Abstract
With unique photochemical properties, graphitic carbon nitride (g-C3N4) has gained significant attention for application in photocatalytic degradation of a wide range of organic pollutants. However, its performance is limited by the rapid electron–hole recombination and the relatively weak redox capability. Substantial progress has been made in the preparation of g-C3N4-based photocatalysts with enhanced photocatalytic activity. This review summarizes the recent advances in strategies to improve the photocatalytic activity of g-C3N4-based photocatalysts and their application in the photocatalytic degradation of organic pollutants. Morphology control, doping, functionalization, metal deposition, dye sensitization, defect engineering, and construction of heterojunctions can be used to improve the photocatalytic activity of g-C3N4 through promoting charge carrier separation, reducing the bandgap, and suppressing charge recombination. Furthermore, a range of oxidants, such as hydrogen peroxide and persulfate, can be coupled with g-C3N4-based photocatalysts to enhance the generation of reactive oxygen species and boost the photocatalytic degradation of organic pollutants. Precise control over the g-C3N4 structure during the synthesis process remains a challenge, and further improvements are required in photocatalyst stability and the mineralization rates of organic pollutants. More research and development effort is needed to address the existing challenges, refine the design of g-C3N4-based photocatalysts to improve their activity, and promote their practical application in pollutant degradation.
1. Introduction
With the sustained global economic growth in recent years, wastewater pollution due to rapid industrialization and urbanization has caused great concern. A wide variety of organic chemicals, including antibiotics, dyes, pharmaceutical and personal care products, pesticides, and flame retardants, contribute to the pollution of wastewater through their production and use in factories [1,2]. These organic pollutants in wastewaters are typically not effectively degraded in the conventional wastewater treatment processes [3]. The development of green and efficient methods for their removal has become a growing area of research. Advanced oxidation processes (AOPs) with superior oxidation capability are considered a promising technology for addressing the issue of organic pollutants in wastewater [4]. The remarkable reactivity of AOPs arises from the generation of reactive oxygen species (ROSs), such as superoxide radicals (•O2−), hydroxyl radicals (•OH), sulfate radicals (SO4•−), and singlet oxygen (1O2). Under the attack of these strong oxidants, most organic pollutants can be broken down into degradation intermediates, and even partially or fully mineralized into H2O and CO2 [5,6].
A variety of AOPs have been put forward to use for the degradation of organic wastewater [7,8]. Benefiting from the plentiful and renewable solar energy on Earth, as well as its cost-effectiveness, environmental friendliness, high efficiency, and mild reaction conditions, photocatalytic oxidation is widely regarded as a highly promising and sustainable solution for degrading organic pollutants [9,10]. As shown in Figure 1a, photocatalytic reactions consist of three key steps. Initially, the photocatalyst absorbs photons with energy (hv) greater than or equal to its bandgap energy (Eg), leading to the generation of electron–hole pairs. Subsequently, the photo-generated charge carriers separate and then migrate to the surface of the photocatalyst. Electrons that move from the valence band (VB) to the conduction band (CB) are referred to as photo-generated electrons (e−), while the remaining holes in the VB are known as photo-generated holes (h+). Finally, they react directly or indirectly with the organic pollutants on the surface of the photocatalysts [11,12]. The stability and efficiency of photocatalytic reactions depend on the effective design and precise fabrication of photocatalysts.
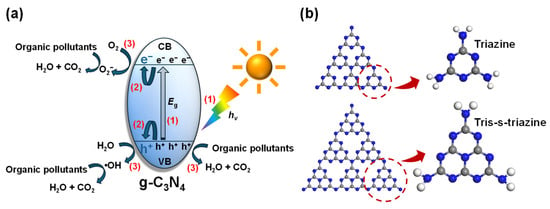
Figure 1.
Schematic illustration of the photocatalytic reaction (a) and the basic structural unit (b) of g-C3N4. The numbers in red denote the three key steps in photocatalytic reactions.
Among various types of photocatalysts, graphitic carbon nitride (g-C3N4), a non-metallic semiconductor, has received significant interest globally. As shown in Figure 1b, triazine (C3N3) rings and tri-s-triazine (C6N7) rings are the basic structural units of g-C3N4. The latter is the most stable phase, making it the typical structural unit that forms g-C3N4. Due to its suitable bandgap (ca. 2.7 eV) and favorable band position, g-C3N4 exhibits high visible light responsiveness. It also offers a range of benefits, such as low cost, excellent stability, and environmental friendliness, making it a promising non-metallic semiconductor photocatalyst [13,14].
Despite its advantages, the pristine g-C3N4 photocatalyst has several limitations, including a relatively small specific surface area (SSA), easy recombination of photo-generated charge carriers, and weak redox capability. These drawbacks greatly limit its large-scale application [15]. To date, various strategies have been employed to boost the photocatalytic performance of g-C3N4 [16,17].
Some excellent reviews on the synthesis and design strategies of g-C3N4-based photocatalysts have been published [15,18,19]. However, strategies to enhance the efficiency of g-C3N4-based photocatalysts for the degradation of organic pollutants have not been systematically reviewed, and the advantages and disadvantages of different design strategies for g-C3N4-based photocatalysts have not been compared. In this review, recent advances in design strategies aimed at boosting the photocatalytic activity of g-C3N4-based photocatalysts are comprehensively discussed, while the strategy of oxidant coupling to further enhance photocatalytic degradation of organic pollutants is also introduced. Finally, the perspectives and challenges of g-C3N4-based photocatalysts in degrading organic pollutants are discussed. By systematically summarizing the major strategies for improving the photocatalytic activity of g-C3N4-based photocatalysts, along with their respective advantages and limitations, we hope that this review can provide valuable guidance and insights for the development of more efficient photocatalysts. We also aim to inspire future work to address the existing challenges, refine the design of g-C3N4-based photocatalysts, and promote their practical application in pollution control.
2. Strategies to Improve Photocatalytic Activity
Numerous strategies have been proposed to enhance the photocatalytic activity of g-C3N4-based photocatalysts through suppressing the recombination of photo-generated carriers, optimizing charge migration pathway, promoting the separation of photo-generated carriers, and adjusting their redox capability. This section systematically introduces these strategies, including morphology control, doping, functionalization, metal deposition, dye sensitization, defect engineering, and construction of heterojunctions. Table 1 compares the major advantages and limitation of these strategies for enhancing the photocatalytic activity of g-C3N4-based photocatalysts. Interestingly, these strategies may work synergistically in practical application. For instance, the formation of heterojunctions may involve the combination of semiconductors with different dimensional morphology, and the incorporation of foreign elements is often associated with the formation of surface defects.

Table 1.
Summary of major advantages and limitations of different strategies to improve photocatalytic activity of g-C3N4-based photocatalysts.
2.1. Morphology Control
g-C3N4 with various morphology has been constructed. According to their dimensions, g-C3N4-based photocatalysts have been fabricated as zero-dimensional (0D) nanostructures (e.g., quantum dots [20]), one-dimensional (1D) nanostructures (e.g., nanorods [21], nanowires [22], nanobelts [23], and nanotubes [24]), two-dimensional (2D) nanostructures (e.g., nanosheets [25]), and three-dimensional (3D) nanostructures (e.g., nanoflowers [26], nanospheres [27], nanocages [28], and hierarchical structures [29]).
Controlled morphology imparts various advantages and properties to g-C3N4. The 0D structure of g-C3N4 generally refers to g-C3N4 quantum dots (QDs). This unique structure endows the photocatalyst with excellent light absorption capability, superior conversion efficiency, and enhanced photocatalytic performance [30,31]. Rajeshwari et al. [20] fabricated a g-C3N4 QD-incorporated MoO3 catalyst, which brought about 98% degradation of p-chlorophenol and 89% degradation of rifampicin after 330 min of visible light irradiation. The improvement in photocatalytic activity through g-C3N4 QD doping was confirmed, which led to a notable narrowing of the band gap, better utilization of visible light, a larger surface area, an improved charge transfer rate, and reduced electron–hole pair recombination.
The formation of 1D tubular structures can provide an effective 1D pathway for photo-generated charge carriers, thereby promoting the charge transfer rate [32]. Zhang et al. [33] prepared a metal-free nanotubular carbon nitride photocatalyst (CN NT) for activating O2 to decompose chloroquine phosphate. Density functional theory (DFT) calculations indicated that the geometric modification of CN NT optimized the surface electronic effect, particularly through enhancing charge separation and transfer properties, which promoted strong electron interactions with O2, thereby improving the O2 adsorption capacity. Furthermore, CN NT exhibited enhanced charge donation and interaction with adsorbed O2, enabling better photocatalytic O2 activation and the generation of ROSs.
The 2D nanosheet structure of g-C3N4 usually results in a larger SSA, providing more active sites for reactant capture and activation, and simultaneously improving mass transfer [34,35]. Additionally, the reduced thickness significantly facilitates the separation of electron–hole pairs, shortens the migration path to the surface of g-C3N4, suppresses their recombination, and improves their overall utilization [36]. Kuate et al. [37] developed a black graphite carbon nitride photocatalyst using a one-step calcination method with urea and phloxine B, and used it for degrading tetracycline (TC) in seawater under visible light. Up to 92% of TC degraded at room temperature after 2 h, which is more than that achieved by pure g-C3N4 under the same conditions. The enhanced photocatalytic performance was attributed to the ultrathin nanosheet structure, which not only minimizes the charge transfer distance but also facilitates more efficient electron–hole separation, thereby improving the overall photocatalytic degradation efficiency.
The 3D structure, with its simple synthesis process, outstanding absorption of visible light, and recyclability, endows g-C3N4 with significant potential for engineering application [30,38]. Gnanaguru et al. [39] fabricated a 3D g-C3N4/WS2/agarose aerogel (GCWAA) using a simple freeze-casting method. The synthesized 3D GCWAA exhibited promising photocatalytic removal of tetracycline, ofloxacin, and sulfamethoxazole, with degradation rates reached 94%, 96%, and 97%, respectively, in 90 min. The lightweight nature and tunable hydrophobicity of the 3D aerogel structure allow it to float on water surfaces, enhancing its ability to absorb the incident light and facilitating recovery and reuse.
In summary, the morphology of g-C3N4 influences its key physical and photochemical properties, such as SSA, light absorption capacity, charge carrier dynamics, and ultimately, photocatalytic performance. For instance, specific morphology increases the SSA of g-C3N4-based photocatalysts and provides more active sites for reactions to enhance the photocatalytic degradation performance. Moreover, the morphology affects the separation and transportation of photo-generated electron–hole pairs. For example, tubular structures provide an effective 1D pathway for photo-generated charge carriers and increase the charge transfer rate, reduce their recombination, and enhance photocatalytic efficiency [32]. Additionally, certain morphology, such as 3D tubular yolk–shell structures, can effectively enhance light harvesting by utilizing multiple scatterings and reflections within the yolk–shell chambers, which increases the photocatalytic activity [40]. To address the diverse needs of practical application, it is necessary to combine g-C3N4 with one or more strategies to achieve synergistic interactions. Structural design and morphology control of g-C3N4 are often adopted together to optimize the integration between components in the photocatalysts.
2.2. Doping
Modifying the bandgap structure and surface of g-C3N4 can drastically improve the photocatalytic efficiency of g-C3N4-based photocatalysts [15]. Elemental doping is considered an effective strategy to modify the bandgap and electronic structure of g-C3N4, as it can efficiently narrow the bandgap, improve light absorption, and adjust the redox potential of g-C3N4 [41,42]. As shown in Figure 2a, doping involves introducing foreign elements into the semiconductor, where the introduced elements can occupy structural voids within g-C3N4 or substitute its carbon or nitrogen atoms [34]. Bandgap engineering through metal doping [43], non-metal doping [44], or their co-doping [45] has proven to be an effective strategy for tailoring g-C3N4 for photocatalytic application [34]. Table 2 summarizes the enhancement in photocatalytic performance of g-C3N4-based photocatalysts through the introduction of common doped elements.
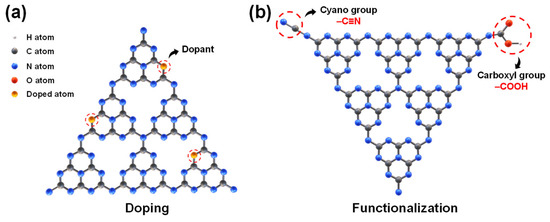
Figure 2.
Schematic diagram of doping (a) and functionalization of g-C3N4 (b).

Table 2.
