Abstract
Manganese-based oxides with good redox properties exhibit high soot oxidation activity. To further enhance their catalytic performance, introducing additional metal elements into manganese-based oxides is considered an effective approach. Herein, two rare earth elements (Sm and Ce)-modified MnOx catalysts were prepared by the co-precipitation method. The synthesized MnOx catalyst primarily consists of the Mn3O4 phase, with trace amounts of Mn5O8. The addition of Sm or Ce maintains the predominance of the Mn3O4 phase, increases the proportion of Mn5O8, and enhances the redox properties, thereby boosting the catalytic activity for NO and soot oxidation. Notably, the coexistence of Sm and Ce achieves optimal soot oxidation activity, with T10 reaching 306 °C. Comprehensive physicochemical characterization elucidates the underlying structure–performance relationships of these catalysts.
1. Introduction
Soot particles are formed from the incomplete combustion of diesel fuel under high-temperature and oxygen-deficient conditions, resulting from the condensation of partially burned carbon elements [,,]. The harmful substances, such as sulfates and polycyclic aromatic hydrocarbons, adsorbed on the surface of soot particles, not only pollute the atmospheric environment but also pose a direct threat to human health [,]. Therefore, it is urgent to limit the emission of soot particles in diesel engine exhaust. Currently, the most effective way to eliminate soot particles is to combine catalytic combustion technology with filter capture technology for external purification [,]. Developing efficient soot oxidation catalysts is the core of this technology. Pt-based catalysts are widely utilized as commercial catalysts. However, Pt, being a rare noble metal, is very expensive. Therefore, it is essential to develop novel non-Pt-based soot oxidation catalysts with high activity.
Mn-based catalysts used for soot oxidation have received widespread attention due to their high catalytic activity, excellent redox properties, and lower costs [,,,]. Anantharaman et al. [] prepared CeO2, SnO2, Pr6O11, and Mn3O4 catalysts using the EDTA-citrate complexation method for soot oxidation. They found that Mn3O4 had the highest soot oxidation activity because of its higher reducibility. Dai et al. [] reported several pure MnOx compounds and evaluated their soot oxidation activity. They believed that the presence of oxygen species over MnOx could capture and oxidize NO, producing more reactive NO2, thereby enhancing soot oxidation. Wagloehner et al. [] evaluated the catalytic activity of various manganese oxide catalysts, such as MnO2, Mn2O3, Mn3O4, and nanosized Mn3O4, synthesized via flame spray pyrolysis (FSP-Mn3O4). They explored the relationship between the physical properties of these catalysts and their effectiveness in oxidizing soot. The findings indicated that FSP-Mn3O4 demonstrated the highest catalytic performance, achieving maximal soot oxidation at 305 °C under a tight contact of catalyst and soot, attributed to its superior oxygen mobility. Although single MnOx demonstrates good catalytic activity in soot oxidation, it has poor thermal stability, leading to agglomeration at high temperatures. To further improve the catalytic performance of MnOx, various metal oxides, including Fe2O3 [], CeO2 [], and Co3O4 [], have been introduced and explored. Among these metal oxides, CeO2 and Sm2O3, considered as environmentally friendly and economically advantageous materials, are quite effective. For example, Liao et al. [] introduced CeO2 into MnO2 using the electrospinning method, which exhibited more active oxygen species and higher oxygen storage capacity. The T50 of soot oxidation was decreased from 439 to 376 °C under tight contact. Sacco et al. [] found the addition of Ce to MnOx remarkably improved the soot oxidation activity due to the synergistic effects of Ce and Mn. Yu et al. [] synthesized CeMnOx composite oxides via a simple hydrothermal process and investigated their effect on soot oxidation. They found that Ce-doped amorphous Ce1MnOx catalysts exhibited higher specific surface area, increased porosity, and enhanced oxygen activation compared to pure MnOx catalysts, while also demonstrating excellent stability and resistance to sulfur and water during soot oxidation. Additionally, Sm, primarily in its trivalent state, when used to modify reducible MnOx, enables a large number of oxygen vacancies. This results in excellent catalytic activity in various oxidation reactions, such as the oxidation of NO and CO.
Inspired by the above, in this work, two rare earth metal elements, namely Sm and Ce were introduced into MnOx by the co-precipitation method for soot oxidation. Adding Sm or Ce into MnOx improved the soot oxidation activity. The synthesized mixed oxide catalysts were thoroughly characterized by means of XRD, SEM, TEM, BET, XPS, and H2-TPR to correlate these properties with the catalytic performance. This work provides new references for the use of rare earth elements in the modification of metal oxide catalysts.
