β-Cyclodextrin Functionalization of Nitrogen-Doped Graphene to Enhance Dispersibility and Activate Persulfate for Trace Antibiotic Degradation in Water
Abstract
1. Introduction
2. Results and Discussion
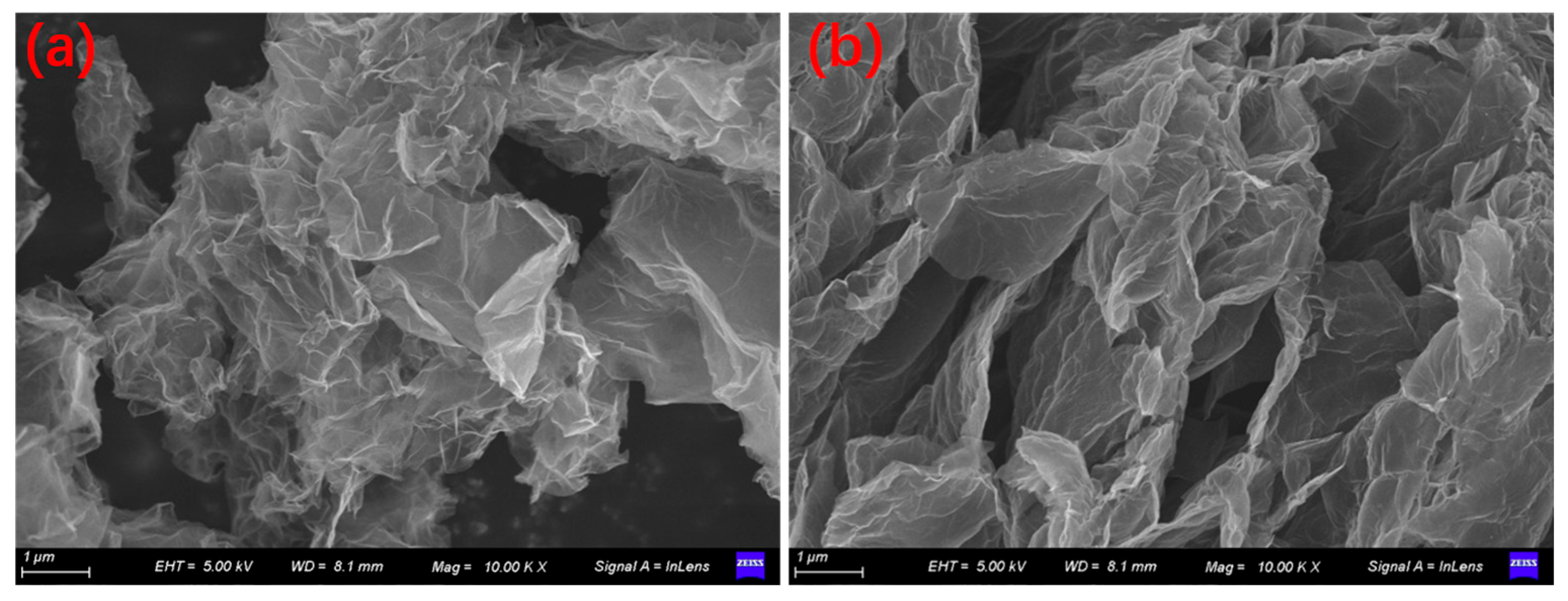
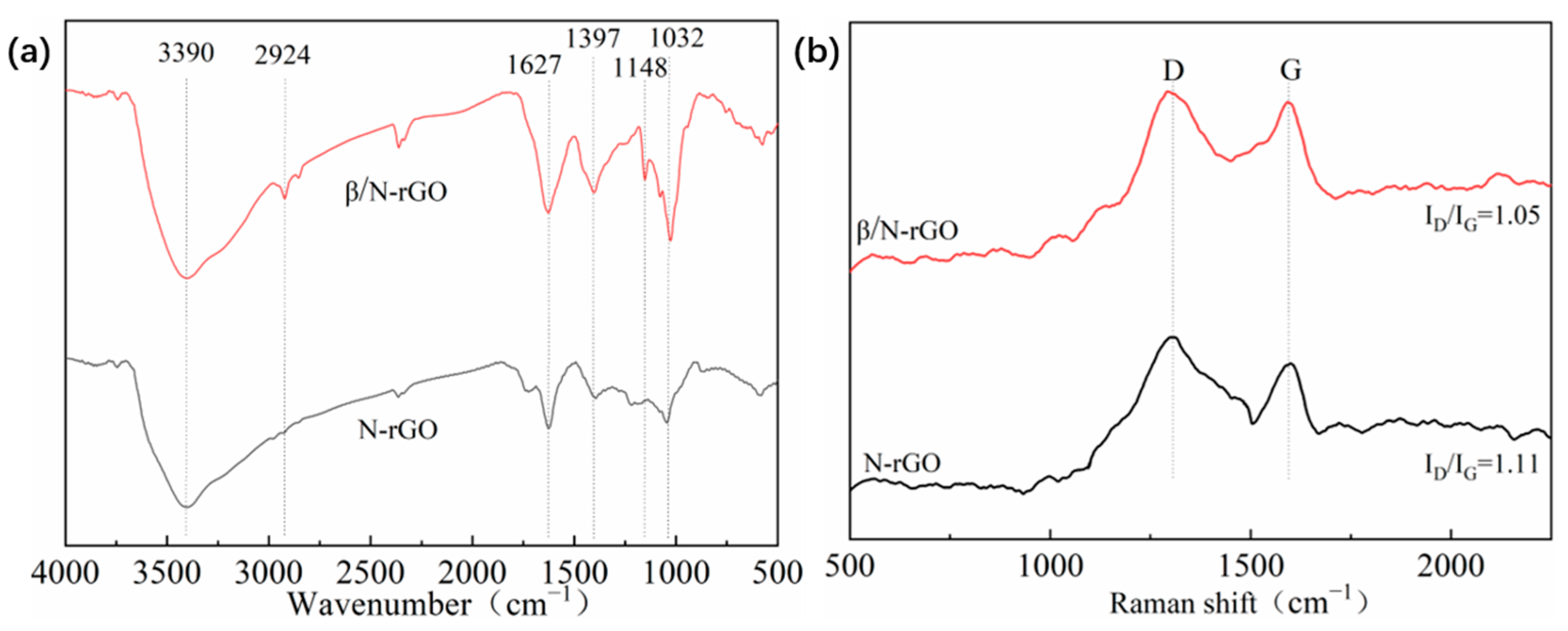
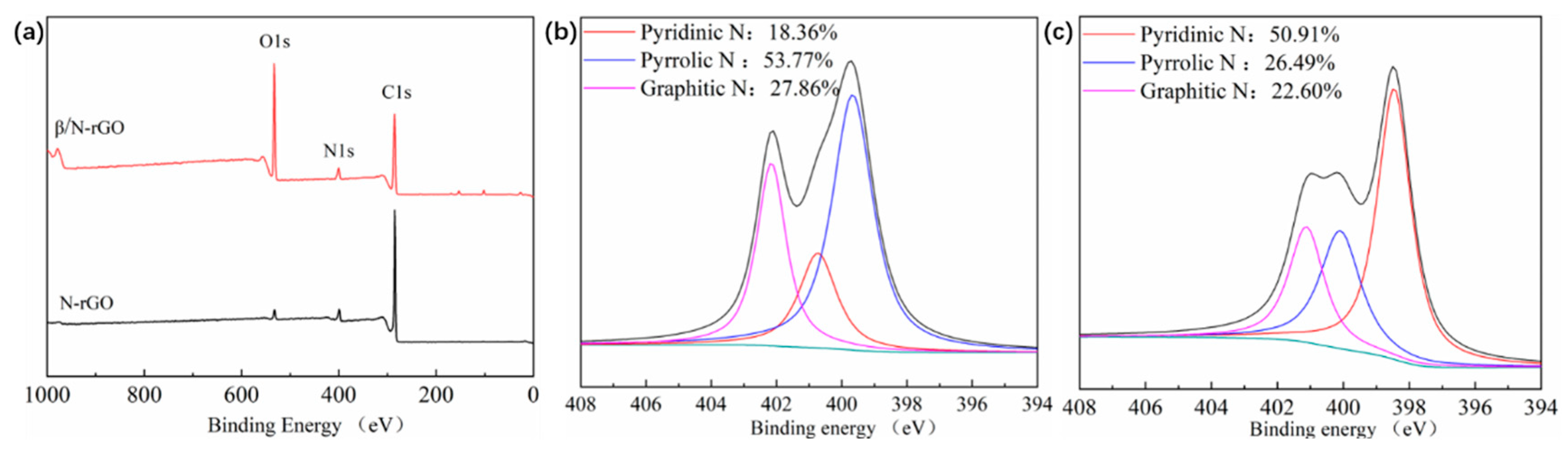
2.1. Properties of Catalytic Materials
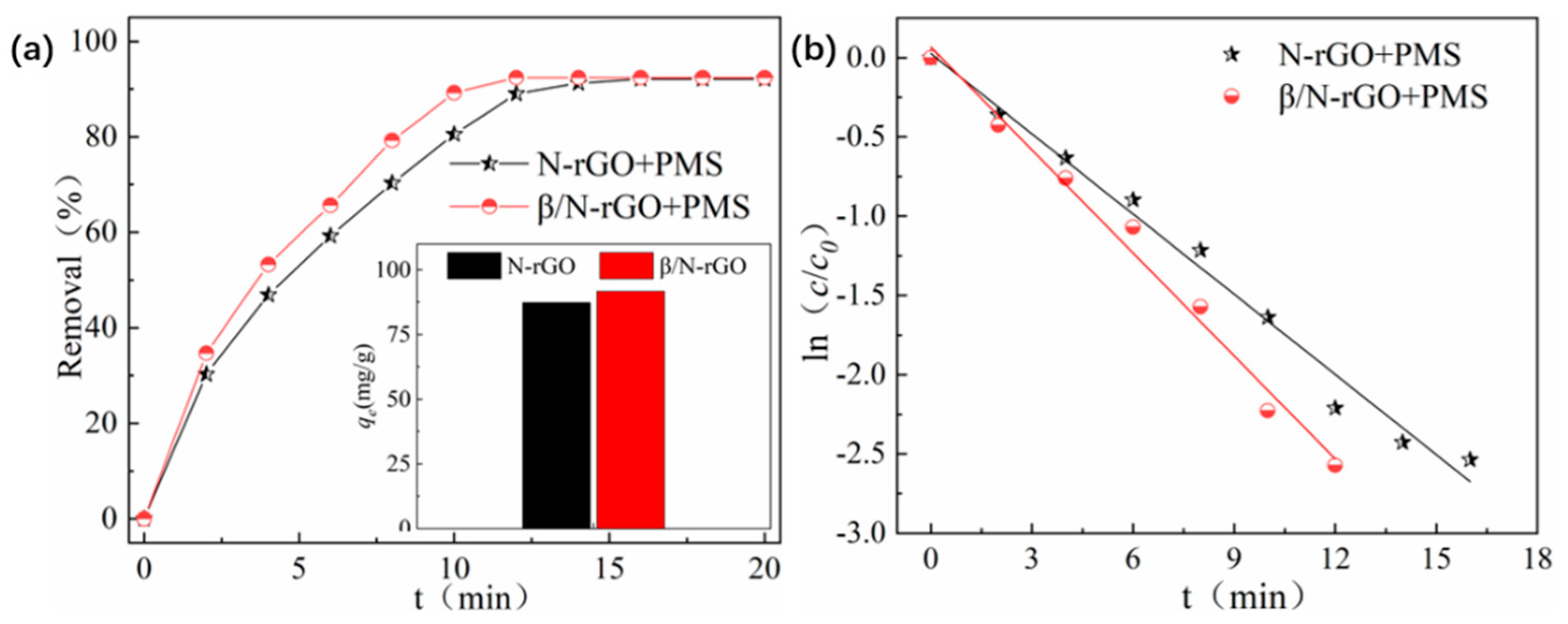
2.2. Degradation Kinetics Analysis
2.3. Analysis of Influencing Factors
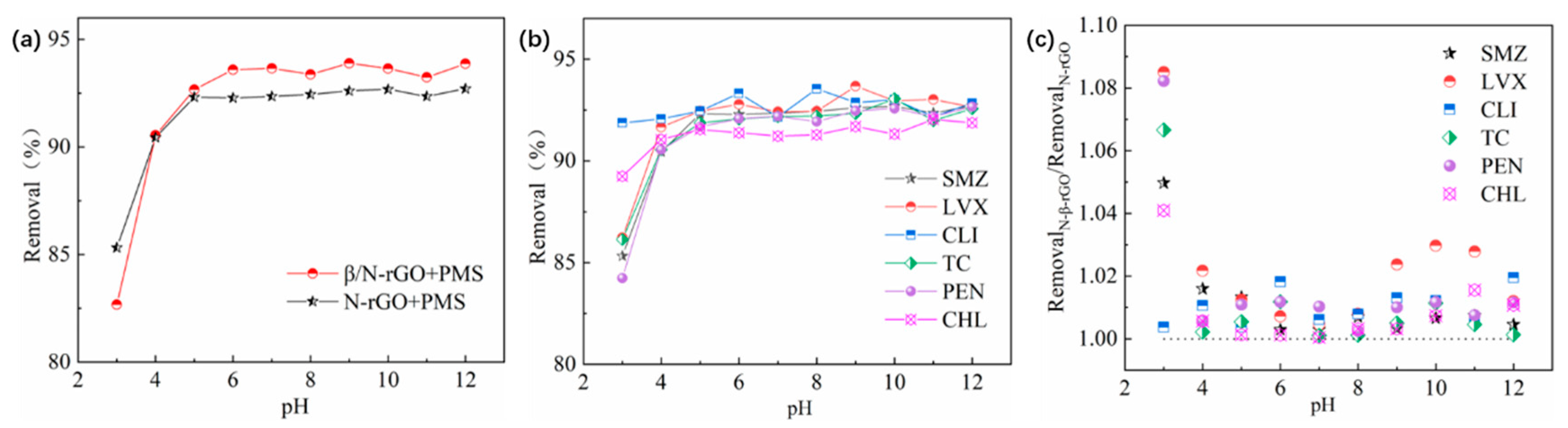
2.3.1. Influence of pH Value
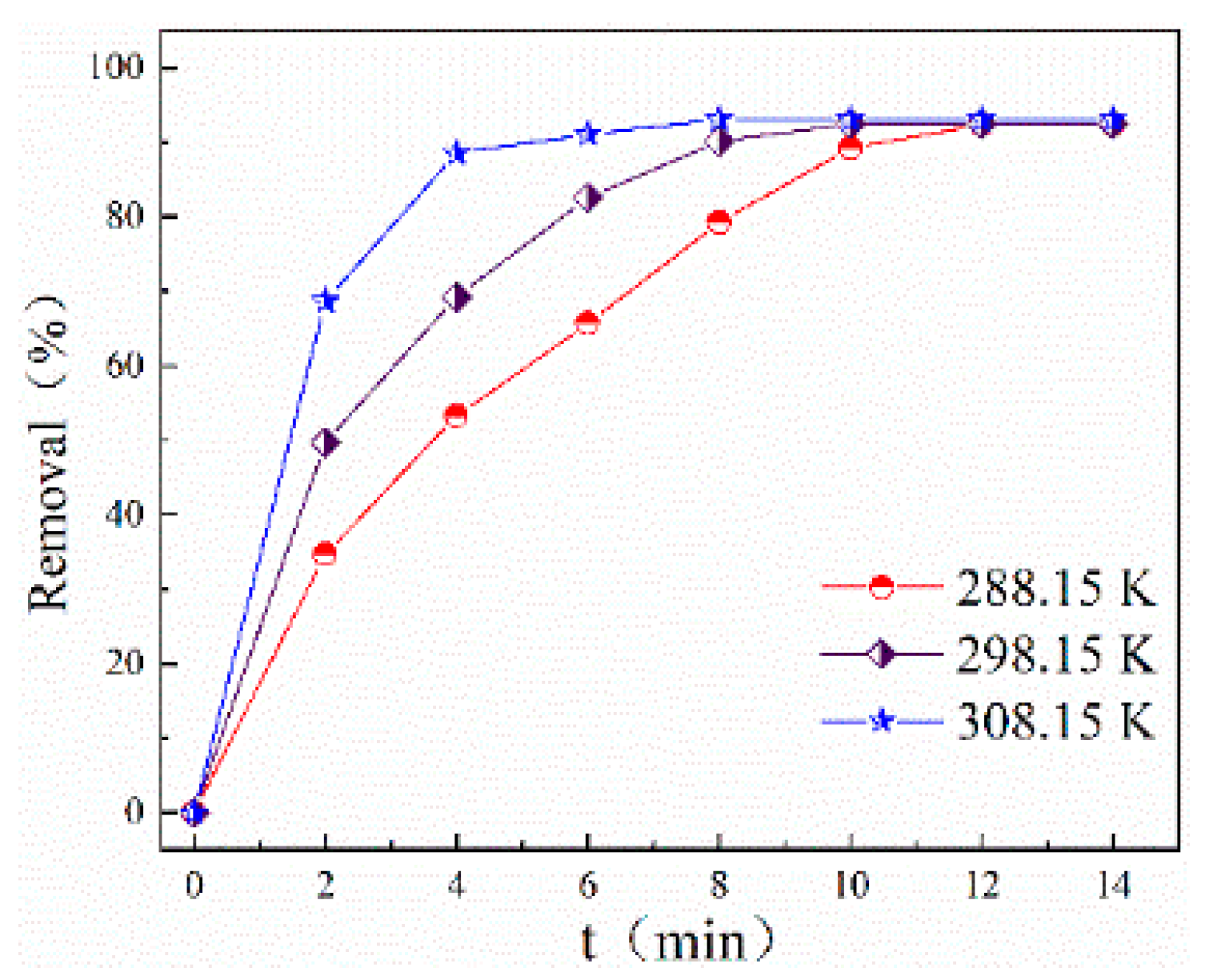
2.3.2. Effect of Temperature
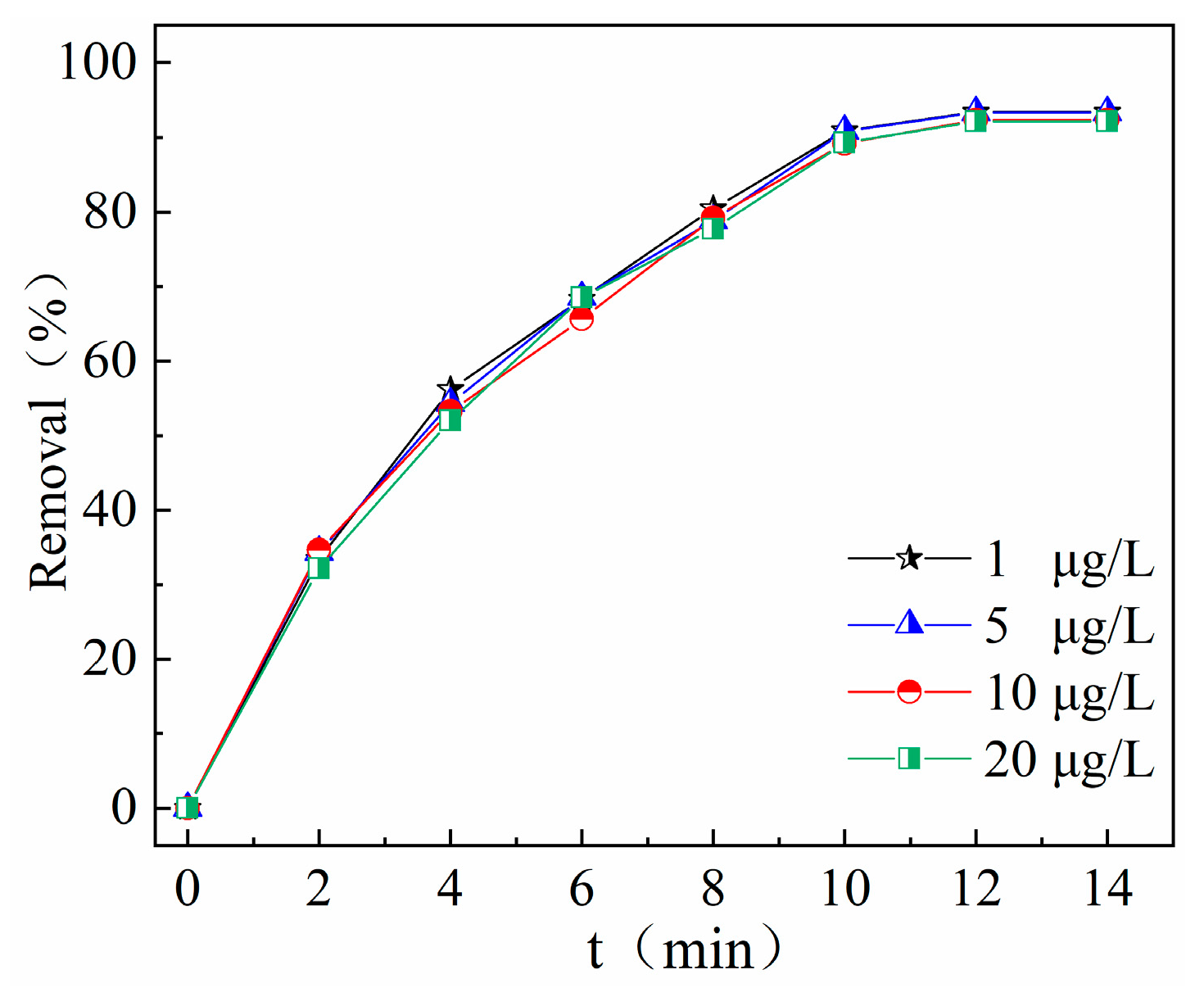
2.3.3. Effect of Initial Antibiotic Concentration on Degradation Effect
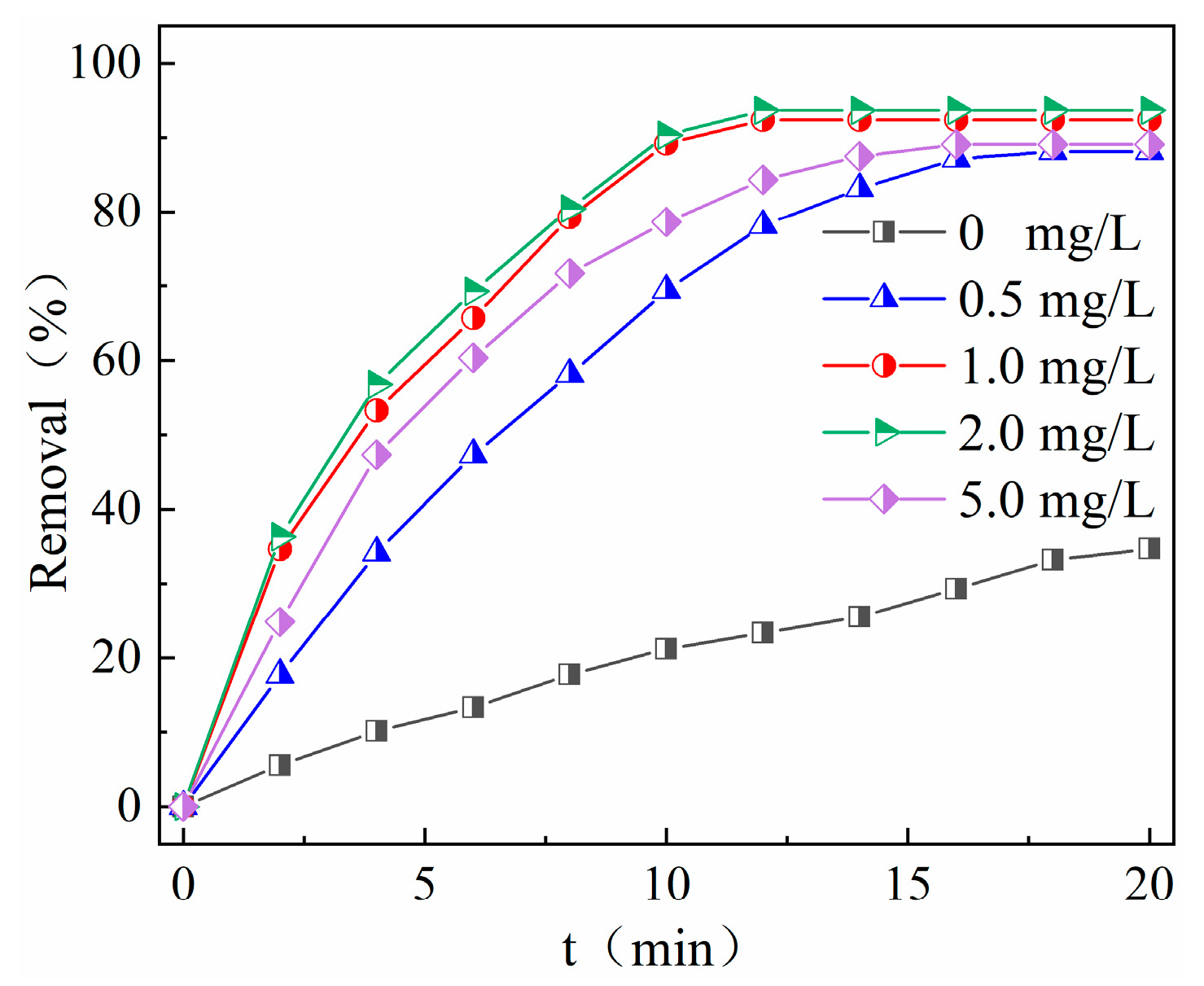
2.3.4. Effect of PMS Concentration
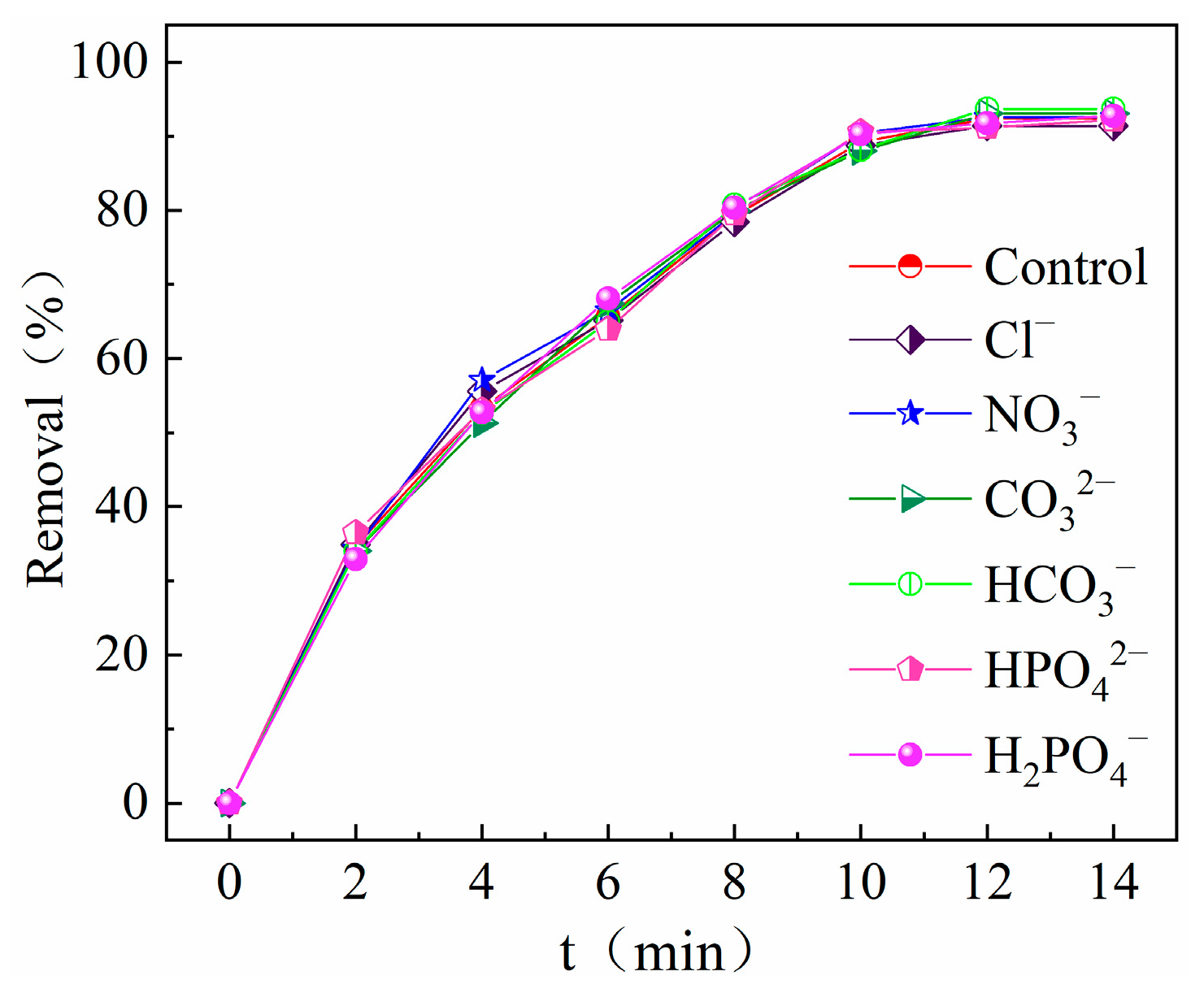
2.3.5. Effect of Anions on Degradation Effect
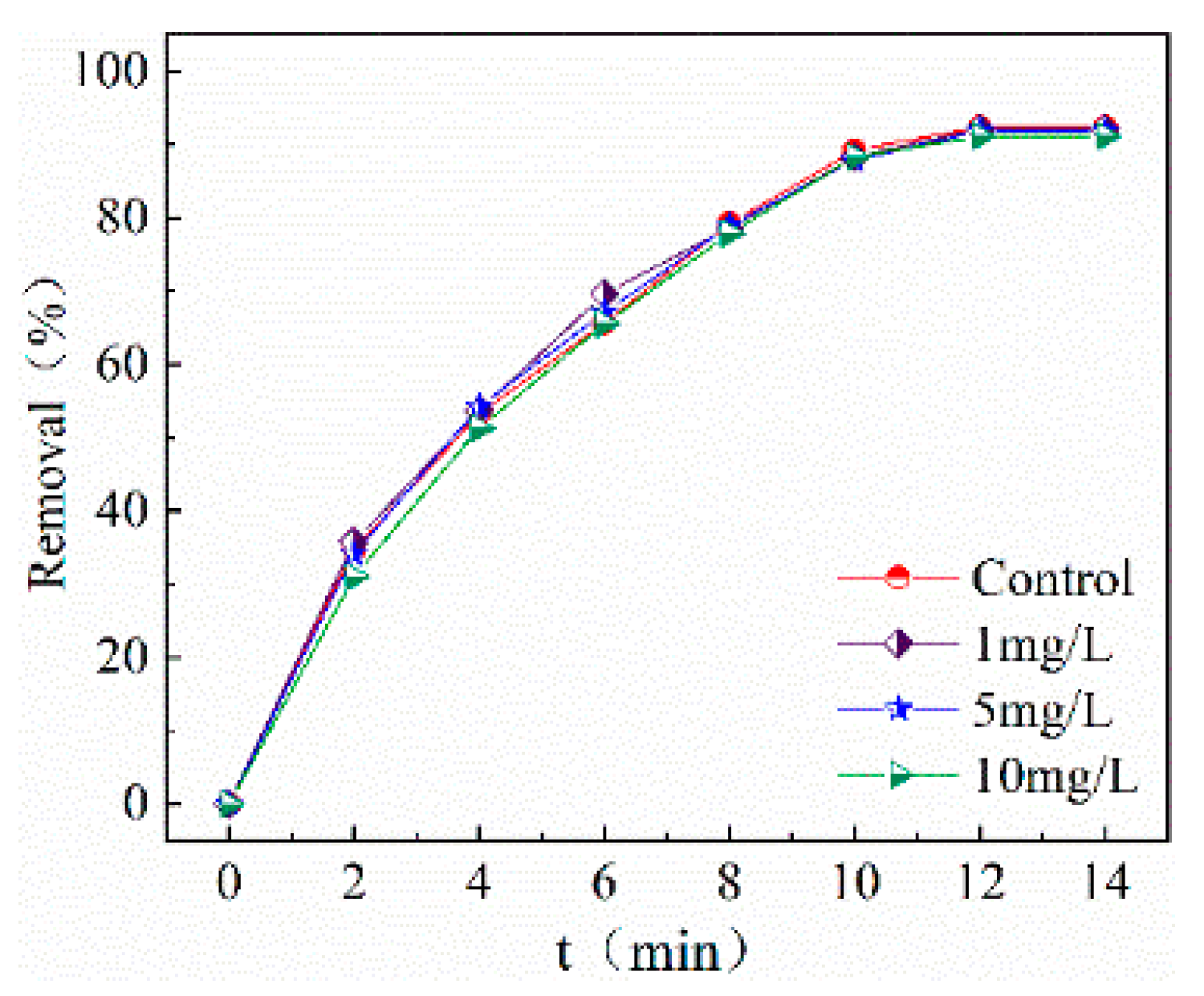
2.3.6. Effect of Humic Acid on Degradation Effects
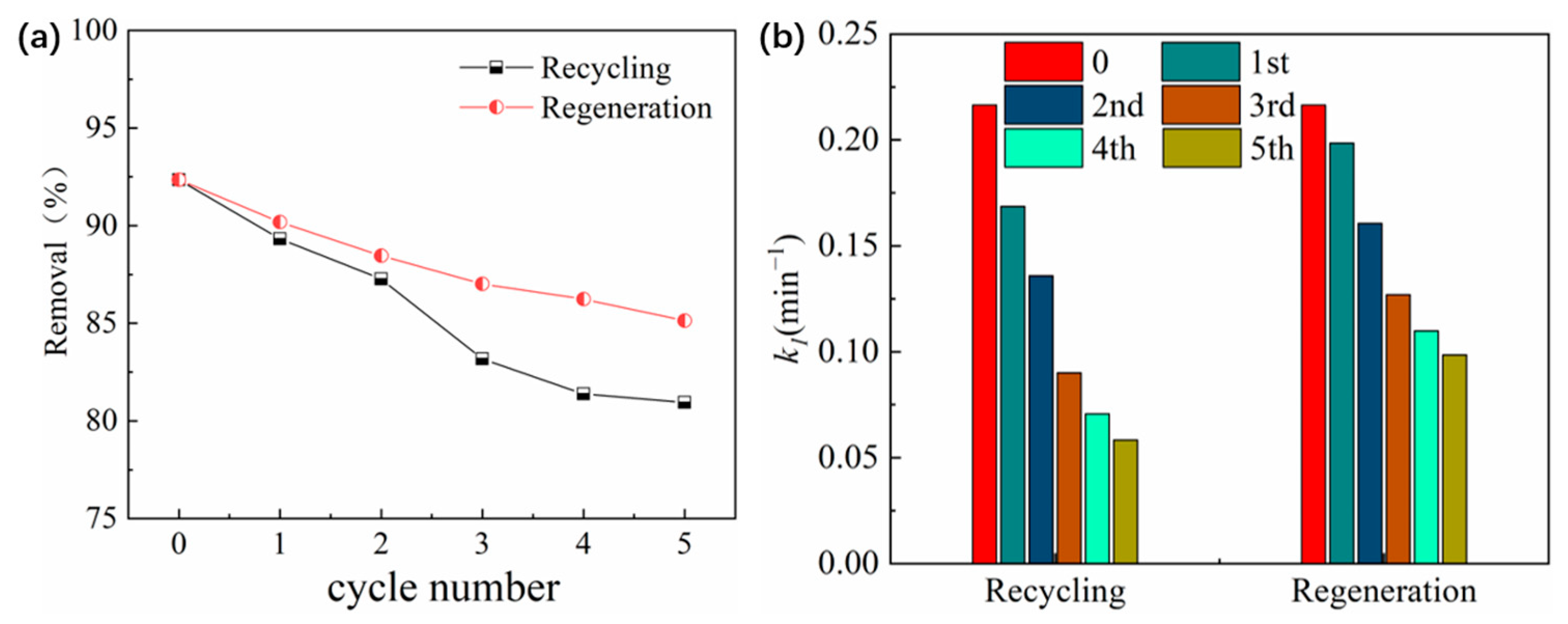
2.3.7. Impacts of Recycling and Regeneration
2.4. Analysis of the Effect of β-CD in Improving Graphene Agglomeration
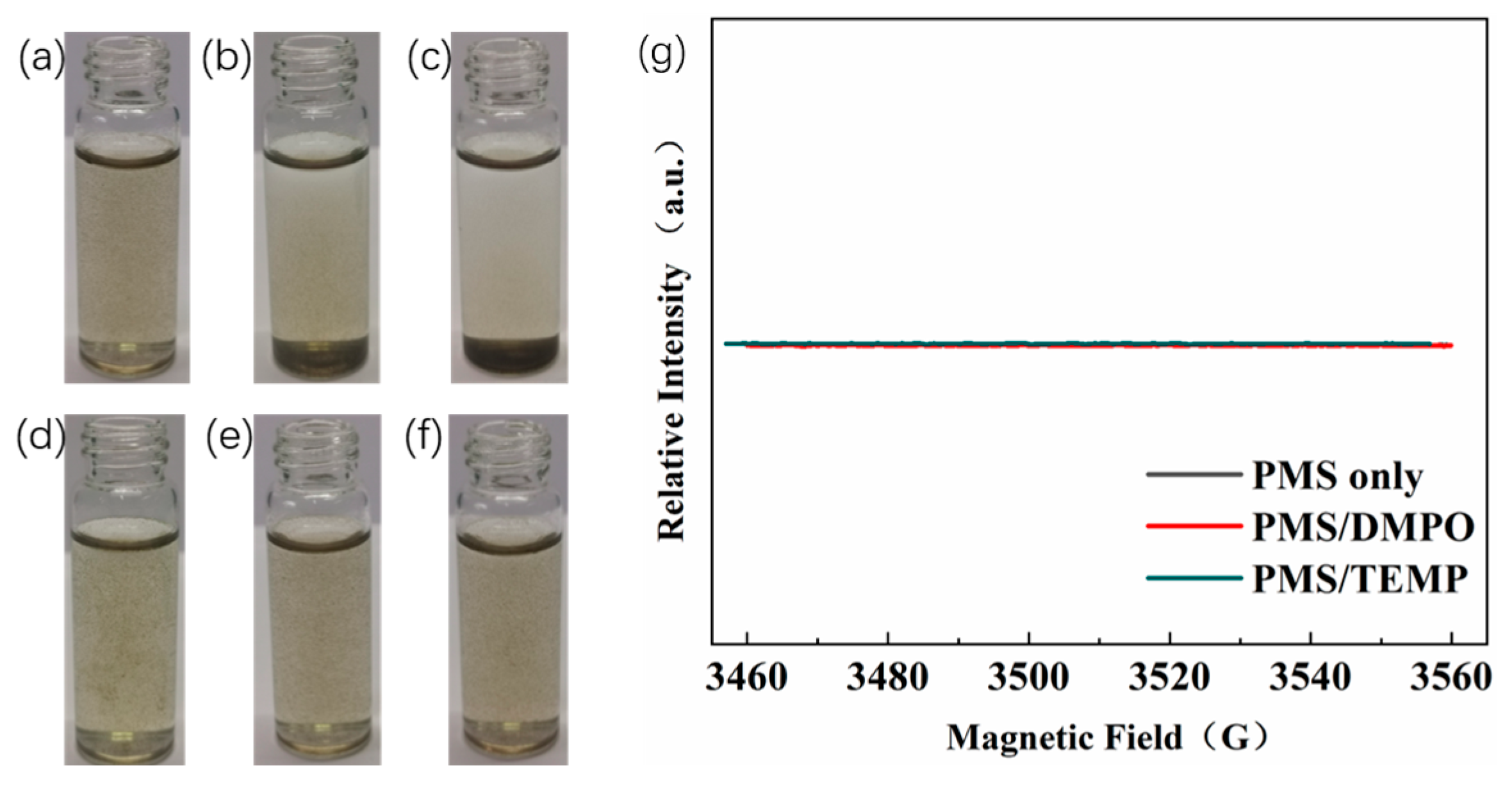
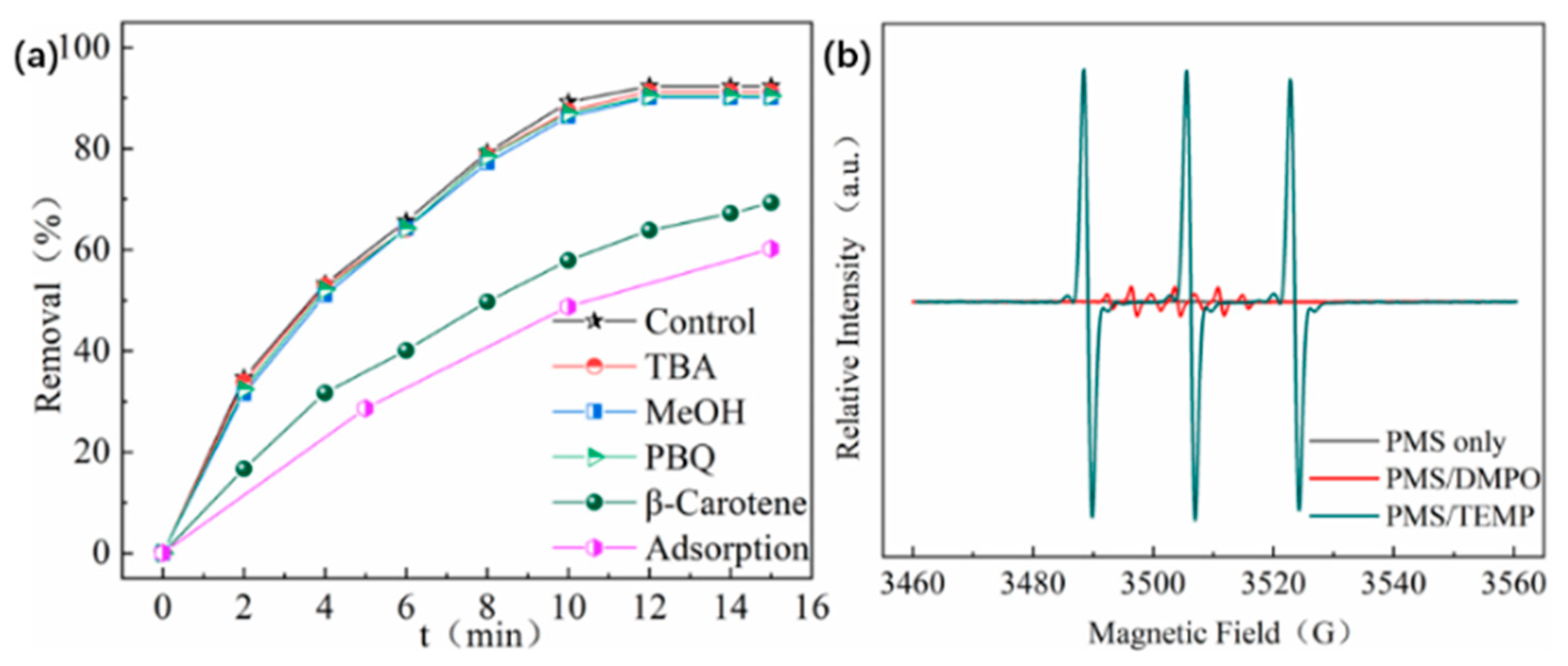
2.5. Quenching Experiments and EPR Spectra Analysis
3. Conclusions
4. Experiments and Methods
4.1. Catalyst Preparation
4.2. Experimental Methods
4.3. Antibiotic Concentration Testing
- (1)
- Sulfamethoxazole (SMZ): The mobile phase consisted of water, acetonitrile, and triethylamine in a volume ratio of 799:200:1. Sodium hydroxide or glacial acetic acid was used to adjust the pH to 5.9. The flow rate was 1 mL/min, and the wavelength was 240 nm.
- (2)
- Levofloxacin (LVX): The mobile phase consisted of 0.05 mol/L potassium dihydrogen phosphate (pH adjusted to 2.8 with phosphoric acid) and acetonitrile (v/v: 82:18). The flow rate was 1 mL/min, and the wavelength was 293 nm.
- (3)
- Clindamycin (CLI): The mobile phase consisted of 0.68 g/L potassium dihydrogen phosphate (pH adjusted to 7.5 with 250 g/L sodium hydroxide) and acetonitrile (v/v: 5:45). The flow rate was 1 mL/min, and the wavelength was 210 nm.
- (4)
- Tetracycline (TC): The mobile phase consisted of methanol and 0.02 mol/L potassium dihydrogen phosphate (pH 2.0 adjusted with nitric acid) in a volume ratio of 12:88. The flow rate was 1 mL/min, and the wavelength was 280 nm.
- (5)
