Abstract
In this work, we have reported a simple synthesis method for a hollow porous organic nanosphere-supported ZnO composite photocatalyst (HPON@ZnO) through a combination of a hyper-crosslinking-mediated self-assembly method and a “ship-in-bottle” strategy. The obtained HPON@ZnO possesses a large specific surface area and hierarchically porous structures, which exhibited exceptionally high catalytic activity in the adsorption and degradation of crystal violet, with the reaction proceeding under mild conditions. Additionally, the catalyst demonstrated degradation activity towards other dyes and featured a good stability and recyclability. This simple strategy provides a new approach for the large-scale synthesis of efficient heterogeneous photocatalysts, and offers an effective dye wastewater treatment technique.
1. Introduction
With the acceleration of industrialization and urbanization, water supply is increasingly challenging with growing demand for water in the world. Approximately 90 million tons of water are used annually in the dye industry, accounting for 20% of industrial wastewater [1]. The discharge of large amounts of dye wastewater into natural water bodies results in a series of ecological and environment problems. Therefore, developing effective dye wastewater treatment techniques is of great significance for water pollution control.
Currently, various wastewater treatment methods have been explored for removal of dyes from wastewater, including biological treatment [2], chemical coagulation [3], chemical oxidation [4,5], adsorption [6,7], electrochemical methods [8,9], and photocatalysis. Photocatalysis has been proven to be a promising method for the treatment of dye wastewater with superior operational conditions, low cost, and high efficiency. Based on the in situ generation of highly reactive transient species (i.e., .OH and .O2−) in the photocatalysis progress, dyes can be degraded and mineralized into CO2 and H2O [10]. So, there are few secondary pollutants generated in photocatalysis without further treatment [11]. Since the Honda–Fujishima effect with TiO2 photoelectrodes was first reported in 1972, numerous breakthroughs have been achieved in developing new semiconductor photocatalysts for various photocatalytic applications [12]. To date, multiphase photocatalytic reduction and oxidation based on semiconductors and solar energy have attracted extensive attention in various fields, such as energy, materials, the environment, and chemistry. Currently, zinc oxide (ZnO) is widely used in the fields of photocatalysis [13,14,15], thermocatalysis [16,17], and electrocatalysis [18,19,20]. Additionally, it has broad application prospects in the fields of antibacterial [21,22,23] and antioxidant agents [24,25,26]. For example, Yi and co-workers reported a silver-modified ZnO nanocatalyst for photocatalytic oxidation of methane [27]. Nanoscale ZnO exhibited high activity for methane oxidation under simulated sunlight, and nano silver decorations further improve the photocatalytic activity via the surface plasmon resonance, even under visible light illumination. Song and partners reported on ZnO-Cu(I) hybrid nanoparticles in aqueous media that converted carbon dioxide into methane with a selectivity of up to 99% [28]. Ansari et al. explored the construction of carbon-doped ZnO by a new furfural complexation method, and the synthesized ZnO photocatalyst showed excellent catalytic activity in the degradation of Rhodamine B [29]. Olga Osmolovskaya and her team synthesized core-shell ZnO nanoparticles combined with Fe3O4, which featured magnetic separability and exhibited good photocatalytic activity in the degradation of Naphthol Green B [30]. To date, ZnO-based composites have been extensively studied for dye photodegradation because of their nontoxicity, chemical stability, and abundant availability [31,32]. However, high surface energy facilitates the aggregation of ZnO nanoparticles during the photocatalysis process, which consequently restricts the photocatalytic activity and hinders the practical application of photocatalysts owing to their poor stability and reusability [33,34,35]. The immobilization of ZnO nanoparticles on suitable substrates is an effective strategy for constructing ZnO photocatalysts with high efficiency and good stability. Porous organic polymers have attracted considerable interest as substrates for heterogeneous catalysts due to their high specific surface areas, abundant porous configurations, and excellent structural designability. Therefore, the immobilization of ZnO on porous organic polymers facilitates the high dispersion of ZnO nanoparticles in ZnO-based composites, which further improves the structural stability of the photocatalysts. Furthermore, hierarchical meso/micropores and the high surface area of the supports is crucial for mass transmission in ZnO-based composites and the accessibility of the ZnO active sites to achieve the effective photodegradation of dyes. Hence, an exploration of porous polymer-supported ZnO composites is necessary for the effective degradation of dyes.
In this work, we have developed a facile synthesis approach for constructing a hollow porous organic nanosphere-supported ZnO composite photocatalyst (HPON@ZnO) through a combination of a hyper-crosslinking-mediated self-assembly method and a “ship-in-bottle” strategy. According to our previous report, HPONs were firstly prepared by the hyper-crosslinking-mediated self-assembly of polylactide-b-polystyrene (PLA-b-PS) diblock copolymers via the Scholl reaction in CHCl3 solvent using AlCl3 as a catalyst. Then, HPONs were used as carriers to encapsulate ZnO nanoparticles through a “ship-in-bottle” approach. The obtained HPON@ZnO possesses a large specific surface area and hierarchically porous structure, which exhibited exceptionally high catalytic activity in the adsorption and degradation of crystal violet, with the reaction proceeding under mild conditions. Additionally, the catalyst demonstrated degradation activity towards other dyes and featured good cyclic stability and recyclability.