2. Results and Discussions
2.1. Characterization of the Catalysts
2.1.1. XRD Analysis
Figure 1 shows the XRD patterns of the catalysts prepared using the precipitation method. The XRD spectrum of MnOx primarily indicates the presence of the Mn3O4 phase. Additionally, a Mn5O8 phase is also detected, evidenced by characteristic diffraction peaks at 21.8°, 46.7°, and 47.9°. The introduction of Sm or Ce results in an obvious decrease in the intensity of the XRD patterns, especially for Sm-Ce-MnOx. This may be attributed to the addition of Sm or Ce, which alters the crystallinity and phase composition of manganese oxide, resulting in smaller crystallite sizes and, consequently, weaker XRD peak intensities. Another effect of adding Sm or Ce is to promote the formation of the Mn5O8 phase. No diffraction peaks for Sm2O3 or CeO2 are detected due to their trace amounts. Compared with MnOx, there is no significant peak shift in the diffraction patterns of Sm-MnOx, Ce-MnOx, and Sm-Ce-MnOx, indicating that the Sm and Ce promoters do not enter the lattice of MnOx.
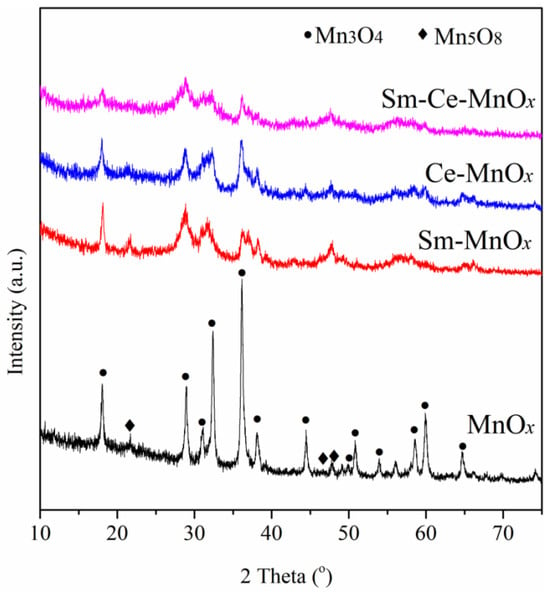
Figure 1.
XRD patterns of the catalysts.
2.1.2. SEM and TEM Observations
Figure 2 displays the SEM images of the catalysts. As shown in Figure 2a, MnOx presents non-uniform aggregated nanoparticles that form distinct clusters. After introducing the Sm element, no significant morphological changes are observed. However, when Ce is introduced, although the catalyst still maintains the aggregated state of nanoparticles, the degree of aggregation is reduced, presenting a more dispersed nanoparticle morphology. This phenomenon is particularly significant when Ce and Sm are simultaneously introduced. This observation is consistent with the findings reported by Yang et al. []. The catalyst after the reaction was also characterized by SEM, and the results are shown in Figure S1. It was found that the sintering of nanoparticles after the reaction was more serious. This may be caused by an additional heat treatment experienced by the catalyst during the catalytic soot oxidation process.
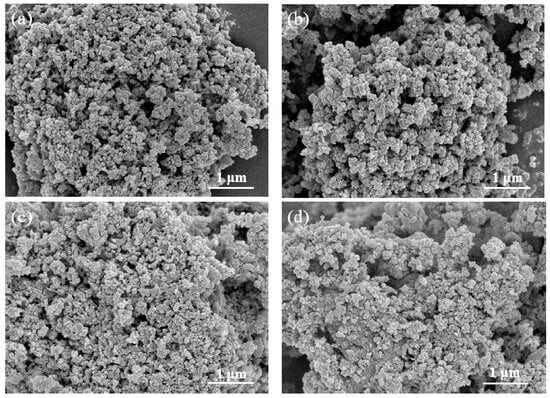
Figure 2.
SEM images of MnOx (a), Sm-MnOx (b), Ce-MnOx (c), and Sm-Ce-MnOx (d).
Figure 3 displays the TEM and HRTEM images of Sm-Ce-MnOx. As shown in Figure 3a, the Sm-Ce-MnOx obtained by the co-precipitation method shows an irregular structure consisting of agglomerated nanoparticles, agreeing with the SEM images. From Figure 3b, clear lattice fringes measuring 0.310 nm and 0.235 nm are evident, corresponding to the (112) plane of Mn3O4 and the (−221) plane of Mn5O8, respectively []. No lattice fringe corresponding to Sm2O3 or CeO2 is detected, which may be attributed to their high dispersion on the surface of Mn3O4.
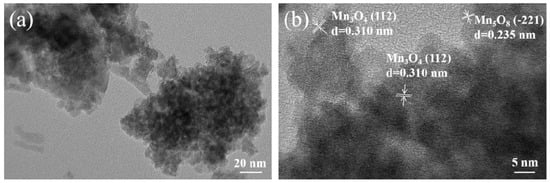
Figure 3.
