Current Progress in Advanced Oxidation Processes for the Removal of Contaminants of Emerging Concern Using Peracetic Acid as an Effective Oxidant
Abstract
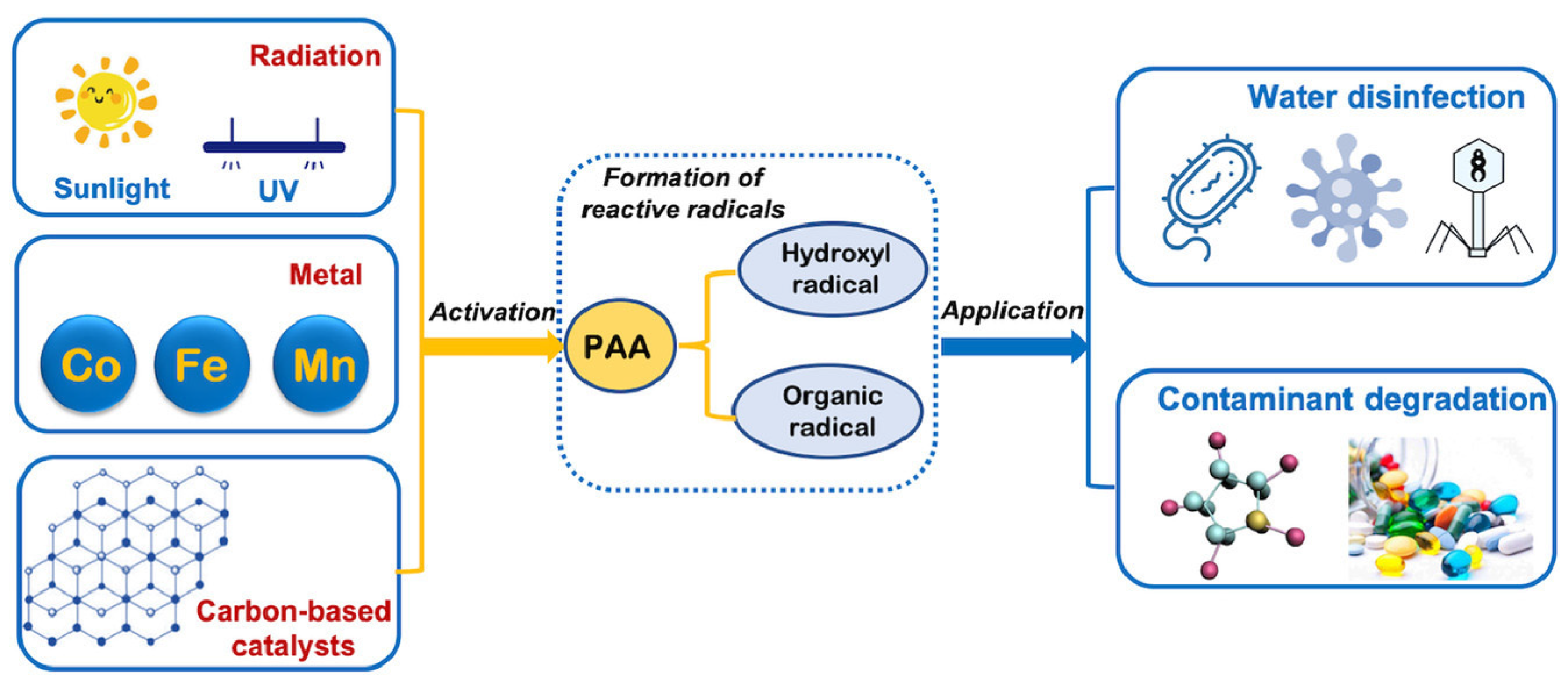
1. Introduction
2. Intrinsic Performance of PAA
3. Proficiency of PAA-Based AOPs in CECs Remediation
- -
- -
- -
- -
- -
3.1. Transition Metal for the Activation of PAA
3.1.1. Iron-Based Catalysis for PAA Activation
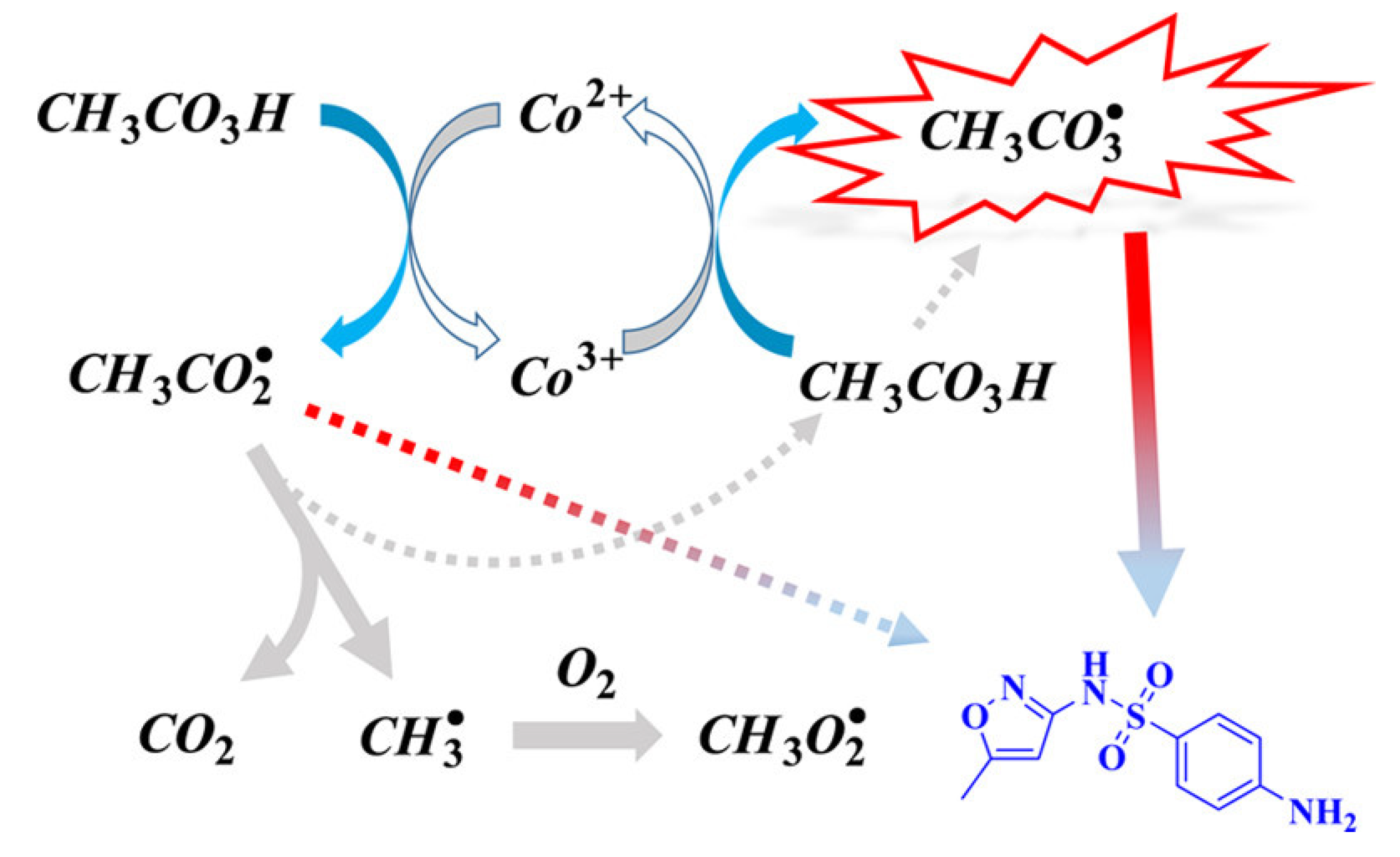
3.1.2. Cobalt-Based Catalysis for PAA Activation
3.1.3. Manganese-Based Catalysis for PAA Activation
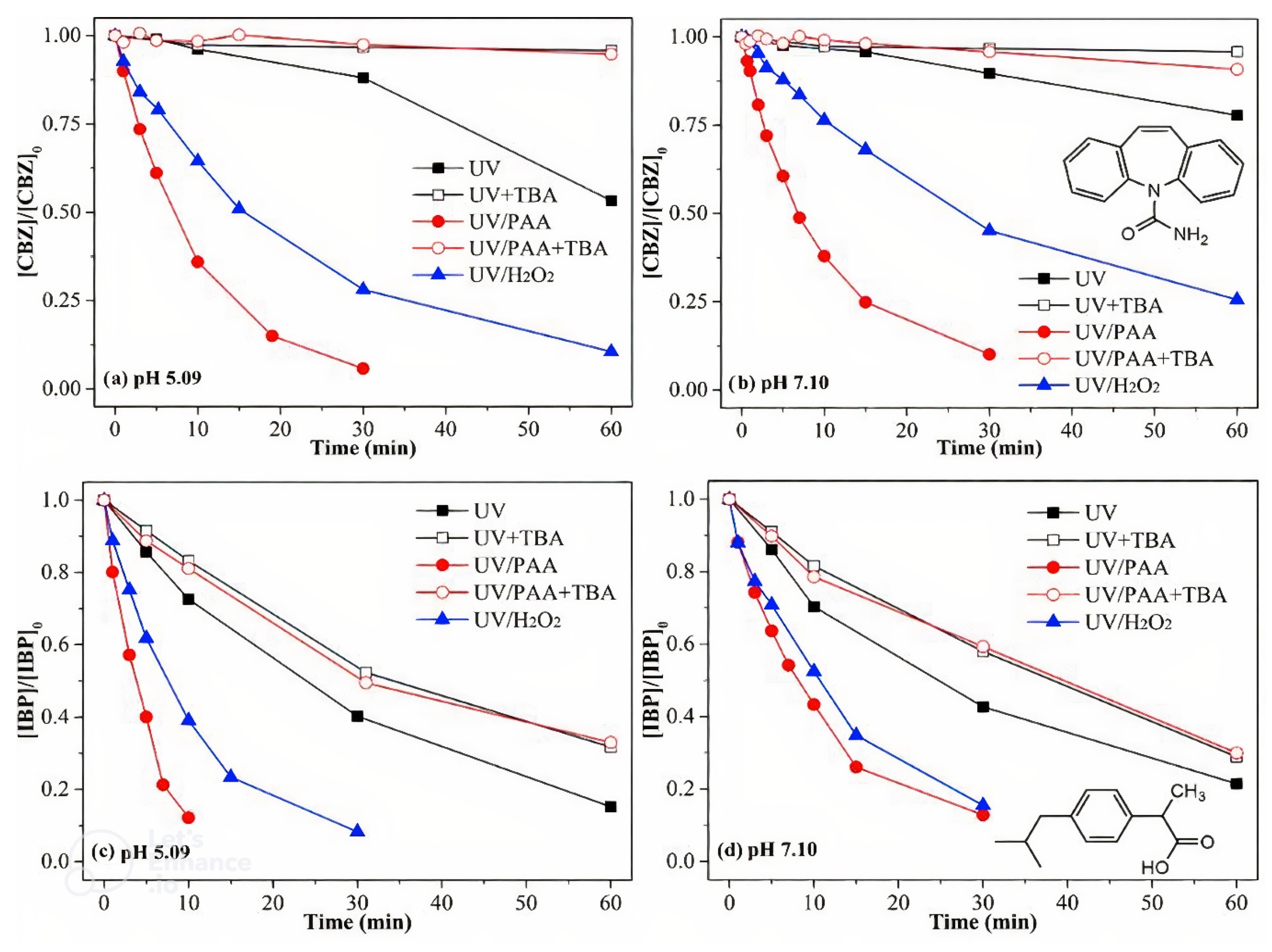
3.2. UV Irradiation-Induced Activation of PAA
| CEC | [CEC]0 | Removal Efficiency (%) | Activator | PAA Dose | pH | Reaction Time | Ref. |
|---|---|---|---|---|---|---|---|
| Fluoxetine | 5 mg·L−1 | 100 | UV (254 nm) irradiation: (647–3502) W·m−3 | 5–100 mg·L−1 | 7 | 30 min | [53] |
| Sulfamethoxazole | 5 mg·L−1 | 100 | UV (254 nm) irradiation: (647–3502) W·m−3 | 5–100 mg·L−1 | 7 | 30 min | [53] |
| Diclofenac | 1 μM | 90 | UV (254 nm) intensity: 2.12 × 10−6 E·L−1·s−1 | 1 mg·L−1 | 7.1 | <5 min | [21] |
| Ibuprofen | 1 μM | 90 | UV (254 nm) intensity: 2.12 × 10−6 E·L−1·s−1 | 1 mg·L−1 | 7.1 | 30 min | [21] |
| Carbamazepine | 1 μM | >90 | UV (254 nm) intensity: 2.12 × 10−6 E·L−1·s−1 | 1 mg·L−1 | 7.1 | 30 min | [21] |
| Naproxen | 1 μM | >95 | UV (254 nm) intensity: 2.12 × 10−6 E·L−1·s−1 | 1 mg·L−1 | 7.1 | 10 min | [21] |
| Chloramphenicol | 25 mg·L−1 | 100 | UV (254 nm) doses: 0~12.5 W·m−2 | 5–50 mg·L−1 | Not mentioned | 120 min | [96] |
| Clofibric acid | 1 μM | >90 | UV (254 nm) intensity: 2.12 × 10−6 E·L−1·s−1 | 1 mg·L−1 | 7.1 | 10 min | [21] |
| Venlafaxine | 5 mg·L−1 | 100 | UV (254 nm) irradiation: (647–3502) W·m−3 | 5–100 mg·L−1 | 7 | 30 min | [53] |
| 4-chlorophenol | 4 μM | >90 | UV (254 nm) | 3042 mg·L−1 | 9.5 | 5 min | [54] |
| Pentachlorophenol | 4 μM | 100 | UV (254 nm) | 3042 mg·L−1 | 9.5 | 5 min | [54] |
| 2,4,6-trichlorophenol | 4 μM | >80 | UV (254 nm) | 3042 mg·L−1 | 9.5 | 5 min | [54] |
| 2,4-dichlorophenol | 4 μM | 100 | UV (254 nm) | 3042 mg·L−1 | 9.5 | 5 min | [54] |
| Bezafibrate | 1 μM | 80 | UV (254 nm) intensity: 2.12 × 10−6 E·L−1·s−1 | 1 mg·L−1 | 7.1 | 120 min | [21] |
| Carbamazepine | 5 mg·L−1 | 100 | UV (254 nm) irradiation: (647–3502) W·m−3 | 5–100 mg·L−1 | 7 | 30 min | [53] |
| Naproxen | 4 μM | 80 | UV (254 nm) intensity: 9.04 × 10−8 E·L−1·s−1 | 20 mg·L−1 | 7 | 14 min | [92] |
3.3. Carbon-Based Catalyst for the Activation of PAA
| CEC | Catalyst | Processes | [CEC]0 | Removal Efficiency (%) | PAA Dose | PH | Reaction Time | Ref. |
|---|---|---|---|---|---|---|---|---|
| Reactive Brilliant Red X-3B | ACFs | ACFs/PAA | 50 μM | 97 | 5 mM | 7 | 45 min | [99] |
| sulfamethoxazole | rGO | rGO/PAA | 10 μM | 100 | 0.1 mM | 5 | 5 min | [98] |