2. Results and Discussion
2.1. Catalyst Synthesis and Characterization
The synthetic route for HPON@ZnO is illustrated in Scheme 1. Following our previously reported work, the PLA-b-PS diblock copolymer was firstly synthesized through a combination of ring-opening polymerization (ROP) and reversible addition–fragmentation chain transfer polymerization (RAFT) (Scheme S1). The successful preparation of the PLA-b-PS diblock copolymer was confirmed by the 1H NMR spectrum (Figure S1). Subsequently, the PLA-b-PS precursor was hyper-crosslinked to prepare hollow porous organic nanospheres (HPONs) through the Scholl reaction using CHCl3 as a solvent and anhydrous AlCl3 as a catalyst. The FT-IR spectra of the PLA-b-PS diblock copolymer and HPONs are illustrated in Figure S2. The characteristic adsorption peak at 1753 cm−1 in the FT-IR spectroscopy for the PLA-b-PS polymer was attributed to the carbonyl stretching vibrations of the PLA block, which demonstrated the existence of PLA in the diblock copolymer. In contrast, the peak at 1753 cm−1 disappeared in the FT-IR spectra for the hyper-crosslinked HPON. The above results indicated that the PLA chains were completely removed during the hyper-crosslinking.
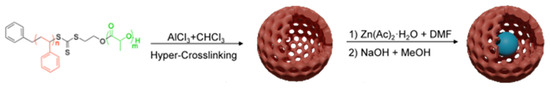
Scheme 1.
Schematic illustration of synthetic process for the HPON@ZnO composites.
As shown in Figure 1, the morphology of the HPON and HPON@ZnO was characterized by transmission electron microscopy (TEM). Hollow cavity structures with a uniform shell thickness (about 20 nm) were observed in the hyper-crosslinked organic polymer HPONs. During hyper-crosslinking, micelles with a PS shell and PLA core were firstly formed. Then, the PS shells were crosslinked and the PLA cores were etched, facilitating the formation of the hollow porous polymer HPONs [36]. Subsequently, the HPON@ZnO composites were prepared by the "ship-in-bottle" method. It was found that ZnO nanoparticles with sizes of 5 nm–10 nm were successfully encapsulated in the cavity of HPONs and the hollow porous structure of the HPONs was maintained after the immobilization of ZnO (Figure 1c,d). Furthermore, the core-shell structure of the HPON@ZnO composites can be further proved by HR SEM-EDS (Figure S3). The high dispersity of the ZnO nanoparticles and excellent chemical stability of the HPONs can facilitate high photocatalytic activity for dye degradation.
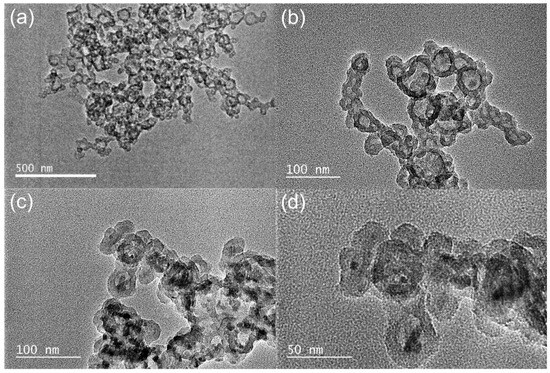
Figure 1.
TEM images of HPONs (a,b) and HPON@ZnO (c,d).
The chemical composition of the HPON@ZnO composites was investigated by powder X-ray diffraction (XRD) analysis. As shown in Figure 2, diffraction peaks at 32.0°, 34.6°, 36.3°, 47.5°, 56.6°, 62.8°, and 68.0° were observed in the XRD pattern, which could be attributed to the (100), (002), (101), (102), (110), (103), and (200) crystal planes of ZnO, respectively. The XRD results demonstrated the existence of ZnO and the successful preparation of the HPON@ZnO. As shown in Figure 3, the chemical composition and state of the elements in the HPON@ZnO was explored by X-ray photoelectron spectroscopy (XPS) analysis. The results showed that the HPON@ZnO was mainly composed of C (66.1 wt%), O (25.9 wt%), and Zn (8.0 wt%) elements (Table S1). The content of Zn obtained from the XPS analysis was found to be lower than that determined by ICP-OES (Table S2). This discrepancy can be attributed to the inherent differences in the analytical capabilities of the two techniques. XPS primarily analyzes the surface properties of a material, while ICP-OES provides insights into the composition of the entire material. The results further demonstrated that the ZnO nanoparticles were encapsulated in the cavity of the HPONs not immobilized on the surfaces of the HPONs. The characteristic peaks at 1023.0 eV and 1046.1 eV are attributed to Zn2+ in the metal oxide ZnO, corresponding to Zn 2p3/2 and Zn 2p1/2, respectively (Figure 3c). In Figure 3d, the corresponding O 1s spectrum is represented with two symmetrical peaks. The characteristic peaks at 532.7 eV in the O 1s band can be attributed to oxygen loosely absorbed on the ZnO surface [37]. The binding energy at 537.2 eV may be related to the presence of a O−2 species, which is coordinated to the Zn-O bond in ZnO [38]. The integration of the deconvolutions of the O 1s and Zn 2p spectra further proved the existence of ZnO in the −HPON@ZnO.
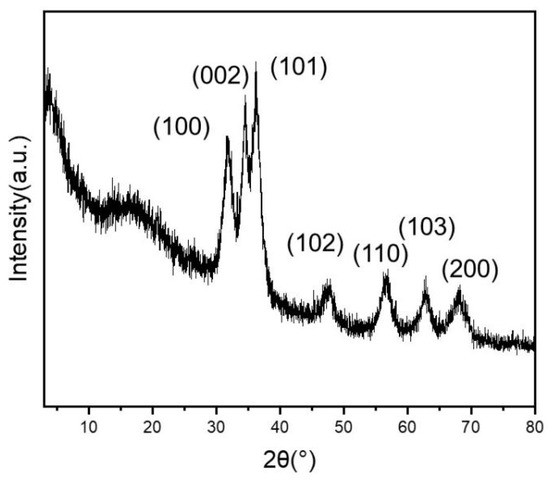
Figure 2.
XRD spectrum of HPON@ZnO.
