Abstract
Herein, we rationally designed a molecular catalytic filter for effective micropollutants decontamination via peroxymonosulfate (PMS) activation. Specifically, iron phthalocanine (FePc) molecules with defined Fe–N4 coordination were immobilized onto carbon nanotubes (CNTs), forming a hybrid catalyst that integrated molecular precision with heterogeneous catalytic properties. The resulting CNT-FePc filter achieved a 98.4% removal efficiency for bisphenol A (10 ppm) in a single-pass operation system, significantly outperforming the CNT/PMS system without FePc (41.6%). Additionally, the CNT-FePc/PMS system demonstrated remarkable resistance to performance inhibition by common water matrix components. Unlike typical radical-dominated PMS activation processes, mechanistic investigations confirmed that the CNT-FePc/PMS system selectively promoted singlet oxygen (1O2) generation as the primary oxidative pathway. Density functional theory (DFT) calculations revealed that PMS exhibited stronger adsorption on FePc (−3.05 eV) compared to CNT (−2.86 eV), and that FePc effectively facilitated O–O bond elongation in PMS, thereby facilitating 1O2 generation. Additionally, seed germination assays indicated a significant reduction in the biotoxicity of the treated effluents. Overall, this work presents a catalyst design strategy that merges molecular-level coordination chemistry with practical flow-through configuration, enabling rapid, selective, and environmentally benign micropollutant removal.
1. Introduction
Water in natural ecosystems undergoes continuous self-purification. However, rapid economic development and population growth have escalated the global demand for clean water, intensifying pressure on water resources. Compounding this challenge, large-scale discharge of industrial and domestic wastewater has substantially reduced freshwater availability, disrupting the supply-demand equilibrium [1,2]. Consequently, developing advanced strategies for efficient and sustainable water purification has become imperative. Among the various treatment technologies, advanced oxidation processes (AOPs) based on peroxymonosulfate (PMS) activation have garnered widespread attention due to their ability to degrade recalcitrant micropollutants through in situ generation of reactive oxygen species (ROS), such as singlet oxygen (1O2), hydroxyl radicals (HO•), sulfate radicals (SO4•−) and high-valent metal-oxo species [3,4,5,6,7]. Among these, 1O2 offers distinct advantages by selectively degrading electron-rich contaminants while mitigating toxic byproducts formation [8,9,10,11]. Nevertheless, conventional catalysts typically favor the production of HO• and SO4•−, relegating 1O2 to a secondary role [12,13,14,15,16]. To address this limitation, single atom catalysts (SACs), which feature atomically dispersed metal centers (typically M–N4 moieties), have emerged as promising platforms for selective 1O2 generation. The unique electronic structure and well-defined active sites of SACs enable efficient PMS activation and promote selective ROS generation [17,18,19]. Notably, Fe-based SACs have demonstrated superior selectivity toward the 1O2 generation during PMS activation [20,21,22]. Despite these advantages, the practical development of SACs remains challenging, primarily due to the thermodynamic instability of isolated metal atoms, which are prone to migration and aggregation into clusters, thereby diminishing catalytic activity and selectivity.
Metal phthalocyanine, a class of molecular catalysts featuring M–N4 structures, represent a structurally precise and thermally stable alternative to traditional SACs. In contrast to pyrolyzed SACs, which often suffer from structural ambiguities, residual defects, and require energy-intensive high-temperature synthesis, molecular iron phthalocyanine (FePc) provides distinct advantages, including atomic-level structural precision, inherent thermal stability, and scalable fabrication via low-temperature solution-based immobilization. These molecular catalysts also possess excellent electron transport properties and have been widely applied in electrocatalytic processes such as oxygen reduction reaction, carbon dioxide reduction reaction and nitrate reduction reaction [23,24,25,26,27]. Nevertheless, despite its prominence in electrocatalysis, FePc remains relatively underexplored in Fenton-like systems. A challenge lies in its intrinsic tendency to aggregate into dimers or polymers via π–π stacking, which compromises its dispersion and limits the catalytic efficiency. To address this issue, anchoring FePc molecules onto high-surface-area substrate has proven effective in enhancing molecular dispersion and maximizing active site accessibility. Another critical consideration in catalyst design is recoverability. While immobilized catalysts can improve structural stability, they often suffer from limited mass transfer and reduced reactivity over time. In this regard, flow-through configuration offers a promising strategy by significantly enhancing contact between reactants and active sites, thus improving reaction kinetics and overall catalytic performance [28,29,30,31]. Carbon nanotubes (CNTs) are particularly well suited as a support material for such systems due to their high surface area, excellent chemical stability, environmental compatibility, and capability to form interconnected fibrous networks [32].
In this work, we developed a molecular catalyst composite by immobilizing FePc molecules onto CNTs via π–π interactions, resulting in a hybrid material referred to as CNT-FePc. This strategy enabled the formation of well-defined active sites without the need for high-temperature pyrolysis. The resulting nanohybrids were further processed into catalytic filters through vacuum filtration, enabling a flow-through operation to overcome mass transfer limitations inherent in batch systems. Comprehensive structural and spectroscopic characterizations confirmed the atomic dispersion of Fe centers in the form of Fe–N4 coordination. Mechanistic investigations revealed that the CNT-FePc/PMS system predominantly generated 1O2 as the main reactive species. Under single-pass filtration conditions, the CNT-FePc/PMS filter exhibited rapid and efficient degradation of micropollutants, with strong resistance to interference from common water constituents and stable performance across a wide pH range. This work presents a catalyst design that integrates the molecular precision of FePc coordination chemistry with the operational advantages of a scalable flow-through membrane reactor, offering a robust and selective strategy for high-performance water purification under realistic environmental conditions.