Summary of enhancement in photocatalytic activity of g-C3N4-based photocatalysts by different doped elements.
The metal dopants that are currently applied to g-C3N4-based photocatalysts are primarily alkali metals and transition metals, which can be further categorized into s-block metals (Li [46], Na [47], K [43], Ca [48], Mg [65]), d-block metals (Fe [66], Co [67], Ni [68]), and ds-block metals (Cu [69], Ag [54]). The incorporation of metal elements plays a key role in boosting charge carrier separation and enhancing visible light absorption, ultimately improving the photocatalytic efficiency [70]. Mao et al. [66] successfully assembled an iron-doped g-C3N4/GO hybrid composite (GO/Fe-GCN) and employed it as an adsorption–photocatalytic Fenton-type heterogeneous catalyst. The experimental results showed that Fe2+ and Fe3+ were captured by the nitrogen-rich g-C3N4, resulting in the formation of well-dispersed active sites. The equilibrium removal rate of Rhodamine B reached 83.6%, which is five times higher than that of the pristine g-C3N4. The hybrid GO/Fe-GCN structure, along with the efficient cycling of Fe2+/Fe3+, plays a crucial role in the synergistic effect of adsorption enrichment and the photocatalytic Fenton reaction. Furthermore, the Fe–N ligands that are formed through Fe doping facilitate charge carrier migration while reduce their recombination.
Numerous non-metal elements have been extensively employed for doping g-C3N4-based photocatalysts, including B [55], C [56], O [71], N [58], S [59], P [60], F [61], Cl [62], Br [63], and I [64]. These elements can adjust the electronic structure of g-C3N4-based photocatalysts, leading to enhanced photocatalytic activity. Meng et al. [71] synthesized O-doped porous g-C3N4 (OCN) through a thermal polymerization method for the activation of peroxymonosulfate (PMS) to remove carbamazepine (CBZ) under visible light and dark conditions. Their findings revealed that the oxygen atoms facilitated electron transfer by tuning the electronic structure of g-C3N4, activating PMS even without light irradiation. Moreover, the incorporation of oxygen atoms narrowed the bandgap, improving the light responsiveness and enhancing the efficiency of electron–hole pair separation, which in turn boosted the photocatalytic activation efficiency of OCN.
Co-doping involves the incorporation of two or more metal or non-metal elements into a material, resulting in enhanced photocatalytic performance through synergistic interactions. Compared to single-element doping, co-doping typically achieves superior photocatalytic activity [45,72,73,74]. Yue et al. [72] synthesized potassium and sodium co-doped carbon nitride (CN-K/Na) through ionothermal polymerization of urea. This co-doping process altered the crystallinity and surface morphology of the carbon nitride. The simultaneous introduction of K and Na significantly promoted the formation of long-range ordered crystalline structure in carbon nitride, outperforming single-element doping. In addition, the CN-K/Na co-doped carbon nitride possessed substantial amount of surface –C≡N and –NH2 functional groups, which play a crucial role in enhancing photocatalytic performance. This improvement in activity is driven by multiple mechanisms: the Na+ ions aid in the transfer of charges within the plane of the material, while K+ ions support charge migration between different planes. In addition, the –C≡N groups are instrumental in capturing and activating O2, while the –NH2 groups facilitate the extraction and release of protons.
2.3. Functionalization
The use of various chemical modifiers for functionalization has proven to be an effective approach to introducing new features into g-C3N4. Through molecular doping, functionalization can optimize the energy band structure, photoelectrochemical properties, and intrinsic conjugation system of g-C3N4. Owing to its conjugated structure, the physicochemical properties of g-C3N4 can be significantly altered by copolymerizing with unique aromatic groups or organic units [75,76,77]. The functionalization of g-C3N4 typically refers to covalent modification, where new functional groups are introduced through stable covalent bonds between the g-C3N4 structure and chemical modifiers. Figure 2b shows a schematic diagram for the functionalization of g-C3N4 using the cyano and carboxyl groups, with the introduction of these groups imparting new properties to g-C3N4.
A common covalent functionalization strategy is oxidation, which incorporates one or more oxygen-containing functional groups, such as hydroxyl groups, carboxyl groups, and others, onto the surface of g-C3N4. This process can adjust its band structure, thereby influencing the types of reactive species that are generated during photocatalysis and controlling the degradation and transformation pathways of pollutants [78,79]. Ming et al. [80] employed a simple hydrothermal method to gradually oxidize g-C3N4, resulting in oxidized g-C3N4 (CNO). Besides the N–O groups, the formation of C=O and C–O groups was also detected in the CNO, and the introduction of these electron-withdrawing groups reduced the VB level of g-C3N4, thereby enhancing its photo-oxidation activity. Furthermore, the photocurrent response of CNO was measured to be approximately 10 times higher than that of raw g-C3N4, and the photocatalytic activity of CNO for the degradation of Acid Orange 7 was found to be approximately 7 times higher than that of g-C3N4. Together, the incorporation of these oxygen-containing functional groups plays a significant role in improving the separation efficiency of photo-generated electrons and holes, thus enhancing the photocatalytic activity of CNO.
In addition, a variety of functional groups, such as cyano groups [81], amino groups [82], aldehyde groups [83], and amidoxime groups [84], have been used for the functionalization of g-C3N4 to regulate its photocatalytic activity. Xu et al. [81] found that the introduction of the cyano group increased the local charge density of g-C3N4, creating a potential well that attracted exciton “holes”, thereby accelerating exciton dissociation. As a result, free radical selectivity up to 97.6% was achieved in the photocatalytic degradation of tetracycline, with the removal of total organic carbon reached 82.1% within 6 h. This approach, which selectively generates radicals with stronger photocatalytic oxidation ability by manipulating charge carrier transfer and exciton dissociation processes, provides a method for the precise control of radical production to mineralize refractory pollutants.
g-C3N4 has also been functionalized using non-covalent methods. Unlike covalent bonding, non-covalent methods primarily rely on physical bonding and intermolecular interactions, including electrostatic interactions, van der Waals interactions, and π-π interactions [85]. Chen et al. [86] utilized an ultrasonic dispersion method to hybridize Cu(II) meso-Tetra (4-carboxyphenyl) porphyrin (CuTCPP) with g-C3N4 through π-π interactions. The resulting composite possessed significantly enhanced photocatalytic activity under visible light compared to pristine g-C3N4. This improvement was attributed to the efficient transfer of electrons from CuTCPP to g-C3N4, which facilitated the separation and migration of photo-generated electron–hole pairs. Notably, the photocatalytic activity of the composite reached its maximum at the CuTCPP content of 0.75%, with the rate constant for phenol degradation being approximately 2.2 times that of g-C3N4. The enhanced photocatalytic performance under visible light irradiation is ascribed not only to the well-matched overlapping band structures, which promote the separation and transfer of electron–hole pairs, but also to the sensitization effect of CuTCPP, which leads to an overall improvement in the photocatalytic activity of g-C3N4 across its full absorption spectrum.
It is noteworthy that non-covalent interactions are weaker than covalent bonds, resulting in composites with relatively poor stability. However, non-covalent methods can retain the inherent properties of both components while incorporating their characteristics [87]. Therefore, in practical application, the choice of an appropriate functionalization method should be based on the specific requirements.
2.4. Metal Deposition
Metal doping incorporates metals into the structure of g-C3N4, whereas metal deposition places metals onto the surface of g-C3N4. Figure 3 schematically illustrates metal deposition on g-C3N4. When the metal interfaces with the g-C3N4 surface, owing to the higher Fermi energy (Ef) of the g-C3N4 compared to the metal, photo-generated electrons will migrate from the higher Ef of g-C3N4 to the metal, forming a Schottky barrier [88]. As a result, the structure comprising of g-C3N4 and the metal is referred to as a Schottky junction. Depositing metal onto the surface of g-C3N4 can promote the separation of photo-generated charge carriers and enhance the photocatalytic activity [89,90]. Additionally, the localized surface plasmon resonance (LSPR) effect leads to the plasmonic electron injecting to g-C3N4, extending photocatalyst’s light absorption range [91,92]. Since they can promote photocatalytic reactions, the doped metals are also referred to as cocatalysts. Based on the aforementioned principles, the deposition of noble metals, such as Au [93], Ag [94], Pt [95], and Pd [96], has been widely studied and proven to be an efficient method for enhancing the solar energy utilization capability of g-C3N4. Nevertheless, the high cost and limited availability of noble metals restrict their practical utilization. Consequently, many researchers have explored the deposition of non-noble metals, such as Ni [97], Cu [98], and Bi [99], to strengthen the photocatalytic performance of g-C3N4-based catalysts. The LSPR effect of non- noble metals can also elevate the separation and migration efficiency of electron–hole pairs and expand the absorption range of photocatalysts. However, their free carrier density is significantly lower compared to the noble metals, and their LSPR frequency corresponds to longer wavelength, resulting in lower photocatalytic activity, which limits their further development [100].
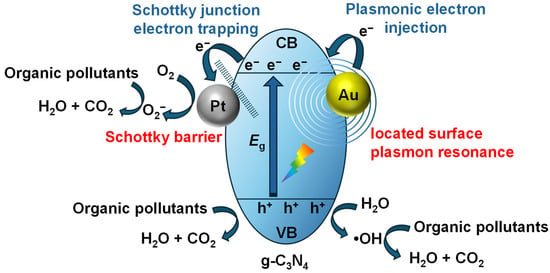
Figure 3.
Schematic illustration of metal deposition (using Pt and Au as examples) for boosting the photocatalytic activity of g-C3N4.
2.5. Dye Sensitization
Dyes possess high tendency for light absorption and can efficiently convert longer wavelength solar light into molecular energy [101]. Therefore, dye sensitization is an effective method for enabling g-C3N4-based photocatalysts to capture solar energy from longer wavelength. As shown in Figure 4, when the CB of g-C3N4 lies between the LUMO and HOMO levels of the dye, the electrons that are generated upon light excitation of the dye on g-C3N4 can transfer from the LUMO orbitals of the dye to the CB of the g-C3N4. This not only broadens the light absorption range, but also suppresses the carrier recombination, thus enhancing the photocatalytic activity [102]. Since dyes are organic molecules that can be designed and synthesized in a controllable manner [102], it is easy to obtain dyes with suitable CB values and tunable absorption range. Two common types of dyes that have been used for sensitization of g-C3N4 are metal-free dyes and metal-based dyes. Bakhtiar et al. [103] utilized a hydrothermal technique to load zinc phthalocyanine (ZnPc) onto the surface of g-C3N4, significantly boosting its photocatalytic capability. Within 1 h of light irradation, 85% of 2,4-dichlorophenol degraded in the presence of the ZnPc-functionalized g-C3N4. Its high photocatalytic activity was attributed to the excellent visible light absorption and efficient generation of superoxide ions and holes, which were generated through charge transfer between g-C3N4 and ZnPc. However, during the photocatalytic degradation process, the free radicals generated may indiscriminately oxidize the sensitizing dye. As a result, dye sensitization is more commonly applied in photocatalytic hydrogen production rather than in the photocatalytic degradation of organic pollutants.
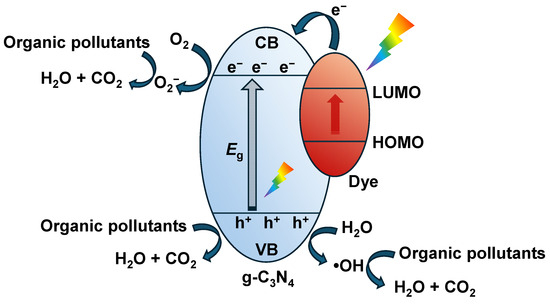
Figure 4.
Mechanisms for sensitizing g-C3N4-based photocatalysts using dyes. The red arrow indicates the transition of electrons from HOMO to LUMO after light excitation of the dye.
