Abstract
This research systematically investigates the influence of high-energy ball-milling (BM) parameters on the acidic and textural properties of zeolite Y. Among the BM parameters, the milling time (MT) exerted a more significant influence on the zeolite degradation than milling speed (MS), primarily affecting particle size and crystallinity. Milling produced nanozeolites with particle sizes ranging from 210 to 430 nm, and their activity was tested in the catalytic cracking of vacuum gas oil (VGO). The highest catalytic activity was observed for the zeolite with a particle size of 397 nm and a crystallinity of 75.9%: the VGO conversion was 69.0%, and the gasoline fraction yield was 33.9%, compared to the parent zeolite’s 62.7% and 22.1%, respectively. It was found that the activity of milled zeolites in catalytic cracking is determined by the accessibility of acid sites, which can be controlled by forming an optimal micro-mesoporous structure.
1. Introduction
The primary direction of oil refinery development is the improvement of technologies aimed at increasing the depth of crude oil processing. On the one hand, these improvements enhance efficiency of crude oil utilization, thereby reducing the carbon footprint of petroleum-derived products. On the other hand, they facilitate the processing of heavier oils, whose share of global reserves continues to grow [1].
Catalytic cracking is one of the most essential oil-refining processes for achieving deeper hydrocarbon refining [2]. Modern catalytic-cracking catalysts are based on zeolites, such as Y and ZSM-5, which exhibit high activity in the conversion of heavy hydrocarbons [3]. However, the involvement of oil residues in the catalytic-cracking process rapidly reduces its efficiency. This decline is attributed to several factors: acceleration of the reversible deactivation of the catalyst, coke formation and pore clogging by high-molecular-weight molecules, and irreversible deactivation of the catalyst due to metal contaminants in the feedstock [4]. Similar issues are observed when components derived from lignocellulosic biomass are involved in the cracking feed (VGO) [5,6]. As catalyst activity diminishes, both feedstock conversion and product selectivity decline, and catalyst deactivation by metals has to be compensated by increased catalyst consumption [7]. Consequently, current research focuses on modifying zeolite catalysts and developing new catalytic systems that meet modern industrial requirements, including high activity, resistance to deactivation, and long-term stability.
One of the promising approaches to modifying zeolite catalysts is reducing their particle size to tens or hundreds of nanometer scale, which decreases diffusion limitations and enhances the availability of acid sites on the surface [8]. Currently, two main techniques exist for obtaining nanozeolites: direct chemical synthesis [9] and high-energy ball milling [10]. The main drawbacks of chemical synthesis include limited control over particle size distribution and inevitable formation of a significant number of coarse particles, which are difficult to separate from the target zeolite fraction, especially in continuous production process. [11]. In contrast, BM circumvents these disadvantages and can efficiently mill large particles (1–5 mm) to sub-micrometer range [12]. Furthermore, BM is widely available, operationally simple, and scalable, making it a viable solution to meet the current global demand for cracking catalysts, which exceeds 850,000 tons/year [3].
Relatively few studies have explored the dependence of the catalytic activity of zeolites on their BM parameters. In research [13], it was shown that BM for up to 30 min increased the conversion of n-hexane, isooctane, and toluene on NaA, CaA, and KL zeolites compared to the parent zeolite. Further increases in MT led to a decline in conversion. The high activity of zeolites was attributed to the increase in the specific surface area due to BM, which exposed additional active sites to the reactant molecules. Similar results were obtained by Kurniawan et al. [14,15], who studied the activity of milled mordenite in the isomerization of n-butane and the conversion of dimethyl ether to olefins. In both cases, BM led to higher product yields compared to the parent zeolite.
Wakihara et al. [16] investigated the activity of ZSM-5 zeolite in the cracking of cumene following two treatment stages: BM and alkali treatment. After milling, the average zeolite particle sizes were 170 and 70 nm at MT of 60 and 120 min, respectively. BM for 60 min resulted in up to 42% increase in the benzene yield. However, extending the MT to 120 min led to a decline in yield, returning to the initial level. The alkali-treated zeolites exhibited considerably higher activity compared to the samples without post-treatment, likely due to the removing of damaged part of the zeolite.
The catalytic activity of the FAU, MFI, and BEA zeolites after 40 min of BM has been studied in the liquid-phase cracking of n-alkanes at 300–350 °C and 7 atm hydrogen pressure [17]. The BEA zeolite catalyst with a SiO2/Al2O3 ratio of 75 exhibited the highest activity. The key factor determining the activity of zeolites is the specific surface area of the catalyst particles smaller than 200 nm.
Inagaki et al. [18] studied the effect of post-treatment of milled ZSM-5 on its catalytic activity in n-hexane cracking at 650 °C. In the absence of a catalyst, the thermal cracking yielded ethylene as the major product (selectivity 45%) at an average n-hexane conversion of 20%. The initial ZSM-5 sample exhibited high activity in the beginning of the process but underwent significant deactivation within 55 min. The major cracking products were ethylene, propylene, and aromatic hydrocarbons.
These studies demonstrated that increasing MT reduces zeolite catalytic activity, which is attributed to structural degradation of commercial zeolite and a corresponding decrease in the number of active sites. Nonetheless, the presented works focused only on model compounds (e.g., n-hexane, cumene, and toluene) and did not examine real industrial feedstocks. Only in [19] was the catalytic-cracking activity of nanozeolites was tested with VGO. The obtained zeolites showed a correlation between their crystal size and catalytic activity, with the highest conversion—approximately 50%—observed for a sample with a particle size of ~25 nm. However, in this work, zeolites were obtained via synthesis.
