Membrane-Based Photocatalytic and Electrocatalytic Systems: A Review
Abstract
1. Introduction
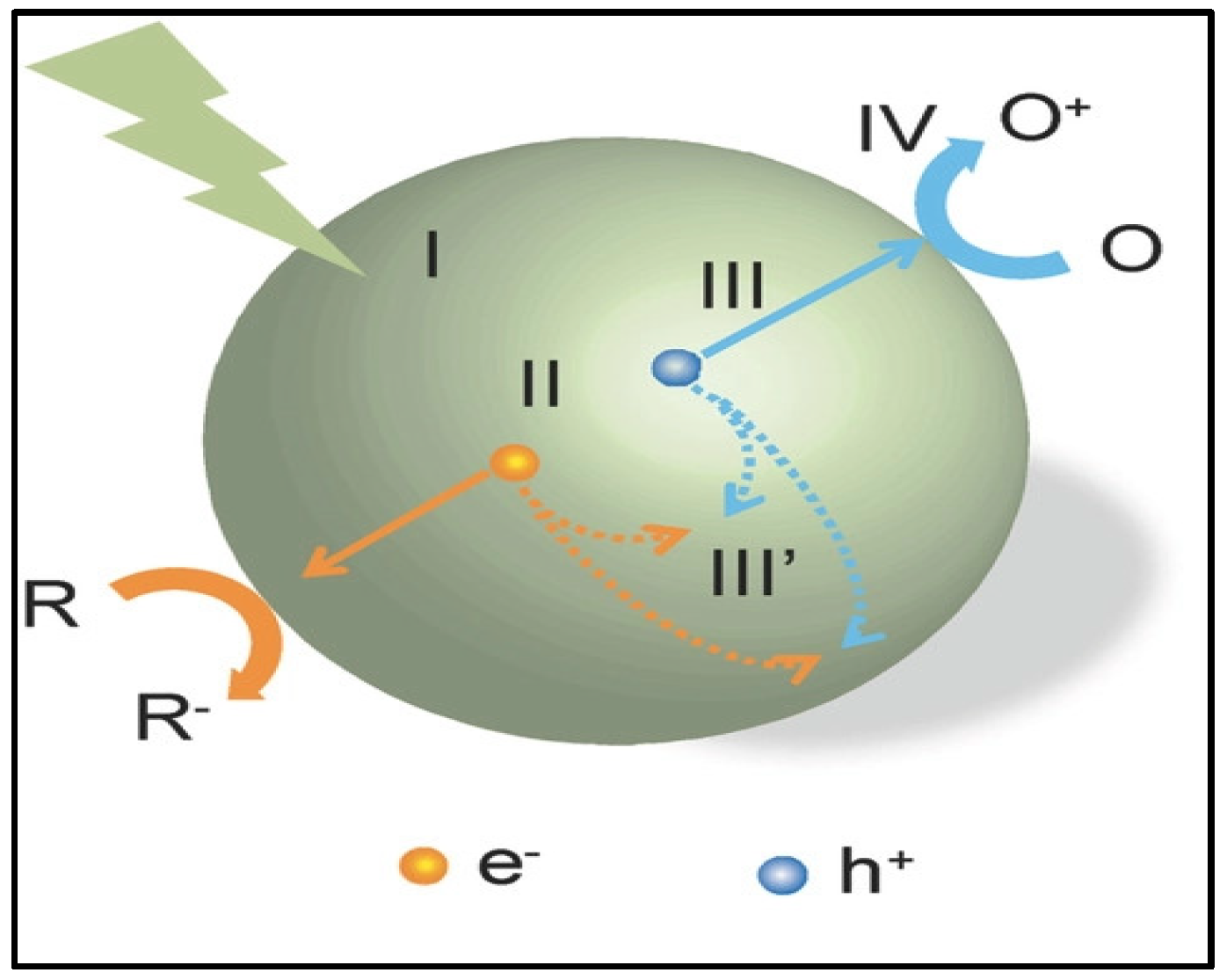
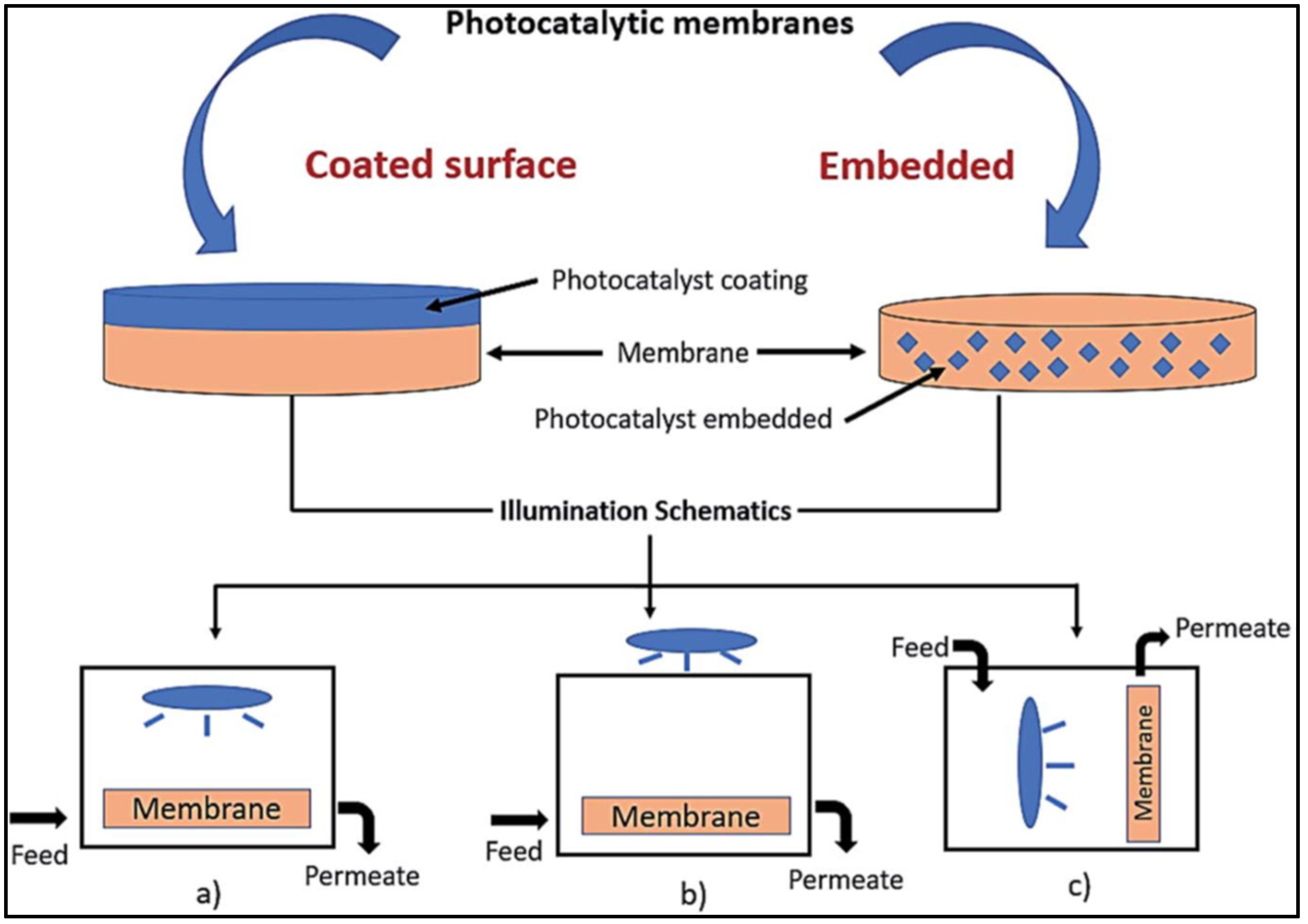
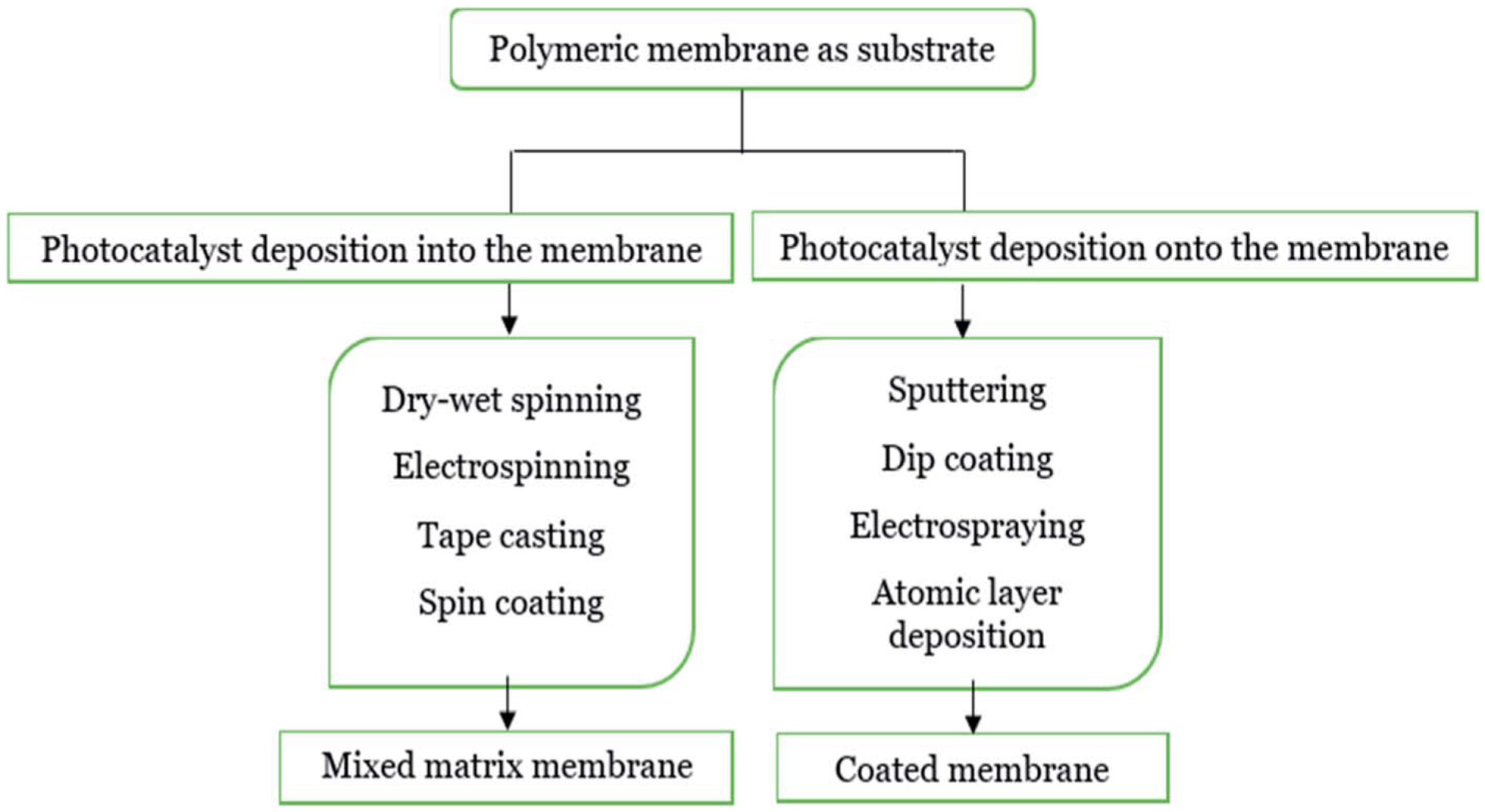
2. Photocatalytic Membranes
- (a)
- Suspension preparation involves combining the starting powders with an appropriate binding liquid.
- (b)
