Revealing the Electronic Effects Between Pt and W on the Performance of Selective Catalytic Reduction of NOx with H2 over Pt-W/SSZ-13
Abstract
1. Introduction
2. Results and Discussion
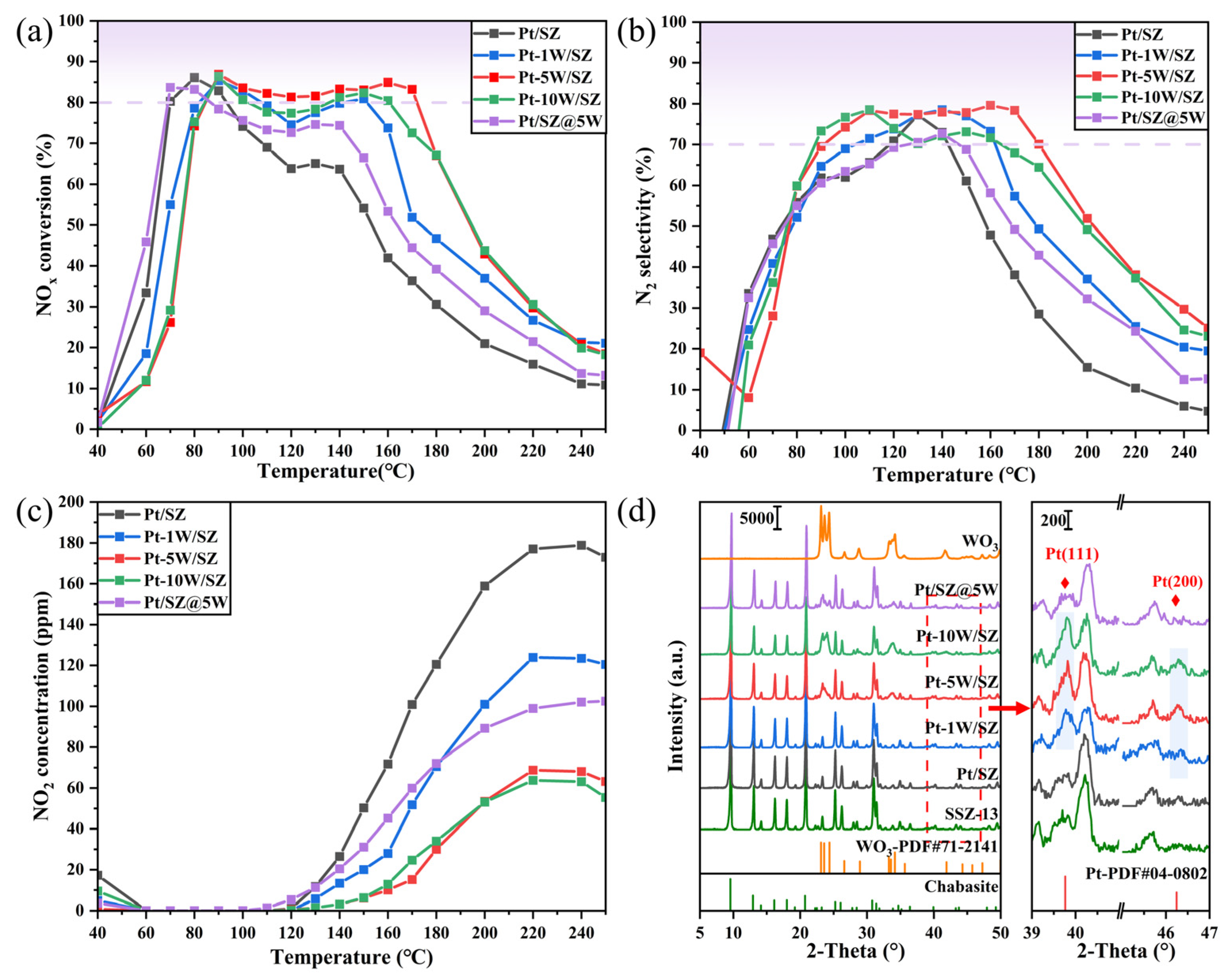
2.1. H2-SCR Performance
2.2. Structural Properties
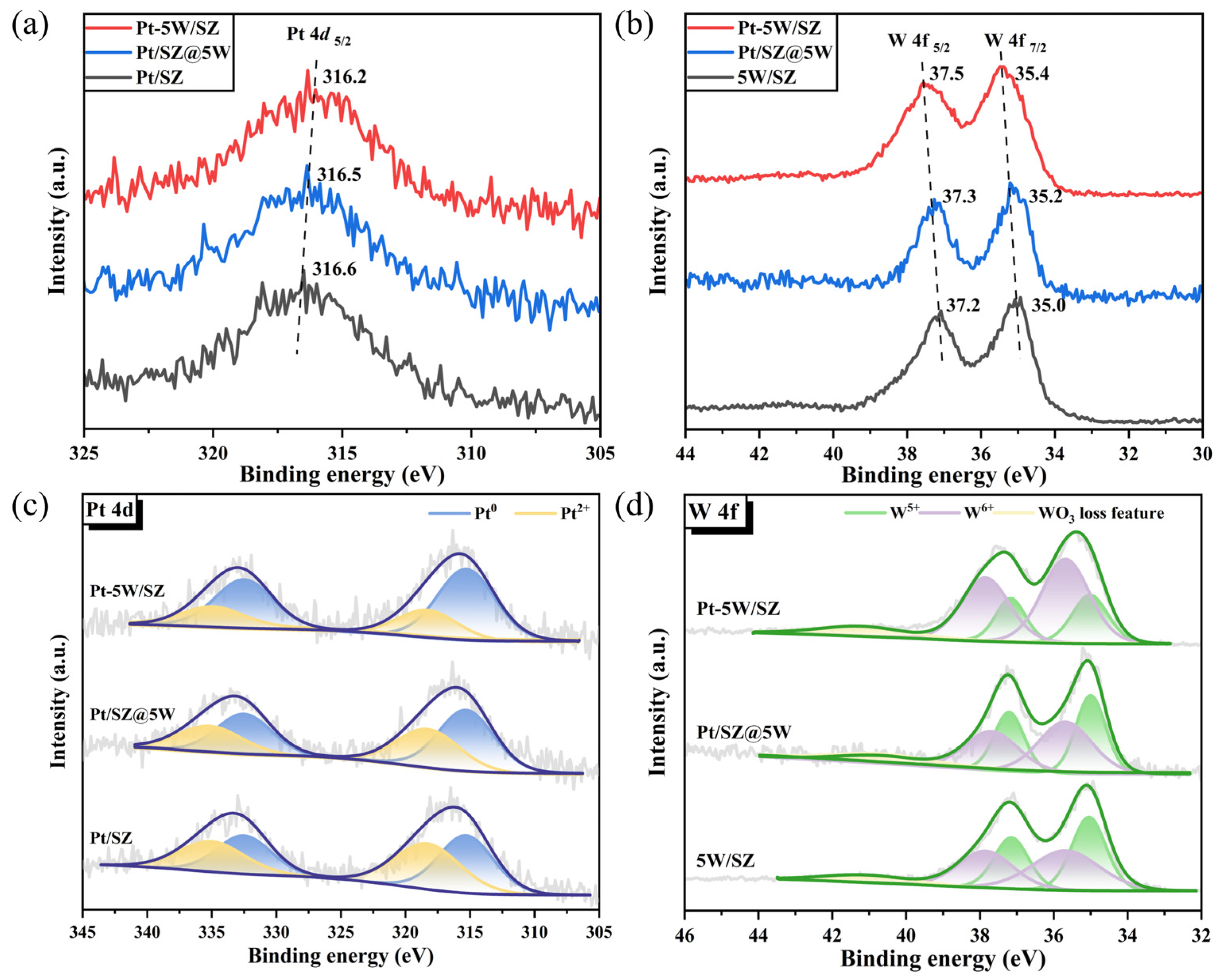
2.3. Chemical States of Pt Species
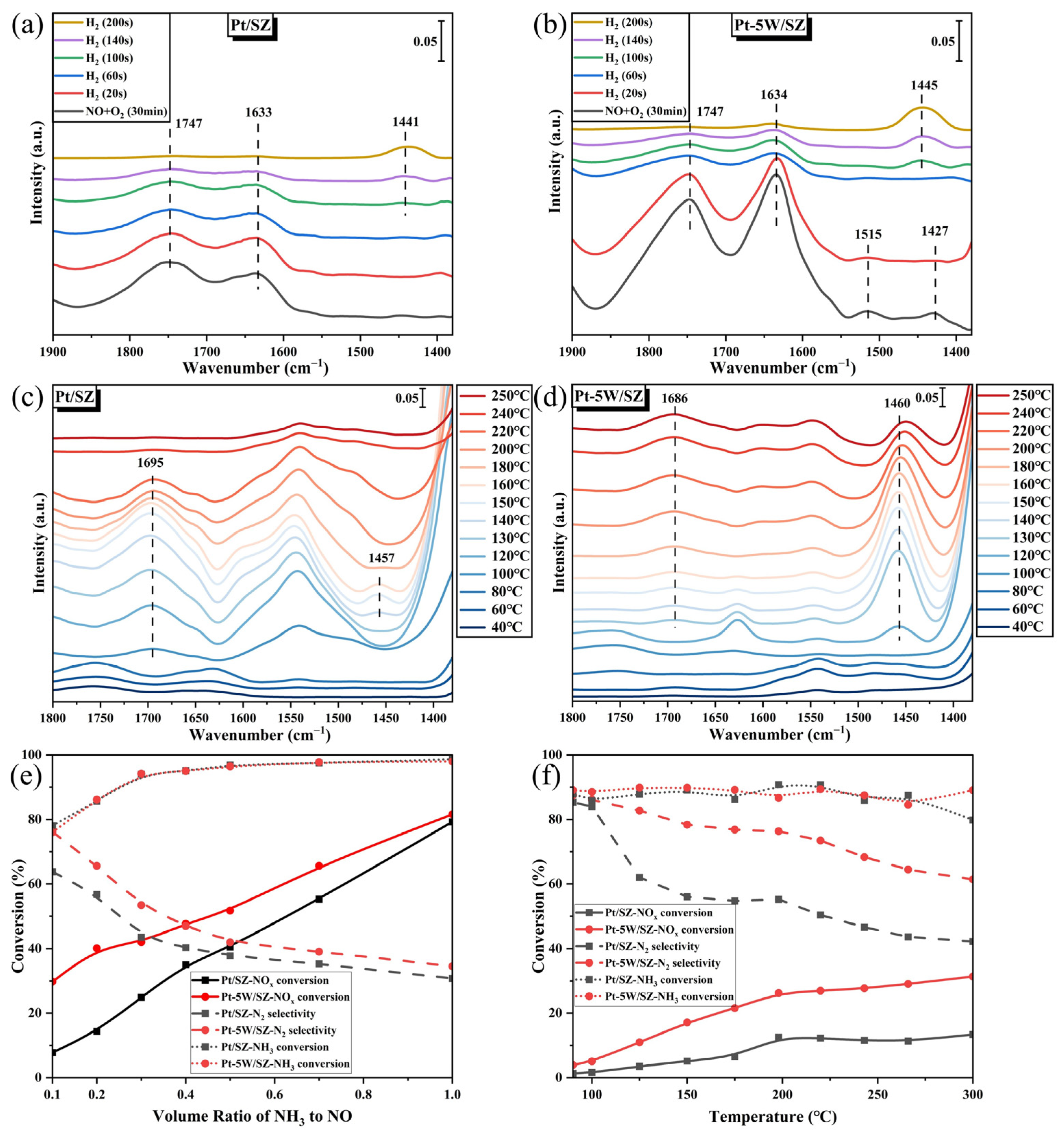
2.4. Formation of NOx− Species
2.5. Reactivity of the Adsorbed NOx− with H2
2.6. In Situ NH4+ Formation and Reactions
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalytic Performance Measurement
3.3. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Le, P.-A.; Trung, V.D.; Nguyen, P.L.; Phung, T.V.B.; Natsuki, J.; Natsuki, T. The current status of hydrogen energy: An overview. RSC Adv. 2023, 13, 28262–28287. [Google Scholar] [CrossRef] [PubMed]
- Evro, S.; Oni, B.A.; Tomomewo, O.S. Carbon neutrality and hydrogen energy systems. Int. J. Hydrogen Energy 2024, 78, 1449–1467. [Google Scholar] [CrossRef]
- Sterlepper, S.; Fischer, M.; Classen, J.; Huth, V.; Pischinger, S. Concepts for Hydrogen Internal Combustion Engines and Their Implications on the Exhaust Gas Aftertreatment System. Energies 2021, 14, 8166. [Google Scholar] [CrossRef]
- Shi, Z.; Peng, Q.; E, J.; Xie, B.; Wei, J.; Yin, R.; Fu, G. Mechanism, performance and modification methods for NH3-SCR catalysts: A review. Fuel 2023, 331, 125885–125907. [Google Scholar] [CrossRef]
- Lian, D.; Chen, M.; Wang, H.; Liu, Y.; Liu, Y.; Li, C.; Liu, B.; Dai, G.; Hou, S.; Zhang, W.; et al. Promising selective catalytic reduction of NOx by CO: Status, Challenges, and perspective. Chem. Eng. J. 2024, 496, 154242. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, R.T. 110th Anniversary: Recent Progress and Future Challenges in Selective Catalytic Reduction of NO by H2 in the Presence of O2. Ind. Eng. Chem. Res. 2019, 58, 10140–10153. [Google Scholar] [CrossRef]
