Solar-Driven Photocatalytic Degradation of Clothianidin Using Green NiO-GO Composite
Abstract
1. Introduction
2. Results
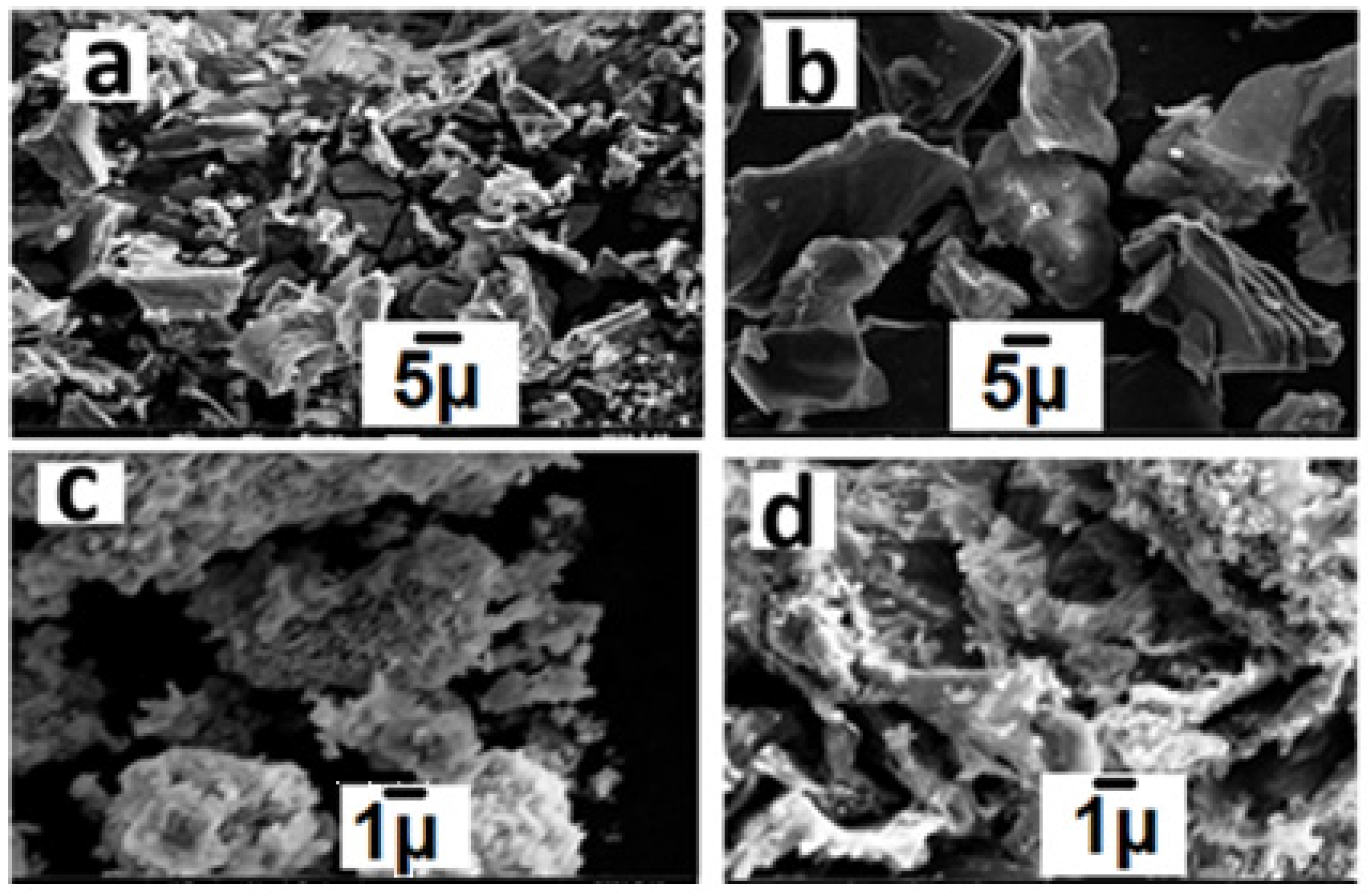