- Forming entails reshaping the prepared suspension using a predefined technique.
- (c)
- Heat treatment is the process of sintering membrane particles together at high temperatures.
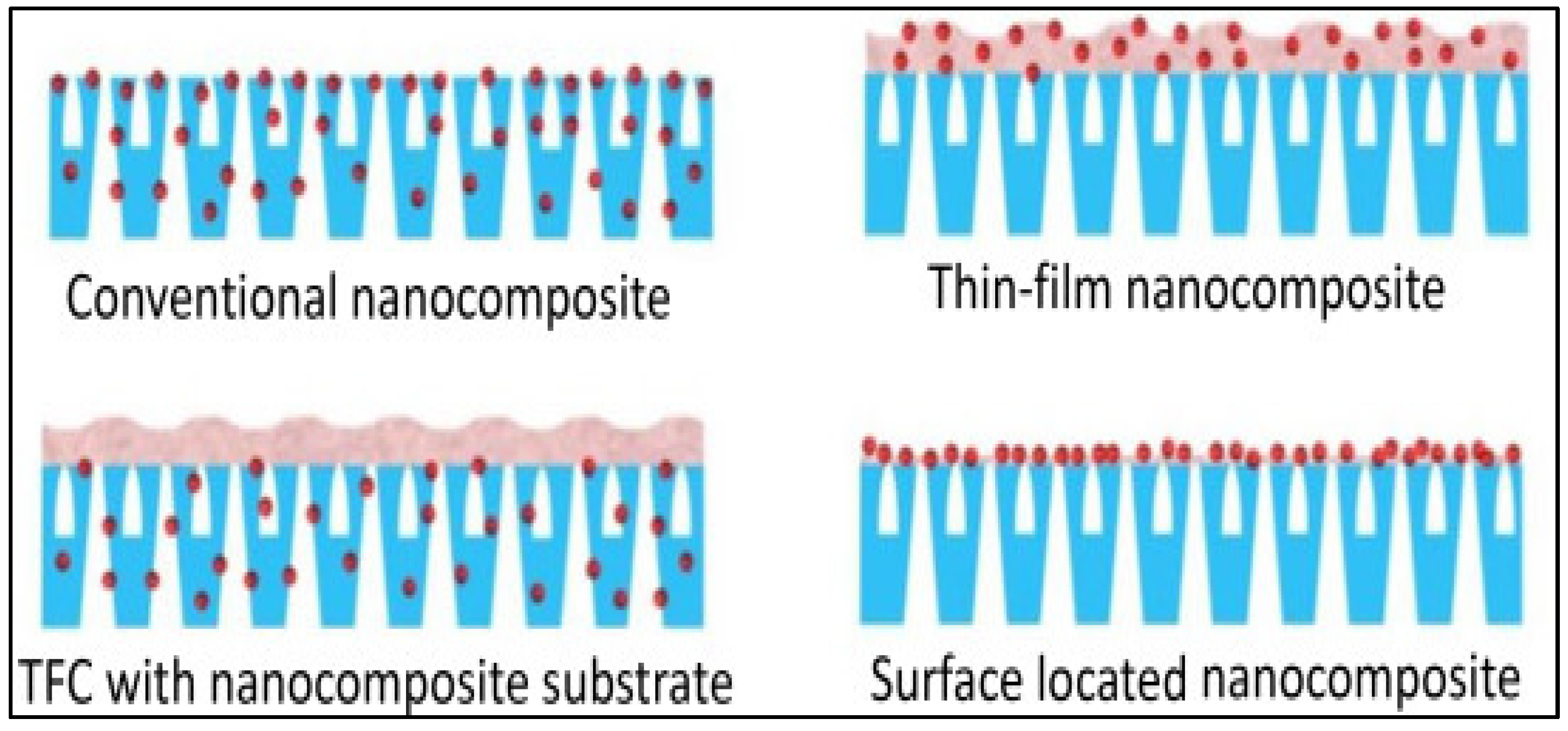
3. Nanocomposite Photocatalytic Membranes
- Standard nanocomposites;
- Nanocomposites with thin films (TFCs);
- Nanocomposite thin film on a nanocomposite substrate;
- Nanocomposites with a surface-based position.