| bisphenol A | CNTs | CNT/PAA | 0.02 mM | 96.4 | 0.25 mM | 7 | 20 min | [97] |
| phenol | CNT950 | CNT950/PAA | 20 μM | 100 | 0.15 mM | 10 | 10 min | [111] |
| sulfamethoxazole | AC600 | AC600/PAA | 20 mg·L−1 | 99.4 | 0.26 mM | 7 | 150 min | [59] |
| sulfamethazine | activated biochar (ABC) | ABC/PAA | 5 mg·L−1 | 72.8 | 0.07 mM | 7 | 100 min | [103] |
| phenol | carbonized polyaniline (CPANI) | CPANI/PAA | 10 μM | 97 | 0.1 mM | 7 | 60 min | [112] |
- -
- The first mechanism involves the generation of singlet oxygen 1, generated through PAA activation by N-doped carbonaceous catalysts. For example, Tian et al. [112] employed carbonized polyaniline (CPANI) as the catalyst, where the C=O group on CPANI activated PAA to generate 1, the main reactive species driving phenol degradation.
- -
- The second mechanism is based on direct electron transfer (DET). In this context, Kong et al. [98] demonstrated the effectiveness of reduced graphene oxide (rGO) in activating PAA for the rapid removal of SMX, achieving a near-complete elimination of the pollutant within just 2 min. Through a combination of quenching experiments, open-circuit potential measurements, and probe-based studies, they confirmed that DET was the dominant degradation pathway. The rGO/PAA system exhibited strong removal performance even in complex water matrices, highlighting the advantages of DET-driven oxidation. Building upon these findings, subsequent research explored other carbon-based materials to enhance DET processes. For instance, Kong et al. [111] emphasized the role of the physicochemical properties of CNTs on organic pollutant removal and PAA activation. The CNT/PAA system was also dominated by the DET oxidation pathway, achieving high MP removal rates. The enhanced catalytic efficiency of surface-regulated CNTs was attributed to reinforced DET, driven by the increased oxidative potential of the CNT/PAA complex and the enhanced electrical conductivity of CNTs. Furthermore, the larger specific surface area and lower oxygen content of CNTs were found to contribute significantly to the elevated oxidative potential, with electrical conductivity closely linked to their degree of graphitization.
- -
- The third degradation mechanism involves the participation of active radicals, as demonstrated by a study on the degradation of 4-chlorophenol (4-CP) via PAA activation using a newly synthesized biochar (P5S5-SDBC) [60]. Mediated electrochemical oxidation and reduction tests revealed that persistent free radicals (PFRs), generated by structural defects in the biochar, acted as electron shuttles, enhancing PAA activation and promoting the formation of reactive species. Electron paramagnetic resonance (EPR) measurements further confirmed that radicals were the primary active species responsible for 4-CP degradation [60].