TEM (a) and HRTEM (b) images of Sm-Ce-MnOx.
2.1.3. Surface Adsorption and Desorption
The porosity and surface characteristics of the catalysts significantly influence their catalytic performance. The N2 adsorption–desorption isotherms and pore size distribution of the samples are depicted in Figure 4. The isotherm (Figure 4a) exhibits a typical Type IV sorption behavior with an H3-type hysteresis loop at high P/P0, indicative of slit-shaped mesopores formed by particle aggregates []. Brunauer–Emmett–Teller (BET) analysis was used to measure the specific surface areas of the catalysts, with results summarized in Table 1. The surface areas for MnOx, Sm-MnOx, Ce-MnOx, and Sm-Ce-MnOx are 28.3, 49.8, 55.0, and 88.6 m2g−1, respectively. These data indicate that introducing Sm and Ce significantly increases the specific surface area of the catalysts. This enhancement may be attributed to the changes in the nanoparticle stacking method caused by these elements, leading to finer pore formation, and thereby, affecting the specific surface area. The specific surface area of the catalyst slightly decreases after the reaction. For example, the specific surface area of the Sm-Ce-MnOx catalyst reduces to 84.96 m2/g post-reaction. The reduction in average pore size, as shown in Table 1, supports this viewpoint. Furthermore, the BJH pore size distribution curves (Figure 4b) reveal peaks primarily between 2 and 50 nm, confirming the presence of mesoporous structures. All BET-BJH parameters are also listed in Table 1. The increased pore volume and surface area provide more active sites for soot oxidation reactions. The soot oxidation reaction is a gas–solid–solid multiphase catalytic process where a high specific surface area facilitates better contact between soot and the catalyst. For these catalysts, Sm- and Ce-modified MnOx exhibit the highest specific surface area among the catalysts, significantly enhancing the interaction between soot and the catalyst. This could promote soot oxidation.
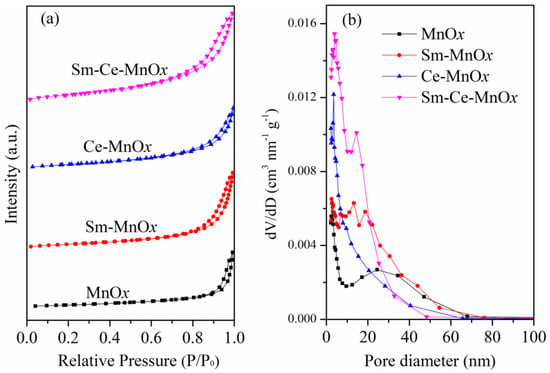
Figure 4.
N2 adsorption–desorption isotherms (a) and the BJH pore size distribution curves (b) of the catalysts.

Table 1.
Results summarized from N2 adsorption and XPS.
2.1.4. XPS Analysis
The surface chemical compositions and elemental valence states of the catalysts were characterized by XPS, and the results are shown in Figure 5. Figure 5a shows the Mn 2p XPS spectra. The Mn 2p3/2 signal exhibits three peaks corresponding to Mn2+, Mn3+, and Mn4+ at binding energies of approximately 640.1 eV, 641.5 eV, and 643.1 eV, respectively [,]. Similarly, the Mn 2p1/2 spectra also show three deconvoluted peaks at binding energies of 652.0 eV (Mn2+), 653.0 eV (Mn3+), and 654.6 eV (Mn4+). The Mn4+/(Mn2+ + Mn3+ + Mn4+) ratios follow the order of Sm-Ce-MnOx (0.31) > Ce-MnOx (0.30) > Ce-MnOx (0.28) > Ce-MnOx (0.22). The higher Mn4+/(Mn2+ + Mn3+ + Mn4+) ratio observed on the surface of the Sm-Ce-MnOx catalyst suggests that more active sites are generated, potentially enhancing their catalytic activity. Figure 5b shows the O 1s spectra of the catalysts. For MnOx, the binding energy peaks at approximately 529.4 eV and 531.0 eV are attributed to lattice oxygen (Olatt) and surface-adsorbed oxygen species (Oads), respectively [,,]. The latter encompasses defect-related oxides (O22− and O−) and hydroxyl groups. Notably, the ratio of Oads/Olatt for Sm-MnOx (0.38), Ce-MnOx (0.41), and Sm-Ce-MnOx (0.53) is markedly higher compared to that of pristine MnOx (0.30), suggesting that the introduction of Sm or Ce elements increases the number of active oxygen species on the surface of MnOx catalysts. The increased proportion of surface-reactive oxygen species, along with a higher concentration of Mn4+ ions can promote soot oxidation. Additionally, it is observed that the XPS peak positions associated with lattice oxygen (Olatt) in the modified MnOx shift toward lower binding energies compared to unmodified MnOx. This shift likely results from a weakened chemical bond between lattice oxygen and Mn, induced by the introduction of other elements [].
