Abstract
Liquid fuels obtained from CO2 and green hydrogen (i.e., e-fuels) are powerful tools for decarbonizing economy. Improvements provided by Process Intensification in the existing conventional reactors aim to decrease energy consumption, increase yield, and ensure more compact and safe processes. This review describes the advances in the production of methanol, dimethyl ether, and hydrocarbons by Fischer–Tropsch using different Process Intensification tools, mainly membrane reactors, sorption-enhanced reactors, and structured reactors. Due to the environmental interest, this review article focused on discussing methanol and dimethyl ether synthesis from CO2 + H2, which also represented the most innovative approach. The use of syngas (CO + H2) is generally preferred for the Fischer–Tropsch process; hence, studies examining this process were included in the present review. Both mathematical models and experimental results are discussed. Achievements in the improvement of catalytic reactor performance are described. Experimental results in membrane reactors show increased performance in e-fuels production compared to the conventional packed bed reactor. The combination of sorption and reaction also increases the single-pass conversion and yield, although this improvement is limited by the saturation capacity of the sorbent in most cases.
1. Introduction
There are many reasons to believe that, in the future, fuels derived from renewable electricity will gradually replace fuels derived from fossil sources. The most relevant reasons are as follows:
- -
- Fossil fuels are being progressively depleted, which is inevitable since they are finite resources. This depletion will reduce the production of fossil fuels and increase their prices.
- -
- Reducing CO2 emissions is desirable as they are the main cause of climate change.
- -
- Renewable electric energy is available at an increasingly lower price, even though renewable sources such as wind and photovoltaics are intermittent and difficult to control. Hence, this availability comes with the drawback of needing to store surplus energy, which increases as the percentage of electrical energy produced from renewable sources increases.
- -
- Using batteries as energy sources in heavy transportation, such as trucks or ships, as well as in aviation, is difficult. In general, the high weight of batteries (i.e., low energy density) makes it unfeasible for them to be used as the primary energy source for these types of vehicles.
- -
- There is a limited amount of biomass available to produce biofuels. Fuels derived from fats and exhausted oils can only cover a small percentage of the energy currently employed in transportation. In most countries, the energy provided by biofuels is only 2–5% of the energy provided by oil. Even if all available biomass, including lignocellulosic residues, was employed, only 10–20% of the energy demand will be obtained [1].
- -
- Electrolysers are decreasing in cost [2]. As the cost has decreased, obtaining hydrogen via electrolysis has become easier. In particular, the cost of electrolysers becomes dominant when they are employed for short-time periods [3], as it would happen if only excess electricity was used (i.e., in periods when the production of renewable energy overcomes the electrical system requirements).
Fuels derived from renewable electrical energy, commonly known as e-fuels, include methanol, dimethyl ether (DME), liquid hydrocarbons obtained by Fischer–Tropsch, and ammonia. Synthetic natural gas, i.e., methane obtained by the Sabatier reaction between CO2 and H2, and hydrogen itself when it is obtained from renewable electricity, can also be considered as e-fuels. The processes for the e-fuels synthesis are commonly addressed as Power-to-X, where X indicates the target fuel, and can generally be distinguished into Power-to-Gas and Power-to-Liquids. Power-to-X is a process chain technology: in all cases, the first step for e-fuels synthesis is to produce renewable hydrogen by electrolysis, using green energy (deriving from renewable sources—according to the general definition—typically solar or wind). The second step is the utilization of green-H2 to produce the desired fuel. To this end, the preferred carbon source is carbon dioxide, as this allows production to be coupled with carbon capture solutions. Carbon capture and storage technologies alone allow CO2 to be extracted from the atmosphere, but they are an expensive alternative. Power-to-X technology, on the other hand, allows CO2 utilization and enables the integration of renewable electricity generation with conventional fossil-based systems by mitigating surplus electricity issues through the conversion of electrical energy into chemical energy carriers. These carriers can be stored, allowing them to be reconverted into electricity on demand, thereby enhancing grid stability and reducing fluctuations.
This review will focus on liquid e-fuels that can be obtained from CO2 and renewable hydrogen, i.e., methanol, dimethyl ether, and Fischer–Tropsch liquid hydrocarbons. While methanol and dimethyl ether can be obtained from direct CO2 hydrogenation, Fischer-Tropsch synthesis has been mostly studied from CO + H2 feeds; it therefore requires a first step of CO2 reduction to CO (Reverse Water Gas Shift reaction). Those compounds would make the transition to renewable fuels easier than employing gaseous fuels (like hydrogen and methane) because they are similar to commonly employed fuels like diesel or gasoline. It is worth specifying that CO2 can be employed in other processes [4,5,6], but the amount that could be consumed in the production of chemicals other than fuels would be quite small compared to the huge amounts of CO2 produced in industrial processes (the so-called “Teraton challenge” [7]).
1.1. Types of E-Fuels
1.1.1. Methanol
The vision of a methanol economy was first proposed by George Olah, the Nobel Prize winner in Chemistry [8], and the advances in this field have been described in successive works by this author and his collaborators [9,10,11]. Professor Centi’s group has also advocated for the use of CO2 as a raw material in numerous works [12,13,14,15,16,17]. Aresta has also described his vision in this field in successive publications [18,19,20,21,22,23]. Figure 1 shows several schemes that allow renewable methanol and its possible derived products to be obtained. The literature contains many articles about suitable catalysts for the synthesis of methanol from CO2, which have been recently reviewed [24,25,26,27,28,29,30]. There are also various reviews available on advances in technology for obtaining fuels and chemical products using CO2 as raw material [5,6,31,32,33,34,35,36]. Many reviews focus on the state-of-the-art catalysts [37,38], while others discuss a particular type of catalyst, such as zeolites [39,40], MOFs [28], or bifunctional catalysts [41]. Artz et al. [42] also considered the Life Cycle Assessment (LCA). Despite the large amount of research on direct CO2 hydrogenation, the current industrial catalysts are similar to the classical Cu/ZnO/Al2O3 employed in methanol production from syngas, with small modifications to improve the thermal stability [6]. However, it is clear that selecting the best catalyst is not enough to obtain the best process, but that the most suitable reactor must also be used. At this point, a relatively new line of work in the field of Chemical Engineering is considered: Process Intensification.
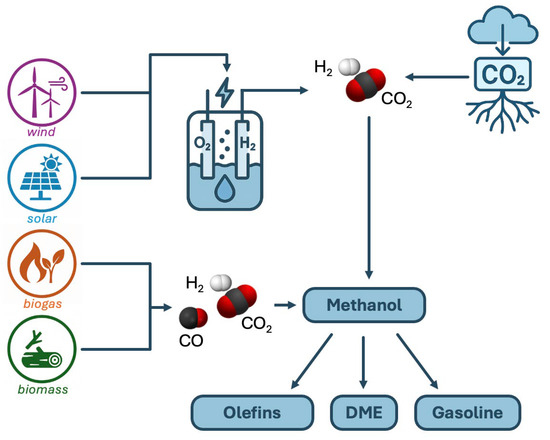
Figure 1.
Methanol production from renewable energy and potential transformation.
Finally, although direct hydrogenation of CO2 to methanol or other liquid fuels will be the focus of this review, another alternative is represented by the two-step synthesis: syngas production from CO2 and hydrogen (a reaction named reverse water–gas shift—rWGS), and then production of methanol from the syngas. The CAMERE Process follows this strategy using methanol [43]. The advantage of using methanol from syngas is that it is a well-established technology, and the reverse water gas shift can be made using conventional catalysts. A comparison of both alternatives provided by Anicic et al. [44] concluded that the direct process is economically preferable.
A track on current methanol projects, including renewable methanol, may be found at the Methanol Institute web page [45]. Among the e-fuels, methanol has historically received the most attention. The reason can be found in the great advantages of methanol: (a) it is a liquid at ambient temperature and pressure, (b) there is no need for further transformations for its use (while Fischer–Tropsch hydrocarbons need additional treatments), and (c) it may be easily converted to other fuels, like dimethyl ether, drop-in hydrocarbons (through the Methanol-to-Gasoline—MTG—process), or olefins (through the Methanol-to-Olefins—MTO—process).
1.1.2. Dimethyl Ether
Dimethyl ether (DME) has several advantages as e-fuel, compared to methanol [46]: higher energy density, high cetane number (which makes it suitable for use in diesel engines), and properties similar to those of LPG (Liquefied Petroleum Gas), which are already used by a significative number of vehicles. It is liquid with a small pressure at ambient temperature. A main advantage of DME over methanol production comes from the higher conversion and yield achievable in thermodynamic equilibrium [47].
1.1.3. Fischer–Tropsch Liquid Hydrocarbons
Fischer–Tropsch is a well-known process for producing hydrocarbons from syngas [48]. The main advantage of hydrocarbons obtained by Fischer–Tropsch is that they can have the same properties as the current diesel or gasoline after suitable treatments, since they are chemically the same. In addition, the diesel obtained by Fischer–Tropsch contains almost no sulfur, and a very small amount of aromatics, which makes it a premium fuel.
It is challenging to foresee which specific e-fuel will dominate the future, or whether a combination of them will be adopted, as it largely depends on the application. Both Dell’Aversano et al. [49] and Dieterich et al. [50] compared different e-fuels.
1.2. Process Intensification
The concept of Process Intensification was described in a book by Stankiewicz and Mouljin [51] and various developments that seek more efficient processes with lower consumption of raw materials and energy, more compact equipment, and less environmental impact have emerged. Many technologies can be considered under the umbrella of Process Intensification. It is often a matter of combining the chemical reaction with a separation, as it occurs in reactive distillation, in membrane reactors, or in reaction with sorption [52]. In other applications, alternative energy sources, such as microwaves or plasma, are employed instead of conventional heating [53,54,55]. Rotating fields are used to increase mass transfer coefficients in multiphase reactions. Structured catalysts may help to achieve better effectiveness and lower pressure drop.
As will be seen in this review, three tools that are widely used in the search for e-fuels synthesis Process Intensification are: membrane reactors, reaction with sorption, and structured catalysts. Membrane reactors seek to combine the chemical reaction with the action of a membrane. The membrane often has the mission of selectively separating one of the reaction products but, as described in the pioneering articles of Armor [56] and Sirkar [57], many other possibilities are available. The membrane can be a way to distribute a reactant, it can have catalytic activity itself, or it can be used to retain a catalyst. Among these and in relation to this review, it is worth highlighting that the relatively recent zeolite membrane reactors offer many opportunities in processes where CO2 is hydrogenated. A complete review of all the developments in the field of membrane reactors would be outside the scope of this article, but interested readers can refer to any of the numerous books that already exist [58,59,60,61,62].
In this work, the use of membrane reactors, reactors with sorption, and structured reactors, as well as some other possibilities for Process Intensification will be reviewed, and the results in the synthesis of methanol, DME and hydrocarbons by Fischer-Tropsch will be discussed. While the indirect production method, from CO2 via the reverse water gas shift, will be considered in all cases, only direct conversion will be considered in the case of methanol and DME, and in the Process Intensification for Fischer–Tropsch, the use of CO + H2 mixtures will be discussed.
2. Membrane Reactors
Membrane reactors represent a class of Process Intensification technologies in which chemical reaction and selective mass transport are coupled within a single unit operation. This integration allows for the dynamic control of reaction equilibria and kinetics, offering significant advantages in terms of conversion, selectivity, and energy efficiency. As briefly discussed in the introduction, membrane reactors utilize a semipermeable membrane—typically made of inorganic (e.g., ceramic, metallic) or advanced polymeric materials—that selectively permits the transport of specific species (reactants or products) based on properties such as molecular size, diffusivity, and chemical potential gradients. The reactor configuration can be categorized broadly into permeation-selective or reactive separation systems, depending on whether the membrane facilitates product removal or reactant dosing.
One of the principal benefits of membrane reactors is their ability to overcome the thermodynamic limitations in equilibrium-limited reactions (Le Châtelier’s principle). With e-fuels synthesis, many reactions are accompanied by water production; water can be efficiently removed from the reacting system using a suitable membrane, shifting the chemical equilibrium towards the products. A schematic representation of the mechanism of a membrane reactor—applied to methanol synthesis—is shown in Figure 2.
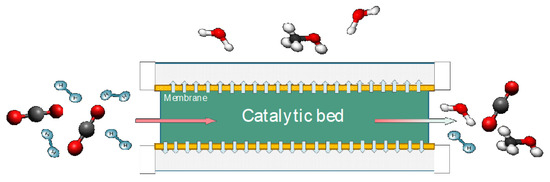
Figure 2.
Schematic representation of the mechanism of a membrane reactor applied to methanol synthesis.
2.1. Membrane Reactors for Methanol
2.1.1. Experimental Works
Membrane reactors are used in CO2 hydrogenation to methanol mainly as a way to remove the water formed in the reaction. In fact, industrial methanol synthesis is carried out from the mixture of CO and hydrogen known as synthesis gas, and this reaction does not produce water as a co-product, according to Equation (1). When CO2 is used instead of CO, on the other hand, the maximum conversion attainable according to thermodynamic equilibrium is lower, which is a serious drawback because it requires an increase in the flow rate of gases that must be recirculated. In addition, a greater quantity of water is formed (Equation (2)) and the usual catalyst in industrial methanol synthesis (Cu/ZnO/Al2O3) is deactivated in the presence of water vapor.
Methanol synthesis in a membrane reactor was initially proposed by Struis et al. [63] by using a Nafion® membrane. They observed, that in methanol synthesis from CO + H2, Nafion® membranes allowed the permeation of methanol selectively compared to CO and hydrogen, thus shifting the equilibrium and allowing a higher yield per step. However, the maximum temperature of use of Nafion® is around 200 °C, which limits its range of applicability, since the kinetics of the reactions are excessively slow at that temperature. The patent by Menendez et al. [64] described a membrane reactor for methanol synthesis in which a zeolite membrane is used instead of Nafion®. Algieri et al. [65] observed that the selectivity of methanol and water vapors versus permanent gases (hydrogen, CO2) of a zeolite membrane was greater when a mixture with water and methanol was fed than when only methanol was present. The first work describing experimental results on the use of a zeolite membrane for the synthesis of methanol from CO2 was published by Gallucci et al. [66], who showed a significant increase in the conversion of CO2 using a membrane reactor. They also observed that the improvement disappeared when the temperature was higher than 240 °C, which is the critical temperature of methanol. In addition, they found that the selectivity to methanol for a given conversion was higher in the membrane reactor. Sea et al. [67] obtained a 150% increase in CO2 conversion by performing CO2 hydrogenation with a silica/alumina ceramic membrane. However, microporous silica membranes are not highly stable under steam, which explains why few further works have been presented with this material. Chen et al. [68] obtained excellent results with a silicone rubber membrane deposited on a ceramic support. This approach could be useful to combine the mechanical strength of a ceramic support with the easy and versatile synthesis of polymeric materials. A very significant advance was the article by Li et al. [69], in which a zeolite membrane was developed from nanoparticles and provided extraordinary water selectivity over the rest of the compounds at the reaction temperatures. This allowed a large increase in yield (e.g., from 13 to 38) and a second important advantage: The methanol obtained in the retentate had a low water content, which would save costs in the subsequent purification stage. In addition, the membrane had a good stability during a 100 h experiment. In addition, those membranes were operated by drawing vacuum on the permeate side, which is much better than the use of a purge in the permeate, as is often found in other works. Seshimo et al. [70] continued the experimental work with a Si-rich LTA zeolite membrane. The advantage of this kind of zeolite compared with conventional LTA comes from the higher stability, a topic that is scarcely considered. For a given set of operating conditions, the conversion in a membrane reactor was 60%, while in the traditional reactor, it was 20%.