3.4. Comparative Analysis of Catalytic System for PAA Activation: Transition Metals, UV Irradiation, and Carbon-Based Catalysts
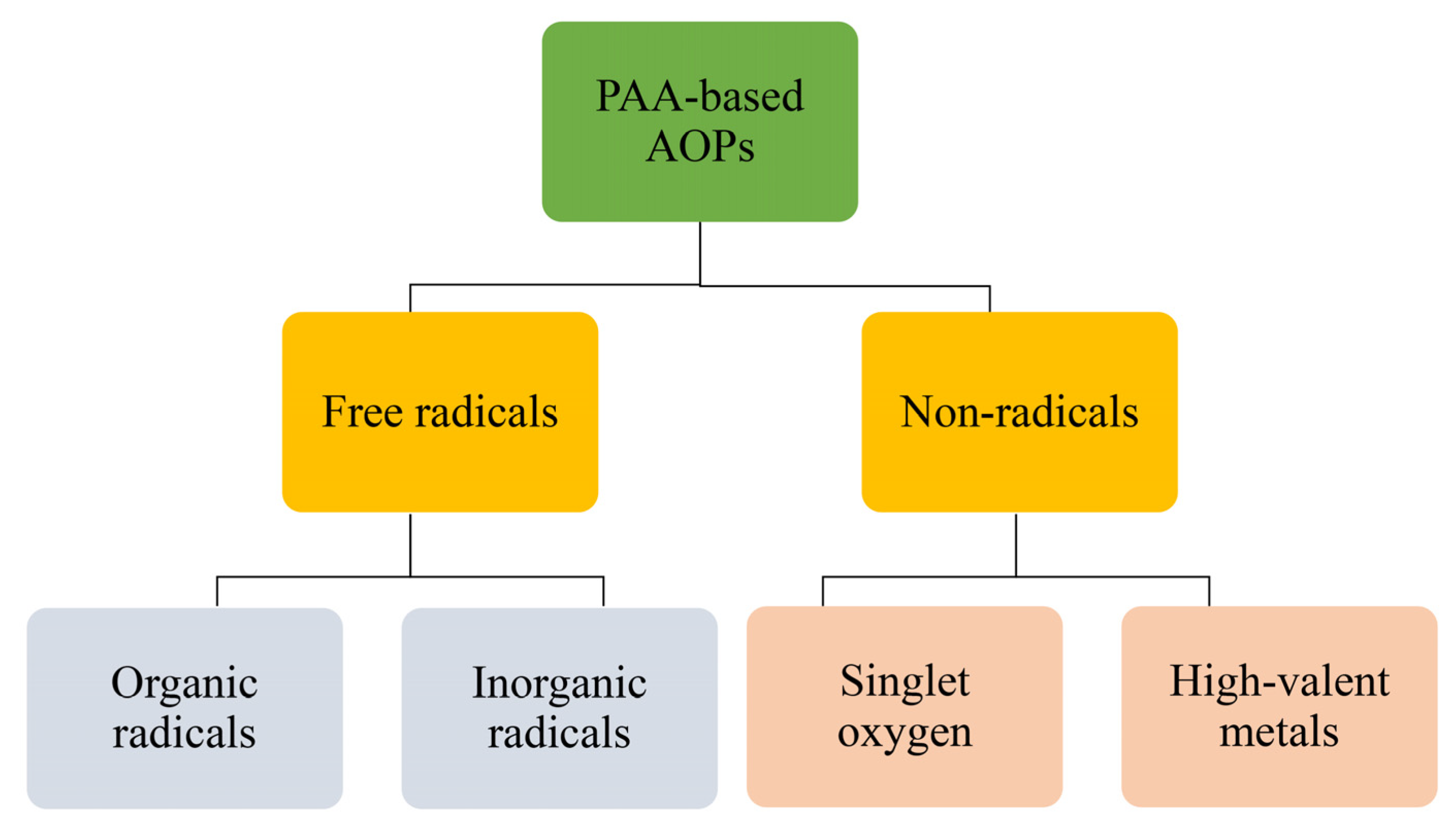
4. Determination of Reactive Species in PAA-Based AOPs for Water Decontamination
4.1. Free Radicals
4.1.1. Organic Radicals: and
4.1.2. Inorganic Radicals:
4.2. Non-Radical Species
4.2.1. Singlet Oxygen (1)
4.2.2. High-Valent Metals (HVMs)
5. By-Products and Hazard Assessment of PAA-Based AOPs
6. Factors Affecting the Removal of CECs in PAA-Based Systems
6.1. Impact of Initial PAA Concentration
6.2. Effects of Catalyst Dosage
6.3. Effects of pH
7. Challenges and Future Perspectives
- (1)
- A comprehensive study of the selective oxidation mechanisms of both non-radical and organic free radical pathways is essential. Non-radical mechanisms, such as 1 and HVMs, have emerged as key areas of interest. Crucial subjects for further investigation involve the precise detection and quantification of and , as well as exploring the oxidation capacity of organic radicals toward pollutants. Additionally, the differences in product accumulation and toxicity alterations between the free radical and non-free radical pathways for the same pollutant require further investigation.
- (2)
- Conducting parameter optimization techniques and their applications; creating analytical approaches for three-dimensional and other multidimensional factors is a key strategy for obtaining more sophisticated parameter optimization.
- (3)
- Investigating affordable and sustainable approaches for activating PAA, such as solar irradiation, holds significant potential for the degradation of CECs. The solar irradiation/PAA process offers advantages like easy accessibility, the absence of the need for additional chemicals, and its renewable nature, making it a promising avenue for further development and application.
- (4)
- Developing catalysts with enhanced catalytic efficiency: PAA-based AOPs primarily focus on metal-derived catalysts, with being the most commonly studied activator. While exhibits high catalytic performance at a neutral pH, its release into the environment poses a secondary source of pollution, necessitating careful consideration in future applications. Therefore, iron activation is considered a more environmentally friendly option. However, iron-based systems are highly pH-sensitive and involve additional chemicals for pH regulation. Moreover, although heterogeneous catalysts release metal ions at lower concentrations, these ions can still accumulate to levels exceeding water treatment standards. To mitigate these issues, encapsulating metal nanoparticles within a carbon or polymer layer offers a promising solution. This encapsulation enables the precise tuning of the electronic structure and work function, while leveraging the electron tunneling effect at the composite interface to optimize catalytic performance. Such advancements could enhance the efficiency and environmental compatibility of PAA-based AOPs, paving the way for more sustainable water treatment technologies.
- (5)
- Given that most current studies use ultrapure water matrices, and few explore real wastewater conditions, the influence of coexisting ions and natural organic matter remains poorly understood. Further research using real water matrices is critically needed to better predict process performance under practical conditions.
- (6)
- Additionally, while the laboratory-scale efficiency of PAA-based AOPs has been demonstrated, few studies have addressed the development of scalable reactor designs or process integration strategies for industrial applications. Future work should focus on conducting pilot-scale studies to evaluate the use of PAA-based AOPs in large-scale systems, focusing on managing multiple pollutants in real-world water treatment processes.
- (7)
- Additionally, efforts should be made to integrate PAA-based AOPs with technologies such as electrochemistry, membrane filtration, and biological treatments to improve overall efficiency.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pal, A.; He, Y.; Jekel, M.; Reinhard, M.; Gin, K.Y.-H. Emerging Contaminants of Public Health Significance as Water Quality Indicator Compounds in the Urban Water Cycle. Environ. Int. 2014, 71, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, J. Drinking Water Quality and Public Health. Expo. Health 2019, 11, 73–79. [Google Scholar] [CrossRef]
- Kotowska, U.; Karpińska, J.; Kiejza, D.; Ratkiewicz, A.; Piekutin, J.; Makarova, K.; Olchowik-Grabarek, E. Oxidation of Contaminants of Emerging Concern by Combination of Peracetic Acid with Iron Ions and Various Types of Light Radiation–Optimization, Kinetics, Removal Efficiency and Mechanism Investigation. J. Mol. Liq. 2023, 369, 120859. [Google Scholar] [CrossRef]
- Richardson, S.D.; Kimura, S.Y. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2016, 88, 546–582. [Google Scholar] [CrossRef]
- Wu, X.; Ren, J.; Xu, Q.; Xiao, Y.; Li, X.; Peng, Y. Priority Screening of Contaminant of Emerging Concern (CECs) in Surface Water from Drinking Water Sources in the Lower Reaches of the Yangtze River Based on Exposure-Activity Ratios (EARs). Sci. Total Environ. 2023, 856, 159016. [Google Scholar] [CrossRef]
- Ahmad, A.; Kurniawan, S.B.; Abdullah, S.R.S.; Othman, A.R.; Hasan, H.A. Contaminants of Emerging Concern (CECs) in Aquaculture Effluent: Insight into Breeding and Rearing Activities, Alarming Impacts, Regulations, Performance of Wastewater Treatment Unit and Future Approaches. Chemosphere 2022, 290, 133319. [Google Scholar] [CrossRef] [PubMed]
- Kasonga, T.K.; Coetzee, M.A.A.; Kamika, I.; Ngole-Jeme, V.M.; Momba, M.N.B. Endocrine-Disruptive Chemicals as Contaminants of Emerging Concern in Wastewater and Surface Water: A Review. J. Environ. Manag. 2021, 277, 111485. [Google Scholar] [CrossRef]
- Gavrilescu, M. Environmental Biotechnology: Achievements, Opportunities and Challenges. Dyn. Biochem. Process Biotechnol. Mol. Biol. 2010, 4, 1–36. [Google Scholar]
- Kumari, P.; Kumar, A. Advanced Oxidation Process: A Remediation Technique for Organic and Non-Biodegradable Pollutant. Results Surf. Interfaces 2023, 11, 100122. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.M.I.; Ong, H.C.; Chia, W.Y. Recent Developments in Physical, Biological, Chemical, and Hybrid Treatment Techniques for Removing Emerging Contaminants from Wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef]
- Khan, J.A.; Sayed, M.; Khan, S.; Shah, N.S.; Dionysiou, D.D.; Boczkaj, G. Advanced Oxidation Processes for the Treatment of Contaminants of Emerging Concern. In Contaminants of Emerging Concern in Water and Wastewater; Elsevier: Amsterdam, The Netherlands, 2020; pp. 299–365. [Google Scholar]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A Review of the Recent Advances on the Treatment of Industrial Wastewaters by Sulfate Radical-Based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Scaria, J.; Nidheesh, P.V. Comparison of Hydroxyl-Radical-Based Advanced Oxidation Processes with Sulfate Radical-Based Advanced Oxidation Processes. Curr. Opin. Chem. Eng. 2022, 36, 100830. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Wang, Y. A Critical Review on Removal of Gaseous Pollutants Using Sulfate Radical-Based Advanced Oxidation Technologies. Environ. Sci. Technol. 2021, 55, 9691–9710. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, X.; Fu, Y.; Dionysiou, D.D. Degradation Kinetics and Mechanism of Oxytetracycline by Hydroxyl Radical-Based Advanced Oxidation Processes. Chem. Eng. J. 2016, 284, 1317–1327. [Google Scholar] [CrossRef]
