A Review on Production of Ethylene Oxide from Epoxidation of Ethylene: Catalysis, Mechanism and Kinetics
Abstract
1. Introduction
2. Role of Catalysts in the Process of Epoxidation of Ethylene
| Entry No. | Catalyst | Mechanisms | Key Feature | EO Selectivity | Catalyst Structure/Type | Operating Conditions | Stability | Promoters/Dopant | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Mn-N4 Porphyrin-like graphene (Mn-N4GP) | Two-step: (1) N2O reduction to form Mn=O site, (2) ethylene epoxidation via alkoxide radical pathway | High selectivity and stability; thermodynamically favorable Mn=O site | 105× higher than acetaldehyde, 104× higher than 5MR species | Mn single-atom on porphyrin-like graphene | DFT modelled, mild | High (DFT shows stability) | None | [22] |
| 2 | Ag on B-vacancy h-BN (Ag–h-BN) | Trimolecular Langmuir–Hinshelwood mechanism with -CH2CH2OOCH2CH2- intermediate | Single-atom catalyst; strong selectivity; thermally stable | High, dominant over acetaldehyde (activation barrier: EO 0.44 eV vs. AC much higher) | Ag single-atom on B-vacancy h-BN | DFT modeled, ambient | High (embedded single atom) | None | [25] |
| 3 | Ag metal (Ag(111), Ag(100)) | OMC (oxometallacycle) mechanism and dominant OMC-dehydrogenation path | Low EO selectivity under low oxygen pressure; selectivity improves with Ag(100) facet | <30% on Ag(111), ~65% on Ag(100) | Metallic Ag surfaces | High temp (500 K), 20 bar | Moderate (metal surface oxidation sensitive) | Cs, Cl, alkali metals | [32] |
| 4 | Group IB metals (Ag, Cu, Au) | Three stages: (i) O2 dissociation, (ii) Oxometallacycle formation, (iii) EO or AA formation | Ag offers best balance of O- and C-binding; Cu oxidizes easily; Au poor O2 dissociation | Ag(100) > Ag(111) > Au/Cu (EO formation barrier higher on Au/Cu) | Bulk Group IB metals (Ag, Cu, Au) | Various surfaces and O2 pressures | Low for Cu/Au due to poor O2 handling | None | [34] |
| 5 | AgOx (surface oxide Ag structures) | Multiple: Langmuir–Hinshelwood, Eley–Rideal; involves OMC intermediate | Diverse mechanisms; phase-dependent activity; AgO_p(4 × 4) surface enables ER | Varies with surface; LH dominant in most AgOx, ER in AgO p(4 × 4) | Ag surface oxides | Varied O2 pressure & surface coverage | Phase-dependent | None | [64] |
| 6 | Doped Ag catalysts (with metal dopants) | Cocatalytic mechanism optimizing oxophilicity; modifies O-affinity via dopants | Improved selectivity beyond pristine Ag by tuning O-binding without altering C-binding | Higher than pristine Ag (specific numbers not stated) | Ag with metal dopants (Co, Cu, etc.) | DFT modeled, optimized O-binding | Enhanced by dopants | Co, Cu, Cs, Cl | [39] |
| 7 | Iridium single-atom on α-MnO2 (Ir1–α-MnO2) | π-coordination enabled OMC intermediate formation | Molecular-like catalysis; π-interaction between Ir and ethylene enhances selectivity | ~99% | Ir single-atom on α-MnO2 | ~200–250 °C, ambient pressure | High | None | [65] |
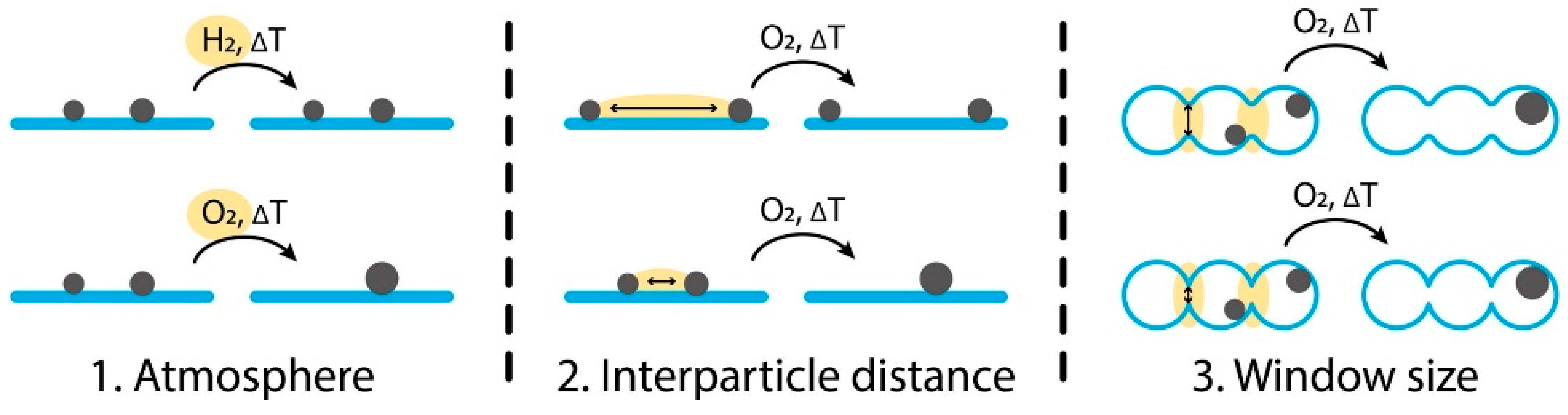
| 8 | Ag on α-alumina | Standard Ag-catalyzed epoxidation; affected by particle size and support structure | Stability via particle size and interparticle distance; Ostwald ripening dominant | ~80% (industrial with Cl/alkali promoters) | Ag on α-Al2O₃ | Industrial ~230–270 °C | Good if particle sintering avoided | Cs, Cl | [43] |
| 9 | Electrochemical systems (e.g., RuO2 mediated with Cl−) | Electrochemical halide-mediated pathway; forms hypobromite intermediates | Green method; improved selectivity via isolated OMC sites | High with halide mediation | RuO2 electrode with halide mediation | ~Room temp, electrochemical | Good (electrolyte optimized) | Cl−, Br− | [66] |
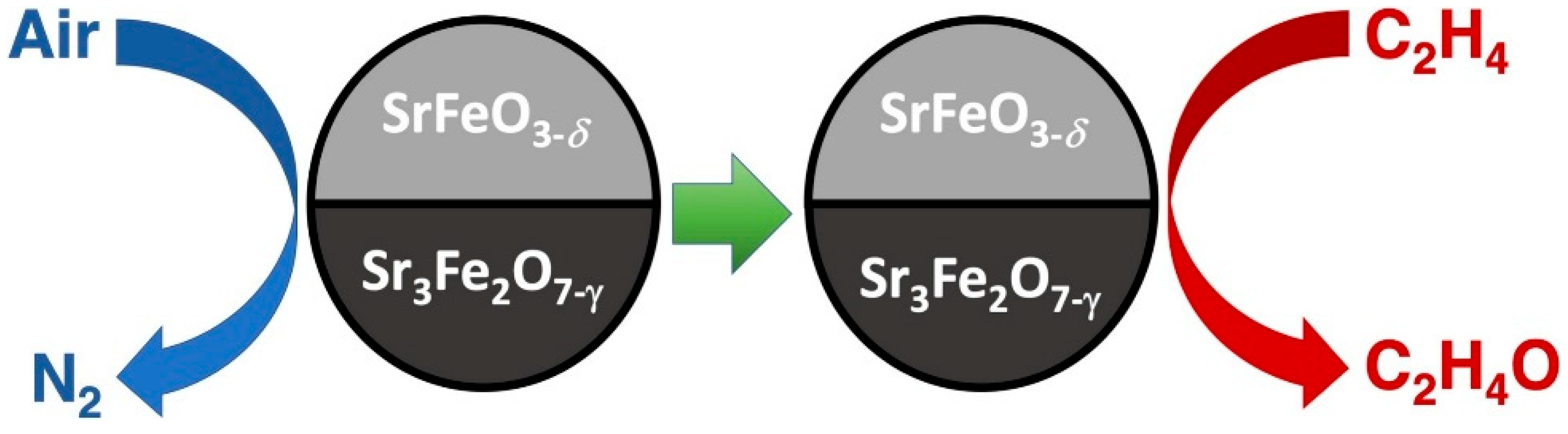
| 10 | Ag on strontium ferrite perovskite (SrFeO3/Sr3Fe2O7 mix) | Chemical looping with lattice oxygen donation | High oxygen capacity; safe oxygen delivery; phase composition influences selectivity | Up to 25%, it improved to 60% with ceria doping | Ag on SrFeO3/Sr3Fe2O7 perovskite | Chemical looping, cyclic oxidation/reduction | Improved with RP-phase mixing | None | [46] |
| 11 | Ru single-atom on beta zeolite | Heterogeneous oxidation via isolated Ru active centers | Highly dispersed Ru atoms on zeolite provide high activity | High | Ru single-atom on Beta Zeolite | ~200–300 °C (lab-scale) | High (zeolite structure stabilizes) | None | [65] |