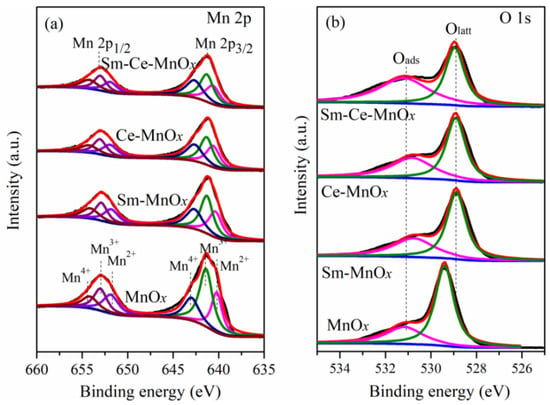
Figure 5.
XPS spectra of (a) Mn 2p and (b) O 1s.
2.1.5. H2-TPR Analysis
For a soot oxidation reaction, the redox performance of the catalyst is crucial. Enhanced redox properties indicate a more favorable environment for catalyzing soot oxidation, leading to improved catalytic activity. To understand the reduction/oxidation behaviors of the catalysts, the H2 temperature-programmed reduction (H2-TPR) profiles of the catalysts are shown in Figure 6. The three peaks detected on the MnOx catalyst are centered at 194, 244, and 393 °C. The first two low-temperature peaks are attributed to the reduction of Mn4+ to Mn3+. The occurrence of peak splitting may be due to the presence of a non-uniform size distribution of the MnOx particles. The higher reduction peak at 393 °C is attributed to the reduction of Mn3+ to Mn2+. With the addition of Sm, the reduction peaks slightly shift toward higher temperatures. However, the area of the reduction peaks has changed. The area of the first reduction peak has significantly increased, while that of the second reduction peak has decreased, indicating that the introduction of Sm is conducive to the presence of high-valence Mn4+. For the Ce-MnOx catalyst, the addition of Ce broadens the temperature ranges of both reduction peaks and shoulder peaks appear within each reduction peak. The presence of these shoulder peaks indicates that the reduction in some Mn4+ and Mn3+ is facilitated by introducing Ce, while other Mn4+ and Mn3+ are not affected by Ce. When Sm and Ce are simultaneously introduced in MnOx, Sm-Ce-MnOx exhibits similar reduction patterns to those of Sm-MnOx. Although there is little difference in the temperature of the first reduction peak, the second reduction peak shifts significantly to lower temperatures. Compared to Ce-MnOx, the two reduction peaks of Sm-Ce-MnOx do not show shoulder peaks, indicating that the simultaneous introduction of Sm and Ce is more effective in promoting the reduction in bulk MnOx.
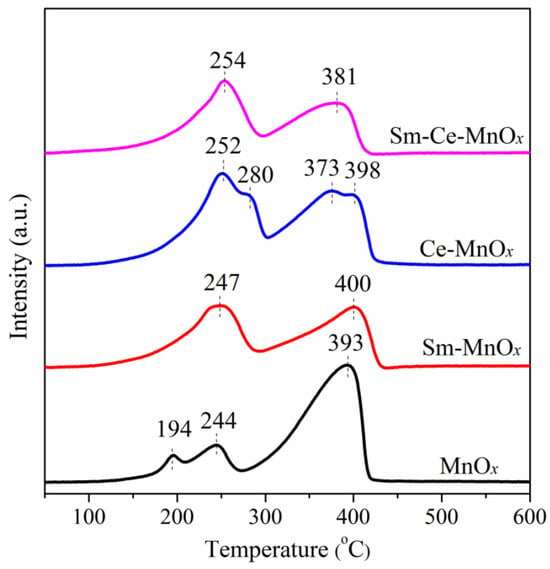
Figure 6.
H2-TPR profiles of the catalysts.