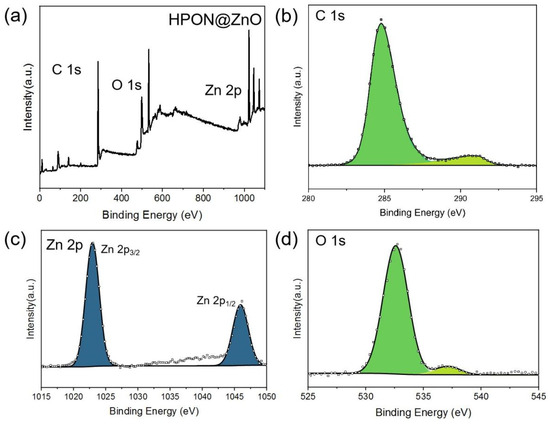
Figure 3.
(a) XPS survey spectra of HPON@ZnO; (b) C 1s; (c) Zn 2p; (d) O 1s.
The thermogravimetric analysis (TGA) results showed that HPON@ZnO retained 30.2% of its weight at 800 °C under the N2 atmosphere (Figure S4), indicating good thermal stability. The slight weight loss observed at 100 °C is attributed to the removal of physically adsorbed water, and the obvious thermal decomposition of the HPONs is found at approximately 400 °C. The weight loss after 700 °C may be attributed to the reduction of zinc oxide by CO and H2 formed in decomposition of the HPONs [39]. Furthermore, the proportion of ZnO in the composite photocatalysts was measured with ICP-OES. As shown in Table S2, the mass fraction of ZnO in HPON@ZnO-1M is 26.45 wt%, that in HPON@ZnO-1.5M is 45.57 wt %, and that in HPON@ZnO-2M is up to 62.80 wt%. This suggests that the HPON@ZnO composites contain a relatively high concentration of the active ZnO catalyst.
As shown in Figure S5, the porosity characteristics of HPON@ZnO were analyzed by N2 adsorption-desorption isotherms. The Brunauer-Emmett-Teller (BET) surface area and pore size distribution were obtained through the BET equation and Non-Local Density Functional Theory (NLDFT) method, respectively. The HPON@ZnO exhibits a typical Type IV isotherm with an H4 hysteresis loop, indicating the presence of both micropores and mesopores in the composite photocatalyst material (Figure S5a). The micropores in the ZnO composites derive from microporous structures in the rigid external shells formed by hyper-crosslinking of the phenylene, and the micropore diameters in the composite photocatalysts are mainly about 0.92 nm and 1.41 nm. Mesopores distributed around 2.19 nm and 5.01 nm are attributed to the cavities within the nanospheres and stacking between the spheres (Figure S5b). Based on the BET calculations, the specific surface area of HPON@ZnO-1M was determined to be 364 m2/g, with a total pore volume of 0.178 cm3/g. These results demonstrate that HPON@ZnO has a large specific surface area and hierarchically porous structures, which is beneficial for dye adsorption and also facilitates mass transfer during the degradation process.
2.2. Photocatalytic Degradation of Dyes
The photocatalytic performance of the HPON@ZnO composites in the degradation of dye was evaluated using crystal violet (CV) as the model dye. Crystal violet, a triphenylmethane dye known as "Methyl Violet 10B", is extensively used in histological staining and Gram staining tests. Owing to its vivid coloration, high stability, and potential carcinogenicity, crystal violet in water has a negative impact on aquatic ecosystems and human health. The effective degradation of crystal violet in wastewater is necessary for water purification.
In this study, the degradation of CV was monitored by a UV-Vis spectrophotometer with a maximum absorption wavelength at 589 nm. More than 98% of the CV was degraded within 30 min via the photocatalysis of HPON@ZnO-1M (Figure 4a). Composite catalysts with different photocatalyst contents (HPON@ZnO-2M for 1 h and HPON@ZnO-1.5M for 4 h) required longer times to degrade CV (Figure S6). It was observed that the adsorption capacity of the HPON@ZnO in the reaction decreased with the increase in the photocatalyst content, which may be due to the photocatalyst blocking the pores in the HPON carrier. When the amount of HPON@ZnO-1M catalyst was reduced to 5 mg, the degradation of more than 98% of the CV (50 ppm) was achieved after 2.5 h (Figure 4b). Figure 4c shows that the adsorption equilibrium was basically reached after stirring for 30 min in a dark environment. It was noted that, without the addition of the HPON@ZnO catalyst, the concentration of crystal violet in the solution did not decrease after 2 h of UV radiation (Figure 4d). In addition, the degradation of methylene blue with HPON@ZnO and ZnO was explored, respectively, to study the synergistic effect of the HPONs and ZnO (Figures S7e and S8). The HPON@ZnO composite demonstrated significantly enhanced photocatalytic degradation of methylene blue, achieving a degradation efficiency of almost 100% within 5 h, whereas pristine ZnO exhibited negligible catalytic activity after 24 h. The above results demonstrate that HPON@ZnO has excellent photocatalytic degradation ability for dyes. Based on the excellent photocatalytic performance of HPON@ZnO-1M, the degradation of mixed dyes was studied to further explore the selectivity of photocatalysts during the degradation of dyes. Firstly, selective adsorption was not found in the mixed solution of CV (50 ppm) and Rhodamine B (50 ppm). Then, both dyes were degraded simultaneously through the photocatalysis of the HPON@ZnO under UV radiation. However, the degradation rate for both dyes in the mixed solution was slower compared to that of single dye solutions, which may result from the limited active sites in the photocatalytic degradation. More than 99% of rhodamine B and CV can be degraded after 2 h, which is much longer than 0.5 h (Figure S6d). These results demonstrate that there is no competitive photocatalytic decolorization with the coexistence of different dyes.