| 12 | CoCu Co-doped Ag(111) with oxygen reconstruction | OMC mechanism with enhanced desorption and reactivity via dual dopants | Dual metal dopants (Co, Cu) optimize oxygen affinity, enhancing EO formation and desorption | Up to 89.5% | CoCu-doped Ag(111) | ~300–400 °C | Improved by dual dopants | Co, Cu | [67] |
| 13 | Pt/ZSM-5 with fluorine promotion | Low temperature oxidation mechanism enhanced by F-modified acid sites | F-doping enhances low temperature activity and selectivity | Improved vs. unmodified Pt/ZSM-5 (quantitative data not provided) | Pt/ZSM-5 with F | ~Low temp | Improved with F | F | [65] |
| 14 | Ag–ZSM-5 | Room temperature catalytic oxidation of ethylene | Supports selective EO formation at mild conditions | 30–40% depending on support acidity and Ag loading | Ag–ZSM-5 | Room temperature | Stable under mild | None | [65] |
| 15 | Electrochemical RuO2 with Cl− | Halide-mediated pathway via Cl-inhibited sites leading to EO production | Inhibits combustion path; enables EO pathway | High selectivity | RuO2 with Cl− | Electrochemical | Good with halide control | Cl− | [68] |
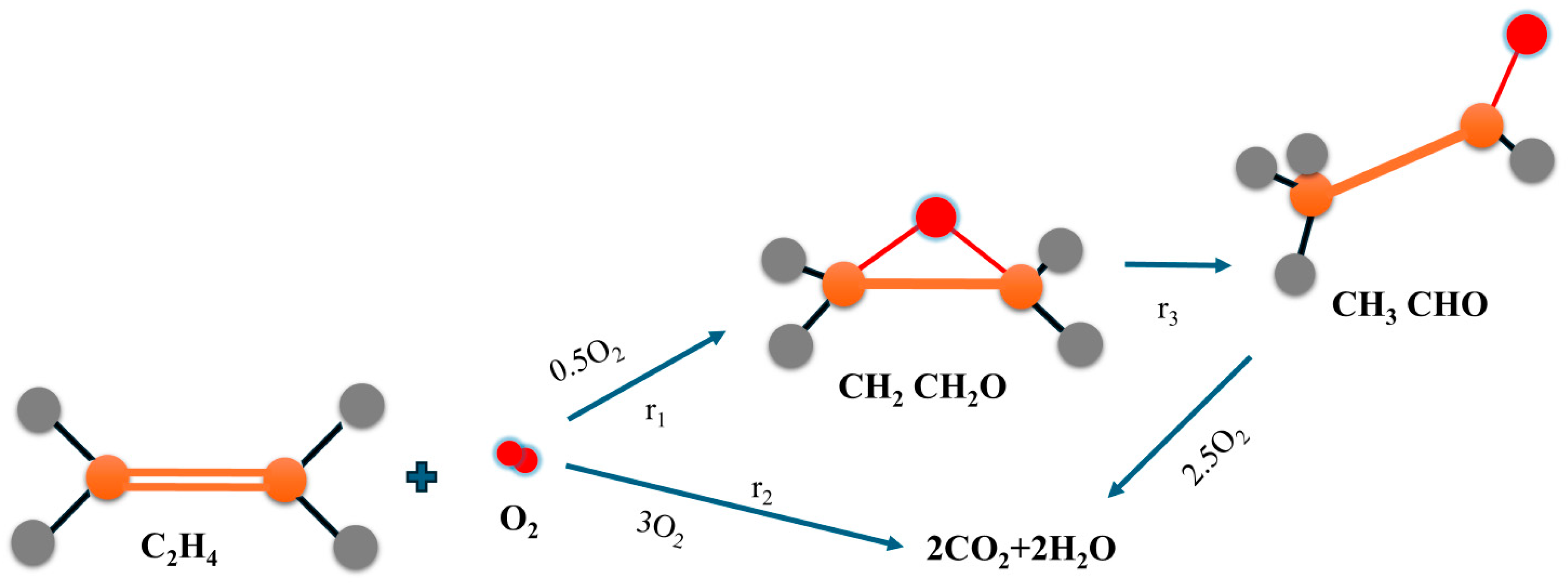
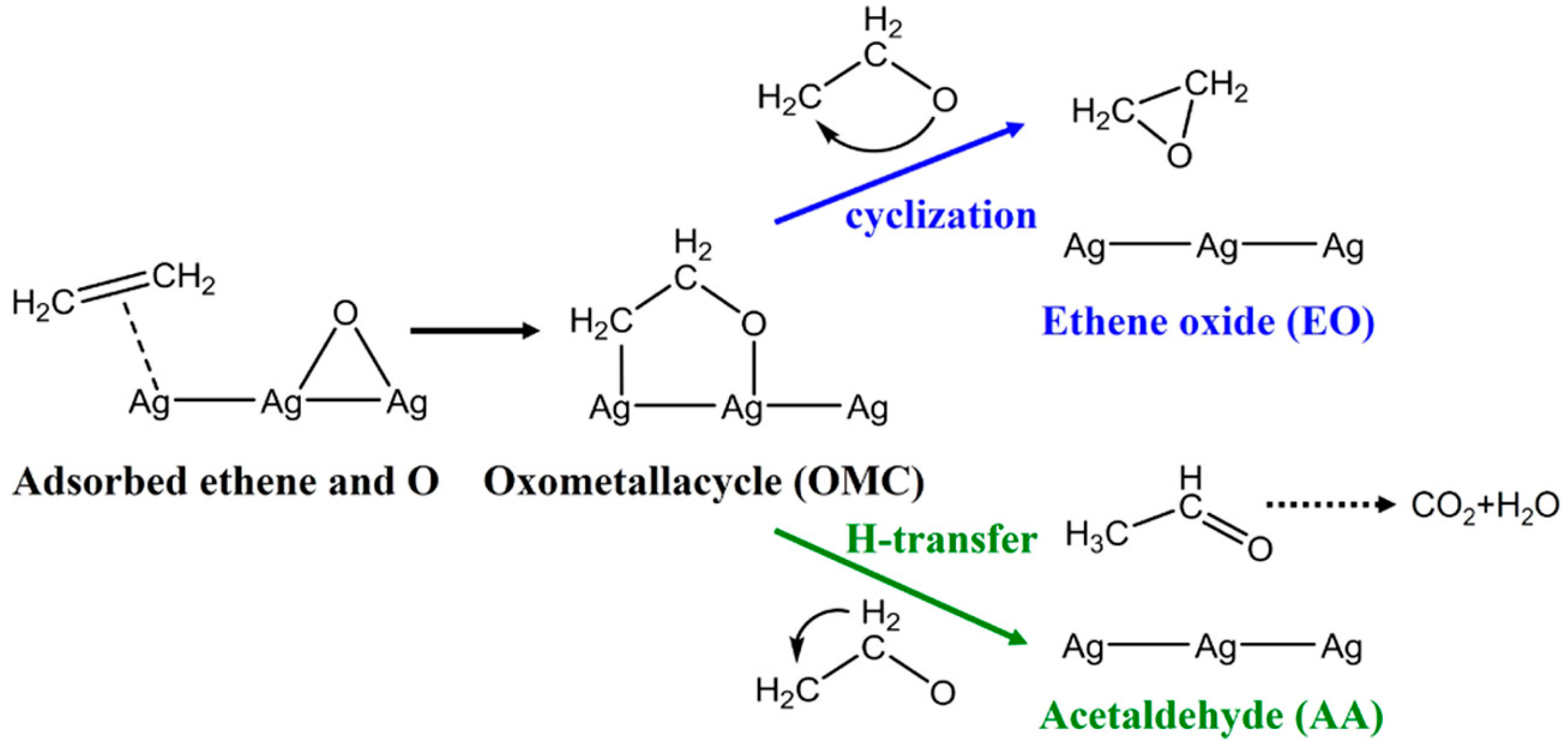
3. Mechanisms of the Process
4. Kinetics of Epoxidation of Ethylene
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EO | ethylene oxide |
| DFT | density functional theory |
| LSPR | localized surface plasmon resonance |
| CLE | chemical looping epoxidation |
| LPE | liquid phase epoxidation |
| MTO | methyltrioxorhenium |
| DOS | density of states analysis |
| OMC–DH | oxometallacycle–dehydrogenation |
| DCE | dichloroethane |
| TM | transition metal |
References
- Pinaeva, L.G.; Noskov, A.S. Prospects for the Development of Ethylene Oxide Production Catalysts and Processes (Review). Pet. Chem. 2020, 60, 1191–1206. [Google Scholar] [CrossRef]
- Maqbool, M.; Parveen, N.; Jaffar, S.; Hassan, S.U.; Mahmood, A.; Al-Masry, W.; Kim, T.; Han, S.K.; Park, C.H.; Razzaque, S.; et al. CO2-Free Ethylene Oxide Production via Liquid-Phase Epoxidation with Fe2O3/MSM Catalyst. Chem. Asian J. 2024, 19, e202400002. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, M.; Akhter, T.; Faheem, M.; Nadeem, S.; Park, C.H.; Mahmood, A. CO2 Free Production of Ethylene Oxide via Liquid Phase Epoxidation of Ethylene Using Niobium Oxide Incorporated Mesoporous Silica Material as the Catalyst. RSC Adv. 2023, 13, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Sirijaraensre, J.; Limtrakul, J. Theoretical Investigation on Reaction Pathways for Ethylene Epoxidation on Ti-Decorated Graphene. Struct. Chem. 2018, 29, 159–170. [Google Scholar] [CrossRef]
- Xue, W.; Quan, L.; Liu, H.; Yu, B.; Chen, X.; Xia, B.Y.; You, B. Bromine-Enhanced Generation and Epoxidation of Ethylene in Tandem CO2 Electrolysis Towards Ethylene Oxide. Angew. Chem. Int. Ed. 2023, 62, e202311570. [Google Scholar] [CrossRef]
- Lockemeyer, J.R.; Lohr, T.L. Ethylene Oxide Catalysis Under Commercial Conditions—A Guide for Researchers. ChemCatChem 2023, 15, e202201511. [Google Scholar] [CrossRef]
- Charchi Aghdam, N.; Chen, N.; Soltan, J. Ozonative Epoxidation of Ethylene: A Novel Process for Production of Ethylene Oxide. Appl. Catal. A Gen. 2023, 661, 119239. [Google Scholar] [CrossRef]
- van Hoof, A.J.F.; van der Poll, R.C.J.; Friedrich, H.; Hensen, E.J.M. Dynamics of Silver Particles during Ethylene Epoxidation. Appl. Catal. B 2020, 272, 118983. [Google Scholar] [CrossRef]
- Pu, T.; Tian, H.; Ford, M.E.; Rangarajan, S.; Wachs, I.E. Overview of Selective Oxidation of Ethylene to Ethylene Oxide by Ag Catalysts. ACS Catal. 2019, 9, 10727–10750. [Google Scholar] [CrossRef]
- Aikaterini, B.; Jose Antonio, M.R. Energy Efficiency and GHG Emissions: Prospective Scenarios for the Chemical and Petrochemical Industry; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar] [CrossRef]
- Chung, M.; Jin, K.; Zeng, J.S.; Manthiram, K. Mechanism of Chlorine-Mediated Electrochemical Ethylene Oxidation in Saline Water. ACS Catal. 2020, 10, 14015–14023. [Google Scholar] [CrossRef]
- Yang, H.; Li, G.; Ma, W.; Hao, B.; Zhang, Z.; Liu, Y.; Hao, Z. Recent Developments in Heterogeneous Catalyzed Epoxidation of Ethylene to Ethylene Oxide. Chem. A Eur. J. 2025, 31, e202404773. [Google Scholar] [CrossRef] [PubMed]