4. Photocatalysis Against Water Pollutants
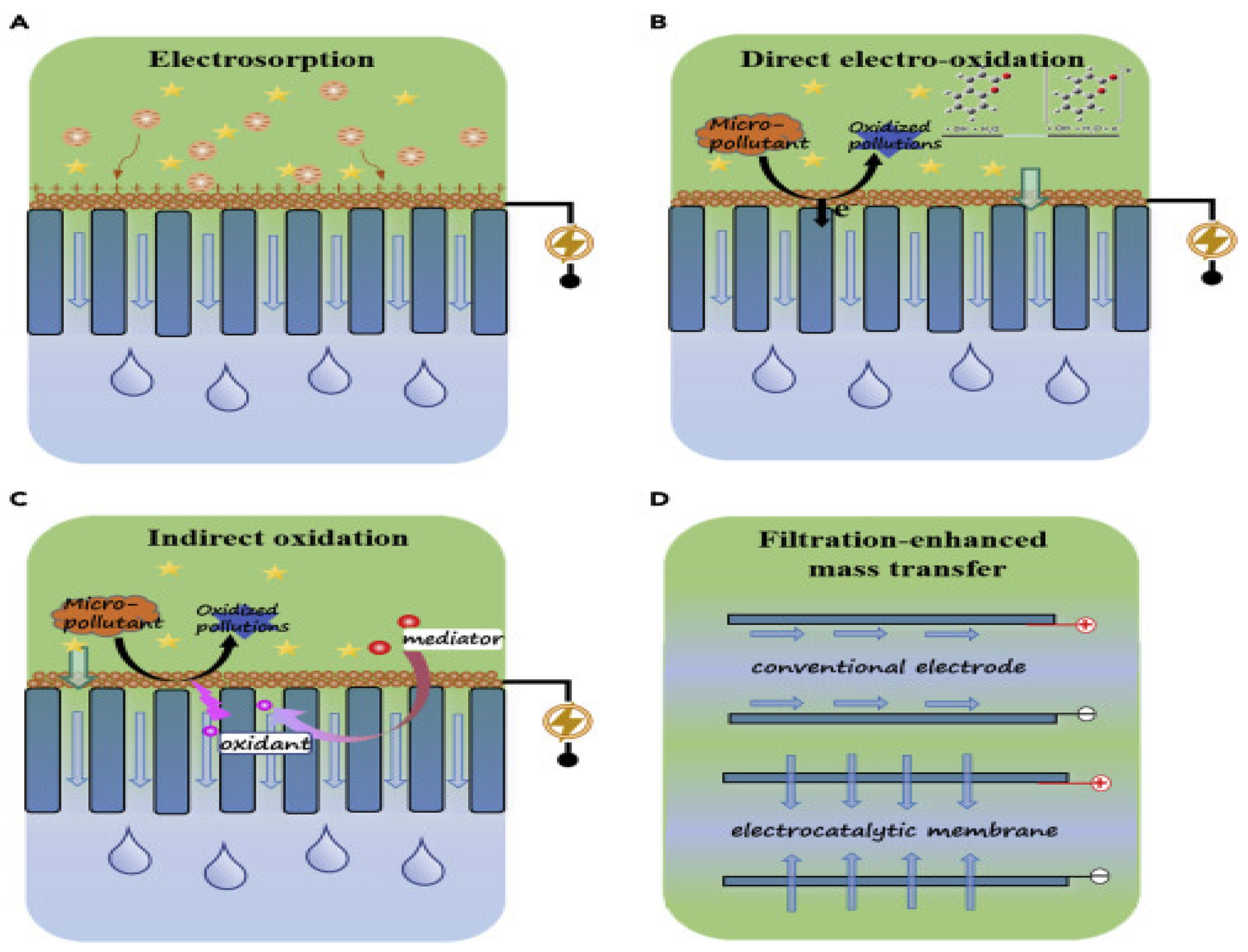
5. Electrocatalytic Membranes
5.1. Electrosorption
5.2. Electrochemical Oxidation
5.2.1. Direct Electro-Oxidation
5.2.2. Indirect Oxidation
5.3. Filtration-Boosted Mass Transfer
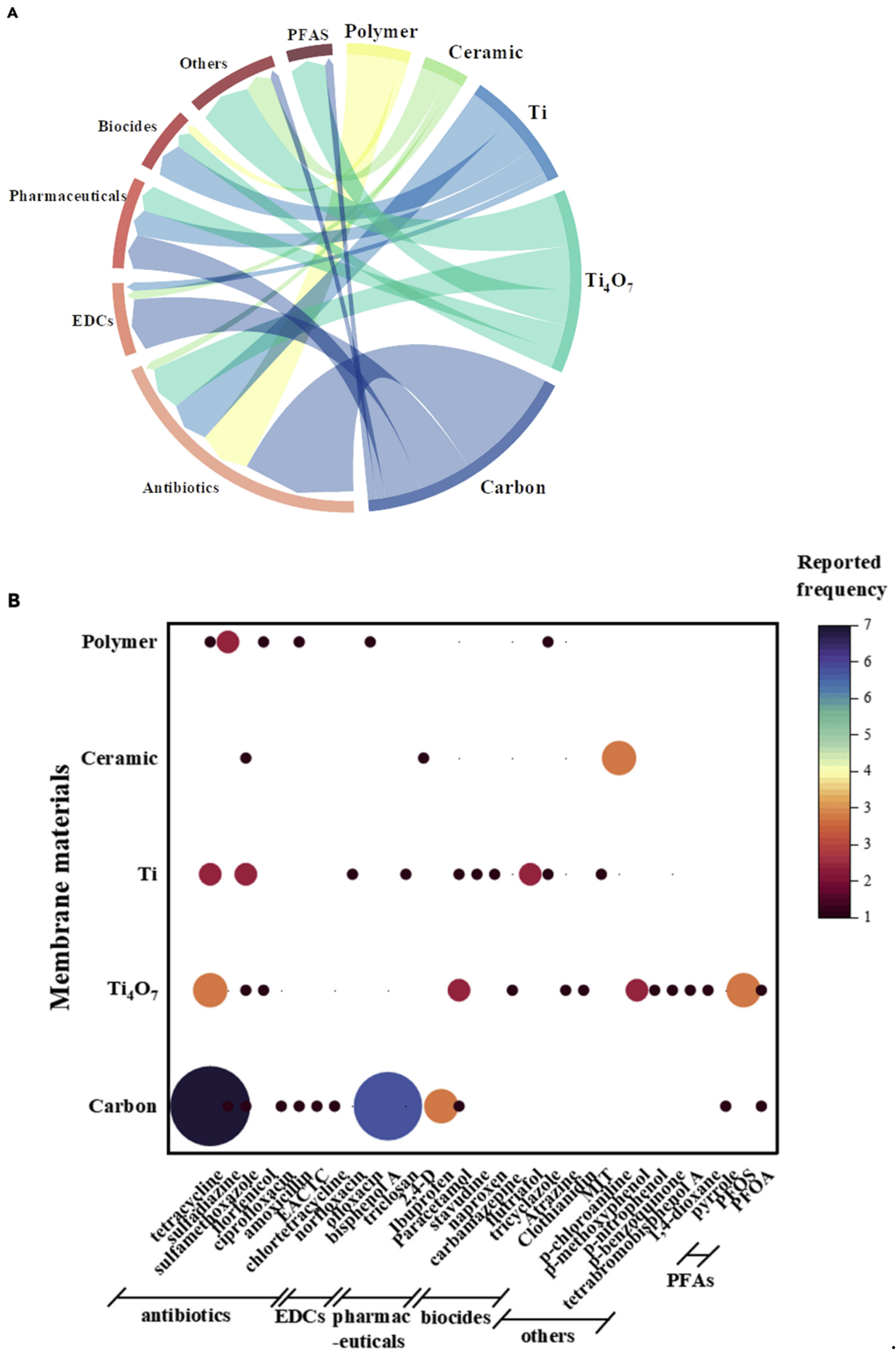
6. Applications of Electrocatalytic Membranes for Micropollutant Removal
6.1. Membrane Materials
6.1.1. Carbon-Based Electrocatalytic Membranes
6.1.2. Porous Ti-Based Electrocatalytic Membranes
6.1.3. Electrocatalytic Membranes Based on Magnéli Phase
6.1.4. Electrochemical Ceramic Membranes
6.1.5. Polymer Composite Membranes
7. Summary and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhayana, P.; Dandekar, T.; Bala, R.; Madaan, R.; Mahajan, P. Role of carbon nanotubes in remediation of pharmaceutical wastewater. Remediation 2024, 34, 21776. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, L.; Ma, K. Microfiber from textile dyeing and printing wastewater of a typical industrial park in China: Occurrence, removal and release. Sci. Total Environ. 2020, 739, 140329. [Google Scholar] [CrossRef]
- Kushniarou, A.; Garrido, I.; Fenoll, J.; Vela, N.; Flores, P.; Navarro, G.; Hellin, P.; Navarro, S. Solar photocatalytic reclamation of agro-waste water polluted with twelve pesticides for agricultural reuse. Chemosphere 2019, 214, 839–845. [Google Scholar] [CrossRef]
- Molinari, R.; Severino, A.; Lavorato, C.; Argurio, P. Which Configuration of Photocatalytic Membrane Reactors Has a Major Potential to Be Used at an Industrial Level in Tertiary Sewage Wastewater Treatment? Catalysts 2023, 13, 1204. [Google Scholar] [CrossRef]
- Jabbar, Z.; Graimed, B.; Ammar, S.; Sabit, D.; Najim, A.; Radeef, A.; Taher, A. The latest progress in the design and application of semiconductor photocatalysis systems for degradation of environmental pollutants in wastewater: Mechanism insight and theoretical calculations. Mater. Sci. Semicond. Process. 2024, 173, 108153. [Google Scholar] [CrossRef]
- Ali, Z.; Saif, M.; Nawaz, F.; Fareed, I.; Khan, M.D.; Saif, A. Exploring the catalytic potential of MnO for photodegradation of organic dyes. MRS Adv. 2025. [Google Scholar] [CrossRef]
- Acharya, R.; Parida, K. A review on TiO2/g-C3N4 visible-light- responsive photocatalysts for sustainable energy generation and environmental remediation. J. Environ. Chem. Eng. 2020, 8, 103896. [Google Scholar] [CrossRef]
- Jo, Y.K.; Lee, J.M.; Son, S.; Hwang, S.J. 2D inorganic nanosheet-based hybrid photocatalysts: Design; applications, and perspectives. J. Photochem. Photobiol. C Photochem. Rev. 2019, 40, 150–190. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chang, T.F.M.; Chen, C.Y.; Sone, M.; Hsu, Y.J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Wu, X.; Lu, J.; Huang, S.; Shen, X.; Cui, S.; Chen, X. Facile fabrication of novel magnetic 3-D ZnFe2O4/ZnO aerogel based heterojunction for photoreduction of Cr(VI) under visible light: Controlled synthesis, facial change distribution, and DFT study. Appl. Surf. Sci. 2022, 594, 153486. [Google Scholar] [CrossRef]
- Dong, J.; Yu, J.; Deng, P.; Qu, D.; Liu, J.; Ji, X.; Zhang, D.; Pu, X.; Cai, P. Highly efficient Bi/BiOCl with oxygen vacancies photocatalyst with synergetic effects of oxygen vacancy, surface plasmon resonance, and electron sink effects of metallic Bi. Appl. Organomet. Chem. 2024, 38, e7633. [Google Scholar] [CrossRef]
- Darowna, D.; Wrobel, R.; Morawski, A.W.; Mozia, S. The influence of feed composition on fouling and stability of a polyethersulfone ultrafiltration membrane in a photocatalytic membrane reactor. Chem. Eng. J. 2017, 310, 360–367. [Google Scholar] [CrossRef]
- Ikrari, K.I.; Hasbullah, H.; Salleh, W.N.W.; Nakagawa, K.; Yoshioka, T. Photocatalytic membrane technologies for removal of recalcitrant pollutants. Mater. Proc. 2022, 65, 3101–3108. [Google Scholar] [CrossRef]
- Baig, U.; Waheed, A.; Abu-Zahra, N.; Aljundi, I.H. A polymeric-ceramic hybrid membrane with a self-cleaning and super-wettable surface decorated with polypyrrole-G-C3N4 photocatalyst for oily wastewater treatment. Sep. Purif. Technol. 2024, 339, 126487. [Google Scholar] [CrossRef]
- Zakria, H.S.; Othman, M.H.D.; Kamaludin, R.; Kadir, S.; Kurniawan, T.A.; Jilani, A. Immobilization techniques of a photocatalyst into and onto a polymer membrane for photocatalytic activity. RSC Adv. 2021, 11, 6985–7014. [Google Scholar] [CrossRef]
- Sarkar, S.; Chakraborty, S. Nanocomposite polymeric membrane a new trend of water and wastewater treatment: A short review. Groundw. Sustain. Dev. 2021, 12, 100533. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Qin, H.; Lin, H.; Chhuon, K. Application of Microfiltration membrane Technology in Water treatment. IOP Conf. Ser. Earth Environ. Sci. 2020, 571, 012158. [Google Scholar] [CrossRef]