2. Results and Discussion
2.1. Characterization of the Nanohybrid Filter
Synthesis of the CNT-FePc nanohybrid was achieved through a controlled π–π interaction between molecular FePc and the graphitic walls of the CNTs. As illustrated in Figure S1, CNT was first purified via acid treatment and subsequently dispersed in dimethylformamide (DMF) together with FePc. Upon mixing, FePc molecules were non-covalently adsorbed onto the CNT surface, enabling uniform molecular distribution and effective exposure of Fe–N4 catalytic sites while preserving the intrinsic coordination structure of FePc. X-ray diffraction (XRD) analysis revealed three additional diffraction peaks in CNT-FePc, corresponding to crystalline FePc, which were absent in the pristine CNT sample (Figure S2). Despite the incorporation of FePc, scanning electron microscope (SEM) images (Figure S3) showed that CNT-FePc retained the characteristic one-dimensional morphology of the CNT scaffold. Transmission electron microscopy (TEM) further confirmed a smooth surface topology with no evidence of aggregated particles (Figure 1a). To probe the atomic dispersion of Fe species, aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) was employed. As depicted in Figure 1b, numerous bright spots, corresponding to individual Fe atoms were clearly observed, indicating atomic-scale dispersion. Elemental mapping using energy-dispersive X-ray spectroscopy (EDX) confirmed the homogeneous distribution of Fe, C, and N within the CNT-FePc (Figure 1c–f). X-ray photoelectron spectroscopy (XPS) analysis of the Fe 2p region (Figure S4) revealed the coexistence of Fe(II) and Fe(III) species, consistent with previous reports [33,34]. To further probe the local coordination environment, X-ray absorption fine structure (XAFS) spectroscopy was performed. The Fe K-edge X-ray absorption near-edge structure (XANES) spectrum (Figure 1g) close resembled to that of the FePc reference, confirming the preservation of Fe–N4 coordination. The edge position, located between those of FeO and Fe2O3, suggested an average oxidation state between +2 and +3. Fourier-transformed extended X-ray absorption fine structure (EXAFS) analysis (Figure 1h) exhibited a prominent peak at ~1.6 Å, assignable first-shell Fe–N scattering. Notably, no peaks corresponding to Fe–Fe (~2.2 Å) or Fe–O (~1.4 Å) were observed, ruling out the presence of Fe clusters or Fe oxides [35]. Quantitative EXAFS fitting (Table S1) yielded a coordination number of 4.0 ± 0.3 for the Fe–N shell, further confirming mononuclear dispersion. Complementary wavelet transform (WT) analysis (Figure 1j–m) reinforced these findings. While Fe foil displayed intensity maxima at 8.1 Å−1 characteristic of Fe–Fe bonding [36], CNT-FePc showed a single intensity contour at 4.9 Å−1, consistent with Fe–N coordination environments [37]. Collectively, these results unambiguously demonstrate the successful immobilization of FePc molecules on CNTs, with Fe centers atomically dispersed in a well-defined Fe–N4 coordination, laying the structural foundation for selective catalytic performance.
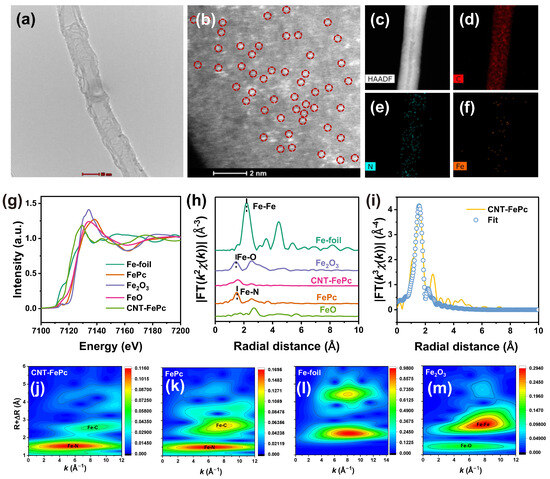
Figure 1.
(a) TEM, (b) HAADF-STEM and (c–f) EDX mappings images of CNT-FePc; (g) Fe K-edge XANES spectra and (h) FT-EXAFS spectra of FePc, Fe foil, FeO, Fe2O3 and CNT-FePc; (i) EXAFS fitting curve in R space of CNT-FePc; (j–m) WT-EXAFS contour plots of different samples.
2.2. Catalytic Activity of the Nanohybrid Filter Toward Organic Degradation
Bisphenol A (BPA) was employed as a model contaminant to evaluate the catalytic performance of the CNT-FePc nanohybrid filter in PMS-mediated organic pollutant degradation. As illustrated in Figure S5, the catalytic activity of the CNT-FePc/PMS system exhibited a strong dependence on FePc loading. Compared to the CNT control (lacking FePc), BPA removal efficiency progressively increased with FePc dosage, reaching near-complete removal (~100%) at 7 mg of FePc, corresponding to an Fe atomic density of 9.33 × 1017 atoms cm−2. Figure 2a shows that PMS alone, CNT alone, or CNT-FePc alone induced negligible BPA degradation. In contrast, the CNT-FePc/PMS system achieved 98.4% BPA removal efficiency under single-pass flow-through conditions, significantly outperforming the CNT/PMS system (41.6%). These results underscore the critical role of FePc in facilitating PMS activation for effective contaminant degradation. The CNT-FePc/PMS system also demonstrated excellent resistance to common water matrix constituents. The presence of coexisting anions (Cl−, SO42−, HPO42− and HCO3−) and natural organic matter (humic acid, HA) had a negligible influence on BPA removal (Figure 2b). Additionally, the system maintained high degradation efficiency (>98%) across a wide pH range (3–11, Figure 2c), highlighting its strong tolerance to pH fluctuations. Crucially, single-pass flow-through operation enabled ultrafast BPA removal (98.4%), far exceeding the performance of traditional batch treatment, which achieved only 32.2% removal in 30 min (Figure S6). This enhancement is attributed to the convective mass transport facilitated by the flow-through configuration. Moreover, the catalytic platform exhibited broad-spectrum reactivity, effectively degrading a variety of electron-rich micropollutants—including BPA, 4-chlorophenol (4-CP), phenol, sulfamethoxazole (SMX), aniline, and tetracycline (TC)—with removal efficiencies exceeding 90% in all cases (Figure 2d, Table S2). The practical applicability of the CNT-FePc/PMS system was further validated using natural water matrices, including lake water, tap water, and river water (Figure 2e). Iron leaching remained minimal (0.031 mg L−1 after 600 min; Figure 2f), affirming the system’s long-term durability. Cyclic stability tests (Figure S7) confirmed excellent recyclability, with degradation efficiency consistently above 95% after 10 consecutive cycles and no observable performance loss. In comparison, the FePc-only/PMS system achieved a lower BPA removal efficiency (~86.4%) and suffered from significant Fe leaching (3.12 mg L−1 after 60 min), nearly 100-fold higher than that of the CNT-FePc system (Figure S8). Post-reaction analysis by TEM and HAADF-STEM (Figure S9) revealed that CNT-FePc retained its nanotubular morphology, with no evidence of Fe-containing nanoparticles or aggregation. Furthermore, XPS analysis of the Fe 2p region (Figure S10) showed negligible changes relative to the fresh sample, indicating the preservation of Fe valence states and coordination environments. Notably, the Fenton-like catalytic performance of CNT-FePc surpasses that of several state-of-the-art heterogeneous catalysts reported in recent literature (Table S3) [38,39,40,41,42,43]. Collectively, these findings indicate that the CNT-FePc/PMS system demonstrates excellent resistance to environmental interferents, high degradation efficiency over a broad pH range, wide pollutant applicability, and outstanding structural stability, highlighting its strong potential for practical applications in advanced water treatment.