- Diao, W.; DiGiulio, C.; Schaal, M.; Ma, S.; Monnier, J. An Investigation on the Role of Re as a Promoter in AgCsRe/α-Al2O3 High-Selectivity, Ethylene Epoxidation Catalysts. J. Catal. 2015, 322, 14–23. [Google Scholar] [CrossRef]
- Yan, W.; Ramanathan, A.; Ghanta, M.; Subramaniam, B. Towards Highly Selective Ethylene Epoxidation Catalysts Using Hydrogen Peroxide and Tungsten- or Niobium-Incorporated Mesoporous Silicate (KIT-6). Catal. Sci. Technol. 2014, 4, 4433–4439. [Google Scholar] [CrossRef]
- Marek, E.J.; García-Calvo Conde, E. Effect of Catalyst Preparation and Storage on Chemical Looping Epoxidation of Ethylene. Chem. Eng. J. 2021, 417, 127981. [Google Scholar] [CrossRef]
- Van den Reijen, J.E.; Versluis, W.C.; Kanungo, S.; d’Angelo, M.F.; de Jong, K.P.; de Jongh, P.E. From Qualitative to Quantitative Understanding of Support Effects on the Selectivity in Silver Catalyzed Ethylene Epoxidation. Catal. Today 2019, 338, 31–39. [Google Scholar] [CrossRef]
- Liu, B.; Ji, J.; Zhang, B.; Huang, W.; Gan, Y.; Leung, D.Y.C.; Huang, H. Catalytic Ozonation of VOCs at Low Temperature: A Comprehensive Review. J. Hazard. Mater. 2022, 422, 126847. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.M.; Williams, F.J.; Cropley, R.L.; Palermo, A. Heterogeneous Alkene Epoxidation: Past, Present and Future. J. Mol. Catal. A Chem. 2005, 228, 27–33. [Google Scholar] [CrossRef]
- Özbek, M.O.; van Santen, R.A. The Mechanism of Ethylene Epoxidation Catalysis. Catal. Lett. 2013, 143, 131–141. [Google Scholar] [CrossRef]
- Shaw, M.S.; Bates, M.R.; Jones, M.D.; Ward, B.D. Metallocene Catalysts for the Ring-Opening Co-Polymerisation of Epoxides and Cyclic Anhydrides. Polym. Chem. 2022, 13, 3315–3324. [Google Scholar] [CrossRef]
- Kurańska, M.; Beneš, H.; Prociak, A.; Trhlíková, O.; Walterová, Z.; Stochlińska, W. Investigation of Epoxidation of Used Cooking Oils with Homogeneous and Heterogeneous Catalysts. J. Clean. Prod. 2019, 236, 117615. [Google Scholar] [CrossRef]
- Impeng, S.; Roongcharoen, T.; Maitarad, P.; Wu, H.; Chitpakdee, C.; Promarak, V.; Shi, L.; Namuangruk, S. High Selective Catalyst for Ethylene Epoxidation to Ethylene Oxide: A DFT Investigation. Appl. Surf. Sci. 2020, 513, 145799. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, K.; Gao, S.; Huang, H.; Xia, G.; Lin, Z.; Jiang, P.; Wang, C.; Wang, H.; Chen, Q. O-, N-Atoms-Coordinated Mn Cofactors within a Graphene Framework as Bioinspired Oxygen Reduction Reaction Electrocatalysts. Adv. Mater. 2018, 30, e1801732. [Google Scholar] [CrossRef]
- Impeng, S.; Siwaipram, S.; Bureekaew, S.; Probst, M. Ethane C-H Bond ActIVation on the Fe(IV)-Oxo Species in a Zn-Based Cluster of Metal-Organic Frameworks: A Density Functional Theory Study. Phys. Chem. Chem. Phys. 2017, 19, 3782–3791. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, M.D.; Janebi, H.; Mousavian, P. Epoxidation of Ethylene over an Ag Atom Embedded B-Vacancy Defective Boron-Nitride Nanosheet via a Trimolecular Langmuir–Hinshelwood Mechanism: A DFT Investigation. Mol. Catal. 2021, 514, 111843. [Google Scholar] [CrossRef]
- García-Mota, M.; Rieger, M.; Reuter, K. Ab Initio Prediction of the Equilibrium Shape of Supported Ag Nanoparticles on α-Al2O3(0 0 0 1). J. Catal. 2015, 321, 1–6. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, J.; Wu, D.; Zhao, X.; Jing, Y.; Zhou, Z. A Ti-Anchored Ti2CO2 Monolayer (MXene) as a Single-Atom Catalyst for CO Oxidation. J. Mater. Chem. A Mater. 2016, 4, 4871–4876. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mousavian, P. The Strengthening Effect of a Halogen, Chalcogen or Pnicogen Bonding on Halogen–π Interaction: A Comparative Ab Initio Study. Mol. Phys. 2018, 116, 526–535. [Google Scholar] [CrossRef]
- Lohr, T.L.; Lockemeyer, J.R.; Bishopp, S.D.; Motagamwala, A.H.; Wells, G.J.; Wermink, T. Ethylene Oxide: A Catalyst and Process Development Success Story. Ind. Eng. Chem. Res. 2024, 63, 18221–18240. [Google Scholar] [CrossRef]
- Tarrat, N.; Rapacioli, M.; Cuny, J.; Morillo, J.; Heully, J.L.; Spiegelman, F. Global Optimization of Neutral and Charged 20- and 55-Atom Silver and Gold Clusters at the DFTB Level. Comput. Theor. Chem. 2017, 1107, 102–114. [Google Scholar] [CrossRef]
- Li, X.T.; Chen, L.; Wei, G.F.; Shang, C.; Liu, Z.P. Sharp Increase in Catalytic Selectivity in Acetylene Semihydrogenation on Pd Achieved by a Machine Learning Simulation-Guided Experiment. ACS Catal. 2020, 10, 9694–9705. [Google Scholar] [CrossRef]