- Shi, F.M.; Ma, Y.X.; Ma, J.; Wang, P.P.; Sun, W.X. An overview on nanostructured TiO2–containing fibers for photocatalytic degradation of organic pollutants in wastewater treatment. J. Water Process Eng. 2012, 389, 522–531. [Google Scholar]
- Pasini, S.M.; Valério, A.; Yin, G.; Wang, J.; Souza, S.M.A.G.U.; Hotza, D.; Souza, A.A.U. Development of advanced nanocomposite membranes using graphene nanoribbons and nanosheets for water treatment. J. Membr. Sci. 2021, 40, 101827. [Google Scholar]
- Khanlari, S.; Tofighy, M.A.; Mohammadi, T. Transport phenomena through nanocomposite membranes. Micro Nano Technol. 2020, 91–112. [Google Scholar] [CrossRef]
- Li, C.C.; Zhang, H.; Wang, F.; Zhu, H.L.; Guo, Y.H.; Chen, M.Y. PVA and CS cross-linking combined with in situ chimeric SiO2 nanoparticle adhesion to enhance the hydrophilicity and antibacterial properties of PTFE flat membranes. RSC Adv. 2019, 9, 19205–19216. [Google Scholar] [CrossRef]
- Li, Y.; Jin, C.L.; Peng, Y.L.; An, Q.F.; Chen, Z.P.; Zhang, J.C.; Ge, L.; Wang, S.B. Fabrication of PVDF hollow fiber membranes via integrated phase separation for membrane distillation. J. Taiwan Inst. Chem. Eng. 2019, 95, 487–494. [Google Scholar] [CrossRef]
- Cai, T.; Zeng, W.G.; Liu, Y.T.; Wang, L.L.; Dong, W.Y.; Chen, H.; Xia, X.N. A promising inorganic-organic Z-scheme photocatalyst Ag3PO4/PDI supermolecule with enhanced photoactivity and photostability for environmental remediation. Appl. Catal. B Environ. 2020, 263, 118327. [Google Scholar] [CrossRef]
- You, J.H.; Guo, Y.Z.; Guo, R.; Liu, X.W. A review of visible light-active photocatalysts for water disinfection: Features and prospects. Chem. Eng. J. 2019, 373, 624–641. [Google Scholar] [CrossRef]
- Zhu, S.S.; Wang, D.W.; Principles, B. Diverse Forms of Implementations and Emerging Scientific Opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef]
- Ibrahim, I.; Kaltzoglou, A.; Athanasekou, C.; Katsaros, F.; Devlin, E.; Kontos, A.G.; Ioannidis, N.; Perraki, M.; Tsakiridis, P.; Sygellou, L.; et al. Magnetically separable TiO2/CoFe2O4/Ag nanocomposites for the photocatalytic reduction of hexavalent chromium pollutant under UV and artificial solar light. Chem. Eng. J. 2020, 381, 122730. [Google Scholar] [CrossRef]
- Singh, R.; Yadav, V.S.K.; Purkait, M.K. Cu2O photocatalyst modified antifouling polysulfone mixed matrix membrane for ultrafiltration of protein and visible light driven photocatalytic pharmaceutical removal. Sep. Purif. Technol. 2019, 212, 191–204. [Google Scholar] [CrossRef]
- Madhavi, V.; Kondaiah, P.; Rao, G.M. Influence of silver nanoparticles on titanium oxide and nitrogen doped titanium oxide thin films for sun light photocatalysis. Appl. Surf. Sci. 2018, 436, 708–719. [Google Scholar]
- Rasch, M.R.; Rossinyol, E.; Hueso, J.L.; Goodfellow, B.W.; Arbiol, J.; Korgel, B.A. Hydrophobic Gold Nanoparticle Self-Assembly with Phosphatidylcholine Lipid: Membrane-Loaded and Janus Vesicles. Nano Lett. 2010, 10, 3733–3739. [Google Scholar] [CrossRef] [PubMed]
- Von White, G.; Chen, Y.J.; Roder-Hanna, J.; Bothun, G.D.; Kitchens, C.L. Structural and Thermal Analysis of Lipid Vesicles Encapsulating Hydrophobic Gold Nanoparticles. ACS Nano 2012, 6, 4678–4685. [Google Scholar] [CrossRef]
- Chen, R.; Pearce, D.J.G.; Fortuna, S.; Cheung, D.L.; Bon, S.A.F. Polymer Vesicles with a Colloidal Armor of Nanoparticles. J. Am. Chem. Soc. 2011, 133, 2151–2153. [Google Scholar] [CrossRef]
- Houga, C.; Giermanska, J.; Lecommandoux, S.; Borsali, R.; Taton, D.; Gnanou, Y.; Le Meins, J.F. Micelles and Polymersomes Obtained by Self-Assembly of Dextran and Polystyrene Based Block Copolymers. Biomacromolecules 2009, 10, 32–40. [Google Scholar] [CrossRef]
- Binder, W.H.; Sachsenhofer, R. Polymersome/silica capsules by ’Click’-chemistry. Macromol. Rapid Commun. 2008, 29, 1097–1103. [Google Scholar] [CrossRef]
- Binder, W.H.; Sachsenhofer, R.; Farnik, D.; Blaas, D. Guiding the location of nanoparticles into vesicular structures: A morphological study. Phys. Chem. Chem. Phys. 2008, 10, 7328. [Google Scholar] [CrossRef]
- Krack, M.; Hohenberg, H.; Kornowski, A.; Lindner, P.; Weller, H.; Forster, S. Nanoparticle-loaded magnetophoretic vesicles. J. Am. Chem. Soc. 2008, 130, 7315–7320. [Google Scholar] [CrossRef]
- Schulz, M.; Olubummo, A.; Binder, W.H. Beyond the lipid-bilayer: Interaction of polymers and nanoparticles with membranes. Soft Matter 2012, 8, 4849–4864. [Google Scholar] [CrossRef]
- Al Harby, N.F.; El-Batouti, M.; Elewa, M.M. Prospects of Polymeric Nanocomposite Membranes for Water Purification and Scalability and their Health and Environmental Impacts: A Review. Nanomaterials 2022, 12, 3637. [Google Scholar] [CrossRef]