- Muhammad Farhan, S.; Pan, W.; Zhijian, C.; JianJun, Y. Innovative catalysts for the selective catalytic reduction of NOx with H2: A systematic review. Fuel 2024, 355, 129364. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, M.; Yang, X.; Zhao, X.; Sun, L.; Deng, W.; Wang, A.; Li, J.; Zhang, T. On the mechanism of H2 activation over single-atom catalyst: An understanding of Pt1/WOx in the hydrogenolysis reaction. Chin. J. Catal. 2020, 41, 524–532. [Google Scholar] [CrossRef]
- Panina, N.S.; Buslaeva, T.M.; Fischer, A.I. Activation of H2 Molecules on Platinum and Platinum-Vanadium Clusters: DFT Quantum Chemical Modeling. Kinet. Catal. 2023, 64, 588–602. [Google Scholar] [CrossRef]
- Ma, F.-Y.; Huang, P.; Zhou, J.; Zeng, H.-W.; Zhang, J.-W.; Zhao, H.; Dong, Y.-M.; Zhu, Y.-F.; Wang, Y. In situ revealing C-C coupling behavior for CO2 electroreduction on tensile strain Ptδ+-Cuδ+ dual sites. Rare Met. 2024, 43, 6436–6446. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Georgiadis, A.G.; Drosou, C.; Charisiou, N.D.; Goula, M.A. Selective Catalytic Reduction of NOx over Perovskite-Based Catalysts Using CxHy(Oz), H2 and CO as Reducing Agents—A Review of the Latest Developments. Nanomaterials 2022, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Liu, Y.; Lv, Q.; Wang, B.; Che, D. Review on the selective catalytic reduction of NOx with H2 by using novel catalysts. J. Environ. Chem. Eng. 2021, 9, 106770–106784. [Google Scholar] [CrossRef]
- Machida, M.; Ikeda, S.; Kurogi, D.; Kijima, T. Low temperature catalytic NOx-H2 reactions over Pt/TiO2-ZrO2 in an excess oxygen. Appl. Catal. B Environ. 2001, 35, 107–116. [Google Scholar] [CrossRef]
- Liu, S.-R.; Luo, S.-T.; Wu, X.-D.; Wang, T.-J.; Ran, R.; Weng, D.; Si, Z.-C.; Liu, S. Application of silica-alumina as hydrothermally stable supports for Pt catalysts for acid-assisted soot oxidation. Rare Met. 2023, 42, 1614–1623. [Google Scholar] [CrossRef]
