S.M.; Leaf, P.A.; Olson, A.C.; Ray, P.Z.; Tarr, M.A. Photolytic and photocatalytic degradation of surface oil from the Deepwater Horizon spill. Chemosphere 2014, 95, 415–422. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Younesi, H.; Zinatizadeh, A.A. Preparation, characterization and photocatalytic application of TiO2/Fe-ZSM-5 nanocomposite for the treatment of petroleum refinery wastewater: Optimization of process parameters by response surface methodology. Chemosphere 2016, 159, 552–564. [Google Scholar] [CrossRef]
- Cheng, C.K.; Derahman, M.; Khan, M. Evaluation of the photocatalytic degradation of pre-treated palm oil mill effluent (POME) over Pt-loaded titania. J. Environ. Chem. Eng. 2015, 3, 261–270. [Google Scholar] [CrossRef]
- He, J.N.; Hu, Z.Y.; Zhu, X.Q.; Liu, Z.H. Enhanced photocatalytic treatment of oilfield wastewater using TiO2/MoS2 nanocomposites prepared via sol-gel and hydrothermal methods. React. Kinet. Mech. Catal. 2025, 138, 1153–1174. [Google Scholar] [CrossRef]
- Yaacob, N.; Sean, G.P.; Nazri, N.A.M.; Ismail, A.F.; Abidin, M.N.Z.; Subramaniam, M.N. Simultaneous oily wastewater adsorption and photodegradation by ZrO2–TiO2 heterojunction photocatalysts. J. Water Process Eng. 2021, 39, 101644. [Google Scholar] [CrossRef]
- Hansen, H.P. Photochemical degradation of petroleum hydrocarbon surface films on seawater. Mar. Chem. 1975, 3, 183–195. [Google Scholar] [CrossRef]
- Mohd, K.; Sapawe, N. A short review on photocatalytic reaction in diesel degradation. Mater. Today Proc. 2020, 31, A33–A37. [Google Scholar]
- Esa, Y.; Sapawe, N. A short review on zinc metal nanoparticles synthesize by green chemistry via natural plant extracts. Mater. Today Proc. 2020, 31, 386–393. [Google Scholar] [CrossRef]
- Hanafi, M.F.; Harun, N.F.C.; Sapawe, N. Electrobiosyntesis of NiO using rambutan leaves for photodegradation of remazol brilliant blue dye. Malays. J. Anal. Sci. 2020, 24, 227–235. [Google Scholar]
- Mohd, M.; Sapawe, N. Removal of methyl orange over low-cost silica nanoparticles extrated from bamboo leaves ash. Mater. Today Proc. 2020, 31, A54–A57. [Google Scholar] [CrossRef]
- Hanafi, M.F.; Sapawe, N. Photocatalytic degradation of remazol brilliant blue dye using zirconia catalyst under visible light irradiation. Mater. Today Proc. 2020, 31, 272–274. [Google Scholar] [CrossRef]
- Hanafi, M.F.; Sapawe, N. Effect of microwave irradiation time on ZnO nanoparticles: Enhance the catalytic activity toward phenol degradation. AIP Conf. Proc. 2024, 2923, 040021. [Google Scholar] [CrossRef]
- Muhammad, F.H.; Sapawe, N. Effect of microwave power on ZnO nanoparticles: Enhance the catalytic activity toward phenol degradation. AIP Conf. Proc. 2024, 2923, 040020. [Google Scholar] [CrossRef]
- Wang, J.W.; Yu, T.; Wang, M.C.; Guo, X.; Chen, Y. A novel biochar-composed TiO2 (BC-Ti) for efficient photocatalytic degradation on arbidol. J. Ind. Eng. Chem. 2024, 134, 537–547. [Google Scholar] [CrossRef]
- Zambrano, J.; García-Encina, P.A.; Jiménez, J.J.; López-Serna, R.; Irusta-Mata, R. Photolytic and photocatalytic removal of a mixture of four veterinary antibiotics. J. Water Process Eng. 2022, 48, 102841. [Google Scholar] [CrossRef]
- Manassero, A.; Alfano, O.M.; Satuf, M.L. Degradation of emerging pollutants by photocatalysis: Radiation modeling and kinetics in packed-bed reactors. Water 2022, 14, 3608. [Google Scholar] [CrossRef]
- Ong, C.S.; Lau, W.J.; Goh, P.S.; Ng, B.C.; Ismail, A.F. Investigation of submerged membrane photocatalytic reactor (sMPR) operating parameters during oily wastewater treatment process. Desalination 2014, 353, 48–56. [Google Scholar] [CrossRef]
