Carbonation Deactivation of Limestone in a Micro-Fluidized Bed Reactor
Abstract
1. Introduction
2. Experimental Methodology
2.1. Limestone Characterization
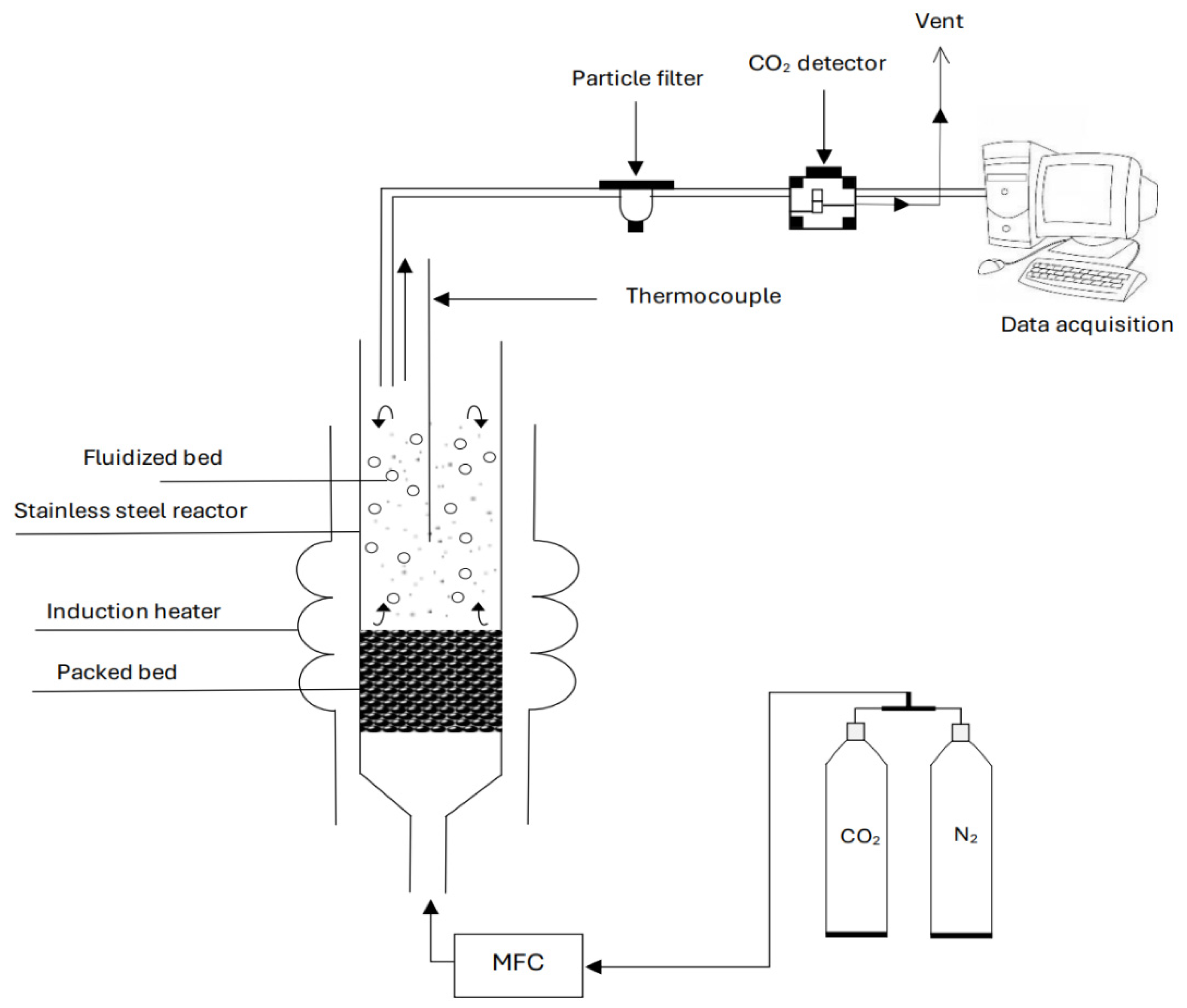