- Li, J.; Yang, T.; Zeng, G.; An, L.; Jiang, J.; Ao, Z.; Ma, J. Ozone-and Hydroxyl Radical-Induced Degradation of Micropollutants in a Novel UVA-LED-Activated Periodate Advanced Oxidation Process. Environ. Sci. Technol. 2023, 57, 18607–18616. [Google Scholar] [CrossRef] [PubMed]
- Liou, S.-Y.; Dodd, M.C. Evaluation of Hydroxyl Radical and Reactive Chlorine Species Generation from the Superoxide/Hypochlorous Acid Reaction as the Basis for a Novel Advanced Oxidation Process. Water Res. 2021, 200, 117142. [Google Scholar] [CrossRef]
- Ao, X.; Eloranta, J.; Huang, C.-H.; Santoro, D.; Sun, W.; Lu, Z.; Li, C. Peracetic Acid-Based Advanced Oxidation Processes for Decontamination and Disinfection of Water: A Review. Water Res. 2021, 188, 116479. [Google Scholar] [CrossRef]
- Kim, J.; Du, P.; Liu, W.; Luo, C.; Zhao, H.; Huang, C.-H. Cobalt/Peracetic Acid: Advanced Oxidation of Aromatic Organic Compounds by Acetylperoxyl Radicals. Environ. Sci. Technol. 2020, 54, 5268–5278. [Google Scholar] [CrossRef]
- Cai, M.; Sun, P.; Zhang, L.; Huang, C.-H. UV/Peracetic Acid for Degradation of Pharmaceuticals and Reactive Species Evaluation. Environ. Sci. Technol. 2017, 51, 14217–14224. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Homlok, R.; Kovács, K.; Tegze, A.; Takács, E. Oxidation and Mineralization Rates of Harmful Organic Chemicals in Hydroxyl Radical Induced Reactions. Ecotoxicol. Environ. Saf. 2024, 281, 116669. [Google Scholar] [CrossRef] [PubMed]
- Krystynik, P. Advanced Oxidation Processes (AOPs)—Utilization of Hydroxyl Radical and Singlet Oxygen. In Reactive Oxygen Species; IntechOpen: London, UK, 2021. [Google Scholar]
- Liu, Z.; Demeestere, K.; Van Hulle, S. Comparison and Performance Assessment of Ozone-Based AOPs in View of Trace Organic Contaminants Abatement in Water and Wastewater: A Review. J. Environ. Chem. Eng. 2021, 9, 105599. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Sun, K.; Lin, C.; Ma, J.; He, M.; Ouyang, W. Persulfate-Based Advanced Oxidation Processes (AOPs) for Organic-Contaminated Soil Remediation: A Review. Chem. Eng. J. 2019, 372, 836–851. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of Peroxymonosulfate and Its Activation Methods for Degradation of Environmental Organic Pollutants. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhan, Y.; Zhang, Y.; Zhao, X.; Yang, M.; Ruan, W.; Zhang, Z.; Liang, X.; Ma, J. Peracetic Acid-Induced Nanoengineering of Fe-Based Metallic Glass Ribbon in Application of Efficient Drinking Water Treatment. Appl. Catal. B Environ. Energy 2024, 355, 124161. [Google Scholar] [CrossRef]
- Qiao, C.; Jia, W.; Tang, J.; Chen, C.; Wu, Y.; Liang, Y.; Du, J.; Wu, Q.; Feng, X.; Wang, H. Advances of Carbon-Based Materials for Activating Peracetic Acid in Advanced Oxidation Processes: A Review. Environ. Res. 2024, 263, 120058. [Google Scholar] [CrossRef]
- Shen, W.; Yang, L.; Zhou, Z.; Gao, H.; Zhou, X.; Zhang, Y.; Chen, J. Activation of Peracetic Acid by Thermally Modified Carbon Nanotubes: Organic Radicals Contribution and Active Sites Identification. Chem. Eng. J. 2023, 474, 145521. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, R.; Liu, Y.; Zhang, L.; Zhang, L.; Fu, Y. Efficient Degradation of Sulfamethoxazole Using Peracetic Acid Activated by Zero-Valent Cobalt. J. Environ. Chem. Eng. 2022, 10, 107783. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Li, Q.; Wang, J.; Cao, L.; Cheng, Y.; Yu, S.; Liu, Z.; Chen, Y.; Yue, S. Non-Radical Activation of Peracetic Acid by Powdered Activated Carbon for the Degradation of Sulfamethoxazole. Environ. Sci. Technol. 2023, 57, 10478–10488. [Google Scholar] [CrossRef]
- Kiejza, D.; Kotowska, U.; Polińska, W.; Karpińska, J. Peracids-New Oxidants in Advanced Oxidation Processes: The Use of Peracetic Acid, Peroxymonosulfate, and Persulfate Salts in the Removal of Organic Micropollutants of Emerging Concern—A Review. Sci. Total Environ. 2021, 790, 148195. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, X.; Du, P.; Zhang, T.; Cai, M.; Sun, P.; Huang, C.-H. Oxidation of β-Lactam Antibiotics by Peracetic Acid: Reaction Kinetics, Product and Pathway Evaluation. Water Res. 2017, 123, 153–161. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Liu, T.; Wang, Q.; Li, N.; Zhang, Y.; Yang, L.; Zhou, X. Oxidation of Tetracycline Antibiotics by Peracetic Acid: Reaction Kinetics, Mechanism, and Antibacterial Activity Change. Chem. Eng. J. 2022, 431, 134190. [Google Scholar] [CrossRef]
- Kim, J.; Huang, C.-H. Reactivity of Peracetic Acid with Organic Compounds: A Critical Review. ACS EST Water 2020, 1, 15–33. [Google Scholar] [CrossRef]
- Hey, G.; Ledin, A.; Jansen, J.L.C.; Andersen, H.R. Removal of Pharmaceuticals in Biologically Treated Wastewater by Chlorine Dioxide or Peracetic Acid. Environ. Technol. 2012, 33, 1041–1047. [Google Scholar] [CrossRef]
- Liu, F.; Zou, Y.; Liang, H.; Hu, J.; Li, Y.; Lin, L.; Li, X.; Li, B. Trace Co (II) Triggers Peracetic Acid Activation in Phosphate Buffer: New Insights into the Oxidative Species Responsible for Ciprofloxacin Removal. J. Hazard. Mater. 2024, 467, 133638. [Google Scholar] [CrossRef] [PubMed]
- Caretti, C.; Lubello, C. Wastewater Disinfection with PAA and UV Combined Treatment: A Pilot Plant Study. Water Res. 2003, 37, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Zhang, T.; Liu, W.; Du, P.; Dobson, J.T.; Huang, C.-H. Advanced Oxidation Process with Peracetic Acid and Fe (II) for Contaminant Degradation. Environ. Sci. Technol. 2019, 53, 13312–13322. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, C.-H. Modeling the Kinetics of UV/Peracetic Acid Advanced Oxidation Process. Environ. Sci. Technol. 2020, 54, 7579–7590. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Guo, W.; Jia, W.; Wang, H.; Zheng, S.; Si, Q.; Zhao, Q.; Luo, H.; Jiang, J.; Ren, N. Insights into the Oxidation of Organic Contaminants by Co (II) Activated Peracetic Acid: The Overlooked Role of High-Valent Cobalt-Oxo Species. Water Res. 2021, 201, 117313. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Li, H.; Qian, J.; Pan, B. New Insights into the Activation of Peracetic Acid by Co (II): Role of Co (II)-Peracetic Acid Complex as the Dominant Intermediate Oxidant. ACS EST Eng. 2021, 1, 1432–1440. [Google Scholar] [CrossRef]
- Rothbart, S.; Ember, E.E.; van Eldik, R. Mechanistic Studies on the Oxidative Degradation of Orange II by Peracetic Acid Catalyzed by Simple Manganese (II) Salts. Tuning the Lifetime of the Catalyst. New J. Chem. 2012, 36, 732–748. [Google Scholar] [CrossRef]
- Wu, J.; Zou, J.; Lin, J.; Li, S.; He, L.; Wu, Z.; Li, Q.; Gong, C.; Ma, J. Overlooked Role of Coexistent Hydrogen Peroxide in Activated Peracetic Acid by Cu (II) for Enhanced Oxidation of Organic Contaminants. Environ. Sci. Technol. 2024, 58, 15741–15754. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Manoli, K.; Kim, J.; Feng, M.; Huang, C.-H.; Sharma, V.K. Peracetic Acid–Ruthenium (III) Oxidation Process for the Degradation of Micropollutants in Water. Environ. Sci. Technol. 2021, 55, 9150–9160. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, T.; Pehkonen, S.O. Peracids in Water Treatment: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1–39. [Google Scholar] [CrossRef]