- Ren, L.; Ma, J.; Chen, M.; Qiao, Y.; Dai, R.; Li, X.; Wang, Z. Recent advances in electrocatalytic membrane for the removal of micropollutants from water and wastewater. Iscience 2022, 25, 104342. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. A short review of activated carbon assisted electrosorption process: An overview, current stage and future prospects. J. Hazard. Mater. 2009, 170, 552–559. [Google Scholar] [CrossRef]
- Radjenovic, J.; Duinslaeger, N.; Avval, S.S.; Chaplin, B.P. Facing the Challenge of Poly- and Perfluoroalkyl Substances in Water: Is Electrochemical Oxidation the Answer? Environ. Sci. Technol. 2020, 54, 14815–14829. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, F.X.; Hu, X.M.; Hua, T. Electrochemical advanced oxidation processes coupled with membrane filtration for degrading antibiotic residues: A review on its potential applications, advances, and challenges. Sci. Total Environ. 2021, 784, 146912. [Google Scholar] [CrossRef]
- Le, T.X.H.; Haflich, H.; Shah, A.D.; Chaplin, B.P. Energy-Efficient Electrochemical Oxidation of Perfluoroalkyl Substances Using a Ti4O7 Reactive Electrochemical Membrane Anode. Environ. Sci. Technol. Lett. 2019, 6, 504–510. [Google Scholar] [CrossRef]
- Zhou, X.Z.; Liu, S.Q.; Xu, A.L.; Wei, K.J.; Han, W.Q.; Li, J.S.; Sun, X.Y.; Shen, J.Y.; Liu, X.D.; Wang, L.J. A multi-walled carbon nanotube electrode based on porous Graphite-RuO2 in electrochemical filter for pyrrole degradation. Chem. Eng. J. 2017, 330, 956–964. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Direct And Mediated Anodic Oxidation of Organic Pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef]
- Huang, D.H.; Wang, K.X.; Niu, J.F.; Chu, C.H.; Weon, S.; Zhu, Q.H.; Lu, J.J.; Stavitski, E.; Kim, J.H. Amorphous Pd-Loaded Ti4O7 Electrode for Direct Anodic Destruction of Perfluorooctanoic Acid. Environ. Sci. Technol. 2020, 54, 10954–10963. [Google Scholar] [CrossRef]
- Feng, J.R.; Johnson, D.C. Electrocatalysis of anodic oxygen-transfer reactions—Fe-doped beta-lead dioxide electrodeposited on noble-metals. J. Electrochem. Soc. 1990, 137, 507–510. [Google Scholar] [CrossRef]
- Martinez-Huitle, C.A.; Rodrigo, M.A.; Sires, I.; Scialdone, O. Single and Coupled Electrochemical Processes and Reactors for the Abatement of Organic Water Pollutants: A Critical Review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef]
- Jiang, W.L.; Xia, X.; Han, J.L.; Ding, Y.C.; Haider, M.R.; Wang, A.J. Graphene Modified Electro-Fenton Catalytic Membrane for in Situ Degradation of Antibiotic Florfenicol. Environ. Sci. Technol. 2018, 52, 9972–9982. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Y.H.; Li, Y.L.; Hu, Z.X.; Zhou, L.; Zhou, M.H. Three-dimensional electrochemical process for wastewater treatment: A general review. Chem. Eng. J. 2013, 228, 455–467. [Google Scholar] [CrossRef]
- Zheng, J.J.; Ma, J.X.; Wang, Z.W.; Xu, S.P.; Waite, T.D.; Wu, Z.C. Contaminant Removal from Source Waters Using Cathodic Electrochemical Membrane Filtration: Mechanisms and Implications. Environ. Sci. Technol. 2017, 51, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Qu, Y.; Kwon, D.; Zhang, H.; Cid, C.A.; Aryanfar, A.; Hoffmann, M.R. Effects of Anodic Potential and Chloride Ion on Overall Reactivity in Electrochemical Reactors Designed for Solar-Powered Wastewater Treatment. Environ. Sci. Technol. 2014, 48, 2377–2384. [Google Scholar] [CrossRef]
- Martinez-Huitle, C.A.; Panizza, M. Electrochemical oxidation of organic pollutants for wastewater treatment. Curr. Opin. Electrochem. 2018, 11, 62–71. [Google Scholar] [CrossRef]
- Chi, Z.X.; Zhao, J.Y.; Zhang, Y.; Yu, H.; Yu, H.B. Coral-like WO3/BiVO4 photoanode constructed via morphology and facet engineering for antibiotic wastewater detoxification and hydrogen recovery. Chem. Eng. J. 2022, 428, 131817. [Google Scholar] [CrossRef]
- Martinez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Sun, M.; Wang, X.X.; Wang, C.; Lu, D.W.; Ma, W.; Kube, S.A.; Ma, J.; Elimelech, M. Janus electrocatalytic flow-through membrane enables highly selective singlet oxygen production. Nat. Commun. 2020, 11, 6228. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, H.G.; Zhang, Y.L.; Wu, X.; Liu, Y. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res. 2017, 113, 80–88. [Google Scholar] [CrossRef]
- Liu, Z.; Ding, H.J.; Zhao, C.; Wang, T.; Wang, P.; Dionysiou, D.D. Electrochemical activation of peroxymonosulfate with ACF cathode: Kinetics, influencing factors, mechanism, and application potential. Water Res. 2019, 159, 111–121. [Google Scholar] [CrossRef]