2.6. Defect Engineering
When the periodic arrangement of atoms or ions in a crystalline structure alters, defects are introduced into the material, which are referred to as crystallographic defects [104]. Since defects can be precisely introduced and characterized, defect engineering can be utilized to optimize the performance of g-C3N4-based photocatalysts by improving charge transfer and separation, enhancing light absorption, or manipulating surface reactions [105]. Generally, defects in g-C3N4 are point defects, which can be categorized into four distinct types: nitrogen vacancies [106], carbon vacancies [107], amino defects [108], and cyano defects [109,110]. Introducing vacancies into semiconductors can narrow their band gap, thereby expanding the photocatalyst’s light absorption range [111]. Li et al. [112] constructed porous g-C3N4 nanosheets with carbon vacancies through a simple annealing process. In the photocatalytic Fenton-like reaction, the degradation efficiency of metronidazole reached 90.7%, and the TOC removal efficiency achieved 62% within 100 min, which was approximately 6 times higher than those achieved by g-C3N4. The results indicated that carbon vacancies could broaden the light absorption range, leading to a negative shift in the energy bands, which in turn promotes the capture of photo-generated electrons and activates the surface-adsorbed molecular oxygen. Defect-induced electronic states, commonly referred to as “trap states”, play a crucial role in expanding light absorption by enabling electron excitation. Additionally, these states provide pathways for the energy relaxation of photo-generated charge carriers within VB and CB. By influencing the dynamics of charge carriers, these states accelerate the efficient separation and transport of electron–hole pairs, ultimately enhancing the photocatalytic activity. Liu et al. [113] synthesized nitrogen-defective g-C3N4 with varying nitrogen defect density for photocatalytic degradation of ciprofloxacin (CIP). The degradation rate constant of CIP in the presence of nitrogen-defective g-C3N4 was approximately an order of magnitude higher than that of g-C3N4. The incorporation of nitrogen defects induced defect states between the CB and VB, thereby modulating the electronic and band structure. These induced defect states shifted downward toward the VB, achieving an optimal nitrogen defect density to facilitate the excitation of electrons. This effectively narrowed the bandgap, broadened the light absorption range, and enhanced the separation and transfer of charge carriers.
2.7. Construction of Heterojunctions
In an isolated g-C3N4 photocatalytic system, photo-generated electrons in the CB are likely to recombine with the holes in the VB, causing electron–hole recombination, which is detrimental to the photocatalytic activity. Hence, the development of g-C3N4-based heterostructures has garnered significant attention for promoting the photocatalytic activity of g-C3N4. These heterostructures can significantly expand the light absorption capacity, increase the SSA, and enhance the density of active sites. Additionally, the incorporation of cocatalysts helps lower the overpotential that is required for catalytic reactions and enhances the separation of photo-generated charge carriers under light excitation. In general, designing and fabricating suitable heterostructures is widely regarded as one of the most effective approaches to improve the efficiency of electron–hole pair separation, yielding a notable increase in photocatalytic activity [12]. Moreover, the introduction of other components, presumably with synergistic interactions, brings several new advantages, including enhanced light absorption capacity, higher surface reactivity, and optimized band positions, all of which benefit the photocatalytic activity. Based on the separation and transfer mechanisms of photo-generated electron–hole pairs, g-C3N4-based heterostructures are grouped into four major types: Type-II heterojunction, Z-scheme heterojunction, S-scheme heterojunction, and p-n heterojunction.
2.7.1. Type-II Heterojunction
A conventional Type-II heterojunction system is formed by establishing a tailored interface between two semiconductor photocatalysts, utilizing the distinct energy band alignment. This design promotes efficient spatial separation of photo-generated charge pairs, significantly enhancing the photocatalytic activity [114]. For pure g-C3N4, due to the Coulombic effect, photo-generated electrons in the CB are likely to recombine with holes in the VB. However, in g-C3N4-based Type II heterojunction systems, the CB potential of g-C3N4, typically around −1.1 eV, is lower than that of the second semiconductor. As a result, photoexcited electrons in the CB of g-C3N4 can rapidly migrate to the CB of photocatalyst II, which has a higher potential. Simultaneously, the photo-generated holes shift in the reverse direction. This separation significantly reduces the recombination of electrons and holes, thereby extending their lifetime. Figure 5a schematically illustrates the separation pathway of photo-generated electron–hole pairs in a type-II heterojunction.
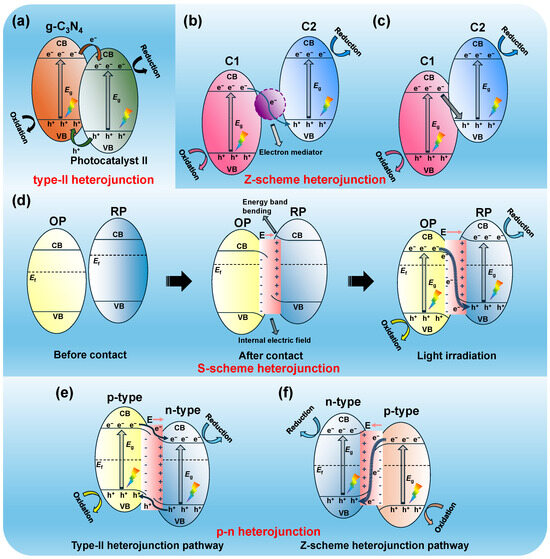
Figure 5.
Schematic illustration of the separation pathways of photo-generated electron–hole pairs in a type-II heterojunction (a), a Z-scheme heterojunction with an electron mediator (b), a direct Z-scheme heterojunction (c), an S-scheme heterojunction (d), depicting the band structure before contact, the formation of a built-in electric field and band bending, and the charge transfer pathway under light irradiation, as well as the different charge migration pathways in the p-n heterojunction: type-II heterojunction pathway (e) and Z-scheme heterojunction pathway (f).
To date, various g-C3N4-based type II heterojunctions have been constructed, exploring different semiconductor materials, including metal oxides (e.g., TiO2 [115], CeO2 [116], Fe2O3 [117], Cu2O [118]), metal sulfides (e.g., MoS2 [119], ZnIn2S4 [120], SnS2 [121]), halides (e.g., BiOBr [122], BiOI [123]), and other semiconductors (e.g., ZnFe2O4 [124], BiVO4 [125], CoTiO3 [126], LDH [127], MOFs [128], COFs [129]). Numerous studies have demonstrated that Type-II heterojunctions are effective in promoting the separation of photo-generated charge carriers [130]. For instance, Song et al. [131] fabricated boron-doped carbon dot (BC-dot)-decorated g-C3N4 (C3N4/BC-dots) photocatalysts through surface deposition. The boron doping treatment transformed the BC-dots from semimetals into semiconductors, leading to the construction of a type II heterojunction between the C3N4 and BC-dots, which facilitated the separation of electrons and holes. The experimental data revealed that BC-dots/C3N4 possessed a large SSA, fast charge transfer rate, and superior visible light absorption. Additionally, construction of type II heterojunctions also enhances the photoelectrochemical properties of g-C3N4 to different extent, depending on the specific characteristics of the second semiconductor material [132].
2.7.2. Z-Scheme Heterojunction
While g-C3N4-based Type-II heterojunction photocatalysts enhance photo-generated electron–hole separation, photocatalytic reactions may occur on semiconductors with lower redox potentials, thereby reducing both the reduction and oxidation capability from a thermodynamic standpoint [12,19,114]. A key challenge in designing g-C3N4-based heterojunction systems is to efficiently separate photo-generated charge carriers while preserving strong redox properties.
To address these issues, Bard et al. [133] introduced the groundbreaking concept of Z-scheme photocatalytic systems in 1979. Based on the natural photosynthesis process in green plants, this unique charge transfer pathway enhances the separation of electron–hole pairs while maintaining strong redox capability. Figure 5b depicts the typical Z-scheme electron–hole pair transfer mechanism between two semiconductors (C1 and C2), facilitated by electron mediators. In this system, photoexcited electrons in the lower CB of C1 migrate to an electron mediator. The mediator then passes these electrons to the VB of C2, where they recombine with photo-generated holes. This process effectively separates the electron–hole pairs in both semiconductors, with electrons remaining in C2 and holes in C1, thereby enhancing the redox ability of both semiconductors.
A conventional Z-scheme system is usually limited to liquid-phase application due to the necessity of redox mediators. Therefore, the development of all-solid-state Z-scheme photocatalytic systems, where solid conductive materials either replace or eliminate the need for mediators, is highly important [134]. Tada et al. [135] proposed an all-solid-state Z-scheme photocatalyst in 2006, which includes two semiconductors and a solid electron mediator between them. These all-solid-state systems can be employed in a variety of phases, including solutions, gases, and solids, thus broadening their scope f application. However, the solid electron mediators that are required for enhancing electron migration in these systems are often costly and scarce, posing a significant barrier to their large-scale deployment.
Building on previous concepts, Yu et al. [136] presented an innovative idea of direct Z-scheme heterojunctions in 2013. Unlike traditional all-solid-state Z-scheme photocatalysts, which require costly electron mediators, direct Z-scheme photocatalysts eliminate this need, greatly reducing the production cost. In such systems, the electrostatic attraction between photo-generated electrons and holes enhances the charge transfer, making it more efficient than in Type II heterojunctions. Specifically, as shown in Figure 5c, the electrostatic interaction facilitates the transport of electrons from the CB of C1 to the VB of C2, which is rich in holes [134]. Wang et al. [137] constructed a Fe-g-C3N4/Bi2MoO6 (FCNB) Z-scheme heterojunction enriched with oxygen vacancies. This structure exhibited excellent activity in photocatalytic Fenton degradation of TC under visible light. The introduction of the Z-scheme heterojunction effectively suppressed the recombination of electron–hole pairs at the interface.
Z-scheme heterojunction systems require the coupling of two semiconductors with appropriate bandgap structures and closely aligned energy bands to achieve favorable charge carrier separation and transfer. This limits the selection of materials for the construction of Z-scheme photocatalysts [138]. Moreover, the complexity of semiconductor interfaces and the high contact resistance could inhibit the transport of charge carriers, leading to inferior photocatalytic activity [139,140]. To mitigate these issues, researchers have constructed dual Z-scheme heterojunction systems, where the combination of multiple components enhances the photon absorption range of photocatalysts [141,142]. Ma et al. [143] synthesized a g-C3N4/Bi2O2CO3/β-Bi2O3 ternary heterojunction via a Bi-based metal–organic framework (Bi-MOF) derivative strategy. This material possessed a high SSA, excellent visible light utilization efficiency, and enhanced charge spatial separation ability due to the electronic transfer pathway via Bi−N bonds and the formation of the heterojunction. Under visible light excitation, approximately 96.7% of TC degraded in the presence of this heterojunction within 120 min, and the reaction rate constant (0.023 min−1) was 4.6 times higher than that of g-C3N4.
2.7.3. S-Scheme Heterojunction
The present Z-scheme heterojunction mechanism has some drawbacks in explaining charge transfer during photocatalysis. In 2019, Yu et al. [144] established a Step-scheme (S-scheme) heterojunction (Figure 5d), which can visually describe the transfer pathway of electrons from lower to higher energy levels, resembling the “steps” of a staircase, thus offering new insights into the charge transfer process in heterojunction photocatalysts [145,146]. This system consists of two specific semiconductors: an oxidation photocatalyst (OP) and a reduction photocatalyst (RP), which can be either n-type or p-type semiconductors. However, the CB position and Ef of the RP must be higher than those of the OP [146]. The contact between the OP and RP causes electrons to migrate from the RP to the OP, driven by the difference in Ef, until an equilibrium is reached. The change in interface charge causes the energy bands of the OP to bend downward, forming an accumulation layer, while the energy bands of the RP bend upward at the interface, resulting in a depletion layer of electrons and creating an internal electric field that promotes the transfer of photo-generated electrons from the OP to the RP. The charge transfer at the interface of the two photocatalysts is driven by the internal electric field that is formed between them [147]. This S-scheme heterojunction effectively enhances charge carrier separation while preserving the redox capability, offering a significant advantage over traditional Type II heterojunctions [114]. Duan et al. [148] fabricated an S-scheme heterojunction photocatalyst by integrating CdS with sulfur-doped carbon nitride (GCNS) using a straightforward solid-state diffusion technique. The photocatalytic degradation rate constant of methyl orange in the presence of this heterojunction was 9.67 and 5.39 times higher than those of GCNS and CdS, respectively. DFT calculations and charge flow tracking revealed that the S-scheme was formed as a result of the unidirectional band edge bending, which facilitates the migration of electrons from CdS to GCNS. This configuration significantly enhanced the light absorption, improved charge separation, and promoted the generation of •O2− species.
Similar to the dual Z-scheme heterojunctions, g-C3N4-based dual S-scheme heterojunctions have also been proposed to address the limitations of single-junction S-scheme structures, including weak interactions and poor multiphase integration [147,149]. Zhao et al. [150] reported a dual S-scheme heterojunction, CeO2/g-C3N4/Bi2O4, which exhibited excellent photocatalytic activity and stability. The dual S-scheme electron migration pathway significantly facilitates the transport of photo-generated charge carriers, suppresses electron–hole recombination, and maintains high redox activity.