- Bell, J.; Wen, Y.; Ma, X.; McDonald, T.J.; Huang, C.-H.; Sharma, V.K. Interaction of Peracetic Acid with Chromium (III): Understanding Degradation of Coexisting Organic Pollutants in Water. J. Hazard. Mater. 2022, 438, 129537. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Cheng, Y.; Cao, L.; Xie, P.; Ma, J. Molybdenum Disulfide (MoS2) Promoted Sulfamethoxazole Degradation in the Fe (III)/Peracetic Acid Process. Sep. Purif. Technol. 2022, 281, 119854. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Cheng, Y.; Cao, L.; Bai, F.; Yue, S.; Xie, P.; Ma, J. Molybdenum Disulfide (MoS2): A Novel Activator of Peracetic Acid for the Degradation of Sulfonamide Antibiotics. Water Res. 2021, 201, 117291. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, X.; Wang, Y.; Liu, H.; Wu, Y.; Jin, X.; Chen, P.; Lv, W.; Liu, G. Activation of Peracetic Acid via Co3O4 with Double-Layered Hollow Structures for the Highly Efficient Removal of Sulfonamides: Kinetics Insights and Assessment of Practical Applications. J. Hazard. Mater. 2022, 431, 128579. [Google Scholar] [CrossRef]
- Yang, K.; Zhai, Z.; Liu, H.; Zhao, T.; Yuan, D.; Jiao, T.; Zhang, Q.; Tang, S. Peracetic Acid Activation by Natural Chalcopyrite for Metronidazole Degradation: Unveiling the Effects of Cu-Fe Bimetallic Sites and Sulfur Species. Sep. Purif. Technol. 2023, 305, 122500. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, B.; Miao, L.; Wang, S.; Xie, P.; Wang, Z.; Ma, J. Applying a Novel Advanced Oxidation Process of Activated Peracetic Acid by CoFe2O4 to Efficiently Degrade Sulfamethoxazole. Appl. Catal. B 2021, 280, 119422. [Google Scholar] [CrossRef]
- Hollman, J.; Dominic, J.A.; Achari, G. Degradation of Pharmaceutical Mixtures in Aqueous Solutions Using UV/Peracetic Acid Process: Kinetics, Degradation Pathways and Comparison with UV/H2O2. Chemosphere 2020, 248, 125911. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mukhopadhyay, M.; Murthy, Z.V.P. UV/Peroxyacetic Acid Mediated Chlorophenol Congener Degradation. CLEAN–Soil Air Water 2014, 42, 276–283. [Google Scholar] [CrossRef]
- Wang, J.; Wan, Y.; Ding, J.; Wang, Z.; Ma, J.; Xie, P.; Wiesner, M.R. Thermal Activation of Peracetic Acid in Aquatic Solution: The Mechanism and Application to Degrade Sulfamethoxazole. Environ. Sci. Technol. 2020, 54, 14635–14645. [Google Scholar] [CrossRef]
- Yao, K.; Fang, L.; Liao, P.; Chen, H. Ultrasound-Activated Peracetic Acid to Degrade Tetracycline Hydrochloride: Efficiency and Mechanism. Sep. Purif. Technol. 2023, 306, 122635. [Google Scholar] [CrossRef]
- Yuan, D.; Yang, K.; Pan, S.; Xiang, Y.; Tang, S.; Huang, L.; Sun, M.; Zhang, X.; Jiao, T.; Zhang, Q. Peracetic Acid Enhanced Electrochemical Advanced Oxidation for Organic Pollutant Elimination. Sep. Purif. Technol. 2021, 276, 119317. [Google Scholar] [CrossRef]
- Dai, Y.; Qi, C.; Cao, H.; Wen, Y.; Zhao, Y.; Xu, C.; Yang, S.; He, H. Enhanced Degradation of Sulfamethoxazole by Microwave-Activated Peracetic Acid under Alkaline Condition: Influencing Factors and Mechanism. Sep. Purif. Technol. 2022, 288, 120716. [Google Scholar] [CrossRef]
- Dai, C.; Li, S.; Duan, Y.; Leong, K.H.; Liu, S.; Zhang, Y.; Zhou, L.; Tu, Y. Mechanisms and Product Toxicity of Activated Carbon/Peracetic Acid for Degradation of Sulfamethoxazole: Implications for Groundwater Remediation. Water Res. 2022, 216, 118347. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, Z.; Cheng, P.; She, Y.; Wang, W.; Tian, Y.; Ma, J.; Sun, Z. Efficient Activation of Peracetic Acid by Mixed Sludge Derived Biochar: Critical Role of Persistent Free Radicals. Water Res. 2022, 223, 119013. [Google Scholar] [CrossRef]
- Correa-Sanchez, S.; Peñuela, G.A. Peracetic Acid-Based Advanced Oxidation Processes for the Degradation of Emerging Pollutants: A Critical Review. J. Water Process Eng. 2022, 49, 102986. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Liu, Y.; Fu, Y. Effective Degradation of Sulfamethoxazole with Fe2+-Zeolite/Peracetic Acid. Sep. Purif. Technol. 2020, 233, 115973. [Google Scholar] [CrossRef]
- Yang, T.; An, L.; Zeng, G.; Jiang, M.; Li, J.; Liu, C.; Jia, J.; Ma, J. Efficient Removal of P-Arsanilic Acid and Arsenite by Fe (II)/Peracetic Acid (Fe (II)/PAA) and PAA Processes. Water Res. 2023, 241, 120091. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, T.; Heyninck, T.; Rämö, J.; Lassi, U. Comparison of Organic Peracids in Wastewater Treatment: Disinfection, Oxidation and Corrosion. Water Res. 2015, 85, 275–285. [Google Scholar] [CrossRef]
- Zhou, G.; Fu, Y.; Zhou, R.; Zhang, L.; Zhang, L.; Deng, J.; Liu, Y. Efficient Degradation of Organic Contaminants by Magnetic Cobalt Ferrite Combined with Peracetic Acid. Process Saf. Environ. Prot. 2022, 160, 376–384. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Zhao, X.; Jing, G.; Zhou, Z. Activation of Peracetic Acid with Zero-Valent Iron for Tetracycline Abatement: The Role of Fe (II) Complexation with Tetracycline. J. Hazard. Mater. 2022, 424, 127653. [Google Scholar] [CrossRef]
- Tian, D.; Wu, W.; Shen, Z.; Huang, T.; Chen, J. Degradation of Organic Dyes with Peracetic Acid Activated by Co (II). Acta Sci. Circumstantiae 2018, 38, 4023–4031. [Google Scholar]
- Wu, W.; Tian, D.; Liu, T.; Chen, J.; Huang, T.; Zhou, X.; Zhang, Y. Degradation of Organic Compounds by Peracetic Acid Activated with Co3O4: A Novel Advanced Oxidation Process and Organic Radical Contribution. Chem. Eng. J. 2020, 394, 124938. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, H.; Zhang, L.; Liang, B.; Sun, X.; Chen, J. Activation of Peracetic Acid with Lanthanum Cobaltite Perovskite for Sulfamethoxazole Degradation under a Neutral PH: The Contribution of Organic Radicals. Molecules 2020, 25, 2725. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, G.; Liu, Y.; Liu, S.; Wang, S.; Fu, Y. Activated Peracetic Acid by Mn3O4 for Sulfamethoxazole Degradation: A Novel Heterogeneous Advanced Oxidation Process. Chemosphere 2022, 306, 135506. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Shao, S.; Yang, Y.; Zhou, Z.; Jing, G.; Zhao, X. Mechanistic Insights into the Efficient Activation of Peracetic Acid by Pyrite for the Tetracycline Abatement. Water Res. 2022, 222, 118930. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Z.; Wang, J.; Cao, L.; Chen, Z.; Chen, Y.; Liu, Z.; Xie, P.; Ma, J. New Insights into the Degradation of Micro-Pollutants in the Hydroxylamine Enhanced Fe (II)/Peracetic Acid Process: Contribution of Reactive Species and Effects of PH. J. Hazard. Mater. 2023, 441, 129885. [Google Scholar] [CrossRef]
- Yang, S.; Liang, Z.-H.; Wen, Y.; He, C.-S.; Xiong, Z.; Du, Y.; Liu, Y.; Zhang, H.; Zhou, P.; Mu, Y. Gallic Acid Accelerates the Oxidation Ability of the Peracetic Acid/Fe (III) System for Bisphenol A Removal: Fate of Various Radicals. ACS EST Eng. 2023, 3, 271–282. [Google Scholar] [CrossRef]
- Lin, J.; Hu, Y.; Xiao, J.; Huang, Y.; Wang, M.; Yang, H.; Zou, J.; Yuan, B.; Ma, J. Enhanced Diclofenac Elimination in Fe (II)/Peracetic Acid Process by Promoting Fe (III)/Fe (II) Cycle with ABTS as Electron Shuttle. Chem. Eng. J. 2021, 420, 129692. [Google Scholar] [CrossRef]
- Li, C.; Yuan, D.; Yang, K.; Wang, H.; Wang, Z.; Zhang, Q.; Tang, S. Reutilization of Pyrite Tailings in Peracetic Acid-Based Advanced Oxidation Process for Water Purification. Sep. Purif. Technol. 2025, 354, 129155. [Google Scholar] [CrossRef]
- Yao, M.; Zhang, S.; Xie, M.; Zhao, L.; Zhao, R.-S. Efficient Activation of Peracetic Acid by Cobalt Modified Nitrogen-Doped Carbon Nanotubes for Drugs Degradation: Performance and Mechanism Insight. Chemosphere 2024, 358, 142277. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Xiong, B.; Bai, F.; Wang, S.; Wan, Y.; Zhang, L.; Xie, P.; Wiesner, M.R. Application of Cobalt/Peracetic Acid to Degrade Sulfamethoxazole at Neutral Condition: Efficiency and Mechanisms. Environ. Sci. Technol. 2019, 54, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Haghshenas, D.F.; Darvishi, D.; Moshefi, S.Z.; Keshavarz, A.E.; Sadrnezhad, S.K. Leaching Recovery of Zinc, Cobalt and Manganese from Zinc Purification Residue. Int. J. Eng. 2007, 20, 133–140. [Google Scholar]