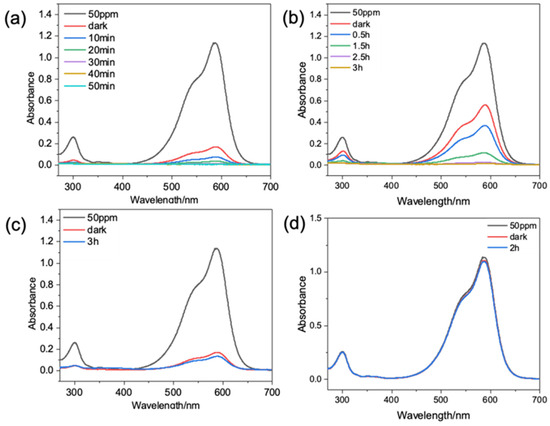
Figure 4.
UV spectra of crystal violet solution degraded by HPON@ZnO-1M: (a) 10 mg of HPON@ZnO-1M with ultraviolet radiation; (b) 5 mg of HPON@ZnO-1M with ultraviolet radiation; (c) 10 mg of HPON@ZnO-1M without ultraviolet radiation. (d) No catalyst with ultraviolet radiation.
To further study the catalytic activity of the HPON@ZnO composites, the degradation kinetics of CV (10 mL, 50 ppm) by the HPON@ZnO-1M (10 mg) were investigated. The pseudo-first-order kinetic equation is as follows:
where Co and Ct are the initial concentration (ppm) and the concentration at time t, respectively, t is the reaction time, and k is the rate constant. The calculated values of ln(Ct/Co) for different times and the reaction time t are fitted to a curve, with the slope of the resulting line representing the specific transformation rate constant.
As shown in Figure 5, the linear regression of the photocatalytic decolorization of CV by the HPON@ZnO composites agrees well with the pseudo-first-order kinetic model. The slope of the ln(Ct/Co) vs. irradiation time graph gives the rate constant (k) for the degradation of CV with a fitted curve R2 > 0.99. The HPON@ZnO-1M shows a high rate constant value of 0.0875 min−1 for the photocatalytic CV degradation. The reason for the high photocatalytic activity of the HPON@ZnO composites is attributed to the interaction between the support and the photocatalyst.
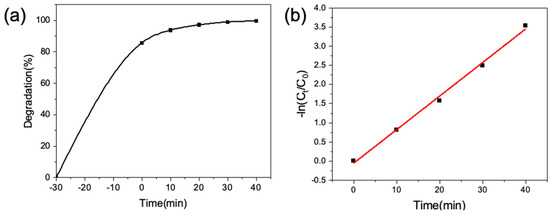
Figure 5.
(a) The degradation rate of CV changes with time; (b) kinetic fitting of pseudo-first-order.
The stability and recyclability of the HPON@ZnO composite photocatalyst was investigated (Figure 6a). There is no decrease in the photocatalytic activity of the HPON@ZnO for the degradation of CV after eight uses. In addition, the hollow porous structures of the HPON@ZnO composites were maintained after reuse, and there was no noticeable aggregation of zinc oxide in the composite materials (Figure 6b). The yolk-shell hollow porous structures confined the zinc oxide within the cavities and further restricted the aggregation-induced inactivation of the photocatalysts, facilitating the reuse of the HPON@ZnO composite photocatalysts. Therefore, the HPON@ZnO-1M exhibited excellent stability and recoverability in the photocatalytic decolorization of CV. In order to explore the universality of the composite photocatalysts, as shown in Figure S7, the degradation efficiency of several organic dyes by the HPON@ZnO-1M composite was studied. Under the same conditions, the composite photocatalyst demonstrated good degradation activity towards various dyes such as bromocresol green, methyl orange, rhodamine b, alizarin red S, methylene blue, and amido black 10B. These results indicate the excellent universality of the HPON@ZnO composite in the photocatalytic degradation of dyes. Compared to other catalysts reported in recent years (Table 1), the HPON@ZnO photocatalyst in this study exhibits a significant advantage in the dye degradation rate. Significantly, the photocatalytic degradation of dyes using HPON@ZnO is relatively mild and environmentally friendly compared to other photocatalysts.
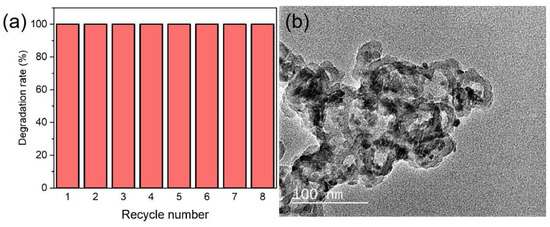
Figure 6.
(a) Cycling test for degradation of CV by HPON@ZnO-1M; (b) TEM image of HPON@ZnO-1M after cycling.

Table 1.
Summary of photocatalysts for degrading CV in the literature.
Density Functional Theory (DFT) calculations were performed on the hyper-crosslinked polystyrene HPONs, ZnO photocatalyst, and the HPON@ZnO composites. Using a model consisting of a hyper-crosslinked molecule cage formed by six styrenes and a photocatalyst molecule, the Highest Occupied Molecular Orbital-Lowest Unoccupied Molecular Orbital (HOMO-LUMO) gaps were analyzed. It was found that compared to ZnO or the HPONs, the HPON@ZnO composites showed a significantly reduced band gap. The original band gap of ZnO was 3.21 eV, which decreased to 2.40 eV after composite formation with HPONs (Figure 7). This suggests that the composites can better absorb photon energy and are more prone to forming electron-hole pairs compared to ZnO, thus facilitating dye decomposition. Therefore, the HPON@ZnO composites exhibit excellent photocatalytic activity in the degradation of dyes.