- Tian, H.; Pan, N.; Thompson, R.L.; Canadell, J.G.; Suntharalingam, P.; Regnier, P.; Davidson, E.A.; Prather, M.; Ciais, P.; Muntean, M.; et al. Global nitrous oxide budget (1980–2020). Earth Syst. Sci. Data 2024, 16, 2543–2604. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Xu, Y.; Liu, Y.; Wang, X. Influence of support properties on H2 selective catalytic reduction activities and N2 selectivities of Pt catalysts. Chin. J. Catal. 2015, 36, 197–203. [Google Scholar] [CrossRef]
- Yu, Q.; Cheng, H.; Tang, X.; Yi, H.; Ren, X.; Li, Z. Progress in the synthesis of small-pore zeolites for purifying NOx from motor vehicle exhaust. J. Clean. Prod. 2022, 381, 135119. [Google Scholar] [CrossRef]
- Li, P.; Xin, Y.; Zhang, H.; Yang, F.; Tang, A.; Han, D.; Jia, J.; Wang, J.; Li, Z.; Zhang, Z. Recent progress in performance optimization of Cu-SSZ-13 catalyst for selective catalytic reduction of NOx. Front. Chem. 2022, 10, 1033255. [Google Scholar] [CrossRef]
- Hong, Z.; Sun, X.; Wang, Z.; Zhao, G.; Li, X.; Zhu, Z. Pt/SSZ-13 as an efficient catalyst for the selective catalytic reduction of NOx with H2. Catal. Sci. Technol. 2019, 9, 3994–4001. [Google Scholar] [CrossRef]
- Shao, J.; Ho, P.H.; Di, W.; Creaser, D.; Olsson, L. Pt-based catalysts for NOx reduction from H2 combustion engines. Catal. Sci. Technol. 2024, 14, 3219–3234. [Google Scholar] [CrossRef]
- Zagaynov, I.V.; Liberman, E.Y.; Naumkin, A.V. Influence of Pt/Pd state on ceria-based support in CO oxidation. J. Rare Earths 2023, 41, 1963–1968. [Google Scholar] [CrossRef]
- Zhou, B.; Ke, Q.; Wen, M.; Ying, T.; Cui, G.; Zhou, Y.; Gu, Z.; Lu, H. Catalytic combustion of toluene on Pt/Al2O3 and Pd/Al2O3 catalysts with CeO2, CeO2-Y2O3 and La2O3 as coatings. J. Rare Earths 2023, 41, 1171–1178. [Google Scholar] [CrossRef]