2.1.2. Mathematical Models
Numerous researchers have developed mathematical models for this reaction. Struis et al. [71] presented a model for the reaction of CO2 with hydrogen. Menéndez et al. [64] calculated the results of fixed bed reactors and compared them with a combination of a fixed bed and a zeolite membrane reactor; the results showed better performance with the second combination. Barbieri et al. [72] presented a mathematical model using water permeation values taken from Piera et al. [73] with a composite mordenite/ZSM-5 membrane. The model was based on a series of thermodynamic equilibrium reactors in series. Gallucci et al. [74] discussed the difference between countercurrent and co-current configurations, finding that countercurrent was preferable. Later, they showed the achievable results as a function of the ratio of methanol and water permeabilities, showing that a dynamic equilibrium was obtained in the membrane reactor, with greater conversion and selectivity than the thermodynamic equilibrium in a traditional reactor [75]. Farse et al. [76,77] presented a model in which the catalyst deactivation was also included, although they assume that only water permeates, which is not the usual experimental result. Hamedi et al. [78], discussed the importance of selectivity in water permeation to obtain improvements in the reactor performance. In addition, they also evaluated the improvements in power, heating, and refrigerant capabilities, obtaining 1.5%, 44.5%, and 69.4% savings, respectively [79]. Seshimo et al. [70] compared the results of his model with their experimental results, obtaining a good agreement. Mathematical models for membrane reactors usually assume plug flow and a constant permeance and selectivity. A few recent models have considered the potential existence of radial concentration or temperature profiles. Ountaksinkul [80] used Comsol® to model a micro-membrane reactor and found that even with a width of 5 mm, the radial profiles of concentration were clearly seen. With a model using CFD (Computational Fluid Dynamics), Hauth et al. [81] showed the existence of radial profiles of temperature in a reactor with a diameter of 10 mm, which is similar to the values employed in experimental works. The techno-economic analyses [82,83] can also be included within the modeling studies.
As may be deduced from the previous discussion, two categories of model have been employed in the literature of membrane reactors for methanol production: 1D and 2D models. While 1D models assume that radial concentration profiles are negligible, 2D models calculate those radial profiles. The first type has been successful in describing the performance of laboratory scale reactors and would be useful to explore the effect of operating conditions more quickly. Meanwhile, 2D models would be needed for a reliable reactor design if membrane reactors with diameters larger than 5–10 mm were considered.
Mathematical models for other types of membrane reactors, which did not use a zeolite membrane for water removal, have also been developed. Rahimpour and Ghader [84] modelled a membrane reactor based on a distributed hydrogen feed to shift the equilibrium, using a Pd membrane for hydrogen addition and with variable CO/CO2 ratio in the feed. In a subsequent work [85], they simulated a two-reactors methanol synthesis in which methanol production occurs partially in the first reactor, and the second stage is a membrane reactor with the hydrogen being permeated from a fresh feed. They compared countercurrent and co-current operation using two sets of reactors. In the first case, the system operated as a fixed bed, and in the second, it operated as a fluidized bed. In the second case, they obtained an enhancement of approximately 4% in methanol yield. Gong et al. [86] modelled a reactor using an ionic liquid as a sweep liquid to selectively remove methanol from the catalyst bed, which was enclosed in a ceramic tubular membrane. Calculations predict large conversion increases, and experimental data agree well with the model predictions.
2.1.3. Developments in Membrane
A fundamental factor in the efficiency of this type of reactor is the performance of the membrane, which should be highly permeable and highly selective in most applications. Although zeolite membranes are a new material, with the first functional zeolite membranes appearing in the 1990s [87,88,89], a great deal of research has been devoted to the use of zeolite membranes in membrane reactors [90,91,92,93,94]. In many cases, zeolite A membranes have been used in the experimental work when the aim was to selectively remove water, since this kind of zeolite is highly hydrophilic. Gorbe et al. [95] showed the effect of temperature and water partial pressure on the flux and separation factor with a zeolite A membrane. In particular, a clear decrease in the separation factor was found when the temperature increased from 200 °C to 260 °C. The separation factor also decreased when the total pressure was different at both sides of the membrane. Lee et al. [96] found that zeolite A was suitable for separating water from H2 mixtures at low temperatures, but the selectivity decreased at 240 °C. They also observed that the membrane was stable under these conditions. Li et al. [97] has presented a membrane composed of an ionic liquid and a MOF membrane, with an excellent separation factor H2O/H2 of 603 at 200 °C. The developments by Chen et al. [68] and Juárez et al. [98] are also interesting, since they show the possibility of using a ceramic material scaffold for a polymer. Pham et al. [99] have also studied polyimide and Matrimid® membranes in the separation of methanol and water from CO2 and hydrogen at high temperature. They found good selectivity, but the maximum temperature studied was 200 °C. Another interesting development is the one proposed by Seshimo et al. [70], who developed a special type of silicon-rich zeolite A membrane. This has the advantage of being more stable to steam than normal zeolite A. Sawamura et al. [100] showed that mordenite membranes also allow selective water permeation in H2/H2O/CH3OH mixtures, and Hirota et al. [101] also observed selective water prevention with ZSM-5 membranes. Sodalite, another hydrophilic zeolite with small pore size, has been proposed for water removal in the synthesis of methanol or DME [102]. That study examined the separation factor in the interval 125–200 °C and found that sodalite membranes provided an H2O/H2 separation factor of approximately 8. An interesting point with this membrane was that H2O/CH3OH or H2O/DME selectivities were quite large, in accordance with the small pore of sodalite. Silica membranes have been employed in membrane reactors [67], but the water/H2 permselectivity was only between 1 and 3. Otherwise, the stability of silica membranes under a steam atmosphere is quite low, since the transport of silicic acid vapors cause pores to grow. Other interesting materials are carbon molecular sieve membranes, such as the one patented by Rahimalimamaghani et al. [103].
A list of experimental results for the separation of vapors (e.g., water, methanol) from permanent gases (e.g., CO2, H2) using zeolite or carbon molecular sieve membranes is provided in Table 1. As Table 1 shows, NaA zeolite membrane are widely employed, although mordenite also provides good results. In fact, the values provided by Sawamura et al. [100] were obtained with a total pressure in the feed of 7 bar and with the permeate at atmospheric pressure, while the operation with different total pressure in feed and retentate often causes a loss of selectivity. Although some good results have been described with zeolite NaA, large differences between different authors can be seen, which reflects the effect of defects at temperatures of interest for membrane reactors. The best results at high temperature correspond to zeolite membranes prepared from zeolite nanoparticles, which have very few defects [66,104].

Table 1.
Experimental results for the separation of vapors from permanent gases using zeolite membranes.
2.2. Membrane Reactors for DME
DME is currently produced from methanol, but it could be produced from CO2/H2 mixtures, according to Equation (3).
This reaction is produced on bifunctional catalysts that include a metallic active site for methanol production and an acid active site for methanol etherification. It is clear from Equation (3) that a significant amount of water is produced, and therefore, the removal of water from the reaction environment will allow higher yield than in a traditional reactor. This idea was firstly proposed by Diban et al. [113], who employed a mathematical model of a membrane reactor using the kinetic model for the reaction previously developed by the same group [114]. After the initial proposal, more sophisticated models have been published. Diban et al. [115] improved the previous mathematical model by including the non-ideality of the membrane, i.e., by considering some methanol permeance.
This concept was experimentally proven by Rodriguez-Vega et al. [116], who used a zeolite A membrane, and Poto et al. [112,117], who used a carbon molecular sieve membrane. Dong et al. [118] presented a prototype of membrane reactor for DME production using a bundle of hollow fiber zeolite membranes. This reactor provided 1.3 kg/day with a set of seven membranes with a length of 15 cm each. The use of hollow fibers will be interesting for industrial application, since they can provide very large permeation area per unit of reactor volume. Poto et al. [110] developed a 2D heterogenous reactor model, showing that intraparticle mass transfer may decrease the reaction rate under some operating conditions and that concentration polarization may decrease the permeation rate through the membrane compared with an ideal system without radial concentration profiles.
Brunetti et al. [119] studied DME production in a membrane reactor, but used methanol as feed. They used two ZSM-5 membranes, with two different supports (TiO2 and Al2O3 membranes). In that case, the membrane was acting as the catalyst for methanol dehydration to DME, and the reactor was operating in a flow-through configuration, i.e., all the feed was permeated through the membrane. This configuration made a more effective use of the catalyst possible, but it also had a higher pressure drop.
2.3. Membrane Reactors for Fischer–Tropsch
Hydrocarbons are produced from syngas (CO-H2 mixture), that is derived from fossil fuels, using the Fischer–Tropsch process by various companies (Sasol and Shell). The production from CO2 is a more recent proposal [36,120], and it would allow CO2 and H2 that were obtained from electrolysis using renewable electricity to be used.
In the classical way, this reaction also produces a large amount of water, as may be seen from Equation (4).
Membrane reactors for Fischer–Tropsch have been proposed considering a feed of syngas (i.e., CO + H2, with minor amounts of CO2). Experimental results in conditions that simulate the environment in this reaction have shown that zeolite membranes can remove water selectively [121,122], and thus, could be employed to reduce the partial pressure of water in the reaction environment. As may be seen from Equation (4), a large amount of water is formed in the reaction (in molar amounts, much more than hydrocarbons), and thus the water partial pressure can be very high. Water has several negative effects on this reaction: it reduces the reaction rate and, in some cases, may deactivate the catalyst. This concept was experimentally tested by Rhode et al. [123], first using a silica membrane and later [124] using a zeolite membrane (hydroxy sodalite, H-SOD).
Forghani et al. [125] and Rahimpour et al. [126] also have simulated the Fischer–Tropsch reaction in a membrane reactor using a hydrogen selective membrane (Pd-based membrane). The basis of this reactor is the permeation of hydrogen to the reaction area and feeding the hydrogen depleted feed to the reaction area, where hydrogen is distributed (Figure 3).
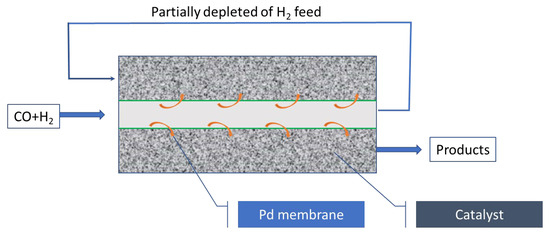
Figure 3.
Membrane reactor for methanol with Pd membrane (selective to H2).
Simulation results show 4.45% enhancement in the yield of gasoline production, 6.16% decrease in the undesired product formations, and a favorable temperature profile along the membrane Fischer–Tropsch reactor in comparison with conventional reactor. Rahimpour et al. [127] proposed a dual-type reactor in which a membrane reactor (with a fixed bed of catalyst in it) with a water selective membrane was followed by fluidized bed membrane reactor with a Pd–Ag membrane (selective to hydrogen). The mathematical model predicted a higher yield to gasoline and lower yield to CO2 in this configuration.
Another type of membrane reactor applicable for Fischer–Tropsch synthesis has been presented by Khassin et al. [128]. These authors call it a “monolith”, but it is a cylindrical pellet with the feed permeating through the pellet, and this could be called a flow-through membrane reactor. They found high values of the “alfa” parameter, which characterizes the Anderson–Schulz–Flory model, and this high value is an indication of high selectivity to C5+.
When each catalyst particle is surrounded by a membrane, this configuration can also be considered as a membrane reactor. It has been tested in Fischer–Tropsch, showing an improved selectivity to C5–C10 hydrocarbons with a Co/SiO2 catalyst covered by a ZSM-5 membrane, since the exit of larger hydrocarbons from the catalyst particle is hindered by the membrane [129]. An extension of this concept used carbon-coated catalysts [130]. The idea of a membrane covering each catalyst particle may be advantageous compared to the traditional concept of a catalytic bed surrounded by a membrane. In the second case, when something affects the membrane (for example, a leak point), it may harm the performance of the whole reactor; however, in the first case, only the performance of the one pellet is affected. A combination of a ZSM-5 membrane covering a Co-Al2O3 monolith [131] showed excellent yield to gasoline while keeping the same conversion as the monolith. In that case, the authors say that the membrane contributes additional catalytic activity (cracking and isomerization). Monoliths are a kind of structured reactor widely employed for Fischer–Tropsch, as will be discussed below.
2.4. Summary and Outlooks for Membrane Reactors
To ensure the successful deployment of this technology, several key areas must be addressed. First, scaling to higher Technology Readiness Levels (TRLs) is essential to transition from laboratory-scale proof of concept to industrial-scale application. This involves not only optimizing system integration but also validating performance under real-world conditions. Second, the long-term stability of materials, particularly the zeolite-based membranes, requires further investigation. While current results demonstrate promising selectivity and efficiency, extended operational testing is necessary to assess durability, resistance to degradation, and the impact of potential fouling agents or contaminants over time. Finally, a comprehensive techno-economic analysis is critical. This includes both Capital Expenditures (CAPEX) and Operational Expenditures (OPEX) to evaluate the cost-competitiveness of the technology compared to conventional alternatives. Lifecycle cost modeling and sensitivity analysis will support informed investment decisions and identify key economic bottlenecks.
3. Sorption-Enhanced Reactors
The concept of sorption-enhanced reactions originated from the need to improve the efficiency of chemical processes by simultaneously conducting reaction and separation in a single unit. Along with water removal through membrane separation, the sorption-enhanced reaction (SER) technologies leverage the well-known Le Châtelier’s principle to improve a process performance. Unlike the previous configuration, water deletion from the reaction environment is produced by its sorption into a specific solid, which is generally put in contact with the catalyst, as shown in Figure 4 (exemplificative scheme in case of methanol synthesis enhanced by water removal).
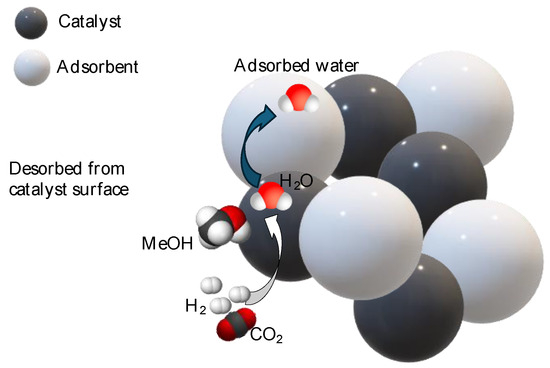
Figure 4.
Scheme of sorption-enhanced processes (example of methanol sorption-enhanced synthesis).
The idea of removing a product from the reaction environment to enhance reaction efficiency is rooted in the principles of pressure-swing adsorption, which allows for the periodic regeneration of the sorbent.
This approach was first explored in processes like the water-gas shift reaction and steam-methane reforming, where the removal of a reaction by-product, such as carbon dioxide, can shift the equilibrium towards the desired products, thereby enhancing conversion rates and product purity. One of the earliest studies on sorption-enhanced reactions involved the equilibrium-controlled reverse water-gas shift reaction for carbon monoxide production. This study demonstrated the feasibility of using a fixed packed column containing a mixture of catalyst and sorbent to selectively remove a reaction by-product from the reaction zone, thereby achieving high conversion rates at lower temperatures than traditional methods [132]. This concept was further developed in the context of hydrogen production via steam-methane reforming, where a chemisorbent was used to selectively remove carbon dioxide, resulting in high-purity hydrogen production [133,134]. Additionally, the sorption-enhanced water-gas shift reaction (SE-WGS) for hydrogen production has been studied, where carbon dioxide is removed through chemisorption on CaO particles, leading to higher-than-equilibrium hydrogen production [135]. This approach has been applied to various processes, including methanol synthesis, where the in-situ removal of water byproduct helps overcome thermodynamic limitations and improve methanol yield [136,137].
In the framework of e-fuels synthesis, water sorption has the benefits already explained in the previous sections. SER processes, intended as the ones in which water removal is produced by its sorption onto a specific solid, can produce the same effects in the enhancement of reaction performance as in the membrane reactor, but with important advantages. To begin with, their frailty is a huge drawback for membranes; once a membrane is damaged in a single point, its separation efficiency is completely disrupted, and it must be entirely substituted. At the same time, the dispersed and non-continuous nature of a sorbent guarantees that even in the case of partial deactivation, the remaining amount of solid would keep its separation function, with a minor repercussion on the whole process. In addition, SER processes are easier to operate and scale up; they do not need different piping for the retentate side, nor a sweep gas for aiding the separation. Furthermore, the spatial limitation that can exist when using a membrane does not exist when working with sorbent solids. Ultimately, membranes are fragile, making them suboptimal in high-pressure processes, and when applied specifically to methanol/DME processes, they must fulfill the requisite of high selectivity toward the permeation of water but not methanol, which is still a significant issue in membranes research [138].
On the other hand, sorption-enhanced processes have their drawbacks as well. Since water is trapped into the sorbent, it undergoes saturation overtime; therefore, the process can suffer from a periodic efficiency decrease and requires regeneration steps to operate. Furthermore, sorption effectiveness is strictly related to the operating temperature, which is often in contrast with the requirements of the chemical reaction carried out during the process. Even though many reactions are conducted at relatively low temperature (and this is the case with methanol and DME synthesis, as well as the Fischer–Tropsch process, which occur below 300 °C), for kinetic reasons, the operating condition cannot involve very low temperatures, which affects the water sorption capacity and time of breakthrough.