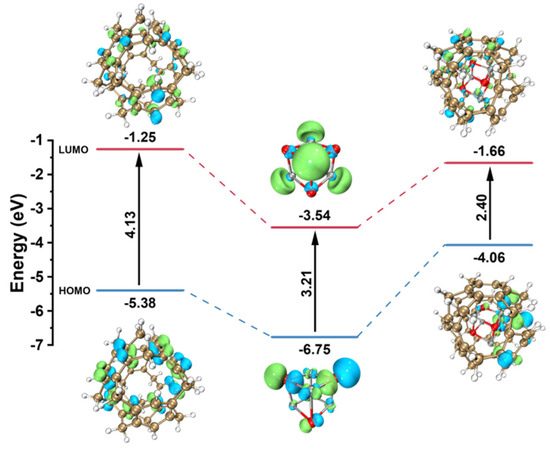
Figure 7.
DFT-calculated HOMO-LUMO gaps of HPON, ZnO, and the HPON@ZnO.
3. Methods and Experiments
3.1. Materials
Unless stated otherwise, all chemicals and reagents in this work were used without further purification. Styrene was purified by passing through the basic alumina (Al2O3) column. Dichloromethane (DCM) and toluene were employed after distillation with CaH2. 2,2-Azoisobutyronitrile (AIBN) and D,L-lactide (LA) used in this work were obtained by recrystallization from methanol and ethyl acetate, respectively.
3.2. Characterization
TEM images were obtained using a JEOL JEM-2100F (JEOL, Tokyo, Japan) instrument working at 200 kV. XRD patterns were recorded on an Ultima IV, Rigaku model D/MAX-50KV system (Rigaku, Tokyo, Japan) (Cu Kα radiation, λ=1.5418 A). XPS analysis was recorded by a Thermo Scientific K-Alpha (Thermo Scientific, Waltham, MA, USA) spectrometer equipped with an Al Kα radiation, and all XPS spectra were calibrated with contaminated C 1 s peak at 284.8 eV. The BET (Brunauer-Emmett-Teller) surface area, N2 sorption isotherms (77 K), and pore size distributions were measured using the Micromeritics ASAP 2460 surface area and porosity analyzer (Micromeritics Instrument Corporation, Norcross, GA, USA) in N2 adsorption and desorption experiments. The Zn contents in different samples were determined with inductively coupled plasma optical emission spectrometer (ICP-OES) on the Optima 8300 (PerkinElmer, Inc, Waltham, MA, USA). Standard curve fitting was achieved by diluting the commercially available samples. TGA was carried out with a Mettler Toledo SDTA851e simultaneous thermal analyzer (Mettler Toledo AG, Greifensee, Switzerland) at a heating rate of 10 °C/min from 25 to 800 °C in a N2 atmosphere. All the reaction solutions were diluted with deionized water and their UV–visible absorption spectra were measured by SOPTOP UV2400 (Sunny Hengping Scientific Instrument Co., Ltd, Shanghai, China). All 1H NMR spectra were acquired by testing on a Bruker AVANCE III 500 instrument (500 MHz) (Bruker Corporation, Billerica, MA, USA). FT-IR analysis was carried out using the Thermo NICOLET iS50 (Thermo Fisher Scientific Inc., Madison, MI, USA).
3.3. Catalyst Synthesis
3.3.1. Synthesis of Hollow Porous Organic Nanospheres (HPONs)
The PLA-b-PS block copolymer was synthesized via a procedure previously reported in the literature [36]. Anhydrous aluminium chloride (AlCl3) (0.63 g) was added to a solution of PLA-b-PS (500 mg) in CHCl3 (50 mL). The mixture was stirred at room temperature for half an hour, and then heated at 80 °C for 24 h. The generated solid was washed with a mixture of ethanol and water (v/v, 4:1), and the obtained brown powder was dried under vacuum at room temperature.
3.3.2. Preparation of Hollow Porous Organic Nanosphere-Supported ZnO Composites (HPON@ZnO)
A mass of 200 mg HPONs was dispersed in a DMF solution of 10 mL zinc acetate (1 M) and the mixture was stirred for 24 h. Then, 10 mL sodium hydroxide ethanol solution (1 M) was added, and the mixture was stirred at 40 °C for 2 h. The prepared solid was washed with ethanol and dried overnight at 80 °C to obtain HPON@ZnO-1M. In particular, samples prepared with ZnO (1 M/1.5 M/2 M) were named HPON@ZnO-xM (HPON@ZnO-1M, HPON@ZnO-1.5M, and HPON@ZnO-2M), respectively.
3.4. Photodegradation Experiment
All reactions were conducted in sample vials. Crystal violet aqueous solution (10 mL, 50 ppm) and 10 mg of catalyst were evenly mixed in the sample vial and stirred in the dark for 30 min to reach adsorption equilibrium. The suspension was then stirred under UV light. A portion of the suspension (0.7 mL) was withdrawn and filtered through a syringe filter (0.22 μm) at regular intervals. The filtrate was diluted with deionized water and measured using a UV-visible spectrophotometer.
In the recovery experiment, the photocatalyst was separated by centrifugation after photodegradation of dyes. The supernatant was analyzed using a UV-visible spectrophotometer, and the solid catalyst was washed with ethanol and dried at 80 °C for subsequent catalytic reactions.