- Li, Y.; He, D.; Zhao, H.; Pei, M.; Fan, Y.; Xu, H.; Wang, J.; Chen, Y. Revealing the roles of Zr on enhanced H2-SCR performances on Pt/TiO2 catalyst. Chem. Eng. J. 2024, 490, 151714–151724. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Liu, Z. Selective catalytic reduction of NOx by hydrogen over PtIr/TiO2 catalyst. Catal. Today 2022, 402, 115–121. [Google Scholar] [CrossRef]
- Hu, M.; Yao, Z.; Li, L.; Tsou, Y.-H.; Kuang, L.; Xu, X.; Zhang, W.; Wang, X. Boron-doped graphene nanosheet-supported Pt: A highly active and selective catalyst for low temperature H2-SCR. Nanoscale 2018, 10, 10203–10212. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, G.; Guo, Y.; Guo, Y.; Zhang, Z.; Wang, Y.; Gong, X.-Q. High Performance and Stability of the Pt-W/ZSM-5 Catalyst for the Total Oxidation of Propane: The Role of Tungsten. Chemcatchem 2013, 5, 2495–2503. [Google Scholar] [CrossRef]
- Zhou, W.; Luo, J.; Wang, Y.; Liu, J.; Zhao, Y.; Wang, S.; Ma, X. WOx domain size, acid properties and mechanistic aspects of glycerol hydrogenolysis over Pt/WOx/ZrO2. Appl. Catal. B Environ. 2019, 242, 410–421. [Google Scholar] [CrossRef]
- Shibata, J.; Hashimoto, M.; Shimizu, K.; Yoshida, H.; Hattori, T.; Satsuma, A. Factors controlling activity and selectivity for SCR of NO by hydrogen over supported platinum catalysts. J. Phys. Chem. B 2004, 108, 18327–18335. [Google Scholar] [CrossRef]
- Cepollaro, E.M.; Cimino, S.; D’Agostini, M.; Gargiulo, N.; Franchin, G.; Lisi, L. 3D-Printed Monoliths Based on Cu-Exchanged SSZ-13 as Catalyst for SCR of NOx. Catalysts 2024, 14, 85. [Google Scholar] [CrossRef]
- Zuo, G.; Xu, Y.; Zheng, J.; Jiang, F.; Liu, X. Investigation on converting 1-butene and ethylene into propene via metathesis reaction over W-based catalysts. RSC Adv. 2018, 8, 8372–8384. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Niu, M.; Wu, J.; Han, Y.; Xu, Y.; Zhu, J.; Wang, Z. Platinum nanoparticles confined in Zn-S-1 for efficient propane dehydrogenation. Chem. Eng. J. 2025, 505, 159748–159758. [Google Scholar] [CrossRef]