3.1. Sorption-Enhanced Methanol Synthesis
3.1.1. Experimental Studies
The pioneering work of Westerterp et al. [139] introduced the countercurrent gas–solid–solid trickle bed reactor as an innovative approach to methanol synthesis. As reported by the authors, the trickle flow principle was already well-known in literature, even though it was mainly applied to the heat exchangers field. In these first applications, the contact between a sand-like solid and a gas was enhanced by a packing material; this is now widely employed in sorption columns. This configuration was called a gas–solid–solid trickle flow (GSSTF) contractor. At the time, the possibility of substituting the inert packing with a reaction-involved solid had never been evaluated. Hence, they proposed creating the column packing using a combination of a pellet catalyst and Rashing rings, and to employ a top-to-bottom sorbent stream in countercurrent with the gas. The study was first performed as a mathematical model, which already assessed the potential of such an application to achieve equilibrium conversion by leveraging in-situ water removal. Further experimental validation [140] confirmed the viability of this system, showing that the GSSTF configuration significantly improved methanol yields. In detail, the authors demonstrated through their experiments that complete conversion in single-pass operation is attainable during methanol synthesis. It worth noticing that these works dealt with methanol synthesis from syngas. According to the stoichiometry of the reaction, methanol is the only product of the process, in the absence of competitive reactions. Therefore, in these earlier studies, methanol, and not water, was actually separated via sorption. Nevertheless, the technology applies the same principle as water separation. These foundational studies set the stage for further advancements in sorption-enhanced methanol synthesis (SEMS).
In the following years, the topic of CO2 utilization has become significant, and the studies on methanol synthesis followed this trend. Methanol production from CO2 hydrogenation involves simultaneous water and methanol generation. In this case, selective separation of one of the two reactants is more complicated, and many aspects favor water sorption instead of methanol. The most conventional sorbents for this application are zeolites, which are generally more selective toward water sorption [141], even though factors other than the simple molecular sieve mechanism drive the sorption selectivity toward water, since the molecular size is comparable [142].
Among recent studies, Terreni et al. [143] provided an early demonstration of SEMS using 13X zeolite as the sorbent for the Process Intensification and highlighted exactly this issue. The authors observed the enhancement of methanol yields by using hydrophilic sorbents, with a maximum increase of 28% in methanol yield. Nevertheless, they reported that, at 200 °C methanol and dimethyl ether, both obtained as products, were completely sorbed onto the zeolite during the sorption-enhanced process. At higher temperatures, the sorption of the products was not total, but it was still competitive with water. With the increase in temperature, the authors were able to recover half of their products previously sorbed into the zeolite. Finally, they concluded that 13X zeolite was appropriate for water sorption and process enhancement, but not ideal for the purpose of SEMS as it also adsorbs other reaction products. Therefore, the Le Châtelier principle is applied to multiple reactions, and controlling the selectivity is difficult. The authors hypothesize that the employment of a zeolite which had pores with a smaller radius could lead to better selectivity management since the intra-crystalline activity would be limited.
It is also worth noting that the incorporation of a zeolite into a methanol synthesis system not only induced products sorption. The presence of strong acid sites—particularly Brønsted-type sites—promotes methanol dehydration and the formation of dimethyl ether. Among the key properties of zeolites, their surface acidity, the nature of their acid sites, and their hydrophobicity play a crucial role in this process. HZSM-5 zeolites are well known for their efficiency as methanol dehydration catalysts, with their hydrophobicity, as reported by Vanoye et al. [144], being a critical factor for achieving high DME productivity. However, other types of zeolites, such as LTA, FER, and SAPO, also exhibit dehydration activity and are employed in methanol dehydration [145]. Moreover, in addition to DME, light olefins can also be produced over zeolites [146].
To disclose the catalytic effect from sorption, Nikolic et al. [147] extended this research by employing inelastic neutron scattering (INS) techniques to investigate the molecular interactions between catalysts and sorbents. Their study provided fundamental insights into how different sorbents influence reaction pathways, demonstrating that zeolites play a dual role in shifting equilibrium and stabilizing intermediate species. The study was performed using a so-called “sorption catalyst”, constituted by a copper-zeolite-supported formulation, and mixture of commercial CZA catalyst with either 3A or 5A zeolite. Other than the different selectivity caused by the employment of one catalytic system or the other (the authors clearly state that methanol selectivity is higher when 3A is employed as the small pores are inaccessible by methanol, preventing further conversion), they observed a similar evolution in time of the process. Water was quickly formed as the reactants entered the catalytic bed as the product of the reverse water-gas shift reaction, and it began to saturate the sorbent. Conversely, the formation of methanol and dimethyl ether occurred at a relatively later stage and was not associated with significant water production. This suggests that a substantial amount of intermediates (CO, methoxy, and methyl groups) forms in the early stages, accumulating within the sorption catalyst before ultimately reacting to yield the final products.
From this study, it appears that sorption-enhancement is not really applied directly to methanol formation; instead, it induces CO2 conversion to CO and other intermediates, and their amount and conversion rate finally determines the yields to methanol and/or dimethyl ether.
Maksimov et al. [137] further studied SEMS via CO2 hydrogenation, offering a more macroscopic observation of the reaction enhancement. Based on their experiments and calculations, the enhancement factor was in the range of 150–290% for methanol and 220–510% for CO. These results are more convincing regarding the efficiency of the SEMS process, but it worth noticing that they were obtained at higher pressures compared to previous studies (20–70 bar). The authors themselves stated that the enhancement factor is remarkably influenced by the operating pressure; furthermore, their result somehow confirms the INS observation, since CO production was promoted to a higher extent compared to methanol synthesis. It is also worth mentioning that the work involved not only the evaluation of SEMS at different pressures, but also at different space velocities and catalyst-to-zeolite ratios. Other than the differences in productivity obtained in each condition, the obtainment of a higher enhancement factor for CO production was not affected by any of the variable. Hence, there does not appear to be a way to tune the system to enhance methanol production instead of CO.
This can lead to the conclusion that the catalyst formulation is mainly responsible for the selectivity of the system, which is also the case in SEMS.
Heracleous et al. [148] conducted a detailed experimental investigation into SEMS for CO2 hydrogenation, further validating the efficacy of sorption-enhanced techniques. Their findings confirmed that selective water removal significantly improves methanol selectivity and yield, reinforcing earlier theoretical predictions. By using high pressure and 13X zeolite, their work can be compared to the previous studies separately, concluding that the 60.5% enhancement observed in this work is halfway between previous results, since the type of zeolite has worse selectivity toward methanol, but high pressures improve the results, as already mentioned by Maksimov et al. In a subsequent study, Heracleous et al. [149] explored the impact of different zeolite types on SEMS performance, revealing that sorbent properties such as pore structure and hydrophilicity are key factors in optimizing the process. With this study, the authors highlighted that the better enhancement factor was obtained with 5A zeolite because of its methanol uptake ability. Nevertheless, the authors did not observe as much of a difference in selective methanol enhancement compared to the study by Maksimov et el. [137], at least for what was considered the first reaction cycle. Afterwards, the authors also evaluated the stability of sorbents and catalyst over three cycles and observed that all zeolites lost sorption efficiency, leading to lower methanol and CO yields. In addition to the catalyst’s decrease in activity, among the sorbents, the 13X zeolite was the one that exhibited the major yield decrease, while the 4A-CZA was found to be the most stable SEMS system. This result is coherent with the study conducted by Gavrilovic et al. [150], who evaluated various catalyst–sorbent pairings, including CaO, which was first considered for SEMS. The study of the sorbents can be considered preliminary, since it only evaluated the sorption ability of all sorbents, concluding that among zeolites, 13X was the one with the highest sorption capacity, but it was overcome by CaO. Despite this result, the CaO stability over saturation/desorption cycles was not investigated, and only zeolites 13X and 4A were tested. The authors reported that 4A zeolite outstood 13X in terms of stability, since it almost did not lose sorption capacity over 100 cycles, while 13X reduced its capacity to the half. These studies mark a critical step toward industrial scalability, demonstrating that careful material selection can lead to substantial process improvements.
From the literature survey, it is possible to observe that SEMS studies have been conducted in a limited range of operating conditions and types of sorbents. Zeolites 3A, 5A and 13X have been studied most frequently, and as reported, 3A zeolite is the most suitable sorbent for SEMS, especially for its selectivity toward methanol. Many authors have attributed this characteristic to a shape selectivity, since the 3 Å pores of the zeolite allows the sorption of water but not methanol. On the other hand, 5A zeolite was demonstrated to be a significant alternative since it has a strong sorption capacity. 13X zeolite, despite being the first choice in former studies, was demonstrated to have a low selectivity and a scarce stability overtime in cycles of sorption/regeneration. Figure 5 shows a bubble graph of the different SEMS studies. This representation highlights the most employed combinations of operating conditions (temperatures mostly between 230 and 260 °C and pressures around 30 bar) together with the magnitude of methanol enhancement factor obtained with each kind of zeolite. The dimension of the bubble is proportional to the magnitude, and the maximum value is indicated in the figure.
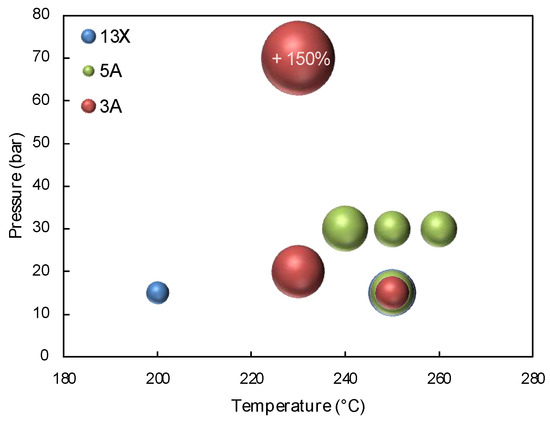
Figure 5.
Methanol enhancement factor in SEMS experimental studies vs. operating conditions (temperature and pressure). The dimension of the bubble represents the magnitude.
As the previous literature review demonstrates, most of the sorption-enhanced applications have been studied so far in fixed bed reactors. These reactors can be mainly operated in two ways, which are shown Figure 6a,b. In the first case, the reactor alternates reaction and regeneration cycles, which is the operating condition of a lab-scale reactor. At higher TRLs, it is necessary to operate as scheme (b), with parallel reactors alternating reaction and regeneration cycles, to avoid affecting the process’s productivity. In recent years, Menéndez et al. [151] proposed and patented a technology to conduct sorption-enhanced reactions in a fluidized bed with continuous flux of one of the solids. This can be referred to as continuous sorbent flow fluidized bed (CSFFB). The proposed technology involved a fluidized bed reactor in which the sorbent and the catalyst have significantly different physical properties—mainly particle size and density—so that they segregate in the fluidized bed. If segregation is optimized, either at the top or to the bottom, a fraction of almost 100% of one of the solids can be reached (100% of flotsam for the top section and 100% of the jetsam for the bottom section). Hence, it is possible to selectively remove either the catalyst or the sorbent from the reactor, to perform the regeneration of the sorbent without damaging the catalyst. Also, if the sorbent is chosen to be the continuous flux and the catalyst remains fluidized into the reactor, it is possible to operate both the process and the regeneration step continuously. This concept is illustrated in Figure 6. A preliminary assessment of this application was presented by Menéndez et al. [152]. In this study, the authors only evaluated the feasibility of the proposed technology by continuously segregating a methanol synthesis catalyst and a sorbent (13X zeolite), demonstrating that with opportune fluidization condition an efficient separation is possible, allowing the technology to be proficiently employed. Furthermore, since zeolite can provide water removal, the proposed system can be used in all the processes which have water as by-product. Methanol synthesis from CO2 is one such process, but it can also be applied to DME synthesis and CO production via reverse water–gas shift.
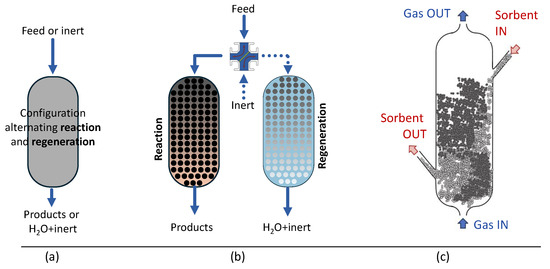
Figure 6.
Continuous sorbent-flow technology proposed by Menéndez et al. [151,152] (c) compared to fixed bed reaction with alternating operations (a) and parallels fixed bed reactors (b).
3.1.2. Mathematical Modelling
Mathematical modelling has limitations in predicting the behavior of experimental systems, mainly due to the assumptions made or the equations chosen. Nevertheless, it remains a powerful tool for saving time and costs in experimental work, gaining insight into phenomena that cannot be directly observed, and supporting the scale-up of technologies. Sorption-enhanced processes are particularly studied with mathematical modeling because of their complex nature and the challenges of experimentally capturing all the interacting phenomena involved. Also, mathematical modelling allows researchers to use a significantly higher amount of data compared to experimental work, offering a better estimation of the process costs.
Arora et al. [153] presented a detailed modelling study on optimizing methanol production through a sorption-enhanced reaction process, aiming to overcome equilibrium limitations that restrict conversion efficiency in conventional methanol synthesis. The authors proposed a periodic SEMS system that integrates in-situ water removal via selective sorption, thereby shifting the reaction equilibrium toward higher methanol yields. The study employed a fixed-bed, multi-tubular reactor where a Cu-ZnO-Al2O3 catalyst was combined with NaX zeolite sorbent, designed to selectively capture water during the reaction. To account for the sorbent finite capacity, the system was modeled to operate in a cyclic manner, alternating between reaction-sorption and regeneration phases. The model considered operating conditions typical of industrial methanol synthesis, i.e., temperatures between 200 and 270 °C, pressures up to 77 bar, and a typical synthesis gas composition (65.9% H2, 9.4% CO2, and 4.6% CO, and smaller amounts of CH4, N2, CH3OH, and H2O). The study found that optimizing the ratio of reactants, particularly adjusting CO2 and H2 levels, significantly influences conversion efficiency. By carefully tuning these parameters, the authors demonstrated that SEMS process achieve remarkable improvements in yield while maintaining competitive production costs. A key finding of the study is the substantial increase in methanol yield compared to conventional processes. Without sorption-enhancement, the base-case methanol yield is reported at 34.02%. When the SEMS process is applied, yield increases to as much as 59.92%, representing a 76.13% improvement. However, this enhancement came with a trade-off. Due to the periodic nature of the sorption–regeneration cycle, overall production capacity is reduced by between 9 and 46%, depending on the specific optimization scenario. Despite this reduction, the authors demonstrated that careful process optimization can mitigate losses, with a 7% yield gain achieved in an industrial reactor setup at the cost of only a 2% decrease in production capacity. With these data, the study further explored the techno-economic feasibility of SEMS, showing that the production cost remains competitive with traditional syngas-to-methanol processes. The authors emphasized that the balance between yield improvement and production capacity reduction is a crucial factor in determining the industrial viability of SEMS. They identify synthesis gas composition, reactor temperature, and pressure as primary determinants of process performance. Additionally, they highlight the importance of optimizing the sorbent regeneration phase to minimize downtime and maintain continuous operation.
Nieminen et al. [154] developed and modeled a full process configuration for CO2-based sorption-enhanced methanol synthesis. The process was considered as a cyclic system with alternated reaction-sorption and sorbent regeneration steps, using either adiabatic packed-bed or isothermal boiling-water reactors. According to their findings, considering an operating pressure of 50 bar and a temperature of 220 °C for the isothermal configuration and initial temperature of 215 °C for the adiabatic one, water sorption shifts the equilibrium toward methanol production, improving per-pass conversion and yield. Many other process parameters, such as flowrate and catalyst/sorbent ratio, were optimized by the authors to achieve a target purity of methanol. As they reported, the model was able to predict a configuration that eliminated the need for distillation by producing high-purity crude methanol (99 wt.%) through condensation and separation of unreacted gases. This result highlighted the feasibility of trapping all the water produced by the reaction into a suitable sorbent, leading to the condensation of almost pure methanol. Economic analysis suggested that cost reductions could be achieved through further optimization in cycle design, reactor configuration, and separation methods.