4. Conclusions
In this work, we propose a facile method for synthesizing a hyper-crosslinked polymer-supported ZnO composite (HPON@ZnO). The HPON@ZnO composites exhibit a good adsorption performance and excellent photocatalytic activity in the degradation of dyes. Their good performance in the adsorption and photodegradation of dyes can be attributed to the hierarchical porous structure and low band gap in the ZnO composites. Furthermore, the catalysts show excellent stability and durability owing to the effective encapsulation of ZnO nanoparticles within the mesoporous cavities in the hyper-crosslinked polymer. In summary, this work provides a feasible strategy for encapsulating ZnO nanoparticles within hollow porous polymers to produce porous organic polymer/ZnO composite photocatalysts with high activity and remarkable stability in dye degradation. Additionally, future work will focus on investigating control over the pore size and shell thickness of the ZnO composites to improve the photocatalytic performance, and exploring further possible mechanisms for optimization of the ZnO composite in the photodegradation of dyes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15060529/s1: Scheme S1: Synthesis of PLA-b-PS diblock copolymer; Figure S1: 1H NMR spectrum of PLA-b-PS; Figure S2: FT-IR spectra of HPONs and PLA-b-PS; Figure S3: TEM-mapping of HPON@ZnO (a–d) and EDS spectra of HPON@ZnO (e); Figure S4: TGA spectrum of HPON@ZnO-1M; Figure S5: (a) N2 adsorption–desorption curve of HPON@ZnO-1M; (b) pore size distribution of HPON@ZnO-1M; Figure S6: UV degradation spectra of HPON@ZnO: (a) 10 mg HPON@ZnO-1M, (b) 10 mg HPON@ZnO-1.5M, (c) 10 mg HPON@ZnO-2M, and (d) rhodamine B and CV mixed dye; Figure S7: UV spectra of degradation of HPON@ZnO-1M for (a) bromocresol green, (b) methyl orange, (c) rhodamine b, (d) alizarin red S, (e) methylene blue, and (f) amido black 10B; Figure S8: UV spectra of methylene blue degraded by ZnO; Table S1: The content of different elements in HPON@ZnO-1M analyzed by XPS; Table S2: The content of ZnO in HPON@ZnO-xM analyzed by ICP-OES.
Author Contributions
Y.L.: writing—original draft, software, investigation. W.Z.: software, data curation. M.P.: data curation, validation. H.Z.: writing—review and editing, supervision, methodology. K.H.: writing—review and editing, supervision, project administration, investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 22375064.
Data Availability Statement
The data presented in this study are available in the manuscript and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Routoula, E.; Patwardhan, S.V. Degradation of Anthraquinone Dyes from Effluents: A Review Focusing on Enzymatic Dye Degradation with Industrial Potential. Environ. Sci. Technol. 2020, 54, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Naseem, S.; Rawal, R.S.; Pandey, D.; Suman, S.K. Immobilized Laccase: An Effective Biocatalyst for Industrial Dye Degradation from Wastewater. Environ. Sci. Pollut. Res. 2023, 30, 84898–84917. [Google Scholar] [CrossRef] [PubMed]
- El-Gaayda, J.; Titchou, F.E.; Oukhrib, R.; Yap, P.-S.; Liu, T.; Hamdani, M.; Ait Akbour, R. Natural Flocculants for the Treatment of Wastewaters Containing Dyes or Heavy Metals: A State-of-the-Art Review. J. Environ. Chem. Eng. 2021, 9, 106060. [Google Scholar] [CrossRef]
- Shaida, M.A.; Sen, A.K.; Dutta, R.K. Alternate Use of Sulphur Rich Coals as Solar Photo-Fenton Agent for Degradation of Toxic Azo Dyes. J. Clean. Prod. 2018, 195, 1003–1014. [Google Scholar] [CrossRef]
- Ertugay, N.; Acar, F.N. Removal of COD and Color from Direct Blue 71 Azo Dye Wastewater by Fenton’s Oxidation: Kinetic Study. Arab. J. Chem. 2017, 10, S1158–S1163. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, C.; Hou, B.; Wang, Y.; Hao, C.; Wu, J. Carbon Composite Lignin-Based Adsorbents for the Adsorption of Dyes. Chemosphere 2018, 206, 587–596. [Google Scholar] [CrossRef]
- Sellaoui, L.; Bouzidi, M.; Franco, D.S.P.; Alshammari, A.S.; Gandouzi, M.; Georgin, J.; Mohamed, N.B.H.; Erto, A.; Badawi, M. Exploitation of Bauhinia Forficata Residual Fruit Powder for the Adsorption of Cationic Dyes. Chem. Eng. J. 2023, 456, 141033. [Google Scholar] [CrossRef]
- Alsaffar, M.A.; Rahman, M.A.; Mageed, A.K.; Ali, S.A.K.; Lutfee, T.; Adnan, S.W.; Shakir, H.A.A. Electrochemical Removal of Dye from a Tanning Process Industrial Wastewater. Chem. Pap. 2023, 77, 6311–6318. [Google Scholar] [CrossRef]
- Xu, X.; Jia, Y.; Xiao, L.; Wu, Z. Strong Vibration-Catalysis of ZnO Nanorods for Dye Wastewater Decolorization via Piezo-Electro-Chemical Coupling. Chemosphere 2018, 193, 1143–1148. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, X.; Zhang, J.; Wang, H.; Jiang, L.; Zeng, G. Photocatalytic Decontamination of Wastewater Containing Organic Dyes by Metal-Organic Frameworks and Their Derivatives. ChemCatChem 2017, 9, 41–64. [Google Scholar] [CrossRef]
