Polyethylene Terephthalate Hydrolysis Catalyzed by Deep Eutectic Solvents: COSMO-RS Screening and Experimental Validation
Abstract
1. Introduction
- i
- In hydrolysis, PET is broken down using water as a solvent under neutral, alkaline, or acidic conditions. The reaction typically requires higher temperature, pressure, and time, but these parameters can be adjusted with the use of an appropriate catalyst. The main monomers produced from this process are the primary PET building block called terephthalic acid (TPA) and ethylene glycol (EG) [12].
- ii
- Methanolysis operates under high pressure (2 to 4 MPa) and high temperatures (180–280 °C), yielding dimethyl terephthalate (DMT) and EG as byproducts [12].
- iii
- Aminolysis uses amine-containing solutions like methylamine or ethanolamine, which then forms diamides of terephthalic acid and EG. Ammonolysis employs anhydrous ammonia in an EG medium under pressure to produce terephthalamide and EG [13].
- iv
- Finally, glycolysis breaks down PET through solvolytic degradation, forming BHET monomers while generating oligomers as by-products [14].
2. Results
2.1. COSMO-RS Screening Results
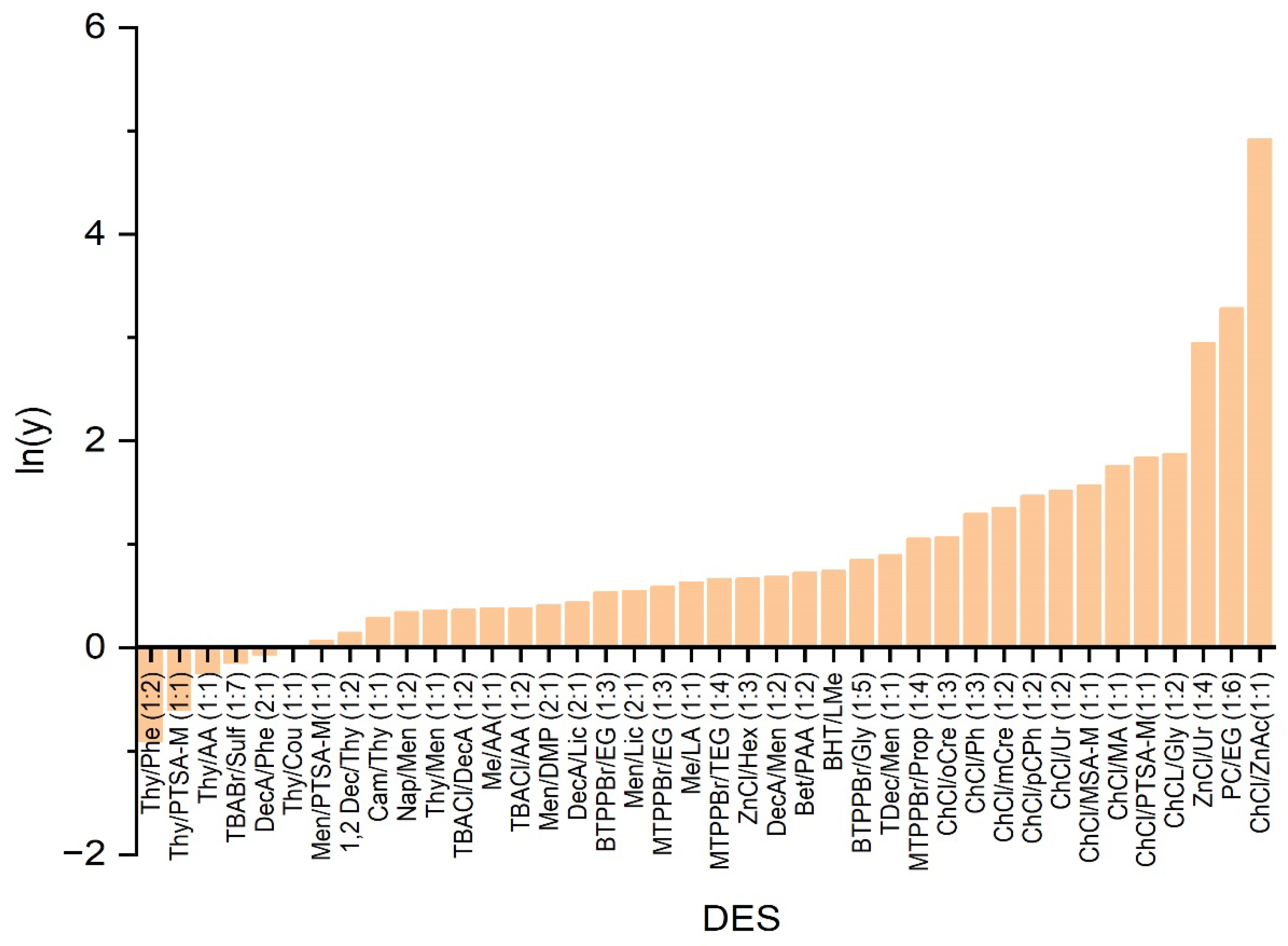
2.1.1. Logarithmic Activity Coefficient of PET in DESs as Predicted Using COSMO-RS
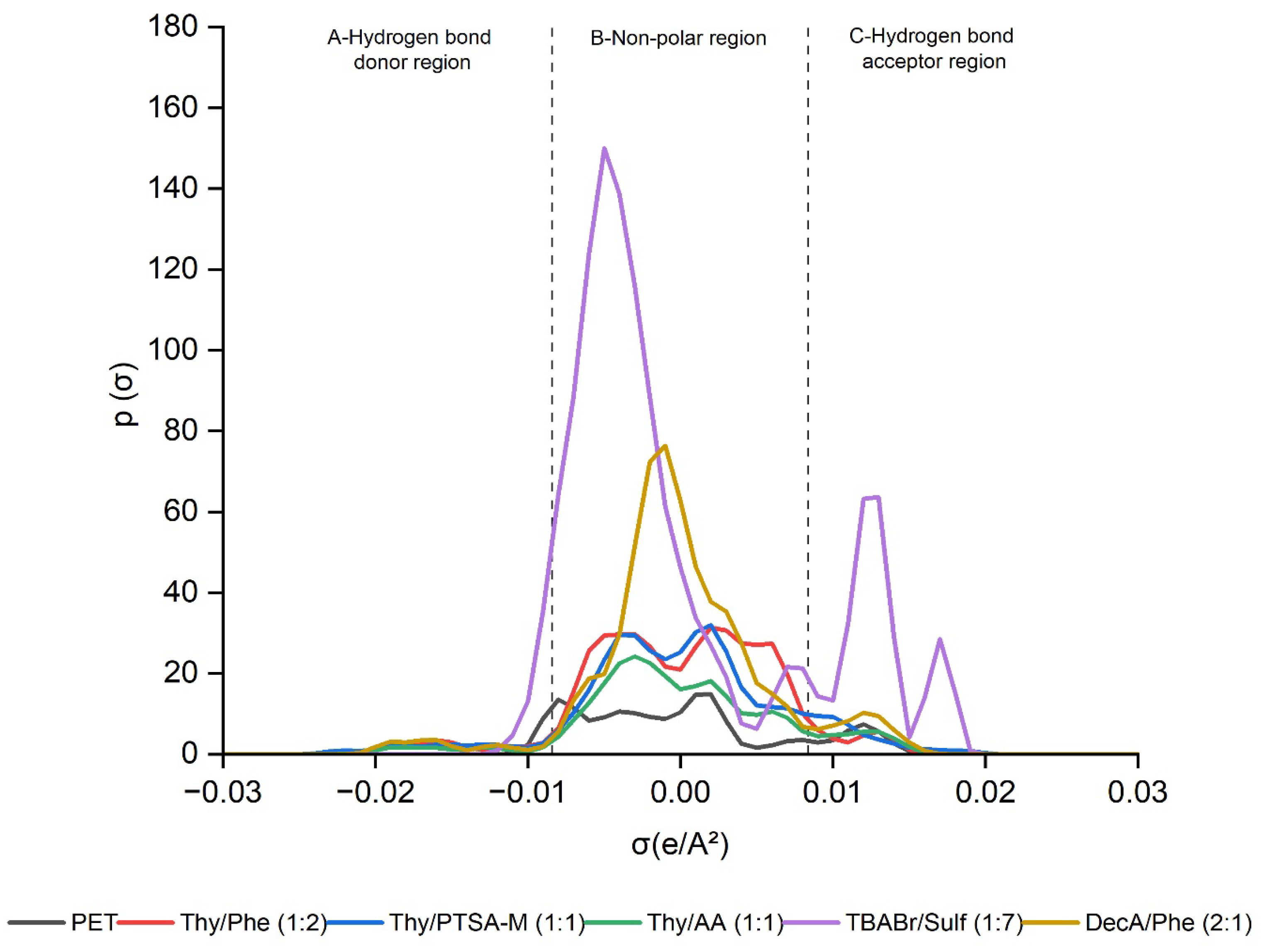
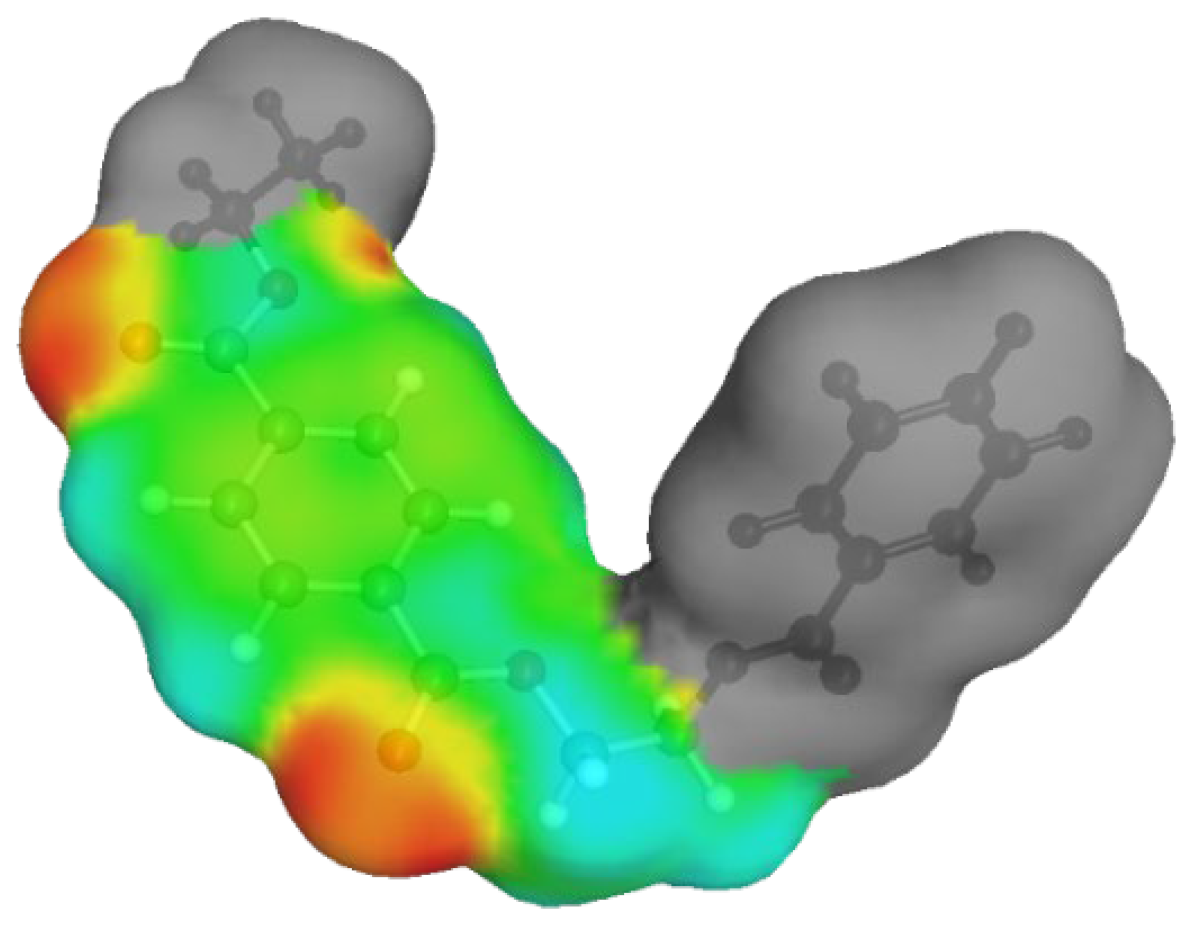
