Abstract
The sustainable valorization of lignocellulosic biomass into high-value platform chemicals presents a crucial pathway for reducing reliance on fossil resources. Gamma (γ)-valerolactone (GVL) has gained recognition as a versatile bio-derived compound with broad applications in renewable energy systems and green chemical synthesis. While conventional GVL production strategies from carbohydrate biomass typically depend on noble metal catalysts paired with high-pressure hydrogen gas, these approaches face substantial technical barriers including catalyst costs, hydrogen storage requirements, and operational safety concerns in large-scale applications. This work develops an innovative catalytic system utilizing earth-abundant iron for in situ hydrogen generation through water splitting, integrated with Raney Ni as the hydrogenation catalyst. The designed two-stage process enables direct conversion of cellulose—first through acid hydrolysis to levulinic acid (LA) followed by catalytic hydrogenation to GVL without intermediate purification. Through systematic parameter optimization, a remarkable 61.9% overall GVL yield from cellulose feedstock was achieved. Furthermore, the methodology’s versatility was demonstrated through wheat straw conversion experiments, yielding 24.6% GVL. This integrated methodology explores a technically feasible pathway for direct cellulose-to-GVL conversion utilizing abundant water as the hydrogen source, effectively overcoming the critical limitations associated with conventional hydrogenation technologies regarding hydrogen infrastructure and process safety.
1. Introduction
The overexploitation of fossil resources and heavy reliance on them have not only triggered energy depletion crises but also precipitated severe environmental degradation, including but not limited to global warming and acid rain deposition. A promising mitigation strategy involves developing utilization of biomass, which is the Earth’s most abundant renewable carbon source. However, biomass possesses relatively low energy density and significant geographical constraints have hindered its large-scale application. Therefore, to truly realize the potential of biomass as a substitute for fossil fuels, it is crucial to develop efficient biomass utilization technologies that can convert this low-energy-density resource into clean, value-added products, such as key platform compounds or biofuels [1,2,3].
Cost-effective catalytic strategies have been systematically developed to accommodate the structural complexity of highly functionalized biomass derivatives, aiming to render the resultant chemicals competitive in both quality and market price compared to petroleum-based analogues [4,5,6]. Over the past decade, γ-valerolactone (GVL) has emerged as a preeminent biomass-derived platform chemical, distinguished by its unique physicochemical profile: low melting point (−31 °C), elevated boiling temperature (207 °C), and favorable open-cup flash point (96 °C) [7]. These thermophysical characteristics, combined with its biodegradable nature and non-toxic properties, facilitate its global logistics in bulk quantities while ensuring storage safety. The versatility of GVL extends beyond its conventional roles as a food-grade additive and green aprotic solvent [8,9]. Recent advancements have demonstrated that GVL can be employed as an octane-enhancing fuel additive with superior oxygen content (32 wt%) [10,11], and a reactive intermediate for synthesizing value-added derivatives including linear alkanes [12], valeric esters [13,14], and dimethyl adipate [15] through cascade catalytic processes. Much attention focusses on direct acid-catalyzed conversion of C6 carbohydrates (e.g., glucose, fructose) and their derivatives (notably levulinic acid, LA) to GVL, where the synergy between Brønsted acid sites and hydrogenation catalysts significantly improves conversion rate and process economics [16,17,18].
Current GVL production methods predominantly utilize two hydrogenation approaches differentiated by hydrogen source: exogenous gaseous hydrogen versus liquid-phase hydrogen donors. The conventional gaseous hydrogen route requires high-pressure H2 injection into sealed reactor vessels, entailing substantial safety risks associated with hydrogen storage, transportation, and high-pressure operations. This methodology necessitates noble metal catalysts (e.g., [Ru(CO)3I3]−, CuAg/Al2O3, Ru/C) to activate molecular hydrogen, as evidenced by prior studies [19,20,21,22]. However, developing cost-effective catalysts with sustained activity remains a critical challenge for practical applications. In contrast, alternative liquid-phase systems employ organic hydrogen donors such as formic acid or alcohols to circumvent gaseous H2 requirements [23]. Heeres [24] obtained the highest GVL yield of 52% from D-fructose using formic acid as a hydrogen donor and Bo [25] achieved a 97% yield of GVL from ethyl levulinate using 2-butanol as a hydrogen donor. Although both formic acid and 2-butanol as hydrogen sources can be derived from biomass, their application as hydrogen donors in hydrogenation reactions necessitates precise control of catalytic conditions, which inevitably complicates large-scale implementation.
Water emerges as a superior sustainable hydrogen donor compared to conventional organic sources, providing three distinct merits: (1) global abundance and environmental benignity; (2) facile hydrogen liberation via spontaneous water splitting by Fe; (3) enhanced hydrogenation efficiency from in situ generated atomic hydrogen exhibiting greater reactivity than molecular H2 [26,27,28]. These inherent properties position water as a potential hydrogen carrier for green GVL synthesis.