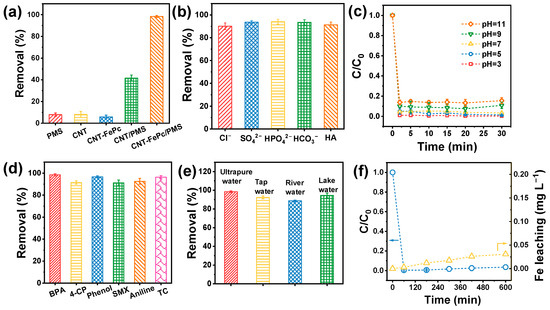
Figure 2.
(a) BPA removal efficiency in different degradation system. Effect of (b) different ions and HA and, (c) initial pH on BPA removal by the CNT-FePc/PMS system. (d) Removal efficiency of various contaminants by the CNT-FePc/PMS system. (e) BPA removal efficiency from different natural waters by the CNT-FePc activated PMS systems. (f) BPA removal efficiency of the CNT-FePc/PMS system over 600 min and the amount of Fe leakage. Reaction conditions: [PMS]0 = 2.0 mM, [Cl−]0 = [SO42−]0 = [HPO42−]0 = [HCO3−]0 = 10 mM, [HA]0 = 10 ppm, Contaminant concentration = 10 ppm, flow rate = 2.0 mL min−1, pH0 = 5, if not otherwise specified.
2.3. Mechanistic Investigations
Electron paramagnetic resonance (EPR) trapping experiments were conducted to directly identify the key ROS generated during PMS activation. As shown in Figure 3a, a characteristic quartet signal with an intensity ratio of 1:2:2:1—corresponding to the DMPO-HO• adduct—was observed in both the CNT-FePc/PMS and CNT/PMS systems. In addition, signals attributable to the DMPO-SO4•− adduct were also detected, confirming the formation of sulfate radicals [44]. When TEMP was used as a spin-trapping agent, a distinct triplet signal with a 1:1:1 intensity ratio, characteristic of the TEMP-1O2 adduct, was observed in both systems (Figure 3b). Notably, the signal intensities of both DMPO-HO• and TEMP-1O2 adducts were markedly stronger in the CNT-FePc/PMS system than in the CNT/PMS system, indicating FePc significantly enhances the ROS generation.
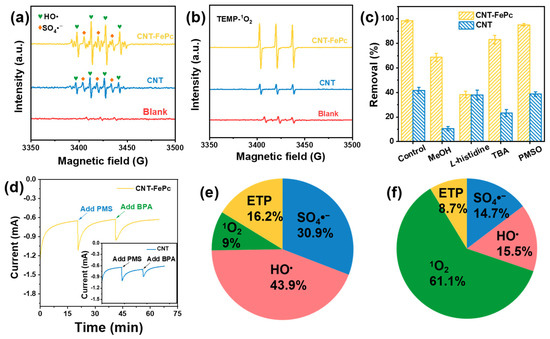
Figure 3.
(a) HO• and SO4•− and (b) 1O2 signals in different systems with PMS. (c) Comparison of BPA removal efficiency by the CNT-FePc/PMS and CNT/PMS systems under different quenching conditions; [PMS]0 = 2.0 mM, flow rate = 2.0 mL min−1, pH0 = 5, [BPA]0 = 10 ppm, [MeOH]0 = [L-histidine]0 = [TBA]0 = [PMSO]0 = 10 mM. (d) I-t curves of CNT-FePc at −0.2 V vs. SCE. Proportions of 1O2, HO•, SO4•− and DET in (e) CNT/PMS, (f) CNT-FePc/PMS.