2.2. Fluidization Rig and Procedures
3. Results and Discussion
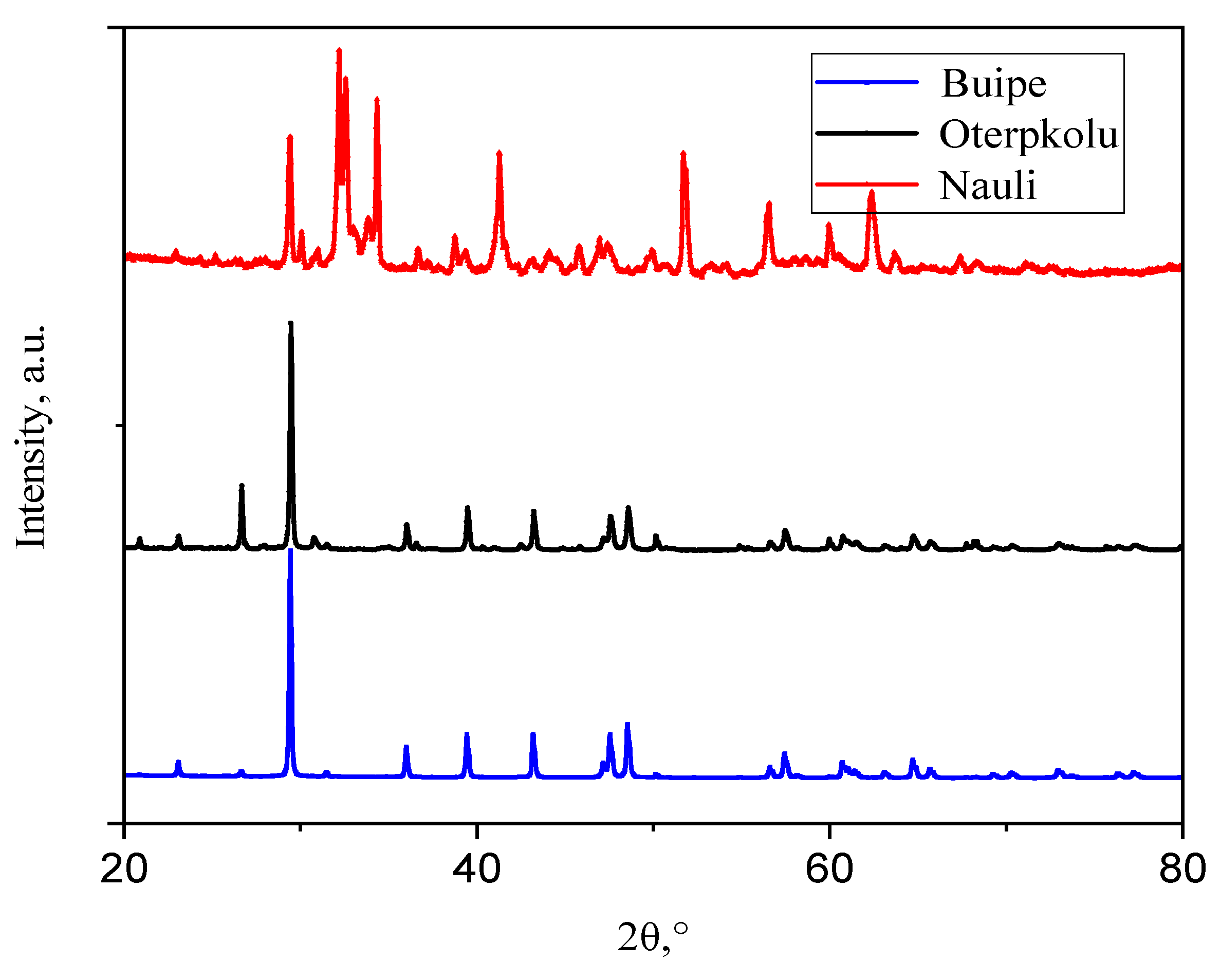
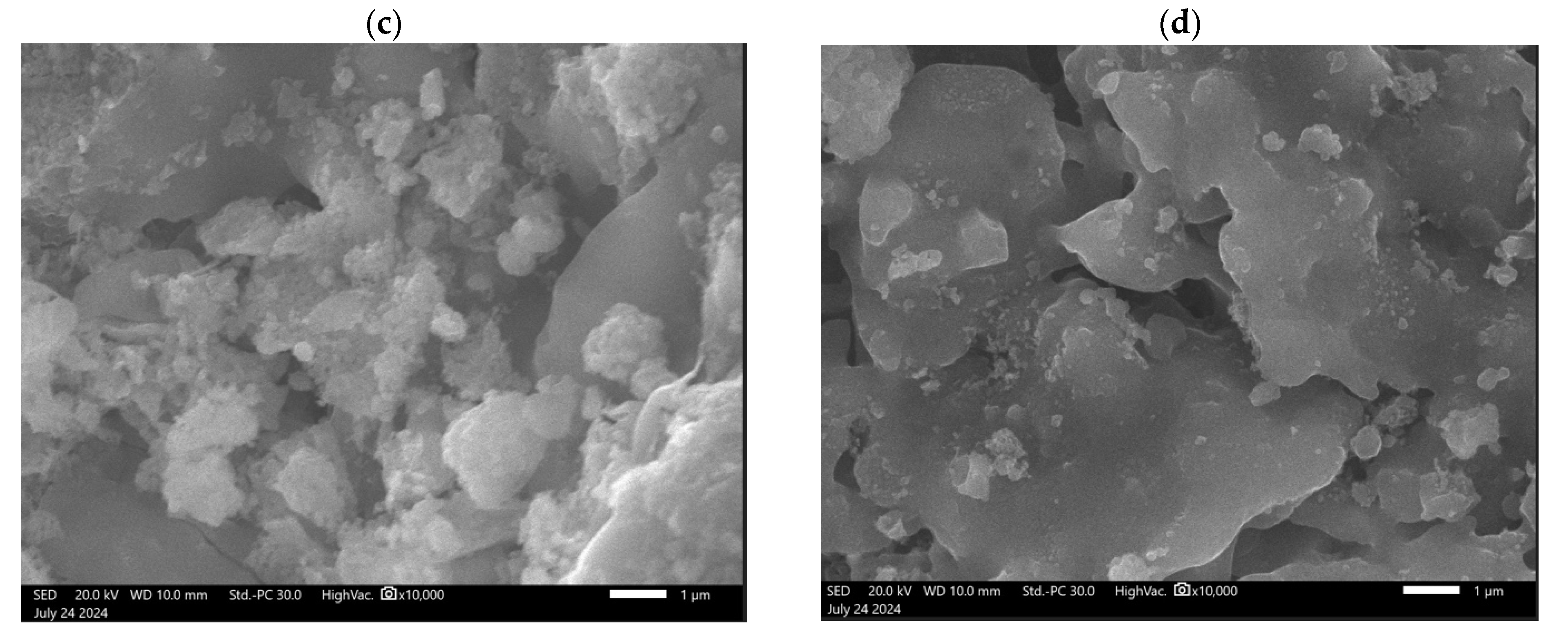
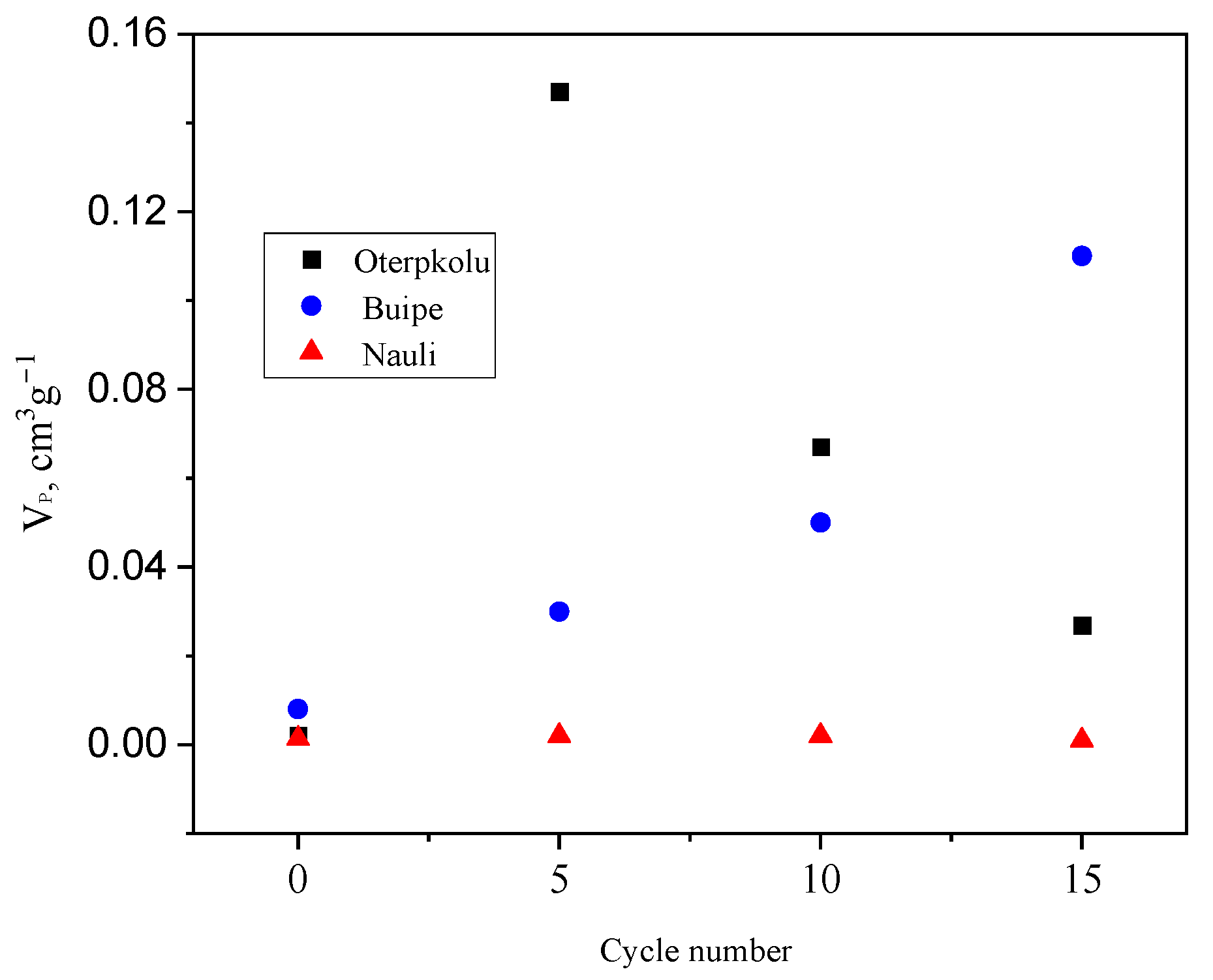