- Moore, T.A.; Black, A.; Centeno, J.A.; Harding, J.S.; Trumm, D.A. Metal Contaminants in New Zealand. Sources, Treatments, and Effects on Ecology and Human Health; Resolutionz Press: Sydney, NSW, Australia, 2005. [Google Scholar]
- Barceloux, D.G.; Barceloux, D. Cobalt. J. Toxicol. Clin. Toxicol. 1999, 37, 201–216. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, G.; Liu, Y.; Wang, S.; Fu, Y. Cobalt Doped Graphitic Carbon Nitride as an Effective Catalyst for Peracetic Acid to Degrade Sulfamethoxazole. RSC Adv. 2022, 12, 13810–13819. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Duan, P.-J.; Zheng, J.-X.; Xie, Y.-Q.; Bai, C.-W.; Sun, Y.-J.; Chen, X.-J.; Chen, F.; Yu, H.-Q. Nano-Island-Encapsulated Cobalt Single-Atom Catalysts for Breaking Activity-Stability Trade-off in Fenton-like Reactions. Nat. Commun. 2025, 16, 115. [Google Scholar] [CrossRef]
- Lv, K.; Ling, L.; Lu, Q.; Lu, J.; Zhou, Y.; Zhou, Y. Reducing Cobalt Leaching in Disinfectant Degradation: Insights from Chitosan-Derived Carbon Encapsulated Co9S8. Sep. Purif. Technol. 2024, 344, 127207. [Google Scholar] [CrossRef]
- Taujale, S.; Baratta, L.R.; Huang, J.; Zhang, H. Interactions in Ternary Mixtures of MnO2, Al2O3, and Natural Organic Matter (NOM) and the Impact on MnO2 Oxidative Reactivity. Environ. Sci. Technol. 2016, 50, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Babuponnusami, A.; Muthukumar, K. A Review on Fenton and Improvements to the Fenton Process for Wastewater Treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Zhao, W.; Duan, Z.; Zheng, Z.; Li, B. Efficient Diclofenac Removal by Superoxide Radical and Singlet Oxygen Generated in Surface Mn (II)/(III)/(IV) Cycle Dominated Peroxymonosulfate Activation System: Mechanism and Product Toxicity. Chem. Eng. J. 2022, 433, 133742. [Google Scholar] [CrossRef]
- Ember, E.; Rothbart, S.; Puchta, R.; van Eldik, R. Metal Ion-Catalyzed Oxidative Degradation of Orange II by H2O2. High Catalytic Activity of Simple Manganese Salts. New J. Chem. 2009, 33, 34–49. [Google Scholar] [CrossRef]
- Zong, Y.; Shao, Y.; Ji, W.; Zeng, Y.; Xu, J.; Liu, W.; Xu, L.; Wu, D. Trace Mn (II)-Catalyzed Periodate Oxidation of Organic Contaminants Not Relying on Any Transient Reactive Species: The Substrate-Dependent Dual Roles of in-Situ Formed Colloidal MnO2. Chem. Eng. J. 2023, 451, 139106. [Google Scholar] [CrossRef]
- Kim, J.; Wang, J.; Ashley, D.C.; Sharma, V.K.; Huang, C.-H. Picolinic Acid-Mediated Catalysis of Mn (II) for Peracetic Acid Oxidation Processes: Formation of High-Valent Mn Species. Environ. Sci. Technol. 2023, 57, 18929–18939. [Google Scholar] [CrossRef]
- Dong, J.; Dong, H.; Xiao, J.; Li, L.; Huang, D.; Zhao, M. Enhanced Degradation of Micropollutants in a Peracetic Acid/Mn (II) System with EDDS: An Investigation of the Role of Mn Species. Environ. Sci. Technol. 2024, 58, 12179–12188. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, R.; Tang, Y.; Li, X.; Xu, L.; Fu, Y. Enhanced Mn (II)/Peracetic Acid by Nitrilotriacetic Acid to Degrade Organic Contaminants: Role of Mn (V) and Organic Radicals. Sci. Rep. 2024, 14, 29686. [Google Scholar] [CrossRef]
- Chen, S.; Cai, M.; Liu, Y.; Zhang, L.; Feng, L. Effects of Water Matrices on the Degradation of Naproxen by Reactive Radicals in the UV/Peracetic Acid Process. Water Res. 2019, 150, 153–161. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Fu, Y. Degradation Kinetics and Mechanism of Diclofenac by UV/Peracetic Acid. RSC Adv. 2020, 10, 9907–9916. [Google Scholar] [CrossRef]
- Paul, A.; Dziallas, C.; Zwirnmann, E.; Gjessing, E.T.; Grossart, H.-P. UV Irradiation of Natural Organic Matter (NOM): Impact on Organic Carbon and Bacteria. Aquat. Sci. 2012, 74, 443–454. [Google Scholar] [CrossRef]
- Sharma, S.; Mukhopadhyay, M.; Murthy, Z.V.P. Degradation of 4-Chlorophenol in Wastewater by Organic Oxidants. Ind. Eng. Chem. Res. 2010, 49, 3094–3098. [Google Scholar] [CrossRef]
- Rizzo, L.; Lofrano, G.; Gago, C.; Bredneva, T.; Iannece, P.; Pazos, M.; Krasnogorskaya, N.; Carotenuto, M. Antibiotic Contaminated Water Treated by Photo Driven Advanced Oxidation Processes: Ultraviolet/H2O2 vs Ultraviolet/Peracetic Acid. J. Clean. Prod. 2018, 205, 67–75. [Google Scholar] [CrossRef]
- Miao, F.; Yue, X.; Cheng, C.; Chen, X.; Ren, W.; Zhang, H. Insights into the Mechanism of Carbocatalysis for Peracetic Acid Activation: Kinetic Discernment and Active Site Identification. Water Res. 2022, 227, 119346. [Google Scholar] [CrossRef]
- Kong, D.; Zhao, Y.; Fan, X.; Wang, X.; Li, J.; Wang, X.; Nan, J.; Ma, J. Reduced Graphene Oxide Triggers Peracetic Acid Activation for Robust Removal of Micropollutants: The Role of Electron Transfer. Environ. Sci. Technol. 2022, 56, 11707–11717. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Lu, C.; Yao, Y.; Sun, L.; Gong, F.; Li, D.; Pei, K.; Lu, W.; Chen, W. Activated Carbon Fibers as an Effective Metal-Free Catalyst for Peracetic Acid Activation: Implications for the Removal of Organic Pollutants. Chem. Eng. J. 2015, 281, 953–960. [Google Scholar] [CrossRef]
- Tan, H.; Li, J.; He, M.; Li, J.; Zhi, D.; Qin, F.; Zhang, C. Global Evolution of Research on Green Energy and Environmental Technologies: A Bibliometric Study. J. Environ. Manag. 2021, 297, 113382. [Google Scholar] [CrossRef]
- Huong, P.T.; Jitae, K.; Al Tahtamouni, T.M.; Tri, N.L.M.; Kim, H.-H.; Cho, K.H.; Lee, C. Novel Activation of Peroxymonosulfate by Biochar Derived from Rice Husk toward Oxidation of Organic Contaminants in Wastewater. J. Water Process Eng. 2020, 33, 101037. [Google Scholar] [CrossRef]
- Hou, J.; Xu, J.; Tang, R.; Min, Y.; Eitssayeam, S.; Shi, P. Activation of Peracetic Acid by Biochar for Sulfamethoxazole Degradation: Revealing Roles of the Active Sites. J. Taiwan. Inst. Chem. Eng. 2023, 152, 105184. [Google Scholar] [CrossRef]
- Zhang, Z.; Duan, Y.; Dai, C.; Li, S.; Chen, Y.; Tu, Y.; Leong, K.H.; Zhou, L. Oxidation of Sulfamethazine by Peracetic Acid Activated with Biochar: Reactive Oxygen Species Contribution and Toxicity Change. Environ. Pollut. 2022, 313, 120170. [Google Scholar] [CrossRef]
- Park, M.; Kim, N.; Lee, J.; Gu, M.; Kim, B.-S. Versatile Graphene Oxide Nanosheets via Covalent Functionalization and Their Applications. Mater. Chem. Front. 2021, 5, 4424–4444. [Google Scholar] [CrossRef]
- Shahzad, A.; Jawad, A.; Ifthikar, J.; Chen, Z.; Chen, Z. The Hetero-Assembly of Reduced Graphene Oxide and Hydroxide Nanosheets as Superlattice Materials in PMS Activation. Carbon 2019, 155, 740–755. [Google Scholar] [CrossRef]
- Jothinathan, L.; Hu, J. Kinetic Evaluation of Graphene Oxide Based Heterogenous Catalytic Ozonation for the Removal of Ibuprofen. Water Res. 2018, 134, 63–73. [Google Scholar] [CrossRef]
- Farajollahi, A.; Marjani, A.P.; Pesyan, N.N.; Alamgholiloo, H. Efficient Degradation of Crystal Violet by GO/CuMn2O4 Nanocomposite via Peroxymonosulfate Activation. Appl. Surf. Sci. 2023, 622, 156903. [Google Scholar] [CrossRef]
- Tshangana, C.; Mubiayi, M.P.; Kuvarega, A.; Mamba, B.; Muleja, A. Advanced Oxidation Processes for Pharmaceutical Degradation and Disinfection of Wastewater: Peracetic Acid and Graphene Oxide Quantum Dots. Int. J. Environ. Sci. Technol. 2023, 20, 11997–12014. [Google Scholar] [CrossRef]
- Choi, J.; Tu, N.D.K.; Lee, S.-S.; Lee, H.; Kim, J.S.; Kim, H. Controlled Oxidation Level of Reduced Graphene Oxides and Its Effect on Thermoelectric Properties. Macromol. Res. 2014, 22, 1104–1108. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I.; Gurmen, S.; Gafarli, I.; Khoei, S.; Safaltin, S.; Ozcelik, D.Y. Oxidative Degradation of Bisphenol A by Carbocatalytic Activation of Persulfate and Peroxymonosulfate with Reduced Graphene Oxide. J. Hazard. Mater. 2018, 360, 141–149. [Google Scholar] [CrossRef]