2.7.4. p-n Heterojunction
In typical p-type semiconductors, holes are the primary charge carriers, whereas in n-type semiconductors, free electrons serve as the dominant charge carriers. g-C3N4, with its –NH/NH2 groups acting as electron donors, is classified as an n-type semiconductor. Thus, g-C3N4 can be coupled with a suitable p-type semiconductor to construct a p-n heterojunction for enhanced photo-generated charge separation [12,151]. Typically, the Ef of p-type semiconductors is close to the VB, while it is near the CB in n-type semiconductors. The contact of these two semiconductors leads to band bending at the interface, forming an internal electric field. This field aids in the transfer of photo-generated electrons from the p-type to the n-type semiconductor, while holes migrate in the reverse direction. This p-n heterojunction structure promotes effective charge separation in g-C3N4, enhancing its photocatalytic activity [114].
Interestingly, although the photo-generated electron transfer mechanism in p-n heterojunctions always involves the movement of electrons from the p-type semiconductor to the n-type semiconductor, the pathway of charge carrier transfer can vary significantly owing to the difference in their Ef values. As shown in Figure 5e, if the Ef of the p-type semiconductor is higher than that of the n-type semiconductor, the photo-generated charge carriers will transfer through the Type-II heterojunction path. The presence of built-in electric field accelerates this process. Conversely, photo-generated charge carriers will transfer through the Z-scheme heterojunction path pathway if the Ef of the p-type semiconductor is lower than that of the n-type semiconductor (Figure 5f), driven by the internal electric field. This mode is analogous to the S-scheme heterojunction that is formed between n-type and p-type semiconductors, which efficiently suppresses the recombination of electron–hole pairs while maintaining strong redox capability.
Recent studies have used various p-type semiconductors, including CuAl2O4 [152], NiO [153], Mn3O4 [154], and BiOI [155], for constructing p-n heterojunctions with g-C3N4. Wang et al. [152] developed an S-scheme p-n heterojunction by coupling p-type MnS with n-type protonated g-C3N4 (PCN) semiconductors for photocatalytic H2O2 production and achieved in situ oxidative degradation of oxytetracycline. The construction of a p-n heterojunction significantly enhanced the photo-generated charge separation and electron transfer efficiency.
3. Oxidant Coupling Strategy to Enhance the Photocatalytic Degradation Efficiency of Organic Pollutants
g-C3N4-based photocatalysts have been applied for the photocatalytic degradation of various organic pollutants, such as dyes [156], antibiotics [157], microplastics [158], pharmaceutical and personal care products [159], and pesticides [59]. Various strategies have been adopted to enhance the photocatalytic activity of g-C3N4 and performance in pollutant degradation. For instance, Liu et al. [160] developed an innovative Z-scheme heterojunction composed of Ag/AgVO3 and carbon-rich g-C3N4 using a simple hydrothermal calcination method, which exhibited excellent solar-driven photocatalytic activity in the degradation of sulfamethazine. The degradation rate constant for the optimal composite was approximately 13 times higher than that of carbon-rich g-C3N4 and 30 times higher than that of Ag/AgVO3. •O2− was revealed to be the key ROS in the Z-scheme photocatalytic system. Notably, due to the difference in redox potential among different ROSs, the degradation efficiency of organic pollutants depends not only on the rate of ROS generation, but also on the types of ROSs produced. However, most of the ROSs generated under light irradiation in typical photocatalytic systems of g-C3N4-based photocatalysts are •O2−, which has a relatively weak oxidizing power.
An increasing number of researchers have begun to employ strategies that couple g-C3N4-based photocatalysts with oxidants to enhance the generation of ROSs, aiming to optimize the photocatalytic degradation efficiency of organic pollutants. Figure 6 briefly summarizes the major oxidants that have been coupled with g-C3N4-based photocatalysts in the degradation of organic pollutants. Qin et al. [161] fabricated carbon-rich graphitic carbon nitride (Fe1/C-CN) containing single-atomic Fe−N4 sites in the interlayer, which exhibited excellent activity in the degradation of p-nitrophenol in the presence of H2O2 as an oxidant during the photo-Fenton-like catalytic oxidation process. The pseudo-first-order degradation rate constant of p-nitrophenol in the coupled oxidation process was 7.5 times greater than that of Fe1/C-CN photocatalysis and 21.1 times higher than that of Fenton-like system, and complete TOC removal was achieved after 4 h of reaction. The enhanced separation of electron–hole pairs was key in the process, which was coupled with the efficient regeneration of ≡Fe(II) and the activation of H2O2 on Fe1/C-CN. The synergistic effect of these factors greatly enhanced the generation of ROSs, including •OH, •O2−, and 1O2, thereby improving the oxidation of p-nitrophenol and its transformation intermediates.
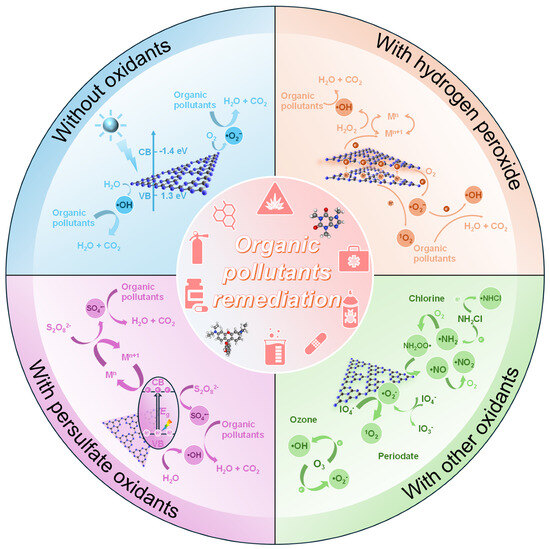
Figure 6.
Summary of the major oxidants that have been coupled with g-C3N4-based photocatalysts in photocatalytic degradation of organic pollutants.
In addition to hydrogen peroxide, photo-assisted AOPs with the addition of persulfate oxidants have also demonstrated superior degradation performance. Nguyen et al. [162] successfully developed a flower-like core–shell heterojunction by integrating manganese dioxide (MnO2) nanosheets with B and S co-doped g-C3N4 (CNBS) nanotubes to create a Z-scheme CNBS@MnO2 photocatalyst. This structure significantly enhanced the photocatalytic degradation of diclofenac (DCF) when combined with peroxymonosulfate (PMS) under visible light (460 nm). Near complete degradation of DCF occurred within just 15 min of light irradiation. The Z-scheme CNBS@MnO2 not only facilitates efficient charge carrier separation, but also activates the PMS, generating a range of reactive radicals, such as h+, •O2−, and SO4•−, which are responsible for the fast degradation of DCF.
Besides hydrogen peroxide and persulfate, g-C3N4-based photocatalysts can also be coupled with a range of other oxidants, such as chlorine-containing oxidants [163], ozone [164], periodate (PI) [165], acetylacetone (AA) [166], permanganate [Mn(VII)] [167], and peracetic acid (PAA) [168], to generate different types of ROSs, to enhance the degradation and mineralization of organic pollutants [169]. For instance, Cheng et al. [163] constructed a visible-light-driven g-C3N4-enabled chlorine advanced oxidation process (VgC-AOP). The pseudo-first-order degradation rate constant of carbamazepine in this process was 16 and 7 times greater than those in the systems without g-C3N4 and HOCl/ClO−, respectively. Additionally, the VgC-AOP system showed stable performance over multiple use cycles. Unlike traditional HOCl/ClO− systems, which only produce radicals under UV light, this process successfully expanded the visible light absorption (>400 nm), thereby enhancing its potential in practical application.
Overall, coupling oxidants with g-C3N4-based photocatalysts to generate multiple highly oxidative ROSs is a promising strategy for enhancing the photocatalytic degradation of pollutants. This approach can be combined with the design strategies of g-C3N4-based photocatalysts discussed in the above section. Nonetheless, overdosing of oxidants poses a potentially significant problem in practical application, as the generation of excessive ROSs may lead to ROS quenching, thereby impairing the degradation efficiency of targeted pollutants. Thus, the selection of appropriate oxidants and their doses should be tailored based on the type and concentration of organic pollutants to be treated.
4. Conclusions and Perspectives
Photocatalytic degradation of organic pollutants has become one of the most effective techniques for harvesting solar energy for pollution control. g-C3N4, with its low cost, strong visible light absorption, and high stability, is considered a promising non-metal semiconductor photocatalyst. However, the easy recombination of photo-generated charge carriers and the relatively weak redox capability limit its large-scale application. This review systematically summarizes the major strategies for enhancing the photocatalytic activity of g-C3N4-based photocatalysts, including morphology control, doping, functionalization, metal deposition, dye sensitization, defect engineering, and construction of heterojunctions. The enhanced photocatalytic activity is attributed to the improved charge carrier separation, reduced bandgap, expanded light absorption, and suppressed charge recombination. Additionally, the strategy of coupling oxidants, such as hydrogen peroxide and persulfate, with g-C3N4-based photocatalysts to enhance the photocatalytic degradation of organic pollutants was also briefly discussed.
Despite the significant progress that has been made through the development of various strategies to enhance the photocatalytic performance of g-C3N4-based photocatalysts, several critical challenges remain, particularly for their practical application in industrial-scale wastewater treatment.
- While many strategies have demonstrated significant performance enhancement in laboratory-scale experiments, ensuring long-term stability of the photocatalysts under harsh industrial conditions remains a pressing issue. Photocatalysts often degrade or lose efficiency with prolonged use, especially when exposed to complex wastewater matrices containing various inorganic and organic components. Additionally, the mineralization rates of organic pollutants are often insufficient, requiring further optimization of the photocatalysts’ oxidizing capability.
- The synergistic or antagonistic effects of combining multiple catalyst design strategies remain poorly understood. In-depth understanding of the inter-relationship between these strategies is essential for designing more efficient and cost-effective photocatalysts.
- Precise control over the structure of g-C3N4 during the synthesis process poses a significant challenge. Some of the aforementioned strategies, such as morphology control and construction of heterojunctions, require precise regulation of the surface structure to optimize photocatalytic performance.