2.1.2. σ-Profile and σ-Potential of DESs in Relation of PET
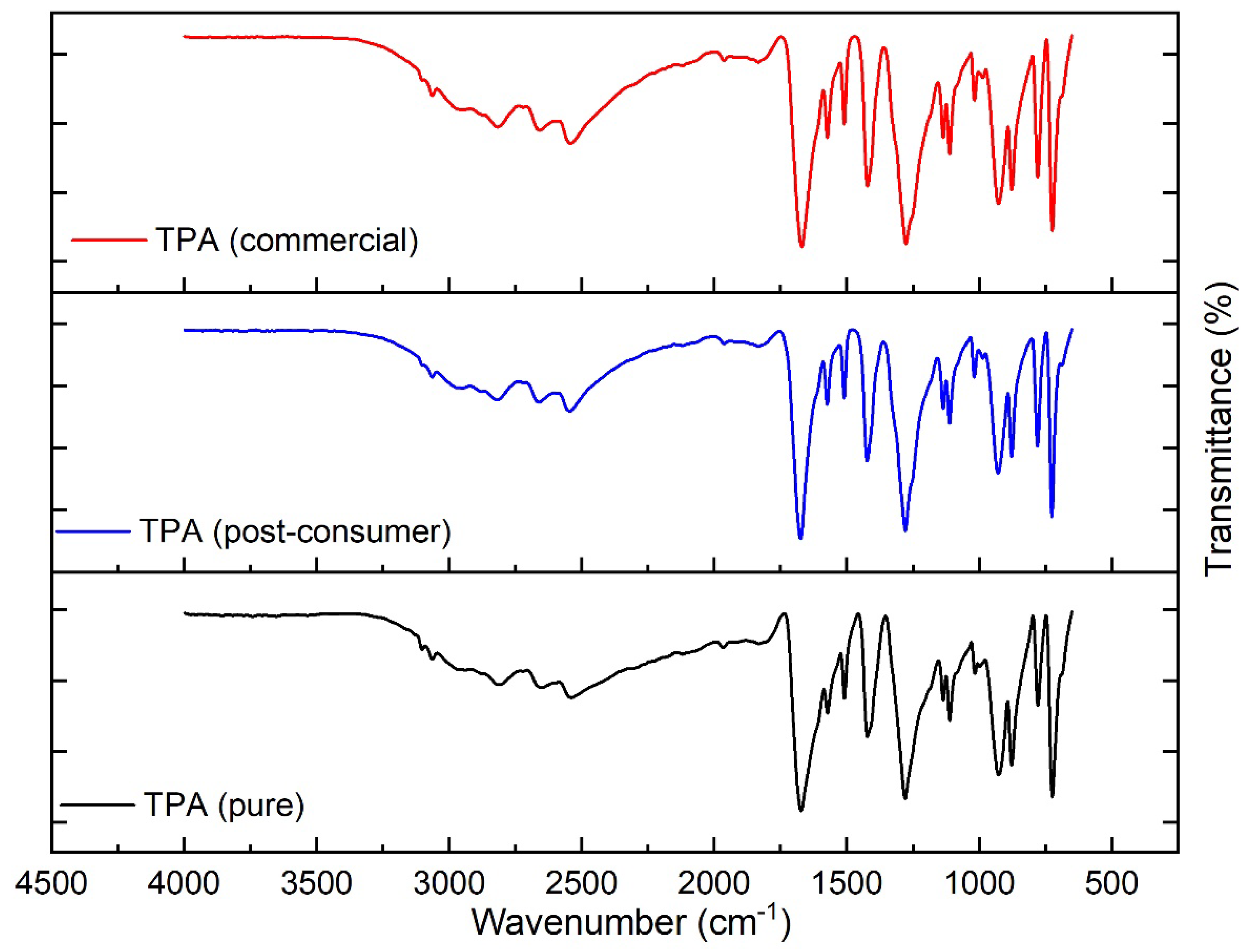
2.2. Experimental Validation for Catalytic Hydrolysis of PET
3. Discussion
4. Materials and Methods
4.1. Geometry Optimization and COSMO-RS Computation
4.2. DES Screening Using COSMO-RS
4.3. Chemicals and Materials Used in This Work
4.4. Experimental Protocol
4.4.1. Synthesis of DES
4.4.2. Depolymerization Experiment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FeCl3.6H2O | Ferric chloride hexahydrate |
| P-TSA | P-toluenesulfonic acid |
| AA | Acetic acid |
| MSA | methanesulfonic acid |
| [Bmim][Cl] | 1-butyl-3-methylimidazolium chloride |
| [HSO3-pmim][HSO4] | 1-methyl-3-(3-sulfopropyl)-imidazolium hydrogen sulfate |
Appendix A
Appendix A.1
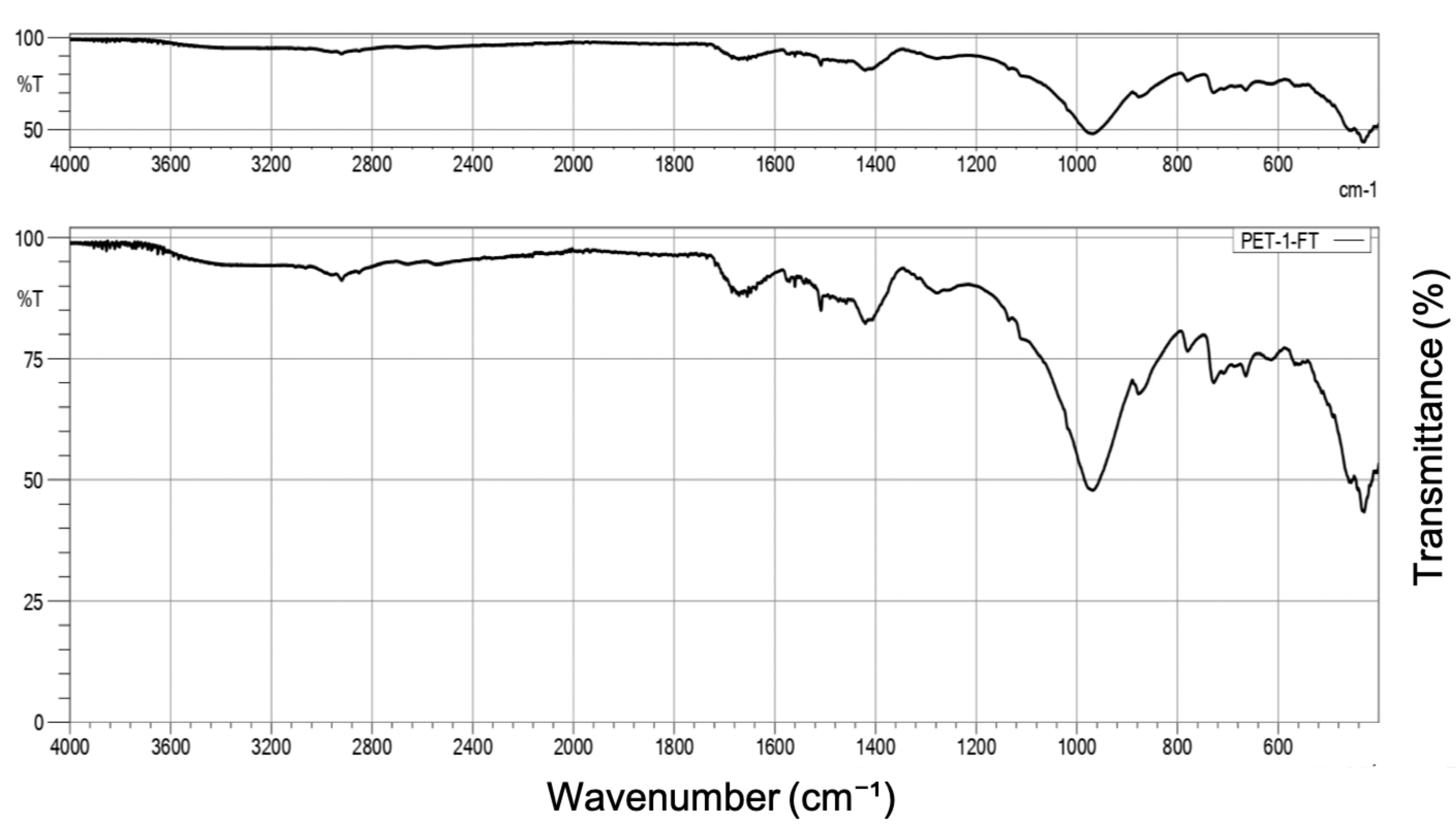
Appendix A.2

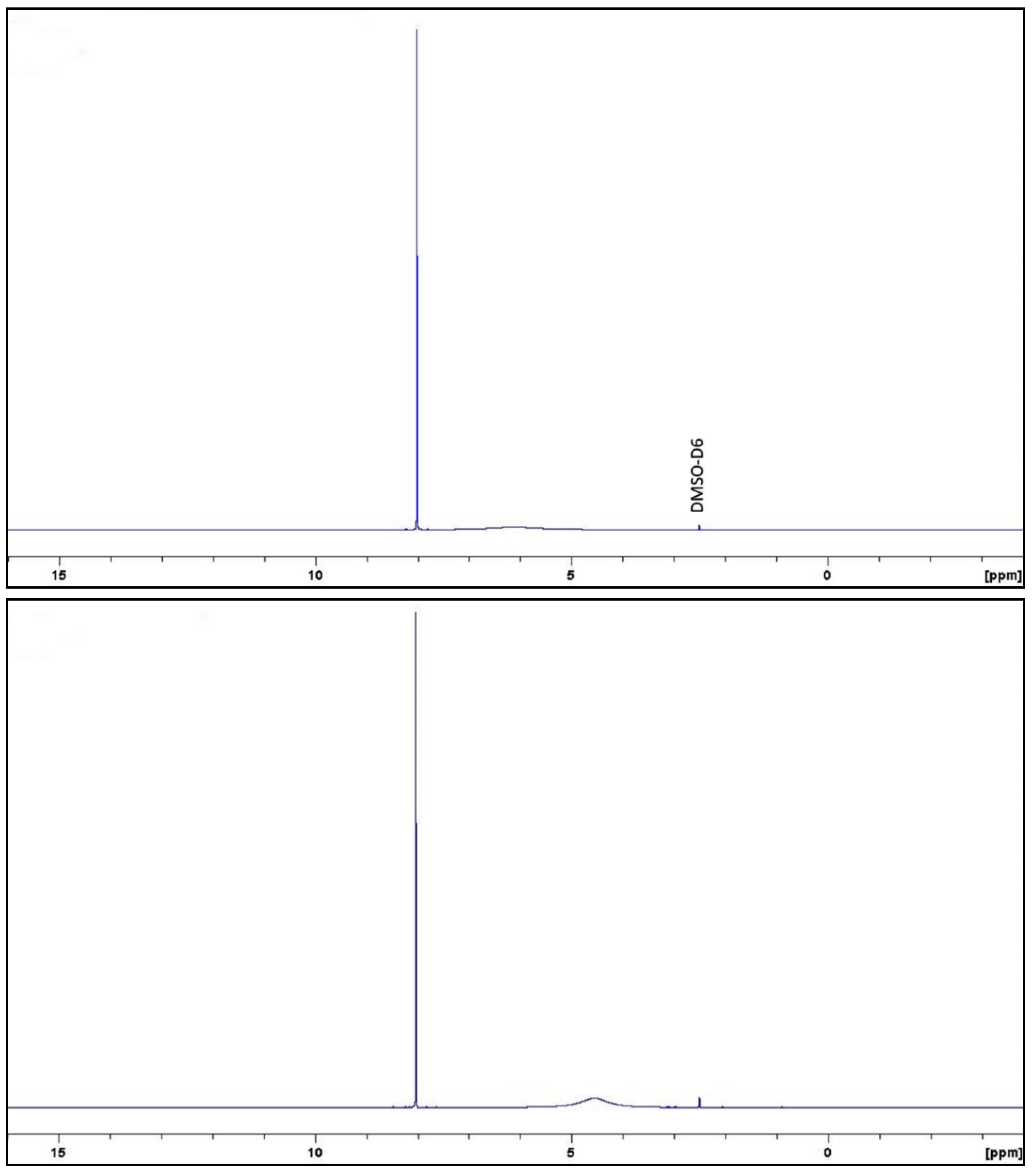
References
- Choudhary, K.; Sangwan, K.S.; Goyal, D. Environment and economic impacts assessment of PET waste recycling with conventional and renewable sources of energy. Procedia CIRP 2019, 80, 422–427. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M.; Mohammadian, M.; Jones, K. Physicochemical aspects of the environmental degradation of poly (ethylene terephthalate). Polym. Degrad. Stab. 1994, 43, 229–237. [Google Scholar] [CrossRef]