- Shahrezaei, F.; Mansouri, Y.; Zinatizadeh, A.A.L.; Akhbari, A. Process modeling and kinetic evaluation of petroleum refinery wastewater treatment in a photocatalytic reactor using TiO2 nanoparticles. Powder Technol. 2012, 221, 203–212. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Ziolli, R.L.; Jardim, W.F. Photocatalytic decompsition of seawater-soluble crude oil fractions using high surface areacolloid nanoparticles of TiO2. J. Photochem. Photobiol. A-Chem. 2002, 147, 205–212. [Google Scholar] [CrossRef]
- Cybula, A.; Nowaczyk, G.; Jarek, M.; Zaleska, A. Preparation and characterization of Au/Pd modified-TiO2 photocatalysts for phenol and toluene degradation under visible light-the effect of calcination temperature. J. Nanomater. 2014, 2014, 918607. [Google Scholar] [CrossRef]
- Hu, D.D. Preparation of ZnO-Based Photocatalysts and Its Application in Marine Oil Pollution. Master’s Thesis, Dalian Ocean University, Dalian, China, 2013. [Google Scholar]
- Liu, L.; Xue, Z.K.; Gao, T.; Zhao, Q.P.; Sun, Y.J.; Wu, Y.Z. Photocatalytic degradation performance of Ag-modified flexible TiO2 nanofiber film. Opt. Mater. 2025, 160, 116720. [Google Scholar] [CrossRef]
- Merdoud, R.; Aoudjit, F.; Mouni, L.; Honavar, V.; Moghadam, R.P.; Palabathuni, M.; Ranade, V.V. Methyl Orange Degradation Using Ag-Doped TiO2, H2O2, and Hydrodynamic Cavitation. ACS Omega 2025, 10, 21377–21390. [Google Scholar] [CrossRef]
- Taufik, A.; Yoko, A.; Han, C.L.; Wahyudiono; Ohara, S.; Adschiri, T. Facet dependent pt adsorption on rutile TiO2 surface for efficient photocatalytic VOCs removal. Small 2025, 21, e2412727. [Google Scholar] [CrossRef]
- Bi, F.K.; Zhang, Y.F.; Zhou, Z.X.; Guo, L.; Zhu, Z.Q.; Liu, B.L.; Zhang, X.D. Electron beam irradiation-induced defects enhance Pt-TiO2 photothermal catalytic degradation in PAEs: A performance and mechanism study. Molecules 2025, 30, 697. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A.; Kowalska, E.; Sobczak, J.W.; Lisowski, W.; Ohtani, B.; Zaleska, A. Preparation and characterization of monometallic (Au) and bimetallic (Ag/Au) modified-titania photocatalysts activated by visible light. Appl. Catal. B Environ. 2011, 101, 504–514. [Google Scholar] [CrossRef]
- Rahman, E.H.; Nair, P.S.; Shinoj, V.K.; Shaji, S.; Bunnell, A.; Jobson, N.; Philip, R.R. Enhanced photocatalytic degradation efficiency of TiO2 nanotubes by potassium doping. J. Mater. Sci.-Mater. Electron. 2025, 36, 236. [Google Scholar] [CrossRef]
- Hajri, E.A.; Alrashedi, N.A.; Alabbad, S.S.; Younas, M.; Malih, R.J.; ALZahrani, A.; Kochkar, H.; Ercan, I. Strontium-modified TiO2 nanotubes for efficient photodegradation of formic acid. Arab. J. Sci. Eng. 2025, 1–15. [Google Scholar] [CrossRef]
- Ángeles-Beltrán, D.; Chavez-Esquivel, G.; Tavizón-Pozos, J.A.; Suárez-Toriello, V.A.; Santolalla-Vargas, C.E.; Aguilar-Martínez, O. Photocatalytic degradation of phenol using cadmium-TiO2/chitosan hybrid catalysts. Mater. Res. Express 2025, 12, 065901. [Google Scholar] [CrossRef]
- Joodi, A.; Allahyari, S.; Rahemi, N.; Hoseini, S.; Tasbihi, M. ZnO-C3N4 solar light-driven nano-photocatalysts on floating recycled PET bottle as support for degradation of oil spill. Ceram. Int. 2020, 46, 11328–11339. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Ji, L.; Song, F.; Li, T.; Fu, X.; Li, Q.; Xing, Y.; Zhang, Q.; Wang, J. Effects of salinity on the biodegradation of polycyclic aromatic hydrocarbons in oilfield soils emphasizing degradation genes and soil enzymes. Front. Microbiol. 2022, 12, 824319. [Google Scholar] [CrossRef]