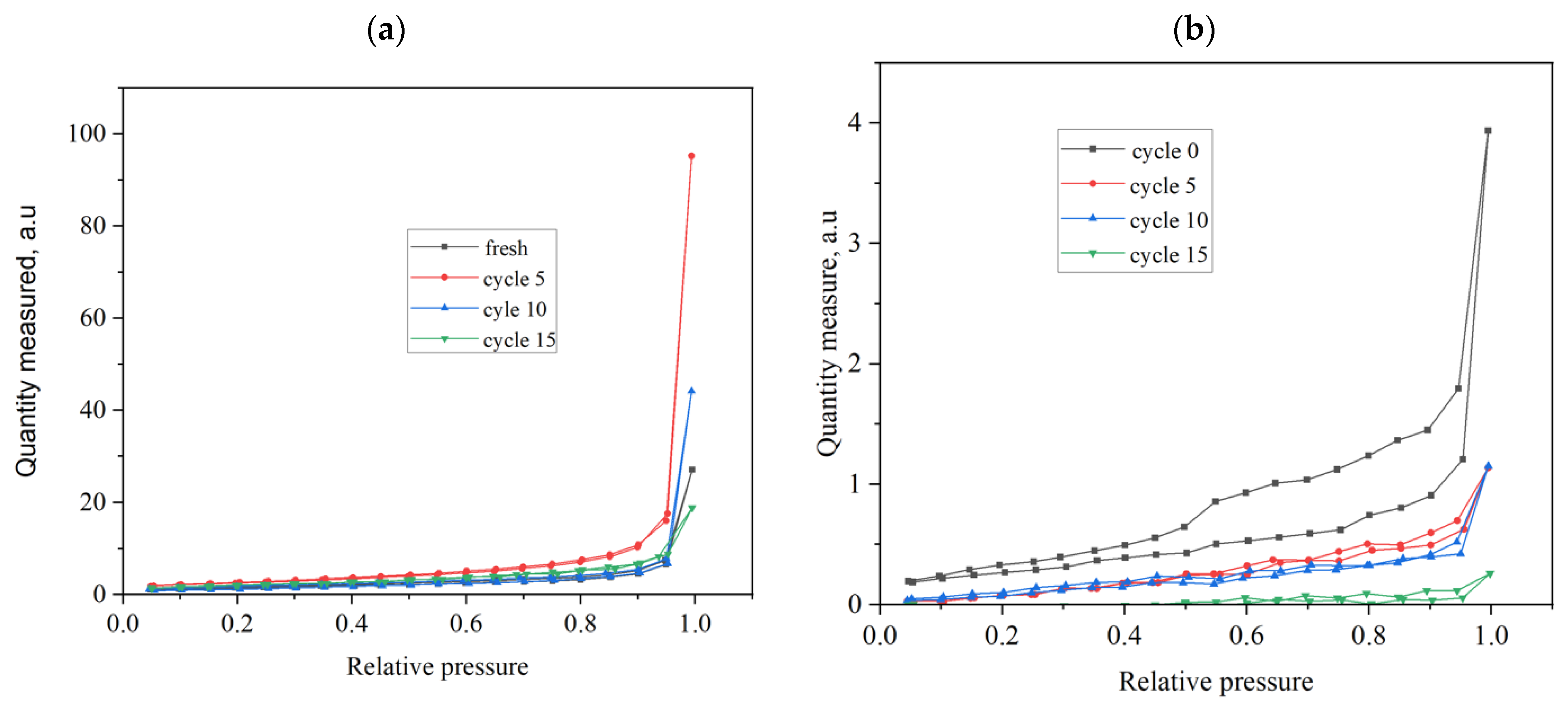
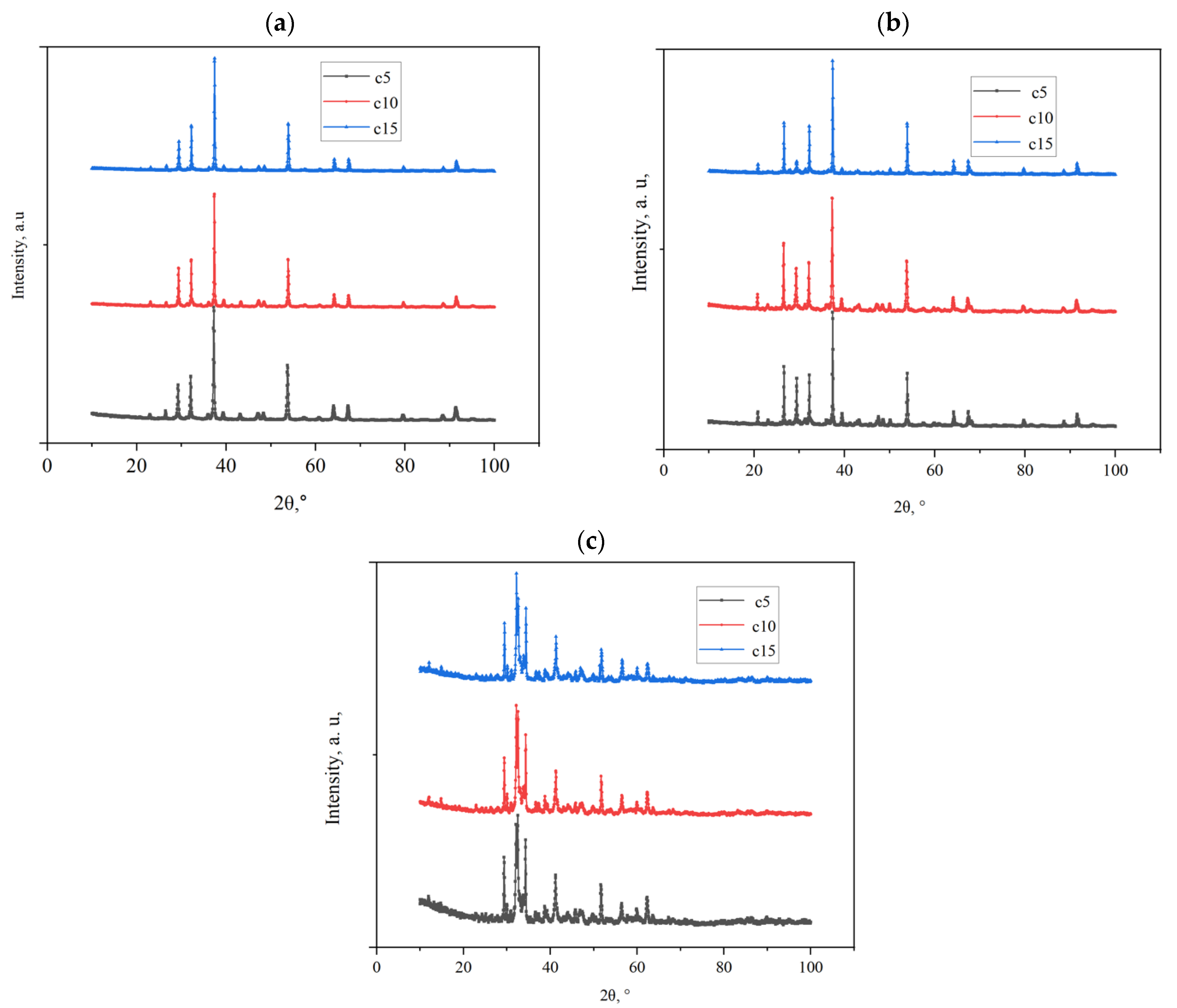
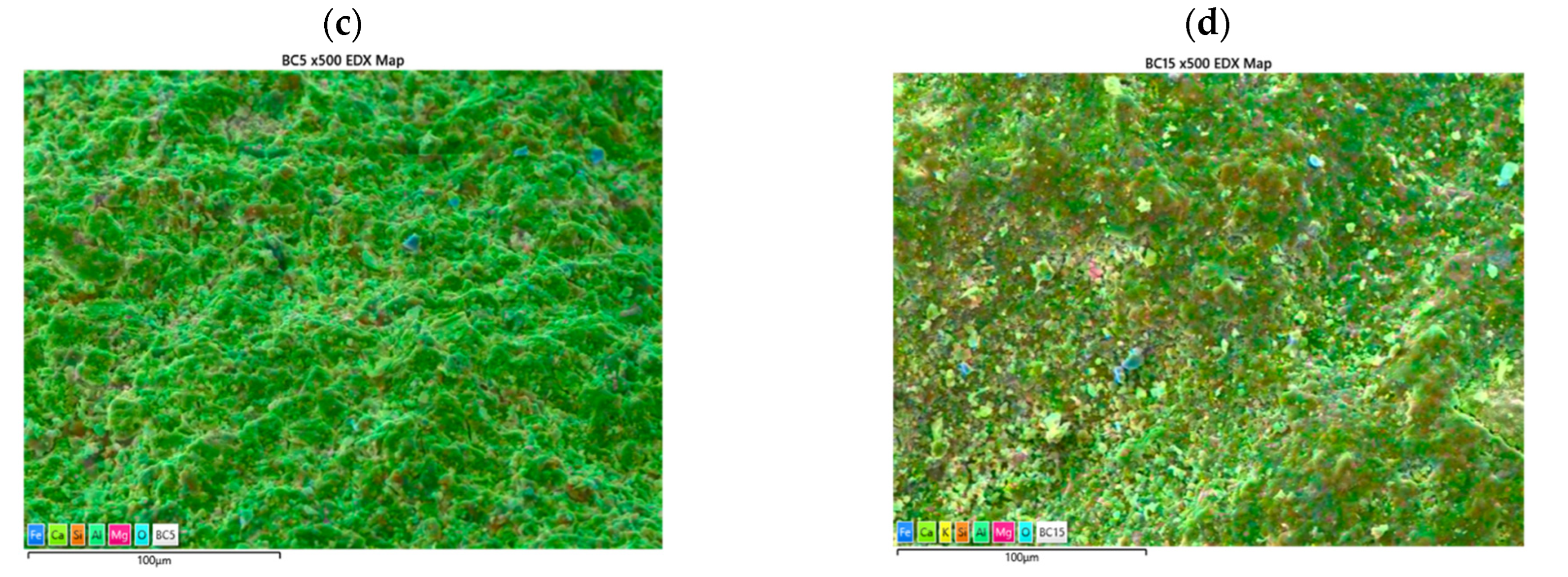
3.1. Limestone Characterization
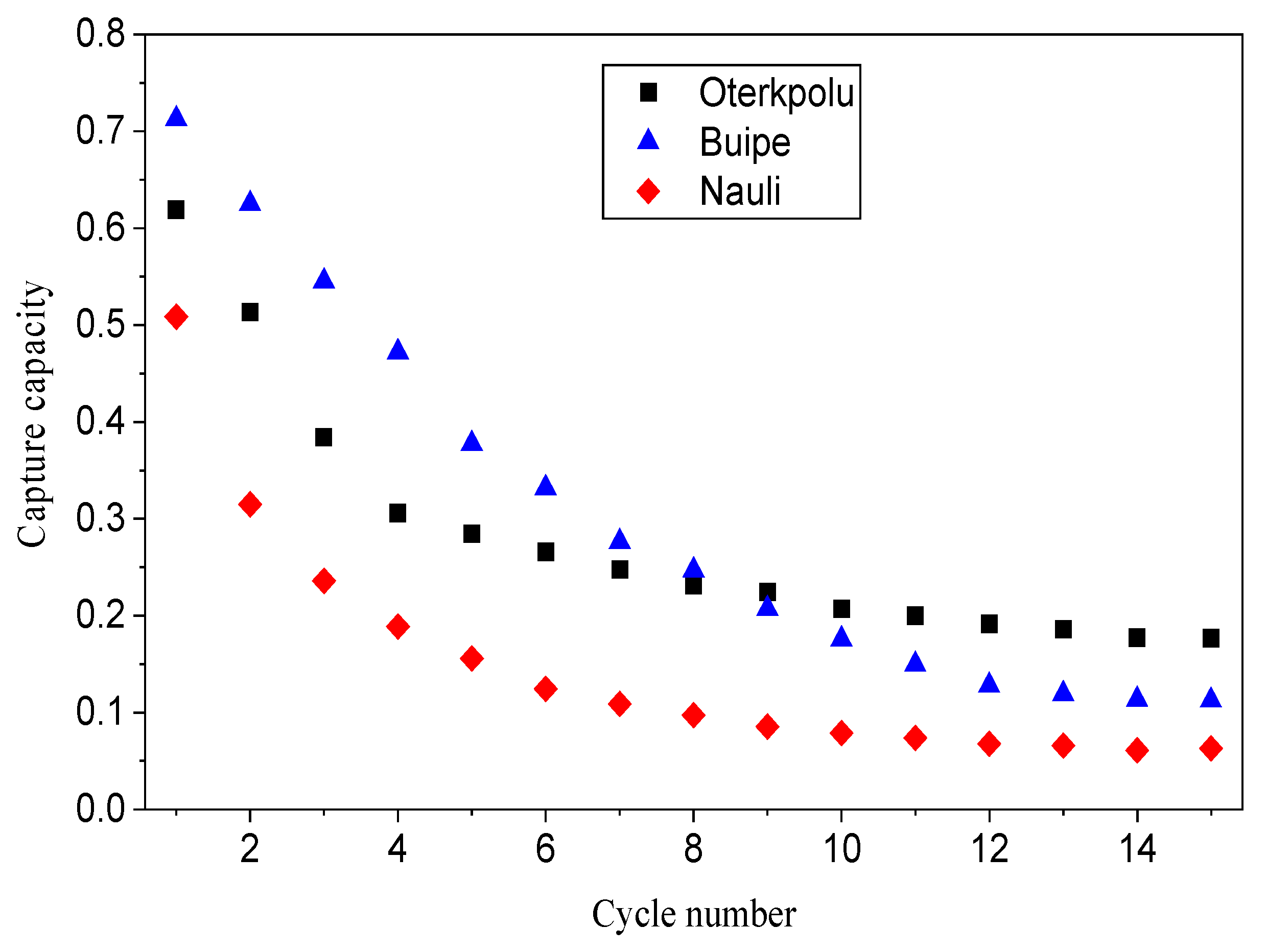
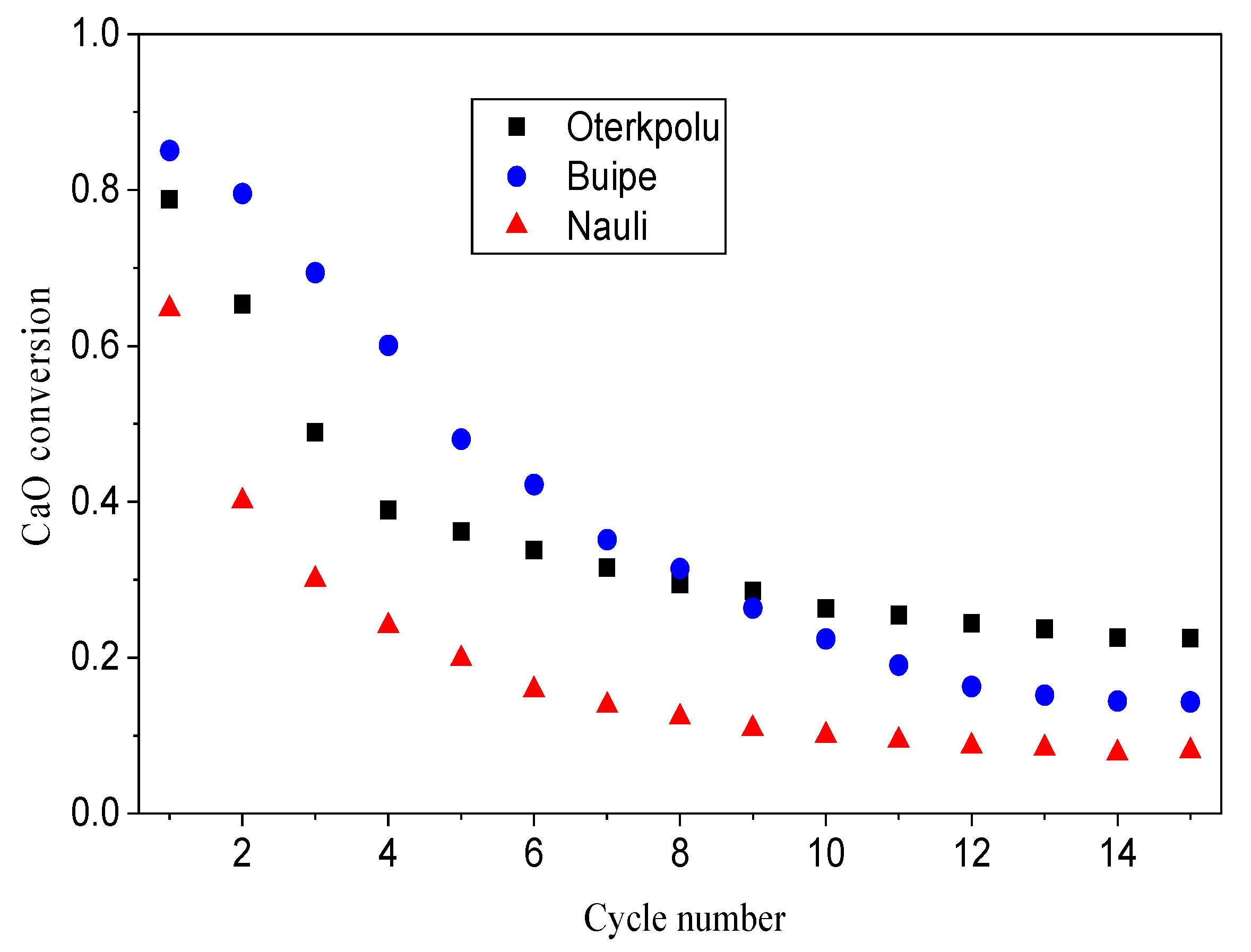
3.2. Reactivity of Limestones
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plakitkina, L.S.; Plakitkin, Y.A.; Dyachenko, K.I. Hydrogen versus coal: Implications of the hydrogen energy engineering advance for the coal industry. Gorn. Zhurnal 2021, 2021, 14–21. [Google Scholar] [CrossRef]
- World Metrological Organization. WMO Confirms 2024 as Warmest Year on Record at About 1.55 °C Above Pre-Industrial Level. 2024. Available online: https://wmo.int/news/media-centre/wmo-confirms-2024-warmest-year-record-about-155degc-above-pre-industrial-level (accessed on 27 February 2025).