We reported the hydrogenation of LA to GVL via zinc-induced water splitting, achieving a 56% GVL yield without adding external catalysts. The results demonstrate that in situ hydrogen generation through metal-enabled water splitting can effectively facilitate the LA-to-GVL conversion [29]. This work extends the strategy toward practical biorefinery integration. Whereas our previous efforts focused on purified LA feedstocks, the direct conversion of native cellulose, which is the predominant constituent of lignocellulosic biomass, to GVL without intermediate isolation represents a critical advancement for industrial feasibility [30]. While existing cellulose-to-GVL systems [31,32] invariably depend on pressurized H2 and noble metal catalysts, our approach implements two key technological innovations (Figure 1): (1) replacement of zinc with economically favorable iron powder for enhancing water-splitting sustainability; (2) deployment of commercial Raney Ni as a robust heterogeneous catalyst for in situ hydrogenation of cellulose-derived LA. Notably, the iron is converted into iron oxides during water splitting for hydrogen production and can be reduced back to metallic iron via biomass-derived reductants such as glycerol, enabling an iron—iron oxide chemical looping [26,33]. This integrated one-pot system successfully couples acid-catalyzed cellulose depolymerization with metal-induced hydrogenation, effectively eliminating energy-intensive separation steps in conventional biomass processing.
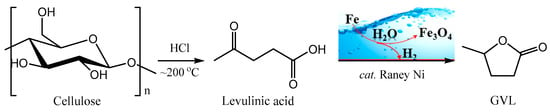
Figure 1.
Synthesis of GVL from cellulose by a two-step approach in this study.
2. Results and Discussion
2.1. Conversion of Cellulose to GVL by One-Step Process
A preliminary feasibility assessment was conducted to evaluate the single-step conversion of microcrystalline cellulose to GVL via an integrated acid hydrolysis–hydrogenation process under mild hydrothermal conditions (200 °C, 1 h). Intriguingly, HPLC analysis (Figure S1a) revealed substantial accumulation of 5-hydroxymethylfurfural (HMF) and organic acids (predominantly LA and formic acid) as primary liquid-phase products. Complementary GC-MS characterization (Figure S1b) confirmed the absence of GVL in the product spectrum, indicating incomplete cascade conversion. This observation aligns with established reaction pathways [34], wherein cellulose-derived glucose undergoes Brønsted acid-catalyzed dehydration to HMF, followed by HMF fragmentation into LA and formic acid.
Critically, the complete lack of GVL formation suggests thermodynamic or kinetic limitations in the hydrogenation step. The reason for no GVL formation in one-step conversion is probably due to preferential HCl-Fe redox interactions (i.e., 2Fe + 6HCl = 2FeCl3 + 3H2 ↑) over cellulose depolymerization. This competitive metal–acid reaction likely depletes proton availability for sustained cellulose hydrolysis and compromises hydrogen generation efficiency, thereby arresting the cascade process at the LA intermediate stage.
2.2. Conversion of Cellulose to GVL by Two-Step Process
2.2.1. Hydrolysis of Cellulose to LA
A sequential two-stage strategy was employed to achieve GVL production from cellulose through (i) acid-catalyzed hydrolysis to LA, followed by (ii) hydrogenation using Raney Ni catalyst with H2 generated via water splitting by Fe. Systematic optimization of reaction parameters for cellulose hydrolysis was first conducted to maximize LA yield. Post-hydrolysis characterization of liquid products by HPLC (Figure S2) and GC-MS (Figure S3) confirmed the formation of LA along with byproducts including HMF, formic acid, and volatile fatty acids.
Time-dependent studies at 200 °C (Figure 2a) revealed that LA yield increased from 30.8% to 45.1% as reaction duration was extended from 60 to 90 min. However, prolonging exposure to 120 min resulted in decreased LA yield (42.5%), indicating LA decomposition under extended hydrothermal conditions [32,34]. Elevated temperature experiments at 250 °C demonstrated comparable yields (44.4% at 60 min; 48% at 90 min), though temporal extension similarly proved detrimental to LA accumulation. Considering both process efficiency and energy consumption, optimal hydrolysis conditions were established at 200 °C for 90 min.
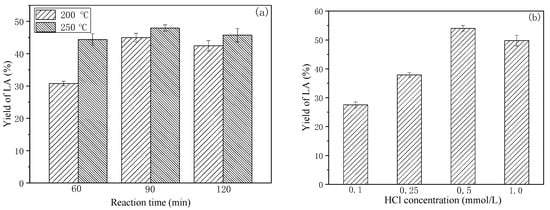
Figure 2.
Effect of reaction temperature and time (a), concentration of HCl (b) on the yield of LA ((a) [H+] 0.5 mmol/L; (b) 200 °C, 90 min).
Subsequent investigation of acid concentration effects (0.1–1.0 mmol/L HCl) identified 0.5 mmol/L as optimal, achieving 54.0% LA yield. The observed inverse correlation between HCl concentration (>0.5 mmol/L) and LA production likely stems from competitive humin formation pathways [32]. This acid-mediated side reaction provides a mechanistic explanation for the counterproductive effects of excessive proton concentrations.