- Malini, B.; Allen Gnana Raj, G. C,N and S-Doped TiO2-Characterization and Photocatalytic Performance for Rose Bengal Dye Degradation under Day Light. J. Environ. Chem. Eng. 2018, 6, 5763–5770. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical Photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef] [PubMed]
- Hasanah, A.U.; Gareso, P.L.; Rauf, N.; Tahir, D. Photocatalytic Performance of Zinc Oxide and Metal-Doped Zinc Oxide for Various Organic Pollutants. ChemBioEng Rev. 2023, 10, 698–710. [Google Scholar] [CrossRef]
- Guzmán-Carrillo, H.R.; Manzano-Ramírez, A.; Garcia Lodeiro, I.; Fernández-Jiménez, A. ZnO Nanoparticles for Photocatalytic Application in Alkali-Activated Materials. Molecules 2020, 25, 5519. [Google Scholar] [CrossRef]
- Shundo, Y.; Tam Nguyen, T.; Akrami, S.; Edalati, P.; Itagoe, Y.; Ishihara, T.; Arita, M.; Guo, Q.; Fuji, M.; Edalati, K. Oxygen Vacancy-Rich High-Pressure Rocksalt Phase of Zinc Oxide for Enhanced Photocatalytic Hydrogen Evolution. J. Colloid. Interfaces Sci. 2024, 666, 22–34. [Google Scholar] [CrossRef]
- Guzmán, H.; Salomone, F.; Bensaid, S.; Castellino, M.; Russo, N.; Hernández, S. CO2 Conversion to Alcohols over Cu/ZnO Catalysts: Prospective Synergies between Electrocatalytic and Thermocatalytic Routes. ACS Appl. Mater. Interfaces 2022, 14, 517–530. [Google Scholar] [CrossRef]
- Ren, L.; Li, Y.Z.; Liu, H.H.; Zhao, C.F.; Zhao, X.J.; Xie, H. Intensitive UV-Vis-IR Driven Catalytic Activity of Pt Supported on Hierarchical ZnO Porous Nanosheets for Benzene Degradation via Novel Photothermocatalytic Synergetic Effect. J. Environ. Chem. Eng. 2022, 10, 107694. [Google Scholar] [CrossRef]
- Aroui, L.; Madani, S.; Bousnoubra, I.; Boublia, A.; Lakikza, I.; Aouni, S.I.; Abdelouahed, L.; Ernst, B.; Alam, M.; Benguerba, Y. Enhanced Degradation of Crystal Violet Using PANI-ZnO Nanocomposites: Electro-Oxidation and Photocatalysis Studies. J. Mol. Liq. 2024, 412, 125818. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Zheng, S.; Xue, H.; Pang, H. N-Doped Mesoporous ZnO with Oxygen Vacancies for Stable Hydrazine Electrocatalysis. ChemNanoMat 2019, 5, 79–84. [Google Scholar] [CrossRef]
- Kelly, S.R.; Shi, X.; Back, S.; Vallez, L.; Park, S.Y.; Siahrostami, S.; Zheng, X.; Nørskov, J.K. ZnO as an Active and Selective Catalyst for Electrochemical Water Oxidation to Hydrogen Peroxide. ACS Catal. 2019, 9, 4593–4599. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Choe, Y.-E.; Shin, S.-J.; Park, J.-H.; Dashnyam, K.; Kim, H.S.; Jun, S.-K.; Knowles, J.-C.; Kim, H.-W.; Lee, J.-H.; et al. Photocatalytic Effect-Assisted Antimicrobial Activities of Acrylic Resin Incorporating Zinc Oxide Nanoflakes. Biomater. Adv. 2022, 139, 213025. [Google Scholar]
- Dobrucka, R.; Długaszewska, J. Biosynthesis and Antibacterial Activity of ZnO Nanoparticles Using Trifolium Pratense Flower Extract. Saudi J. Biol. Sci. 2016, 23, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Anitha, R.; Ramesh, K.V.; Ravishankar, T.N.; Sudheer Kumar, K.H.; Ramakrishnappa, T. Cytotoxicity, Antibacterial and Antifungal Activities of ZnO Nanoparticles Prepared by the Artocarpus Gomezianus Fruit Mediated Facile Green Combustion Method. J. Sci. -Adv. Mater. Dev. 2018, 3, 440–451. [Google Scholar] [CrossRef]
- Suresh, D.; Nethravathi, P.C.; Udayabhanu; Rajanaika, H.; Nagabhushana, H.; Sharma, S.C. Green Synthesis of Multifunctional Zinc Oxide (ZnO) Nanoparticles Using Cassia Fistula Plant Extract and Their Photodegradative, Antioxidant and Antibacterial Activities. Mat. Sci. Semicon. Proc. 2015, 31, 446–454. [Google Scholar] [CrossRef]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. In Vitro Antioxidant and Antidiabetic Activities of Zinc Oxide Nanoparticles Synthesized Using Different Plant Extracts. Bioprocess Biosyst. Eng. 2017, 40, 943–957. [Google Scholar] [CrossRef]
- Hafez, A.; Nassef, E.; Fahmy, M.; Elsabagh, M.; Bakr, A.; Hegazi, E. Impact of Dietary Nano-Zinc Oxide on Immune Response and Antioxidant Defense of Broiler Chickens. Environ. Sci. Pollut. Res. 2020, 27, 19108–19114. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Pan, X.; Cortie, D.; Huang, X.; Yi, Z. Photocatalytic Oxidation of Methane over Silver Decorated Zinc Oxide Nanocatalysts. Nat. Commun. 2016, 7, 12273. [Google Scholar] [CrossRef]
- Bae, K.-L.; Kim, J.; Lim, C.K.; Nam, K.M.; Song, H. Colloidal Zinc Oxide–Copper(I) Oxide Nanocatalysts for Selective Aqueous Photocatalytic Carbon Dioxide Conversion into Methane. Nat. Commun. 2017, 8, 1156. [Google Scholar] [CrossRef]