To further elucidate the ROS generation pathways and underlying reaction mechanisms, a series of radical scavenging experiments were performed. Methanol (MeOH), a non-selective quencher of both HO• and SO4•−, was used alongside L-histidine (a selective scavenger for 1O2), tert-butyl alcohol (TBA, a selective HO• scavenger), and methyl phenyl sulfoxide (PMSO, a scavenger of high-valent Fe–oxo species) [45,46,47]. As shown in Figure 3c, the addition of MeOH significantly decreased BPA removal efficiency by the CNT-FePc/PMS system from 98.4% to 68.7%. An even more pronounced decrease was observed in the CNT/PMS system, where BPA removal dropped from 41.6% to 10.7%, suggesting that HO• and SO4•− were the predominant ROS in the absence of FePc. In contrast, the addition of L-histidine led to a substantial reduction in BPA removal efficiency in the CNT-FePc/PMS system (down to 38.4%), but had minimal effect in the CNT/PMS system. This observation indicates that 1O2 played a major role in pollutant degradation when FePc was present. Furthermore, the addition of TBA resulted in only a slight decrease in BPA removal efficiency in the CNT-FePc/PMS system, further supporting the conclusion that HO• was not the dominant ROS in this system.
It is well established that PMSO can be selectively oxidized by high-valent Fe–oxo species to its corresponding sulfone (PMSO2) via an oxygen atom transfer pathway [48]. However, the addition of PMSO had no discernible inhibitory effect on BPA degradation in either the CNT/PMS or the CNT-FePc/PMS systems. This observation suggests that high-valent Fe–oxo species were scarcely generated under the applied reaction conditions. Beyond ROS-mediated pathways, the electron transfer process (ETP) has also been recognized as a potential route for pollutant degradation in carbon-based catalyst/PMS systems [49]. ETP involves direct electron transfer from the contaminant to PMS, and its occurrence was evaluated using chronoamperometric measurements. As shown in Figure 3d, the current density of CNT-FePc increased by 0.46 mA upon PMS addition and by 0.32 mA upon subsequent BPA introduction. In comparison, the pristine CNT exhibited lower increases of 0.34 mA and 0.19 mA, respectively, under the same conditions. These results confirm the involvement of ETP in both systems, with CNT-FePc exhibiting enhanced electron transfer capability. Based on the collective experimental evidence, the relative contributions of different ROS species and ETP to BPA degradation were quantitatively estimated. As illustrated in Figure 3e, in the CNT/PMS system, HO• and SO4•− were the predominant reactive species, contributing 74.8 ± 0.8% to BPA degradation, while ETP accounted for 16.2 ± 0.7%. In contrast, in the CNT-FePc/PMS system (Figure 3f), 1O2 emerged as the dominant oxidizing species, contributing 61.1 ± 1.0%, which was a significant increase compared to its contribution in the CNT/PMS system (9.0 ± 0.5%). Meanwhile, the contributions of HO• and SO4•− in the CNT-FePc/PMS system were relatively minor. These findings collectively demonstrate that the incorporation of FePc significantly alters the PMS activation pathway, shifting the mechanism from a radical-dominated process toward a non-radical, 1O2-mediated oxidation route. This molecular-level modulation of the reaction pathway underscores the advantage of using FePc as a selective catalyst for environmentally benign and efficient micropollutant degradation.
To gain deeper insight into the origin of the enhanced catalytic performance of CNT-FePc, DFT calculations were carried out. The optimized adsorption configurations of PMS on CNT and FePc are shown in Figure 4a,b, respectively. In both cases, the terminal oxygen atom of PMS preferentially interacts with the Fe center in FePc and with hydroxyl groups on the CNT surface, which is consistent with previous findings [12,27]. The calculated adsorption energy of PMS on CNT-FePc was −3.05 eV (Figure 4c), which is more negative than that on CNT alone (−2.86 eV), indicating a stronger binding affinity between PMS and the Fe center in the CNT-FePc hybrid. Notably, upon adsorption onto FePc, the O–O bond length of PMS was significantly elongated from 1.33 Å (on CNT) to 1.49 Å, implying a weakened bond that is more susceptible to cleavage. This bond elongation is a key structural indicator of PMS activation toward 1O2 generation. Collectively, these DFT results reveal that the superior catalytic activity of CNT-FePc originates from its dual capability to strongly adsorb PMS and to promote O–O bond activation. This synergistic interaction at the molecular level facilitates efficient 1O2 production, accounting for the observed enhancement in contaminant degradation performance.
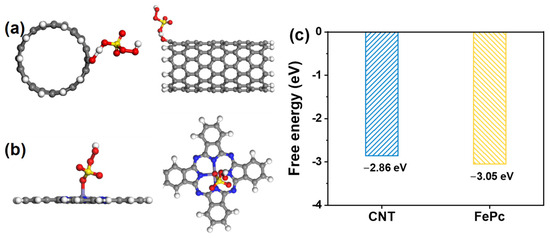
Figure 4.
DFT-optimized adsorption configurations of PMS on (a) CNT and (b) FePc. (c) The adsorption energy of PMS on CNT and FePc.
2.4. Biotoxicity Assessment
To assess the ecological risks of BPA before and after degradation, phytotoxicity assays were performed using mung bean seed germination. As shown in Figure S11 and Figure 5a–c, the BPA solution (10 ppm) had a negligible effect on germination rate but significantly inhibited bud elongation compared to the ultrapure water control. Remarkably, seeds exposed to the degraded BPA solution exhibited growth performance comparable to those in ultrapure water. On day 3, germination rates were 93.5 ± 2.1% for ultrapure water, 79.0 ± 3.5% for the BPA solution, and 96.7 ± 1.8% for the degraded BPA solution (Figure 5d), with all groups showing increased germination over time. As depicted in Figure 5e, BPA-treated seedlings consistently exhibited suppressed bud growth—for instance, on day 5, the average bud length was 0.40 ± 0.05 cm in the BPA group, significantly lower than that in the ultrapure water (1.00 ± 0.08 cm) and degraded BPA (0.85 ± 0.06 cm) groups.
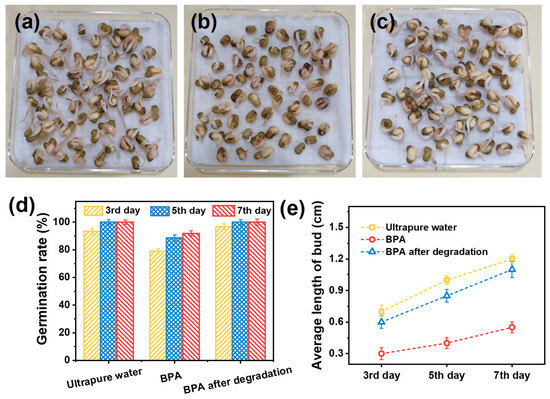
Figure 5.