- Fattah, A.N.; Hizaddin, H.F.; Hashim, M.A.; Basirun, W.J.; Salleh, M.M.Z. Evaluation of Deep Eutectic Solvent Performance for the Extraction of Free 3-Monochloropropane-1, 2-diol (3-MCPD) from Model Oil–COSMO-RS and Experimental Validation. Eur. J. Lipid Sci. Technol. 2022, 124, 2100165. [Google Scholar] [CrossRef]
- Johansen, M.R.; Christensen, T.B.; Ramos, T.M.; Syberg, K. A review of the plastic value chain from a circular economy perspective. J. Environ. Manag. 2022, 302, 113975. [Google Scholar] [CrossRef]
- Garcia, J.M.; Robertson, M.L. The future of plastics recycling. Science 2017, 358, 870–872. [Google Scholar] [CrossRef]
- Lamtai, A.; Elkoun, S.; Robert, M.; Mighri, F.; Diez, C. Mechanical Recycling of Thermoplastics: A Review of Key Issues. Waste 2023, 1, 860–883. [Google Scholar] [CrossRef]
- Kassab, A.; Al Nabhani, D.; Mohanty, P.; Pannier, C.; Ayoub, G.Y. Advancing plastic recycling: Challenges and opportunities in the integration of 3d printing and distributed recycling for a circular economy. Polymers 2023, 15, 3881. [Google Scholar] [CrossRef]
- Quicker, P.; Seitz, M.; Vogel, J. Chemical recycling: A critical assessment of potential process approaches. Waste Manag. Res. 2022, 40, 1494–1504. [Google Scholar] [CrossRef]
- Chemical Recycling in Circular Perspective. Available online: https://circulareconomy.europa.eu/platform/sites/default/files/2023-08/Chemical%20Recycling%20in%20Circular%20Perspective.pdf (accessed on 4 July 2025).