2.2. Catalyst Oxidation Activities
NO2 is a key constituent of nitrogen oxides (NOx), predominantly formed by the oxidation of NO. In diesel exhaust, NOx primarily stems from the combustion-induced reaction between N2 and O2 within the engine, initially yielding NO. Due to its instability, NO rapidly reacts with ambient oxygen to produce NO2, which is unavoidable during exhaust emissions, particularly at elevated temperatures that accelerate NO oxidation, thereby increasing NO2 levels. NO2 with a stronger oxidation ability than O2 is critical to soot catalytic oxidation in the presence of NO [,,]. Therefore, the ability for NO oxidation to NO2 is an important factor for catalytic soot combustion. To evaluate the ability of NO oxidation to NO2 of the as-prepared catalysts, the NO oxidation experiments were carried out and the results are shown in Figure 7a. From Figure 7a, the catalysts display the different abilities for NO oxidation to NO2. Sm-Ce-MnOx exhibits a lower activation temperature and higher abilities for NO oxidation to NO2. The NO2 production of the catalysts during the TPO follows the order of Sm-Ce-MnOx > Ce-MnOx > Sm-MnOx > MnOx. This finding suggests that the dual-promoter modification of MnOx with Sm and Ce enhances the NO oxidation capability of MnOx, thereby significantly boosting the soot oxidation reaction. The synergistic effect of Sm and Ce promoters is effective in boosting the catalytic performance of MnOx for soot oxidation.
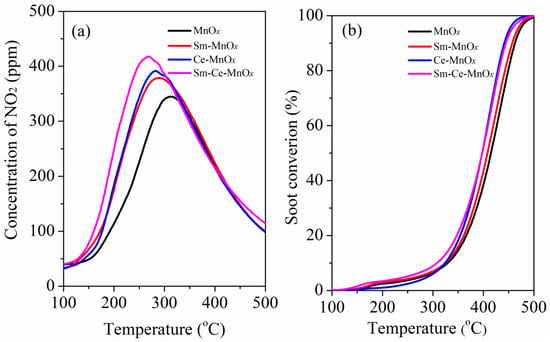
Figure 7.
(a) NO2 profiles during temperature-programmed oxidation of NO and (b) soot-TPO curves in 500 ppm NO/10% O2/N2.
Figure 7b shows the TPO profiles for soot oxidation over the prepared catalysts, with the corresponding T10, T50, and T90 values listed in Table 2. As shown in Table 2, the T10, T50, and T90 values for soot oxidation over MnOx are 340 °C, 416 °C, and 461 °C, respectively. In contrast, MnOx modified with Sm or Ce exhibits higher soot oxidation activity. From Table 2, although the T50 and T90 values for Ce-MnOx are similar to those of Sm-Ce-MnOx, the ignition activity of Sm-Ce-MnOx is much higher than that of other catalysts with the T10 = 306 °C. According to T10, the activity of the catalyst follows a similar order as NO-TPO, indicating that the simultaneous introduction of Sm and Ce into MnOx increases the number of high-valence Mn ions, thereby promoting NO oxidation and further enhancing the soot oxidation reaction. The catalyst was tested over three cycles, and it was found that its activity remained essentially unchanged with repeated use, indicating excellent stability (Figure S2). Additionally, the CO2 selectivity of the catalysts during the oxidation of soot was investigated. The SCO2 value for all three catalysts exceeds 99%, indicating that the production of CO is minimal during the process of soot oxidation, thereby effectively avoiding secondary pollution.

Table 2.
Catalytic features of the catalysts for soot oxidation.
3. Experiment
3.1. Preparation of Catalysts
Sm- or Ce-modified MnOₓ mixed oxides were prepared via a co-precipitation method. Taking Sm and Ce co-modified MnOₓ as an example, Ce(NO3)3·6H2O (purity 99.9%), Mn(NO3)2 (50 wt%), and Sm(NO3)3·6H2O (purity 99.9%) were used as precursors. These precursors were dissolved in deionized water and stirred until fully dissolved. NH3∙H2O solution was then added dropwise to the mixed solution until its pH value reached 9. The precipitate was subsequently washed several times via centrifugation, dried overnight in an oven at 60 °C, and finally calcined at 500 °C for 4 h. The other catalysts were prepared using the same method while adding different precursors according to the requirements. The resulting catalysts were designated as Sm-MnOₓ, Ce-MnOₓ, and Sm-Ce-MnOₓ, respectively. Importantly, within this series of catalysts, the amounts of Sm and Ce added were in a ratio of 0.1:1 with respect to MnOx.
3.2. Characterization
The crystalline phases of the catalysts were characterized by powder X-ray diffraction (XRD) using the Kα radiation of Cu (λ = 1.5406 Å) at 40 kV and 40 mA, from 10.0° to 80.0° (PANalytica X’Pert PRO MPD, Almelo, The Netherlands). The morphology of the catalyst was characterized by scanning electron microscopy (SEM) (JEM-6700F, JEOL, Tokyo, Japan) and transmission electron microscopy (TEM) (JEM-2010, JEOL, Tokyo, Japan) at a working voltage of 200 KV, respectively. The specific surface area was determined by employing the Brunauer–Emmett–Teller (BET) method on a JW–BK122 F (JWGB, Beijing, China) instrument. Prior to the analysis, the samples were degassed at 220 °C under vacuum for 1 h. The specific surface area of the samples was obtained from the Brunauer–Emmett–Teller (BET) equation.