Bayat et al. [155] introduced a Fluidized Bed Sorption-Enhanced Thermally Coupled Reactor (FSE-TCR) to enhance methanol synthesis. The system integrates methanol production with in-situ water sorption in a fluidized bed while using the exothermic heat to drive the dehydrogenation of cyclohexane on the endothermic side. The study employs a multi-objective optimization approach using the NSGA-II algorithm to maximize methanol production and selectivity. The FSE-TCR reactor was further optimized in terms of temperature profile to achieve the maximum enhancement in methanol yield. As reported, optimization results indicate significant improvements in methanol yield (214.3 ton/day) and CO2 removal (280.5 ton/day) compared to conventional methanol synthesis reactors. Furthermore, temperature-optimized FSE-TCR ensured a 55.97 ton/day higher methanol yield compared to the non-optimized configuration. Even though the results can be seen as hardly replicable in an experimental methanol synthesis system, they are still of high interest since they highlight the feasibility and the potentiality of the proposed technology.
Abashar and Al-Rabiah [156] investigated the performance of a circulating fast fluidized-bed reactor (CFFBR) for sorption-enhanced methanol synthesis. The study employed a mathematical model, validated using industrial data from a quasi-isothermal Lurgi reactor. The authors simulated reactor conditions with a pressure of 50 bar, a temperature of 201.75 °C, and a stoichiometric feed ratio of H2/CO2 = 3:1, evaluating different sorbent-to-catalyst mass fractions. The results demonstrated that the CFFBR significantly enhanced methanol production, with a 14 times higher yield compared to the process without water sorption conducted in the same type of reactor. The study further revealed that CFFBR offers superior performances compared to fluidized bed reactor, which the authors attributed to bubble bypass and backmixing effects.
In a further study [157], the authors demonstrated that CFFBR has better performances compared to a reactor with in-situ condensation (IC), with an enhancement of approximately 88% for the former and 60% for the latter. If, on one hand, the sorption was more efficient than condensation in displacing the chemical equilibrium, the authors also demonstrated that excessive sorption inhibited the WGS equilibrium, causing it to favor the CO existence and lower the process’s selectivity to methanol.
Moioli and Schildhauer [158] conducted a computational investigation into small-scale SEMS to evaluate the difference in the type of reactor employed for the process. The study evaluated a fixed-bed, an entrained-flow, and a fluidized-bed/entrained reactor. The latter application recalls the one previously described Menéndez et al.’s patent [151]. The patent claims to segregate the solids while keeping them both in a fluidized bed regime; this technology involves the full entrainment of one of the solids, specifically the sorbent, which flows across a fluidized/almost-suspended bed of catalyst. The models considered a CO2/H2 feed ratio of 1:3, a pressure of 30 bar, and a temperature range of 220–300 °C, simulating conditions relevant to small-scale methanol production. The fixed-bed reactor was modeled as a dynamically operated system, where one reactor was in reaction mode while another underwent regeneration. Results indicated that while the water removal significantly shifted the thermodynamic equilibrium toward methanol formation, the process was limited by the need for frequent reactor switching, making continuous operation complex. The entrained-flow reactor, in contrast, allowed for continuous operation due to simultaneous catalyst and sorbent exchange. However, the short residence time reduced methanol selectivity, favoring the competing reverse water–gas shift (rWGS) reaction, leading to an increased CO yield. The fluidized-bed/entrained reactor, which combined entrained sorbent flow with a suspended catalyst bed, demonstrated the most balanced performance, achieving higher CO2 conversion and methanol selectivity while maintaining continuous operation. The work is highly valuable, especially to validate the idea of selective removal of one of the solids from the reactor. Nevertheless, it surely can be considered a difficult application because of the complications of experimentally realizing the suspension of one solid and the entrainment of another. Furthermore, many other factors, such as diffusion, which can limit both catalysis and sorption, will occur experimentally and will probably lead to worse performances compared to the theoretical study.
Nevertheless, comparing the SEMS simulation results with the experimental data in Figure 7 shows that the predicted enhancement factors are reasonably close to the experimental values, suggesting that the simulations provide good predictive capability for existing systems.
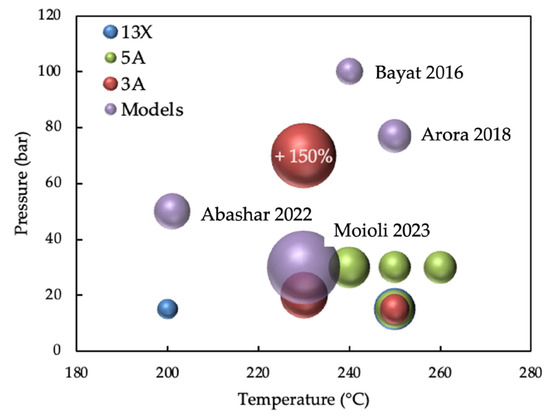
Figure 7.
Methanol enhancement factor in SEMS studies vs. operating conditions (temperature and pressure) for experimental and mathematical studies [136,155,157,158]. The dimension of the bubble represents the magnitude.
3.2. Sorption-Enhanced DME Synthesis
The sorption-enhanced approach has recently been extended to dimethyl ether synthesis as well. Although interest in DME production emerged later than in methanol or CO synthesis, it has steadily gained a growing share of research attention over the years. Similar to methanol synthesis, DME production benefits significantly from water removal; moreover, due to the higher water-to-DME ratio compared to methanol, the enhancement effect could be even more pronounced.
It is clear, however, that sorption-enhanced DME synthesis (SEDMES) is more complicated than SEMS. Indeed, DME is produced via methanol dehydration, and to perform a single-step conversion from CO/CO2, the systems need two co-working catalysts, one dedicated to methanol synthesis and the other to its dehydration [159]. The properties of these catalysts are significantly different, meaning that there is no way to conduct a one-step process on a single formulation. Typically, basic sites are needed for CO2 activation while acid sites are necessary for methanol dehydration [160]. In most cases, this requires the coexistence of two solids in the reactor, and the presence of a water sorbent would add a third. The mechanism of reaction + sorption would be the one depicted in Figure 8. Hence, aspects like internal diffusion or heat management must be related to three different components. Some authors have reported the use of a single solid, addressed as bifunctional catalyst, to conduct the direct DME synthesis [161,162]; the employment of such a solution would make the SEDMES process more similar to SEMS.
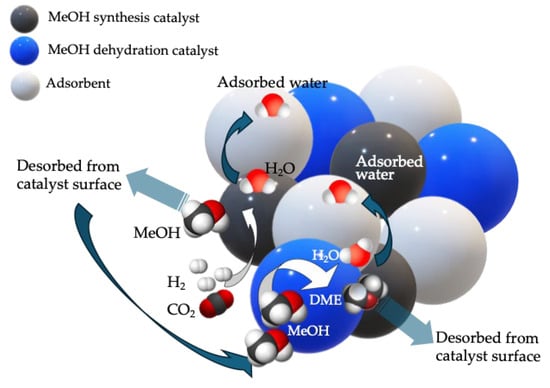
Figure 8.
Scheme of sorption-enhanced DME synthesis in presence of three solids: the methanol synthesis catalyst, the methanol dehydration catalyst and the water sorbent.
3.2.1. Experimental
Sorption-enhanced DME synthesis conducted in the presence of sorbents physically mixed with the catalyst is a novel topic, and there are not many studies on this subject, to the best of our knowledge. One of the earliest studies on SEDMES was conducted by Liuzzi et al. [163]. This study investigated the direct synthesis of dimethyl ether from CO2-rich biomass-derived syngas using a dual catalytic bed composed of Cu/ZnO/Al2O3 (CZA) for methanol synthesis and γ-Al2O3 for methanol dehydration. Experiments were conducted with a syngas mixture featuring a CO2/CO molar ratio of 1.9, reflecting typical biomass gasification outputs. In their study, the authors first observed that the addition of promoters (ZrO2 and Ga2O3) to the methanol synthesis catalyst increased the extent of the side reactions, mainly rWGS, leading to higher CO and water production. Then, they reported that the water-rich atmosphere of the system was detrimental for catalyst stability, and therefore, the enhancement obtained by doping the catalyst actually led to a worsening of the performances over time. To mitigate the detrimental effects of water, zeolite 3A was incorporated as an in-situ water sorbent, establishing a sorption-enhanced process. The inclusion of the zeolite shifted reaction equilibria, enhancing both methanol and DME production. Operating at 250 °C and 20 bar, the DME yield increased dramatically, from 8.7% without sorption to 70% with the zeolite. Carbon conversion surpassed conventional equilibrium limits due to the continuous water removal, confirming the viability of SEDMES in intensifying DME production from CO2-rich syngas.
Similar results were obtained by Altinsoy et al. [164], who explored direct CO2 hydrogenation to DME using a physical mixture of commercial Cu–ZnO/Al2O3 (CZA) and synthesized phosphotungstic acid (PTA)/γ-Al2O3 catalysts. Both methanol synthesis and dehydrogenation catalysts are similar to the previously revised study, but the authors modified the γ-Al2O3 to ensure better dehydrogenation performances. Reaction intensification was achieved by incorporating in-situ steam separation via zeolite 3A sorption. Experiments were conducted at 225 °C, 30 bar, H2:CO2 ratio of 3:1, and a gas hourly space velocity (GHSV) of 1750 h−1 based on the CZA catalyst volume. The study optimized PTA loading, identifying 30 wt.% as optimum concentration. By operating the system with a CZA:PTA/γ-Al2O3 mass ratio of 1:2 as the best configuration, achieving ~21% CO2 conversion and 6.3% DME yield. Integration of 3A zeolite, tested at catalyst-to-sorbent mass ratios of 1:0.33 to 1:4, elevated CO2 conversion and DME yield beyond equilibrium values. At the ratio of 1:4, they found that catalyst productivity increased from 5.5 × 10−3 to 2 × 10−2 kgDME h−1 kgcat−1. Both catalysts and sorbent demonstrated stability over multiple pressure swing sorption–regeneration cycles, showcasing the potential of sorption-enhanced systems in overcoming thermodynamic limitations of CO2 hydrogenation.
Van Kampen et al. [165,166] experimentally validated the SEDMES process at bench-scale under industrially relevant conditions using pressure swing regeneration (PSR). The system operated between 250–300 °C and 25 bar with a catalyst-to-sorbent mass ratio of 1:4. Also, in the case of this study, a CZA catalyst and γ-Al2O3 dehydration catalyst were mixed with zeolite 3A for in-situ water sorption. The study demonstrated that PSR allowed for high-performance water removal, achieving over 80% single-pass carbon selectivity to DME and a fourfold increase in productivity. The authors developed and validated a 1D dynamic reactor model integrating Graaf’s methanol synthesis kinetics [167], Berčič’s methanol dehydration kinetics [168], and a Sips isotherm for water sorption [169]. The Sips isotherm is a hybrid sorption model that combines features of both the Langmuir isotherm (which assumes homogeneous sorption sites with a maximum capacity) and the Freundlich isotherm (which accounts for surface heterogeneity). Cycle optimization indicated that PSR enabled shorter cycles, improving productivity while maintaining high selectivity, confirming SEDMES’s potential for efficient CO2 utilization and intensification.
Ozcan et al. [170] also reported enhancement in DME production using sorption-enhanced processes, although the application used was different. They evaluated the effect of CO2 sorption onto Huntite, a carbonate mineral with the chemical formula Mg3Ca(CO3)4. They reported an enhancement in DME selectivity from 56% to over 60% due to the removal of CO2. Nevertheless, this result cannot be compared to the effect of Le Châtelier equilibrium displacement obtained with water removal. Indeed, to the best of our knowledge, this is the only study that evaluated the sorption of CO2 instead of water.
As can be observed, according to the comments in this paragraph regarding introductive section, all these studies involve the utilization of three solids: a methanol synthesis catalyst (typically CZA), a methanol dehydration catalyst (typically γ-Al2O3), and a water sorbent (mainly 3A zeolite). So far, no studies have been reported using a bifunctional catalyst for the one-step DME synthesis and a water sorbent; hence, this can be considered one of the challenges for future works on the topic.
The concept of continuous sorbent flow fluidized bed reactors described for sorption-enhanced methanol synthesis [151,152] can also be applied to SEDMES, as long as the solids can be reduced to two. This can be completed using bifunctional catalysts or a sorbent which can also address methanol dehydration. A preliminary assessment of this application has been conducted by the same research group in [171].
3.2.2. Model
As with SEMS, many theoretical studies have been published regarding SEDMES processes, with the same limitation. Nevertheless, all the previously discussed aspects on the potentiality of the mathematical models also apply also in this case. Guffanti et al. [172] developed a detailed 2D + 1D heterogeneous dynamic model to investigate the performance of a multitubular fixed-bed reactor for SEDMES. The model described the coupled reaction and sorption steps and accounted for axial and radial gradients in species concentrations and temperature. The expression “2D + 1D model” means that a 2D model was employed for solving mass and energy balances for the gas phase, while a 1D model was adopted to describe the radial concentration profile into each particle; for this model, only the mass balance was performed, as particles were assumed to be isothermal. Validation was performed using experimental data from a bench-scale unit (2 m length, 3.8 cm internal diameter) operated with a CO/CO2/H2 mixture. The system operated at 250 °C and 25 bar, utilizing a physical mixture of Cu/ZnO/Al2O3 (CZA) and γ-Al2O3 catalysts with zeolite 3A as the water sorbent. A catalyst-to-sorbent weight ratio of 1:4 was also employed in this case, and 1:1 methanol and dehydration catalysts weight ratio was adopted. Simulations revealed that effective in-situ water removal enabled DME yields of 65–70%, largely independent of the CO/CO2 ratio in the feed. Increasing CO content slightly raised peak temperatures due to accelerated reaction kinetics. Nonetheless, the use of sorbent material effectively controlled maximum temperatures, maintaining them 20–30 °C below the 300 °C catalyst stability threshold. Furthermore, the study demonstrated that reactor tubes up to 46.6 mm in diameter could be utilized with less than 2% loss in DME yield, offering potential scale-up benefits. This modeling framework provided essential insights for designing industrial-scale SEDMES reactors with optimized thermal management and performance. A subsequent study [162] performed a comprehensive analysis of the interplay between kinetics, sorption capacity, and transport phenomena in SEDMES using the 2D + 1D dynamic model already validated. The model simulated the cyclic sorption/reaction step under isobaric conditions at 25 bar and 250 °C with external cooling. The reactor was packed with a physical mixture or advanced structured catalysts (hybrid and core-shell designs) of CZA and γ-Al2O3, combined with zeolite 3A as the water sorbent. Various sorbent-to-catalyst ratios (2:1 to 16:1 by weight) and pellet configurations were examined to quantify their impact on DME yield, productivity, and thermal behavior. Results demonstrated that increasing the sorbent ratio enhanced water removal, sustaining higher DME yields (>70%), but reduced productivity due to catalyst dilution. Conversely, low sorbent ratios led to faster water saturation, diminishing DME yields. Intraparticle diffusion limitations were significant, particularly in conventional pellet mixtures, but were mitigated by decreasing catalyst particle sizes or adopting hybrid/core-shell structures, which improved mass transfer and DME productivity. Thermal analyses showed that maximum temperatures were well-controlled due to the thermal buffering effect of the sorbent.
Iliuta et al. [173] developed a two-scale, isothermal, unsteady-state model to investigate sorption-enhanced dimethyl ether synthesis with in-situ H2O removal. The fixed-bed reactor was considered packed with a homogeneous mixture of bifunctional catalysts and zeolite 4A sorbent, with axial dispersion considered due to the low gas velocity. The model coupled bulk-scale mass, momentum, and energy balances with particle-scale diffusion and reaction equations and used the hypothesis of single species sorption: only water is considered to be sorbed by zeolite. The Unilan isotherm [107] described water sorption. Simulations demonstrated that in-situ water removal remarkably favored methanol and DME yields, and that the unconverted methanol fraction is reduced during the SEDMES process compared to conventional DME synthesis. The effect of enhancing DME selectivity was found to be particularly relevant with higher CO2 feed concentrations due to increased water production and removal.
A recent study by Tyraskis et al. [174] reported the optimization of a plant for conducting SEDMES using a cycle design based on TNO’s 2023 pilot plant (Petten, The Netherlands) as reference. The impact of pressure, cycle duration, amount of inert gases, tube geometry and feed flow rate were analyzed. Since the conventional DME synthesis without water sorption was not taken into account, this study cannot provide an enhancement factor, but it focuses on detailed optimization of the SEDMES process. The authors demonstrated how the system was tolerant to high concentrations of inert gas into the reactant mixture (up to 50%), and that a minor increase in the tube diameter could effectively reduce the gas velocity, thus increasing the contact time and improving the DME selectivity.