This work focuses on optimizing the synthesis parameters for highly active nanozeolites via high-energy ball milling, and on studying acid and textural characteristics, as well as their catalytic activity in catalytic cracking of VGO of synthesized nanozeolites.
2. Results and Discussion
2.1. Synthesis of Nanozeolites
The optimization of zeolite BM parameters was carried out for two characteristics: the particle size and the crystallinity. The response surface as a function of the zeolite CBV-760 particle size after milling is shown in Figure 1. The particle size decreases with increasing MT (τ) and MS (rpm), consistent with literature data [20]. The maximum degradation rate of the zeolite particle size is observed at shot MT (<50 min); further increase in MT to 120 min reduce particle size to 200–210 nm, while longer MT results in particle aggregation. MS has a negligible effect on the particle size.
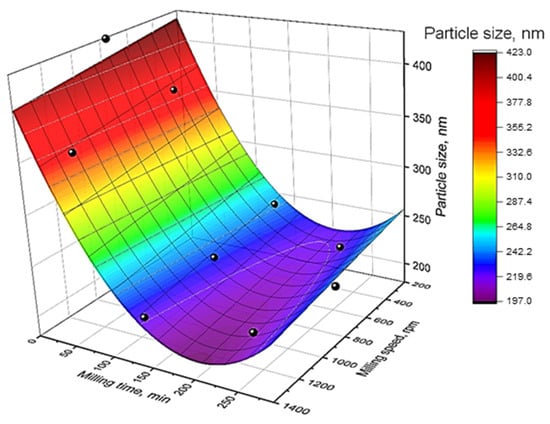
Figure 1.
The particle size of zeolite CBV-760 (nm) response surface as a function of milling time (τ) and milling speed (rpm).
Data processing (Table S1) enabled derivation of Equation (1) describing the effects of MT and MS on the particle size of zeolite CBV-760:
where n is the particle size (nm), ω is MS (rpm), and τ is MT (min).
The difference in the values of regression coefficients at the linear terms of the equation (βi) by almost nine times (69.80/7.77) indicates that MT has the greatest effect on the reduction of the zeolite particle size. Nevertheless, the influence of the MS parameter is also statistically significant, as confirmed by experimental data [20].
Increasing MS has a minor effect on the motion of zeolite particles with respect to the balls, preventing sufficient particle degradation and size reduction. In contrast, increasing MT enhances the probability of ball–zeolite collisions, even at low MS, making MT the dominant factor in particle-size reduction. Notably, higher MS reduces the particle aggregation tendencies during prolonged milling within the investigated MS range.
According to [21], zeolite catalyst activity depends not only on particle size but also on the crystallinity, as milling destroys the crystal structure of the zeolite, leading to active site degradation and a corresponding decrease in catalytic activity. The response surface illustrating the zeolite crystallinity after BM is shown in Figure 2.
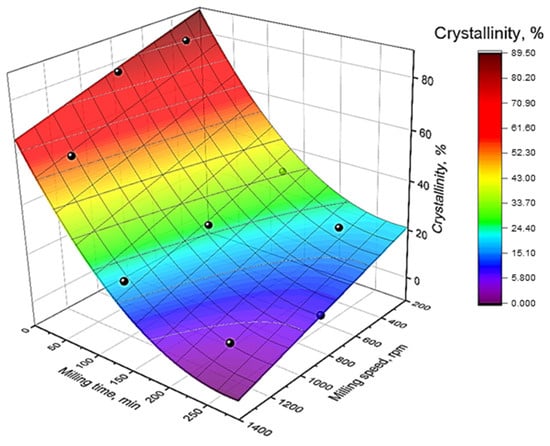
Figure 2.
The crystallinity of zeolite CBV-760 (%) response surface as a function of milling time (τ) and milling speed (rpm).
The crystallinity decreases monotonically with increasing MT and MS—the surface lacks inflection points or local minima/maxima. Even the shortest MT durations (5–15 min) reduce the crystallinity by 20–30% compared to the parent zeolite, while extending the duration to 200 min of BM at 1000 rpm leads to complete structural collapse of the sample to amorphous phase. The highest crystallinity samples (>55%) were obtained at MT below 30 min. A more significant reduction in the crystallinity was observed at high MS (>800 rpm), while the effect is less pronounced in the 300–600 rpm range and MT below 30 min. Similar to Equation (1), the empirical Equation (2) was derived to describe the influence of MT and MS on the zeolite crystallinity (Table S2):
where k is the crystallinity (%), ω is MS (rpm), and τ is MT (min).
As with particle size, MT has a greater influence on the crystallinity reduction, as indicated by the regression coefficient ratio (26.58/7.61 = 3.49). However, the effect of MS on the crystallinity is approximately three times greater than its influence on particle size.
Based on the regression analysis, BM conditions were selected to investigate the dependence of catalytic-cracking activity on particle size and crystallinity. Zeolites were milled at a constant MS of 600 rpm for varying durations: 0 min of milling (parent zeolite CBV-760)—CBV-0; 15 min of milling—CBV-15; 60 min of milling—CBV-60; and 240 min of milling—CBV-240.