Author Contributions
Conceptualization, Writing—original draft preparation, and Writing—review and editing, Y.R.; Conceptualization, Writing—review and editing, Supervision, and Funding acquisition, Y.H.; Conceptualization, Writing—original draft preparation, Writing—review and editing, Supervision, and Funding acquisition, H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the Natural Science Foundation of China (Grant Nos. U23A2005, 42477251, and 42277226).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bisaria, K.; Sinha, S.; Singh, R.; Iqbal, H.M.N. Recent advances in structural modifications of photo-catalysts for organic pollutants degradation-A comprehensive review. Chemosphere 2021, 284, 131263. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.K.; Badru, R.; Singh, P.P.; Kaushal, S. Photodegradation of organic pollutants using heterojunctions: A review. J. Environ. Chem. Eng. 2020, 8, 103666. [Google Scholar]
- Li, Z.; Zheng, T.; Li, M.; Liu, X. Organic contaminants in the effluent of Chinese wastewater treatment plants. Environ. Sci. Pollut. Res. 2018, 25, 26852–26860. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Kong, L.; Zhong, Y.; Diao, Z.; Shih, K.; Hou, L.A.; Wang, S.; Chen, D. Review on the synthesis and activity of iron-based catalyst in catalytic oxidation of refractory organic pollutants in wastewater. J. Clean Prod. 2021, 321, 128924. [Google Scholar] [CrossRef]
- Shanmugavel, S.P.; Kumar, G.; Yogalakshmi, K.N.; Gunasekaran, M.; Banu, J.R. Recent progress in mineralization of emerging contaminants by advanced oxidation process: A review. Environ. Pollut. 2024, 341, 122842. [Google Scholar]
- Huang, S.; Tian, F.; Dai, J.; Tian, X.; Li, G.; Liu, Y.; Chen, Z.; Chen, R. Highly efficient degradation of chlorophenol over bismuth oxides upon near-infrared irradiation: Unraveling the effect of Bi-O-Bi-O defects cluster and 1O2 involved process. Appl. Catal. B-Environ. 2021, 298, 120576. [Google Scholar] [CrossRef]
- Zhong, Y.; Shih, K.; Diao, Z.; Song, G.; Su, M.; Hou, L.A.; Chen, D.; Kong, L. Peroxymonosulfate activation through LED-induced ZnFe2O4 for levofloxacin degradation. Chem. Eng. J. 2021, 417, 129225. [Google Scholar] [CrossRef]
- Tan, W.; Ruan, Y.; Diao, Z.; Song, G.; Su, M.; Hou, L.A.; Chen, D.; Kong, L.; Deng, H. Removal of levofloxacin through adsorption and peroxymonosulfate activation using carbothermal reduction synthesized nZVI/carbon fiber. Chemosphere 2021, 280, 130626. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial photosynthesis for solar water-splitting. Nat. Photonics 2012, 6, 511–518. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.; Tan, L.-L.; Ng, Y.H.; Yong, S.; Chai, S. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Guo, R.; Wang, J.; Bi, Z.; Chen, X.; Hu, X.; Pan, W. Recent advances and perspectives of g-C3N4-based materials for photocatalytic dyes degradation. Chemosphere 2022, 295, 133834. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, G.; Qin, L.; Liu, J.; Li, B.; Hu, Y.; Cheng, H. Z-scheme g-C3N4-AQ-MoO3 photocatalyst with unique electron transfer channel and large reduction area for enhanced sunlight photocatalytic hydrogen production. Appl. Catal. B-Environ. Energy 2021, 288, 120025. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, H. Self-introduction of carbon nitride quantum dots into carbon nitride planar structure for enhanced photocatalytic hydrogen production. Appl. Catal. B-Environ. 2023, 339, 123101. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci. Total Environ. 2021, 767, 144896. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Raaja Rajeshwari, M.; Okla, M.K.; Kokilavani, S.; Abdel-Maksoud, M.A.; Saleh, I.A.; Abu-Harirah, H.A.; AlRamadneh, T.N.; Khan, S.S. Synergistic visible light assisted photocatalytic degradation of p-chlorophenol and rifampicin from aqueous solution using a novel g-C3N4 quantum dots incorporated alpha-MoO3 nanohybrid—Mechanism, pathway and toxicity studies. Chemosphere 2023, 339, 139529. [Google Scholar] [CrossRef]
- Li, C.; Wu, J.; Wang, X.; Cai, Y.; Jia, R.; Wang, W.; Xia, S.; Li, L.; Chen, Z.; Jin, C.-C. One-step preparation of Z-scheme g-C3N4 nanorods/TiO2 microsphere with improved photodegradation of antibiotic residue. Ceram. Int. 2024, 50, 42127–42134. [Google Scholar] [CrossRef]
- Cui, D.; Yang, X.; Liu, Y.; Ou, T.; Kong, X.; Zhang, Y.; Zhang, J.; Li, F. Strategy of constructing D-A structure and accurate active sites over graphitic carbon nitride nanowires for high efficient photocatalytic nitrogen fixation. J. Colloid Interface Sci. 2025, 678, 955–969. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Shan, R.; Gu, J.; Wang, S.; Cheng, L.; Yuan, H.; Chen, Y. Unraveling the impact of three coordinate nitrogen (N3c) vacancies in porous carbon nitride nanobelt for boosted photocatalytic degradation of microplastics and antibiotics. Appl. Catal. B-Environ. 2024, 358, 124402. [Google Scholar] [CrossRef]
- Dai, J.; Liu, Z.; Song, Y.; Liu, Y.; Nghiem, L.D.; Wang, Q.; Liu, W.; Sun, X.; Cai, Z. Heterocyclic polymerization modified g-C3N4 nanotube with advanced charge separation for solar light driven degradation of ciprofloxacin. Sep. Purif. Technol. 2024, 348, 127692. [Google Scholar] [CrossRef]
- Altendji, K.; Hamoudi, S. Efficient Photocatalytic Degradation of Aqueous Atrazine over Graphene-Promoted g-C3N4 Nanosheets. Catalysts 2023, 13, 1265. [Google Scholar] [CrossRef]
- Liang, H.; Zhao, J.; Wang, A.; Kannan, P.; Jing, S.; Chen, F.; Tsiakaras, P. Synthesis of novel nanoflowers-like P, K co-doped graphitic carbon nitride for efficient H2O2 photoproduction. J. Colloid Interface Sci. 2025, 677, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Tai, Z.; Zhang, J.; Cheng, B.; Yu, H.; Yu, J. Bifunctional g-C3N4 nanospheres/CdZnS QDs S-scheme photocatalyst with boosted H2 evolution and furfural synthesis mechanism. Appl. Catal. B-Environ. 2024, 358, 124459. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, B.; Zhang, H.; Huang, T.; Wang, Y.; Huang, W.; Hu, W.; Pan, A.; Fan, X.; Huang, G. A host-guest self-assembly strategy to enhance π-electron densities in ultrathin porous carbon nitride nanocages toward highly efficient hydrogen evolution. Chem. Eng. J. 2022, 430, 132880. [Google Scholar] [CrossRef]
- Xiao, S.; Yin, R.; Wu, L.; Wu, S.; Tian, G.; Shalom, M.; Wang, L.; Wang, Y.; Pu, F.; Barad, H.; et al. Hierarchically Porous Few-Layer Carbon Nitride and Its High H plus Selectivity for Efficient Photocatalytic Seawater Splitting. Nano Lett. 2023, 23, 4390–4398. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Li, B.; Sun, H.; Wang, S.; Duan, X. Morphology-dependent photocatalysis of graphitic carbon nitride for sustainable remediation of aqueous pollutants: A mini review. J. Environ. Chem. Eng. 2022, 10, 107438. [Google Scholar] [CrossRef]
- Wang, A.; Guo, S.; Zheng, Z.; Wang, H.; Song, X.; Zhu, H.; Zeng, Y.; Lam, J.; Qiu, R.; Yan, K. Highly dispersed Ag and g-C3N4 quantum dots co-decorated 3D hierarchical Fe3O4 hollow microspheres for solar-light-driven pharmaceutical pollutants degradation in natural water matrix. J. Hazard. Mater. 2022, 434, 128905. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Huang, H.; Du, X.; Dong, F.; Zhang, Y. Rational nanostructure design of graphitic carbon nitride for photocatalytic applications. J. Mater. Chem. A 2019, 7, 11584–11612. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Yu, P.; Yu, Y.; Fan, X.; Zhang, J.; Yu, Y.; Zheng, H.; Sun, Y. Photocatalytic O2 activation by metal-free carbon nitride nanotube for rapid reactive species generation and organic contaminants degradation. J. Hazard. Mater. 2023, 456, 131715. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Maigbay, M.A.; Li, M.; Qiu, X. Synthesis and modification strategies of g-C3N4 nanosheets for photocatalytic applications. Adv. Powder Mater. 2024, 3, 100150. [Google Scholar] [CrossRef]
- Panneri, S.; Ganguly, P.; Mohan, M.; Nair, B.N.; Mohamed, A.A.P.; Warrier, K.G.; Hareesh, U.S. Photoregenerable, Bifunctional Granules of Carbon-Doped g-C3N4 as Adsorptive Photocatalyst for the Efficient Removal of Tetracycline Antibiotic. ACS Sustain. Chem. Eng. 2017, 5, 1610–1618. [Google Scholar] [CrossRef]
- Huang, Y.; Li, D.; Feng, S.; Jia, Y.; Guo, S.; Wu, X.; Chen, M.; Shi, W. Pt Atoms/Clusters on Ni-phytate-sensitized Carbon Nitride for Enhanced NIR-light-driven Overall Water Splitting beyond 800 nm. Angew. Chem.-Int. Edit. 2022, 61, e202212234. [Google Scholar] [CrossRef]
- Kuate, L.J.N.; Chen, Z.; Lu, J.; Wen, H.; Guo, F.; Shi, W. Photothermal-Assisted Photocatalytic Degradation of Tetracycline in Seawater Based on the Black g-C3N4 Nanosheets with Cyano Group Defects. Catalysts 2023, 13, 1147. [Google Scholar] [CrossRef]
- Nguyen, T.; Sherpa, K.; Chen, C.; Chen, L.; Thao Ho, P.N.; Dong, C. Enhancing photocatalytic reduction of Cr(VI) in water through morphological manipulation of g-C3N4 photocatalysts: A comparative study of 1D, 2D, and 3D structures. Chemosphere 2024, 362, 142787. [Google Scholar] [CrossRef]
- Gnanaguru, M.V.L.; Dey, D.; Ghangrekar, M.M.; Sen, R.; Chowdhury, S. 3D g-C3N4/WS2/Agarose Aerogel Photocatalyst for Near-Complete Degradation of Broad-Spectrum Antibiotics in Batch and Continuous Flow Modes. ACS EST Eng. 2024. [Google Scholar] [CrossRef]
- Tian, N.; Xiao, K.; Zhang, Y.; Lu, X.; Ye, L.; Gao, P.; Ma, T.; Huang, H. Reactive sites rich porous tubular yolk-shell g-C3N4 via precursor recrystallization mediated microstructure engineering for photoreduction. Appl. Catal. B-Environ. 2019, 253, 196–205. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, H. Coupled adsorption and photocatalysis of g-C3N4 based composites: Material synthesis, mechanism, and environmental applications. Chem. Eng. J. 2023, 453, 139755. [Google Scholar] [CrossRef]
- Bhagat, B.R.; Dashora, A. Understanding the synergistic effect of Co-loading and B-doping in g-C3N4 for enhanced photocatalytic activity for overall solar water splitting. Carbon 2021, 178, 666–677. [Google Scholar] [CrossRef]
- Lu, W.; Wang, A.; Zhang, Y.; Ren, S.; Zhang, Z. Insights into enhanced degradation of antibiotic trimethoprim in water using a novel K doped g-C3N4 photocatalyst under vacuum-UV irradiation: Performance and mechanisms. Chem. Eng. J. 2024, 495, 153192. [Google Scholar] [CrossRef]
- Zhou, Z.; Zeng, H.; Li, L.; Tang, R.; Feng, C.; Gong, D.; Huang, Y.; Deng, Y. Methyl contributes to the directed phosphorus doping of g-C3N4: pH-dependent selective reactive oxygen species enable customized degradation of organic pollutants. Water Res. 2024, 255, 121521. [Google Scholar] [CrossRef]
- Bai, Y.; Hu, X.; Tian, T.; Cai, B.; Li, Y. Carbon pre-protected iron strategy to construct Fe, C-codoped g-C3N4 for effective photodegradation of organic pollutants via hole oxidation mechanism. J. Clean Prod. 2024, 437, 140739. [Google Scholar] [CrossRef]