3.3. Sorption-Enhanced Fischer–Tropsch Process
A consistent theme across the research on the Fischer–Tropsch process is the detrimental effect of water, which is inherently produced during the reaction, on catalyst stability, conversion efficiency, and selectivity. In detail, in case of cobalt-base catalysts, which are a typical formulation in this reaction, the presence of a significant amount of water induces oxidation of the metal, reducing the number of active sites and causing an irreversible deactivation of the catalyst [175]. The same effect has also been observed in the presence of iron-based catalysts, in which water presence induced the active iron carbides into less active Fe3O4 phases, leading to deactivation [176]. Some recent studies have explored the concept of Sorption-Enhanced Fischer–Tropsch Synthesis (SE-FTS), focusing on the integration of water removal strategies to improve the catalyst performance and the process efficiency.
Asbahr et al. [177] developed and experimentally evaluated a cold flow model of two interconnected slurry bubble columns designed for SE-FTS. Their study addressed the hydrodynamics of a system to enable continuous operation by integrating in-situ water removal and sorbent regeneration. By employing artificial intelligence models, they established that the liquid circulation rate (LCR) strongly depended on gas holdup, with minimal influence from the liquid level. This reactor concept demonstrated potential for overcoming the adverse kinetic and thermodynamic impacts of water in Fischer–Tropsch synthesis, thus contributing to Process Intensification strategies.
Gavrilović et al. [178] experimentally validated the SE-FTS concept using zeolite sorbents (13X and 4A) for water removal under reactive conditions. Their findings revealed that the presence of zeolites enhanced CO conversion by 10% over 65 h of continuous operation, improved C5+ hydrocarbon selectivity, and mitigated catalyst deactivation. However, they also observed a significant decline in the sorption capacity of zeolite 13X after repeated cycles, attributable to structural degradation. This result agrees well with the stability studies of Heracleous et al. [149], which also reported the weak stability of this type of zeolite in their research on SEMS. Therefore, it can be assumed that the presence of a slurry phase does not significantly impact the stability of the zeolite, since the same sorption capacity loss is observed in gas-phase processes.
Bayat et al. [179] contributed to the evaluation of SE-FTS with a mathematical model, somehow recalling the initial idea of SEMS provided by Westerterp et al. [139]. The authors considered a trickle bed flow in which both the gas and the sorbent flows across a packed bed of catalyst pellets (gas-flowing solids-fixed bed reactor—GFSFBR—concept). The sorbent motion allowed for continuous sorbent regeneration, determining the obtainment of a notable advancement over conventional fixed-bed systems. Their simulations predicted a 45% increase in gasoline yield, and the thermal coupling of this reactor with a cyclohexane dehydrogenation gave place to a 57% rise in hydrogen production. These enhancements were attributed to selective in-situ water removal, which shifted the equilibrium and reduced catalyst deactivation. In a subsequent work [180], the authors modeled a system combining membrane and sorption enhanced reaction, with a sorbent composed by fine particles that fall through the spaces of a fixed bed of catalyst.
Collectively, these studies substantiated the necessity of managing water during FT synthesis. Sorption-enhanced approaches demonstrated considerable promise in mitigating water-induced deactivation, enhancing conversion rates, and improving product selectivity. Future research was recommended to optimize sorbent stability, reactor design, and operating conditions to realize the full potential of SE-FTS for sustainable fuel production.
3.4. Summary and Outlooks for Sorption-Enhanced Reaction
The technology of sorption-enhanced reaction, both for methanol and DME synthesis, has shown outstanding results. Not many studies have been reported so far for these applications, and reviewing the current literature shows a specific lack of experimental studies. Nevertheless, the mathematical studies on the topic are a powerful tool to fill the gap and select the range of operating conditions in which experimental studies could be conducted. Mathematical studies also allow the large-scale behavior and/or the economy of these applications to be predicted. Fewer studies have been published regarding the Fischer–Tropsch sorption-enhanced reaction, although those which have been published are particularly encouraging regarding the feasibility and the advantages that this application could provide.
Over the years, various sorbents and contact strategies have been investigated to optimize materials and process integration based on specific reaction requirements. For the choice of sorbents, while a broad range of materials are suitable for the sorption of water, sorption-enhanced methanol and DME synthesis typically relies on a limited selection of solids. For instance, metal oxides such as CaO are commonly employed in CO2 hydrogenation to methane [181,182], but only one study has, thus far, reported its application in the sorption-enhanced synthesis of methanol and DME, whereas zeolites are predominantly used for these processes. The absence of comparative studies makes it unclear whether the preference for zeolites is solely driven by their superior water sorption capacity or if other factors have contributed to the limited use of alkaline earth oxides. Between them, zeolite 3A appears to be ideal for sorption-enhanced processes. However, challenges such as sorbent saturation, reduced long-term stability (notably with 13X), and competition with methanol sorption remain limiting factors. Additionally, the dual role of zeolites—acting as both sorbent and dehydration catalyst—complicates selectivity control due to unintended methanol-to-DME conversion. For DME synthesis, sorption-enhanced processes further increase complexity due to the required synergy between methanol synthesis and dehydration catalysts.
An increase in complexity is also produced in the SE-FTS, due to the co-existence of three phases that generate a slurry in which the sorbent is immersed. However, the loss in sorption capacity that was observed in the experimental studies is coherent with the findings of other sorption-enhanced applications.
Looking ahead, the industrial viability of SEMS, SEDMES and SE-FTS depends on optimizing material selection, cycle design, and reactor configurations. Emerging concepts, such as continuous sorbent circulation in fluidized beds and advanced catalysts, show potential to address these challenges by improving mass transfer, thermal management, and operational flexibility. Future research should focus on long-term sorbent stability, the economic evaluation of large-scale systems, and the development of materials offering tailored sorption properties without compromising catalytic performance.
4. Structured Catalyst Reactors
One tool available for Process Intensification is a structured catalyst reactor. This includes any system where the catalyst is not deposited as a random set of particles filling the reactor, but the systems employed most often are microreactors and monoliths. Microreactors are characterized by having at least one dimension below 1 mm. This provides a surface to volume ratio much larger than conventional reactors, and therefore, a great capacity for heat transfer. This characteristic improves the temperature control, which is clearly a great advantage for exothermic reactions [183].
Microreactors have been employed in Fischer–Tropsch by Velocys [184], which has raised the interest of the study [185,186,187]. CompactGTL (an English Provider of gas-to-liquids services) has built a pilot plant for Petrobras (a Brazilian petrochemical industry) using microreactors and has plans for other plants.
Monoliths are composed by many parallel channels in a single block of a solid. They are widely employed in the catalytic converters of cars as they ensure a lower pressure drop compared to a conventional catalytic bed. The use of monoliths for Fischer–Tropsch (using CO + H2 as feed) has been studied by several groups since the elevated pressure drop is a problem in fixed bed multitubular reactors. Hilmen et al. [188] prepared Co-Re/Al2O3 monoliths showing that the selectivity to C5+ was similar to powder catalysts of 38–53 μm. Guettel and Turek [189] found that a Co-Ru monolith catalyst provided better conversion than the same catalyst as a slurry. The explanation was the excellent mass transfer obtained in monoliths with gas slugs. Deugd et al. [190] claimed (using a mathematical model) that a monolith catalyst operating in a recirculation loop would avoid some problems of conventional reactors, such as catalyst attrition and separation, backmixing and large diffusion distances. They also showed that monolith catalysts have a clear decrease in activation energy (from c.a. 100 to c.a. 50 kJ/mol) when the washcoat layer thickness was increased from 20 to 110 nm, which is a clear indication of mass transfer limitations. Although this is generally undesirable, in the case of Fischer–Tropsch synthesis, a small concentration gradient inside the catalytic layer may have a positive effect: since the diffusivity of hydrogen is higher than that of CO, the H2/CO ratio would be higher inside the catalytic layer than in the gas phase, and the selectivity to hydrocarbons in the gasoline range could be increased [191]. A problem with monoliths is the difficulty of heat removal. This could be solved with a high velocity in the monolith combined with a recirculation loop and an external heat exchanger, or by using metallic monoliths [192].
Periodic Ordered Catalysts (POCs) are another alternative in the search for low pressure drop. Visconti et al. [193] demonstrated their performance in a pilot plant of highly conductive packed POCS with a 99 mm long and 28 mm o.d. POCs sample.
Almeida et al. [185] compared microchannels, aluminum foams, and monoliths. They found that the thickness of the washcoat layer was a critical factor affecting the selectivity. The C5 selectivity of microchannels was higher than that of structured supports, which is explained by the better temperature control.
5. Other Process Intensification Systems
An alternative method, not industrially employed, for methanol synthesis is to use reaction in liquid phase. Ghosh and Seethamraju [194] combined this reaction with distillation in what is commonly referred to as reactive distillation.
Mathematical models also predict that Fischer–Tropsch would provide higher selectivity to middle distillate products by using reactive distillation [195].
Another way to increase conversion beyond the thermodynamic equilibrium in a conventional reactor is to condense the products. This has been applied by van Bennekom et al. [196] with a syngas of composition H2/CO/CO2/CH4 = 0.65/0.25/0.05/0.05. At a suitable temperature, total conversion was achieved in a batch reactor.
The combination of Fischer-Tropsch with hydrocracking in a microstructed reactor was experimentally studied by Kirsch et al. [197].
Another technique that sometimes provides improvements in the performance of catalytic reactors is non-steady state operation. In the case of Fischer–Tropsch dynamic operation, hydrogen pulsing in particular seems to be a potentially beneficial operating technique to remove accumulated liquid products, restore initial catalyst activity, and increase diesel-range productivity [198].
6. Conclusions
The production of liquid e-fuels is generating increasing interest among researchers and industry. E-fuels include methanol, DME, and hydrocarbons from Fischer–Tropsch synthesis, which could be obtained from CO2 and renewable hydrogen. Direct hydrogenation of CO2 is usually considered, although in the case of Fischer–Tropsch synthesis, an intermediate step of rWGS to produce syngas (CO + H2) would probably be convenient. The incorporation of Process Intensification tools to the technologies employed in the production of liquid fuels from CO2 will be a way to decrease the cost of the final product and reduce the environmental impact derived from their production. Many promising alternatives have been identified, and the most encouraging ones are membrane reactors, sorption enhanced reactors, and structured catalysts. As demonstrated in this review, the exploitation of Le Chatelier principle is highly convenient in the production of e-fuels since it can increment the single-pass conversion and the selectivity toward the desired products. At the same time, innovative technologies like microreactors and structured catalysts can offer exclusive properties to tune the selectivity and ensure an adequate heat management of the system.
Author Contributions
Conceptualization, S.R. and M.M.; writing—original draft preparation, S.R. and M.M.; writing—review and editing, S.R. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research is part of the grant no. PLEC2022–009239 funded by the Spanish Ministry of Science and Innovation MCIN/AEI/10.13039/501,100,011,033 and EU through the program NextGenerationEU/PRTR. The consolidated research group Catalysis and Reactor Engineering Group (CREG, T43–23 R) has the financial support of Gobierno de Aragón through the European Social Fund—FEDER. S.R. acknowledges the Spanish Ministry of Science, Innovation and University MCIU/AEI/10.13039/501,100,011,033 and FSE+ for the Juan de la Cierva fellowship (Grant no. JDC2023-052947-I).
Data Availability Statement
No new data were created for this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CCS | Carbon Capture and Storage |
| CFFBR | Circulating Fast Fluidized-Bed Reactor |
| CZA | Copper-zinc-alumina |
| DME | Dimethyl Ether |
| GSSTF | Gas–Solid–Solid Trickle Flow |
| LPG | Liquefied Petroleum Gas |
| MTG | Methanol-to-Gasoline |