- Chen, D.; Kang, P.L.; Liu, Z.P. Active Site of Catalytic Ethene Epoxidation: Machine-Learning Global Pathway Sampling Rules out the Metal Sites. ACS Catal. 2021, 11, 8317–8326. [Google Scholar] [CrossRef]
- Li, H.; Nørskov, J.K. Effects of a Conductive Support on the Bonding of Oxygen Containing Molecules to Transition Metal Oxide Surfaces. Phys. Chem. Chem. Phys. 2020, 22, 26216–26222. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, A.; Nørskov, J.K. Understanding Trends in Ethylene Epoxidation on Group IB Metals. ACS Catal. 2021, 11, 12052–12057. [Google Scholar] [CrossRef]
- Liu, J.X.; Su, Y.; Filot, I.A.W.; Hensen, E.J.M. A Linear Scaling Relation for CO Oxidation on CeO2-Supported Pd. J. Am. Chem. Soc. 2018, 140, 4580–4587. [Google Scholar] [CrossRef]
- Wu, S.; Tatarchuk, B.J.; Adamczyk, A.J. Ethylene Oxidation on Unpromoted Silver Catalysts: Reaction Pathway and Selectivity Analysis Using DFT Calculations. Surf. Sci. 2021, 708, 121834. [Google Scholar] [CrossRef]
- Van Hoof, A.J.F.; Filot, I.A.W.; Friedrich, H.; Hensen, E.J.M. Reversible Restructuring of Silver Particles during Ethylene Epoxidation. ACS Catal. 2018, 8, 11794–11800. [Google Scholar] [CrossRef]
- Greiner, M.T.; Jones, T.E.; Johnson, B.E.; Rocha, T.C.R.; Wang, Z.J.; Armbrüster, M.; Willinger, M.; Knop-Gericke, A.; Schlögl, R. The Oxidation of Copper Catalysts during Ethylene Epoxidation. Phys. Chem. Chem. Phys. 2015, 17, 25073–25089. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhu, L.; Nan, Y.; Xie, Y.; Cheng, D. Revisit the Role of Metal Dopants in Enhancing the Selectivity of Ag-Catalyzed Ethylene Epoxidation: Optimizing Oxophilicity of Reaction Site via Cocatalytic Mechanism. ACS Catal. 2021, 11, 3371–3383. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, D.; Gao, Y.; Zeng, X.C. Assessment of Catalytic Activities of Gold Nanoclusters with Simple Structure Descriptors. ACS Catal. 2018, 8, 9702–9710. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Loffreda, D.; Koper, M.T.M.; Sautet, P. Introducing Structural Sensitivity into Adsorption-Energy Scaling Relations by Means of Coordination Numbers. Nat. Chem. 2015, 7, 403–410. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Zeng, X.C.; Chen, Z. Exploration of High-Performance Single-Atom Catalysts on Support M1/FeOx for CO Oxidation via Computational Study. ACS Catal. 2015, 5, 544–552. [Google Scholar] [CrossRef]
- Keijzer, P.H.; van den Reijen, J.E.; Keijzer, C.J.; de Jong, K.P.; de Jongh, P.E. Influence of Atmosphere, Interparticle Distance and Support on the Stability of Silver on α-Alumina for Ethylene Epoxidation. J. Catal. 2022, 405, 534–544. [Google Scholar] [CrossRef]
- Hu, S.; Li, W.X. Influence of Particle Size Distribution on Lifetime and Thermal Stability of Ostwald Ripening of Supported Particles. ChemCatChem 2018, 10, 2900–2907. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, T.; Gong, J. Advances in Electrochemical Oxidation of Olefins to Epoxides. CCS Chem. 2023, 5, 1028–1042. [Google Scholar] [CrossRef]
- Gabra, S.; Marek, E.J.; Poulston, S.; Williams, G.; Dennis, J.S. The Use of Strontium Ferrite Perovskite as an Oxygen Carrier in the Chemical Looping Epoxidation of Ethylene. Appl. Catal. B 2021, 286, 119821. [Google Scholar] [CrossRef]
- Lau, C.Y.; Dunstan, M.T.; Hu, W.; Grey, C.P.; Scott, S.A. Large Scale in Silico Screening of Materials for Carbon Capture through Chemical Looping. Energy Environ. Sci. 2017, 10, 818–831. [Google Scholar] [CrossRef]
- Marek, E.J.; Gabra, S.; Dennis, J.S.; Scott, S.A. High Selectivity Epoxidation of Ethylene in Chemical Looping Setup. Appl. Catal. B 2020, 262, 118216. [Google Scholar] [CrossRef]
- Hossain, S.T.; Azeeva, E.; Zhang, K.; Zell, E.T.; Bernard, D.T.; Balaz, S.; Wang, R. A Comparative Study of CO Oxidation over Cu-O-Ce Solid Solutions and CuO/CeO2 Nanorods Catalysts. Appl. Surf. Sci. 2018, 455, 132–143. [Google Scholar] [CrossRef]
- Suarez, A.; Ramirez, A.; Bueno-Alejo, C.; Hueso, J. Silver-Copper Oxide Heteronanostructures for the Plasmonic-Enhanced Photocatalytic Oxidation of N-Hexane in the Visible-NIR Range. Materials 2019, 12, 3858. [Google Scholar] [CrossRef]
- Cropley, R.L.; Williams, F.J.; Urquhart, A.J.; Vaughan, O.P.H.; Tikhov, M.S.; Lambert, R.M. Efficient Epoxidation of a Terminal Alkene Containing Allylic Hydrogen Atoms: Trans-Methylstyrene on Cu{111}. J. Am. Chem. Soc. 2005, 127, 6069–6076. [Google Scholar] [CrossRef]