- Mazoyer, C.; Vanneste, H.; Dufresne, C.; Ourmières, Y.; Magaldi, M.G.; Molcard, A. Impact of wind-driven circulation on contaminant dispersion in a semi-enclosed bay. Estuar. Coast. Shelf Sci. 2020, 233, 106529. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, S.; Sathiyaseelan, A.; Zhang, L.; Lu, Y.; Chen, Y.; Jin, T.; Wang, M.-H. Recent advances in photocatalytic nanomaterials for environmental remediation: Strategies, mechanisms, and future directions. Chemosphere 2024, 364, 143142. [Google Scholar] [CrossRef]
- Samuel, O.; Khan, A.U.; Othman, M.H.D.; Kurniawan, T.A.; Kamaludin, R.; Matsuura, T.; Imtiaz, A.; Rushdan, A.I. Visible light-driven TiO2-WO3@ GO photocatalyst with catalytic memory for round-the-clock photocatalytic degradation of oilfield-produced water. Ceram. Int. 2024, 50, 18205–18219. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, X.C.; Nie, Z.W.; Guo, M.C.; Liu, J.H.; Wang, L.P. Preparation of ZnO/SnO2 composite nanometer photocatalyst and photocatalytic treatment of marine diesel pollution. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Kunming, China, 27–29 October 2017; Volume 281, p. 12015. [Google Scholar]
- Duan, Y.K.; Wang, C.; Yang, S.L.; Liu, L.F. Critical roles of Co-doped g-C3N4/Bi2WO6 in photocatalytic removal of organic pollutants and treating oil field wastewater in electrocatalysis-microbial fuel cells. J. Chem. Technol. Biotechnol. 2023, 98, 2554–2567. [Google Scholar] [CrossRef]
- Pandey, S.K.; Romac, S.; Zjačić, J.P.; Roković, M.K.; Kovačić, M.; Kušić, H.; Žener, B.; Genorio, B.; Štangar, U.L.; Božić, A.L. SnS2-TiO2 heterojunction designed for reductive degradation of contaminants of emerging concern. Nanomaterials 2025, 15, 969. [Google Scholar] [CrossRef]
- Natali Sora, I.; Bertolotti, B.; Pelosato, R.; Lucotti, A.; Tommasini, M.; Muscetta, M. TiO2/LaFeO3 Composites for the efficient degradation of benzoic acid and hydrogen production. Molecules 2025, 30, 1526. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, V.; Lavanya, V.; Karthik, P.; Sarala, S.; Prakash, N.; Mukkannan, A. Enhanced photocatalytic degradation of organic pollutants via TiO2-integrated 2D MoS2 nanostructures. Surf. Interfaces 2025, 72, 107089. [Google Scholar] [CrossRef]
- Dai, J.Z.; Wu, Y.H.; Yao, Y.H.; Zhang, B. ZnO/TiO2 photocatalysts for degradation of methyl orange by low-power irradiation. Sci. Prog. 2025, 108, 368504251322606. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhao, Z.; Ma, J.; Kong, W.; Wang, Z.; Guo, S.; Shen, B. Photothermal synergistic catalysis: CeO2/MnO2/TiO2 ternary heterojunction for enhanced toluene degradation. Fuel 2025, 404, 136150. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Tran, Q.B.; Nguyen, X.C.; Hai, L.T.; Ho, T.T.T.; Shokouhimehr, M.; Vo, D.-V.N.; Lam, S.S.; Nguyen, H.P.; Hoang, C.T.; et al. Submerged photocatalytic membrane reactor with suspended and immobilized N-doped TiO2 under visible irradiation for diclofenac removal from wastewater. Process Saf. Environ. Prot. 2020, 142, 229–237. [Google Scholar] [CrossRef]
- Ding, J.; Han, Z.; Yang, X.; Liu, J.; Guo, K.; Fan, H.; Hu, P.; Teng, F. Multifunctional ZnO/PVDF hybrid fiber membrane: Enhanced photocatalytic degradation performance and oil/water separation application. Mater. Sci. Semicond. Process. 2025, 185, 108942. [Google Scholar] [CrossRef]