- Beghetto, V.; Gatto, V.; Samiolo, R.; Scolaro, C.; Brahimi, S.; Facchin, M.; Visco, A. Plastics today: Key challenges and EU strategies towards carbon neutrality: A review. Environ. Pollut. 2023, 334, 122102. [Google Scholar] [CrossRef]
- The Circular Economy for Plastics—A European Analysis 2024. Available online: https://plasticseurope.org/knowledge-hub/the-circular-economy-for-plastics-a-european-analysis-2024/ (accessed on 27 June 2025).
- Grigore, M.E. Methods of recycling, properties and applications of recycled thermoplastic polymers. Recycling 2017, 2, 24. [Google Scholar] [CrossRef]
- Shojaei, B.; Abtahi, M.; Najafi, M. Chemical recycling of PET: A stepping-stone toward sustainability. Polym. Adv. Technol. 2020, 31, 2912–2938. [Google Scholar] [CrossRef]
- Aguado, A.; Becerra, L.; Martínez, L. Glycolysis optimisation of different complex PET waste with recovery and reuse of ethylene glycol. Chem. Pap. 2023, 77, 3293–3303. [Google Scholar] [CrossRef]
- Jiang, X.; Bateer, B. A systematic review of plastic recycling: Technology, environmental impact and economic evaluation. Waste Manag. Res. 2025, 43, 1159–1178. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Walker, T.R. Plastic recycling: A panacea or environmental pollution problem. npj Mater. Sustain. 2024, 2, 17. [Google Scholar] [CrossRef]
- Ügdüler, S.; Van Geem, K.M.; Denolf, R.; Roosen, M.; Mys, N.; Ragaert, K.; De Meester, S. Towards closed-loop recycling of multilayer and coloured PET plastic waste by alkaline hydrolysis. Green Chem. 2020, 22, 5376–5394. [Google Scholar] [CrossRef]
- Shi, K.; Guo, L.; Zheng, R.; Wang, H.; Chen, Y. Preparation of diacid comprising ionic liquid catalyst and its application in catalytic degradation of PET. Catal. Lett. 2022, 152, 1182–1193. [Google Scholar] [CrossRef]
- Wang, R.; Wang, T.; Yu, G.; Chen, X. A new class of catalysts for the glycolysis of PET: Deep eutectic solvent@ ZIF-8 composite. Polym. Degrad. Stab. 2021, 183, 109463. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Achilias, D.S.; Redhwi, H.H.; Bikiaris, D.N.; Katsogiannis, K.A.G.; Karayannidis, G.P. Hydrolytic depolymerization of PET in a microwave reactor. Macromol. Mater. Eng. 2010, 295, 575–584. [Google Scholar] [CrossRef]
- Cho, J.Y.; Choi, H.-M.; Oh, K.W. Rapid hydrophilic modification of poly (ethylene terephthalate) surface by using deep eutectic solvent and microwave irradiation. Text. Res. J. 2016, 86, 1318–1327. [Google Scholar] [CrossRef]
- Bhogle, C.S.; Pandit, A.B. Ultrasound-assisted alkaline hydrolysis of waste poly (ethylene terephthalate) in aqueous and non-aqueous media at low temperature. Indian Chem. Eng. 2018, 60, 122–140. [Google Scholar] [CrossRef]
- Giraldo-Narcizo, S.; Guenani, N.; Sánchez-Pérez, A.M.; Guerrero, A. Accelerated Polyethylene Terephthalate (PET) Enzymatic Degradation by Room Temperature Alkali Pre-treatment for Reduced Polymer Crystallinity. ChemBioChem 2023, 24, e202200503. [Google Scholar] [CrossRef]
- Attallah, O.A.; Janssens, A.; Azeem, M.; Fournet, M.B. Fast, high monomer yield from post-consumer polyethylene terephthalate via combined microwave and deep eutectic solvent hydrolytic depolymerization. ACS Sustain. Chem. Eng. 2021, 9, 17174–17185. [Google Scholar] [CrossRef]
- Rollo, M.; Perini, M.A.; Sanzone, A.; Polastri, L.; Tiecco, M.; Torregrosa-Chinillach, A.; Martinelli, E.; Ciancaleoni, G. Effect of chloride salts and microwaves on polyethylene terephthalate (PET) hydrolysis by iron chloride/acetic acid Lewis/Brønsted acidic deep eutectic solvent. RSC Sustain. 2024, 2, 187–196. [Google Scholar] [CrossRef]
- Attallah, O.A.; Azeem, M.; Nikolaivits, E.; Topakas, E.; Fournet, M.B. Progressing ultragreen, energy-efficient biobased depolymerization of poly (ethylene terephthalate) via microwave-assisted green deep eutectic solvent and enzymatic treatment. Polymers 2021, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Attallah, O.A.; Tas, C.E.; Fournet, M.B. All Green Microwave Assisted 99% Depolymerisation of Polyethylene Terephthalate into Value Added Products via Glycerol Pre-treatment and Hydrolysis Reaction. J. Polym. Environ. 2024, 32, 303–315. [Google Scholar] [CrossRef]
- Azeem, M.; Fournet, M.B.; Attallah, O.A. Ultrafast 99% Polyethylene terephthalate depolymerization into value added monomers using sequential glycolysis-hydrolysis under microwave irradiation. Arab. J. Chem. 2022, 15, 103903. [Google Scholar] [CrossRef]
- Liu, F.; Cui, X.; Yu, S.; Li, Z.; Ge, X. Hydrolysis reaction of poly (ethylene terephthalate) using ionic liquids as solvent and catalyst. J. Appl. Polym. Sci. 2009, 114, 3561–3565. [Google Scholar] [CrossRef]
- Xiao, C.; Fusheng, L.; Zhuo, L.; Shitao, Y. Hydrolysis of poly (ethylene terephthalate) to recover terephthalic acid in ionic liquids. Chem. Eng. 2010, 38, 40–44. [Google Scholar]
- Zheng, X.; Feng, J.; Lu, Y.; Li, R.; Cavaco-paulo, A.; Fu, J. Deep eutectic solvent as an additive to improve enzymatic hydrolysis of polyethylene terephthalate (PET). J. Polym. Environ. 2024, 32, 5936–5951. [Google Scholar] [CrossRef]
- Rollo, M.; Raffi, F.; Rossi, E.; Tiecco, M.; Martinelli, E.; Ciancaleoni, G. Depolymerization of polyethylene terephthalate (PET) under mild conditions by Lewis/Brønsted acidic deep eutectic solvents. Chem. Eng. J. 2023, 456, 141092. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Vigier, K.D.O.; Chatel, G.; Jerome, F. Contribution of deep eutectic solvents for biomass processing: Opportunities, challenges, and limitations. Chem. Commun. 2015, 7, 1250–1260. [Google Scholar] [CrossRef]
- Mohan, M.; Keasling, J.D.; Simmons, B.A.; Singh, S. In silico COSMO-RS predictive screening of ionic liquids for the dissolution of plastic. Green Chem. 2022, 24, 4140–4152. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, J.; Wang, P.; Ji, Z.; Ling, Z.; Yong, Q. Microwave-assisted fast co-production of fermentable sugar and nanocellulose via tunable zinc acetate based deep eutectic solvents treatments. Ind. Crops Prod. 2023, 191, 115965. [Google Scholar] [CrossRef]