- Ramirez, A.; Hueso, J.L.; Suarez, H.; Mallada, R.; Ibarra, A.; Irusta, S.; Santamaria, J. A Nanoarchitecture Based on Silver and Copper Oxide with an Exceptional Response in the Chlorine-Promoted Epoxidation of Ethylene. Angew. Chem. Int. Ed. Engl. 2016, 55, 11158–11161. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Dang, K.; Wang, J.; Chen, C.; Zhao, J.; Zhang, Y. Solar-Driven Green Synthesis of Epoxides. Sci. China Chem. 2023, 66, 3415–3425. [Google Scholar] [CrossRef]
- Dorchies, F.; Serva, A.; Crevel, D.; De Freitas, J.; Kostopoulos, N.; Robert, M.; Sel, O.; Salanne, M.; Grimaud, A. Controlling the Hydrophilicity of the Electrochemical Interface to Modulate the Oxygen-Atom Transfer in Electrocatalytic Epoxidation Reactions. J. Am. Chem. Soc. 2022, 144, 22734–22746. [Google Scholar] [CrossRef] [PubMed]
- Sayama, K. Production of High-Value-Added Chemicals on Oxide Semiconductor Photoanodes under Visible Light for Solar Chemical-Conversion Processes. ACS Energy Lett. 2018, 3, 1093–1101. [Google Scholar] [CrossRef]
- Hardwick, T.; Qurashi, A.; Shirinfar, B.; Ahmed, N. Interfacial Photoelectrochemical Catalysis: Solar-Induced Green Synthesis of Organic Molecules. ChemSusChem 2020, 13, 1967–1973. [Google Scholar] [CrossRef]
- Svintsitskiy, D.A.; Lazarev, M.K.; Slavinskaya, E.M.; Fedorova, E.A.; Kardash, T.Y.; Cherepanova, S.V.; Boronin, A.I. Room Temperature Epoxidation of Ethylene over Delafossite-Based AgNiO2 Nanoparticles. Phys. Chem. Chem. Phys. 2023, 25, 20892–20902. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, T.; Liu, K.; Du, H.; Podkolzin, S.G. Atomic, Molecular and Hybrid Oxygen Structures on Silver. Langmuir 2021, 37, 11603–11610. [Google Scholar] [CrossRef]
- Prins, R. Eley–Rideal, the Other Mechanism. Top. Catal. 2018, 61, 714–721. [Google Scholar] [CrossRef]
- Pu, T.; Setiawan, A.; Foucher, A.C.; Guo, M.; Jehng, J.M.; Zhu, M.; Ford, M.E.; Stach, E.A.; Rangarajan, S.; Wachs, I.E. Revealing the Nature of Active Oxygen Species and Reaction Mechanism of Ethylene Epoxidation by Supported Ag/α-Al2O3 Catalysts. ACS Catal. 2024, 14, 406–417. [Google Scholar] [CrossRef]
- Harris, J.W.; Arvay, J.; Mitchell, G.; Delgass, W.N.; Ribeiro, F.H. Propylene Oxide Inhibits Propylene Epoxidation over Au/TS-1. J. Catal. 2018, 365, 105–114. [Google Scholar] [CrossRef]
- Smidstrup, S.; Pedersen, A.; Stokbro, K.; Jónsson, H. Improved Initial Guess for Minimum Energy Path Calculations. J. Chem. Phys. 2014, 140, 214106. [Google Scholar] [CrossRef] [PubMed]
- Kumer, A.; Sarker, M.N.; Paul, S. The Theoretical Investigation of HOMO, LUMO, Thermophysical Properties and QSAR Study of Some Aromatic Carboxylic Acids Using HyperChem Programming. Int. J. Chem. Technol. 2019, 3, 26–37. [Google Scholar] [CrossRef]
- Liu, J.X.; Lu, S.; Ann, S.B.; Linic, S. Mechanisms of Ethylene Epoxidation over Silver from Machine Learning-Accelerated First-Principles Modeling and Microkinetic Simulations. ACS Catal. 2023, 13, 8955–8962. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Liu, Q.; Huang, A.; Zhang, X.; Yu, Y.; Zhuang, Z.; Li, G.; Li, Y.; Peng, Q.; et al. Heterogeneous Iridium Single-Atom Molecular-like Catalysis for Epoxidation of Ethylene. J. Am. Chem. Soc. 2023, 145, 6658–6670. [Google Scholar] [CrossRef]
- Li, X.; Chen, G.; Liu, Y.; Lu, R.; Ma, C.; Wang, Z.; Han, Y.; Wang, D. Optimal Selection of RuO2 for Durable Oxygen Evolution Reactions in Acidic Media by Continuous Regulation of Ru–O Covalency. Energy Environ. Sci. 2025, 18, 4200–4209. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, L.; Nan, Y.; Xie, Y.; Cheng, D. Revisit the Role of Chlorine in Selectivity Enhancement of Ethylene Epoxidation. Ind. Eng. Chem. Res. 2019, 58, 21403–21412. [Google Scholar] [CrossRef]
- Liang, N.N.; Choi, W.; Suk Han, D.; Park, H. Electrocatalytic Conversion of Ethylene to Ethylene Oxide Mediated by Halide Oxidation: Chloride vs. Bromide vs. Iodide. Chem. Eng. J. 2024, 494, 153042. [Google Scholar] [CrossRef]
- Jones, T.E.; Wyrwich, R.; Böcklein, S.; Carbonio, E.A.; Greiner, M.T.; Klyushin, A.Y.; Moritz, W.; Locatelli, A.; Menteş, T.O.; Niño, M.A.; et al. The Selective Species in Ethylene Epoxidation on Silver. ACS Catal. 2018, 8, 3844–3852. [Google Scholar] [CrossRef]
- Ma, W.; Yuan, H.; Wang, H.; Zhou, Q.; Kong, K.; Li, D.; Yao, Y.; Hou, Z. Identifying Catalytically Active Mononuclear Peroxoniobate Anion of Ionic Liquids in the Epoxidation of Olefins. ACS Catal. 2018, 8, 4645–4659. [Google Scholar] [CrossRef]