2.2.2. Hydrogenation of LA to GVL Without Additional Catalyst
Preliminary evaluation of cellulose hydrolysate hydrogenation in an exogenous catalyst-free environment revealed the system’s inherent catalytic capacity. Chromatographic analysis (Figure S4) demonstrated distinct GVL formation alongside residual LA and formic acid, confirming the feasibility of in situ H2 utilization from water splitting without exogenous catalysts. Solid-phase product analysis via XRD (Figure S5) identified metallic iron and magnetite as dominant species, suggesting partial iron oxidation during hydrothermal processing.
A dose-dependent relationship between metallic iron loading (5–25 mmol) and GVL yield was established (Figure 3). Maximum GVL production (13.6%) occurred at 25 mmol Fe (200 °C, 4 h). More Fe facilitating the GVL production could be attributed to the enhancement of hydrogen production by Fe reduction of water. The generated hydrogen participates in LA hydrogenation possibly through the proton-coupled electron transfer processes.
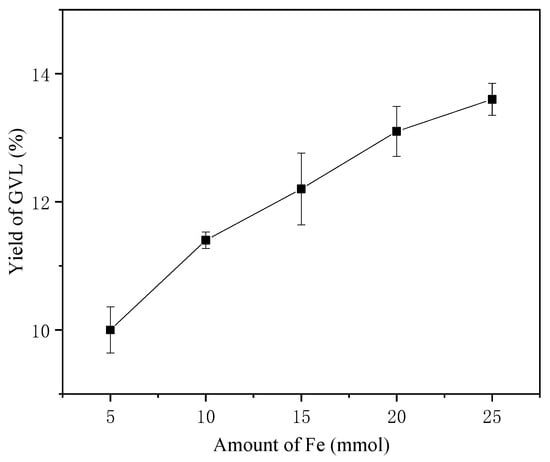
Figure 3.
Effect of Fe amount on the yield of GVL (200 °C, 4 h).
2.2.3. Hydrogenation of LA to GVL with Catalyst
Systematic catalyst screening was performed to enhance GVL productivity through optimized hydrogenation processes. Commonly used metal catalysts (Cu, Ni, Co, Mo, Sn, Cr) powder were comparatively evaluated using H2 formed in situ via water splitting. Post-reaction XRD analysis (Figure S6) confirmed the structural integrity of supplemental metals, with all additives retaining metallic phases except iron species that transformed into Fe3O4 through oxidation.
Catalytic performance assessment (Figure 4) identified the Ni catalyst as exhibiting exceptional LA-to-GVL conversion efficiency, achieving a peak GVL yield of 16.1%—representing a marked 41.2% enhancement relative to non-catalyzed conditions (11.4% yield). This pronounced Ni effect aligns with its known hydrogen spillover capacity and optimal d-band electronic structure for carbonyl group activation. Thus, porous Ni which possessed higher surface area was selected as the hydrogenation catalyst in the following study.
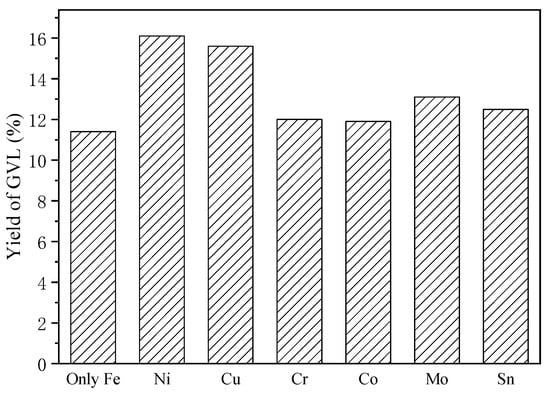
Figure 4.
Screening of catalysts for GVL production (Fe 10 mmol, cat. 30 mmol, 200 °C, 4 h).
To obtain the optimal conditions of hydrogenation of LA with Raney Ni, the effect of Fe amount on the yield of GVL was initially studied. As shown in Figure 5a, the increase of Fe dosage from 5 to 25 mmol led to the remarkable growth of the GVL yield from 38.9% to 55.7% with 3 mmol Raney Ni at 200 °C. This represents a 4.3-fold enhancement in GVL yield compared to the system without added catalyst (Figure 3, 13.1% yield). The GVL yield rose slowly with levels of Fe above 15 mmol; GVL increased by 1.4% with 25 mmol Fe over the level at 20 mmol Fe, suggesting hydrogen saturation at active sites. Thus, 20 mmol Fe was selected for the following investigation.
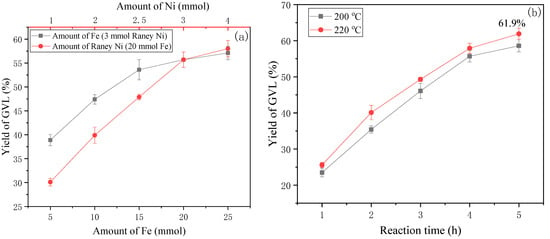
Figure 5.
Effect of amounts of Fe and Raney Ni (a), reaction time and temperature (b) on the yields of GVL ((a) 200 °C, 4 h; (b) 3 mmol Raney Ni, 20 mmol Fe).