- Parangusan, H.; Bhadra, J.; Ahmad, Z.; Al-Maadeed, A.S.M.A.; Al-Mohannadi, A.M.A.A.; Al-Thani, N. Electrospun PVDF/ZnO based composite fibers for oil absorption and photocatalytic degradation of organic dyes from waste water. Fibers Polym. 2022, 23, 1217–1224. [Google Scholar] [CrossRef]
- Chiulan, I.; Dumitru, F.-D.; Matei, M.; György, D.; Izhar, T.N.T. Removal of oil from wastewater: Sources and advances in polymeric photocatalytic membranes. E3s Web Conf. 2024, 589, 6003. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, Z.; Yang, Z.; Wang, J.; Zhu, Q.X.X. Application of in-situ microbubble method on SEP@MnO2/RGO composite membrane for efficient and long-acting treatment of oil field wastewater. Diam. Relat. Mater. 2022, 130, 109499. [Google Scholar] [CrossRef]
- Wang, J.; Yu, B.; Hou, K.; Ji, Y.; Li, X.; Li, C.; Yao, C.; Cai, Z. PH-responsive fiber membrane with high flux and photocatalytic performance for oily wastewater. J. Environ. Chem. Eng. 2025, 13, 115322. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.; Luo, P.; Ma, L.; Li, S.; Nie, Y.; Zhong, F.; Wang, Y.; Chen, L. Photocatalytic GO/M88A “interceptor plate” assembled nanofibrous membrane with photo-Fenton self-cleaning performance for oil/water emulsion separation. Chem. Eng. J. 2022, 427, 130948. [Google Scholar] [CrossRef]
- Tian, S.; He, Y.; Zhang, L.; Li, S.; Bai, Y.; Wang, Y.; Wu, J.; Yu, J.; Guo, X. CNTs/TiO2-loaded carbonized nanofibrous membrane with two-type self-cleaning performance for high efficiency oily wastewater remediation. Colloids Surf. A-Physicochem. Eng. Asp. 2023, 656, 130306. [Google Scholar] [CrossRef]
- Qu, J.; Xue, J.; Wu, Z.; Wu, T.; Huang, K.; Wang, M.; Ma, S. Self-assembled supramolecular/Fe-MOF functionalized nanofibrous membrane with switchable superwettability and photo-Fenton activity for on-demand oily wastewater treatment. Sep. Purif. Technol. 2025, 368, 133092. [Google Scholar] [CrossRef]
- Chaithra, K.P.; Varghese, A.; Vinod, T.P.; Sunaja Devi, K.R. Multifunctional electrospun membranes incorporated with metal oxide nanoparticles, cellulose acetate, and polyvinylpyrrolidone for wastewater treatment: Oil/water separation, dye adsorption, and dye degradation. Chem. Eng. J. 2024, 499, 156049. [Google Scholar] [CrossRef]
- Lu, W.; Duan, C.; Zhang, Y.; Gao, K.; Dai, L.; Shen, M.; Wang, W.; Wang, J.; Ni, Y. Cellulose-based electrospun nanofiber membrane with core-sheath structure and robust photocatalytic activity for simultaneous and efficient oil emulsions separation, dye degradation and Cr (VI) reduction. Carbohydr. Polym. 2021, 258, 117676. [Google Scholar] [CrossRef] [PubMed]
- Gunatilake, U.B.; Bandara, J. Efficient removal of oil from oil contaminated water by superhydrophilic and underwater superoleophobic nano/micro structured TiO2 nanofibers coated mesh. Chemosphere 2017, 171, 134–141. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Zhao, Y.; Wang, H. Bifunctional TiO2-coated SSM for efficient emulsion separation and photocatalytic degradation of soluble organic pollutants. Langmuir 2024, 40, 22787–22793. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, S.; Sun, S.; Chen, Y. TiO2 coated stainless steel mesh with high separation efficiency and flux to separate dispersed and emulsified oil in oil-in-water solution. Sep. Purif. Technol. 2025, 373, 133605. [Google Scholar] [CrossRef]
- Wang, D.; Huang, L.; Sun, H.; Li, S.; Wang, G.; Zhao, R.; Zhou, S.; Sun, X. Enhanced photogenic self-cleaning of superhydrophilic Al2O3@GO-TiO2 ceramic membranes for efficient separation of oil-in-water emulsions. Chem. Eng. J. 2024, 486, 150211. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, L.; Wu, W.; An, H.; Feng, M.; Sun, M.; Zhao, Y.; Chen, L. S-doped TiO2 nanotubes decorated whisker mullite-based hollow fiber membrane with anti-fouling and photocatalytic self-cleaning properties for high-efficient separation of emulsified oily wastewater. Sep. Purif. Technol. 2025, 373, 133615. [Google Scholar] [CrossRef]