Photos of mung bean seeds on the seventh day treated with (a) ultrapure water, (b) BPA and (c) BPA after degradation. (d) Germination rate and (e) average length of buds of mung bean seeds treated with different solutions for a week.
To elucidate the degradation pathway, UHPLC-MS analysis was conducted to identify major transformation products (TPs) (Figure S12). Initial ROS attack on the phenolic –OH groups of BPA yielded TP1, which underwent further oxidation at the carboxyl group to form TP2. Decarboxylation subsequently produced TP3 and TP4, whose aliphatic –CH2/–CH3 side chains were oxidized into the higher m/z product TP5. TP5 was further transformed into p-hydroxybenzoic acid, followed by aromatic ring cleavage to generate maleic acid, succinic acid, and acetic acid, eventually leading to complete mineralization into CO2 and H2O. These results confirm that BPA primarily exerts phytotoxic effects through inhibition of seedling growth, which can be substantially alleviated following degradation by the CNT-FePc/PMS system.
3. Materials and Methods
3.1. Materials and Catalyst Preparation
All chemicals and reagents used were of analytical grade and employed without further purification. Detailed information is provided in Text S1. The CNT-FePc nanohybrid filter was synthesized based on previously reported protocols with slight modifications [28,50]. As illustrated in Figure S1, pristine CNTs were first stirred in 6 M HCl for 12 h to remove residual metal impurities. After thorough washing with deionized water, the solids were vacuum-dried at 60 °C overnight. Subsequently, 30 mg of purified CNT powder was ultrasonically dispersed in 30 mL of DMF. Separately, 25 mg of FePc was dissolved in 25 mL of DMF and then added to the CNT suspension in varying volumes (0, 1, 4, 7, or 10 mL). The mixture was stirred at room temperature for 12 h, followed by vacuum filtration through a polytetrafluoroethylene (PTFE) membrane. The resulting hybrid was washed sequentially with ethanol and water to remove unbound species. For comparison, CNT filters without FePc were synthesized using an identical procedure. Characterization of the synthesized catalysts is described in Texts S2 and S3. The analytical methods used for the quantification of organic pollutants are detailed in Text S4. The calculation of Fe atomic density is provided in Text S5.
3.2. Activity Evaluation
The catalytic activity was evaluated using a commercially available Whatman polycarbonate filtration unit loaded with the prepared filters (Figure S13). Prior to testing, all filters were pre-saturated with water to minimize adsorption effects on organic pollutant removal. In a typical experiment, 100 mL of a 10 ppm pollutant solution containing 2 mM PMS was pumped through the filter at a flow rate of 2.0 mL min−1. The solution pH was adjusted using NaOH or H2SO4. At designated time intervals, 1.0 mL of the effluent was collected and immediately quenched with 0.05 mL of 0.1 M sodium hyposulfite solution. To compare performance under flow-through and batch modes, the CNT-FePc filter was placed in a 100 mL beaker containing 80 mL of 10 ppm pollutant solution and 2 mM PMS under magnetic stirring. For cycling experiments, the used filters were thoroughly rinsed with large volumes of ultrapure water after each run. All catalytic experiments were conducted in at least duplicate, and the results are presented as mean ± standard deviation.
3.3. Theoretical Calculations
First-principles calculations under the framework of DFT were performed using Quantum ESPRESSO (QE) software (version 7.4.1) [51,52,53,54,55]. Further computational details are provided in Text S6 of the Supplementary Material.
4. Conclusions
In this study, a facile and effective strategy was developed to construct a molecular catalyst (CNT-FePc) featuring a well-defined Fe–N4 coordination structure for the activation of PMS to achieve efficient micropollutant degradation. The CNT-FePc/PMS system exhibited outstanding oxidative performance across a broad pH range (3–11) under single-pass filtration conditions, highlighting its potential for practical water treatment applications. Comprehensive experimental analyses revealed that 1O2 was the predominant ROS responsible for pollutant degradation, whereas HO• and SO4•− played secondary roles. DFT calculations further demonstrated that FePc exhibited stronger adsorption affinity for PMS than CNT alone, thereby facilitating PMS activation and enhancing ROS generation. The system also displayed strong resistance to common environmental interferences, including ubiquitous anions (Cl−, SO42−, HPO42−, and HCO3−) and natural organic matter, maintaining high catalytic efficiency under realistic conditions. Long-term stability and environmental safety were confirmed through repeated cycling experiments and tests in natural water matrices. Notably, iron leaching was minimal, with concentrations as low as 0.031 mg L−1 after 600 min of operation, which was well below environmental risk thresholds. Furthermore, seed germination assays demonstrated a significant reduction in the biotoxicity of BPA following treatment by the CNT-FePc/PMS system, underscoring the environmental compatibility of the degradation products. Overall, this work offers valuable insights into the rational design of heterogeneous Fenton-like catalysts based on molecular coordination structures and presents a promising strategy for sustainable and efficient water purification, contributing to the mitigation of global water resource challenges.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15080747/s1, Figure S1: Schematic procedure for the synthesis of CNT-FePc filter; Figure S2: The XRD patterns of CNT and CNT-FePc; Figure S3: The SEM image of (a) CNT and (b) CNT-FePc; Figure S4: The Fe 2p XPS spectra of CNT-FePc; Figure S5: The effect of FePc dosage on BPA removal efficiency in PMS systems; Figure S6: Effect of (a) flow rate and (b) PMS concentration on the removal of BPA in the CNT-FePc/PMS system; Figure S7: Cycle stability experiments of the CNT-FePc; Figure S8: Comparison of BPA removal and Fe leaching in the CNT-FePc/PMS and FePc/PMS systems; Figure S9: (a) TEM and (b) STEM images of CNT-FePc after reaction; Figure S10: Fe 2p XPS of CNT-FePc before and after reaction; Figure S11: Photos of mung bean seeds treated by (a–c) water, (d–f) BPA solution and (g–i) degraded PBA solution (Time: 3rd, 5th and 7th day); Figure S12: The possible BPA degradation pathways over CNT-FePc/PMS system; Figure S13: Photo of continuous flow Whatman polycarbonate filtration: (a) Water inlet side, (b) water outlet side, (c) overall schematic diagram, (d) fixing nut and (e) CNT-FePc membrane; Table S1: EXAFS fitting parameters at the Fe K–edge for various samples; Table S2: Relative parameters of different pollutants; Table S3: Comparison of the BPA removal performance of proposed system with reported systems; Table S4: HPLC operation parameters for different substrates; Text S1: Chemicals; Text S2: Characterization; Text S3: The details for collecting Fe K-edge XAFS spectra; Text S4: Analytical methods; Text S5: Calculation of Fe atomic density; Text S6: Computational details.