| MTO | Methanol-to-Olefins |
| POC | Periodic Ordered Catalysts |
| PTA | Phosphotungstic Acid |
| rWGS | Reverse Water Gas Shift |
| SE-WGS | Sorption-Enhanced Water–Gas Shift |
| SEDMES | Sorption-enhanced Dimethyl Ether Synthesis |
| SEMS | Sorption-enhanced Methanol Synthesis |
| SER | Sorption-enhanced Reaction |
| WGS | Water–Gas Shift |
References
- IEA. IEA Bioenergy Annual Report; International Energy Agency: Paris, France, 2021. [Google Scholar]
- Saba, S.M.; Müller, M.; Robinius, M.; Stolten, D. The Investment Costs of Electrolysis—A Comparison of Cost Studies from the Past 30 Years. Int. J. Hydrogen Energy 2018, 43, 1209–1223. [Google Scholar] [CrossRef]
- Proost, J. State-of-the Art CAPEX Data for Water Electrolysers, and Their Impact on Renewable Hydrogen Price Settings. Int. J. Hydrogen Energy 2019, 44, 4406–4413. [Google Scholar] [CrossRef]
- Kamkeng, A.D.N.; Wang, M.; Hu, J.; Du, W.; Qian, F. Transformation Technologies for CO2 Utilisation: Current Status, Challenges and Future Prospects. Chem. Eng. J. 2021, 409, 128138. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Vo, D.V.N.; Jeevanantham, S.; Bhuvaneswari, V.; Anantha Narayanan, V.; Yaashikaa, P.R.; Swetha, S.; Reshma, B. A Comprehensive Review on Different Approaches for CO2 Utilization and Conversion Pathways. Chem. Eng. Sci. 2021, 236, 116515. [Google Scholar] [CrossRef]
- Busca, G.; Spennati, E.; Riani, P.; Garbarino, G. Mechanistic and Compositional Aspects of Industrial Catalysts for Selective CO2 Hydrogenation Processes. Catalysts 2024, 14, 95. [Google Scholar] [CrossRef]
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The Teraton Challenge. A Review of Fixation and Transformation of Carbon Dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Olah, G.A. Beyond Oil and Gas: The Methanol Economy. Angew. Chem. Int. Ed. 2005, 44, 2636–2639. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Chemical Recycling of Carbon Dioxide to Methanol and Dimethyl Ether: From Greenhouse Gas to Renewable, Environmentally Carbon Neutral Fuels and Synthetic Hydrocarbons. J. Org. Chem. 2009, 74, 487–498. [Google Scholar] [CrossRef]
- Olah, G.A. Towards Oil Independence through Renewable Methanol Chemistry. Angew. Chem. Int. Ed. 2013, 52, 104–107. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; Jones, J.-P.; Surya Prakash, G.K.; Olah, G.A. Recycling of Carbon Dioxide to Methanol and Derived Products—Closing the Loop. Chem. Soc. Rev. 2014, 43, 7995–8048. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Heterogeneous Catalytic Reactions with CO2: Status and Perspectives. Stud. Surf. Sci. Catal. 2004, 153, 1–8. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Opportunities and Prospects in the Chemical Recycling of Carbon Dioxide to Fuels. Catal. Today 2009, 148, 191–205. [Google Scholar] [CrossRef]
- Quadrelli, E.A.; Centi, G.; Duplan, J.L.; Perathoner, S. Carbon Dioxide Recycling: Emerging Large-Scale Technologies with Industrial Potential. ChemSusChem 2011, 4, 1194–1215. [Google Scholar] [CrossRef]
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 Conversion: A Key Technology for Rapid Introduction of Renewable Energy in the Value Chain of Chemical Industries. Energy Environ. Sci. 2013, 6, 1711–1731. [Google Scholar] [CrossRef]
- Perathoner, S.; Centi, G. CO2 Recycling: A Key Strategy to Introduce Green Energy in the Chemical Production Chain. ChemSusChem 2014, 7, 1274–1282. [Google Scholar] [CrossRef]
- Ampelli, C.; Carreon, M.L.; Liu, Y. New Paths and Research Directions in CO2 Conversion by Electro-, Photo- and Plasma Catalysis. J. Energy Chem. 2024, 99, 300–301. [Google Scholar] [CrossRef]
- Aresta, M. My Journey in the CO2-Chemistry Wonderland. Coord. Chem. Rev. 2017, 334, 150–183. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. The Changing Paradigm in CO2 Utilization. J. CO2 Util. 2013, 3–4, 65–73. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Quaranta, E. State of the Art and Perspectives in Catalytic Processes for CO2 Conversion into Chemicals and Fuels: The Distinctive Contribution of Chemical Catalysis and Biotechnology. J. Catal. 2016, 343, 2–45. [Google Scholar] [CrossRef]
- Arakawa, H.; Aresta, M.; Armor, J.N.; Barteau, M.A.; Beckman, E.J.; Bell, A.T.; Bercaw, J.E.; Creutz, C.; Dinjus, E.; Dixon, D.A.; et al. Catalysis Research of Relevance to Carbon Management: Progress, Challenges, and Opportunities. Chem. Rev. 2001, 101, 953–996. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A. Utilisation of CO2 as a Chemical Feedstock: Opportunities and Challenges. Dalton Trans. 2007, 28, 2975–2992. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef] [PubMed]
- Tursunov, O.; Kustov, L.; Kustov, A. A Brief Review of Carbon Dioxide Hydrogenation to Methanol Over Copper and Iron Based Catalysts. Oil Gas Sci. Technol.–Revue IFP Energ. Nouv. 2017, 72, 30. [Google Scholar] [CrossRef]
- Tursunov, O.; Kustov, L.; Tilyabaev, Z. Methanol Synthesis from the Catalytic Hydrogenation of CO2 over CuO–ZnO Supported on Aluminum and Silicon Oxides. J. Taiwan Inst. Chem. Eng. 2017, 78, 416–422. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A Short Review of Recent Advances in CO2 Hydrogenation to Hydrocarbons over Heterogeneous Catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef]
- Guil-López, R.; Mota, N.; Llorente, J.; Millán, E.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis. Materials 2019, 12, 3902. [Google Scholar] [CrossRef]
- Din, I.U.; Usman, M.; Khan, S.; Helal, A.; Alotaibi, M.A.; Alharthi, A.I.; Centi, G. Prospects for a Green Methanol Thermo-Catalytic Process from CO2 by Using MOFs Based Materials: A Mini-Review. J. CO2 Util. 2021, 43, 101361. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Song, C.; Guo, X. Catalytic Conversion of Carbon Dioxide to Methanol: Current Status and Future Perspective. Front. Energy Res. 2021, 8, 621119. [Google Scholar] [CrossRef]
- Darji, H.R.; Kale, H.B.; Shaikh, F.F.; Gawande, M.B. Advancement and State-of-Art of Heterogeneous Catalysis for Selective CO2 Hydrogenation to Methanol. Coord. Chem. Rev. 2023, 497, 215409. [Google Scholar] [CrossRef]
- Alaba, P.A.; Abbas, A.; Daud, W.M.W. Insight into Catalytic Reduction of CO2: Catalysis and Reactor Design. J. Clean. Prod. 2017, 140, 1298–1312. [Google Scholar] [CrossRef]
- Sick, V. Spiers Memorial Lecture: CO2utilization: Why, Why Now, and How? Faraday Discuss. 2021, 230, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Guo, X.; He, Y.; Tsubaki, N. CO2 Heterogeneous Hydrogenation to Carbon-Based Fuels: Recent Key Developments and Perspectives. J. Mater. Chem. A Mater. 2023, 11, 11637–11669. [Google Scholar] [CrossRef]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Peppel, T.; Seeburg, D.; Kondratenko, V.A.; Kalevaru, N.; Martin, A.; Wohlrab, S. Methane Conversion into Different Hydrocarbons or Oxygenates: Current Status and Future Perspectives in Catalyst Development and Reactor Operation. Catal. Sci. Technol. 2017, 7, 366–381. [Google Scholar] [CrossRef]
- Dorner, R.W.; Hardy, D.R.; Williams, F.W.; Willauer, H.D. Heterogeneous Catalytic CO2 Conversion to Value-Added Hydrocarbons. Energy Environ. Sci. 2010, 3, 884. [Google Scholar] [CrossRef]
- Cui, L.; Liu, C.; Yao, B.; Edwards, P.P.; Xiao, T.; Cao, F. A Review of Catalytic Hydrogenation of Carbon Dioxide: From Waste to Hydrocarbons. Front. Chem. 2022, 10, 1037997. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, X.; Wu, Z.; Liang, B.; Huang, Y.; Zhang, T. State of the Art and Perspectives in Heterogeneous Catalysis of CO2 Hydrogenation to Methanol. Chem. Soc. Rev. 2020, 49, 1385–1413. [Google Scholar] [CrossRef]
- Velty, A.; Corma, A. Advanced Zeolite and Ordered Mesoporous Silica-Based Catalysts for the Conversion of CO2 to Chemicals and Fuels. Chem. Soc. Rev. 2023, 52, 1773–1946. [Google Scholar] [CrossRef]
- Yan, P.; Peng, H.; Vogrin, J.; Rabiee, H.; Zhu, Z. Selective CO2 Hydrogenation over Zeolite-Based Catalysts for Targeted High-Value Products. J. Mater. Chem. A Mater. 2023, 11, 17938–17960. [Google Scholar] [CrossRef]
- Dokania, A.; Ramirez, A.; Bavykina, A.; Gascon, J. Heterogeneous Catalysis for the Valorization of CO2: Role of Bifunctional Processes in the Production of Chemicals. ACS Energy Lett. 2019, 4, 167–176. [Google Scholar] [CrossRef]
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef] [PubMed]
- Joo, O.S.; Jung, K.D.; Moon, I.; Rozovskii, A.Y.; Lin, G.I.; Han, S.H.; Uhm, S.J. Carbon Dioxide Hydrogenation to Form Methanol via a Reverse-Water-Gas- Shift Reaction (the CAMERE Process). Ind. Eng. Chem. Res. 1999, 38, 1808–1812. [Google Scholar] [CrossRef]
- Anicic, B.; Trop, P.; Goricanec, D. Comparison between Two Methods of Methanol Production Fromcarbon Dioxide. Energy 2014, 77, 279–289. [Google Scholar] [CrossRef]
- Methanol Institute. Available online: https://www.methanol.org/renewable (accessed on 17 March 2025).
- Fleisch, T.H.; Basu, A.; Sills, R.A. Introduction and Advancement of a New Clean Global Fuel: The Status of DME Developments in China and Beyond. J. Nat. Gas Sci. Eng. 2012, 9, 94–107. [Google Scholar] [CrossRef]
- Ateka, A.; Rodriguez-Vega, P.; Ereña, J.; Aguayo, A.T.; Bilbao, J. A Review on the Valorization of CO2. Focusing on the Thermodynamics and Catalyst Design Studies of the Direct Synthesis of Dimethyl Ether. Fuel Process. Technol. 2022, 233, 107310. [Google Scholar] [CrossRef]
- Dry, M.E. The Fischer-Tropsch Process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Dell’Aversano, S.; Villante, C.; Gallucci, K.; Vanga, G.; Di Giuliano, A. E-Fuels: A Comprehensive Review of the Most Promising Technological Alternatives towards an Energy Transition. Energies 2024, 17, 3995. [Google Scholar] [CrossRef]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-Liquid via Synthesis of Methanol, DME or Fischer–Tropsch-Fuels: A Review. Energy Environ. Sci. 2020, 13, 3207–3252. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Moulijin, J. Re-Engineering the Chemical Processing Plant: Process Intensification; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Stankiewicz, A. Reactive Separations for Process Intensification: An Industrial Perspective. Chem. Eng. Technol. 2003, 42, 137–144. [Google Scholar] [CrossRef]
- Meloni, E.; Cafiero, L.; Renda, S.; Martino, M.; Pierro, M.; Palma, V. Ru- and Rh-Based Catalysts for CO2 Methanation Assisted by Non-Thermal Plasma. Catalysts 2023, 13, 488. [Google Scholar] [CrossRef]
- Meloni, E.; Iervolino, G.; Ruocco, C.; Renda, S.; Festa, G.; Martino, M.; Palma, V. Electrified Hydrogen Production from Methane for PEM Fuel Cells Feeding: A Review. Energies 2022, 15, 3588. [Google Scholar] [CrossRef]
- Muccioli, O.; Meloni, E.; Renda, S.; Martino, M.; Brandani, F.; Pullumbi, P.; Palma, V. NiCoAl-Based Monolithic Catalysts for the N2O Intensified Decomposition: A New Path towards the Microwave-Assisted Catalysis. Processes 2023, 11, 1511. [Google Scholar] [CrossRef]
- Armor, J.N. Membrane Catalysis: Where Is It Now, What Needs to Be Done? Catal. Today 1995, 25, 199–207. [Google Scholar] [CrossRef]
- Sirkar, K.K. Membrane Separation Technologies: Current Developments. Chem. Eng. Commun. 1997, 157, 145–184. [Google Scholar] [CrossRef]
- Sanchez-Marcano, J.G.; Tsotsis, T.T. Catalytic Membranes and Membrane Reactors; Wiley-VCH: Hoboken, NJ, USA, 2002. [Google Scholar]
- Tan, X.; Li, K. Inorganic Membrane Reactors: Fundamentals and Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Basile, A.; Gallucci, F. Membranes for Membrane Reactors: Preparation, Optimization and Selection; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Basile, A. Handbook of Membrane Reactors; Woodhead Publishing: Sawston, UK, 2013. [Google Scholar]
- Judd, S.; Judd, C. The MBR Book Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Struis, R.P.W.J.; Stucki, S.; Wiedorn, M. A Membrane Reactor for Methanol Synthesis. J. Memb. Sci. 1996, 113, 93–100. [Google Scholar] [CrossRef]
- Menendez, M.; Piera, E.; Coronas, J.; Santamaría, J. Zeolite Membrane Reactor for the Production of Methanol and Other Alcohols Form Synthesis Gas. OEPM Patent no. 2164544, 22 July 1999. [Google Scholar]
- Algieri, C.; Bruno, A.; Gomez, O.; Mallada, R.; Aguado, S.; Bernal, P.; Golemme, G.; Menéndez, M.; Santamaría, J.; Granato, T.; et al. Zeolite membranes for methanol synthesis in a membrane reactor (Comunicazione a convegno). In Proceedings of the 4th European Congress of Chemical Engineering, Granada, Spain, 21–25 September 2003; p. O-7.2-002. [Google Scholar]
- Gallucci, F.; Paturzo, L.; Basile, A. An Experimental Study of CO2 Hydrogenation into Methanol Involving a Zeolite Membrane Reactor. Chem. Eng. Proc. 2004, 43, 1029–1036. [Google Scholar] [CrossRef]
- Sea, B.; Lee, K. Hydrogen Using a Ceramic Membrane Reactor. React. Kinet. Catal. Lett. 2003, 80, 33–38. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, Q. Methanol Synthesis from CO2 Using a Silicone Rubber/Ceramic Composite Membrane Reactor. Sep. Purif. Technol. 2004, 34, 227–237. [Google Scholar] [CrossRef]
- Li, H.; Qiu, C.; Ren, S.; Dong, Q.; Zhang, S.; Zhou, F.; Liang, X.; Wang, J.; Li, S.; Yu, M. Na+–Gated Water-Conducting Nanochannels for Boosting CO2 Conversion to Liquid Fuels. Science 2020, 367, 667–671. [Google Scholar] [CrossRef]
- Seshimo, M.; Liu, B.; Lee, H.R.; Yogo, K.; Yamaguchi, Y.; Shigaki, N.; Mogi, Y.; Kita, H.; Nakao, S.I. Membrane Reactor for Methanol Synthesis Using Si-rich LTA Zeolite Membrane. Membranes 2021, 11, 505. [Google Scholar] [CrossRef]
- Struis, R.P.W.J.; Stucki, S. Verification of the Membrane Reactor Concept for the Methanol Synthesis. Appl. Catal. A Gen. 2001, 216, 117–129. [Google Scholar] [CrossRef]
- Barbieri, G.; Marigliano, G.; Golemme, G.; Drioli, E. Simulation of CO2 Hydrogenation with CH3OH Removal in a Zeolite Membrane Reactor. Chem. Eng. Commun. 2002, 85, 53–59. [Google Scholar] [CrossRef]
- Piera, E.; Salomón, M.A.; Coronas, J.; Menéndez, M.; Santamaría, J. Synthesis, Characterization and Separation Properties of a Composite Mordenite/ZSM-5/Chabazite Hydrophilic Membrane. J. Memb. Sci. 1998, 149, 99–114. [Google Scholar] [CrossRef]