Subsequent Ni loading studies mirrored this threshold behavior: GVL yield surged from 30.1% (1 mmol Ni) to 55.7% (3 mmol Ni), followed by marginal enhancement to 58.0% at 4 mmol Ni. Economic evaluation justified 3 mmol Ni as optimal, balancing catalytic performance and resource utilization. For the effect of reaction temperature and time, as shown in Figure 5b, the change of GVL yield with the increase of reaction time showed rapid growth followed by slow increase, with little change discernible between 200 °C and 220 °C. The GVL yield at 220 °C was slightly higher than that at 200 °C and a 61.9% yield was reached for 5 h. Interestingly, GVL yields were universally slightly higher than LA yields, indicating the kinetic competition between precursor generation and consumption.
Finally, the reusability of the Raney Ni catalyst was investigated through consecutive recycling experiments (Figure 6). Cyclic performance analysis revealed an initial activity reduction (4.8% yield) over two cycles, followed by stabilized catalytic behavior with less than 2% performance fluctuation in subsequent cycles. This stabilization phenomenon may arise from surface reconstruction eliminating metastable active sites while preserving the catalyst’s core nickel crystallites.
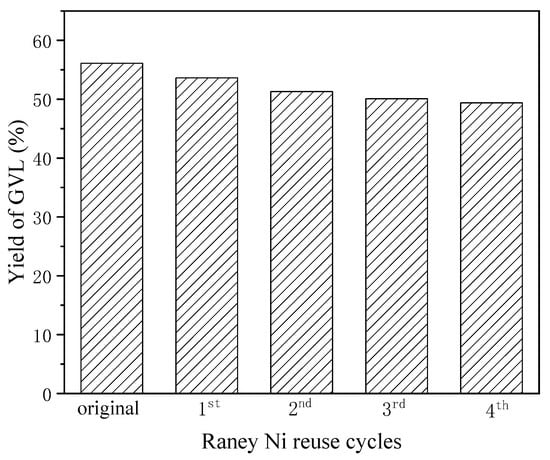
Figure 6.
Effect of reusing Raney Ni on the yield of GVL (200 °C, 4 h, 20 mmol Fe, 3 mmol Raney Ni).
2.3. Proposed Pathway of GVL Synthesis from Cellulose
Based on the experimental results and the previous study [29] on GVL synthesis, a possible pathway of production of GVL from cellulose using hydrogen from water splitting by Fe was proposed, as illustrated in Figure 7. The cascade reaction initiates with acid-catalyzed cellulose depolymerization generating glucose intermediates, which undergo successive dehydration to HMF and rehydration to LA under hydrothermal conditions. Parallel iron oxidation drives proton-coupled electron transfer through the water-splitting reaction: The in situ formed hydrogen was adsorbed and activated on the surface of Raney Ni. Meanwhile, the keto carbonyl group of LA was adsorbed on the Ni surface. Then, the activated hydrogen attacked the carbon of the keto carbonyl of LA, leading to the reduction of the keto carbonyl group. Finally, the formed O- in the keto carbonyl attacked the carbon in the carboxyl, resulting in hydroxyl and GVL formation with H2O.
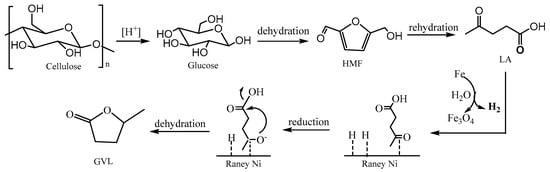
Figure 7.
Proposed pathway of GVL synthesis from cellulose using water as hydrogen source.
2.4. Conversion of Wheat Straw to GVL by Two-Step Process
Straw, a cellulose-rich biomass, has attracted sustained attention for its resource valorization. To validate the applicability of the proposed one-pot GVL production method, wheat straw conversion experiments were conducted.
Initially, the optimization of LA production via straw acid hydrolysis was investigated. As shown in Figure 8, the yields of LA and FA at 250 °C under varying acid concentrations (0.1, 0.5, and 1 mmol/L) exhibited an initial increase followed by a decline with prolonged reaction time. This trend was consistent with the cellulose hydrolysis pattern for LA production (Figure 2). The maximum LA yield of 23.9% was achieved at 90 min with 1 mmol/L HCl. Furthermore, the effect of reaction temperature on LA yield was investigated under 1 mmol/L acid concentration (Figure 8d). Results indicated that both LA and FA yields initially increased and subsequently decreased with rising temperature, likely due to thermal decomposition of LA and FA at excessive temperatures. Consequently, the optimized conditions for LA production were determined as 240 °C, 90 min, and 1 mmol/L acid concentration, achieving an LA yield of 24.1%. HPLC analysis (Figure S7) revealed that the liquid-phase products from straw acid hydrolysis contained oxalic acid, acetic acid, propionic acid, and acrylic acid, in addition to LA and FA. This observation is consistent with previous findings in biomass conversion studies [23,34].