Author Contributions
C.X.: data curation, methodology, writing—original draft; Y.R.: characterization, investigation, writing—original draft; Y.L.: conceptualization, writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Natural Science Foundation of Shanghai (23ZR1401300).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Author C.X. Xie was employed by the company CenerTech Tianjin Chemical Research & Design Institute Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, H.M.N. Antibiotics traces in the aquatic environment: Persistence and adverse environmental impact. Curr. Opin. Environ. Sci. Health 2020, 13, 68–74. [Google Scholar] [CrossRef]
- Dias, I.M.; Mourão, L.C.; Andrade, L.A.; Souza, G.B.M.; Viana, J.C.V.; Oliveira, S.B.; Alonso, C.G. Degradation of antibiotic amoxicillin from pharmaceutical industry wastewater into a continuous flow reactor using supercritical water gasification. Water Res. 2023, 234, 119826. [Google Scholar] [CrossRef]
- Wang, A.; Du, M.; Ni, J.; Liu, D.; Pan, Y.; Liang, X.; Liu, D.; Ma, J.; Wang, J.; Wang, W. Enhanced and synergistic catalytic activation by photoexcitation driven S−scheme heterojunction hydrogel interface electric field. Nat. Commun. 2023, 14, 6733. [Google Scholar] [CrossRef]
- Wei, S.; Sun, Y.; Qiu, Y.Z.; Li, A.; Chiang, C.Y.; Xiao, H.; Qian, J.; Li, Y. Self-carbon-thermal-reduction strategy for boosting the Fenton-like activity of single Fe-N4 sites by carbon-defect engineering. Nat. Commun. 2023, 14, 7549. [Google Scholar] [CrossRef]
- Wei, Y.; Miao, J.; Ge, J.; Lang, J.; Yu, C.; Zhang, L.; Alvarez, P.J.J.; Long, M. Ultrahigh peroxymonosulfate utilization efficiency over CuO nanosheets via heterogeneous Cu(III) formation and preferential electron transfer during degradation of phenols. Environ. Sci. Technol. 2022, 56, 8984–8992. [Google Scholar] [CrossRef]
- Zhou, Q.; Song, C.; Wang, P.; Zhao, Z.; Li, Y.; Zhan, S. Generating dual-active species by triple-atom sites through peroxymonosulfate activation for treating micropollutants in complex water. Proc. Natl. Acad. Sci. USA 2023, 120, e2300085120. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; You, S.; Li, F.; Liu, Y. Engineering carbon nanocatalysts towards efficient degradation of emerging organic contaminants via persulfate activation: A review. Chin. Chem. Lett. 2022, 33, 1–10. [Google Scholar] [CrossRef]
- Ghogare, A.A.; Greer, A. Using singlet oxygen to synthesize natural products and drugs. Chem. Rev. 2016, 116, 9994–10034. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Song, J.; Tian, R.; Yang, Z.; Yu, G.; Lin, L.; Zhang, G.; Fan, W.; Zhang, F.; Niu, G.; et al. Activatable singlet oxygen generation from lipid hydroperoxide nanoparticles for cancer therapy. Angew. Chem. Int. Ed. 2017, 56, 6492–6496. [Google Scholar] [CrossRef]
- Jin, L.; Duan, X.; Sun, M.; Vecitis, C.; Yu, H.; Liu, Y. A general strategy to synthesize fluidic single atom electrodes for selective reactive oxygen species production. ACS Nano 2023, 17, 12875–12883. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou Taconnat, L.; Triantaphylidès, C.; Havaux, M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5535–5540. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, M.; Sun, H.; Ao, Z.; Wang, S.; Liu, S. Understanding of the oxidation behavior of benzyl alcohol by peroxymonosulfate via carbon nanotubes activation. ACS Catal. 2020, 10, 3516–3525. [Google Scholar] [CrossRef]
- Ke, M.; Huang, G.; Mei, S.; Wang, Z.; Zhang, Y.; Hua, T.; Zheng, L.; Yu, H. Interface-promoted direct oxidation of p-arsanilic acid and removal of total arsenic by the coupling of peroxymonosulfate and Mn-Fe-mixed oxide. Environ. Sci. Technol. 2021, 55, 7063–7071. [Google Scholar] [CrossRef]
- Liu, Z.; Ding, H.; Zhao, C.; Wang, T.; Wang, P.; Dionysiou, D.D. Electrochemical activation of peroxymonosulfate with ACF cathode: Kinetics, influencing factors, mechanism, and application potential. Water Res. 2019, 159, 111–121. [Google Scholar] [CrossRef]
- Yang, Y.; Banerjee, G.; Brudvig, G.W.; Kim, J.-H.; Pignatello, J.J. Oxidation of organic compounds in water by unactivated peroxymonosulfate. Environ. Sci. Technol. 2018, 52, 5911–5919. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Wang, J. Peroxymonosulfate activation by Fe–Co–O-codoped graphite carbon nitride for degradation of sulfamethoxazole. Environ. Sci. Technol. 2020, 54, 10361–10369. [Google Scholar] [CrossRef]