- Penicillin G sodium (PEN): The mobile phase consisted of 0.1 mol/L potassium dihydrogen phosphate (pH 2.5 adjusted with phosphoric acid) and acetonitrile in a volume ratio of 70:30. The flow rate was 1 mL/min, and the wavelength was 225 nm.
- (6)
- Chloramphenicol (CHL): The mobile phase consisted of methanol and water in a volume ratio of 6:4. The flow rate was 0.5 mL/min, and the wavelength was 272 nm.
4.4. Characterization Methods
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, M.; Yuan, H.; Ni, K.; Ye, C.; Pan, F.; Xiong, J.; Zhu, Y. Structural repair of reduced graphene oxide promoted by single-layer graphene. Adv. Sci. 2025, 12, 2410088. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Wang, X.; Shi, X.; Zhao, Z.; Wang, Y.; Liu, J.; Tang, C.; Wang, G.; Li, L. Graphene nanoribbons enhancing the electronic conductivity and ionic diffusion of graphene electrodes for high-performance microsupercapacitors. Energy Environ. Mater. 2024, 7, e12681. [Google Scholar] [CrossRef]
- Fan, X.; He, C.; Ding, J.; Gao, Q.; Ma, H.; Lemme, M.C.; Zhang, W. Graphene MEMS and NEMS. Microsyst. Nanoeng. 2024, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Gronkiewicz, K.; Richter, L.; Knechtel, F.; Pyrcz, P.; Leidinger, P.; Günther, S.; Ploetz, E.; Tinnefeld, P.; Kamińska, I. Expanding the range of graphene energy transfer with multilayer graphene. Nanoscale 2024, 16, 13464–13470. [Google Scholar] [CrossRef]
- Gu, W.; Yang, M.; Chen, Z.; Cao, T.; Zhang, Y.; Li, Y.; Zhang, R. New insights into enhanced electrochemical advanced oxidation mechanism of B-doped graphene aerogel: Experiments, molecular dynamics simulations and DFT. J. Hazard. Mater. 2023, 443, 130331. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. A sustainable and metal-free advanced oxidation process for efficient water purification by electrified reduced graphene oxide membrane. Chem. Catal. 2024, 4, 101056. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Yang, X. Probing the formation mechanisms of reactive oxygen species in graphene oxide-catalyzed ozone advanced oxidation processes. Carbon 2025, 233, 119831. [Google Scholar] [CrossRef]
- Su, P.; Zhou, M.; Lu, X.; Yang, W.; Ren, G.; Cai, J. Electrochemical catalytic mechanism of N-doped graphene for enhanced H2O2 yield and in-situ degradation of organic pollutant. Appl. Catal. B Environ. 2019, 245, 583–595. [Google Scholar] [CrossRef]