- Sun, M.; Wang, S.; Li, Y.; Xu, H.; Chen, Y. Promotion of catalytic performance by adding W into Pt/ZrO2 catalyst for selective catalytic oxidation of ammonia. Appl. Surf. Sci. 2017, 402, 323–329. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, M.-W.; Jang, J.S.; Lee, S.H.; Lee, K.-Y. Enhanced activity of a WOx-incorporated Pt/Al2O3 catalyst for the dehydrogenation of homocyclic LOHCs: Effects of impregnation sequence on Pt-WOx interactions. Fuel 2022, 313, 122654–122667. [Google Scholar] [CrossRef]
- Wu, Q.; Jing, M.; Wei, Y.; Zhao, Z.; Zhang, X.; Xiong, J.; Liu, J.; Song, W.; Li, J. High-efficient catalysts of core-shell structured Pt@transition metal oxides (TMOs) supported on 3DOM-Al2O3 for soot oxidation: The effect of strong Pt-TMO interaction. Appl. Catal. B Environ. 2019, 244, 628–640. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhang, C.; Huang, Z.; Han, J.; Nie, X.; Wang, F. Insight into the strong Bronsted acid sites on isolated WOx-modified Pt/zirconium phosphate for glycerol efficient hydrodeoxygenation. Appl. Catal. B Environ. 2023, 325, 122342. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Lin, Q.; Xu, H.; Wang, J.; Chen, Y. Engineering CeZrOx-Cu/SSZ-13 coupled catalysts to synergistically enhance the low-temperature NH3-SCR activity. Chem. Eng. J. 2023, 476, 146767. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Yu, H.; Wang, X. The functions of Pt located at different positions of HZSM-5 in H2-SCR. Chem. Eng. J. 2019, 355, 470–477. [Google Scholar] [CrossRef]
- Machida, A.; Watanabe, T. Effect of Na-addition on catalytic activity of Pt-ZSM-5 for low-temperature NO-H2-O2 reactions. Appl. Catal. B Environ. 2004, 52, 281–286. [Google Scholar] [CrossRef]