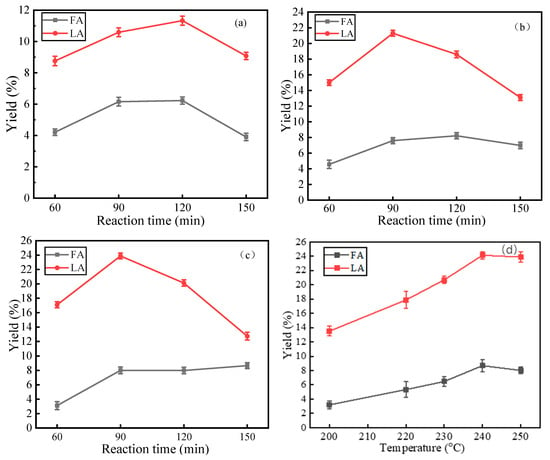
Figure 8.
Effect of reaction time, concentration of HCl and reaction temperature on the yield of LA and FA. ((a) 0.1 mmol/L, (b) 0.5 mmol/L, (c) 1 mmol/L, (d) 1 mmol/L).
Subsequently, the effects of Fe and Ni loading on GVL yield during LA hydrogenation were investigated. As illustrated in Figure 9, a low Fe dosage (<10 mmol) was unfavorable for LA-to-GVL conversion, which aligned with the GVL production trend observed in cellulose-derived systems. The GVL yield gradually increased with higher Fe loading, reaching 22.5% at 25 mmol Fe. According to the proposed cellulose conversion pathway (Figure 7), increased catalyst dosage could provide more reactive sites to enhance hydrogenation efficiency. For hydrogenation of LA to GVL, the influence of Raney Ni loading was further examined (Figure 9b). The addition of Raney Ni significantly promoted GVL generation during the reduction step. Specifically, the GVL yield exhibited a progressive increase with elevated Raney Ni loading. However, when the catalyst dosage exceeded 0.25 g, the GVL yield showed negligible improvement, suggesting that the catalytic sites on the catalyst surface were sufficiently available to meet the reaction demands at this threshold.
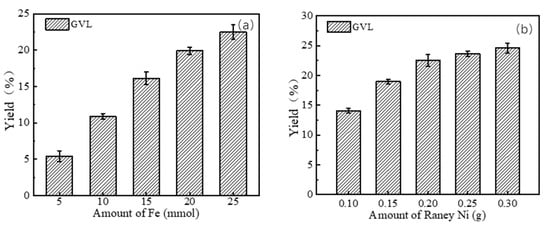
Figure 9.
Effect of amount of Fe ((a) Raney Ni: 0.2 g; 240 °C; 6 h) and Raney Ni ((b) Fe: 25 mmol; 240 °C; 6 h) on the yield of GVL from straw.
Finally, the effects of reaction temperature and time on two-step GVL production from straw were investigated. As demonstrated in Figure 10, the GVL yield exhibited an initial increase followed by a decline with prolonged reaction time across all tested temperatures. Notably, the maximum GVL yields were achieved at 5 h (250 °C), 6 h (240 °C), and 7 h (220 °C), respectively, indicating that higher temperatures favor accelerated GVL formation. Under the optimized conditions (0.25 g Raney Ni, 25 mmol Fe, 240 °C, 6 h), the GVL yield reached 24.6% from straw, demonstrating the feasibility of the proposed two-step strategy.
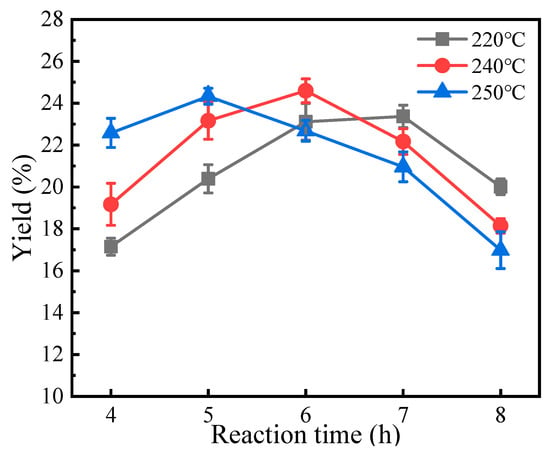
Figure 10.
Effect of reaction temperature and time on the yield of GVL (Raney Ni: 0.2 g; 240 °C; 6 h).
3. Experimental Section
3.1. Materials
Formic acid (FA, ≥96%, Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China), levulinic acid (99.8%, Sigma-Aldrich (Shanghai) Trading Co., Ltd., Shanghai, China), HCl (36–38%, Sino-pharm Chemical Reagent Co., Ltd.), α-cellulose (powder, Sigma-Aldrich) and γ-valerolactone (98%, Sino-pharm Chemical Reagent Co., Ltd.) were used in this study without further purification. The particle size of Raney Ni ordered from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) was 100-mesh, with a specific surface area of 56 m2/g. Other metal powders (Fe, Ni, Cu, Cr, Mo, Co, Sn) were 200-mesh and purchased from Sino-pharm Chemical Reagent Co., Ltd. (Shanghai, China). Wheat straw was sourced from Zhengzhou, Henan Province, China. After drying, the straw material was ball-milled and sieved through a 100-mesh stainless steel sieve.