- Long, Y.; Dai, J.; Zhao, S.; Su, Y.; Wang, Z.; Zhang, Z. Atomically dispersed cobalt sites on graphene as efficient periodate activators for selective organic pollutant degradation. Environ. Sci. Technol. 2021, 55, 5357–5370. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Yan, Y.; Wang, F.; Feng, L.; Chen, Y. Nitrogen-doped graphene for tetracycline removal via enhancing adsorption and non-radical persulfate activation. Environ. Res. 2023, 235, 116642. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y. Nitrogen-doped reduced graphene oxide (N-rGO) three-dimensional electrode electrochemically activates persulfate for the degradation of tetracycline. Carbon Lett. 2024, 34, 865–34879. [Google Scholar] [CrossRef]
- Hirani, R.A.K.; Asif, A.H.; Rafique, N.; Shi, L.; Zhang, S.; Saunders, M.; Tian, W.; Wang, S.; Sun, H. Heterogeneous activation of persulfate by macroscopic nitrogen-doped graphene oxide cubes for the degradation of antibiotic contaminants in water. Sep. Purif. Technol. 2023, 319, 124110. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, R.; Yang, Q.; Guo, W.; Ren, X. Surface-regulated FexOy/NG catalysts enhancing persulfate activation to remove tetracycline via a singlet oxygen-dominated pathway. Sep. Purif. Technol. 2025, 361, 131570. [Google Scholar] [CrossRef]
- Luo, J.; Yang, L.; Sun, D.; Gao, Z.; Jiao, K.; Zhang, J. Graphene oxide “surfactant”-directed tunable concentration of graphene dispersion. Small 2020, 16, 2003426. [Google Scholar] [CrossRef]
- Sonin, A.S.; Churochkina, N.A.; Kaznacheev, A.V.; Golovanov, A.V. Mesomorphism of graphene oxide dispersions. Colloid J. 2021, 83, 161–182. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, P.; Yang, Z.; Fang, Y.; Zhang, Z. Physical dispersion method and mechanism of graphene. J. Superhard Mater. 2023, 45, 186–191. [Google Scholar] [CrossRef]
- Li, L.; Zhou, M.; Jin, L.; Mo, Y.; Xu, E.; Chen, H.; Liu, L.; Wang, M.; Chen, X.; Zhu, H. Green preparation of aqueous graphene dispersion and study on its dispersion stability. Materials 2020, 13, 4069. [Google Scholar] [CrossRef]
- Wang, Q.; Zhan, D.; Qi, G.; Wang, Y.; Zheng, H. Impact of the microstructure of polycarboxylate superplasticizers on the dispersion of graphene. New Carbon Mater. 2020, 35, 547–558. [Google Scholar] [CrossRef]
- Qamar, S.; Ramzan, N.; Aleem, W. Graphene dispersion, functionalization techniques and applications: A review. Synth. Met. 2024, 307, 117697. [Google Scholar] [CrossRef]