- Chaplin, B.P. Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ. Sci. Process. Impacts 2014, 16, 1182–1203. [Google Scholar] [CrossRef]
- Trellu, C.; Chaplin, B.P.; Coetsier, C.; Esmilaire, R.; Cerneaux, S.; Causserand, C.; Cretin, M. Electro-oxidation of organic pollutants by reactive electrochemical membranes. Chemosphere 2018, 208, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, C.; Zhao, X.; Wang, Y.C.; Zhang, W.Q.; Chen, Z.F.; Meng, X.Y.; Luo, J.M.; Crittenden, J. Development of a highly efficient electrochemical flow -through anode based on inner in -site enhanced TiO2-nanotubes array. Environ. Int. 2020, 140, 105813. [Google Scholar] [CrossRef]
- Patil, J.J.; Jana, A.; Getachew, B.A.; Bergsman, D.S.; Gariepy, Z.; Smith, B.D.; Lu, Z.M.; Grossman, J.C. Conductive carbonaceous membranes: Recent progress and future opportunities. J. Mater. Chem. A 2021, 9, 3270–3289. [Google Scholar] [CrossRef]
- Liu, Z.M.; Zhu, M.F.; Wang, Z.; Wang, H.; Deng, C.; Li, K. Novel antimony doped tin oxide/carbon aerogel as efficient electrocatalytic filtration membrane. AIP Adv. 2016, 6, 055015. [Google Scholar] [CrossRef]
- Liu, Z.M.; Zhu, M.F.; Wang, Z.; Wang, H.; Deng, C.; Li, K. Effective Degradation of Aqueous Tetracycline Using a Nano-TiO2/Carbon Electrocatalytic Membrane. Materials 2016, 9, 364. [Google Scholar] [CrossRef]
- Li, X.H.; Shao, S.L.; Yang, Y.; Mei, Y.; Qing, W.H.; Guo, H.; Peng, L.E.; Wang, P.; Tang, C.Y.Y. Engineering Interface with a One-Dimensional RuO2/TiO2 Heteronanostructure in an Electrocatalytic Membrane Electrode: Toward Highly Efficient Micropollutant Decomposition. ACS Appl. Mater. Interfaces 2020, 12, 21596–21604. [Google Scholar] [CrossRef]
- Tan, T.Y.; Zeng, Z.T.; Zeng, G.M.; Gong, J.L.; Xiao, R.; Zhang, P.; Song, B.; Tang, W.W.; Ren, X.Y. Electrochemically enhanced simultaneous degradation of sulfamethoxazole, ciprofloxacin and amoxicillin from aqueous solution by multi-walled carbon nanotube filter. Sep. Purif. Technol. 2020, 235, 116167. [Google Scholar] [CrossRef]
- Yang, S.N.; Liu, Y.B.; Shen, C.S.; Li, F.; Yang, B.; Huang, M.H.; Yang, M.; Wang, Z.W.; Sand, W. Rapid decontamination of tetracycline hydrolysis product using electrochemical CNT filter: Mechanism, impacting factors and pathways. Chemosphere 2020, 244, 125525. [Google Scholar] [CrossRef]
- Bakr, A.R.; Rahaman, M.S. Removal of bisphenol A by electrochemical carbon-nanotube filter: Influential factors and degradation pathway. Chemosphere 2017, 185, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Bahadur, N.; Cretin, M.; Kong, L.X.; O’Dell, L.A.; Merenda, A.; Dumee, L.F. Electro-catalytic membrane reactors for the degradation of organic pollutants—A review. React. Chem. Eng. 2021, 6, 1508–1526. [Google Scholar] [CrossRef]
- Li, Z.Z.; Shen, C.S.; Liu, Y.B.; Ma, C.Y.; Li, F.; Yang, B.; Huang, M.H.; Wang, Z.W.; Dong, L.M.; Wolfgang, S. Carbon nanotube filter functionalized with iron oxychloride for flow-through electro-Fenton. Appl. Catal. B Environ. 2020, 260, 118204. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.Q.; Liu, Z.M.; Deng, C.; Xu, H.M.; Wang, Y.; Zhu, M.F. Carbon nanotube-based electrocatalytic filtration membrane for continuous degradation of flow-through Bisphenol A. Sep. Purif. Technol. 2021, 265, 118503. [Google Scholar] [CrossRef]
- Pan, Z.L.; Yu, F.P.; Li, L.; Song, C.W.; Yang, J.W.; Wang, C.L.; Pan, Y.Q.; Wang, T.H. Electrochemical microfiltration treatment of bisphenol A wastewater using coal-based carbon membrane. Sep. Purif. Technol. 2019, 227, 115695. [Google Scholar] [CrossRef]
- Olvera-Vargas, H.; Rouch, J.C.; Coetsier, C.; Cretin, M.; Causserand, C. Dynamic cross-flow electro-Fenton process coupled to anodic oxidation for wastewater treatment: Application to the degradation of acetaminophen. Sep. Purif. Technol. 2018, 203, 143–151. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, H.; Li, K.L.; Wang, Y.J.; Wang, J.; Zhang, X.Y. Ibuprofen removal from drinking water by electro-peroxone in carbon cloth filter. Chem. Eng. J. 2021, 415, 127618. [Google Scholar] [CrossRef]
- Zhao, W.; Xing, J.T.; Chen, D.H.; Bai, Z.L.; Xia, Y.S. Study on the performance of an improved Ti/SnO2-Sb2O3/PbO2 based on porous titanium substrate compared with planar titanium substrate. RSC Adv. 2015, 5, 26530–26539. [Google Scholar] [CrossRef]
- Wang, Y.P.; Zhou, C.Z.; Wu, J.H.; Niu, J.F. Insights into the electrochemical degradation of sulfamethoxazole and its metabolite by Ti/SnO2-Sb/Er-PbO2 anode. Chin. Chem. Lett. 2020, 31, 2673–2677. [Google Scholar] [CrossRef]
- Li, F.; Duan, J.; Tian, S.T.; Ji, H.D.; Zhu, Y.M.; Wei, Z.S.; Zhao, D.Y. Short-chain per- and polyfluoroalkyl substances in aquatic systems: Occurrence, impacts and treatment. Chem. Eng. J. 2020, 380, 122506. [Google Scholar] [CrossRef]