2.2. Nanozeolite Characterization
The crystallinity and zeolite particle size (Table 1) for the obtained samples are consistent with the central composite rotatable design (CCRD). The diffraction patterns are shown in Figure S1. Short MT (15 min) has the least effect on the zeolite structure, and crystallinity decreases by less than 25% compared to the parent zeolite. As reported in previous studies [11,13], a loss in crystallinity typically leads to a reduction in zeolite activity in catalytic processes. Therefore, the optimal conditions for producing the most active zeolite catalysts correspond to an MT below 30 min combined with a low MS.

Table 1.
Characteristics of the milled zeolites at milling speed 600 rpm.
The analysis of zeolite acidity shows that the highest acidity was observed for CBV-15, with temperature-programmed desorption of ammonia peaks shifted toward stronger acid sites (Figure S2). The total acidity increased by approximately 15% compared to the parent zeolite. At short milling durations, the dispersion of zeolite grains occurs primarily with an increase in the specific surface, which leads to the appearance of additional acid sites. The CBV-240 sample does not exhibit a pronounced peak for medium-strength acid sites, and the distribution of sites is broader. Thereby, prolonged milling significantly reduces acidity, because of the degradation of the crystal lattice, as confirmed by scanning electron microscope images of the milled samples (Figure 3). Thus, the parent zeolite shows clearly defined boundaries of zeolite particles, which have a regular rounded shape. After 15 min of milling, it is possible to notice insignificant changes in the shape of particles—particles become less ordered and sharply angular. After four hours of milling, individual particles are no longer identifiable, which likely is associated with significant amorphization of the zeolite structure.
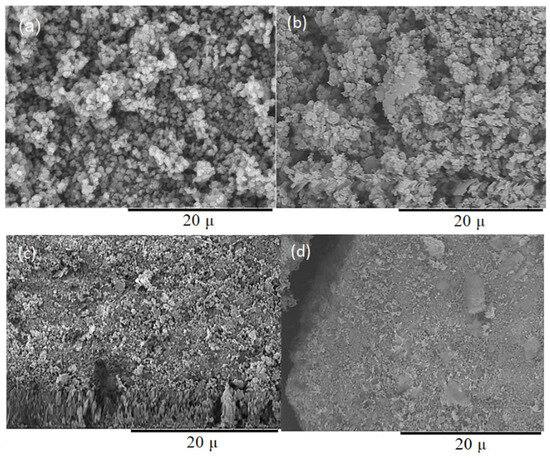
Figure 3.
Micrographs of the zeolite samples: (a) CBV-0; (b) CBV-15; (c) CBV-60; (d) CBV-240.
The surface characteristics of the investigated zeolites are given in Table 2.

Table 2.
Textural characteristics of the milled zeolites at milling speed 600 rpm.
The obtained isotherms (Figure S3), classified according to the IUPAC classification [22], display type I behavior at low pressures and type IV behavior at high pressures, indicating that the studied samples contain both micropores and mesopores. According to the de Boer classification [23], the samples exhibit type H4 hysteresis, suggesting the presence of narrow slit-like pores in their structure.
BET analysis was used to evaluate the effect of BM on the specific surface area as well as the pore volume of zeolites (Table 2). The results indicate that the surface area decreases from 923 to 277 m2/g as the MT increases to 240 min, reflecting sample amorphization. The same trend was observed for the micropore volume. BM increases the proportion of mesopore volume from 39 to 77% for the parent zeolite and CBV-240, respectively. This effect results in a multiporous structure with better diffusion characteristics across the series.
Increasing the MT leads to a shift in the distribution peak to the region of large pore sizes from 9.8 to 64.2 nm, likely due to the degradation of pores from micro- to mesosize, which may explain the reason for the decrease in the surface area determined by BET. Notably, after 240 min of milling, the micropore volume decreases sharply to 0.086 cm3/g, indicating near-complete degradation of zeolite’s pore structure.
2.3. Catalytic Activity of Milled Zeolites
The results of the microactivity tests of milled zeolites in the catalytic cracking of VGO are shown in Figure 4. Activity decreases in the following order: CBV-15 > CBV-0 > CBV-60 > CBV-240. The most active sample, CBV-15, achieved a VGO conversion of 69.0%, a gasoline fraction (IBP-200 °C) yield of 33.9%, and a gas yield of approximately 13%. In contrast, CBV-240 exhibited only 31.5% VGO conversion and 10.2% gasoline fraction yield. Thus, the CBV-240 sample is inferior to the parent zeolite in both activity and selectivity, despite having the smallest particle size (~200 nm). The calculation of the zeolite activity per m2 (BET surface area) shows that CBV-15 also has the highest yield for gas and liquid products.
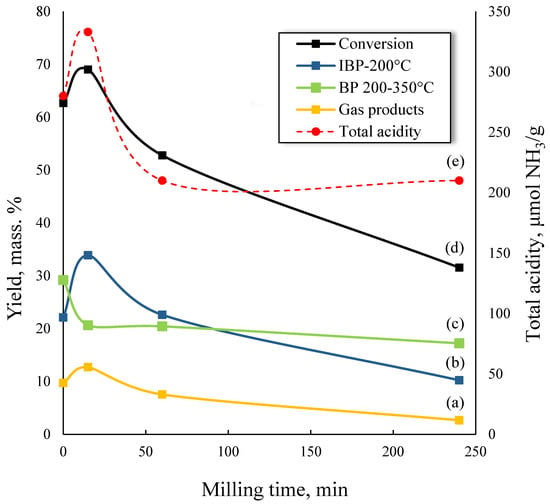
Figure 4.