- Ali Ansari, S.; Parveen, N.; Aljaafari, A.; Alshoaibi, A.; Alsulaim, G.M.; Waqas Alam, M.; Zahid Ansari, M. Novel Furfural-Complexed Approach to Synthesizing Carbon-Doped ZnO with Breakthrough Photocatalytic Efficacy. J. Adv. Res. 2024. [Google Scholar] [CrossRef]
- Tkachenko, D.; Zheltova, V.; Meshina, K.; Vorontsov-Velyaminov, P.; Emelianova, M.; Bobrysheva, N.; Osmolowsky, M.; Voznesenskiy, M.; Osmolovskaya, O. Fe3O4@ZnO Core-Shell Nanoparticles-a Novel Facile Fabricated Magnetically Separable Photocatalyst. Appl. Surf. Sci. 2024, 672, 160873. [Google Scholar] [CrossRef]
- Ghamarpoor, R.; Fallah, A.; Jamshidi, M. A Review of Synthesis Methods, Modifications, and Mechanisms of ZnO/TiO2-Based Photocatalysts for Photodegradation of Contaminants. ACS Omega 2024, 9, 25457–25492. [Google Scholar] [CrossRef] [PubMed]
- Bayram, Ü.; Özer, Ç.; Yilmaz, E. Comparison of Photocatalytic and Adsorption Properties of ZnS@ZnO, CdS@ZnO, and PbS@ZnO Nanocomposites to Select the Best Material for the Bifunctional Removal of Methylene Blue. ACS Omega 2025, 10, 9986–10003. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Q.Y.; EI-Din, M.G.; Zhang X., H. Immobilization of Photocatalytic ZnO Nanocaps on Planar and Curved Surfaces for the Photodegradation of Organic Contaminants in Water. ACS ES&T Water 2023, 3, 2740–2752. [Google Scholar]
- Dehghani, M.; Nadeem, H.; Raghuwanshi, V.S.; Mahdavi, H.; Holl, M.M.B.; Batchelor, W. ZnO/Cellulose Nanofiber Composites for Sustainable Sunlight-Driven Dye Degradation. ACS Appl. Nano Mater. 2020, 3, 10284–10295. [Google Scholar] [CrossRef]
- Hussain M., Z.; Schneemann, A.; Fischer, R.A.; Zhu Y., Q.; Xia Y., D. MOF Derived Porous ZnO/C Nanocomposites for Efficient Dye Photodegradation. ACS Appl. Energy Mater. 2018, 1, 4695–4707. [Google Scholar] [CrossRef]
- He, Z.D.; Zhou, M.H.; Wang, T.Q.; Xu, Y.; Yu, W.; Shi, B.Y.; Huang, K. Hyper-Cross-Linking Mediated Self-Assembly Strategy to Synthesize Hollow Microporous Organic Nanospheres. ACS Appl. Mater. Interfaces 2017, 9, 35209–35217. [Google Scholar] [CrossRef]
- Aier, I.; Purkayastha, D.D. Hierarchical 0D CuO Wrapped by Petal-like 2D ZnO: A Strategic Approach of Superhydrophobic Melamine Sponge toward Wastewater Treatment. Langmuir 2024, 40, 9702–9716. [Google Scholar] [CrossRef]
- Phogat, P.; Shreya; Jha, R.; Singh, S. Synthesis of Novel ZnO nanoparticles with Optimized Band Gap of 1.4 eV for High-sensitivity Photo Electrochemical Detection. Mater. Today Sustain. 2024, 27, 100823. [Google Scholar] [CrossRef]
- Maciel, A.V.M.; Job, A.Z.; Mussel, W.N.; Brito, W.; Pasa, V.M.D. Bio-hydrogen Production Based on Catalytic Reforming of Volatiles Generated by Cellulose Pyrolysis: An Integrated Process for ZnO Reduction and Zinc Nanostructures Fabrication. Biomass Bioenergy 2011, 35, 1121–1129. [Google Scholar] [CrossRef]
- Karthik, P.; Ravichandran, S.; Mukkannan, A.; Rajesh, J. Plant-Mediated Biosynthesis of Zinc Oxide Nanoparticles from Delonix Elata: A Promising Photocatalyst for Crystal Violet Degradation. Inorg. Chem. Commun. 2022, 146, 110122. [Google Scholar] [CrossRef]
- Gokhale, T.A.; Sarda, T.J.; Bhanage, B.M. Sunlight Driven Rapid and Efficient Photodegradation of Crystal Violet Using Magnesium Doped Zinc Oxide Nanostructures. Mater. Chem. Phys. 2023, 295, 127075. [Google Scholar] [CrossRef]
- Munir, S.; Farooq Warsi, M.; Zulfiqar, S.; Ayman, I.; Haider, S.; Alsafari, I.A.; Agboola, P.O.; Shakir, I. Nickel Ferrite/Zinc Oxide Nanocomposite: Investigating the Photocatalytic and Antibacterial Properties. J. Saudi Chem. Soc. 2021, 25, 101388. [Google Scholar] [CrossRef]
- Abarna, B.; Preethi, T.; Rajarajeswari, G.R. Lemon Peel Guided Sol-Gel Synthesis of Visible Light Active Nano Zinc Oxide. J. Environ. Chem. Eng. 2019, 7, 102742. [Google Scholar] [CrossRef]
- Shen, H.; Qin, L.; Gao, X.; Wang, Q.; Zhang, T.; Kang, S.Z.; Li, X. A Novel Core-Shell Heterostructure of Zinc Oxide/Metal-Organic Frameworks Anchored Silver Nanoparticles for Enhanced SERS and Photocatalytic Performance. J. Environ. Chem. Eng. 2023, 11, 111526. [Google Scholar] [CrossRef]
- Rostamzadeh, D.; Sadeghi, S. Ni Doped Zinc Oxide Nanoparticles Supported Bentonite Clay for Photocatalytic Degradation of Anionic and Cationic Synthetic Dyes in Water Treatment. J. Photoch. Photo. A -Chem. 2022, 431, 113947. [Google Scholar] [CrossRef]
- Kanagamani, K.; Muthukrishnan, P.; Saravanakumar, K.; Shankar, K.; Kathiresan, A. Photocatalytic Degradation of Environmental Perilous Gentian Violet Dye Using Leucaena-Mediated Zinc Oxide Nanoparticle and Its Anticancer Activity. Rare Met. 2019, 38, 277–286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).