- Silbaugh, T.L.; Devlaminck, P.; Sofranko, J.A.; Barteau, M.A. Selective Oxidation of Ethanol over Ag, Cu and Au Nanoparticles Supported on Li2O/Γ-Al2O3. J. Catal. 2018, 364, 40–47. [Google Scholar] [CrossRef]
- Greiner, M.T.; Jones, T.E.; Klyushin, A.; Knop-Gericke, A.; Schlögl, R. Ethylene Epoxidation at the Phase Transition of Copper Oxides. J. Am. Chem. Soc. 2017, 139, 11825–11832. [Google Scholar] [CrossRef] [PubMed]
- Greiner, M.T.; Cao, J.; Jones, T.E.; Beeg, S.; Skorupska, K.; Carbonio, E.A.; Sezen, H.; Amati, M.; Gregoratti, L.; Willinger, M.G.; et al. Phase Coexistence of Multiple Copper Oxides on AgCu Catalysts during Ethylene Epoxidation. ACS Catal. 2018, 8, 2286–2295. [Google Scholar] [CrossRef]
- Carbonio, E.A.; Rocha, T.C.R.; Klyushin, A.Y.; Píš, I.; Magnano, E.; Nappini, S.; Piccinin, S.; Knop-Gericke, A.; Schlögl, R.; Jones, T.E. Are Multiple Oxygen Species Selective in Ethylene Epoxidation on Silver? Chem. Sci. 2018, 9, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Huš, M.; Hellman, A. Ethylene Epoxidation on Ag(100), Ag(110), and Ag(111): A Joint Ab Initio and Kinetic Monte Carlo Study and Comparison with Experiments. ACS Catal. 2019, 9, 1183–1196. [Google Scholar] [CrossRef]
- Van den Reijen, J.E.; Kanungo, S.; Welling, T.A.J.; Versluijs-Helder, M.; Nijhuis, T.A.; de Jong, K.P.; de Jongh, P.E. Preparation and Particle Size Effects of Ag/A-Al2O3 Catalysts for Ethylene Epoxidation. J. Catal. 2017, 356, 65–74. [Google Scholar] [CrossRef]
- Ren, D.; Cheng, G.; Li, J.; Li, J.; Dai, W.; Sun, X.X.; Cheng, D. Effect of Rhenium Loading Sequence on Selectivity of Ag–Cs Catalyst for Ethylene Epoxidation. Catal. Lett. 2017, 147, 2920–2928. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, D.; Cao, D.; Zeng, X.C. A Universal Principle for a Rational Design of Single-Atom Electrocatalysts. Nat. Catal. 2018, 1, 339–348. [Google Scholar] [CrossRef]
- Medford, A.J.; Vojvodic, A.; Hummelshøj, J.S.; Voss, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T.; Nilsson, A.; Nørskov, J.K. From the Sabatier Principle to a Predictive Theory of Transition-Metal Heterogeneous Catalysis. J. Catal. 2015, 328, 36–42. [Google Scholar] [CrossRef]
- Cui, X.; Li, W.; Ryabchuk, P.; Junge, K.; Beller, M. Bridging Homogeneous and Heterogeneous Catalysis by Heterogeneous Single-Metal-Site Catalysts. Nat. Catal. 2018, 1, 385–397. [Google Scholar] [CrossRef]
- Yang, H.; Ma, C.; Zhang, X.; Li, Y.; Cheng, J.; Hao, Z. Understanding the Active Sites of Ag/Zeolites and Deactivation Mechanism of Ethylene Catalytic Oxidation at Room Temperature. ACS Catal. 2018, 8, 1248–1258. [Google Scholar] [CrossRef]
- Yang, H.; Ma, C.; Li, Y.; Wang, J.; Zhang, X.; Wang, G.; Qiao, N.; Sun, Y.; Cheng, J.; Hao, Z. Synthesis, Characterization and Evaluations of the Ag/ZSM-5 for Ethylene Oxidation at Room Temperature: Investigating the Effect of Water and Deactivation. Chem. Eng. J. 2018, 347, 808–818. [Google Scholar] [CrossRef]
- Lamoth, M.; Jones, T.; Plodinec, M.; Machoke, A.; Wrabetz, S.; Krämer, M.; Karpov, A.; Rosowski, F.; Piccinin, S.; Schlögl, R.; et al. Nanocatalysts Unravel the Selective State of Ag. ChemCatChem 2020, 12, 2977–2988. [Google Scholar] [CrossRef]
- Hess, C. New Advances in Using Raman Spectroscopy for the Characterization of Catalysts and Catalytic Reactions. Chem. Soc. Rev. 2021, 50, 3519–3564. [Google Scholar] [CrossRef] [PubMed]
- Pu, T.; Setiawan, A.; Mosevitzky Lis, B.; Zhu, M.; Ford, M.E.; Rangarajan, S.; Wachs, I.E. Nature and Reactivity of Oxygen Species on/in Silver Catalysts during Ethylene Oxidation. ACS Catal. 2022, 12, 4375–4381. [Google Scholar] [CrossRef]
- Goodman, E.D.; Schwalbe, J.A.; Cargnello, M. Mechanistic Understanding and the Rational Design of Sinter- Resistant Heterogeneous Catalysts. ACS Catal. 2017, 7, 7156–7173. [Google Scholar] [CrossRef]
- Jirkovský, J.S.; Busch, M.; Ahlberg, E.; Panas, I.; Krtil, P. Switching on the Electrocatalytic Ethene Epoxidation on Nanocrystalline RuO2. J. Am. Chem. Soc. 2011, 133, 5882–5892. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, R.; Peng, W.; Tang, G.; Gao, X. Inverse CeO2 [Sbnd]Fe2O3 Catalyst for Superior Low-Temperature CO Conversion Efficiency. Appl. Surf. Sci. 2017, 416, 911–917. [Google Scholar] [CrossRef]