Catalytic-cracking characteristics of VGO over milled zeolites at different milling times: (a) yield of gas products, % mass.; (b) yield of IBP-200 °C fraction, % mass.; (c) yield of BP 200−350 °C fraction, % mass.; (d) conversion of VGO, % mass.; (e) total acidity of zeolite sample, μmol NH3/g.
On the other hand, the observed activity reduction during BM may be attributed to pore structure collapse and clogging by amorphous phases. This effect negatively impacts zeolite diffusion properties and limits the contact between reactants and active sites. However, catalytic activity of milled zeolites indicates that this effect is less pronounced at short MTs, and the CBV-15 activity exceeds that of the parent sample.
Typically, catalyst activity in gas-phase reactions primarily depends on the acidity and textural characteristics of the catalyst while the mesopore surface area contributes less [19]. Analysis of the zeolites’ characteristics before and after BM (Table 1 and Table 2) shows that VGO conversion primarily depends on the acidity of the samples, which is determined by the porous structure of the zeolite rather than its Si/Al ratio. The Si/Al ratio remains unchanged during the BM, which is related to the identical peak positions in the XRD patterns before and after the BM (Figure S1). This demonstrates that the lattice parameters remain constant, and, consequently, the zeolite structure is preserved. Thus, mechanical impact is not sufficient to induce such structural modifications in zeolite.
The CBV-15 sample has an increased mesopore volume (0.256 cm3/g compared to 0.210 cm3/g for CBV-0), while the micropore volume differs from that of the parent zeolite by less than 15%. This suggests that the activity of zeolites in catalytic cracking is influenced by an optimal ratio of micropores to mesopores, which determines the accessibility of acid sites: mesopores facilitate reactions involving larger molecules, whereas micropores are more accessible to smaller molecules. For the most active sample, the micro/mesopore ratio is 47/53.
More information about hydrocarbon transformations on the zeolite surface can be inferred from the ratios of unsaturated/saturated and isomerized/linear hydrocarbons in the cracking products (Table 3). These ratios reflect the intensity of hydrogen transfer reactions and isomerization, respectively.

Table 3.
Cracking gas properties.
The olefin/paraffin and isomerized/linear hydrocarbons ratios increased for short MT compared to the parent zeolite. This is clearly demonstrated for C4 products, since the participation of C4 intermediates can comprehensively describe reactions occurring on the Y-zeolite surface [24]. It is known that the hydrogen transfer reactions are bimolecular and largely depend on the acid site’s density on the catalyst surface [25], whereas isomerization reactions are monomolecular [26]. The increase in the C4=/C4 ratio with increasing MT indicates a suppression of hydrogen transfer reactions in favor of isomerization reactions. Consequently, while the overall activity of the catalyst increased compared to unmilled zeolite, the quantity of acid sites per g zeolite decreased. With increasing MT to 15 min, the iso-C4/n-C4 ratio rose from 2.55 to 3.63; however, further milling continuously reduced it to 0.82. The increased ratios of iso-C4/n-C4 and C2–4=/n-C2–4 (gas products) and the gasoline fraction can be attributed to an increase in weak zeolites acidic sites (Table 1) during BM. The composition of the cracking gas on the CBV-240 sample is closer to that of thermal cracking gas, likely due to the absence of specific activity. A similar trend was observed for the olefin/paraffin ratios in C2-C3 molecules: after an initial increase, the values approached the thermal cracking parameters. Additionally, the formation of carbon deposits on the catalyst was investigated. The carbon concentration exhibited a decreasing trend from 1.6% mass. (CBV-15) to 1.1% mass. (CBV-240) with increasing duration of BM and reduced activity, which correlated with high intensity of hydrogen transfer reactions that facilitated coke formation.
Gas product yields, similarly to liquid products, prevails for the CBV-15 sample and reaches 12.7% (Figure 4). With increasing zeolite activity, the process selectivity shifted towards C4+ gases (Table 4). The main increase was attributed to higher yields and selectivity of BBF and propylene. Nevertheless, the distribution of cracking products varied significantly. While dry gas yield decreased from CBV-0 to CBV-240, its selectivity showed a minimum for CBV-15. The highest dry gas selectivity (34.9%) was observed for CBV-240.

Table 4.
Composition of cracking gas products on milled zeolites (% mass.).
As a result, short MT does not lead to obtaining the smallest zeolite particle size. However, an optimal balance of crystallinity, acidity, and textural characteristics allowed achievement of the highest yields of liquid and gas products compared to the parent zeolite. This result can be explained by a significant reduction of diffusion limitations and greater accessibility of the internal surface of the particles and active sites for hydrocarbon molecules.