- Paparella, A.N.; Perrone, S.; Salomone, A.; Messa, F.; Cicco, L.; Capriati, V.; Perna, F.M.; Vitale, P. Use of Deep Eutectic solvents in Plastic depolymerization. Catalysts 2023, 13, 1035. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, K.; Zhu, Y.; Zhu, R.; Ye, F.; Song, N.; Xu, Y. Physicochemical properties of deep eutectic solvents formed by choline chloride and phenolic compounds at T = (293.15 to 333.15) K: The influence of electronic effect of substitution group. J. Mol. Liq. 2017, 232, 182–187. [Google Scholar] [CrossRef]
- Ferreira, C.; Sarraguça, M. A Comprehensive Review on Deep Eutectic Solvents and Its Use to Extract Bioactive Compounds of Pharmaceutical Interest. Pharmaceuticals 2024, 17, 124. [Google Scholar] [CrossRef]
- Yao, C.; Liu, H.; Wu, H.; Song, X.; Wang, X.; Ren, S.; Wu, W. Comparative study on the deep eutectic solvents formed by choline chloride and cresol isomers from theoretical and experimental perspectives. J. Mol. Liq. 2022, 367, 120420. [Google Scholar] [CrossRef]
- Rodriguez Rodriguez, N.; Machiels, L.; Binnemans, K. p-Toluenesulfonic acid-based deep-eutectic solvents for solubilizing metal oxides. ACS Sustain. Chem. Eng. 2019, 7, 3940–3948. [Google Scholar] [CrossRef]
- Hizaddin, H.F.; Ramalingam, A.; Hashim, M.A.; Hadj-Kali, M.K. Evaluating the performance of deep eutectic solvents for use in extractive denitrification of liquid fuels by the conductor-like screening model for real solvents. J. Chem. Eng. Data 2014, 59, 3470–3487. [Google Scholar] [CrossRef]
- Xu, G.; Shi, M.; Zhang, P.; Tu, Z.; Hu, X.; Zhang, X.; Wu, Y. Tuning the composition of deep eutectic solvents consisting of tetrabutylammonium chloride and n-decanoic acid for adjustable separation of ethylene and ethane. Sep. Purif. Technol. 2022, 298, 121680. [Google Scholar] [CrossRef]
- Bhupathi, H.K.; Kundu, D.; Pugazhenthi, G.; Banerjee, T. Insight into the structural and transport properties of methyl and benzyl triphenyl phosphonium based deep eutectic solvents using molecular dynamics simulations. J. Indian Chem. Soc. 2023, 100, 100998. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Florindo, C.; Iff, L.C.; Coelho, M.A.; Marrucho, I.M. Menthol-based eutectic mixtures: Hydrophobic low viscosity solvents. ACS Sustain. Chem. Eng. 2015, 3, 2469–2477. [Google Scholar] [CrossRef]
- Van Osch, D.J.; Dietz, C.H.; Van Spronsen, J.; Kroon, M.C.; Gallucci, F.; van Sint Annaland, M.; Tuinier, R. A search for natural hydrophobic deep eutectic solvents based on natural components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, Q.; Abbas, U.L.; Liu, J.; Zheng, Y.; Jones, C.; Shao, Q.; Shi, J. Lignin derived hydrophobic deep eutectic solvents as sustainable extractants. J. Clean. Prod. 2023, 388, 135808. [Google Scholar] [CrossRef]
- Busato, M.; Mannucci, G.; Rocchi, L.A.; Di Pietro, M.E.; Capocefalo, A.; Zorzi, E.; Casu, P.; Veclani, D.; Castiglione, F.; Mele, A. The complex story behind a deep eutectic solvent formation as revealed by L-menthol mixtures with butylated hydroxytoluene derivatives. ACS Sustain. Chem. Eng. 2023, 11, 8988–8999. [Google Scholar] [CrossRef]
- Padilla, N.; Delso, I.; Bergua, F.; Lafuente, C.; Artal, M. Characterization of camphor: Thymol or dl-menthol eutectic mixtures; Structure, thermophysical properties, and lidocaine solubility. J. Mol. Liq. 2024, 405, 125069. [Google Scholar] [CrossRef]
- Siani, G.; Tiecco, M.; Di Profio, P.; Guernelli, S.; Fontana, A.; Ciulla, M.; Canale, V. Physical absorption of CO2 in betaine/carboxylic acid-based Natural Deep Eutectic Solvents. J. Mol. Liq. 2020, 315, 113708. [Google Scholar] [CrossRef]
- Prausnitz, J.M.; Lichtenthaler, R.N.; De Azevedo, E.G. Molecular Thermodynamics of Fluid-Phase Equilibria, 3rd ed.; Pearson Education: London, UK, 1998. [Google Scholar]
- Mohan, M.; Goud, V.V.; Banerjee, T. Solubility of glucose, xylose, fructose and galactose in ionic liquids: Experimental and theoretical studies using a continuum solvation model. Fluid Phase Equilib. 2015, 395, 33–43. [Google Scholar] [CrossRef]
- Diedenhofen, M.; Klamt, A. COSMO-RS as a tool for property prediction of IL mixtures—A review. Fluid Phase Equilib. 2010, 294, 31–38. [Google Scholar] [CrossRef]
- Salleh, Z.; Wazeer, I.; Mulyono, S.; El-blidi, L.; Hashim, M.A.; Hadj-Kali, M.K. Efficient removal of benzene from cyclohexane-benzene mixtures using deep eutectic solvents—COSMO-RS screening and experimental validation. J. Chem. Thermodyn. 2017, 104, 33–44. [Google Scholar] [CrossRef]
- Eckert, F.; Klamt, A. Fast solvent screening via quantum chemistry: COSMO-RS approach. AIChE J. 2002, 48, 369–385. [Google Scholar] [CrossRef]
- Jiang, C.; Cheng, H.; Qin, Z.; Wang, R.; Chen, L.; Yang, C.; Qi, Z.; Liu, X. COSMO-RS prediction and experimental verification of 1, 5-pentanediamine extraction from aqueous solution by ionic liquids. Green Energy Environ. 2021, 6, 422–431. [Google Scholar] [CrossRef]
- Quaid, T.; Reza, T. COSMO prediction of siloxane compounds absorption on type 3 and type 5 deep eutectic solvents. Chem. Eng. J. Adv. 2023, 14, 100489. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, J.; Wang, J. Chemical degradation and recycling of polyethylene terephthalate (PET): A review. RSC Sustain. 2025, 3, 2111–2133. [Google Scholar] [CrossRef]
- Hu, H.; Wu, Y.; Zhu, Z. Optimization of microwave-assisted preparation of TPA from waste PET using response surface methodology. J. Polym. Environ. 2018, 26, 375–382. [Google Scholar] [CrossRef]
- Wazeer, I.; Hadj-Kali, M.K.; Al-Nashef, I.M. Utilization of deep eutectic solvents to reduce the release of hazardous gases to the atmosphere: A critical review. Molecules 2020, 26, 75. [Google Scholar] [CrossRef]
- Eckert, F.; Klamt, A. COSMOtherm, version C3.0; Release 16.01; COSMOlogic GmbH & Co. KG: Leverkusen, Germany, 2015. [Google Scholar]
- Loschen, C.; Klamt, A. Prediction of solubilities and partition coefficients in polymers using COSMO-RS. Ind. Eng. Chem. Res. 2014, 53, 11478–11487. [Google Scholar] [CrossRef]
- Kahlen, J.; Masuch, K.; Leonhard, K. Modelling cellulose solubilities in ionic liquids using COSMO-RS. Green Chem. 2010, 12, 2172–2181. [Google Scholar] [CrossRef]
- Eckert, F.; Klamt, A. COSMOtherm, version C30_1401; COSMOlogic GmbH &, Co. KG: Leverkusen, Germany, 2013.