- Kong, D.; Zhao, Y.; Guo, H.; Han, M.; Fan, X.; Li, J.; He, X.; Ma, J. Unveiling the Direct Electron Transfer Regime of Peracetic Acid Activation: Quantitative Structure–Activity Relationship Analysis of Carbon Nanotube Catalysis. ACS EST Eng. 2023, 3, 1030–1041. [Google Scholar] [CrossRef]
- Tian, X.; Liu, S.; Zhang, B.; Wang, S.; Dong, S.; Liu, Y.; Feng, L.; Zhang, L. Carbonized Polyaniline-Activated Peracetic Acid Advanced Oxidation Process for Organic Removal: Efficiency and Mechanisms. Environ. Res. 2023, 219, 115035. [Google Scholar] [CrossRef]
- Li, D.; Duan, X.; Sun, H.; Kang, J.; Zhang, H.; Tade, M.O.; Wang, S. Facile Synthesis of Nitrogen-Doped Graphene via Low-Temperature Pyrolysis: The Effects of Precursors and Annealing Ambience on Metal-Free Catalytic Oxidation. Carbon 2017, 115, 649–658. [Google Scholar] [CrossRef]
- Chen, F.; Liu, L.; Wu, J.; Rui, X.; Chen, J.; Yu, Y. Single-atom Iron Anchored Tubular G-C3N4 Catalysts for Ultrafast Fenton-like Reaction: Roles of High-valency Iron-oxo Species and Organic Radicals. Adv. Mater. 2022, 34, 2202891. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, J.; Mei, Q.; Wei, B.; An, Z.; Cao, H.; Zhang, C.; Xie, J.; Zhan, J.; Wang, W. Acetaminophen Degradation by Hydroxyl and Organic Radicals in the Peracetic Acid-Based Advanced Oxidation Processes: Theoretical Calculation and Toxicity Assessment. J. Hazard. Mater. 2021, 416, 126250. [Google Scholar] [CrossRef]
- Mortensen, A. Scavenging of Acetylperoxyl Radicals and Quenching of Triplet Diacetyl by β-Carotene: Mechanisms and Kinetics. J. Photochem. Photobiol. B 2001, 61, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, J.; Zhang, Y.; Xu, Y.; Zheng, T.; Zhou, X. Highly Efficient Activation of Peracetic Acid by Nano-CuO for Carbamazepine Degradation in Wastewater: The Significant Role of H2O2 and Evidence of Acetylperoxy Radical Contribution. Water Res. 2022, 216, 118322. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ma, Z.; Tang, J.; Yan, N.; Du, Y.; Xi, S.; Wang, K.; Zhang, W.; Wen, H.; Wang, J. Immediate Hydroxylation of Arenes to Phenols via V-Containing All-Silica ZSM-22 Zeolite Triggered Non-Radical Mechanism. Nat. Commun. 2018, 9, 2931. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Zhang, Y.; Yu, Z.; Ji, R.; Zhou, X. Activation of Peracetic Acid with Cobalt Anchored on 2D Sandwich-like MXenes (Co@ MXenes) for Organic Contaminant Degradation: High Efficiency and Contribution of Acetylperoxyl Radicals. Appl. Catal. B 2021, 297, 120475. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Zheng, T.; Xu, Y.; Liu, T.; Yin, W.; Zhang, Y.; Zhou, X. Co–Mn Spinel Oxides Trigger Peracetic Acid Activation for Ultrafast Degradation of Sulfonamide Antibiotics: Unveiling Critical Role of Mn Species in Boosting Co Activity. Water Res. 2023, 229, 119462. [Google Scholar] [CrossRef]
- Chen, L.; Duan, J.; Du, P.; Sun, W.; Lai, B.; Liu, W. Accurate Identification of Radicals by In-Situ Electron Paramagnetic Resonance in Ultraviolet-Based Homogenous Advanced Oxidation Processes. Water Res. 2022, 221, 118747. [Google Scholar] [CrossRef]
- Lee, J.; Von Gunten, U.; Kim, J.-H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Wang, X.; Si, D.; Li, Y.; Chen, N.; Fang, G.; Zhu, C.; Zhou, D. Alcohols Radicals Can Efficiently Reduce Recalcitrant Perfluorooctanoic Acid. Water Res. 2023, 245, 120557. [Google Scholar] [CrossRef]
- Wang, X.; Qin, Y.; Zhu, L.; Tang, H. Nitrogen-Doped Reduced Graphene Oxide as a Bifunctional Material for Removing Bisphenols: Synergistic Effect between Adsorption and Catalysis. Environ. Sci. Technol. 2015, 49, 6855–6864. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, J.; Yan, Y.; Zhao, X.; Yan, J.; Zhang, Y.; Lai, B.; Chen, X.; Song, L. Catalytic Degradation of Sulfamethoxazole through Peroxymonosulfate Activated with Expanded Graphite Loaded CoFe2O4 Particles. Chem. Eng. J. 2019, 369, 403–413. [Google Scholar] [CrossRef]
- Chen, N.; Fang, G.; Zhu, C.; Wu, S.; Liu, G.; Dionysiou, D.D.; Wang, X.; Gao, J.; Zhou, D. Surface-Bound Radical Control Rapid Organic Contaminant Degradation through Peroxymonosulfate Activation by Reduced Fe-Bearing Smectite Clays. J. Hazard. Mater. 2020, 389, 121819. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Wang, X.; Zhang, Y.; Xu, J.; Sun, C. Reactive Species in Peracetic Acid-Based AOPs: A Critical Review of Their Formation Mechanisms, Identification Methods and Oxidation Performances. Water Res. 2024, 122917. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of Sulfate Radical through Heterogeneous Catalysis for Organic Contaminants Removal: Current Development, Challenges and Prospects. Appl. Catal. B 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, X.; Zhong, Z.; Tian, L.; Sun, Q.; Cui, Y.; Lu, X.; Zou, J.; Luo, S. Carbon Nitride Supported High-loading Fe Single-atom Catalyst for Activation of Peroxymonosulfate to Generate 1O2 with 100% Selectivity. Angew. Chem. Int. Ed. 2021, 60, 21751–21755. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Liang, X.; Lu, Y.; Feng, H.; Wang, J.; Deng, L.; Zou, J.; Zhu, X. Non-Radical Oxidation in Environmental Catalysis: Recognition, Identification, and Perspectives. Chem. Eng. J. 2022, 433, 134385. [Google Scholar] [CrossRef]
- Meng, L.; Dong, J.; Chen, J.; Li, L.; Huang, Q.; Lu, J. Activation of Peracetic Acid by Spinel FeCo2O4 Nanoparticles for the Degradation of Sulfamethoxazole. Chem. Eng. J. 2023, 456, 141084. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.-D.; Lim, T.-T. Graphene-and CNTs-Based Carbocatalysts in Persulfates Activation: Material Design and Catalytic Mechanisms. Chem. Eng. J. 2018, 354, 941–976. [Google Scholar] [CrossRef]
- Zeng, Y.; Fang, G.; Fu, Q.; Peng, F.; Wang, X.; Dionysiou, D.D.; Guo, J.; Gao, J.; Zhou, D.; Wang, Y. Mechanistic Study of the Effects of Agricultural Amendments on Photochemical Processes in Paddy Water during Rice Growth. Environ. Sci. Technol. 2022, 56, 4221–4230. [Google Scholar] [CrossRef]
- Wu, Z.; Xiong, Z.; Liu, R.; He, C.; Liu, Y.; Pan, Z.; Yao, G.; Lai, B. Pivotal Roles of N-Doped Carbon Shell and Hollow Structure in Nanoreactor with Spatial Confined Co Species in Peroxymonosulfate Activation: Obstructing Metal Leaching and Enhancing Catalytic Stability. J. Hazard. Mater. 2022, 427, 128204. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Guo, W.; Jia, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Jiang, J.; Ren, N. Novel Nonradical Oxidation of Sulfonamide Antibiotics with Co (II)-Doped g-C3N4-Activated Peracetic Acid: Role of High-Valent Cobalt–Oxo Species. Environ. Sci. Technol. 2021, 55, 12640–12651. [Google Scholar] [CrossRef]
- Xie, Z.-H.; He, C.-S.; Pei, D.-N.; Dong, Y.; Yang, S.-R.; Xiong, Z.; Zhou, P.; Pan, Z.-C.; Yao, G.; Lai, B. Review of Characteristics, Generation Pathways and Detection Methods of Singlet Oxygen Generated in Advanced Oxidation Processes (AOPs). Chem. Eng. J. 2023, 468, 143778. [Google Scholar] [CrossRef]
- Huang, D.; Ren, Y.; Ren, M.; Wang, G.; Du, L.; Li, R.; Xu, W.; Huang, H.; Li, S.; Shen, L. Insights into the Relationship between Regulation of Oxygen Vacancy and Singlet Oxygen Generation in Peracetic Acid Activation. Sep. Purif. Technol. 2025, 359, 130810. [Google Scholar] [CrossRef]
- Li, W.; Tang, R.; Xiong, S.; Li, L.; Zhou, Z.; Su, L.; Gong, D.; Deng, Y. High-Valent Metal-Oxo Species in Catalytic Oxidations for Environmental Remediation and Energy Conversion. Coord. Chem. Rev. 2024, 510, 215840. [Google Scholar] [CrossRef]
- Zhou, L.; Sleiman, M.; Ferronato, C.; Chovelon, J.-M.; Richard, C. Reactivity of Sulfate Radicals with Natural Organic Matters. Environ. Chem. Lett. 2017, 15, 733–737. [Google Scholar] [CrossRef]