- Zhang, D.; Kawada, T.; Yoshioka, F.; Machida, M. Oxygen Gateway Effect of CeO2/La2O2SO4 Composite Oxygen Storage Materials. ACS Omega 2016, 1, 789–798. [Google Scholar] [CrossRef]
- Li, J.L.; Li, Y.F.; Liu, Z.P. In Situ Structure of a Mo-Doped Pt-Ni Catalyst during Electrochemical Oxygen Reduction Resolved from Machine Learning-Based Grand Canonical Global Optimization. JACS Au 2023, 3, 1162–1175. [Google Scholar] [CrossRef]
- McNeil, L.A.; Mutch, G.A.; Iacoviello, F.; Bailey, J.J.; Triantafyllou, G.; Neagu, D.; Miller, T.S.; Papaioannou, E.I.; Hu, W.; Brett, D.J.L.; et al. Dendritic Silver Self-Assembly in Molten-Carbonate Membranes for Efficient Carbon Dioxide Capture. Energy Environ. Sci. 2020, 13, 1766–1775. [Google Scholar] [CrossRef]
- Shi, L.; Yin, Y.; Wang, S.; Sun, H. Rational Catalyst Design for N2Reduction under Ambient Conditions: Strategies toward Enhanced Conversion Efficiency. ACS Catal. 2020, 10, 6870–6899. [Google Scholar] [CrossRef]
- Urbiztondo, M.; Ramirez, A.; Hueso, J.L.; Santamaria, J.; Ruiz-Salvador, A.R.; Hamad, S. Unravelling the Key Factors in the Chlorine-Promoted Epoxidation of Ethylene over a Silver-Copper Oxide Nanocatalyst. Nanoscale 2022, 14, 7332–7340. [Google Scholar] [CrossRef]
- Chung, M.; Jin, K.; Zeng, J.S.; Ton, T.N.; Manthiram, K. Tuning Single-Atom Dopants on Manganese Oxide for Selective Electrocatalytic Cyclooctene Epoxidation. J. Am. Chem. Soc. 2022, 144, 17416–17422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Duan, M.; Deng, C.; Yang, J.; Yang, S.; Zhang, Y.; Sheng, H.; Li, Y.; Chen, C.; Zhao, J. Br−/BrO−-Mediated Highly Efficient Photoelectrochemical Epoxidation of Alkenes on α-Fe2O3. Nat. Commun. 2023, 14, 1943. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.; Mortensen, H.; Meldgaard, S.; Kolsbjerg, E.; Jacobsen, T.; Sørensen, K.; Hammer, B. Atomistic Structure Learning. J. Chem. Phys. 2019, 151, 054111. [Google Scholar] [CrossRef]
- Huš, M.; Grilc, M.; Teržan, J.; Gyergyek, S.; Likozar, B.; Hellman, A. Going Beyond Silver in Ethylene Epoxidation with First-Principles Catalyst Screening. Angew. Chem. Int. Ed. 2023, 62, e202305804. [Google Scholar] [CrossRef]
- Wexler, R.B.; Qiu, T.; Rappe, A.M. Automatic Prediction of Surface Phase Diagrams Using Ab Initio Grand Canonical Monte Carlo. J. Phys. Chem. C 2019, 123, 2321–2328. [Google Scholar] [CrossRef]
- Chen, D.; Chen, L.; Zhao, Q.C.; Yang, Z.X.; Shang, C.; Liu, Z.P. Square-Pyramidal Subsurface Oxygen [Ag4OAg] Drives Selective Ethene Epoxidation on Silver. Nat. Catal. 2024, 7, 536–545. [Google Scholar] [CrossRef]
- Mukda, N.; Autthanit, C.; Praserthdam, P.; Jongsomjit, B. Production of Acetaldehyde via Oxidative Dehydrogenation of Ethanol over AgLi/SiO2 Catalysts. Bull. Chem. React. Eng. Catal. 2020, 15, 714–725. [Google Scholar] [CrossRef]
- Christopher, P.; Linic, S. Engineering Selectivity in Heterogeneous Catalysis: Ag Nanowires as Selective Ethylene Epoxidation Catalysts. J. Am. Chem. Soc. 2008, 130, 11264–11265. [Google Scholar] [CrossRef]
- Özbek, M.O.; Önal, I.; Vansanten, R.A. Ethylene Epoxidation Catalyzed by Silver Oxide. ChemCatChem 2011, 3, 150–153. [Google Scholar] [CrossRef]
- Xu, H.; Xu, H.; Cheng, D. Resolving the Reaction Mechanism for Oxidative Hydration of Ethylene toward Ethylene Glycol by Titanosilicate Catalysts. ACS Catal. 2022, 12, 9446–9457. [Google Scholar] [CrossRef]
- Nie, X.; Ren, X.; Ji, X.; Chen, Y.; Janik, M.J.; Guo, X.; Song, C. Mechanistic Insight into Propylene Epoxidation with H2O2 over Titanium Silicalite-1: Effects of Zeolite Confinement and Solvent. J. Phys. Chem. B 2019, 123, 7410–7423. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, S.; Zhang, J.; Li, S.; Yang, B. Propagating DFT Uncertainty to Mechanism Determination, Degree of Rate Control, and Coverage Analysis: The Kinetics of Dry Reforming of Methane. J. Phys. Chem. C 2019, 123, 30389–30397. [Google Scholar] [CrossRef]
- Pang, J.; Zheng, M.; Sun, R.; Wang, A.; Wang, X.; Zhang, T. Synthesis of Ethylene Glycol and Terephthalic Acid from Biomass for Producing PET. Green. Chem. 2016, 18, 342–359. [Google Scholar] [CrossRef]
- Chen, C.J.; Harris, J.W.; Bhan, A. Kinetics of Ethylene Epoxidation on a Promoted Ag/α-Al2O3 Catalyst—The Effects of Product and Chloride Co-Feeds on Rates and Selectivity. Chem. A Eur. J. 2018, 24, 12405–12415. [Google Scholar] [CrossRef]