- Roche. Collaborating for a Lasting Future-Global Status of CCC 2024; Roche: Basel, Switzerland, 2024. [Google Scholar]
- Blamey, J.; Anthony, E.; Wang, J.; Fennell, P. The calcium looping cycle for large-scale CO2 capture. Prog. Energy Combust Sci. 2010, 36, 260–279. [Google Scholar] [CrossRef]
- Alie, C.; Backham, L.; Croiset, E.; Douglas, P. Simulation of CO2 capture using MEA scrubbing: A flowsheet decomposition method. Energy Convers. Manag. 2005, 46, 475–487. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.S. A Technical, Economic, and Environmental Assessment of Amine-Based CO2 Capture Technology for Power Plant Greenhouse Gas Control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zahra, M.R.; Schneiders, L.H.; Niederer, J.P.; Feron, P.H.; Versteeg, G.F. CO2 capture from power plants Part I. A parametric study of the technical performance based on monoethanolamine. Int. J. Greenh. Gas Control 2007, 1, 37–46. [Google Scholar] [CrossRef]
- Nabavi, S.A.; Erans, M.; Manović, V. Demonstration of a kW-scale solid oxide fuel cell-calciner for power generation and production of calcined materials. Appl. Energy 2019, 255, 113731. [Google Scholar] [CrossRef]
- Amirkhosrow, M.; Lay, E.N. Simulation model evaluation of desorber column in CO2 capture process by MEA scrubbing: A rigorous rate-based model for kinetic model and mass transfer correlations analysis. Fuel Process. Technol. 2020, 203, 106390. [Google Scholar] [CrossRef]
- Alvarez, D.; Abanades, J.C. Determination of the critical product layer thickness in the reaction of CaO with CO2. Ind. Eng. Chem. Res. 2005, 44, 5608–5615. [Google Scholar] [CrossRef]
- Sun, P.; Lim, C.J.; Grace, J.R. Cyclic CO2 capture by limestone-derived sorbent during prolonged calcination/carbonation cycling. AIChE J. 2008, 54, 1668–1677. [Google Scholar] [CrossRef]
- Marques, L.; Mota, S.; Teixeira, P.; Pinheiro, C.; Matos, H. Ca-looping process using wastes of marble powders and limestones for CO2 capture from real flue gas in the cement industry. J. CO2 Util. 2023, 71, 102450. [Google Scholar] [CrossRef]
- Abreu, M.; Teixeira, P.; Filipe, R.M.; Domingues, L.; Pinheiro, C.I.; Matos, H.A. Modeling the deactivation of CaO-based sorbents during multiple Ca-looping cycles for CO2 post-combustion capture. Comput. Chem. Eng. 2020, 134, 106679. [Google Scholar] [CrossRef]
- Valverde, J.; Sanchez-Jimenez, P.; Perez-Maqueda, L. Ca-looping for postcombustion CO2 capture: A comparative analysis on the performances of dolomite and limestone. Appl. Energy 2015, 138, 202–215. [Google Scholar] [CrossRef]
- Bosoaga, A.; Masek, O.; Oakey, J.E. CO2 Capture Technologies for Cement Industry. Energy Procedia 2009, 1, 133–140. [Google Scholar] [CrossRef]
- Anthony, E.J.; Wang, J.; Oakey, J.E. Integrating CO2 Capture with CaO. Environ. Sci. Technol. 2005, 39, 2861–2866. [Google Scholar]
- Grasa, G.S.; Abanades, J.C. CO2 capture capacity of CaO in long series of carbonation/calcination cycles. Ind. Eng. Chem. Res. 2006, 45, 8846–8851. [Google Scholar] [CrossRef]
- Zhou, L.; Duan, L.; Anthony, E.J. A calcium looping process for simultaneous CO2 capture and peak shaving in a coal-fired power plant. Appl. Energy 2019, 235, 480–486. [Google Scholar] [CrossRef]
- Manovic, V.; Charland, J.-P.; Blamey, J.; Fennell, P.S.; Lu, D.Y.; Anthony, E.J. Influence of calcination conditions on carrying capacity of CaO-based sorbent in CO2 looping cycles. Fuel 2009, 88, 1893–1900. [Google Scholar] [CrossRef]
- Abanades, J.C.; Alvarez, D. Conversion limits in the reaction of CO2 with lime. Energy Fuels 2003, 17, 308–315. [Google Scholar] [CrossRef]
- Sun, P.; Grace, J.R.; Lim, C.J.; Anthony, E.J. The effect of CaO sintering on cyclic CO2 capture in energy systems. AIChE J. 2007, 53, 2432–2442. [Google Scholar] [CrossRef]
- Teixeira, P.; Fernandes, A.; Ribeiro, F.; Pinheiro, C.I.C. Blending wastes of marble powder and dolomite sorbents for calcium-looping CO2 capture under realistic industrial calcination conditions. Materials 2021, 14, 4379. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.W.; Lu, D.; Anthony, E.J.; Wu, Y. Improved long-term conversion of limestone derived sorbents for in situ capture of CO2 in a fluidised bed combustor. Ind. Eng. Chem. Res. 2004, 43, 5529–5539. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C.; Chen, H.; Duan, L.; Chen, X. CO2 Capture Behavior of Shell during Calcination/Carbonation Cycles. Chem. Eng. Technol. 2009, 32, 1176–1182. [Google Scholar] [CrossRef]