- Gallucci, F.; Basile, A. Co-Current and Counter-Current Modes for Methanol Steam Reforming Membrane Reactor. Int. J. Hydrogen Energy 2006, 31, 2243–2249. [Google Scholar] [CrossRef]
- Gallucci, F.; Basile, A. A Theoretical Analysis of Methanol Synthesis from CO2 and H2 in a Ceramic Membrane Reactor. Int. J. Hydrogen Energy 2007, 32, 5050–5058. [Google Scholar] [CrossRef]
- Farsi, M.; Jahanmiri, A. Application of Water Vapor-Permselective Alumina-Silica Composite Membrane in Methanol Synthesis Process to Enhance CO2 Hydrogenation and Catalyst Life Time. J. Ind. Eng. Chem. 2012, 18, 1088–1095. [Google Scholar] [CrossRef]
- Farsi, M.; Jahanmiri, a. Dynamic Modeling of a H2O-Permselective Membrane Reactor to Enhance Methanol Synthesis from Syngas Considering Catalyst Deactivation. J. Nat. Gas Chem. 2012, 21, 407–414. [Google Scholar] [CrossRef]
- Hamedi, H.; Brinkmann, T.; Shishatskiy, S. Membrane-Assisted Methanol Synthesis Processes and the Required Permselectivity. Membranes 2021, 11, 596. [Google Scholar] [CrossRef]
- Hamedi, H.; Brinkmann, T. Rigorous and Customizable 1D Simulation Framework for Membrane Reactors to, in Principle, Enhance Synthetic Methanol Production. ACS Sustain. Chem. Eng. 2021, 9, 7620–7629. [Google Scholar] [CrossRef]
- Ountaksinkul, K.; Vas-Umnuay, P.; Kasempremchit, N.; Bumroongsakulsawat, P.; Kim-Lohsoontorn, P.; Jiwanuruk, T.; Assabumrungrat, S. Performance Comparison of Different Membrane Reactors for Combined Methanol Synthesis and Biogas Upgrading. Chem. Eng. Proc. 2019, 136, 191–200. [Google Scholar] [CrossRef]
- Hauth, T.; Pielmaier, K.; Dieterich, V.; Wein, N. Energy Advances Design Parameter Optimization of a Membrane Reactor for Methanol Synthesis Using a Sophisticated CFD Model. Energy Adv. 2025, 4, 565–577. [Google Scholar] [CrossRef]
- Bazmi, M.; Gong, J.; Jessen, K.; Tsotsis, T. Waste CO2 Capture and Utilization for Methanol Production via a Novel Membrane Contactor Reactor Process: Techno-Economic Analysis (TEA), and Comparison with Other Existing and Emerging Technologies. Chem. Eng. Proc. 2024, 201, 109825. [Google Scholar] [CrossRef]
- Dieterich, V.; Wein, N.; Spliethoff, H.; Fendt, S. Performance Requirements of Membrane Reactors for the Application in Renewable Methanol Synthesis: A Techno-Economic Assessment. Adv. Sust. Systems 2022, 6, 2200254. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Ghader, S. Theoretical Investigation of a Pd-Membrane Reactor for Methanol Synthesis. Chem. Eng. Technol. 2003, 26, 902–907. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Bayat, M.; Rahmani, F. Enhancement of Methanol Production in a Novel Cascading Fluidized-Bed Hydrogen Permselective Membrane Methanol Reactor. Chem. Eng. J. 2010, 157, 520–529. [Google Scholar] [CrossRef]
- Gong, J.; Sadat-Zebarjad, F.; Jessen, K.; Tsotsis, T. An Experimental and Modeling Study of the Application of Membrane Contactor Reactors to Methanol Synthesis Using Pure CO2 Feeds. Chem. Eng. Process. 2023, 183, 109241. [Google Scholar] [CrossRef]
- Jia, M.D.; Chen, B.; Noble, R.D.; Falconer, J.L. Ceramic-Zeolite Composite Membranes and Their Application for Separation of Vapor/Gas Mixtures. J. Memb. Sci. 1994, 90, 1–10. [Google Scholar] [CrossRef]
- Kita, H.; Horii, K.; Ohtoshi, Y.; Tanaka, K.; Okamoto, K.-I. Synthesis of a Zeolite NaA Membrane for Pervaporation of Water/Organic Liquid Mixtures. J. Mater. Sci. Lett. 1995, 14, 206–208. [Google Scholar] [CrossRef]
- Casanave, D.; Giroir-Fendler, A.; Sanchez, J.; Loutaty, R.; Dalmon, J.A. Control of Transport Properties with a Microporous Membrane Reactor to Enhance Yields in Dehydrogenation Reactions. Catal. Today 1995, 25, 309–314. [Google Scholar] [CrossRef]
- Coronas, J.; Santamaria, J. State-of-the-Art in Zeolite Membrane Reactors. Top. Catal. 2004, 29, 29–44. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M. Zeolite Membranes—Recent Developments and Progress. Microporous Mesoporous Mater. 2008, 115, 215–233. [Google Scholar] [CrossRef]
- Téllez, C.; Menéndez, M. Zeolite Membrane Reactors. In Membranes for Membrane Reactors: Preparation, Optimization and Selection; Basile, A., Gallucci, F., Eds.; Wiley: Hoboken, NJ, USA, 2011; pp. 243–273. [Google Scholar]
- Wenten, I.G.; Khoiruddin, K.; Mukti, R.R.; Rahmah, W.; Wang, Z.; Kawi, S. Zeolite Membrane Reactors: From Preparation to Application in Heterogeneous Catalytic Reactions. React. Chem. Eng. 2021, 6, 401–417. [Google Scholar] [CrossRef]
- Gorgojo, P.; de la Iglesia, Ó.; Coronas, J. Preparation and Characterization of Zeolite Membranes. Membr. Sci. Technol. 2008, 13, 135–175. [Google Scholar]
- Gorbe, J.; Lasobras, J.; Francés, E.; Herguido, J.; Menéndez, M.; Kumakiri, I.; Kita, H. Preliminary Study on the Feasibility of Using a Zeolite A Membrane in a Membrane Reactor for Methanol Production. Sep. Purif. Technol. 2018, 200, 164–168. [Google Scholar] [CrossRef]
- Lee, S.M.; Xu, N.; Grace, J.R.; Li, A.; Lim, C.J.; Kim, S.S.; Fotovat, F.; Schaadt, A.; White, R.J. Structure, Stability and Permeation Properties of NaA Zeolite Membranes for H2O/H2 and CH3OH/H2 Separations. J. Eur. Ceram. Soc. 2018, 38, 211–219. [Google Scholar] [CrossRef]
- Li, Z.; Deng, Y.; Wang, Z.; Hu, J.; Haw, K.G.; Wang, G.; Kawi, S. A Superb Water Permeable Membrane for Potential Applications in CO2 to Liquid Fuel Process. J. Memb. Sci. 2021, 639, 119682. [Google Scholar] [CrossRef]
- Juarez, E.; Lasobras, J.; Soler, J.; Herguido, J.; Menéndez, M. Polymer–Ceramic Composite Membranes for Water Removal in Membrane Reactors. Membranes 2021, 11, 472. [Google Scholar] [CrossRef]
- Pham, Q.H.; Goudeli, E.; Scholes, C.A. Selective Separation of Water and Methanol from Hydrogen and Carbon Dioxide at Elevated Temperature through Polyimide and Polyimidazole Based Membranes. J. Memb. Sci. 2023, 686, 121990. [Google Scholar] [CrossRef]
- Sawamura, K.I.; Shirai, T.; Takada, M.; Sekine, Y.; Kikuchi, E.; Matsukata, M. Selective Permeation and Separation of Steam from Water–Methanol–Hydrogen Gas Mixtures through Mordenite Membrane. Catal. Today 2008, 132, 182–187. [Google Scholar] [CrossRef]
- Hirota, Y.; Nakai, M.; Tani, K.; Sakane, K.; Ikeda, A.; Hasegawa, Y.; Araki, S. Gas and Steam Permeation Properties of Cation-Exchanged ZSM-5 Membrane. Membranes 2025, 15, 70. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Huang, A.; Caro, J. Hydrophilic SOD and LTA Membranes for Membrane-Supported Methanol, Dimethylether and Dimethylcarbonate Synthesis. Microporous Mesoporous Mater. 2015, 207, 33–38. [Google Scholar] [CrossRef]
- Rahimalimamaghani, A.; Gallucci, F.; Pacheco-Tanaka, D.A.; Llosa Tanco, M.A. Carbon Molecular Sieve Membrane Prepared from Hydroquinone and the Method of Manufacturing. Patent: US20240261736A1. U.S. Patent Application No. 18/566,48, 2022. [Google Scholar]
- Maghsoudi, H. Defects of Zeolite Membranes: Characterization, Modification and Post-treatment Techniques. Sep. Pur. Rev. 2015, 45, 169–192. [Google Scholar] [CrossRef]
- Aoki, K.; Kusakabe, K.; Morooka, S. Separation of Gases with an A-Type Zeolite Membrane. Ind. Eng. Chem. Res. 2000, 39, 2245–2251. [Google Scholar] [CrossRef]
- Bernal, M.P.; Piera, E.; Coronas, J.; Menéndez, M.; Santamaría, J. Mordenite and ZSM-5 Hydrophilic Tubular Membranes for the Separation of Gas Phase Mixtures. Catal. Today 2000, 56, 221–227. [Google Scholar] [CrossRef]
- Zhu, W.; Gora, L.; Van Den Berg, A.W.C.; Kapteijn, F.; Jansen, J.C.; Moulijn, J.A. Water Vapour Separation from Permanent Gases by a Zeolite-4A Membrane. J. Memb. Sci. 2005, 253, 57–66. [Google Scholar] [CrossRef]
- Rezai, S.A.S.; Lindmark, J.; Andersson, C.; Jareman, F.; Möller, K.; Hedlund, J. Water/Hydrogen/Hexane Multicomponent Selectivity of Thin MFI Membranes with Different Si/Al Ratios. Microporous Mesoporous Mater. 2008, 108, 136–142. [Google Scholar] [CrossRef]
- Poto, S.; Endepoel, J.G.H.; Llosa-Tanco, M.A.; Pacheco-Tanaka, D.A.; Gallucci, F.; Neira d’Angelo, M.F. Vapor/Gas Separation through Carbon Molecular Sieve Membranes: Experimental and Theoretical Investigation. Int. J. Hydrog. Energy 2022, 47, 11385–11401. [Google Scholar] [CrossRef]
- Poto, S.; van den Bogaard, H.; Gallucci, F.; Fernanda Neira d’Angelo, M. Evaluation of the Relevant Mass and Heat Transfer Phenomena in a Packed Bed Membrane Reactor for the Direct Conversion of CO2 to Dimethyl Ether. Fuel 2023, 350, 128783. [Google Scholar] [CrossRef]
- Poto, S.; Vink, T.; Oliver, P.; Gallucci, F.; Neira D’angelo, M.F. Techno-Economic Assessment of the One-Step CO2 conversion to Dimethyl Ether in a Membrane-Assisted Process. J. CO2 Util. 2023, 69, 102419. [Google Scholar] [CrossRef]
- Poto, S.; Llosa Tanco, M.A.; Pacheco Tanaka, D.A.; Neira d′Angelo, M.F.; Gallucci, F. Experimental Investigation of a Packed Bed Membrane Reactor for the Direct Conversion of CO2 to Dimethyl Ether. J. CO2 Util. 2023, 72, 102513. [Google Scholar] [CrossRef]
- Diban, N.; Urtiaga, A.M.; Ortiz, I.; Ereña, J.; Bilbao, J.; Aguayo, A.T.; Ereña, J.; Bilbao, J.; Aguayo, A.T. Influence of the Membrane Properties on the Catalytic Production of Dimethyl Ether with in Situ Water Removal for the Successful Capture of CO2. Chem. Eng. J. 2013, 234, 140–148. [Google Scholar] [CrossRef]
- Aguayo, A.T.; Ereña, J.; Mier, D.; Arandes, J.M.; Olazar, M.; Bilbao, J. Kinetic Modeling of Dimethyl Ether Synthesis in a Single Step on a CuO-ZnO-Al2O3/γ-Al2O3 Catalyst. Ind. Eng. Chem. Res. 2007, 46, 5522–5530. [Google Scholar] [CrossRef]
- Diban, N.; Urtiaga, A.M.; Ortiz, I.; Ereña, J.; Bilbao, J.; Aguayo, A.T. Improved Performance of a Pbm Reactor for Simultaneous CO2 Capture and DME Synthesis. Ind. Eng. Chem. Res. 2014, 53, 19479–19487. [Google Scholar] [CrossRef]
- Rodriguez-Vega, P.; Ateka, A.; Kumakiri, I.; Vicente, H.; Ereña, J.; Aguayo, A.T.; Bilbao, J. Experimental Implementation of a Catalytic Membrane Reactor for the Direct Synthesis of DME from H2+CO/CO2. Chem. Eng. Sci. 2021, 234, 116396. [Google Scholar] [CrossRef]
- Poto, S.; Gallucci, F.; Neira d’Angelo, M.F. Direct Conversion of CO2 to Dimethyl Ether in a Fixed Bed Membrane Reactor: Influence of Membrane Properties and Process Conditions. Fuel 2021, 302, 121080. [Google Scholar] [CrossRef]
- Dong, Q.; Xu, W.L.; Fan, X.; Li, H.; Klinghoffer, N.; Pyrzynski, T.; Meyer, H.S.; Liang, X.; Yu, M.; Li, S. Prototype Catalytic Membrane Reactor for Dimethyl Ether Synthesis via CO2 Hydrogenation. Ind. Eng. Chem. Res. 2022, 61, 14656–14663. [Google Scholar] [CrossRef]
- Brunetti, A.; Migliori, M.; Cozza, D.; Catizzone, E.; Giordano, G.; Barbieri, G. Methanol Conversion to Dimethyl Ether in Catalytic Zeolite Membrane Reactors. ACS Sustain. Chem. Eng. 2020, 8, 10471–10479. [Google Scholar] [CrossRef]
- Guo, L.; Sun, J.; Ge, Q.; Tsubaki, N. Recent Advances in Direct Catalytic Hydrogenation of Carbon Dioxide to Valuable C2+ Hydrocarbons. J. Mater. Chem. A Mater. 2018, 6, 23244–23262. [Google Scholar] [CrossRef]
- Espinoza, R.L.; Santamaría, J.; Menendez, M.; Coronas, J.; Irusta, S. Production of Hydrocarbons. US Patent no. US6403660B1, 5 December 2000. [Google Scholar]
- Espinoza, R.L.; du Toit, E.; Santamaria, J.; Menendez, M.; Coronas, J.; Irusta, S. Use of Membranes in Fischer-Tropsch Reactors. In Studies in Surface Science and Catalysis 130; Corma, A., Melo, F.V., Mendioroz, S., Fierro, J.L.G., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2000; pp. 389–394. [Google Scholar]
- Rohde, M.P.; Unruh, D.; Schaub, G. Membrane Application in Fischer-Tropsch Synthesis to Enhance CO2 Hydrogenation. Ind. Eng. Chem. Res. 2005, 44, 9653–9658. [Google Scholar] [CrossRef]
- Rohde, M.P.; Schaub, G.; Khajavi, S.; Jansen, J.C.; Kapteijn, F. Fischer-Tropsch Synthesis with in Situ H2O Removal—Directions of Membrane Development. Microporous Mesoporous Mater. 2008, 115, 123–136. [Google Scholar] [CrossRef]
- Forghani, A.A.; Elekaei, H.; Rahimpour, M.R. Enhancement of Gasoline Production in a Novel Hydrogen-Permselective Membrane Reactor in Fischer-Tropsch Synthesis of GTL Technology. Int. J. Hydrog. Energy 2009, 34, 3965–3976. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Jokar, S.M.; Jamshidnejad, Z. A Novel Slurry Bubble Column Membrane Reactor Concept for Fischer-Tropsch Synthesis in GTL Technology. Chem. Eng. Res. Des. 2012, 90, 383–396. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Mirvakili, A.; Paymooni, K. A Novel Water Perm-Selective Membrane Dual-Type Reactor Concept for Fischer-Tropsch Synthesis of GTL (Gas to Liquid) Technology. Energy 2011, 36, 1223–1235. [Google Scholar] [CrossRef]
- Khassin, A.A.; Yurieva, T.M.; Sipatrov, A.G.; Kirillov, V.A.; Chermashentseva, G.K.; Parmon, V.N. Fischer-Tropsch Synthesis Using a Porous Catalyst Packing: Experimental Evidence of an Efficient Use of Permeable Composite Monoliths as a Novel Type of the Fischer-Tropsch Synthesis Catalyst. Catal. Today 2003, 79–80, 465–470. [Google Scholar] [CrossRef]
- He, J.; Xu, B.; Yoneyama, Y.; Nishiyama, N.; Tsubaki, N. Designing a New Kind of Capsule Catalyst and Its Application for Direct Synthesis of Middle Isoparaffins from Synthesis Gas. Chem. Lett. 2005, 34, 148–149. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, B.; Chen, C.; Jia, L.; Ma, Z.; Wang, Q.; Li, D. Carbon Coated Cobalt Catalysts for Direct Synthesis of Middle N-Alkanes from Syngas. Fuel 2022, 327, 124889. [Google Scholar] [CrossRef]
- Zhu, C.; Bollas, G.M. Gasoline Selective Fischer-Tropsch Synthesis in Structured Bifunctional Catalysts. Appl. Catal. B 2018, 235, 92–102. [Google Scholar] [CrossRef]
- Carvill, B.T.; Hufton, J.R.; Anand, M.; Sircar, S. Sorption-Enhanced Reaction Process. AIChE J. 1996, 42, 2756–2772. [Google Scholar] [CrossRef]
- Hufton, J.R.; Mayorga, S.; Sircar, S. Sorption-Enhanced Reaction Process for Hydrogen Production. AIChE J. 1999, 45, 248–256. [Google Scholar] [CrossRef]
- Xiu, G.-H.; Soares, J.L.; Li, P.; Rodrigues, A.E. Simulation of Five-Step One-Bed Sorption-Enhanced Reaction Process. AIChE J. 2002, 48, 2817–2832. [Google Scholar] [CrossRef]
- Živković, L.A.; Pohar, A.; Likozar, B.; Nikačević, N.M. Kinetics and Reactor Modeling for CaO Sorption-Enhanced High-Temperature Water–Gas Shift (SE–WGS) Reaction for Hydrogen Production. Appl. Energy 2016, 178, 844–855. [Google Scholar] [CrossRef]