- Feng, B.; Wang, Z.; Suo, W.; Wang, Y.; Wen, J.; Li, Y.; Suo, H.; Liu, M.; Ma, L. Performance of graphene dispersion by using mixed surfactants. Mater. Res. Express 2020, 7, 095009. [Google Scholar] [CrossRef]
- Wilczewski, S.; Skórczewska, K.; Tomaszewska, J.; Lewandowski, K.; Szulc, J.; Runka, T. Manufacturing homogenous PVC/graphene nanocomposites using a novel dispersion agent. Polym. Test. 2020, 91, 106868. [Google Scholar] [CrossRef]
- Zhao, W.; Sugunan, A.; Gillgren, T.; Larsson, J.A.; Zhang, Z.; Zhang, S.; Nordgren, N.; Sommertune, J.; Ahniyaz, A. Surfactant-free stabilization of aqueous graphene dispersions using starch as a dispersing agent. ACS Omega 2021, 6, 12050–12062. [Google Scholar] [CrossRef]
- Sun, L.; Xu, G.; Tu, Y.; Zhang, W.; Hu, X.; Yang, P.; Wu, D.; Liang, Y.; Wei, D.; Li, A.; et al. Multifunctional porous β-cyclodextrin polymer for water purification. Water Res. 2022, 222, 118917. [Google Scholar] [CrossRef]
- Bie, W.; Zhang, S.; Zhang, L.; Li, H.; Sun, X.; Cai, T.; Wang, Z.; Kong, F.; Wang, W. Thioether-functionalized porous β-cyclodextrin polymer for efficient removal of heavy metal ions and organic micropollutants from water. Carbohydr. Polym. 2024, 324, 121509. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, P.; Liu, D.; Zhao, Q.; Zou, B.; Zhou, L.; Ye, Z. Method to fabricate porous multifunction β-cyclodextrin modified resin for ultrafast and efficient removal of Cu(II) and bisphenol A. J. Taiwan Inst. Chem. E 2021, 119, 286–297. [Google Scholar] [CrossRef]
- Xiong, T.; Zou, C.; Lin, S. β-Cyclodextrin magnetic graphene oxide (β-CD@MGO) for efficient removal of Ni(II) and phenol: Adsorption performance, reusability and adsorption mechanism studies. Diam. Relat. Mater. 2023, 139, 110269. [Google Scholar] [CrossRef]
- Verma, M.; Hong, Y.; Kumar, V.; Bisht, B.M.; Vlaskin, S.; Chae, S.; Shah, K.J.; Kim, H. Enhanced removal and stepwise recovery of inorganic and organic micropollutants from water using novel graphene oxide doped multifunctional β−cyclodextrin chitosan polymer. J. Phys. Chem. Solids 2023, 182, 111615. [Google Scholar] [CrossRef]