- Liu, Z.; Jia, B.; Zhang, Y.; Haneda, M. Engineering the Metal-Support Interaction on Pt/TiO2 Catalyst to Boost the H2-SCR of NOx. Ind. Eng. Chem. Res. 2020, 59, 13916–13922. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Li, J.; He, E.; Liu, Z. Unraveling the Promotional Effect of Co on the Pd/TiO2 Catalyst for H2-SCR of NOx in the Presence of Oxygen. J. Phys. Chem. C 2023, 127, 7248–7256. [Google Scholar] [CrossRef]
- Xie, S.; Li, Y.; Wang, C.; Low, K.-B.; Ye, K.; Kim, D.; Zhang, X.; Li, Y.; Zhang, Y.; Shi, F.; et al. Silica modulated palladium catalyst with superior activity for the selective catalytic reduction of nitrogen oxides with hydrogen. Appl. Catal. B Environ. 2023, 327, 122437. [Google Scholar] [CrossRef]
- Kim, G.J.; Shin, J.H.; Kim, S.B.; Hong, S.C. The role of Pt valence state and La doping on titanium supported Pt-La/TiO2 catalyst for selective catalytic reduction with H2. Appl. Surf. Sci. 2023, 608, 155040–155052. [Google Scholar] [CrossRef]
- Fu, W.; Yin, C.; Feng, Y.; Zhang, L.; Cheng, F.; Fang, Z.; Zhu, C.; Tang, T. Synergistic catalysis of the Bronsted acid and highly dispersed Cu on the mesoporous Beta zeolite in the intermolecular aminoazidation of styrene. Appl. Catal. A Gen. 2021, 609, 117907. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I. Identification of neutral and charged NxOy surface species by IR spectroscopy. Catal. Rev. Sci. Eng. 2000, 42, 71–144. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, S.; Pei, M.-M.; Li, J.-Y.; Xu, H.-D.; Chen, Y.-Q. Unveiling H2O2-optimized NOx adsorption-selective catalytic reduction (AdSCR) performance of WO3/CeZrO2 catalyst. Rare Met. 2023, 42, 3755–3765. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Haller, G.; Li, Y. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-Chabazite catalysts. Appl. Catal. B Environ. 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Yang, J.B.; Fu, O.Z.; Wu, D.Y.; Wang, S.D. DRIFTS study of NO-H2 reaction over Pd/Al2O3 with excess oxygen. Appl. Catal. B Environ. 2004, 49, 61–65. [Google Scholar] [CrossRef]