- Arora, A.; Iyer, S.S.; Bajaj, I.; Faruque Hasan, M.M. Optimal Methanol Production via Sorption-Enhanced Reaction Process. Ind. Eng. Chem. Res. 2018, 57, 14143–14161. [Google Scholar] [CrossRef]
- Maksimov, P.; Laari, A.; Ruuskanen, V.; Koiranen, T.; Ahola, J. Methanol Synthesis through Sorption Enhanced Carbon Dioxide Hydrogenation. Chem. Eng. J. 2021, 418, 129290. [Google Scholar] [CrossRef]
- Cholewa, T.; Semmel, M.; Mantei, F.; Güttel, R.; Salem, O. Process Intensification Strategies for Power-to-X Technologies. ChemEngineering 2022, 6, 13. [Google Scholar] [CrossRef]
- Westerterpt, K.R.; Kuczynski, M. A Model for a Countercurrent Gas-Solid Trickle Flow Reactor for Equilibrium Reactions. The Methanol Synthesis. Chem. Eng. Sci. 1987, 42, 1871–1885. [Google Scholar] [CrossRef][Green Version]
- Kuczynski, M.; Oyevaar, M.H.; Pieters, R.T.; Westerterpt, K.R. Methanol Synthesis in a Countercurrent Gas-Solid-Solid Trickle Flow Reactor. An Experimental Study. Chem. Eng. Sci. 1987, 42, 1887–1898. [Google Scholar] [CrossRef]
- Ison, A.; Gorte, R.J. The Adsorption of Methanol and Water on H-ZSM-5. J. Catal. 1984, 89, 150–158. [Google Scholar] [CrossRef]
- Ullah, A.; Hashim, N.A.; Rabuni, M.F.; Mohd Junaidi, M.U. A Review on Methanol as a Clean Energy Carrier: Roles of Zeolite in Improving Production Efficiency. Energies 2023, 16, 1482. [Google Scholar] [CrossRef]
- Terreni, J.; Trottmann, M.; Franken, T.; Heel, A.; Borgschulte, A. Sorption-Enhanced Methanol Synthesis. Energy Technol. 2019, 7, 1801093. [Google Scholar] [CrossRef]
- Vanoye, L.; Favre-Réguillon, A.; Munno, P.; Rodríguez, J.F.; Dupuy, S.; Pallier, S.; Pitault, I.; De Bellefon, C. Methanol Dehydration over Commercially Available Zeolites: Effect of Hydrophobicity. Catal. Today 2013, 215, 239–242. [Google Scholar] [CrossRef]
- Jia Le, N.; Yin Fong, Y. Catalytic Conversion of CO2 to Dimethyl Ether: A Review of Recent Advances in Catalysts and Water Selective Layer. J. Ind. Eng. Chem. 2024, 140, 88–102. [Google Scholar] [CrossRef]
- Zapater, D.; Lasobras, J.; Soler, J.; Herguido, J.; Menéndez, M. Counteracting SAPO-34 Catalyst Deactivation in MTO Process Using a Two Zone Fluidized Bed Reactor: Reactor Testing and Process Viability. Catal. Today 2021, 362, 155–161. [Google Scholar] [CrossRef]
- Nikolic, M.; Daemen, L.; Ramirez-Cuesta, A.J.; Xicohtencatl, R.B.; Cheng, Y.; Putnam, S.T.; Stadie, N.P.; Liu, X.; Terreni, J.; Borgschulte, A. Neutron Insights into Sorption Enhanced Methanol Catalysis. Top. Catal. 2021, 64, 638–643. [Google Scholar] [CrossRef]
- Heracleous, E.; Koidi, V.; Lappas, A.A. Experimental Investigation of Sorption-Enhanced CO2 Hydrogenation to Methanol. ACS Sustain. Chem. Eng. 2023, 11, 9684–9695. [Google Scholar] [CrossRef]
- Heracleous, E.; Koidi, V.; Lappas, A.A. Effect of Zeolite Type in Sorption-Enhanced CO2 Hydrogenation to Methanol. Chem. Eng. J. 2024, 502, 157846. [Google Scholar] [CrossRef]
- Gavrilovic, L.; Kazi, S.S.; Cordero-Lanzac, T.; Olsbye, U. Sorption-Enhanced Methanol Synthesis. In Proceedings of the 17th Greenhouse Gas Control Technologies Conference (GHGT-17), Calgary, AB, Canada, 20–24 October 2024. [Google Scholar]
- Menéndez, M.; Herguido, J.; Soler, J.; Lasobras, J. Patent application: Reactor System for Sorption-Enhanced Catalytic Reactions with Continuous Regeneration of Sorbent and Related Methods. WO 2025/008320A1, 9 January 2025. [Google Scholar]
- Menéndez, M.; Ciércoles, R.; Lasobras, J.; Soler, J.; Herguido, J. A Preliminary Assessment of Sorption-Enhanced Methanol Synthesis in a Fluidized Bed Reactor with Selective Addition/Removal of the Sorbent. Catalysts 2024, 14, 409. [Google Scholar] [CrossRef]
- Arora, A.; Iyer, S.S.; Hasan, M.M.F. GRAMS: A General Framework Describing Adsorption, Reaction and Sorption-Enhanced Reaction Processes. Chem. Eng. Sci. 2018, 192, 335–358. [Google Scholar] [CrossRef]
- Nieminen, H.; Maksimov, P.; Laari, A.; Väisänen, V.; Vuokila, A.; Huuhtanen, M.; Koiranen, T. Process Modelling and Feasibility Study of Sorption-Enhanced Methanol Synthesis. Chem. Eng. Proc. 2022, 179, 109052. [Google Scholar] [CrossRef]
- Bayat, M.; Heravi, M.; Rahimpour, M.R. Sorption Enhanced Process by Integrated Heat-Exchanger Reactor Assisted by Fluidization Concept for Methanol Synthesis. Chem. Eng. Proc. 2016, 110, 30–43. [Google Scholar] [CrossRef]
- Abashar, M.E.E.; Al-Rabiah, A.A. Investigation of the Efficiency of Sorption-Enhanced Methanol Synthesis Process in Circulating Fast Fluidized Bed Reactors. Fuel Proc. Technol. 2018, 179, 387–398. [Google Scholar] [CrossRef]
- Abashar, M.E.E.; Al-Rabiah, A.A. Highly Efficient CO2 Hydrogenation to Methanol via In-Situ Condensation and Sorption in a Novel Multi-Stage Circulating Fast Fluidized Bed Reactor. Chem. Eng. J. 2022, 439, 135628. [Google Scholar] [CrossRef]
- Moioli, E.; Schildhauer, T. Tailoring the Reactor Properties in the Small-Scale Sorption-Enhanced Methanol Synthesis. Chem. Ing. Tech. 2023, 95, 631–641. [Google Scholar] [CrossRef]
- Krim, K.; Sachse, A.; Le Valant, A.; Pouilloux, Y.; Hocine, S. One Step Dimethyl Ether (DME) Synthesis from CO2 Hydrogenation over Hybrid Catalysts Containing Cu/ZnO/Al2O3 and Nano-Sized Hollow ZSM-5 Zeolites. Catal. Lett. 2023, 153, 83–94. [Google Scholar] [CrossRef]
- Frusteri, F.; Cordaro, M.; Cannilla, C.; Bonura, G. Multifunctionality of Cu-ZnO-ZrO2/H-ZSM5 Catalysts for the One-Step CO2-to-DME Hydrogenation Reaction. Appl. Catal. B 2015, 162, 57–65. [Google Scholar] [CrossRef]
- Bonura, G.; Cordaro, M.; Cannilla, C.; Mezzapica, A.; Spadaro, L.; Arena, F.; Frusteri, F. Catalytic Behaviour of a Bifunctional System for the One Step Synthesis of DME by CO2 Hydrogenation. Catal. Today 2014, 228, 51–57. [Google Scholar] [CrossRef]
- Guffanti, S.; Visconti, C.G.; Groppi, G. Model Analysis of the Role of Kinetics, Adsorption Capacity, and Heat and Mass Transfer Effects in Sorption Enhanced Dimethyl Ether Synthesis. Ind. Eng. Chem. Res. 2021, 60, 6767–6783. [Google Scholar] [CrossRef]
- Liuzzi, D.; Peinado, C.; Peña, M.A.; Van Kampen, J.; Boon, J.; Rojas, S. Increasing Dimethyl Ether Production from Biomass-Derived Syngas: Via Sorption Enhanced Dimethyl Ether Synthesis. Sustain. Energy Fuels 2020, 4, 5674–5681. [Google Scholar] [CrossRef]
- Altinsoy, N.S.; Avci, A.K. Sorption Enhanced DME Synthesis by One-Step CO2 Hydrogenation. Chem. Eng. Proc. 2024, 203, 109874. [Google Scholar] [CrossRef]
- van Kampen, J.; Boon, J.; Vente, J.; van Sint Annaland, M. Sorption Enhanced Dimethyl Ether Synthesis under Industrially Relevant Conditions: Experimental Validation of Pressure Swing Regeneration. React. Chem. Eng. 2021, 6, 244–257. [Google Scholar] [CrossRef]
- Van Kampen, J.; Booneveld, S.; Boon, J.; Vente, J.; Van Sint Annaland, M. Experimental Validation of Pressure Swing Regeneration for Faster Cycling in Sorption Enhanced Dimethyl Ether Synthesis. Chem. Comm. 2020, 56, 13540–13542. [Google Scholar] [CrossRef]
- Graaf, G.H.; Stamhuis, E.J.; Beenackers, A.A.C.M. Kinetics of Low-Pressure Methanol Synthesis. Chem. Eng. Sci. 1988, 43, 3185–3195. [Google Scholar] [CrossRef]
- Berčič, G.; Levec, J. Intrinsic and Global Reaction Rate of Methanol Dehydration over γ-Al2O3 Pellets. Ind. Eng. Chem. Res. 1992, 31, 1035–1040. [Google Scholar] [CrossRef]
- Kim, K.M.; Oh, H.T.; Lim, S.J.; Ho, K.; Park, Y.; Lee, C.H. Adsorption Equilibria of Water Vapor on Zeolite 3A, Zeolite 13X, and Dealuminated y Zeolite. J. Chem. Eng. Data 2016, 61, 1547–1554. [Google Scholar] [CrossRef]
- Ozcan, M.C.; Karaman, B.P.; Oktar, N.; Dogu, T. Dimethyl Ether from Syngas and Effect of CO2 Sorption on Product Distribution over a New Bifunctional Catalyst Pair Containing STA@SBA-15. Fuel 2022, 330, 125607. [Google Scholar] [CrossRef]
- Renda, S.; Soler, J.; Herguido, J.; Menéndez, M. Effect of Particles Size and Density on the Segregation of Catalyst-Sorbent Mixtures for Direct Sorption-Enhanced DME Synthesis: Experimental and Mathematical Study. Biomass Bioenergy 2025, 197, 107764. [Google Scholar] [CrossRef]
- Guffanti, S.; Visconti, C.G.; van Kampen, J.; Boon, J.; Groppi, G. Reactor Modelling and Design for Sorption Enhanced Dimethyl Ether Synthesis. Chem. Eng. J. 2021, 404, 126573. [Google Scholar] [CrossRef]
- Iliuta, I.; Iliuta, M.C.; Larachi, F. Sorption-Enhanced Dimethyl Ether Synthesis—Multiscale Reactor Modeling. Chem. Eng. Sci. 2011, 66, 2241–2251. [Google Scholar] [CrossRef]
- Tyraskis, I.; Capa, A.; Skorikova, G.; Sluijter, S.N.; Boon, J. Performance Optimization of Sorption-Enhanced DME Synthesis (SEDMES) from Captured CO2 and Renewable Hydrogen. Front. Chem. Eng. 2025, 7, 1521374. [Google Scholar] [CrossRef]
- Bertole, C.J.; Mims, C.A.; Kiss, G. The Effect of Water on the Cobalt-Catalyzed Fischer-Tropsch Synthesis. J. Catal. 2002, 210, 84–96. [Google Scholar] [CrossRef]
- Pendyala, V.R.R.; Jacobs, G.; Mohandas, J.C.; Luo, M.; Hamdeh, H.H.; Ji, Y.; Ribeiro, M.C.; Davis, B.H. Fischer-Tropsch Synthesis: Effect of Water over Iron-Based Catalysts. Catal. Lett. 2010, 140, 98–105. [Google Scholar] [CrossRef]
- Asbahr, W.; Lamparter, R.; Rauch, R. A Cold Flow Model of Interconnected Slurry Bubble Columns for Sorption-Enhanced Fischer–Tropsch Synthesis. ChemEngineering 2024, 8, 52. [Google Scholar] [CrossRef]
- Gavrilović, L.; Kazi, S.S.; Oliveira, A.; Encinas, O.L.I.; Blekkan, E.A. Sorption-Enhanced Fischer-Tropsch Synthesis—Effect of Water Removal. Catal. Today 2024, 432, 114614. [Google Scholar] [CrossRef]
- Bayat, M.; Hamidi, M.; Dehghani, Z.; Rahimpour, M.R.; Shariati, A. Sorption-Enhanced Reaction Process in Fischer-Tropsch Synthesis for Production of Gasoline and Hydrogen: Mathematical Modeling. J. Nat. Gas Sci. Eng. 2013, 14, 225–237. [Google Scholar] [CrossRef]
- Bayat, M.; Dehghani, Z.; Rahimpour, M.R. Sorption-Enhanced Methanol Synthesis in a Dual-Bed Reactor: Dynamic Modeling and Simulation. J. Taiwan Inst. Chem. Eng. 2014, 45, 2307–2318. [Google Scholar] [CrossRef]
- Coppola, A.; Massa, F.; Salatino, P.; Scala, F. Evaluation of Two Sorbents for the Sorption-Enhanced Methanation in a Dual Fluidized Bed System. Biomass Convers. Biorefin. 2021, 11, 111–119. [Google Scholar] [CrossRef]
- Agirre, I.; Acha, E.; Cambra, J.F.; Barrio, V.L. Water Sorption Enhanced CO2 Methanation Process: Optimization of Reaction Conditions and Study of Various Sorbents. Chem. Eng. Sci. 2021, 237, 116546. [Google Scholar] [CrossRef]
- Renda, S.; Ricca, A.; Palma, V. Insights in the Application of Highly Conductive Structured Catalysts to CO2 Methanation: Computational Study. Int. J. Hydrog. Energy 2023, 48, 37473–37488. [Google Scholar] [CrossRef]
- Leviness, S.; Deshmukh, S.R.; Richard, L.A.; Robota, H.J. Velocys Fischer-Tropsch Synthesis Technology—New Advances on State-of-the-Art. Top. Catal. 2014, 57, 518–525. [Google Scholar] [CrossRef]
- Almeida, L.C.; Echave, F.J.; Sanz, O.; Centeno, M.A.; Arzamendi, G.; Gandía, L.M.; Sousa-Aguiar, E.F.; Odriozola, J.A.; Montes, M. Fischer-Tropsch Synthesis in Microchannels. Chem. Eng. J. 2011, 167, 536–544. [Google Scholar] [CrossRef]
- Ying, X.; Zhang, L.; Xu, H.; Ren, Y.L.; Luo, Q.; Zhu, H.W.; Qu, H.; Xuan, J. Efficient Fischer-Tropsch Microreactor with Innovative Aluminizing Pretreatment on Stainless Steel Substrate for Co/Al2O3 Catalyst Coating. Fuel Process. Technol. 2016, 143, 51–59. [Google Scholar] [CrossRef]
- Almeida, L.C.; Sanz, O.; Merino, D.; Arzamendi, G.; Gandía, L.M.; Montes, M. Kinetic Analysis and Microstructured Reactors Modeling for the Fischer-Tropsch Synthesis over a Co-Re/Al2O3 Catalyst. Proc. Catal. Today 2013, 215, 103–111. [Google Scholar] [CrossRef]
- Hilmen, A.-M.M.; Bergene, E.; Lindvåg, O.A.; Schanke, D.; Eri, S.; Holmen, A. Fischer-Tropsch Synthesis on Monolithic Catalysts of Different Materials. Catal. Today 2001, 69, 227–232. [Google Scholar] [CrossRef]
- Guettel, R.; Turek, T. Comparison of Different Reactor Types for Low Temperature Fischer–Tropsch Synthesis: A Simulation Study. Chem. Eng. Sci. 2009, 64, 955–964. [Google Scholar] [CrossRef]
- De Deugd, R.M.; Kapteijn, F.; Moulijn, J.A. Using Monolithic Catalysts for Highly Selective Fischer-Tropsch Synthesis. Catal. Today 2003, 79–80, 495–501. [Google Scholar] [CrossRef]
- Ibáñez, M.; Sanz, O.; Egaña, A.; Reyero, I.; Bimbela, F.; Gandía, L.M.; Montes, M. Performance Comparison between Washcoated and Packed-Bed Monolithic Reactors for the Low-Temperature Fischer-Tropsch Synthesis. Chem. Eng. J. 2021, 425, 130424. [Google Scholar] [CrossRef]
- Visconti, C.G.; Tronconi, E.; Groppi, G.; Lietti, L.; Iovane, M.; Rossini, S.; Zennaro, R. Monolithic Catalysts with High Thermal Conductivity for the Fischer-Tropsch Synthesis in Tubular Reactors. Chem. Eng. J. 2011, 171, 1294–1307. [Google Scholar] [CrossRef]
- Visconti, C.G.; Panzeri, M.; Groppi, G.; Tronconi, E. Heat Transfer Intensification in Compact Tubular Reactors with Cellular Internals: A Pilot-Scale Assessment of Highly Conductive Packed-POCS with Skin Applied to the Fischer-Tropsch Synthesis. Chem. Eng. J. 2024, 481, 148469. [Google Scholar] [CrossRef]
- Ghosh, S.; Seethamraju, S. Feasibility of Reactive Distillation for Methanol Synthesis. Chem. Eng. Proc. 2019, 145, 889–899. [Google Scholar] [CrossRef]
- Srinivas, S.; Malik, R.K.; Mahajani, S.M. Feasibility of Reactive Distillation for Fischer—Tropsch Synthesis. Ind. Eng. Chem. Res. 2008, 47, 889–899. [Google Scholar] [CrossRef]
- Van Bennekom, J.G.; Venderbosch, R.H.; Assink, D.; Lemmens, K.P.J.; Heeres, H.J. Bench Scale Demonstration of the Supermethanol Concept: The Synthesis of Methanol from Glycerol Derived Syngas. Chem. Eng. J. 2012, 207–208, 245–253. [Google Scholar] [CrossRef]
- Kirsch, H.; Lochmahr, N.; Staudt, C.; Pfeifer, P.; Dittmeyer, R. Production of CO2-Neutral Liquid Fuels by Integrating Fischer-Tropsch Synthesis and Hydrocracking in a Single Micro-Structured Reactor: Performance Evaluation of Different Configurations by Factorial Design Experiments. Chem. Eng. J. 2020, 393, 124553. [Google Scholar] [CrossRef]
- Wentrup, J.; Pesch, G.R.; Thöming, J. Dynamic Operation of Fischer-Tropsch Reactors for Power-to-Liquid Concepts: A Review. Renew. Sustain. Energy Rev. 2022, 162, 112454. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).