- Almajidi, Y.Q.; Majeed, A.A.; Ali, E.; Abdullaev, S.; Koka, N.A.; Bisht, Y.S.; Fenjan, M.N.; Alawadi, A.; Alsalamy, A.; Saleh, L.H. A versatile magnetic nanocomposite based on cellulose-cyclodextrin hydrogel embedded with graphene oxide and Cu2O nanoparticles for catalytic application. Int. J. Biol. Macromol. 2024, 260, 129367. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liang, H.; Gong, X. A novel magnetic β-cyclodextrin-modified graphene oxide and chitosan composite as an adsorbent for trace extraction of four bisphenol pollutants from environmental water samples and food samples. Environ. Res. 2024, 29, 867. [Google Scholar] [CrossRef]
- Ouyang, J.; Gao, J.; Shen, J.; Wei, Y.; Wang, C. Preparation of magnetic graphene/β-cyclodextrin polymer composites and their application for removal of organic pollutants from wastewater. J. Environ. Chem. Eng. 2023, 11, 110944. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Zhou, J.; Bao, L.; Zhang, L.; Yang, L.; Wu, J.; Hao, C. Novel carboxymethyl cellulose-based hydrogel embedded with metal organic framework for efficient cationic dye removal from water. Int. J. Biol. Macromol. 2024, 282, 137387. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Guo, Q.; Xia, H.; Song, M.; Qiu, Z.; Fan, S.; Chen, Z.; Zhang, S.; Zhang, S.; Li, B. Cyclodextrin self-assembled graphene oxide aerogel microspheres as broad-spectrum adsorbent for removing dyes and organic micropollutants from water. J. Environ. Chem. Eng. 2021, 9, 104749. [Google Scholar] [CrossRef]
- Zang, X.; Liang, L.; Wang, X.; Li, C.; Li, P.; Shao, Q.; Cao, N. β-Cyclodextrin anchor NiCo2S4 on graphene to enhance electrochemical performance of supercapacitor. Energy Technol. 2022, 10, 2200490. [Google Scholar] [CrossRef]
- Qu, Y.; Li, H.; Yakub, I.; He, W.; Dong, W.; Barawi, M.H.; Wang, S.; Ma, H.; Zhu, Z. Synthesis and characterization of efficient adsorbents for methylene blue based on graphene oxide/β-cyclodextrin composites. Water Air Soil Pollut. 2025, 56, 236. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Y.; Qi, Y.; Zheng, M.; Xie, F.; Wang, Q.; Xiao, X.; Xiao, D. Multifunctional bionanohybrids of graphene oxide/β-cyclodextrin for applications in methylene blue adsorption, solvent response, and smart light-driven. J. Mol. Liq. 2024, 404, 124977. [Google Scholar] [CrossRef]
- Wang, J.; Sha, X.; Chen, X.; Zhuo, H.; Xie, W.; Zhou, Z.; He, X.; Wu, L.; Li, B. Removal and distribution of antibiotics and resistance genes in conventional and advanced drinking water treatment processes. J. Water Process Eng. 2022, 50, 103217. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, C.; Liu, J.; Zhang, Y.; Fu, W. Occurrence, fate, and risk assessment of antibiotics in conventional and advanced drinking water treatment systems: From source to tap. J. Environ. Manag. 2024, 358, 120746. [Google Scholar] [CrossRef]