- Valverde, J.M.; Perejon, A.; Perez-Maqueda, L.A. Enhancement of Fast CO2 Capture by a Nano-SiO2/CaO Composite at Ca-Looping Conditions. Environ. Sci. Technol. 2012, 46, 6401–6408. [Google Scholar] [CrossRef] [PubMed]
- Glasson, D.R.; O’Neill, P. Reactivity of lime and related oxides. XVII. Sulphating of lime with magnesium and aluminium sulphates. J. Appl. Chem. 1967, 17, 96–101. [Google Scholar] [CrossRef]
- Lu, H.; Khan, A.; Pratsinis, S.E.; Smirniotis, P.G. Flame-Made Durable Doped-CaO Nanosorbents for CO2 Capture. Energy Fuels 2009, 23, 1093–1100. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ellis, N.; Lim, C.J.; Grace, J.R. Effect of calcination/carbonation and oxidation/reduction on attrition of binary solid species in sorption-enhanced chemical looping reforming. Fuel 2020, 271, 117665. [Google Scholar] [CrossRef]
- Maya, J.C.; Chejne, F.; Gómez, C.A.; Bhatia, S.K. Effect of the CaO sintering on the calcination rate of CaCO3 under atmospheres containing CO2. AIChE J. 2018, 64, 3638–3648. [Google Scholar] [CrossRef]
- Pinheiro, C.I.C.; Fernandes, A.; Freitas, C.; Santos, E.T.; Ribeiro, M.F. Waste Marble Powders as Promising Inexpensive Natural CaO-Based Sorbents for Post-Combustion CO2 Capture. Ind. Eng. Chem. Res. 2016, 55, 7860–7872. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Alvarez, D.; Abanades, J.C. Pore-Size and Shape Effects on the Recarbonation Performance of Calcium Oxide Submitted to Repeated Calcination/Recarbonation Cycles. Energy Fuels 2005, 19, 270–278. [Google Scholar] [CrossRef]
- Asiedu-Boateng, P.; Asiedu, N.; Zivkovic, V.; Patience, G. Attrition characteristics of limestone in gas-solid fluidized beds. Powder Technol. 2023, 419, 118342. [Google Scholar] [CrossRef]
- Asiedu-Boateng, P.; Legros, R.; Patience, G. Attrition resistance of calcium oxide-copper oxide-cement sorbents for post-combustion carbon dioxide capture. Adv. Powder Technol. 2016, 27, 786–795. [Google Scholar] [CrossRef]
- Perreault, P.; Patience, G.-S. Carbonation and deactivation kinetics of a mixed calcium oxide–copper oxide sorbent/oxygen carrier for post-combustion carbon dioxide capture. Chem. Eng. J. 2016, 306, 726–733. [Google Scholar] [CrossRef]






| Property/Material | Oterpkolu | Buipe | Nauli |
|---|---|---|---|
| Particle density, kg m−3 | 2560 | 2450 | 2430 |
| BET surface area, m2 g−1 | 3.2 | 2.0 | 0.2 |
| Pore volume, mL g−1 | 0.12 | 0.015 | 0.08 |
| D50, µm | 520 | 475 | 330 |
| Mean size, µm | 500 | 420 | 370 |
| Composition | Oterpkolu | Buipe | Nauli |
|---|---|---|---|
| MgO | 3.4 | 0.8 | 2 |
| Al2O3 | 5.0 | 2.4 | 4.1 |
| SiO2 | 27 | 7.7 | 14 |
| P2O5 | 0.1 | 0.4 | - |
| SO3 | 0.5 | 0.4 | 1 |
| K2O | 1.0 | 0.4 | 1.2 |
| CaO | 60 | 87 | 74 |
| TiO2 | 1.03 | 0.16 | 0.12 |
| MnO | 0.11 | 0.54 | 0.40 |
| Fe2O3 | 2.39 | 1.33 | 3.11 |
| BaO | - | - | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asiedu-Boateng, P.; Asiedu, N.Y.; Patience, G.S.; McDonough, J.R.; Zivkovic, V. Carbonation Deactivation of Limestone in a Micro-Fluidized Bed Reactor. Catalysts 2025, 15, 697. https://doi.org/10.3390/catal15080697
Asiedu-Boateng P, Asiedu NY, Patience GS, McDonough JR, Zivkovic V. Carbonation Deactivation of Limestone in a Micro-Fluidized Bed Reactor. Catalysts. 2025; 15(8):697. https://doi.org/10.3390/catal15080697
Chicago/Turabian StyleAsiedu-Boateng, P., N. Y. Asiedu, G. S. Patience, J. R. McDonough, and V. Zivkovic. 2025. "Carbonation Deactivation of Limestone in a Micro-Fluidized Bed Reactor" Catalysts 15, no. 8: 697. https://doi.org/10.3390/catal15080697
APA StyleAsiedu-Boateng, P., Asiedu, N. Y., Patience, G. S., McDonough, J. R., & Zivkovic, V. (2025). Carbonation Deactivation of Limestone in a Micro-Fluidized Bed Reactor. Catalysts, 15(8), 697. https://doi.org/10.3390/catal15080697