| Catalysts | Surface Area (m2/g) | Total Pore Volume (mL/g) | Average Pore Size (nm) | Dispersion a |
|---|---|---|---|---|
| SSZ-13 | 695 | 0.31 | 1.87 | - |
| Pt/SZ | 668 | 0.30 | 1.68 | 27% |
| Pt-1W/SZ | 663 | 0.28 | 1.81 | 21% |
| Pt-5W/SZ | 635 | 0.28 | 1.78 | 16% |
| Pt-10W/SZ | 596 | 0.27 | 1.87 | 11% |
| Catalysts | Pt0/(Pt0 + Pt2+) | W5+/(W5+ + W6+) | Pt (At%) | W (At%) |
|---|---|---|---|---|
| Pt/SZ | 51% | - | 0.78% | - |
| Pt-5W/SZ | 71% | 31% | 0.75% | 1.42% |
| 5W/SZ | - | 48% | - | 1.15% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Li, Y.; Huang, Y.; Wang, J.; Chen, Y.; Xu, H. Revealing the Electronic Effects Between Pt and W on the Performance of Selective Catalytic Reduction of NOx with H2 over Pt-W/SSZ-13. Catalysts 2025, 15, 269. https://doi.org/10.3390/catal15030269
Zhao H, Li Y, Huang Y, Wang J, Chen Y, Xu H. Revealing the Electronic Effects Between Pt and W on the Performance of Selective Catalytic Reduction of NOx with H2 over Pt-W/SSZ-13. Catalysts. 2025; 15(3):269. https://doi.org/10.3390/catal15030269
Chicago/Turabian StyleZhao, Hongyan, Yan Li, Yan Huang, Jianli Wang, Yaoqiang Chen, and Haidi Xu. 2025. "Revealing the Electronic Effects Between Pt and W on the Performance of Selective Catalytic Reduction of NOx with H2 over Pt-W/SSZ-13" Catalysts 15, no. 3: 269. https://doi.org/10.3390/catal15030269
APA StyleZhao, H., Li, Y., Huang, Y., Wang, J., Chen, Y., & Xu, H. (2025). Revealing the Electronic Effects Between Pt and W on the Performance of Selective Catalytic Reduction of NOx with H2 over Pt-W/SSZ-13. Catalysts, 15(3), 269. https://doi.org/10.3390/catal15030269