- Ashish, K.; Rajinder, K.; Shashikala, V.; Samer, S. Antimicrobials and antibiotic resistance genes in water bodies: Pollution, risk, and control. Front. Environ. Sci. 2022, 10, 830861. [Google Scholar] [CrossRef]
- Jerry, O.; Timothy, A.; Damian, C. Mineralization of antibiotics in wastewater via photocatalysis. Water Air Soil Pollut. 2021, 232, 219. [Google Scholar] [CrossRef]
- Xie, J.; Li, X.; Ma, W.; Wang, L.; Lu, Y.; Li, S.; Li, J.; Guo, X.; Li, Z.; Ding, C. Fate of dissolved organic matter and antibiotics in conventional treatment system and constructed wetlands system applied to source water pretreatment: Removal efficiency and risk assessment. Water Air Soil Pollut. 2024, 235, 24. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, X.; Zhang, X.; Yang, K.; Li, Y. Simultaneous degradation of trace antibiotics in water by adsorption and catalytic oxidation induced by N-doped reduced graphene oxide (N-rGO): Synergistic mechanism. Mater. Res. Express 2022, 9, 065601. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, B.; Wu, N. Active sites and structure regulation of non-radical pathways in N-doped graphene-activated persulfate. Catal. Commun. 2024, 187, 106910. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Feng, M.; Huang, C.; Sun, P. Abiotic transformation and ecotoxicity change of sulfonamide antibiotics in environmental and water treatment processes: A critical review. Water Res. 2021, 202, 117463. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, S.; Ren, J.; Zhai, Y.; Dong, S.; Wang, E. Cyclodextrin functionalized graphene nanosheets with high supramolecular recognition capability: Synthesis and host−Guest inclusion for enhanced electrochemical performance. ACS Nano 2010, 4, 4001–4010. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Yin, D.; Meng, Y.; Jafari, S.; Sillanpää, M. EDTA-cross-linked β-cyclodextrin: An environmentally friendly bifunctional adsorbent for simultaneous adsorption of metals and cationic dyes. Environ. Sci. Technol. 2015, 49, 10570–10580. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Chen, B. Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ. Sci. Technol. 2014, 48, 4817–4825. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Z.; Zhang, L.; Cai, Q.; Hu, J.; Wang, W.; Ju, X.; Xie, R.; Chu, L. Functional graphene oxide nanosheets modified with cyclodextrins for removal of Bisphenol A from water. Chin. J. Chem. Eng. 2021, 39, 79–87. [Google Scholar] [CrossRef]