3.2. Experimental Procedure
All experiments were conducted in a Teflon-lined stainless-steel batch reactor with an inner volume of 28 mL. When conducting reactions in the oven, the vessel can rotate around its central axis at 1 r/s to enhance reactant mixing. The integrated process comprised three consecutive stages: (1) acid-catalyzed cellulose depolymerization to monosaccharides and subsequent LA formation; (2) in situ hydrogen generation via Fe–water redox reaction; (3) catalytic hydrogenation of LA to GVL. According to our previous study on the conversion of glucose to GVL, HCl is a suitable inorganic acid for acidic hydrolysis of carbohydrate biomass. A typical experimental procedure is as follows. First, 1 mmol cellulose and HCl (0.1~1 mmol/L) were put into the reactor and heated in the oven to a set temperature (200~250 °C) with water (~35% filling rate) for hydrolysis. Second, the reactor was cooled to room temperature naturally, after which Fe (5~25 mmol) and Ni (1~4 mmol) powder were added to the reactor for the subsequent reaction. Then, the reactor was heated for 1~5 h for hydrogenation. Finally, the reactor was taken out of the oven and cooled naturally again. The liquid products were collected and filtered with a 0.22 μm filter membrane. The collected solid samples were washed once with deionized water and then twice with absolute ethanol to remove impurities, and dried at 40 °C for 6 h in the vacuum oven. The catalyst reuse experiments were conducted by enclosing the catalyst with an SUS316 mesh for separation of Fe powder. The reaction time was defined as the time that the reactor was placed in the oven. The yield of GVL was defined as the ratio of moles of GVL in the product to moles of glucose (unit of cellulose) in the feedstock input (Equation (1)). Reported values represent mean yields from duplicate experiments with relative standard deviations <5%.
3.3. Analysis Methods
Liquid samples were analyzed by high performance liquid chromatography (HPLC, Agilent 1200 LC, Agilent Technologies (China) Inc., Beijing, China) equipped with a UV detector and two series Shodex KC-811 columns. The mobile phase was 2 mmol/L HClO4 and the flow rate was 1 mL/min. The gas chromatography-mass spectroscopy (GC-MS) analysis was conducted on an Agilent 7890A GC system equipped with a 5975C inert MSD. The samples were separated by a HP-INNOWax capillary column with dimensions of 30 m × 250 μm × 0.25 μm and carried by helium gas. Details on the test conditions of HPLC and GC-MS were provided elsewhere [23]. The solid samples were analyzed by X-ray diffraction (XRD, Shimadzu X-ray Diffractometer 6100, Shimadzu Enterprise Management (China) Co., Ltd, Beijing, China) using Cu Kα radiation to determine the solid phase compositions, employing a scanning rate of 2°/min and 2θ angle in the range of 10° to 80°. The accelerating voltage was set at 40 KV with 30 mA flux. Diffraction patterns were compared with reference data in the ICDD PDF-2 database.
4. Conclusions
This work develops an effective catalytic system enabling direct cellulose-to-GVL conversion in a single reactor through acidic hydrolysis and in situ hydrogenation without intermediate separation. The integrated process features iron-driven hydrogen evolution via water splitting, while a Raney Ni catalyst facilitates selective LA hydrogenation to GVL. Under optimized conditions (220 °C, 5 h), a remarkable 61.9% GVL yield was achieved. Furthermore, the methodology’s versatility was demonstrated through wheat straw conversion experiments, yielding 24.6% GVL (240 °C, 6 h). Critically, this methodology explores an efficient biorefinery method that circumvents hazardous gaseous H2 operation and utilizes Earth-abundant water as hydrogen donor via two-stage cascade reactions in one pot. The demonstrated system holds promise for scalable valorization of lignocellulosic waste, providing a technically robust and economically competitive route toward sustainable production of drop-in biofuels and biobased platform chemicals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15060530/s1, Figure S1: HPLC (a) and GC-MS (b) chromatographs of liquid samples by one-step reaction ([H+]: 0.5 mmol/L, Fe: 10 mmol, Raney Ni: 3 mmol, 200 °C, 1 h); Figure S2: HPLC chromatograph of the liquid samples of cellulose hydrolysis (200 ℃, [H+]: 0.5 mmol/L, (a) 60 min, (b) 90 min); Figure S3: GC-MS chromatograph of the liquid samples of cellulose hydrolysis (200 ℃, [H+]: 0.5 mmol/L, (a) 60 min, (b) 90 min); Figure S4: HPLC (a) and GC-MS (b) chromatograph of liquid samples after two-step process (200 °C, 4 h); Figure S5: XRD patterns of solid sample (Fe: 10 mmol, 200 °C, 4 h); Figure S6: XRD patterns of solid samples (200 °C, 4 h, Fe: 10 mmol, cat. 30 mmol); Figure S7: HPLC chromatograph of the liquid samples of wheat straw hydrolysis (240 ℃, [H+]: 1 mmol/L, 90 min).