- Qi, H.; Yang, J.; Liu, F.; Zhang, L.; Yang, J.; Liu, X.; Li, L.; Su, Y.; Liu, Y.; Hao, R.; et al. Highly selective and robust single-atom catalyst Ru1/NC for reductive amination of aldehydes/ketones. Nat. Commun. 2021, 12, 3295. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, H.; Zhu, X.; Qu, Y.; Xiong, C.; Xue, Z.; Zhang, Q.; Liu, X.; Zhou, F.; Mou, X.; et al. Simultaneous oxidative and reductive reactions in one system by atomic design. Nat. Catal. 2021, 4, 134–143. [Google Scholar] [CrossRef]
- Yin, K.; Shang, Y.; Chen, D.; Gao, B.; Yue, Q.; Xu, X. Redox potentials of pollutants determining the dominate oxidation pathways in manganese single-atom catalyst (Mn-SAC)/peroxymonosulfate system: Selective catalytic mechanisms for versatile pollutants. Appl. Catal. B Environ. 2023, 338, 123029. [Google Scholar] [CrossRef]
- Zhang, L.S.; Jiang, X.-H.; Zhong, Z.-A.; Tian, L.; Sun, Q.; Cui, Y.T.; Lu, X.; Zou, J.P.; Luo, S.L. Carbon nitride supported high-loading Fe single-atom catalyst for activation of peroxymonosulfate to generate 1O2 with 100 % selectivity. Angew. Chem. Int. Ed. 2021, 60, 21751–21755. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fu, Y.; Chen, S.; Zhang, W.; Xiang, K.; Shen, F.; Xiao, R.; Chai, L.; Zhao, F. A layered g-C3N4 support single-atom Fe-N4 catalyst derived from hemin to activate PMS for selective degradation of electron-rich compounds via singlet oxygen species. Chem. Eng. J. 2023, 474, 145571. [Google Scholar] [CrossRef]
- Long, Y.; Cao, Z.; Wu, W.; Liu, W.; Yang, P.; Zhan, X.; Chen, R.; Liu, D.; Huang, W. Rational modulation of Fe single-atom electronic structure in a Fe-N2B4 configuration for preferential 1O2 generation in Fenton-like reactions. Appl. Catal. B Environ. 2024, 344, 123643. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, L.; He, J.; Ding, D.; Wang, T.; Li, J.; Li, M.; Liu, Y.; Li, Y.; Yuan, M.; et al. Regulating the spin State of FeIII enhances the magnetic effect of the molecular catalysis mechanism. J. Am. Chem. Soc. 2022, 144, 8204–8213. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, Y.; Lin, Z.; Yuan, Y.; Zhang, X.; Tang, Y.; Wang, H.; Li, H.; Jin, C.; Liang, Y. Molecular electrocatalysts for rapid and selective reduction of nitrogenous waste to ammonia. Energy Environ. Sci. 2023, 16, 2239–2246. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Gu, M.; Wang, M.; Zhang, Z.; Pan, W.; Jiang, Z.; Zheng, H.; Lucero, M.; Wang, H.; et al. Molecular engineering of dispersed nickel phthalocyanines on carbon nanotubes for selective CO2 reduction. Nat. Energy 2020, 5, 684–692. [Google Scholar] [CrossRef]
- Xu, S.; Ding, Y.; Du, J.; Zhu, Y.; Liu, G.; Wen, Z.; Liu, X.; Shi, Y.; Gao, H.; Sun, L.; et al. Immobilization of iron phthalocyanine on pyridine-functionalized carbon nanotubes for efficient nitrogen reduction reaction. ACS Catal. 2022, 12, 5502–5509. [Google Scholar] [CrossRef]
- Huang, B.; Ren, X.; Zhao, J.; Wu, Z.; Wang, X.; Song, X.; Li, X.; Liu, B.; Xiong, Z.; Lai, B. Modulating Electronic structure engineering of atomically dispersed cobalt catalyst in Fenton-like reaction for efficient degradation of organic pollutants. Environ. Sci. Technol. 2023, 57, 14071–14081. [Google Scholar] [CrossRef]
- Guo, D.; Yao, Y.; You, S.; Jin, L.; Lu, P.; Liu, Y. Ultrafast degradation of micropollutants in water via electro-periodate activation catalyzed by nanoconfined Fe2O3. Appl. Catal. B Environ. 2022, 309, 121289. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, G.; Vecitis, C.D. Prospects of an electroactive carbon nanotube membrane toward environmental applications. Acc. Chem. Res. 2020, 53, 2892–2902. [Google Scholar] [CrossRef]
- Li, F.; Peng, X.; Liu, Y.; Mei, J.; Sun, L.; Shen, C.; Ma, C.; Huang, M.; Wang, Z.; Sand, W. A chloride-radical-mediated electrochemical filtration system for rapid and effective transformation of ammonia to nitrogen. Chemosphere 2019, 229, 383–391. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, P.; Liu, F.; Li, F.; An, X.; Liu, J.; Wang, Z.; Shen, C.; Sand, W. Electroactive modified carbon nanotube filter for simultaneous detoxification and sequestration of Sb(III). Environ. Sci. Technol. 2019, 53, 1527–1535. [Google Scholar] [CrossRef]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, X.; Akiyama, K.; Bingham, P.A.; Kubuki, S. Elucidating the mechanistic origin of a spin state-dependent FeNx–C catalyst toward organic contaminant oxidation via peroxymonosulfate activation. Environ. Sci. Technol. 2022, 56, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Ding, L.; Zhang, X.; Wang, K.; Wang, X.; Yang, F.; Han, G. Biaxially-srained phthalocyanine at polyoxometalate@carbon nanotube heterostructure boosts oxygen reduction catalysis. Angew. Chem. Int. Ed. 2023, 62, e202309545. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ji, S.; Wang, Y.; Dong, J.; Chen, W.; Li, Z.; Shen, R.; Zheng, L.; Zhuang, Z.; Wang, D.; et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2017, 56, 6937–6941. [Google Scholar] [CrossRef]