X-ray photoelectron spectroscopy (XPS) analysis was conducted on a Model VG ESCALAB 250 spectrometer (Thermo Electron, London, UK) using a non-monochromatized Al-Ka X-ray source (hv = 1486.6 eV). The binding energies (BE) were calibrated using the adventitious C 1s peak (284.8 eV) as a standard.
The reducibility of catalysts was investigated through H2 temperature-programmed reduction (H2-TPR). For each measurement, a 50 mg sample placed in a U-shaped quartz tube was pretreated in air (20 mL/min) at 500 °C for 1 h. After cooling to 30 °C, the gas flow was switched to 10 vol.% H2/Ar (50 mL/min), and then the catalyst was heated to 900 °C at a heating rate of 10 °C/min under this atmosphere using a Micromeritics Auto Chem II 2920 (Micromeritics, Norcross, GA, USA) apparatus. During the tests, H2 consumption was monitored as a TCD signal by the chemisorption analyzer.
3.3. Catalytic Activity Measurements
The catalytic oxidation of NO was evaluated using an infrared spectrometer (Nicolet iS10, Thermo Corp, Madison, WI, USA). A total of 100 mg of catalyst and 300 mg of silica pellets were mixed evenly with a spatula and then loaded into the quartz reactor (i.d. 5 mm). The reactant gas mixture was 500 ppm NO/10%O2/N2 with a total flow rate of 500 mL/min. After the gas concentration was stable for 10 min, the temperature was increased from room temperature to 600 °C at a heating rate of 5 °C/min for NO oxidation.
The catalytic performance of soot oxidation was evaluated on the same infrared spectrometer. Prior to the experiment, 10 mg of soot was mixed with 100 mg of catalyst using a spatula for 3 min, followed by the addition of 300 mg of silica to prevent uncontrolled reactions. The reactor system was purged with 500 ppm NO/10% O2/N2 with a total flow rate of 500 mL/min and then heated to 600 °C at a rate of 5 °C/min. Each soot-TPO test required approximately 2 h. The temperatures corresponding to the soot conversion of 10%, 50%, and 90% were defined as the T10, T50 and T90, respectively. The selectivity of CO2 (SCO2) was calculated by SCO2 = CCO2/(CCO + CCO2), where CCO and CCO2 were defined as the total CO and CO2 release in the outlet gas during the soot oxidation, respectively, which were obtained by integrating the outlet COx concentrations over time.
4. Conclusions
In conclusion, MnOx catalysts modified with the rare earth element Sm (Sm-MnOx), Ce (Ce-MnOx), and co-modified with Sm and Ce (Sm-Ce-MnOx) were obtained via the co-precipitation method. The introduction of Sm or Ce does not induce significant phase changes and MnOx predominantly retains its Mn3O4 phase. Compared with pure MnOx, the rare earth element-modified MnOx catalysts exhibit improved NO and soot oxidation activity, especially for Sm-Ce-MnOx. The reason for the increased activity of the modified MnOx catalyst is mainly due to the enhanced redox property, a higher concentration of active oxygen species, increased surface area, and an elevated Mn4+/Mn3+ ratio. These improvements collectively create a more favorable environment for catalytic soot oxidation reactions. This work provides a novel reference for rare earth element-modified transition metal MnOx in synergistically promoting soot oxidation reaction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15020149/s1, Figure S1: SEM images of the fresh (a) and used (b) Sm-Ce-MnOx catalyst; Figure S2: Cycled soot oxidation profiles of Sm-Ce-MnOx.