Author Contributions
Conceptualization, G.Y.; Methodology, B.J. and G.Y.; Validation, Y.G. and Z.M.; Formal analysis, L.M.; Investigation, Y.G., Z.M., B.J. and G.Y.; Resources, L.M.; Writing—original draft, Y.G.; Writing—review and editing, B.J. and G.Y.; Supervision, G.Y.; Funding acquisition, G.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Jiaxing Municipal Bureau of Science and Technology (Jiaxing Public Welfare Research Project, 2024AY110039 & 2023AY11050) and Ministry of Science and Technology of the People’s Republic of China (National Key R&D Project of China, 2018YFC1902103).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, N.; Yan, K.; Rukkijakan, T.; Liang, J.; Liu, Y.; Wang, Z.; Nie, H.; Muangmeesri, S.; Castiella-Ona, G.; Pan, X.; et al. Selective lignin arylation for biomass fractionation and benign bisphenols. Nature 2024, 630, 381. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, J.; Shan, R.; Yuan, H.; Chen, Y. Advances in thermochemical valorization of biomass towards carbon neutrality. Resour. Conserv. Recycl. 2025, 212, 107905. [Google Scholar] [CrossRef]
- Zhang, B.; Biswal, B.K.; Zhang, J.; Balasubramanian, R. Hydrothermal Treatment of Biomass Feedstocks for Sustainable Production of Chemicals, Fuels, and Materials: Progress and Perspectives. Chem. Rev. 2023, 123, 7193–7294. [Google Scholar] [CrossRef]
- Cormos, C.-C. Green hydrogen production from decarbonized biomass gasification: An integrated techno-economic and environmental analysis. Energy 2023, 270, 126926. [Google Scholar] [CrossRef]
- Wang, F.; Ouyang, D.; Zhou, Z.; Page, S.J.; Liu, D.; Zhao, X. Lignocellulosic biomass as sustainable feedstock and materials for power generation and energy storage. J. Energy Chem. 2021, 57, 247–280. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fabos, V.; Boda, L.; Mika, L.T. gamma-Valerolactone - a sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Chen, J.; Chen, L. Hydrogenation of levulinic acid into γ-valerolactone over ruthenium catalysts supported on metal–organic frameworks in aqueous medium. Catal. Lett. 2016, 146, 2041–2052. [Google Scholar] [CrossRef]
- Chia, M.; Dumesic, J.A. Liquid-phase catalytic transfer hydrogenation and cyclization of levulinic acid and its esters to gamma-valerolactone over metal oxide catalysts. Chem. Commun. 2011, 47, 12233–12235. [Google Scholar] [CrossRef]
- Sen, S.M.; Henao, C.A.; Braden, D.J.; Dumesic, J.A.; Maravelias, C.T. Catalytic conversion of lignocellulosic biomass to fuels: Process development and technoeconomic evaluation. Chem. Eng. Sci. 2012, 67, 57–67. [Google Scholar]
- Sun, W.; Li, H.; Wang, X.; Liu, A. Cascade upgrading of biomass-derived furfural to γ-valerolactone over Zr/Hf-based catalysts. Front. Chem. 2022, 10, 863674. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Zhang, X.; Liu, J.; Zhang, Q.; Ma, L. Catalytic production of long-chain hydrocarbons suitable for aviation turbine fuel from biomass-derived levulinic acid and furfural. Fuel 2023, 334, 126665. [Google Scholar] [CrossRef]
- Figueredo, K.G.M.; Martinez, F.A.; Segobia, D.J.; Bertero, N.M. Valeric Biofuels from Biomass-Derived γ-Valerolactone: A Critical Overview of Production Processes. Chempluschem 2023, 88, e202300381. [Google Scholar] [CrossRef]
- Pothu, R.; Gundeboyina, R.; Boddula, R.; Perugopu, V.; Ma, J. Recent advances in biomass-derived platform chemicals to valeric acid synthesis. New J. Chem. 2022, 46, 5907–5921. [Google Scholar] [CrossRef]
- Marckwordt, A.; El Ouahabi, F.; Amani, H.; Tin, S.; Kalevaru, N.V.; Kamer, P.C.J.; Wohlrab, S.; de Vries, J.G. Nylon intermediates from bio-based levulinic acid. Angew. Chem.-Int. Ed. 2019, 58, 3486–3490. [Google Scholar] [CrossRef]
- Lang, M.; Li, H. Sustainable routes for the synthesis of renewable adipic acid from biomass derivatives. Chemsuschem 2022, 15, e202101531. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Farooq, U.; Bary, G.; Azim, M.M.; Zhao, X. Sustainable production of levulinic acid and its derivatives for fuel additives and chemicals: Progress, challenges, and prospects. Green Chem. 2021, 23, 9198–9238. [Google Scholar] [CrossRef]