- Long, X.; Li, Z.; Gao, G.; Sun, P.; Wang, J.; Zhang, B.; Zhong, J.; Jiang, Z.; Li, F. Graphitic phosphorus coordinated single Fe atoms for hydrogenative transformations. Nat. Commun. 2020, 11, 4074. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Wang, X.-S.; He, Y.; Wang, Q.; Li, Y.; Li, M.; Yang, G.; Yi, J.; Lin, H.; et al. Facile top-down strategy for direct metal atomization and coordination achieving a high turnover number in CO2 photoreduction. J. Am. Chem. Soc. 2020, 142, 19259–19267. [Google Scholar] [CrossRef]
- Di, L.; Wang, T.; Lu, Q.; Lu, J.; Zhang, Y.; Zhou, Y.; Zhou, Y. Efficient PMS activation toward degradation of bisphenol A by metal-free nitrogen-doped hollow carbon spheres. Sep. Purif. Technol. 2024, 339, 126740. [Google Scholar] [CrossRef]
- Narendra Kumar, A.V.; Shin, W.S. Yolk-shell Fe2O3@mesoporous hollow carbon sphere hybrid sub-micro reactors for effective degradation of organic contaminants. Chem. Eng. J. 2023, 465, 142922. [Google Scholar] [CrossRef]
- Bai, X.; Shi, J.; Xu, L.; Jin, X.; Shi, X.; Jin, P. Fe-g-C3N4/reduced graphene oxide lightless application for efficient peroxymonosulfate activation and pollutant mineralization: Comprehensive exploration of reactive sites. Sci. Total Environ. 2023, 855, 158799. [Google Scholar] [CrossRef]
- Zhou, X.; Ke, M.K.; Huang, G.X.; Chen, C.; Chen, W.; Liang, K.; Qu, Y.; Yang, J.; Wang, Y.; Li, F.; et al. Identification of Fenton-like active Cu sites by heteroatom modulation of electronic density. Proc. Natl. Acad. Sci. USA 2022, 119, e2119492119. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Gao, B.; Duan, P.; Guo, K.; Akram, M.; Xu, X.; Yue, Q.; Gao, Y. Improving peroxymonosulfate activation by copper ion-saturated adsorbent-based single atom catalysts for the degradation of organic contaminants: Electron-transfer mechanism and the key role of Cu single atoms. J. Mater. Chem. A 2021, 9, 11604–11613. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, X.Y.; Xu, F.; Su, R.D.; Li, B.; Zhang, F.; Xu, X.; Wang, Y.; Ma, D.F.; Gao, B.Y.; et al. Diatomic “catalytic/co-catalytic” Fe/Mo catalysts promote Fenton-like reaction to treat organic wastewater through special interfacial reaction enhancement mechanism. Water Res. 2025, 274, 123147. [Google Scholar] [CrossRef]
- Chen, L.; Duan, J.; Du, P.; Sun, W.; Lai, B.; Liu, W. Accurate identification of radicals by in-situ electron paramagnetic resonance in ultraviolet-based homogenous advanced oxidation processes. Water Res. 2022, 221, 118747. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, Z.; Shi, H.; Wu, Z.; Huang, B.; Zhang, H.; Zhou, P.; Pan, Z.; Liu, W.; Lai, B. Switching the reaction mechanisms and pollutant degradation routes through active center size-dependent Fenton-like catalysis. Appl. Catal. B Environ. 2023, 329, 122569. [Google Scholar] [CrossRef]
- Zheng, W.; You, S.; Chen, Z.; Ding, B.; Huang, Y.; Ren, N.; Liu, Y. Copper nanowire networks: An effective electrochemical peroxymonosulfate activator toward nitrogenous pollutant abatement. Environ. Sci. Technol. 2023, 57, 10127–10134. [Google Scholar] [CrossRef]
- Qian, K.; Chen, H.; Li, W.; Ao, Z.; Wu, Y.-N.; Guan, X. Single-atom Fe catalyst outperforms its homogeneous counterpart for activating peroxymonosulfate to achieve effective degradation of organic contaminants. Environ. Sci. Technol. 2021, 55, 7034–7043. [Google Scholar] [CrossRef]
- Cheng, C.; Ren, W.; Miao, F.; Chen, X.; Chen, X.; Zhang, H. Generation of FeIV=O and its contribution to Fenton-like reactions on a single-atom iron−N−C catalyst. Angew. Chem. Int. Ed. 2023, 135, e202218510. [Google Scholar] [CrossRef]
- Ren, W.; Nie, G.; Zhou, P.; Zhang, H.; Duan, X.; Wang, S. The intrinsic nature of persulfate activation and N-doping in carbocatalysis. Environ. Sci. Technol. 2020, 54, 6438–6447. [Google Scholar] [CrossRef]
- Su, J.; Musgrave, C.B.; Song, Y.; Huang, L.; Liu, Y.; Li, G.; Xin, Y.; Xiong, P.; Li, M.M.J.; Wu, H.; et al. Strain enhances the activity of molecular electrocatalysts via carbon nanotube supports. Nat. Catal. 2023, 6, 818–828. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Blöchl, P.E.; Jepsen, O.; Andersen, O.K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.C.; Teter, M.P.; Allan, D.C.; Arias, T.A.; Joannopoulos, J.D. Iterative minimization techniques for ab initio total-energy calculations: Molecular dynamics and conjugate gradients. Rev. Mod. Phys. 1992, 64, 1045–1097. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).