- Akrami, M.R.; Sadeghian, Z.; Ashrafizadeh, S.N. Oily wastewater treatment via a visible-light-responsive slurry membrane photocatalytic reactor incorporating Bi2WO6-based photocatalysts. Chem. Eng. Process.-Process Intensif. 2025, 215, 110365. [Google Scholar] [CrossRef]
- Bao, Z.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Superamphiphilic and underwater superoleophobic membrane for oil/water emulsion separation and organic dye degradation. J. Membr. Sci. 2020, 598, 117804. [Google Scholar] [CrossRef]
- Medeiros, A.D.M.D.; Silva Junior, C.J.G.D.; Durval, I.J.B.; Souza, T.C.D.; Cavalcanti, Y.D.F.; Costa, A.F.D.S.; Sarubbo, L.A. Treatment of oily effluents using a bacterial cellulose membrane as the filter bed. Processes 2024, 12, 1542. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, H.Y.; Wang, R.; Zhang, J.J.; Wei, K.X.; Li, X.F. Robust chitosan sponges for highly efficient aggregation-induced demulsification separation of emulsified oil from surfactant-polymer oily sewage. J. Mater. Sci. 2024, 59, 12885–12898. [Google Scholar] [CrossRef]
- Bande, R.M.; Prasad, B.; Mishra, I.M.; Wasewar, K.L. Oil field effluent water treatment for safe disposal by electroflotation. Chem. Eng. J. 2008, 137, 503–509. [Google Scholar] [CrossRef]
- Ben, M.L.; Chalbi, S. Removal of oil from oil/water emulsions using electroflotation process. J. Appl. Electrochem. 2006, 36, 577–581. [Google Scholar] [CrossRef]
- Mostefa, N.M.; Tir, M. Coupling flocculation with electroflotation for waste oil/water emulsion treatment: Optimization of the operating conditions. Desalination 2004, 161, 115–121. [Google Scholar] [CrossRef]
- Su, B.; Ma, X.; Ran, L.; Wu, S.; Hou, B. Improving the photodegradation efficiency of POPs in oily sewage: Design and synthesis of G-C3N4-based photocatalysts incorporating a pickering emulsion catalytic strategy. J. Environ. Chem. Eng. 2023, 11, 111297. [Google Scholar] [CrossRef]
- Wang, T.S.; Shi, P.B.; Wang, M.Y.; Zhang, S.J. Preparation of AuNP-CQD/PDA/GO anode for MFC and its treatment of oily sewage from ships. Environ. Sci. Pollut. Res. 2023, 30, 66427. [Google Scholar]
- Lu, H.; Zhang, Y.; Zhang, B.; Jiang, S.; Qu, N.; Xiao, C.; Li, L.; Li, G.; Chen, L. Preparation of newly polymer-coated microbial pellets and their adsorption and degradation properties for oil-containing wastewater. Langmuir 2024, 40, 11239–11250. [Google Scholar] [CrossRef]
- Rachel, I.; Debarun, D.P. A cauliflower-like MXene based membrane with light trapping ability for oily wastewater treatment. Chem. Phys. Lett. 2024, 852, 41502. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, Y.; Zhang, D.; Guan, Y.; Wang, Z.; Bao, M.; Li, Y. High-efficiency treatment of oily wastewater by photocatalytic in-situ Fenton oxidation under visible light. Chem. Eng. J. 2024, 500, 156848. [Google Scholar] [CrossRef]
- Yasmina, M.; Mourad, K.; Zineb, A. Combined photocatalytic and Fenton oxidation for oily wastewater treatment. Appl. Water Sci. 2019, 9, 35. [Google Scholar] [CrossRef]
- Song, G.; Du, X.; Zheng, Y.; Su, P.; Tang, Y.; Zhou, M. A novel electro-Fenton process coupled with sulfite: Enhanced Fe3+ reduction and TOC removal. J. Hazard. Mater. 2022, 422, 126888. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).