- Ghanbari, F.; Giannakis, S.; Lin, K.-Y.A.; Wu, J.; Madihi-Bidgoli, S. Acetaminophen Degradation by a Synergistic Peracetic Acid/UVC-LED/Fe (II) Advanced Oxidation Process: Kinetic Assessment, Process Feasibility and Mechanistic Considerations. Chemosphere 2021, 263, 128119. [Google Scholar] [CrossRef]
- Yun, E.-T.; Lee, J.H.; Kim, J.; Park, H.-D.; Lee, J. Identifying the Nonradical Mechanism in the Peroxymonosulfate Activation Process: Singlet Oxygenation versus Mediated Electron Transfer. Environ. Sci. Technol. 2018, 52, 7032–7042. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Liebman, J.F. The Oxidizing Nature of the Hydroxyl Radical. A Comparison with the Ferryl Ion (FeO2+). J. Phys. Chem. 1984, 88, 99–101. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Zhang, J. High-Valent Metals in Advanced Oxidation Processes: A Critical Review of Their Identification Methods, Formation Mechanisms, and Reactivity Performance. Chem. Eng. J. 2023, 460, 141796. [Google Scholar] [CrossRef]
- Popova, T.V.; Aksenova, N.V. Complexes of Copper in Unstable Oxidation States. Russ. J. Coord. Chem. 2003, 29, 743–765. [Google Scholar] [CrossRef]
- Kang, H.; Lee, D.; Lee, K.-M.; Kim, H.-H.; Lee, H.; Kim, M.S.; Lee, C. Nonradical Activation of Peroxymonosulfate by Hematite for Oxidation of Organic Compounds: A Novel Mechanism Involving High-Valent Iron Species. Chem. Eng. J. 2021, 426, 130743. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, H.; Li, S.; Wang, D.; Yang, B. A Review on the Role of High-Valent Metals in Peracetic Acid-Based Advanced Oxidation Processes. Desalination Water Treat. 2024, 100182. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, W.; Pang, S.; Guo, Q.; Guan, C.; Jiang, J. Aqueous Iron (IV)–Oxo Complex: An Emerging Powerful Reactive Oxidant Formed by Iron (II)-Based Advanced Oxidation Processes for Oxidative Water Treatment. Environ. Sci. Technol. 2022, 56, 1492–1509. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, W.; Sun, S.-P. Peracetic Acid-Based UVA Photo-Fenton Reaction: Dominant Role of High-Valent Iron Species toward Efficient Selective Degradation of Emerging Micropollutants. J. Hazard. Mater. 2023, 454, 131448. [Google Scholar] [CrossRef]
- Zong, Y.; Guan, X.; Xu, J.; Feng, Y.; Mao, Y.; Xu, L.; Chu, H.; Wu, D. Unraveling the Overlooked Involvement of High-Valent Cobalt-Oxo Species Generated from the Cobalt (II)-Activated Peroxymonosulfate Process. Environ. Sci. Technol. 2020, 54, 16231–16239. [Google Scholar] [CrossRef] [PubMed]
- Pestovsky, O.; Bakac, A. Reactivity of Aqueous Fe (IV) in Hydride and Hydrogen Atom Transfer Reactions. J. Am. Chem. Soc. 2004, 126, 13757–13764. [Google Scholar] [CrossRef]
- Jacobsen, F.; Holcman, J.; Sehested, K. Reactions of the Ferryl Ion with Some Compounds Found in Cloud Water. Int. J. Chem. Kinet. 1998, 30, 215–221. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, J.; Pang, S.; Zhou, Y.; Guan, C.; Gao, Y.; Li, J.; Yang, Y.; Qiu, W.; Jiang, C. Is Sulfate Radical Really Generated from Peroxydisulfate Activated by Iron (II) for Environmental Decontamination? Environ. Sci. Technol. 2018, 52, 11276–11284. [Google Scholar] [CrossRef]
- Ao, X.; Wang, W.; Sun, W.; Lu, Z.; Li, C. Degradation and Transformation of Norfloxacin in Medium-Pressure Ultraviolet/Peracetic Acid Process: An Investigation of the Role of PH. Water Res. 2021, 203, 117458. [Google Scholar] [CrossRef]
- Kim, K.-H.; Jahan, S.A.; Lee, J.-T. Exposure to Formaldehyde and Its Potential Human Health Hazards. J. Environ. Sci. Health Part. C 2011, 29, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, T.; Mejia-Tickner, B.; Kissel, J.; Xie, X.; Huang, C.-H. Inactivation of Bacteria by Peracetic Acid Combined with Ultraviolet Irradiation: Mechanism and Optimization. Environ. Sci. Technol. 2020, 54, 9652–9661. [Google Scholar] [CrossRef] [PubMed]
- Gasim, M.F.; Bao, Y.; Elgarahy, A.M.; Osman, A.I.; Ala’’a, H.; Rooney, D.W.; Yap, P.-S.; Oh, W.-D. Peracetic Acid Activation Using Heterogeneous Catalysts for Environmental Decontamination: A Review. Catal. Commun. 2023, 180, 106702. [Google Scholar] [CrossRef]
- Yao, G.; Xiao, S.; Qian, Y.; Liu, T.; Shi, Y.; Zhang, Y.; Chen, J.; Zhou, X. Non-Metallic Lewis Acid Sites Enhance the Activation of Peracetic Acid by Cobalt-Iron Spinel for Micropollutant Decontamination: The Critical Role of Lewis Acid Sites as Electron Shuttles. Chem. Eng. J. 2024, 496, 153924. [Google Scholar] [CrossRef]
- Zheng, L.; Fu, J.; Hua, B.; Wu, Y.; Gu, Y.; Qin, N.; Li, F. Hierarchical Porous Bimetallic FeMn Metal–Organic Framework Gel for Efficient Activation of Peracetic Acid in Antibiotic Degradation. ACS Environ. Au 2023, 4, 56–68. [Google Scholar] [CrossRef]
| CEC | Processes | [CEC]0 | Removal Efficiency (%) | PAA Dose | Catalyst Dosage | pH | Reaction Time | Ref. |
|---|---|---|---|---|---|---|---|---|
| P-arsanilic acid | Fe2+/PAA | 5 µM | 98% | 400 µM | 200 µM | 3 | 20 s | [63] |
| Bisphenol-A | Fe2+/PAA | 60 mg·L−1 | 100 | 526 µM | 400 µM | 3.5 | 10 min | [64] |
| Naproxen | Fe2+/PAA | 15 µM | 100 | 100 μM | 100 μM | 3–8.2 | 120 min | [39] |
| Rhodamine B | CoFe2O4/PAA | 20 mg·L−1 | 95 | 800 μM | 2131.1 μM | 7 | 10 min | [65] |
| Sulfamethoxazole | Fe2+-zeolite/PAA | 5 µM | 100 | 400 μM | 150 mg·L−1 | 7 | 50 min | [62] |
| Tetracycline | Fe0/PAA | 10 μM | 95 | 100 μM | 1074.4 µM | 3.5 | 30 min | [66] |
| Sulfamethoxazole | Co2+/PAA | 10 μM | 100 | 100 μM | 10 μM | 3.5 | 20 min | [41] |
| Carbamazepine | Co2+/PAA | 10 μM | 84 | 100 μM | 10 μM | 3.5 | 30 min | [41] |
| Bisphenol-A | Co2+/PAA | 10 μM | 100 | 100 μM | 10 μM | 3.5 | 15 min | [41] |
| Atrazine | Co2+/PAA | 10 μM | 20 | 100 μM | 10 μM | 3.5 | 30 min | [41] |
| Naproxen | Co2+/PAA | 15 µM | 100 | 100 μM | 10 μM | 3–8.1 | 3 min | [20] |
| Crystal Violet | Co2+/PAA | 0.06 mM | 67 | 200 μM | 10 μM | 7 | 60 min | [67] |
| Congo red | Co2+/PAA | 0.05 mM | 98 | 200 μM | 10 μM | 7 | 60 min | [67] |
| Acid orange 7 | Co2+/PAA | 0.05 mM | 92 | 200 μM | 10 μM | 7 | 60 min | [67] |
| Orange G | Co3O4/PAA | 0.05 mM | 100 | 6574.6 μM | 415.3 μM | 7 | 90 min | [68] |
| Sulfamethoxazole | CoFe2O4/PAA | 10 μM | 85 | 100–200 μM | 426.2 μM | 7 | 30 min | [52] |
| Sulfamethoxazole | LaCoO3/PAA | 50 μM | 100 | 263 μM | 660 μM | 7 | 60 min | [69] |
| Sulfamethoxazole | Co0/PAA | 5 μM | 99 | 50 μM | 1696.8 μM | 7 | 5 min | [30] |
| Sulfamethoxazole | Mn3O4/PAA | 1 μM | 100 | 1000 μM | 218.5 μM | 6.5 | 12 min | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzayani, B.; Elaoud, S.C.; Sanromán, M.Á. Current Progress in Advanced Oxidation Processes for the Removal of Contaminants of Emerging Concern Using Peracetic Acid as an Effective Oxidant. Catalysts 2025, 15, 469. https://doi.org/10.3390/catal15050469
Bouzayani B, Elaoud SC, Sanromán MÁ. Current Progress in Advanced Oxidation Processes for the Removal of Contaminants of Emerging Concern Using Peracetic Acid as an Effective Oxidant. Catalysts. 2025; 15(5):469. https://doi.org/10.3390/catal15050469
Chicago/Turabian StyleBouzayani, Bakhta, Sourour Chaâbane Elaoud, and Maria Ángeles Sanromán. 2025. "Current Progress in Advanced Oxidation Processes for the Removal of Contaminants of Emerging Concern Using Peracetic Acid as an Effective Oxidant" Catalysts 15, no. 5: 469. https://doi.org/10.3390/catal15050469
APA StyleBouzayani, B., Elaoud, S. C., & Sanromán, M. Á. (2025). Current Progress in Advanced Oxidation Processes for the Removal of Contaminants of Emerging Concern Using Peracetic Acid as an Effective Oxidant. Catalysts, 15(5), 469. https://doi.org/10.3390/catal15050469