The study results demonstrated that BM effectively enhanced zeolite activity in the catalytic cracking of VGO. Despite this, a direct comparison between the activity of milled zeolites and FCC catalysts is impractical, as FCC catalysts contain a matrix component that plays an active role in VGO conversion. Thus, the activity of milled zeolite regarding conversion is expected to be lower than that of the commercial ones [19]. However, this work primarily focuses on the activity of the main catalytic-cracking component—the zeolite. As noted earlier, similar studies [13,14,15,16,17,18] (cracking of VGO on milled zeolites) were conducted only with model compounds (e.g., n-hexane, cumene, and toluene), which do not provide a complete understanding of the cracking process due to the synergistic effects occurring from the complex composition of VGO. The studies most closely related to this work focus on the catalytic activity of nanozeolites obtained via synthesis. Several studies [27,28] examined VGO conversion over ZSM-5 zeolites to enhance olefin yields. Only one study [19] investigated a similar FAU-type zeolite (with an amorphous silica matrix) to assess the influence of particle size on catalytic-cracking activity for VGO. The results indicated that reducing particle size increases the conversion and selectivity of liquid and gaseous products. A direct comparison of zeolite activity reveals that BM leads to higher VGO conversion: milled zeolite—69.0% mass. and synthesized zeolite—50.0% mass. A similar trend was observed for the gasoline fraction, where the increase was approximately 6%, while gas product yields remained comparable. Thus, the BM method produces nanozeolites with optimal characteristics, demonstrating high catalytic activity and potential for applications in catalytic processes.
3. Materials and Methods
3.1. Ball Milling
BM of zeolites was conducted using an HDDM-01 atrittor (Union Processes, Akron, OH, USA) equipped with 1400 cm3 of zirconia jar. The milling was carried out using 250 cm3 of zirconia balls with a diameter of 1 mm. In the typical experiment, 12 g of calcined (4 h at 550 °C) FAU zeolite CBV-760 with SiO2/Al2O3 ratio of 60 (Zeolyst International, Exton, PA, USA) was milled for varying durations of 0–283 min in 120 cm3 of distillated water. The MS was varied in the range of 440–1355 rpm. The temperature of the jar was maintained at 13 °C using a cooling water jacket. After milling, the zeolite suspension was extracted from the jar and separated from the balls on a sieve. The obtained sample was dried for 3 h at 120 °C, followed by calcination for 4 h at 550 °C.
3.2. Design of Experiments to Optimize Ball-Milling Parameters
A two-factor central composite rotatable design was used to optimize the parameters of the ball-milling experiment (milling time and milling/rotation speed of the atrittor). The rotatable plan was characterized by identical accuracy of the response function in all directions. The size of radius α of the rotatable plan can be found by Equation (3):
where k is the number of factors.
For a two-factor CCRD, radius α is 1.41. The placement of experimental points on the coordinate plane of the factors is shown in Figure S4 and Table S3. CCRD consists of points corresponding to the complete factorial experiment (points lying on a square inscribed in a circle), boundary points of the studied range of parameters, and a set of central points [2].
3.3. Response Surface Methodology
Experimental data are presented as response surfaces. The RSM is the statistical processing of experimental data, whose result is a mathematical dependence that most accurately describes the data array [29]. Response surfaces are described by Equation (4):
where βn is the regression coefficient, and Xk is the investigated parameter. A least square method was used to approximate experimental data with a response surface.
3.4. Characterization of the Zeolites
The crystallinity of zeolite powders was analyzed using X-ray diffraction. Diffraction patterns were obtained with a TD-3700 diffractometer (Dandong Tongda Science & Technology Co., Ltd., Dandong, China) equipped with a sealed copper anode X-ray tube and a Ni-filter in front of the linear strip detector (CuKα radiation wavelength = 0.1542 nm). The Bragg–Brentano geometry was used with continuous θ-2θ scanning in the angular range 2θ = 4–50° with a step size of 0.02°. The crystallinity of the zeolite samples was evaluated based on the relative intensity of peaks at reference 2θ angles in the range 22–25° relative to the parent zeolite, in accordance with ASTM D 5758 [30].
Particle size distribution was determined by dynamic light scattering on a Zetasizer Nano ZS (Malvern Instruments Ltd., Worcestershire, UK). The light source was a helium-neon laser with a wavelength of 633 nm. Pretreatment of the sample included the dispersing 0.1 g of milled zeolite in 3 g of distilled water to form a suspension, followed by homogenization in an ultrasonic bath (Elmasonic P30H, Singen, Germany) for 10 min at 80 kHz and 100 W power. The sample was then transferred to a cuvette and analyzed. The average of five parallel measurements was taken as the result.
The textural characteristics of zeolites were investigated using low-temperature nitrogen adsorption-desorption with a Belsorp Mini X (Microtrac, Osaka, Japan). A zeolite sample of 0.2 g in a glass cuvette was subjected to thermal vacuum treatment at 300 °C and 10 Pa for 8 h. The adsorption capacity of the zeolite was analyzed by sequentially introducing nitrogen (99.999%) until equilibrium pressure was achieved in the system at 77 K within a relative pressure range of P/P0 = 0–0.993. The total specific surface area (SBET) of the zeolite samples was determined in using the BET method according to ISO 9277:2022 [31] (Annex B). The t-plot method was used to determine external surface area; sum of the external surface area and mesopore area (Sexternal + Smeso); and micropore volume. The micropore area was calculated by subtracting the sum of the external surface area and the mesopore area from the total specific surface area. The mesopore size distribution (Figure S5) was determined based on the desorption isotherm using the Barrett–Joyner–Halenda (BJH) theory. The total specific pore volume was determined as the volume of adsorbate adsorbed by the adsorbent at a relative pressure of P/P0 = 0.95.