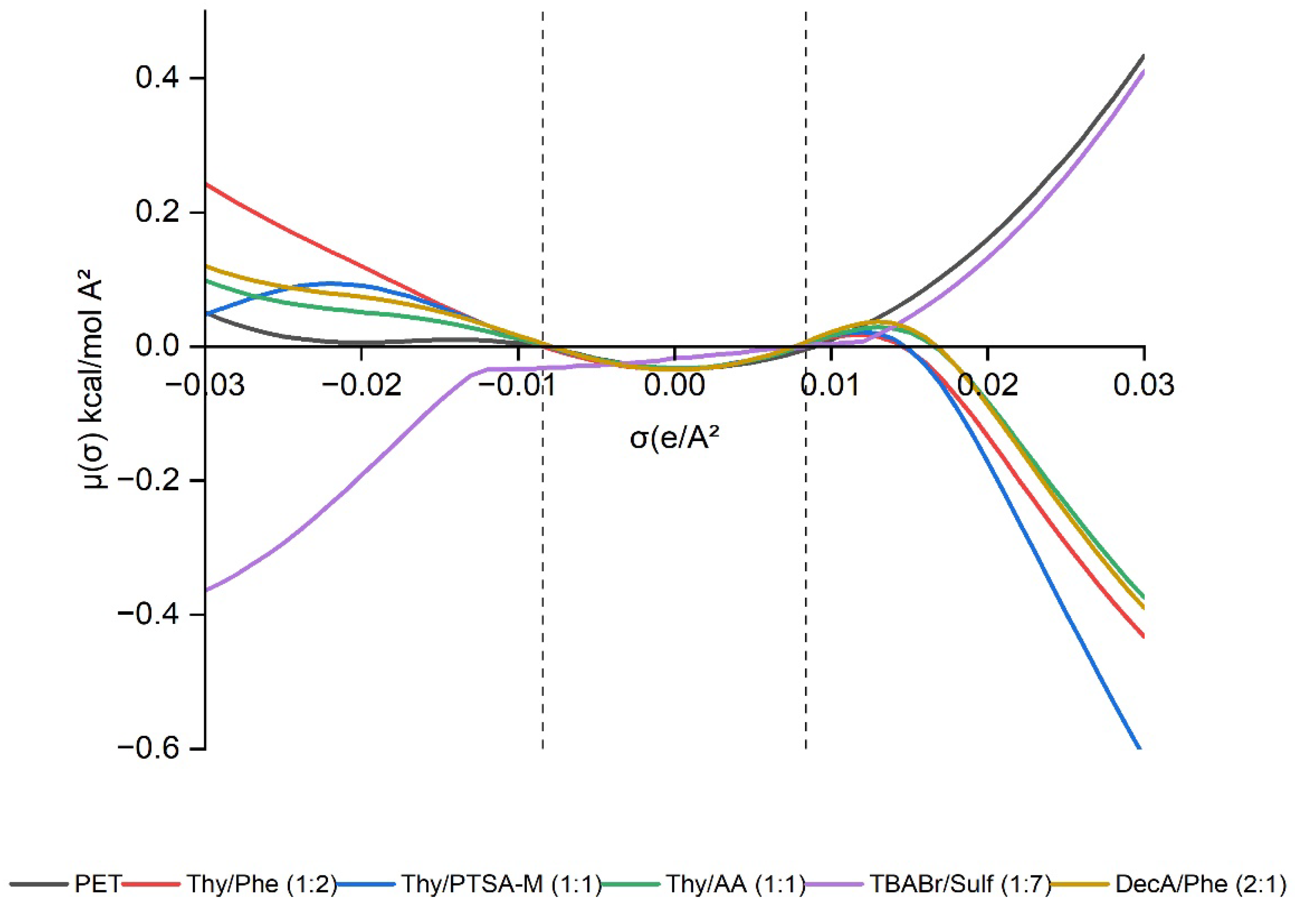
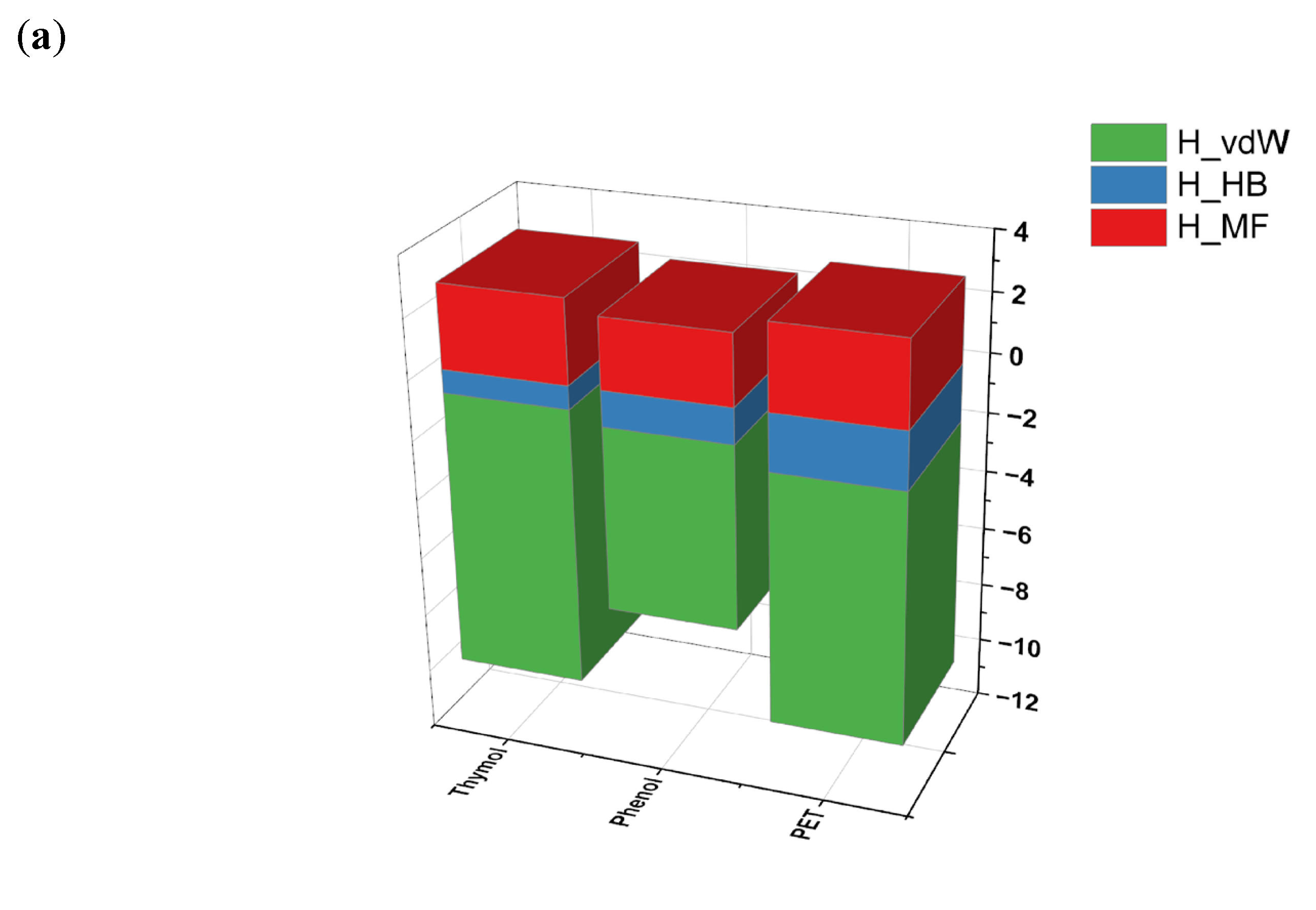
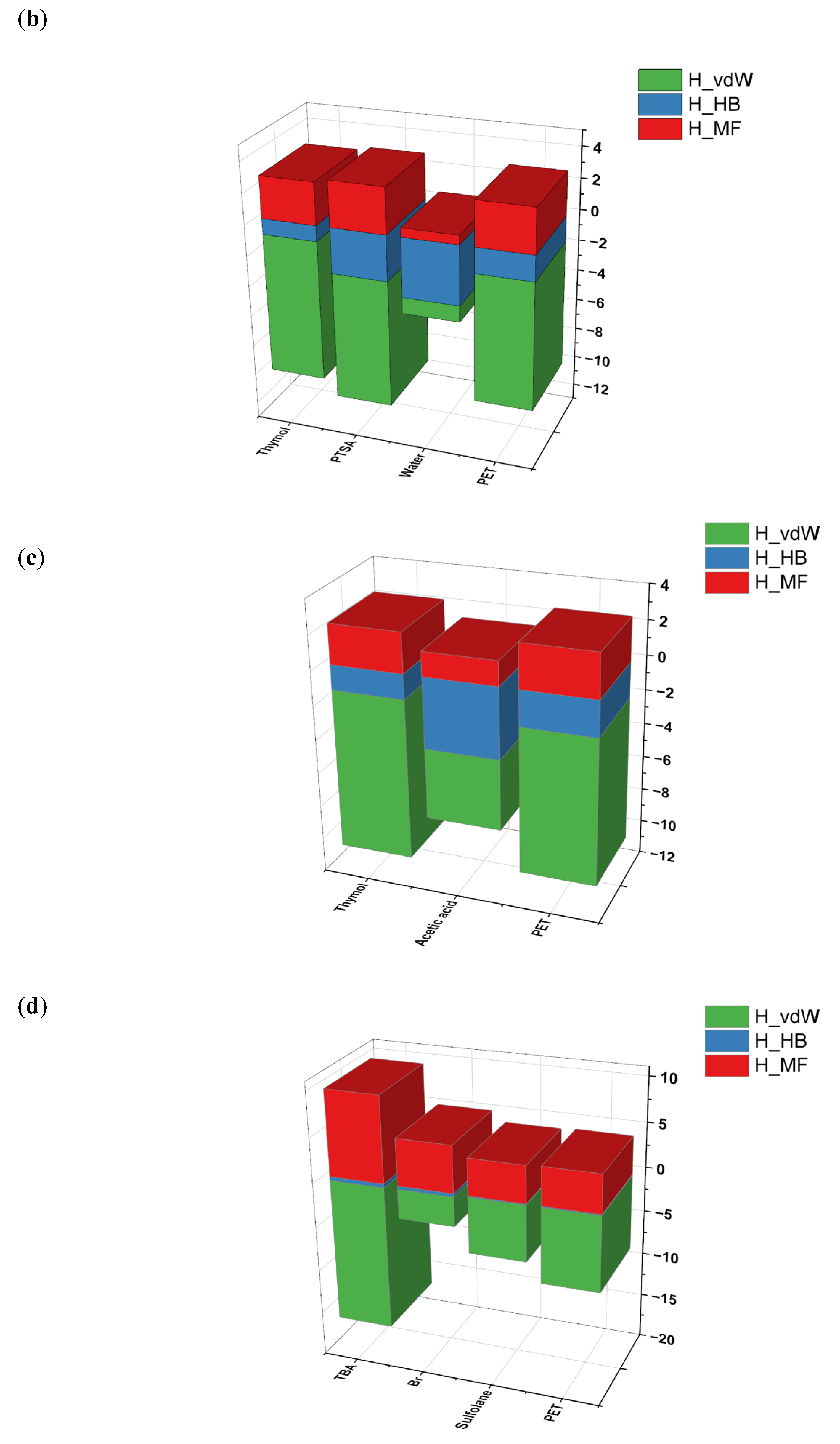
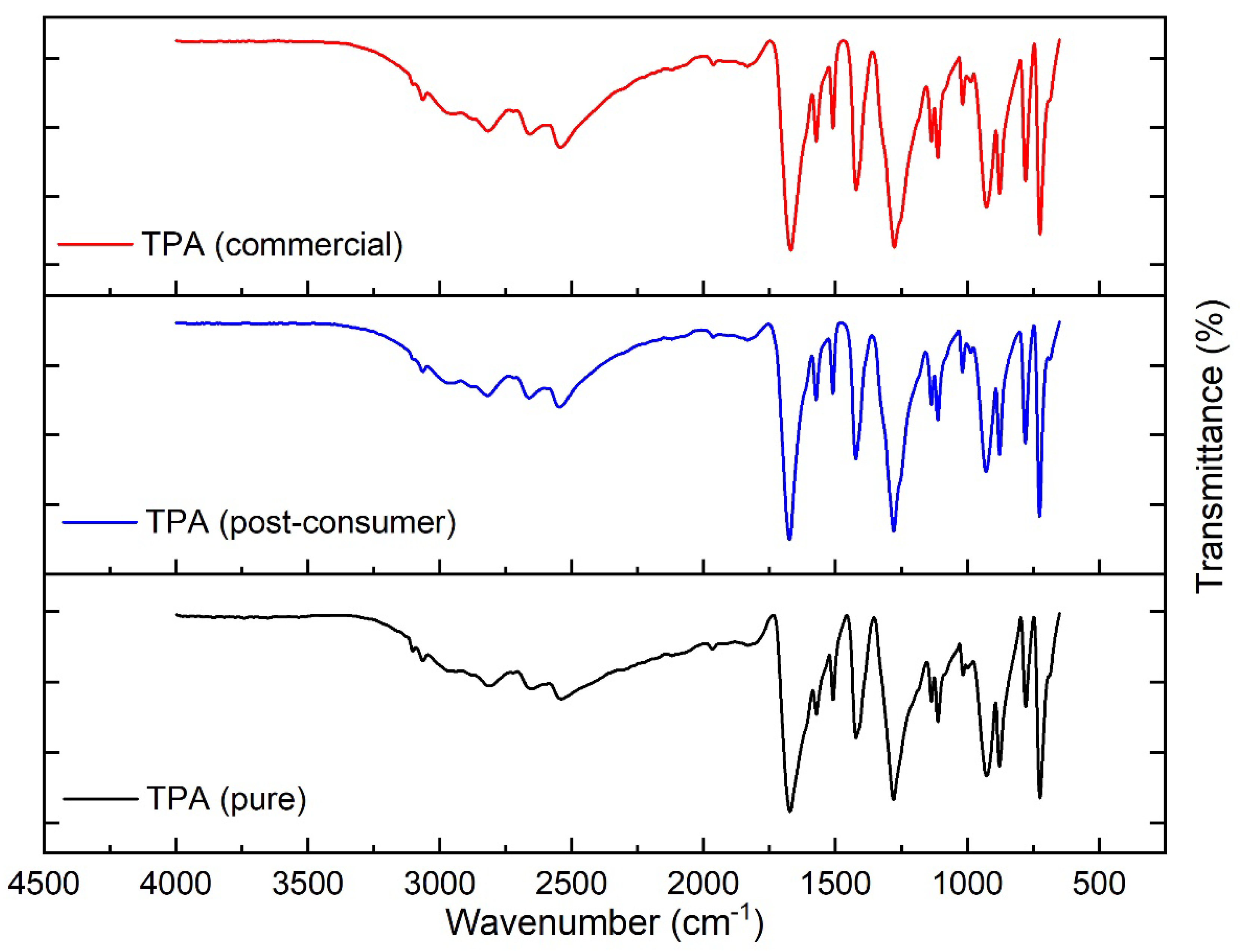
| No. | HBA | HBD | Ratio | Abbreviations | Ref. | |
|---|---|---|---|---|---|---|
| HBA | HBD | |||||
| 1 | Choline chloride | Zinc acetate | 1 | 1 | ChCl/ZnAc(1:1) | [38] |
| 2 | Choline chloride | M-cresol | 1 | 2 | ChCl/mCre (1:2) | [39] |
| 3 | Choline chloride | P-chlorophenol | 1 | 2 | ChCl/pCPh (1:2) | [40] |
| 4 | Choline chloride | Phenol | 1 | 3 | ChCl/Ph (1:3) | [41] |
| 5 | Choline chloride | O-cresol | 1 | 3 | ChCl/oCre (1:3) | [42] |
| 6 | Choline chloride | Urea | 1 | 2 | ChCl/Ur (1:2) | [39] |
| 7 | Choline chloride | Glycerol | 1 | 2 | ChCL/Gly (1:2) | [41] |
| 8 | Choline chloride | Malonic acid | 1 | 1 | ChCl/MA (1:1) | [41] |
| 9 | Choline chloride | P-toluenesulfonic acid monohydrate | 1 | 1 | ChCl/PTSA-M(1:1) | [43] |
| 10 | Choline chloride | Methanesulfonic acid monohydrate | 1 | 1 | ChCl/MSA-M (1:1) | ** |
| 11 | Zinc chloride | Urea | 1 | 4 | ZnCl/Ur (1:4) | [39] |
| 12 | Zinc chloride | Hexanediol | 1 | 3 | ZnCl/Hex (1:3) | [3] |
| 13 | Methyltriphenylphosphonium bromide | Triethylene glycol | 1 | 4 | MTPPBr/TEG (1:4) | [44] |
| 14 | Methyltriphenylphosphonium bromide | Ethylene glycol | 1 | 3 | MTPPBr/EG (1:3) | [44] |
| 15 | Methyltriphenylphosphonium bromide | 1,2- propanediol | 1 | 4 | MTPPBr/Prop (1:4) | ** |
| 16 | Tetrabutylammonium bromide | Sulfolane | 1 | 7 | TBABr/Sulf (1:7) | [3] |
| 17 | Tetrabutylammonium chloride | Acetic acid | 1 | 2 | TBACl/AA (1:2) | [44] |
| 18 | Tetrabutylammonium chloride | Decanoic acid | 1 | 2 | TBACl/DecA (1:2) | [45] |
| 19 | Benzyltriphenylphosphonium bromide | Glycerol | 1 | 5 | BTPPBr/Gly (1:5) | [46] |
| 20 | Benzyltriphenylphosphonium bromide | Ethylene glycol | 1 | 3 | BTPPBr/EG (1:3) | [46] |
| 21 | Menthol | Acetic acid | 1 | 1 | Me/AA (1:1) | [47] |