Author Contributions
Conceptualization, L.T. and D.H.; Data curation, B.J., W.W. and Z.J.; Formal analysis, L.T., B.J. and Q.S.; Funding acquisition, B.J.; Investigation, L.T., X.G., Z.L. and X.G.; Methodology, L.T. and B.J.; Project administration, L.T. and B.J.; Resources, L.T., D.H. and W.W.; Supervision, Z.J.; Visualization, Q.S. and X.G.; Validation, Z.L.; Writing—original draft, B.J.; Writing—review and editing, L.T., B.J., W.W., Z.J., Q.S., X.G. and Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Heilongjiang Province of China (No. LH2023B015), the Natural Science Foundation of Hebei Province (B2024107002), National Natural Science Foundation of China (22266028), Natural Science Foundation of Hainan Province (823MS062), and the Key Laboratory of Advanced Materials of Ministry of Education of China (Advmat-2413).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tian, X.; Gong, Y.; Liu, J.; Guo, Q.; Liu, Y.; Yu, G. Experimental investigation on the soot formation characteristics of diesel impinging flame in an opposed multi-burner gasifier. Fuel 2025, 385, 134152. [Google Scholar] [CrossRef]
- Ni, Z.; Song, E.; Qiao, Y.; Dong, Q. Optical investigation on diesel-methane dual-fuel combustion using high-pressure direct injection. Int. J. Hydrogen Energy 2024, 87, 606–619. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodríguez-Fernández, L.; Sánchez-Valdepeñ, J. Soot reactivity analysis and implications on diesel filter regeneration. Prog. Energy Combust. Sci. 2020, 78, 100833. [Google Scholar] [CrossRef]
- Liu, Y.; He, G.; Chu, B.; Ma, Q.; He, H. Atmospheric heterogeneous reactions on soot: A review. Fundam. Res. 2023, 3, 579–591. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Zeng, H. Interactions of particulate matter and pulmonary surfactant: Implications for human health. Adv. Colloid Interface Sci. 2020, 284, 102244. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, Z.; Ye, Y.; Huang, H.; Cao, C. Review of particle filters for internal combustion engines. Processes 2022, 10, 993. [Google Scholar] [CrossRef]
- Feng, R.; Hu, X.; Li, G.; Sun, Z.; Deng, B. A comparative investigation between particle oxidation catalyst (POC) and diesel particulate filter (DPF) coupling aftertreatment system on emission reduction of a non-road diesel engine. Ecotoxicol. Environ. Saf. 2022, 238, 113576. [Google Scholar] [CrossRef]
- Li, C.; Li, R.; Wang, Y.; Niu, R.; Guo, Q.; Zhang, C. Transition metal modified manganese-based catalysts for soot oxidation promoted by noncompetitive adsorption of oxygen: Experiments and DFT calculations. J. Ind. Eng. Chem. 2023, 126, 454–464. [Google Scholar] [CrossRef]
- Khaskheli, A.; Xu, L.; Liu, D. Manganese oxide-based catalysts for soot oxidation: A review on the recent advances and future directions. Energy Fuel 2022, 36, 7362–7381. [Google Scholar] [CrossRef]
- Li, R.; Zheng, H.; Zhao, K.; Li, J.; Chen, Y.; Yu, X.; Liu, J.; Zhao, Z. 3DOM Mn-based perovskite catalysts modified by potassium: Facile synthesis and excellent catalytic performance for simultaneous catalytic elimination of soot and NOx from diesel engines. J. Phys. Chem. C 2021, 125, 25545–25564. [Google Scholar] [CrossRef]
- Matos, A.; Sériob, S.; Lopesc, M.E.; Nunesd, M.R.; Melo Jorged, M.E. Effect of the sintering temperature on the properties of nanocrystalline Ca1−xSmxMnO3 (0 ≤ x ≤ 0.4) powders. J. Alloys Compd. 2011, 509, 9617–9626. [Google Scholar] [CrossRef]
- Anantharman, A.; Dasari, H.; Lee, J.; Dasari, H.; Babu, G. Soot oxidation activity of redox and non-redox metal oxides synthesised by EDTA-citrate method. Catal. Lett. 2017, 147, 3004–3016. [Google Scholar] [CrossRef]
- Dai, F.; Meng, M.; Zhang, J.; Zheng, L.; Hu, T. Effects of synthesis routes on the states and catalytic performance of manganese oxides used for diesel soot combustion. Catal. Lett. 2014, 144, 1210–1218. [Google Scholar] [CrossRef]
- Wagloehner, S.; Nitzer-Noski, N.; Kureti, S. Oxidation of soot on manganese oxide catalysts. Chem. Eng. J. 2015, 259, 492–504. [Google Scholar] [CrossRef]
- Niu, R.; Zhang, C.; Liu, P.; Jiang, N.; Li, C. Constructing asymmetric active Fe3+-OV-Mn4+ sites at the Fe2O3-MnO2 interface for low-temperature soot combustion. Appl. Catal. B Environ. 2024, 358, 124365. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, J.; Meng, Z.; Pu, P.; Zhang, Q.; Yi, C.; Pan, S.; Li, Y. Catalytic activity and influence factors of Mn-Ce mixed oxides by hydrothermal method on diesel soot combustion. Mol. Catal. 2022, 524, 112334. [Google Scholar] [CrossRef]