The acidity of the samples was characterized by temperature-programmed desorption of ammonia on a USGA-101 chemisorption analyzer (Unisit, Moscow, Russia). The analysis was performed using a gas mixture of ammonia (5% vol.) and helium (95% vol.). The sample pretreatment consisted of heating in a helium flow (20 mL/min) at a rate of 20 °C/min up to 512 °C; then, the zeolite was heated for 40 min at 512 °C. After heating, the sample was cooled at a rate of 15 °C/min to 60 °C, maintained at 60 °C for 21 min, and saturated with ammonia (40 mL/min) at 60 °C for 24 min. Subsequently, the sample was heated (5 °C/min) to 102 °C to desorb ammonia under a helium flow (30 mL/min) for 1 h and finally cooled (20 °C/min) back to 60 °C. Ammonia temperature-programmed desorption spectra were recorded in a temperature range of 60–700 °C under a heating rate of 7 °C/min in a helium stream (30 mL/min). Desorbed ammonia was recorded using a thermal conductivity detector. The amount of desorbed ammonia was calculated with allowance for the pre-calibration of the USGA-101 instrument. Acid sites with ammonia desorption temperatures below and above 300 °C were attributed to weak and medium sites, respectively.
Electron microscopic images of the samples were obtained using a scanning electron microscope TM3030 (Hitachi, Tokyo, Japan). Prior to analysis, the zeolite samples were sputter-coated with Au using a Desk Sputter Coater (DSR1, Vac Coat Ltd., London, UK).
3.5. Catalytic Testing Procedure
Before the catalytic experiments, zeolite samples (after milling and calcination) were steam-deactivated in accordance with ASTM D 4463 [32] at a UPSK-10 unit (LinteL, Ufa, Russia) for 5 h at 815 °C.
The catalytic-cracking activity of milled zeolite was determined by a microactivity test on a laboratory unit MAK-10 (LinteL, Ufa, Russia) with a fixed-bed reactor in accordance with ASTM D 5154 [33]. Milled zeolite (2 g) was mixed 1:1 by volume with quartz balls (0.2–0.4 mm) to avoid clogging of the reactor. VGO was used as a feedstock, and the characteristics of the VGO are shown in Table 5. The mass of the VGO was 1.33 g, and the injection time was 75 s. The reactor and product recovery system was purged by nitrogen flow at 30 mL/min during 15 min after injection of the VGO. Additionally, a blank test was conducted without a catalyst to assess the contribution of thermal cracking.

Table 5.
VGO characteristics.
The boiling range distribution of the liquid cracking products was determined according to ASTM D 2887 [34] using a CG-1000 (Chromos, Dzerzhinsk, Russia) chromatograph with a flame ionization detector. An Agilent HP5-MS capillary column (15 m length × 0.25 mm diameter × 1.00 μm film thickness) was used. Helium was the carrier gas.
The gaseous reaction products were collected in a gas burette and analyzed using a Crystallux-4000M (Meta-Chrom, Yoshkar-Ola, Russia) chromatograph through parallel runs on two chromatographic columns. One was a packed column with molecular sieves CaX (3 m length × 3 mm diameter), which was designed to determine the non-hydrocarbon components of the gas (H2 and N2) and methane. The second column was an Agilent HP-PLOT Al2O3/Na2SO4 capillary column (50 m length × 0.32 mm diameter) to determine the hydrocarbon composition of the reaction gas. The flame ionization detector and thermal conductivity detector were used in parallel; both detectors were calibrated for accurate quantitative analysis. Argon was the carrier gas.
The carbon concentration in a representative zeolite sample after testing was determined by passing a 20% O2/ 80% N2 gas mixture (10 mL/min) over a known amount of the spent zeolite at 550 °C for 1 h. Formed CO2 was adsorbed in a glass tube containing ascarite. Formed CO was pre-oxidized to CO2 in the reactor with CuO at 550 °C. The total carbon content was calculated from the difference in the ascarite mass before and after analysis.
4. Conclusions
The catalytic activity of nanozeolites synthesized for varying durations of CBV-760 zeolite in the catalytic cracking of VGO was investigated. Milling time had a more pronounced effect on zeolite degradation than milling speed. Ball milling affected the physicochemical characteristics of zeolites, thereby impacting their catalytic activity. The highest activity was observed for the sample with a particle size of 397 nm and 75.9% crystallinity, obtained after 15 min of milling at 600 rpm. For this sample, the VGO conversion was 69.0%, and the yield of gasoline fraction was 33.9%, compared to 62.7% and 22.1% for the parent zeolite. In addition to crystallinity and particle size, the increase in activity correlated with a redistribution of the pore ratio. Zeolite milled for 15 min exhibited a micro-/mesopore ratio of 47/53%. The increase in acidity was a 15% increase in acidity relative to the parent zeolite, which indicated enhanced accessibility of acid sites attributed to mechanical impact on the structure.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15060596/s1, Figure S1: Diffraction pattern of CBV-760 zeolite milled for different durations at 600 rpm; Figure S2: Isotherms of temperature-programmed desorption of ammonia of CBV-760 zeolite milled for different durations at 600 rpm; Figure S3: Isotherm adsorption-desorption profile of CBV-760 zeolite milled for different durations at 600 rpm; Figure S4: Location of experimental points on the CCRD coordinate plane of factors; Figure S5: Pore size distribution for the parent and milled samples of zeolite CBV-760 zeolite milled for different durations at 600 rpm; Table S1: Data of mathematical modelling of the effect of the milling speed and the milling time on the particle size of CBV-760 zeolite particles, % (y); Table S2: Data of mathematical modelling of the effect of the milling speed and the milling time on the crystallinity of CBV-760 zeolite crystallinity, % (y); Table S3: Values of the variables and the corresponding coordinates of the factors (x1, x2) relative to the center point of the plan (0.0).