- Niu, B.; Xiao, J.; Xu, Z. Recycling Spent LiCoO2 Battery as a High-efficient Lithium-doped Graphitic Carbon Nitride/Co3O4 Composite Photocatalyst and Its Synergistic Photocatalytic Mechanism. Energy Environ. Mater. 2023, 6, e12312. [Google Scholar] [CrossRef]
- Liu, Z.; Ruan, Z.; Yang, X.; Huang, Y.; Xing, J. Enhancing Performance of Organic Pollutant Degradation via Building Heterojunctions with ZnO Nanowires and Na Doped Conjugated 2,4,6-Triaminopyrimidin-g-C3N4. Molecules 2024, 29, 3240. [Google Scholar] [CrossRef]
- Yan, W.; Yan, L.; Jing, C. Impact of doped metals on urea-derived g-C3N4 for photocatalytic degradation of antibiotics: Structure, photoactivity and degradation mechanisms. Appl. Catal. B-Environ. 2019, 244, 475–485. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Jeong, Y.J.; Cho, I.S.; Park, S.-J.; Lee, C.-G.; Alvarez, P.J.J. Facile synthesis of N vacancy g-C3N4 using Mg-induced defect on the amine groups for enhanced photocatalytic •OH generation. J. Hazard. Mater. 2023, 449, 131046. [Google Scholar] [CrossRef]
- Nguyen Van, M.; Mai, O.L.; Pham Do, C.; Lam Thi, H.; Pham Manh, C.; Nguyen Manh, H.; Pham Thi, D.; Do Danh, B. Fe-Doped g-C3N4: High-Performance Photocatalysts in Rhodamine B Decomposition. Polymers 2020, 12, 1963. [Google Scholar] [CrossRef]
- Yang, X.; Tian, Z.; Chen, Y.; Huang, H.; Hu, J.; Wen, B. In situ synthesis of 2D ultrathin cobalt doped g-C3N4 nanosheets enhances photocatalytic performance by accelerating charge transfer. J. Alloys Compd. 2021, 859, 157754. [Google Scholar] [CrossRef]
- Nguyen, O.T.K.; Nguyen, V.H.; Nong, L.X.; Doan, Q.-M.T.; Hoang, L.-A.T.; Nam, K.H.; Lee, T.; Nguyen, T.D. Transition metal nickel nanoparticle-decorated g-C3N4 photocatalyst for improving tetracycline hydrochloride degradation. J. Photochem. Photobiol. A-Chem. 2025, 459, 116042. [Google Scholar] [CrossRef]
- Lv, Z.; Feng, J.; Zhao, R.; Shen, J.; Yang, W. Visible-light-driven photocatalytic degradation of ibuprofen by Cu-doped tubular C3N4: Mechanisms, degradation pathway and DFT calculation. Chemosphere 2024, 358, 142106. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Li, D.; Zhang, C.; Han, W.; Xue, Y.; Zhang, W.; Sun, H.; Wang, S.; Duan, X. Synergistic silver doping and N vacancy promoting photocatalytic performances of carbon nitride for pollutant oxidation and hydrogen production. Chem. Eng. J. 2024, 479, 147676. [Google Scholar] [CrossRef]
- Jing, M.; Zhao, H.; Jian, L.; Pan, C.; Dong, Y.; Zhu, Y. Coral-like B-doped g-C3N4 with enhanced molecular dipole to boost photocatalysis-self-Fenton removal of persistent organic pollutants. J. Hazard. Mater. 2023, 449, 131017. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Hu, C.; Li, X.; Zhang, P.; Chen, Z. Porous carbon-doped g-C3N4 with tunable band structure for boosting photocatalytic H2O2 production with simultaneous pollutants removal. J. Environ. Chem. Eng. 2022, 10, 107116. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, X.; Liu, C.; Wang, L.; Xia, Y.; Zhang, S.; Luo, S.; Pei, Y. Scalable one-step production of porous oxygen-doped g-C3N4 nanorods with effective electron separation for excellent visible-light photocatalytic activity. Appl. Catal. B-Environ. 2018, 224, 1–9. [Google Scholar] [CrossRef]
- Xing, J.; Huang, X.; Yong, X.; Li, X.; Li, J.; Wang, J.; Wang, N.; Hao, H. N-doped synergistic porous thin-walled g-C3N4 nanotubes for efficient tetracycline photodegradation. Chem. Eng. J. 2023, 455, 140570. [Google Scholar] [CrossRef]
- Zheng, A.; Xie, S.; Li, K.; Zhang, C.; Shi, H. Performance and mechanism investigation on the enhanced photocatalytic removal of atrazine on S-doped g-C3N4. Chemosphere 2024, 347, 140663. [Google Scholar] [CrossRef]
- Bi, L.; Fu, G.; Cai, D.; Shen, J.; Zhang, J.; Kang, J.; Cheng, Y.; Yan, P.; Zhou, Y.; Chen, Z. Electron rich P doped g-C3N4 for photodegradation of 2,4-dichlorophenoxyacetic acid under visible light by improving oxygen adsorption: Performance and catalytic mechanism. Sep. Purif. Technol. 2023, 306, 122562. [Google Scholar] [CrossRef]
- Phoon, B.L.; Yang, T.C.K.; Leo, B.F.; Lai, C.W.; Phang, S.W.; Juan, J.C. Mesoporous semi-ionic F-doped g-C3N4 as efficient photocatalyst for tetracycline removal under visible light. Environ. Technol. Innov. 2023, 32, 103303. [Google Scholar] [CrossRef]
- Guo, F.; Li, M.; Ren, H.; Huang, X.; Shu, K.; Shi, W.; Lu, C. Facile bottom-up preparation of Cl-doped porous g-C3N4 nanosheets for enhanced photocatalytic degradation of tetracycline under visible light. Sep. Purif. Technol. 2019, 228, 115770. [Google Scholar] [CrossRef]
- Hong, J.; Hwang, D.K.; Selvaraj, R.; Kim, Y. Facile synthesis of Br-doped g-C3N4 nanosheets via one-step exfoliation using ammonium bromide for photodegradation of oxytetracycline antibiotics. J. Ind. Eng. Chem. 2019, 79, 473–481. [Google Scholar] [CrossRef]
- Phoon, B.L.; Lai, C.W.; Chen, C.; Boonyuen, S.; Tang, W.K.; Juan, J.C. Mesoporous I-doped-g-C3N4 with C vacancies as superior visible-light-driven photocatalyst for removal of tetracycline antibiotic. J. Alloys Compd. 2024, 1009, 176772. [Google Scholar] [CrossRef]
- Duan, Y.; Li, J.; Jia, D.; Yao, H.; Shang, X.; Li, C. One-step synthesis of Mg-doped g-C3N4 nanosheets for efficient photo-Fenton-like catalysis. Diamond Relat. Mater. 2022, 129, 109313. [Google Scholar] [CrossRef]
- Mao, H.; Wang, L.; Zhang, Q.; Chen, F.; Song, Y.; Gui, H.; Cui, A.; Yao, C. Synergetic Adsorption-Photocatalytic Activated Fenton System via Iron-Doped g-C3N4/GO Hybrid for Complex Wastewater. Catalysts 2023, 13, 88. [Google Scholar] [CrossRef]
- Xie, M.; Tang, J.; Kong, L.; Lu, W.; Natarajan, V.; Zhu, F.; Zhan, J. Cobalt doped g-C3N4 activation of peroxymonosulfate for monochlorophenols degradation. Chem. Eng. J. 2019, 360, 1213–1222. [Google Scholar] [CrossRef]
- Song, T.; Zhang, X.; Xie, C.; Yang, P. N-doped carbon nanotubes enhanced charge transport between Ni nanoparticles and g-C3N4 nanosheets for photocatalytic H2 generation and 4-nitrophenol removal. Carbon 2023, 210, 118052. [Google Scholar] [CrossRef]
- Bao, J.; Bai, W.; Wu, M.; Gong, W.; Yu, Y.; Zheng, K.; Liu, L. Template-mediated copper doped porous g-C3N4 for efficient photodegradation of antibiotic contaminants. Chemosphere 2022, 293, 133607. [Google Scholar] [CrossRef]
- Ma, S.; Zhan, S.; Jia, Y.; Shi, Q.; Zhou, Q. Enhanced disinfection application of Ag-modified g-C3N4 composite under visible light. Appl. Catal. B-Environ. 2016, 186, 77–87. [Google Scholar] [CrossRef]
- Meng, Y.; Li, Z.; Tan, J.; Li, J.; Wu, J.; Zhang, T.; Wang, X. Oxygen-doped porous graphitic carbon nitride in photocatalytic peroxymonosulfate activation for enhanced carbamazepine removal: Performance, influence factors and mechanisms. Chem. Eng. J. 2022, 429, 130860. [Google Scholar] [CrossRef]
- Yue, J.; Yang, H.; Zhou, L.; Liu, C.; Wang, S.; Kang, X. At least five: Benefit origins of potassium and sodium co-doping on carbon nitride for integrating pharmaceuticals degradation and hydrogen peroxide production. Appl. Catal. B-Environ. Energy 2025, 361, 124599. [Google Scholar] [CrossRef]
- Zhang, P.; Cao, X.; Gu, L.; Yu, H.; Wu, F.; Liu, Y.; Liu, X.; Gao, Y.; Zhang, H. Embedded iron and nitrogen co-doped carbon quantum dots within g-C3N4 as an exceptional PMS photocatalytic activator for sulfamethoxazole degradation: The key role of Fe-N bridge. Sep. Purif. Technol. 2024, 342, 126975. [Google Scholar] [CrossRef]
- Deng, X.; Yang, H.; Zhao, D.; Guan, J.; Gu, Z.; Zhang, L. Mn-Cu co-doped graphitic carbon nitride accelerates permonosulfate to generate more singlet oxygen in pollutant degradation. J. Water Process. Eng. 2024, 64, 105755. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, L.; Liao, Y.; Xiang, Q. Effect of Functional Group Modifications on the Photocatalytic Performance of g-C3N4. Small 2023, 19, 2300109. [Google Scholar] [CrossRef]
- Zhou, C.; Almatrafi, E.; Tang, X.; Shao, B.; Xia, W.; Song, B.; Xiong, W.; Wang, W.; Guo, H.; Chen, S.; et al. Investigation on the structure-performance of phthalic acid carboxyl position and carbon nitride towards efficient photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2022, 286, 120464. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, H. In-plane electric field induced by cyano groups and graphitic carbon structure for enhancing photocatalytic hydrogen production of carbon nitride. Sep. Purif. Technol. 2024, 330, 125260. [Google Scholar] [CrossRef]
- Wang, H.; Xu, X.; Labidi, A.; Ren, H.; Allam, A.A.; Rady, A.; Huang, Y.; Wei, S.; Padervand, M.; Ghasemi, S.; et al. Cyano/Hydroxyl Groups Co-Functionalized g-C3N4 for Photocatalytic NO Removal: A Synergistic Strategy towards Inhibition of Toxic Intermediate NO2. Catalysts 2023, 13, 1433. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Guo, Y.; Zhao, H.; Dong, Y.; Zhu, Y. Efficient Photocatalytic Selective Oxidation of Benzylamines over Cobalt Molecular Catalyst Covalently Bonded to Carboxyl Functionalized Carbon Nitride. Adv. Energy Sustain. Res. 2023, 4, 2300114. [Google Scholar] [CrossRef]
- Ming, L.; Yue, H.; Xu, L.; Chen, F. Hydrothermal synthesis of oxidized g-C3N4 and its regulation of photocatalytic activity. J. Mater. Chem. A 2014, 2, 19145–19149. [Google Scholar] [CrossRef]
- Xu, M.; Wang, R.; Fu, H.; Shi, Y.; Ling, L. Harmonizing the cyano- group and Na to enhance selective photocatalytic O2 activation on carbon nitride for refractory pollutant degradation. Proc. Natl. Acad. Sci. USA 2024, 121, e2318787121. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Y.; Sun, T.; Xue, B. Cyano and terminal amino group co-modified polymeric carbon nitride with boosted photocatalytic activity for degradation of dyes and antibiotics. Mater. Lett. 2020, 277, 128315. [Google Scholar] [CrossRef]
- Xing, W.; Zhang, T.; Shao, W.; Zhang, Y.; Li, P.; Han, J.; Wu, G.; Chen, G. Boosting surface charge transfer by aldehyde group grafted on loofah-sponge-like carbon nitride for visible light H2 evolution. Appl. Surf. Sci. 2023, 609, 155227. [Google Scholar] [CrossRef]
- Yu, K.; Li, Y.; Cao, X.; Wang, R.; Zhou, L.; Wu, L.; He, N.; Lei, J.; Fu, D.; Chen, T.; et al. In-situ constructing amidoxime groups on metal-free g-C3N4 to enhance chemisorption, light absorption, and carrier separation for efficient photo-assisted uranium(VI) extraction. J. Hazard. Mater. 2023, 460, 132356. [Google Scholar] [CrossRef]
- Majdoub, M.; Anfar, Z.; Amedlous, A. Emerging Chemical Functionalization of g-C3N4: Covalent/Noncovalent Modifications and Applications. ACS Nano 2020, 14, 12390–12469. [Google Scholar] [CrossRef]
- Chen, D.; Wang, K.; Hong, W.; Zong, R.; Yao, W.; Zhu, Y. Visible light photoactivity enhancement via CuTCPP hybridized g-C3N4 nanocomposite. Appl. Catal. B-Environ. 2015, 166–167, 366–373. [Google Scholar] [CrossRef]