- Cao, C.; Yang, H.; Xiao, J.; Yang, X.; Ren, B.; Xu, L.; Liu, G.; Li, X. Catalytic diesel soot elimination over potassium promoted transition metal oxide (Co/Mn/Fe) nanosheets monolithic catalysts. Fuel 2021, 305, 121446. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, P.; Zhang, J.; Wang, C.; Chen, L.; Yan, D.; Ren, Q.; Liang, X.; Fu, M.; Suib, S.; et al. Chemosphere Electrospun Ce-Mn oxide as an efficient catalyst for soot combustion: Ce-Mn synergy, soot-catalyst contact, and catalytic oxidation mechanism. Chemosphere 2023, 334, 138995. [Google Scholar] [CrossRef]
- Sacco, N.; Bortolozzi, J.; Milt, V.; Miró, E.; Banús, E. One step citric acid-assisted synthesis of Mn-Ce mixed oxides and their application to diesel soot combustion. Fuel 2022, 322, 124201. [Google Scholar] [CrossRef]
- Yu, D.; Peng, C.; Yu, X.; Wang, L.; Li, K.; Zhao, Z.; Li, Z. Facile preparation of amorphous CenMnOx catalysts and their good catalytic performance for soot combustion. Fuel 2022, 307, 121803. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Kang, J.; Liu, W.; Kuang, Y.; Tan, H.; Yu, Z.; Yang, L.; Yang, X.; Yu, K.; et al. Preparation of Ce-MnOx composite oxides via coprecipitation and their catalytic performance for CO oxidation. Nanomaterials 2023, 13, 2158. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zheng, C.; Qu, R.; Wang, J.; Liu, X.; Wu, W.; Gao, X. Non-thermal plasma assisted preparation of MnCeOx, MnOx and CeO2 catalysts for enhancement of surface active oxygen and NO oxidation activity. Aerosol Air Qual. Res. 2019, 19, 945–958. [Google Scholar] [CrossRef]
- Stepova, K.; Fediv, I.; Mazeikienè, A.; Šarko, J.; Mažeika, J. Adsorption of ammonium ions and phosphates on natural and modified clinoptilolite: Isotherm and breakthrough curve measurements. Water 2023, 15, 1933. [Google Scholar] [CrossRef]
- Plavniece, A.; Kaare, K.; Simkunaitè, K.; Balciunaite, A.; Jasulaitiene, V.; Niaura, G.; Volperts, A.; Dobele, G.; Colmenares-Rausseo, L.; Kruusenberg, D.; et al. Manganese- and nitrogen-doped biomass-based carbons as catalysts for the oxygen reduction reaction. Catalysts 2024, 14, 92. [Google Scholar] [CrossRef]
- Motha, S.; Mahomed, A.; Singh, S.; Friedrich, H. Highly active cerium oxide supported solution combustion Cu/Mn catalysts for CO-PrOx in a hydrogen-rich stream. Catalysts 2024, 14, 603. [Google Scholar] [CrossRef]
- Song, F.; Gao, J.; Yang, B.; Cao, Y.; Liu, H.; Xu, Q. Cu2In alloy-embedded ZrO2 catalysts for efficient CO2 hydrogenation to methanol: Promotion of plasma modification. Front. Chem. 2023, 11, 1187762. [Google Scholar] [CrossRef]
- Liu, R.; Trinh, M.; Chuang, H.; Chang, M. Ozone catalytic oxidation of low-concentration formaldehyde over ternary Mn-Ce-Ni oxide catalysts modified with FeOx. Environ. Sci. Pollut. Res. 2023, 30, 32696–32709. [Google Scholar] [CrossRef]
- Akimchenko, J.; Rutkowski, S.; Tran, T.; Dubinenko, G.; Petrov, V.; Kozelskaya, A.; Tverdokhlebov, S. Polyether ether ketone coated with ultra-thin films of titanium oxide and zirconium oxide fabricated by DC magnetron sputtering for biomedical application. Materials 2022, 15, 8029. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, R.; Dai, M.; Liu, H.; Jian, W.; Bai, F.; Wu, X. Constructing high Efficiency CoZnxMn2−xO4 electrocatalyst by regulating the electronic structure and surface reconstruction. Small 2022, 18, 2107268. [Google Scholar] [CrossRef]
- Luo, J.; Zhu, X.; Chen, G.; Hong, Y.; Zhou, Z. Enhanced catalytic soot oxidation over Co-based metal oxides: Effects of transition metal doping. Molecules 2024, 29, 41. [Google Scholar] [CrossRef]
- Álvarez-Docio, C.; Portela, R.; Reinosa, J.; Rubio-Marcos, F.; Granados-Miralles, C.; Pascual, L.; Fernández, J. Pt-free CoAl2O4 catalyst for soot combustion with NOx/O2. Appl. Catal. A Gen. 2020, 591, 117404. [Google Scholar] [CrossRef]
- Jin, B.; Zhao, B.; Liu, S.; Li, Z.; Ran, R.; Si, Z.; Weng, D.; Wu, X. SmMn2O5 catalysts modified with silver for soot oxidation: Dispersion of silver and distortion of mullite. Appl. Catal. B Environ. 2020, 273, 119058. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).