Author Contributions
The project was conceived and designed by P.K. and K.D. and V.M. carried out the materials syntheses/characterization and the catalytic experiments. P.K. and K.D. supervised this project. All authors contributed to the discussion on the project. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out within the State program of TIPS RAS.
Data Availability Statement
Data will be made available on request.
Acknowledgments
This work was performed using the equipment of the Shared Research Center “Analytical Center of Deep Oil Processing and Petrochemistry of TIPS RAS”.
Conflicts of Interest
The authors declare no competing interests.
Abbreviations
The following abbreviations are used in this manuscript:
| BBF | Butane-butylene fraction |
| BET | Brunauer–Emmett–Teller theory |
| BJH | Barrett–Joyner–Halenda theory |
| BM | High-energy ball milling |
| CCRD | Central composite rotatable design |
| FBP | Final boiling point |
| IBP | Initial boiling point |
| IUPAC | International Union of Pure and Applied Chemistry |
| MS | Milling speed |
| MT | Milling time |
| PPF | Propane-propylene fraction |
| RES | Response surface methodology |
| VGO | Vacuum gas oil |
References
- Xie, Y.; Zhang, Y.; He, L.; Jia, C.Q.; Yao, Q.; Sun, M.; Ma, X. Anti-deactivation of zeolite catalysts for residue fluid catalytic cracking. Appl. Catal. A 2023, 657, 119159. [Google Scholar] [CrossRef]
- Dement’ev, K.I.; Sagaradze, A.D.; Kuznetsov, P.S.; Palankoev, T.A.; Maximov, A.L. Selective production of light olefins from Fischer-Tropsch synthetic oil by catalytic cracking. Ind. Eng. Chem. Res. 2020, 59, 15875–15883. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef] [PubMed]
- Guisnet, M.; Magnoux, P. Deactivation by coking of zeolite catalysts. Prevention of deactivation. Optimal conditions for regeneration. Catal. Today 1997, 36, 477–483. [Google Scholar] [CrossRef]
- Khadzhiev, S.N.; Dement’ev, K.I.; Gerzeliev, I.M. Catalytic cracking of alternative raw materials and their mixtures with petroleum fractions over microspherical zeolite-containing catalysts. Pet. Chem. 2014, 54, 1–9. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, R.; Ma, W.; Liu, B.; Li, X.; Yan, B.; Cheng, Z.; Wang, T. Catalytic cracking of model compounds of bio-oil over HZSM-5 and the catalyst deactivation. Sci. Total Environ. 2018, 631–632, 1611–1622. [Google Scholar] [CrossRef]
- Almas, Q.; Naeem, M.A.; Baldanza, M.A.S.; Solomon, J.; Kenvin, J.C.; Müller, C.R.; da Silva, V.T.; Jones, C.W.; Sievers, C. Transformations of FCC catalysts and carbonaceous deposits during repeated reaction-regeneration cycles. Catal. Sci. Technol. 2019, 9, 6977–6992. [Google Scholar] [CrossRef]
- Ponomareva, O.A.; Mal’tseva, A.A.; Maerle, A.A.; Rodionova, L.I.; Pavlov, V.S.; Dobryakova, I.V.; Belova, M.V.; Ivanova, I.I. Production of isobutylene from acetone over micro-mesoporous catalysts. Pet. Chem. 2016, 56, 253–258. [Google Scholar] [CrossRef]
- Kamali, M.; Vaezifar, S.; Kolahduzan, H.; Malekpour, A.; Abdi, M.R. Synthesis of nanozeolite A from natural clinoptilolite and aluminum sulfate; Optimization of the method. Powder Technol. 2009, 189, 52–56. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Yu, C.; Gu, X.; Xu, N. Ball-milled NaA zeolite seeds with submicron size for growth of NaA zeolite membranes. J. Membr. Sci. 2012, 392–393, 18–28. [Google Scholar] [CrossRef]
- Kuznetsov, P.S.; Dementiev, K.I.; Palankoev, T.A.; Kalmykova, D.S.; Malyavin, V.V.; Sagaradze, A.D.; Maximov, A.L. Synthesis of highly active nanozeolites using methods of mechanical milling, recrystallization, and dealumination (a review). Pet. Chem. 2021, 61, 649–662. [Google Scholar] [CrossRef]
- Koch, C.C. The synthesis and structure of nanocrystalline materials produced by mechanical attrition: A review. Nanostruct. Mater. 1993, 2, 109–129. [Google Scholar] [CrossRef]
- Zielinski, P.A.; Van Neste, A.; Akolekar, D.B.; Kaliaguine, S. Effect of high-energy ball milling on the structural stability, surface and catalytic properties of small-, medium- and large-pore zeolites. Microporous Mater. 1995, 5, 123–133. [Google Scholar] [CrossRef]
- Kurniawan, T.; Muraza, O.; Miyake, K.; Hakeem, A.S.; Hirota, Y.; Al-Amer, A.M.; Nishiyama, N. Conversion of dimethyl ether to olefins over nanosized mordenite fabricated by a combined high-energy ball milling with recrystallization. Ind. Eng. Chem. Res. 2017, 56, 4258–4266. [Google Scholar] [CrossRef]