- Chu, D.; Ma, J.; Liu, Q.; Fu, J.; Yin, H. Effects of zeolite porosity and acidity on catalytic conversion of carbohydrates to bio-based chemicals: A review. Catal. Sci. Technol. 2024, 14, 6980–7001. [Google Scholar] [CrossRef]
- Braca, G.; Galletti, A.R.; Sbrana, G. Anionic ruthenium iodocarbonyl complexes as selective dehydroxylation catalysts in aqueous solution. J. Organomet. Chem. 1991, 417, 41–49. [Google Scholar] [CrossRef]
- Deng, L.; Li, J.; Lai, D.; Fu, Y.; Guo, Q.X. Catalytic conversion of biomass-derived carbohydrates into gamma-valerolactone without using an external H2 supply. Angew. Chem. Int. Ed. 2009, 48, 6529–6532. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, J.M.; Hwang, D.W.; Halligudi, S.B.; Hwang, Y.K.; Chang, J.-S. Selective hydrogenation of levulinic acid to γ-valerolactone over carbon-supported noble metal catalysts. J. Ind. Eng. Chem. 2011, 17, 287–292. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, J.B.; Li, S.M.; Yin, J.M.; Sun, X.D.; Guo, X.W.; Song, C.S.; Zhou, J.X. Hydrogenation of levulinic acid into γ-valerolactone over in situ reduced CuAg bimetallic catalyst: Strategy and mechanism of preventing Cu leaching. Appl. Catal. B Environ. 2018, 232, 1–10. [Google Scholar] [CrossRef]
- Jin, F.; Zhou, Z.; Moriya, T.; Kishida, H.; Higashijima, H.; Enomoto, H. Controlling hydrothermal reaction pathways to improve acetic acid production from carbohydrate biomass. Environ. Sci. Technol. 2005, 39, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Heeres, H.; Handana, R.; Chunai, D.; Rasrendra, C.B.; Girisuta, B.; Heeres, H.J. Combined dehydration/(transfer)-hydrogenation of C6-sugars (D-glucose and D-fructose) to γ-valerolactone using ruthenium catalysts. Green Chem. 2009, 11, 1247–1255. [Google Scholar] [CrossRef]
- Cai, B.; Zhou, X.C.; Miao, Y.C.; Luo, J.Y.; Pan, H.; Huang, Y.B. Enhanced catalytic transfer hydrogenation of ethyl levulinate to γ-valerolactone over a robust Cu–Ni bimetallic catalyst. ACS Sustain. Chem. Eng. 2016, 5, 1322–1331. [Google Scholar] [CrossRef]
- Jin, F.M.; Gao, Y.; Jin, Y.; Zhang, Y.; Cao, J.; Wei, Z.; Smith, R.L., Jr. High-yield reduction of carbon dioxide into formic acid by zero-valent metal/metal oxide redox cycles. Energy Environ. Sci. 2011, 4, 881–884. [Google Scholar] [CrossRef]
- Jin, F.M.; Zeng, X.; Liu, J.; Jin, Y.; Wang, L.; Zhong, H.; Yao, G.; Huo, Z.B. Highly efficient and autocatalytic H2O dissociation for CO2 reduction into formic acid with zinc. Sci. Rep. 2014, 4, 4503. [Google Scholar] [CrossRef]
- Lyu, L.; Zeng, X.; Yun, J.; Wei, F.; Jin, F. No Catalyst Addition and highly efficient dissociation of H2O for the reduction of CO2 to formic acid with Mn. Environ. Sci. Technol. 2014, 48, 6003–6009. [Google Scholar] [CrossRef]
- Zhong, H.; Li, Q.; Liu, J.; Yao, G.; Wang, J.; Zeng, X.; Huo, Z.; Jin, F. New method for highly efficient conversion of biomass-derived levulinic acid to gamma-valerolactone in water without precious metal catalysts. ACS Sustain. Chem. Eng. 2017, 5, 6517–6523. [Google Scholar] [CrossRef]
- Llatance-Guevara, L.; Flores, N.E.; Barrionuevo, G.O.; Casillas, J.L.M. Waste biomass selective and sustainable photooxidation to high-added-value products: A review. Catalysts 2022, 12, 1091. [Google Scholar] [CrossRef]
- Ren, H.F.; Zhu, D.; Li, J.F.; Liu, C.L.; Yang, R.Z.; Dong, W.S. One-pot conversion of carbohydrates into gamma-valerolactone under the coordination of heteropoly acid based ionic liquid and Ru/ZrO2 in water media. J. Chem. Technol. Biotechnol. 2019, 94, 2355–2363. [Google Scholar] [CrossRef]
- Ding, D.; Wang, J.; Xi, J.; Liu, X.; Lu, G.; Wang, Y. High-yield production of levulinic acid from cellulose and its upgrading to gamma-valerolactone. Green Chem. 2014, 16, 3846–3853. [Google Scholar] [CrossRef]
- De Vos, Y.; Jacobs, M.; Van Der Voort, P.; Van Driessche, I.; Snijkers, F.; Verberckmoes, A. Development of stable oxygen carrier materials for chemical looping processes—A review. Catalysts 2020, 10, 926. [Google Scholar] [CrossRef]
- Jin, F.; Enomoto, H. Rapid and highly selective conversion of biomass into value-added products in hydrothermal conditions: Chemistry of acid/base-catalysed and oxidation reactions. Energy Environ. Sci. 2011, 4, 382–397. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).