- Zhao, Z.; Long, X.; Millan, M.; Yuan, G.; Cui, Z.; Dong, Z.; Cong, Y.; Zhang, J.; Li, X. The influence of carbon supports and their surface modification on aqueous phase highly selective hydrogenation of phenol to cyclohexanol over different Ni/carbon catalysts. Carbon 2023, 213, 118227. [Google Scholar] [CrossRef]
- Tan, Y.; Zhao, N.; Song, Q.; Ling, H. Alkali synergistic sulfide-modified nZVI activation of persulfate for phenanthrene removal. Chem. Eng. J. 2023, 11, 109923. [Google Scholar] [CrossRef]
- Cheng, N.; Deng, F.; Wang, J.; Hu, L.; Yang, J.; Jiang, S.; Wang, H.; Ma, X.; Zhao, L.; Li, G.; et al. Simultaneous alkali recovery, coagulant recycling and organics removal from textile wastewater via membrane electrochemical system. Sep. Purif. Technol. 2025, 354, 129448. [Google Scholar] [CrossRef]
- Wang, C.; Kang, J.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. One-pot synthesis of N-doped graphene for metal-free advanced oxidation processes. Carbon 2016, 102, 279–287. [Google Scholar] [CrossRef]
- Zhao, W.; Ye, C.; Li, J.; Yu, X. Increased risk of antibiotic resistance in surface water due to global warming. Environ. Res. 2024, 263, 120149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, N. Adsorption characteristics of N-rGO for multiple representative trace antibiotics in water. J. Environ. Qual. 2022, 51, 1298–1309. [Google Scholar] [CrossRef]
- Li, T.; Zhu, P.; Wang, D.; Zhang, Z.; Zhou, L. Efficient utilization of the electron energy of antibiotics to accelerate Fe(III)/Fe(II) cycle in heterogeneous Fenton reaction induced by bamboo biochar/schwertmannite. Environ. Res. 2022, 209, 112830. [Google Scholar] [CrossRef]
- Luo, Y.; Su, R. Cobalt-based MOF material activates persulfate to degrade residual ciprofloxacin. Water 2024, 16, 2299. [Google Scholar] [CrossRef]
- Carmen, S.; Luis, M. p-Nitrophenol degradation by activated persulfate. Environ. Technol. Innov. 2021, 21, 101265. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Deng, Y.; Zhang, Y.; Sui, M.; Deng, J.; Zhou, S. Degradation of antipyrine by UV, UV/H2O2 and UV/PS. J. Hazard. Mater. 2013, 260, 1008–1016. [Google Scholar] [CrossRef]