- Fazeli, A.; Naseri, A.; Eslamjamal, F. Kinetic Models of Ethylene Oxide Production on Ag Catalysts: A Review. Kinet. Catal. 2020, 61, 603–612. [Google Scholar] [CrossRef]
- Partopour, B.; Dixon, A.G. Resolved-Particle Fixed Bed CFD with Microkinetics for Ethylene Oxidation. AIChE J. 2017, 63, 87–94. [Google Scholar] [CrossRef]
- Ghanta, M.; Ruddy, T.; Fahey, D.; Busch, D.; Subramaniam, B. Is the Liquid-Phase H2O2-Based Ethylene Oxide Process More Economical and Greener than the Gas-Phase O2-Based Silver-Catalyzed Process? Ind. Eng. Chem. Res. 2013, 52, 18–29. [Google Scholar] [CrossRef]
- Wang, B.; Han, H.; Ge, B.; Ma, J.; Zhu, J.; Chen, S. An Efficient Hydrophobic Modification of TS-1 and Its Application in the Epoxidation of Propylene. New J. Chem. 2019, 43, 10390–10397. [Google Scholar] [CrossRef]
- Alvear, M.; Fortunato, M.E.; Russo, V.; Eränen, K.; Di Serio, M.; Lehtonen, J.; Rautiainen, S.; Murzin, D.; Salmi, T. Continuous Liquid-Phase Epoxidation of Ethylene with Hydrogen Peroxide on a Titanium-Silicate Catalyst. Ind. Eng. Chem. Res. 2021, 60, 9429–9436. [Google Scholar] [CrossRef]
- Alvear, M.; Eranen, K.; Murzin, D.Y.; Salmi, T. Study of the Product Distribution in the Epoxidation of Propylene over TS-1 Catalyst in a Trickle-Bed Reactor. Ind. Eng. Chem. Res. 2021, 60, 2430–2438. [Google Scholar] [CrossRef]
- Sulimov, A.V.; Danov, S.M.; Ovcharova, A.V.; Ovcharov, A.A.; Flid, V.R. Kinetics of Propylene Epoxidation with Hydrogen Peroxide Catalyzed by Extruded Titanium Silicalite in Methanol. Kinet. Catal. 2016, 57, 466–473. [Google Scholar] [CrossRef]
- Alvear, M.; Fortunato, M.E.; Russo, V.; Salmi, T.; Serio, M. Di Modelling of Transient Kinetics in Trickle Bed Reactors: Ethylene Oxide Production via Hydrogen Peroxide. Chem. Eng. Sci. 2022, 248, 117156. [Google Scholar] [CrossRef]
- Russo, V.; Santacesaria, E.; Tesser, R.; Turco, R.; Vitiello, R.; Di Serio, M. Validation of the Kinetics of the Hydrogen Peroxide Propene Oxide Process in a Dynamic Continuous Stirred Tank Reactor. Ind. Eng. Chem. Res. 2018, 57, 16201–16208. [Google Scholar] [CrossRef]
- Harris, J.W.; Wang, L.; Dewilde, J.F. Kinetic Modeling of Ethylene Epoxidation Kinetics as a Function of Chlorine Coverage over a Highly Promoted Silver Catalyst. Ind. Eng. Chem. Res. 2019, 58, 19061–19071. [Google Scholar] [CrossRef]
- Majumdar, P.; Greeley, J. Generalized Scaling Relationships on Transition Metals: Influence of Adsorbate-Coadsorbate Interactions. Phys. Rev. Mater. 2018, 2, 45801. [Google Scholar] [CrossRef]
- Xu, B.; Hartigan, E.M.; Feula, G.; Huang, Z.; Lumb, J.P.; Arndtsen, B.A. Simple Copper Catalysts for the Aerobic Oxidation of Amines: Selectivity Control by the Counterion. Angew. Chem. Int. Ed. 2016, 55, 15802–15806. [Google Scholar] [CrossRef]
- Rocha, T.C.R.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R. Promoters in Heterogeneous Catalysis: The Role of Cl on Ethylene Epoxidation over Ag. J. Catal. 2014, 312, 12–16. [Google Scholar] [CrossRef]
- Brandão, L.; Reece, C. Non-Steady State Validation of Kinetic Models for Ethylene Epoxidation over Silver Catalysts. Catal. Sci. Technol. 2024, 14, 3596–3608. [Google Scholar] [CrossRef]




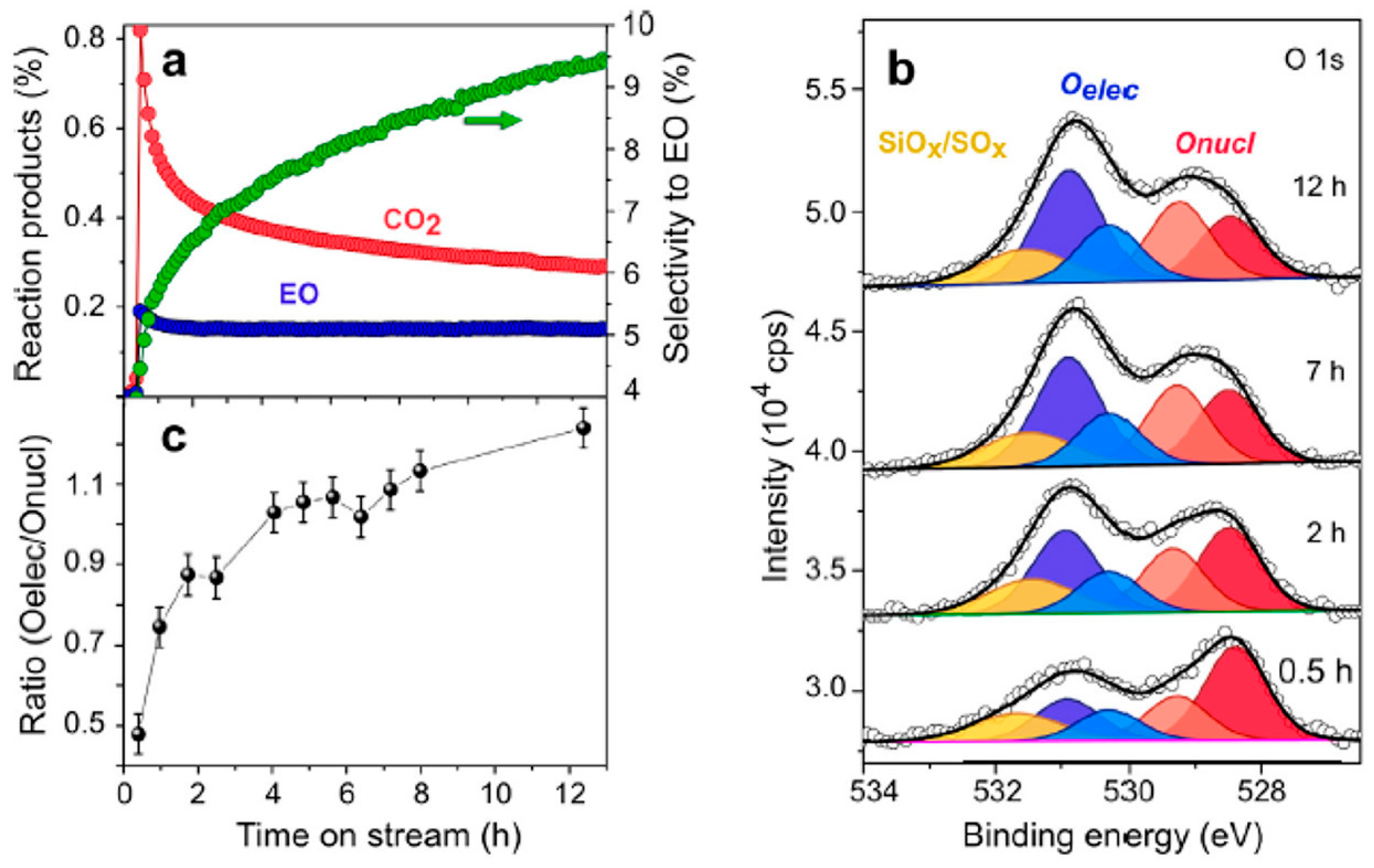
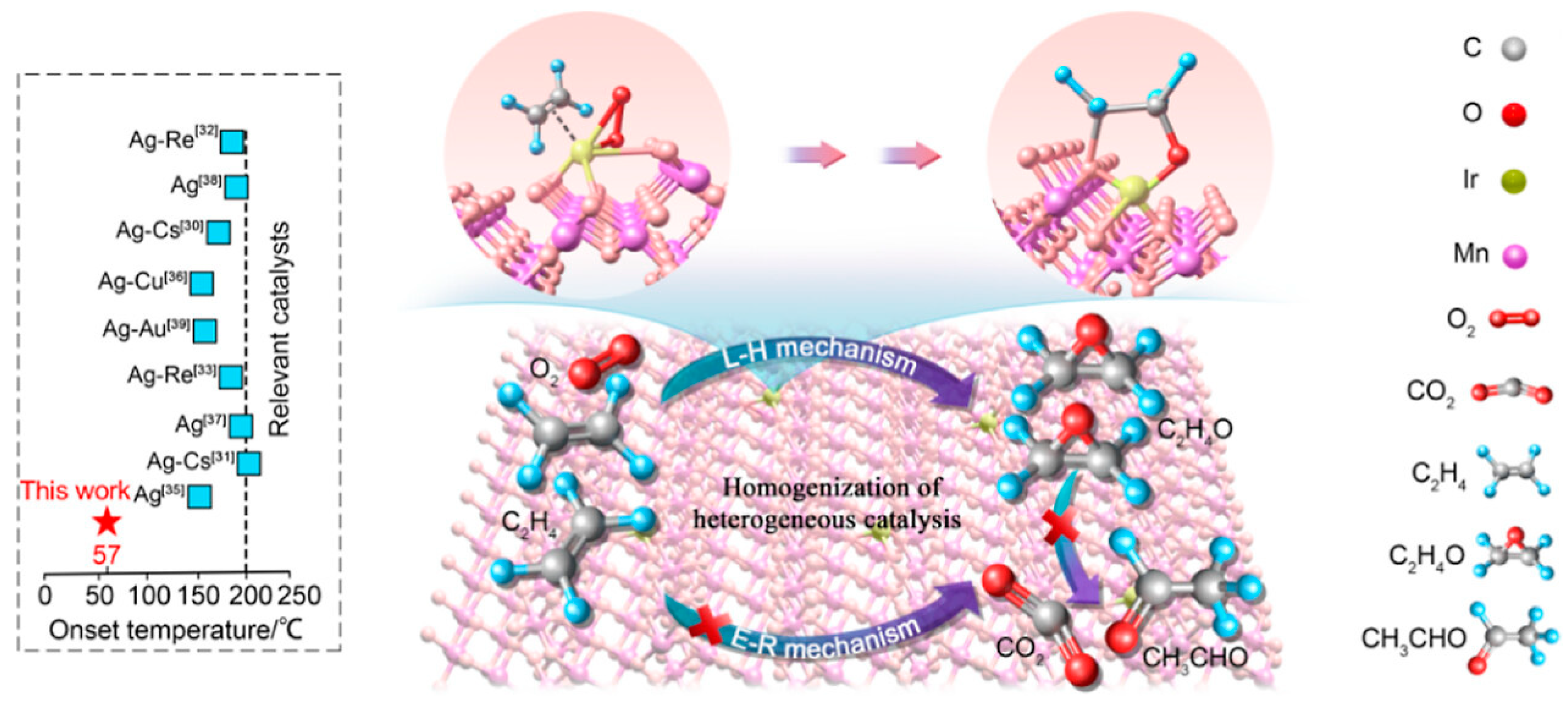

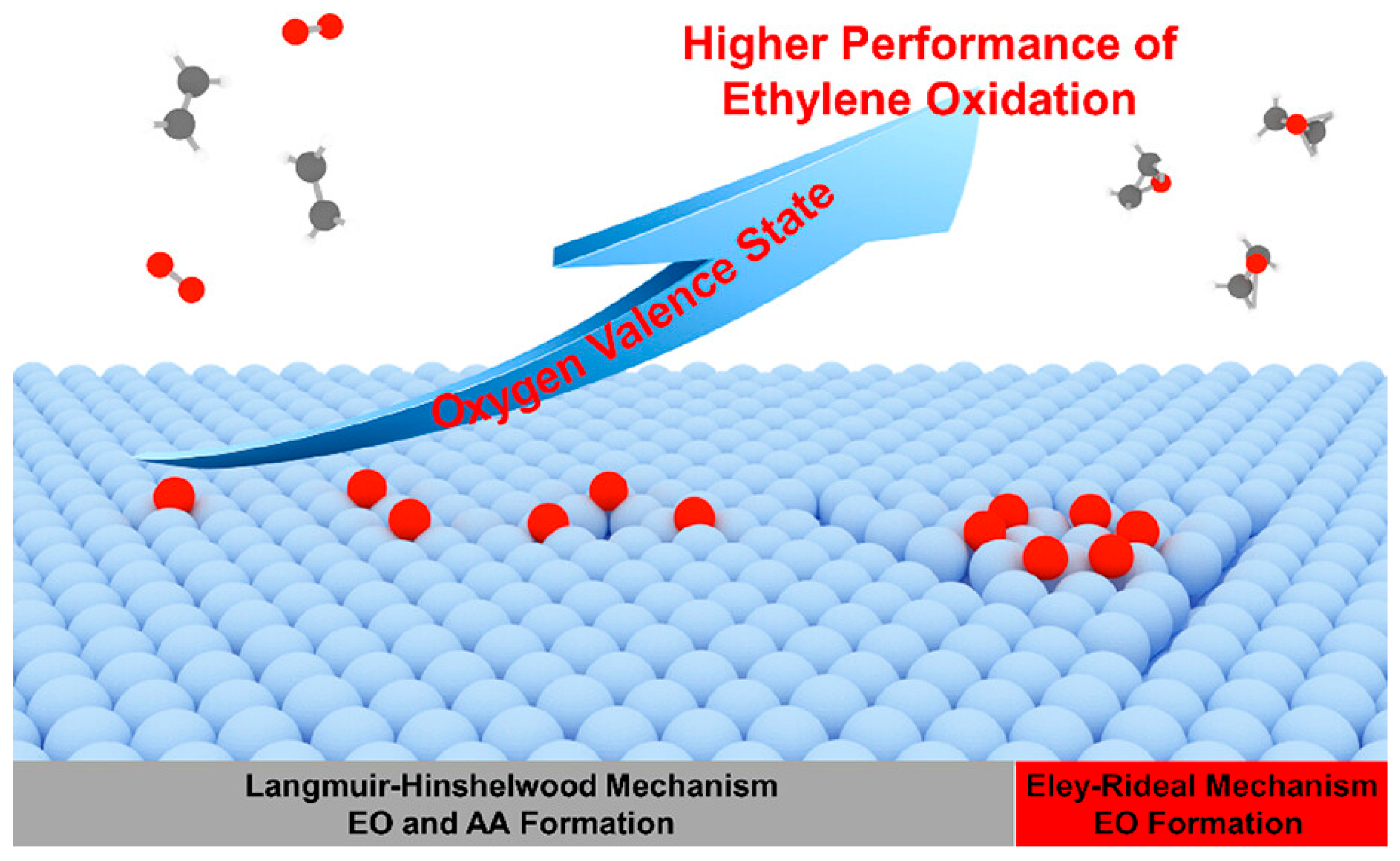
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saritala, M.A.; Muzammil, M.; Quddus, M.R.; Razzak, S.A.; Hossain, M.M. A Review on Production of Ethylene Oxide from Epoxidation of Ethylene: Catalysis, Mechanism and Kinetics. Catalysts 2025, 15, 560. https://doi.org/10.3390/catal15060560
Saritala MA, Muzammil M, Quddus MR, Razzak SA, Hossain MM. A Review on Production of Ethylene Oxide from Epoxidation of Ethylene: Catalysis, Mechanism and Kinetics. Catalysts. 2025; 15(6):560. https://doi.org/10.3390/catal15060560
Chicago/Turabian StyleSaritala, Mahammad Ali, Mohammed Muzammil, Mohammad R. Quddus, Shaikh Abdur Razzak, and Mohammad M. Hossain. 2025. "A Review on Production of Ethylene Oxide from Epoxidation of Ethylene: Catalysis, Mechanism and Kinetics" Catalysts 15, no. 6: 560. https://doi.org/10.3390/catal15060560
APA StyleSaritala, M. A., Muzammil, M., Quddus, M. R., Razzak, S. A., & Hossain, M. M. (2025). A Review on Production of Ethylene Oxide from Epoxidation of Ethylene: Catalysis, Mechanism and Kinetics. Catalysts, 15(6), 560. https://doi.org/10.3390/catal15060560