- Qian, X.B.; Xu, L.; Zhu, Y.Q.; Yu, H.Y.; Niu, J.F. Removal of aqueous triclosan using TiO2 nanotube arrays reactive membrane by sequential adsorption and electrochemical degradation. Chem. Eng. J. 2021, 420, 127615. [Google Scholar] [CrossRef]
- Yang, K.; Xu, J.L.; Lin, H.; Xie, R.Z.; Wang, K.; Lv, S.H.; Liao, J.B.; Liu, X.H.; Chen, J.; Yang, Z.F. Developing a low-pressure and super stable electrochemical tubular reactive filter: Outstanding efficiency for wastewater purification. Electrochim. Acta 2020, 335, 135634. [Google Scholar] [CrossRef]
- Ren, L.H.; Chen, M.; Ma, J.X.; Li, Y.; Wang, Z.W. Pd-O2 interaction and singlet oxygen formation in a novel reactive electrochemical membrane for ultrafast sulfamethoxazole oxidation. Chem. Eng. J. 2022, 428, 131194. [Google Scholar] [CrossRef]
- Liu, S.Q.; Wang, Y.; Zhou, X.Z.; Han, W.Q.; Li, J.S.; Sun, X.Y.; Shen, J.Y.; Wang, L.J. Improved degradation of the aqueous flutriafol using a nanostructure macroporous PbO2 as reactive electrochemical membrane. Electrochim. Acta 2017, 253, 357–367. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Wei, K.J.; Han, W.Q.; Sun, X.Y.; Li, J.S.; Shen, J.Y.; Wang, L.J. Improved electrochemical oxidation of tricyclazole from aqueous solution by enhancing mass transfer in a tubular porous electrode electrocatalytic reactor. Electrochim. Acta 2016, 189, 1–8. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, J.J.; Dai, R.B.; Wu, Z.C.; Wang, Z.W. Preferential removal of 2,4-dichlorophenoxyacetic acid from contaminated waters using an electrocatalytic ceramic membrane filtration system: Mechanisms and implications. Chem. Eng. J. 2020, 387, 124132. [Google Scholar] [CrossRef]
- Zhou, C.Z.; Wang, Y.P.; Chen, J.; Niu, J.F. Porous Ti/SnO2-Sb anode as reactive electrochemical membrane for removing trace antiretroviral drug stavudine from wastewater. Environ. Int. 2019, 133, 105157. [Google Scholar] [CrossRef]
- Xu, L.; Ma, X.; Niu, J.F.; Chen, J.; Zhou, C.Z. Removal of trace naproxen from aqueous solution using a laboratory-scale reactive flow-through membrane electrode. J. Hazard. Mater. 2019, 379, 120692. [Google Scholar] [CrossRef]
- Gayen, P.; Spataro, J.; Avasarala, S.; Ali, A.M.; Cerrato, J.M.; Chaplin, B.P. Electrocatalytic Reduction of Nitrate Using Magneli Phase TiO2 Reactive Electrochemical Membranes Doped with Pd-Based Catalysts. Environ. Sci. Technol. 2018, 52, 370–9379. [Google Scholar] [CrossRef]
- Liang, S.T.; Lin, H.; Yan, X.F.; Huang, Q.G. Electro-oxidation of tetracycline by a Magneli phase Ti4O7 porous anode: Kinetics, products, and toxicity. Chem. Eng. J. 2018, 332, 628–636. [Google Scholar] [CrossRef]
- Qaseem, S.; Dlamini, D.S.; Zikalala, S.A.; Tesha, J.M.; Husain, M.D.; Wang, C.H.; Jiang, Y.M.; Wei, X.; Vilakati, G.D.; Li, J.X. Electro-catalytic membrane anode for dye removal from wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125270. [Google Scholar] [CrossRef]
- Ganzenko, O.; Sistat, P.; Trellu, C.; Bonniol, V.; Rivallin, M.; Cretin, M. Reactive electrochemical membrane for the elimination of carbamazepine in secondary effluent from wastewater treatment plant. Chem. Eng. J. 2021, 419, 129467. [Google Scholar] [CrossRef]
- Trellu, C.; Rivallin, M.; Cerneaux, S.; Coetsier, C.; Causserand, C.; Oturan, M.A.; Cretin, M. Integration of sub-stoichiometric titanium oxide reactive electrochemical membrane as anode in the electro-Fenton process. Chem. Eng. J. 2020, 400, 125936. [Google Scholar] [CrossRef]
- Misal, S.N.; Lin, M.H.; Mehraeen, S.; Chaplin, B.P. Modeling electrochemical oxidation and reduction of sulfamethoxazole using electrocatalytic reactive electrochemical membranes. J. Hazard. Mater. 2020, 384, 121420. [Google Scholar] [CrossRef]
- Wang, J.B.; Zhi, D.; Zhou, H.; He, X.W.; Zhang, D.Y. Evaluating tetracycline degradation pathway and intermediate toxicity during the electrochemical oxidation over a Ti/Ti4O7 anode. Water Res. 2018, 137, 324–334. [Google Scholar] [CrossRef]
- Zhi, D.; Wang, J.B.; Zhou, Y.Y.; Luo, Z.R.; Sun, Y.Q.; Wan, Z.H.; Luo, L.; Tsang, D.C.W.; Dionysiou, D.D. Development of ozonation and reactive electrochemical membrane coupled process: Enhanced tetracycline mineralization and toxicity reduction. Chem. Eng. J. 2020, 383, 123149. [Google Scholar] [CrossRef]
- Gayen, P.; Chen, C.; Abiade, J.T.; Chaplin, B.P. Electrochemical Oxidation of Atrazine and Clothianidin on Bi-doped SnO2-TinO2n−1 Electrocatalytic Reactive Electrochemical Membranes. Environ. Sci. Technol. 2018, 52, 12675–12684. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Niu, J.F.; Liang, S.T.; Wang, C.; Wang, Y.J.; Jin, F.Y.; Luo, Q.; Huang, Q.G. Development of macroporous Magneli phase Ti4O7 ceramic materials: As an efficient anode for mineralization of poly- and perfluoroalkyl substances. Chem. Eng. J. 2018, 354, 1058–1067. [Google Scholar] [CrossRef]