| 22 | Menthol | Lauric acid | 1 | 1 | Me/LA (1:1) | [47] |
| 23 | Menthol | Lidocaine | 2 | 1 | Men/Lic (2:1) | [48] |
| 24 | Menthol | Dimethoxyphenol | 2 | 1 | Men/DMP (2:1) | [49] |
| 25 | 1-tetradecanol | Menthol | 1 | 2 | TDec/Men (1:1) | [48] |
| 26 | Thymol | Menthol | 1 | 1 | Thy/Men (1:1) | [48] |
| 27 | Butylated hydroxytoluene | Menthol | 1 | 3 | BHT/LMe | [50] |
| 28 | Decanoic acid | Menthol | 1 | 2 | DecA/Men (1:2) | [48] |
| 29 | 1-napthol | Menthol | 1 | 2 | Nap/Men (1:2) | [48] |
| 30 | Menthol | P-toluenesulfonic acid monohydrate | 1 | 1 | Men/PTSA-M(1:1) | ** |
| 31 | Thymol | Coumarin | 1 | 1 | Thy/Cou (1:1) | [48] |
| 32 | Camphor | Thymol | 1 | 1 | Cam/Thy (1:1) | [51] |
| 33 | Thymol | Phenol | 1 | 2 | Thy/Phe (1:2) | ** |
| 34 | Thymol | Acetic Acid | 1 | 1 | Thy/AA (1:1) | ** |
| 35 | Thymol | P-toluenesulfonic acid monohydrate | 1 | 1 | Thy/PTSA-M (1:1) | ** |
| 36 | Potassium carbonate | Ethylene glycol | 1 | 6 | PC/EG (1:6) | [39] |
| 37 | N,N,N-trimethylglycine/Betaine | Phenyl acetic acid | 1 | 2 | Bet/PAA (1:2) | [52] |
| 38 | 1,2-decanediol | Thymol | 1 | 2 | 1,2 Dec/Thy (1:2) | [48] |
| 39 | Decanoic acid | Phenol | 2 | 1 | DecA/Phe (2:1) | ** |
| 40 | Decanoic acid | Lidocaine | 2 | 1 | DecA/Lic (2:1) | [48] |
| Components | Molar Ratio | Abbreviation | Stability |
|---|---|---|---|
| Thymol:phenol | 1:2 | Thy/Phe (1:2) | Homogeneous |
| Thymol:p-toluenesulfonic acid monohydrate | 1:2 | Thy/PTSA-M (1:1) | Unstable |
| Thymol:acetic acid | 1:1 | Thy/AA (1:1) | Unstable |
| Tetrabutylammonium bromide:sulfolane | 1:7 | TBABr/Sulf (1:7) | Homogeneous |
| Choline chloride:m-cresol (Ref) | 1:2 | ChCl/mCre (1:2) | Homogeneous |
| DES | Initial Weight (g) | Final Weight (g) | Solubility (%) |
|---|---|---|---|
| Thy/Phe (1:2) | 0.61 | 0 | 100 |
| TBABr/Sulf (1:7) | 0.60 | 0 | 100 |
| ChCl/mCre (1:2) | 0.61 | 0.61 | ~0 |