- Kurniawan, T.; Muraza, O.; Hakeem, A.S.; Bakare, I.A.; Nishitoba, T.; Yokoi, T.; Al Amer, A.M. Selective isomerization of n-butane over mordenite nanoparticles fabricated by a sequential ball milling–recrystallization–dealumination route. Energy Fuels 2017, 31, 12691–12700. [Google Scholar] [CrossRef]
- Wakihara, T.; Sato, K.; Inagaki, S.; Tatami, J.; Komeya, K.; Meguro, T.; Kubota, Y. Fabrication of fine zeolite with improved catalytic properties by bead milling and alkali treatment. ACS Appl. Mater. Interfaces 2010, 2, 2715–2718. [Google Scholar] [CrossRef]
- Dement’ev, K.I.; Palankoev, T.A.; Kuznetsov, P.S.; Abramova, D.S.; Romazanova, D.A.; Makhin, D.Y.; Maksimov, A.L. Effect of size factor on the activity of zeolites in the liquid-phase cracking of hydrocarbons. Pet. Chem. 2020, 60, 30–38. [Google Scholar] [CrossRef]
- Inagaki, S.; Shinoda, S.; Hayashi, S.; Wakihara, T.; Yamazaki, H.; Kondo, J.N.; Kubota, Y. Improvement in the catalytic properties of ZSM-5 zeolite nanoparticles via mechanochemical and chemical modifications. Catal. Sci. Technol. 2016, 6, 2598–2604. [Google Scholar] [CrossRef]
- Vuong, G.T.; Hoang, V.T.; Nguyen, D.T.; Do, T.O. Synthesis of nanozeolites and nanozeolite-based FCC catalysts, and their catalytic activity in gas oil cracking reaction. Appl. Catal. A 2010, 382, 231–239. [Google Scholar] [CrossRef]
- Saepurahman; Hashaikeh, R. Insight into ball milling for size reduction and nanoparticles production of H-Y zeolite. Mater. Chem. Phys. 2018, 220, 322–330. [Google Scholar] [CrossRef]
- Kharitonov, A.S.; Fenelonov, V.B.; Voskresenskaya, T.P.; Rudina, N.A.; Molchanov, V.V.; Plyasova, L.M.; Panov, G.I. Mechanism of FeZSM-5 milling and its effect on the catalytic performance in benzene to phenol oxidation. Zeolites 1995, 15, 253–258. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Broekhoff, J.C.P. Mesopore determination from nitrogen sorption isotherms: Fundamentals, scope, limitations. Stud. Surf. Sci. Catal. 1979, 3, 663–684. [Google Scholar]
- Yaluris, G.; Madon, R.J.; Rudd, D.F.; Dumesic, J.A. Catalytic cycles and selectivity of hydrocarbon cracking on Y-zeolite-based catalysts. Ind. Eng. Chem. Res. 1991, 30, 1013–1022. [Google Scholar] [CrossRef]
- Meusinger, J.; Corma, A. Influence of zeolite composition and structure on hydrogen transfer reactions from hydrocarbons and from hydrogen. J. Catal. 1996, 159, 353–360. [Google Scholar] [CrossRef]
- Abbot, J.; Wojciechowski, B.W. The mechanism of paraffin reactions on HY zeolite. J. Catal. 1989, 115, 1–15. [Google Scholar] [CrossRef]
- Kang, N.Y.; Woo, S.I.; Lee, Y.J.; Bae, J.; Choi, W.C.; Park, Y.-K. Enhanced hydrothermal stability of ZSM-5 formed from nanocrystalline seeds for naphtha catalytic cracking. J. Mater. Sci. 2016, 51, 3735–3749. [Google Scholar] [CrossRef]
- Konno, H.; Tago, T.; Nakasaka, Y.; Ohnaka, R.; Nishimura, J.; Masuda, T. Effectiveness of nano-scale ZSM-5 zeolite and its deactivation mechanism on catalytic cracking of representative hydrocarbons of naphtha. Microporous Mesoporous Mater. 2013, 175, 25–33. [Google Scholar] [CrossRef]
- Keyvanloo, K.; Towfighi, J. Comparing the catalytic performances of mixed molybdenum with cerium and lanthanide oxides supported on HZSM-5 by multiobjective optimization of catalyst compositions using nondominated sorting genetic algorithm. J. Anal. Appl. Pyrolysis 2010, 87, 224–234. [Google Scholar] [CrossRef]
- ASTM D 5758:2001; Standard Test Method for Determination of Relative Crystallinity of Zeolite ZSM-5 by X-Ray Diffraction. ASTM: West Conshohocken, PA, USA, 2001.
- ISO 9277:2022; Determination of the Specific Surface Area of Solids by Gas Adsorption—BET Method. ISO: Geneva, Switzerland, 2022.
- ASTM D4463-96(2006); Standard Guide for Metals Free Steam Deactivation of Fresh Fluid Cracking Catalysts. ASTM: West Conshohocken, PA, USA, 2006.
- ASTM D 5154:2010; Standard Test Method for Determining Activity and Selectivity of Fluid Catalytic Cracking (FCC) Catalysts by Microactivity Test. ASTM: West Conshohocken, PA, USA, 2010.
- ASTM D2887-24; Standard Test Method for Boiling Range Distribution of Petroleum Fractions by Gas Chromatography. ASTM: West Conshohocken, PA, USA, 2024.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).