- Hou, Y.; Cui, C.; Zhang, E.; Wang, J.; Li, Y.; Zhang, Y.; Zhang, Y.; Wang, Q.; Jiang, J. A hybrid of g-C3N4 and porphyrin-based covalent organic frameworks via liquid-assisted grinding for enhanced visible-light-driven photoactivity. Dalton Trans. 2019, 48, 14989–14995. [Google Scholar] [CrossRef]
- Xing, J.; Wang, N.; Li, X.; Wang, J.; Taiwaikuli, M.; Huang, X.; Wang, T.; Zhou, L.; Hao, H. Synthesis and modifications of g-C3N4-based materials and their applications in wastewater pollutants removal. J. Environ. Chem. Eng. 2022, 10, 108782. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Li, C.; Gao, M.; Cao, N.; Zhao, X.; Li, W.; Ding, X.; Li, Z.; Du, X.; et al. Facile fabrication metal Cu-decorated g-C3N4 photocatalyst with Schottky barrier for efficient pollutant elimination. Diamond Relat. Mater. 2022, 126, 109116. [Google Scholar] [CrossRef]
- Chen, M.; Guo, C.; Hou, S.; Wu, L.; Lv, J.; Hu, C.; Zhang, Y.; Xu, J. In-situ fabrication of Ag/P-g-C3N4 composites with enhanced photocatalytic activity for sulfamethoxazole degradation. J. Hazard. Mater. 2019, 366, 219–228. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Xu, Z.; Guo, F.; Li, Y.; Shi, W. Synergistic coupling of piezoelectric and plasmonic effects regulates the Schottky barrier in Ag nanoparticles/ultrathin g-C3N4 nanosheets heterostructure to enhance the photocatalytic activity. Appl. Surf. Sci. 2023, 616, 156466. [Google Scholar] [CrossRef]
- Xin, S.; Ma, X.; Lu, J.; Zhang, G.; Huo, S.; Gao, M.; Xu, P.; Liu, W.; Fu, W. Enhanced visible light photoelectrocatalytic degradation of o-chloronitrobenzene through surface plasmonic Au nanoparticles and g-C3N4 co-modified TiO2 nanotube arrays photoanode. Appl. Catal. B-Environ. Energy 2023, 323, 122174. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, H.; Yao, B.; Xiao, K.; Wang, Y. Hollow g-C3N4@Ag3PO4 Core-Shell Nanoreactor Loaded with Au Nanoparticles: Boosting Photothermal Catalysis in Confined Space. Small 2024, 20, 2308032. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, L.; Sun, S.; Guo, X.; Tian, H.; Xia, Z.; Li, X.; Yan, X.; Jiao, Z. Photodeposition synthesis of hollow g-C3N4/Ag nanotubes with high oxidation properties to enhance visible-light photocatalytic performance. Mater. Res. Bull. 2024, 172, 112658. [Google Scholar] [CrossRef]
- Huang, Z.; Cai, X.; Zang, S.; Li, Y.; Zheng, D.; Li, F. Strong Metal Support Effect of Pt/g-C3N4 Photocatalysts for Boosting Photothermal Synergistic Degradation of Benzene. Int. J. Mol. Sci. 2023, 24, 6872. [Google Scholar] [CrossRef]
- Lei, M.; Wang, Z.; Zhu, L.; Nie, W.; Tang, H. Complete debromination of 2,2′,4,4′-tetrabromodiphenyl ether by visible-light photocatalysis on g-C3N4 supported Pd. Appl. Catal. B-Environ. 2020, 261, 118236. [Google Scholar] [CrossRef]
- Jin, L.; Tanzeel, Q.; Arif, U.; Ali, F.; Ali, N.; Haotian, C.; Mehmood, S.; Akbar, Y.; Raziq, F. Efficient photodegradation of organic dyes from industrial wastewater using novel Ni-decorated g-C3N4-TiO2 photocatalysts. Colloid Polym. Sci. 2024, 302, 487–502. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Review Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Li, N.; Gao, H.; Wang, X.; Zhao, S.; Lv, D.; Yang, G.; Gao, X.; Fan, H.; Gao, Y.; Ge, L. Novel indirect Z-scheme g-C3N4/Bi2MoO6/Bi hollow microsphere heterojunctions with SPR-promoted visible absorption and highly enhanced photocatalytic performance. Chin. J. Catal. 2020, 41, 426–434. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Chen, M. Non-Noble Metal and Nonmetallic Plasmonic Nanomaterials with Located Surface Plasmon Resonance Effects: Photocatalytic Performance and Applications. Catalysts 2023, 13, 940. [Google Scholar] [CrossRef]
- Gonuguntla, S.; Kamesh, R.; Pal, U.; Chatterjee, D. Dye sensitization of TiO2 relevant to photocatalytic hydrogen generation: Current research trends and prospects. J. Photochem. Photobiol. C-Photochem. Rev. 2023, 57, 100621. [Google Scholar] [CrossRef]
- Zhang, N.; Wen, L.; Yan, J.; Liu, Y. Dye-sensitized graphitic carbon nitride (g-C3N4) for photocatalysis: A brief review. Chem. Pap. 2020, 74, 389–406. [Google Scholar] [CrossRef]
- Bakhtiar, S.U.H.; Zada, A.; Raziq, F.; Ali, S.; Shah, M.I.A.; Ateeq, M.; Khan, M.; Alei, D.; Fazil, P.; Naeem, M.; et al. Zinc phthalocyanine sensitized g-C3N4 photocatalyst for exceptional photocatalytic hydrogen evolution and pollutant degradation. Int. J. Hydrogen Energy 2023, 48, 16320–16329. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Shen, Y.; Liu, S.; Zhang, Y. Molecular engineering of polymeric carbon nitride: Advancing applications from photocatalysis to biosensing and more. Chem. Soc. Rev. 2018, 47, 2298–2321. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yang, J.; Yuan, X.; Guo, J.; Liang, J.; Tang, W.; Chen, Y.; Li, X.; Wang, H.; Chu, W. Defect engineering in polymeric carbon nitride photocatalyst: Synthesis, properties and characterizations. Adv. Colloid Interface Sci. 2021, 296, 102523. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Lan, C.; Dong, X.; Zhang, X. New insight into enhanced photocatalytic selectivity of g-C3N4 by nitrogen vacancy introduction: Experimental study and theoretical calculation. Environ. Res. 2022, 212, 113390. [Google Scholar]
- Li, Y.; Gu, M.; Shi, T.; Cui, W.; Zhang, X.; Dong, F.; Cheng, J.; Fan, J.; Lv, K. Carbon vacancy in C3N4 nanotube: Electronic structure, photocatalysis mechanism and highly enhanced activity. Appl. Catal. B-Environ. 2020, 262, 118281. [Google Scholar] [CrossRef]
- Song, X.; Yang, Q.; Jiang, X.; Yin, M.; Zhou, L. Porous graphitic carbon nitride nanosheets prepared under self-producing atmosphere for highly improved photocatalytic activity. Appl. Catal. B-Environ. 2017, 217, 322–330. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, K.; Cui, M.; Liu, X.; Zhang, Q. Co-modification of carbon and cyano defect in g-C3N4 for enhanced photocatalytic peroxymonosulfate activation: Combined experimental and theoretical analysis. Sep. Purif. Technol. 2023, 316, 123844. [Google Scholar] [CrossRef]
- Ma, Z.; Ma, X.; Zhang, L.; Cheng, H.; Shi, F.-N. 1T-WS2/NCN photocatalyst with unique trion behavior and cyano-defects: Photocatalytic hydrogen evolution and mechanistic insights. Sep. Purif. Technol. 2023, 325, 124743. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect engineering in photocatalytic materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Li, R.; Zheng, M.; Zhou, X.; Zhang, D.; Shi, Y.; Li, C.; Yang, M. Carbon vacancies in porous g-C3N4 nanosheets induced robust H2O2 production for highly efficient photocatalysis-self-Fenton system for metronidazole degradation. Chem. Eng. J. 2023, 464, 142584. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Kamali, M.; Rossi, B.; Appels, L.; Dewil, R. Unraveling the Presence and Positions of Nitrogen Defects in Defective g-C3N4 for Improved Organic Photocatalytic Degradation: Insights from Experiments and Theoretical Calculations. Adv. Funct. Mater. 2024, 34, 2405741. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Cheng, B.; Shao, Y. Recent advances in g-C3N4-based heterojunction photocatalysts. J. Mater. Sci. Technol. 2020, 56, 1–17. [Google Scholar] [CrossRef]
- Yadav, S.M.; Desai, M.A.; Sartale, S.D. Superoxide (•O2−) radical species driven type II TiO2/g-C3N4 heterojunction photocatalyst for RhB dye degradation. J. Mater. Sci.-Mater. Electron. 2023, 34, 1651. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, S.; Li, L.; Gu, P.; Wen, T.; Khan, A.; Li, S.; Li, B.; Wang, S.; Wang, X. Enhanced Visible-Light-Induced Photoactivity of Type-II CeO2/g-C3N4 Nanosheet toward Organic Pollutants Degradation. ACS Sustain. Chem. Eng. 2019, 7, 9699–9708. [Google Scholar] [CrossRef]
- Ge, F.; Li, X.; Wu, M.; Ding, H.; Li, X. A type II heterojunction α-Fe2O3/g-C3N4 for the heterogeneous photo-Fenton degradation of phenol. RSC Adv. 2022, 12, 8300–8309. [Google Scholar] [CrossRef]
- Mehtab, A.; Mao, Y.; Alshehri, S.M.; Ahmad, T. Photo/electrocatalytic hydrogen evolution using Type-II Cu2O/g-C3N4 Heterostructure: Density functional theory addresses the improved charge transport efficiency. J. Colloid Interface Sci. 2023, 652, 1467–1480. [Google Scholar] [CrossRef]
- Raj, M.G.; Vijayakumar, E.; Preetha, R.; Narendran, M.G.; Jennifer, G.A.; Varathan, E.; Neppolian, B.; Ganesh, V.K.; Bosco, A.J. Experimental investigation into the π-conjugated HT-g-C3N4/MoS2 (X) evokes the electron transport in type-II heterojunction to achieve high photocatalytic antibiotic removal under visible-light irradiation. Sep. Purif. Technol. 2022, 292, 121028. [Google Scholar]
- Wang, Y.; Li, J.; Chen, S.; Xie, Y.; Ma, Y.; Luo, Y.; Huang, J.; Ling, Y.; Ye, J.; Liang, Y.; et al. In situ loading of ZnIn2S4 nanosheets onto S doped g-C3N4 nanosheets to construct type II heterojunctions for improving photocatalytic hydrogen production. J. Alloys Compd. 2022, 924, 166569. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Wang, L.; Shou, Q.; Gao, M.; Wang, H.; Nazir, A.; Huo, P. Fabrication of g-C3N4/SnS2 type-II heterojunction for efficient photocatalytic conversion of CO2. J. Mater. Sci.-Mater. Electron. 2023, 34, 350. [Google Scholar] [CrossRef]
- Vinoth, S.; Pandikumar, A. Ni integrated S-gC3N4/BiOBr based Type-II heterojunction as a durable catalyst for photoelectrochemical water splitting. Renew. Energy 2021, 173, 507–519. [Google Scholar] [CrossRef]
- Vinoth, S.; Rajaitha, P.M.; Pandikumar, A. Modulating photoelectrochemical water splitting performance by constructing a type-II heterojunction between g-C3N4 and BiOI. New J. Chem. 2021, 45, 2010–2018. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Xu, Z.; Sun, H.; Amin, S.; Guo, F.; Shi, W.; Li, Y. Engineering of 2D/3D architectures type II heterojunction with high-crystalline g-C3N4 nanosheets on yolk-shell ZnFe2O4 for enhanced photocatalytic tetracycline degradation. Mater. Res. Bull. 2022, 150, 111789. [Google Scholar] [CrossRef]
- Li, Y.; Qin, T.; Chen, W.; Huang, M.; Xu, J.; Lv, J. Construction of a Switchable g-C3N4/BiVO4 Heterojunction from the Z-Scheme to the Type II by Incorporation of Pyromellitic Diimide. Cryst. Growth Des. 2022, 22, 1645–1653. [Google Scholar] [CrossRef]
- Sundararaj, S.B.; Amir, H.; Viswanathan, C.; Thangavelu, S. Photoelectrochemical Water Splitting: A Visible-Light-Driven CoTiO3@g-C3N4-Based Photoanode Interface Follows the Type II Heterojunction Scheme. Langmuir 2024, 40, 16582–16594. [Google Scholar] [CrossRef]
- Maridevaru, M.C.; Nagaveni, M.; Kumari, M.M.; Shankar, M.V.; Khraisheh, M.; Selvaraj, R. Unravelling the efficiency of CoAl-LDH/g-C3N4 nanosheets: Type-II heterojunction for photocatalytic hydrogen production and dye degradation under solar light irradiation. Int. J. Hydrogen Energy 2024, 89, 1112–1125. [Google Scholar] [CrossRef]