- Shi, H.H.; Wang, Y.Y.; Li, C.G.; Pierce, R.; Gao, S.X.; Huang, Q.G. Degradation of Perfluorooctanesulfonate by Reactive Electrochemical Membrane Composed of Magneli Phase Titanium Suboxide. Environ. Sci. Technol. 2019, 53, 14528–14537. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.M.; Chaplin, B.P. Porous Substoichiometric TiO2 Anodes as Reactive Electrochemical Membranes for Water Treatment. Environ. Sci. Technol. 2013, 47, 6554–6563. [Google Scholar] [CrossRef]
- Pei, S.Z.; Shi, H.; Zhang, J.N.; Wang, S.L.; Ren, N.Q.; You, S.J. Electrochemical removal of tetrabromobisphenol A by fluorine-doped titanium suboxide electrochemically reactive membrane. J. Hazard. Mater. 2021, 419, 126434. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiao, R.L.; Lin, H.; Yang, K.; He, K.C.; Yang, L.H.; Pu, M.J.; Li, M.Y.; Lv, S.H. Electro-activation of peroxymonosulfate by a graphene oxide/iron oxide nanoparticle-doped Ti4O7 ceramic membrane: Mechanism of singlet oxygen generation in the removal of 1,4-dioxane. J. Hazard. Mater. 2022, 424, 127342. [Google Scholar] [CrossRef]
- Fu, W.C.; Wang, X.Y.; Zheng, J.J.; Liu, M.X.; Wang, Z.W. Antifouling performance and mechanisms in an electrochemical ceramic membrane reactor for wastewater treatment. J. Membr. Sci. 2019, 570, 355–361. [Google Scholar] [CrossRef]
- Zheng, J.J.; Wang, Z.W.; Ma, J.X.; Xu, S.P.; Wu, Z.C. Development of an Electrochemical Ceramic Membrane Filtration System for Efficient Contaminant Removal from Waters. Environ. Sci. Technol. 2018, 52, 4117–4126. [Google Scholar] [CrossRef]
- Xu, S.P.; Zheng, J.J.; Wu, Z.C.; Liu, M.X.; Wang, Z.W. Degradation of p-chloroaniline using an electrochemical ceramic microfiltration membrane with built-in electrodes. Electrochim. Acta 2018, 292, 655–666. [Google Scholar] [CrossRef]
- Zheng, J.J.; Xu, S.P.; Wu, Z.C.; Wang, Z.W. Removal of p-chloroaniline from polluted waters using a cathodic electrochemical ceramic membrane reactor. Sep. Purif. Technol. 2019, 211, 753–763. [Google Scholar] [CrossRef]
- Ahmed, F.; Lalia, B.S.; Kochkodan, V.; Hilal, N.; Hashaikeh, R. Electrically conductive polymeric membranes for fouling prevention and detection: A review. Desalination 2016, 391, 1–15. [Google Scholar] [CrossRef]
- Liu, M.L.; Li, L.; Sun, Y.X.; Fu, Z.J.; Cao, X.L.; Sun, S.P. Scalable conductive polymer membranes for ultrafast organic pollutants removal. J. Membr. Sci. 2021, 617, 118644. [Google Scholar] [CrossRef]
- Chen, M.; Xu, J.; Dai, R.B.; Wu, Z.C.; Liu, M.X.; Wang, Z.W. Development of a moving-bed electrochemical membrane bioreactor to enhance removal of low-concentration antibiotic from wastewater. Bioresour. Technol. 2019, 293, 122022. [Google Scholar] [CrossRef]
- Sun, J.C.; Wang, Q.Y.; Zhang, J.; Wang, Z.W.; Wu, Z.C. Degradation of sulfadiazine in drinking water by a cathodic electrochemical membrane filtration process. Electrochim. Acta 2018, 277, 77–87. [Google Scholar] [CrossRef]





| Membrane Fabrication Techniques | Advantages | Disadvantages |
|---|---|---|
| Phase inversion |
|
|
| Electrospinning |
|
|
| Sintering |
|
|
| Stretching |
|
|
| Track-etching |
|
|
| Template leaching |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, I.; Elseman, A.M.; Sadek, H.; Eliwa, E.M.; Abusaif, M.S.; Kyriakos, P.; Belessiotis, G.V.; Mudgal, M.M.; Abdelbasir, S.M.; Elsayed, M.H.; et al. Membrane-Based Photocatalytic and Electrocatalytic Systems: A Review. Catalysts 2025, 15, 528. https://doi.org/10.3390/catal15060528
Ibrahim I, Elseman AM, Sadek H, Eliwa EM, Abusaif MS, Kyriakos P, Belessiotis GV, Mudgal MM, Abdelbasir SM, Elsayed MH, et al. Membrane-Based Photocatalytic and Electrocatalytic Systems: A Review. Catalysts. 2025; 15(6):528. https://doi.org/10.3390/catal15060528
Chicago/Turabian StyleIbrahim, Islam, Ahmed Mourtada Elseman, Hassan Sadek, Essam M. Eliwa, Moustafa S. Abusaif, Periklis Kyriakos, George V. Belessiotis, Mukesh Madan Mudgal, Sabah M. Abdelbasir, Mohamed Hammad Elsayed, and et al. 2025. "Membrane-Based Photocatalytic and Electrocatalytic Systems: A Review" Catalysts 15, no. 6: 528. https://doi.org/10.3390/catal15060528
APA StyleIbrahim, I., Elseman, A. M., Sadek, H., Eliwa, E. M., Abusaif, M. S., Kyriakos, P., Belessiotis, G. V., Mudgal, M. M., Abdelbasir, S. M., Elsayed, M. H., Mohamed, G. G., & Salama, T. M. (2025). Membrane-Based Photocatalytic and Electrocatalytic Systems: A Review. Catalysts, 15(6), 528. https://doi.org/10.3390/catal15060528