- Lou, X.; Wu, L.; Guo, Y.; Chen, C.; Wang, Z.; Xiao, D.; Fang, C.; Liu, J.; Zhao, J.; Lu, S. Peroxymonosulfate activation by phosphate anion for organics degradation in water. Chemosphere 2014, 117, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Guo, C.; Hou, S.; Lv, J.; Zhang, Y.; Feng, Q.; Zhang, Y.; Xu, J. Kinetic and mechanistic investigation on the decomposition of ketamine by UV-254 nm activated persulfate. Chem. Eng. J. 2019, 370, 19–26. [Google Scholar] [CrossRef]
- Dan, J.; Wang, Q.; Mu, K.; Rao, P.; Dong, L.; Zhang, X.; He, Z.; Gaod, N.; Wang, J. Degradation of sulfachloropyridazine by UV-C/persulfate: Kinetics, key factors, degradation pathway. Environ. Sci. Water Res. 2020, 6, 2510–2520. [Google Scholar] [CrossRef]
- Fan, Z.; Feng, T.; Wu, S.; Wang, S.; Tan, Y.; Yu, Q.; Huang, R.; Zhang, X. Chitin-derived biochar with nitrogen doping to activate persulfate for phenol degradation: Application potential and electron transfer pathway in system. Chemosphere 2023, 330, 138641. [Google Scholar] [CrossRef] [PubMed]
| C, At, % | O, At, % | N, At, % | |
|---|---|---|---|
| β/N-rGO | 63.73 | 2.93 | 32.78 |
| N-rGO | 61.85 | 2.99 | 34.75 |
| β/N-rGO + PMS | N-rGO + PMS | |
|---|---|---|
| k1 (min−1) | 0.2165 | 0.1686 |
| t1/2 | 3.20 | 4.11 |
| R2 | 0.9859 | 0.9842 |
| Temperature (K) | E kJ/mol | R2 | |||
|---|---|---|---|---|---|
| 288.15 | 298.15 | 308.15 | |||
| k1 (min−1) | 0.2165 | 0.2625 | 0.3293 | 4.15 | 0.9844 |
| R2 | 0.9882 | 0.9893 | 0.8964 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, M.; Wu, N. β-Cyclodextrin Functionalization of Nitrogen-Doped Graphene to Enhance Dispersibility and Activate Persulfate for Trace Antibiotic Degradation in Water. Catalysts 2025, 15, 541. https://doi.org/10.3390/catal15060541
Yao M, Wu N. β-Cyclodextrin Functionalization of Nitrogen-Doped Graphene to Enhance Dispersibility and Activate Persulfate for Trace Antibiotic Degradation in Water. Catalysts. 2025; 15(6):541. https://doi.org/10.3390/catal15060541
Chicago/Turabian StyleYao, Min, and Nan Wu. 2025. "β-Cyclodextrin Functionalization of Nitrogen-Doped Graphene to Enhance Dispersibility and Activate Persulfate for Trace Antibiotic Degradation in Water" Catalysts 15, no. 6: 541. https://doi.org/10.3390/catal15060541
APA StyleYao, M., & Wu, N. (2025). β-Cyclodextrin Functionalization of Nitrogen-Doped Graphene to Enhance Dispersibility and Activate Persulfate for Trace Antibiotic Degradation in Water. Catalysts, 15(6), 541. https://doi.org/10.3390/catal15060541