- Wang, C.; Kong, X.; Yu, Z.; Tao, X.; Huang, L.; Shang, S. Construction of g-C3N4/Fe-MOFs Type-II heterojunction promotes photo-Fenton degradation of doxycycline. Sep. Purif. Technol. 2023, 326, 124790. [Google Scholar] [CrossRef]
- Omori, F.; Tateishi, I.; Katsumata, H.; Furukawa, M.; Kaneco, S. Fabrication of pyridine incorporated and O-doped g-C3N4/COF: A type-II heterojunction for enhanced hydrogen production under visible light irradiation. J. Solid State Chem. 2025, 341, 125057. [Google Scholar] [CrossRef]
- Gao, C.; Sun, Y.; Yu, S.; Liu, L.; Liu, C.; Li, Y.; Wang, H.; Chang, X. Type-II heterojunction Se-g-C3N4/ZnO coupled MnCo2O4 photocatalyst for enhanced levofloxacin hydrochloride degradation activated by peroxymonosulfate. Chem. Eng. J. 2024, 500, 156944. [Google Scholar] [CrossRef]
- Song, B.; Wang, Q.; Wang, L.; Lin, J.; Wei, X.; Murugadoss, V.; Wu, S.; Guo, Z.; Ding, T.; Wei, S. Carbon nitride nanoplatelet photocatalysts heterostructured with B-doped carbon nanodots for enhanced photodegradation of organic pollutants. J. Colloid Interface Sci. 2020, 559, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Balu, S.; Chen, Y.-L.; Chen, S.-W.; Yang, T.C.K. Rational synthesis of BixFe1-xVO4 heterostructures impregnated sulfur-doped g-C3N4: A visible-light-driven type-II heterojunction photo(electro)catalyst for efficient photodegradation of roxarsone and photoelectrochemical OER reactions. Appl. Catal. B-Environ. 2022, 304, 120852. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. J. Photochem. 1979, 10, 59–75. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Tada, H.; Mitsui, T.; Kiyonaga, T.; Akita, T.; Tanaka, K. All-solid-state Z-scheme in CdS-Au-TiO2 three-component nanojunction system. Nat. Mater. 2006, 5, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Meng, S.; Li, J.; Guo, D.; Fu, S.; Zhang, D.; Yang, X.; Sui, G. Oxygen Vacancy Engineering and Constructing Built-In Electric Field in Fe-g-C3N4/Bi2MoO6 Z-Scheme Heterojunction for Boosting Photo-Fenton Catalytic Degradation Performance of Tetracycline. Small 2024, 20, 2406125. [Google Scholar] [CrossRef]
- Zheng, D.; Pang, C.; Wang, X. The function-led design of Z-scheme photocatalytic systems based on hollow carbon nitride semiconductors. Chem. Commun. 2015, 51, 17467–17470. [Google Scholar] [CrossRef]
- Liu, X.; Cai, L. A novel double Z-scheme BiOBr-GO-polyaniline photocatalyst: Study on the excellent photocatalytic performance and photocatalytic mechanism. Appl. Surf. Sci. 2019, 483, 875–887. [Google Scholar] [CrossRef]
- Iwase, A.; Ng, Y.H.; Ishiguro, Y.; Kudo, A.; Amal, R. Reduced Graphene Oxide as a Solid-State Electron Mediator in Z-Scheme Photocatalytic Water Splitting under Visible Light. J. Am. Chem. Soc. 2011, 133, 11054–11057. [Google Scholar] [CrossRef]
- Li, X.; Sun, H.; Xie, Y.; Liang, Y.; Gong, X.; Qin, P.; Jiang, L.; Guo, J.; Liu, C.; Wu, Z. Principles, synthesis and applications of dual Z-scheme photocatalysts. Coord. Chem. Rev. 2022, 467, 214596. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, H. Recyclable MXene-bridged Z-scheme NiFe2O4/MXene/Bi2WO6 heterojunction with enhanced charge separation for efficient sonocatalytic removal of ciprofloxacin. Sci. Total Environ. 2023, 901, 165833. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Su, Y.; Li, C.; Lv, X.; Li, W.; Yang, J.; Zhang, W.; Wang, H. Novel MOF-derived dual Z-scheme g-C3N4/Bi2O2CO3/β-Bi2O3 heterojunctions with enhanced photodegradation of tetracycline hydrochloride. Sep. Purif. Technol. 2025, 354, 128580. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B-Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Zhu, B.; Cheng, B.; Fan, J.; Ho, W.; Yu, J. g-C3N4-Based 2D/2D Composite Heterojunction Photocatalyst. Small Struct. 2021, 2, 2100086. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-Scheme Photocatalyst. Adv. Mater. 2022, 34, 2107668. [Google Scholar] [CrossRef]
- Hao, P.; Chen, Z.; Yan, Y.; Shi, W.; Guo, F. Recent advances, application and prospect in g-C3N4-based S-scheme heterojunction photocatalysts. Sep. Purif. Technol. 2024, 330, 125302. [Google Scholar] [CrossRef]
- Duan, X.; Yang, J.; Zhu, J.; Li, H.; Fang, Y.; Liu, R.; Yang, C.; Liu, W.; Ding, C.; Liu, Q.; et al. Activated CdS/sulfur doped g-C3N4 photocatalyst for dye and antibiotic degradation: Experimental and DFT verification of S-scheme heterojunction. Environ. Res. 2025, 266, 120487. [Google Scholar] [CrossRef]
- Kumar, A.; Khosla, A.; Sharma, S.K.; Dhiman, P.; Sharma, G.; Gnanasekaran, L.; Naushad, M.; Stadler, F.J. A review on S-scheme and dual S-scheme heterojunctions for photocatalytic hydrogen evolution, water detoxification and CO2 reduction. Fuel 2023, 333, 126267. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, J.; Zhang, C.; Chen, F.; Song, Y.; Tang, Y. Construction of a novel double S-scheme heterojunction CeO2/g-C3N4/Bi2O4 for significantly boosted degradation of tetracycline: Insight into the dual charge transfer mode. Chem. Eng. J. 2024, 479, 147333. [Google Scholar] [CrossRef]
- Che, L.; Pan, J.; Cai, K.; Cong, Y.; Lv, S.-W. The construction of p-n heterojunction for enhancing photocatalytic performance in environmental application: A review. Sep. Purif. Technol. 2023, 315, 123708. [Google Scholar] [CrossRef]
- Chen, W.; Huang, J.; He, Z.; Ji, X.; Zhang, Y.-F.; Sun, H.; Wang, K.; Su, Z. Accelerated photocatalytic degradation of tetracycline hydrochloride over CuAl2O4/g-C3N4 p-n heterojunctions under visible light irradiation. Sep. Purif. Technol. 2021, 277, 119461. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Zhang, J.; Tao, F.; Xu, J. Construction of NiO/g-C3N4 p-n heterojunctions for enhanced photocatalytic CO2 reduction. J. Solid State Chem. 2022, 308, 122878. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Z.; Su, R.; Chen, Y.; Wang, Y.; Ma, D.; Li, Q. Revealing the reaction mechanism of the novel p-n heterojunction Mn3O4-C3N4 in efficient activation of chlorite to degrade organic pollutants under piezoelectric catalysis. Appl. Catal. B-Environ. 2025, 362, 124714. [Google Scholar] [CrossRef]
- Yang, S.; Wu, T.; Li, K.; Huang, P.; Li, W.; Zhuo, Y.; Liu, K.; Yang, Z.; Han, D. Photocatalytic Enhancement and Recyclability in Visible-Light-Responsive 2D/2D g-C3N4/BiOI p-n Heterojunctions via a Z-Scheme Charge Transfer Mechanism. Molecules 2024, 29, 5418. [Google Scholar] [CrossRef]
- Dharani, S.; Gnanasekaran, L.; Arunachalam, S.; Zielinska-Jure, A.; Almoallim, H.S.; Soto-Moscoso, M. Photodegrading rhodamine B dye with cobalt ferrite-graphitic carbon nitride (CoFe2O4/g-C3N4) composite. Environ. Res. 2024, 258, 119484. [Google Scholar] [CrossRef]
- Zhan, X.; Zeng, Y.; Zhang, Z.; Xia, Y.; Xu, J.; Hong, B.; Wang, X. G-C3N4 with gradient vacancies to enhance spatial charge carriers transfer and separation for photodegrading antibiotics under visible light. Chem. Eng. J. 2023, 474, 145948. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Z.; Jiang, J.; Li, R.; Xiong, J. Preparation of heterojunction C3N4/WO3 photocatalyst for degradation of microplastics in water. Chemosphere 2023, 337, 139206. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, D.; Chang, Y.; Feng, Q.; Liu, Z.; Pang, M.; Meng, D.; Feng, Y.; Fan, C. Anchoring single-atom Cu on tubular g-C3N4 with defect engineering for enhanced Fenton-like reactions to efficiently degrade carbamazepine: Performance and mechanism. Chem. Eng. J. 2024, 479, 147841. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Sun, X.; Ji, H.; Liu, W.; Cai, Z. Construction of Z-scheme Ag/AgVO3/carbon-rich g-C3N4 heterojunction for enhanced photocatalytic degradation of sulfamethiadiazole: DFT calculation and mechanism study. Chem. Eng. J. 2022, 433, 133604. [Google Scholar] [CrossRef]
- Qin, L.; Meng, J.; Yang, G.; Pan, Y.; Gao, X.; Yang, Y.; Guo, Y. Interlayer single-atomic Fe-N4 sites on carbon-rich graphitic carbon nitride for notably enhanced photo-Fenton-like catalytic oxidation processes towards recalcitrant organic micropollutants. Appl. Catal. B-Environ. Energy 2024, 345, 123695. [Google Scholar] [CrossRef]
- Manh Dung, N.; Thanh Binh, N.; Linh Hai, T.; Thuy Giang, N.; Fatimah, I.; Kuncoro, E.P.; Doong, R.-A. Z-scheme S, B co-doped g-C3N4 nanotube@MnO2 heterojunction with visible-light-responsive for enhanced photodegradation of diclofenac by peroxymonosulfate activation. Chem. Eng. J. 2023, 452, 139249. [Google Scholar] [CrossRef]
- Cheng, Z.; Ling, L.; Wu, Z.; Fang, J.; Westerhoff, P.; Shang, C. Novel Visible Light-Driven Photocatalytic Chlorine Activation Process for Carbamazepine Degradation in Drinking Water. Environ. Sci. Technol. 2020, 54, 11584–11593. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Xie, Y.; Rabeah, J.; Brueckner, A.; Cao, H. Visible-Light Photocatalytic Ozonation Using Graphitic C3N4 Catalysts: A Hydroxyl Radical Manufacturer for Wastewater Treatment. Acc. Chem. Res. 2020, 53, 1024–1033. [Google Scholar] [CrossRef]
- Sun, Y.; Fang, Z.; Huang, X.; Bai, C.; Zhu, K.; Chen, X.; Zhang, B.; Zhang, Y.; Yang, Q.; Zheng, J.; et al. Efficient photo-switchable activation of periodate by nitrogen-vacancy-rich carbon nitride for organic contaminant removal: Theoretical predictions and experimental validations. Appl. Catal. B-Environ. 2023, 337, 122994. [Google Scholar] [CrossRef]
- You, Q.; Zhang, C.; Cao, M.; Chen, P.; Wang, B.; Wang, Y.; Huang, J.; Deng, S.; Yu, G. Coordinating the exciton-mediated and carrier-mediated photocatalytic mechanisms of carbon nitride using acetylacetone for enhancing emerging contaminants removal. Appl. Catal. B-Environ. Energy 2023, 338, 123025. [Google Scholar] [CrossRef]
- Zhang, B.; Xia, S.; Wang, Z.; Li, W.; Li, B.; Zhang, H.; Xin, Y.; Wu, K.; Ma, J.; He, X. Enhanced permanganate activation by g-C3N4 under visible light irradiation: Unraveled mechanism involving Mn(V) and photo-induced electron. Appl. Catal. B-Environ. Energy 2024, 349, 123861. [Google Scholar] [CrossRef]
- Yu, Z.; Wu, J.; Zhang, J.; Chen, X.; Wang, Z.; Zhang, Y.; Li, D.; Chen, J.; Liu, H.; Chen, P.; et al. Carbon nitride nanotubes anchored with Cu(I) triggers peracetic acid activation with visible light for removal of antibiotic contaminants: Probing mechanisms of non-radical pathways and identifying active sites. J. Hazard. Mater. 2023, 460, 132401. [Google Scholar] [CrossRef]
- Zhang, B.; Kong, L.; Yan, X.; Zhang, H.; Wang, Z.; Xia, S.; Han, Z.; Xin, Y.; Ding, A.; Ma, J.; et al. Recent progress in graphitic carbon nitride-based catalysts for water treatment: Contaminant elimination, disinfection and membrane applications. Sep. Purif. Technol. 2025, 354, 129420. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).