| Catalyst/Solvent, DES or IL | Hydrolysis Conditions | PET Conversion (%) | TPA Yield (%) | Ref |
|---|---|---|---|---|
| FeCl3∙6H2O/P-TSA | T = 100 °C t = 60 min | 98.0 | 88.6 | [32] |
| FeCl3∙6H2O/AA | T = 120 °C t = 180 min | 100 | 99.7 | [32] |
| FeCl3∙6H2O/MSA | T = 100 °C t = 30 min | 100 | 61.0 | [32] |
| [Bmim][Cl] and [HSO3-mim][HSO4] | T = 170 °C t = 240 min | 100 | 88.0 | [29] |
| Thy/Phe (1:2) | T = 130 °C t = 25 min | 100 (commercial) 100 (post-consumer) | 86.0 94.5 | This work |
| TBABr/Sulf (1:7) | T = 130 °C t = 40 min | 68.0 (commercial) 93.8 (post-consumer) | 86.6 94.1 | This work |
| ChCl/mCre (1:2) | T = 130 °C t = 40 min | 3.3 (commercial) 45.4 (post-consumer) | Non-quantitative 25.9 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Fattah, N.; Mat Salleh, M.Z.; Yuhana, N.Y.; Dambatta, Y.S.; Hadj-Kali, M.K. Polyethylene Terephthalate Hydrolysis Catalyzed by Deep Eutectic Solvents: COSMO-RS Screening and Experimental Validation. Catalysts 2025, 15, 1154. https://doi.org/10.3390/catal15121154
Abdul Fattah N, Mat Salleh MZ, Yuhana NY, Dambatta YS, Hadj-Kali MK. Polyethylene Terephthalate Hydrolysis Catalyzed by Deep Eutectic Solvents: COSMO-RS Screening and Experimental Validation. Catalysts. 2025; 15(12):1154. https://doi.org/10.3390/catal15121154
Chicago/Turabian StyleAbdul Fattah, Nurasyqin, Muhammad Zulhaziman Mat Salleh, Nor Yuliana Yuhana, Yusuf Suleiman Dambatta, and Mohamed Kamel Hadj-Kali. 2025. "Polyethylene Terephthalate Hydrolysis Catalyzed by Deep Eutectic Solvents: COSMO-RS Screening and Experimental Validation" Catalysts 15, no. 12: 1154. https://doi.org/10.3390/catal15121154
APA StyleAbdul Fattah, N., Mat Salleh, M. Z., Yuhana, N. Y., Dambatta, Y. S., & Hadj-Kali, M. K. (2025). Polyethylene Terephthalate Hydrolysis Catalyzed by Deep Eutectic Solvents: COSMO-RS Screening and Experimental Validation. Catalysts, 15(12), 1154. https://doi.org/10.3390/catal15121154