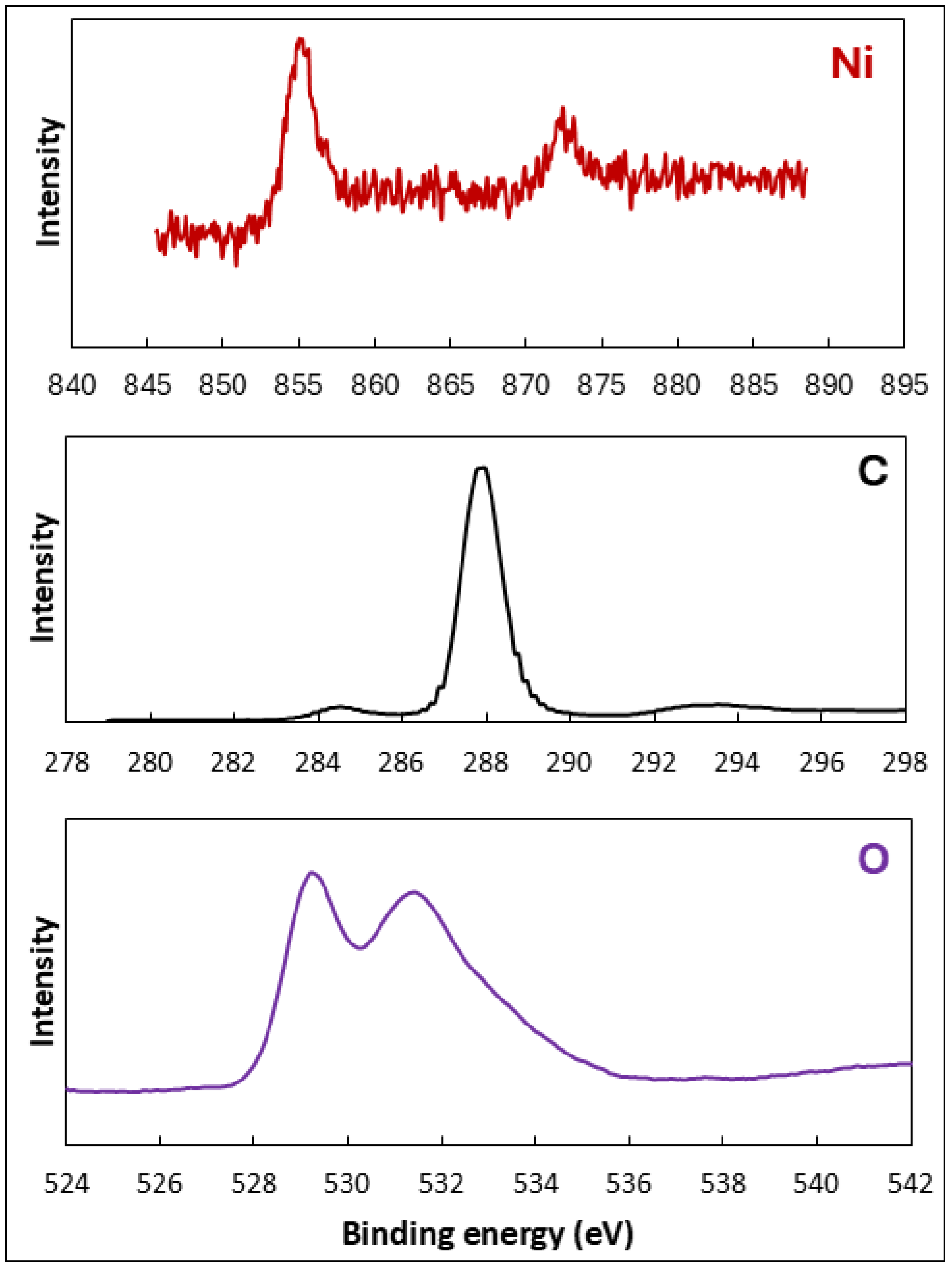
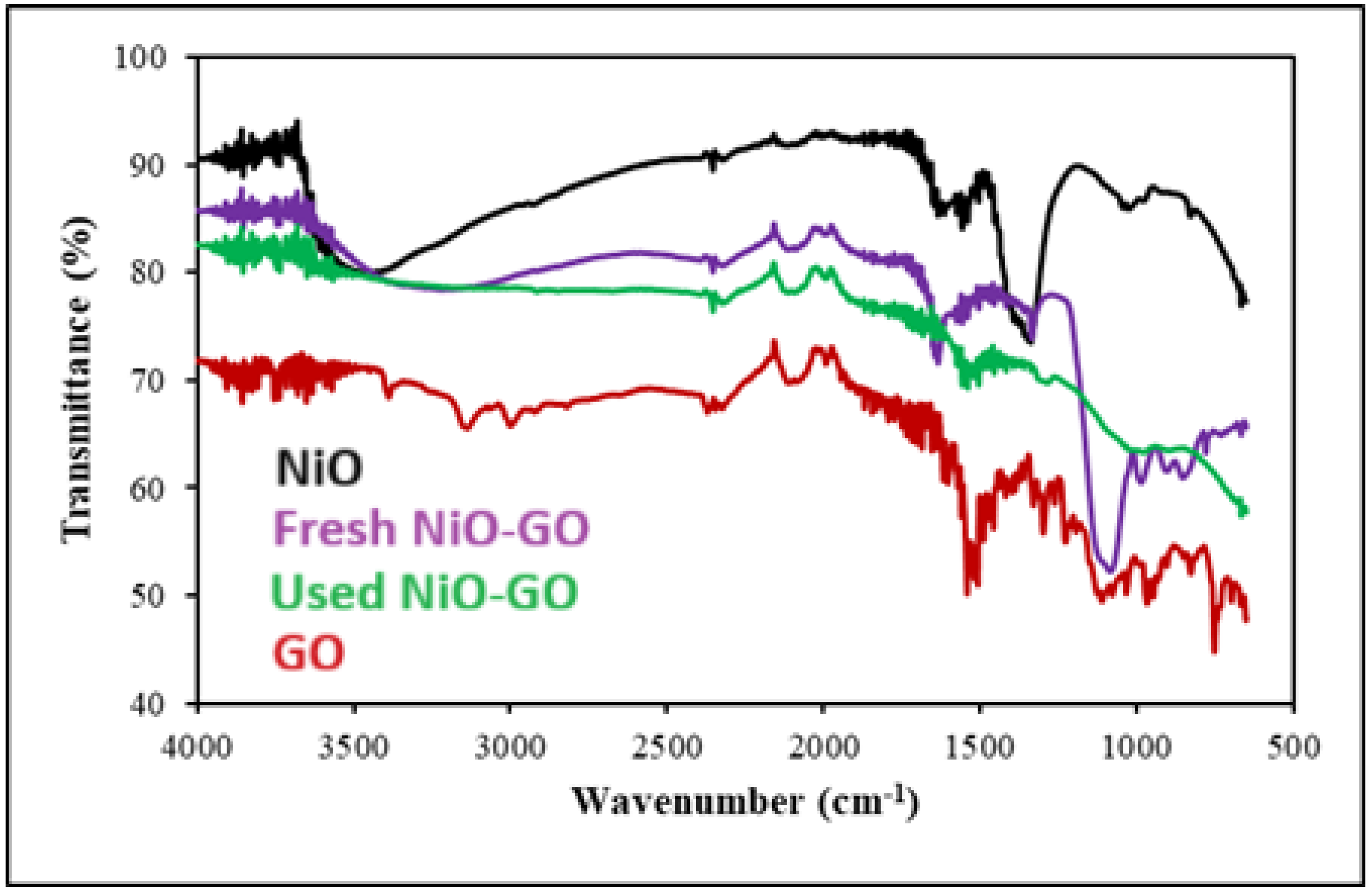
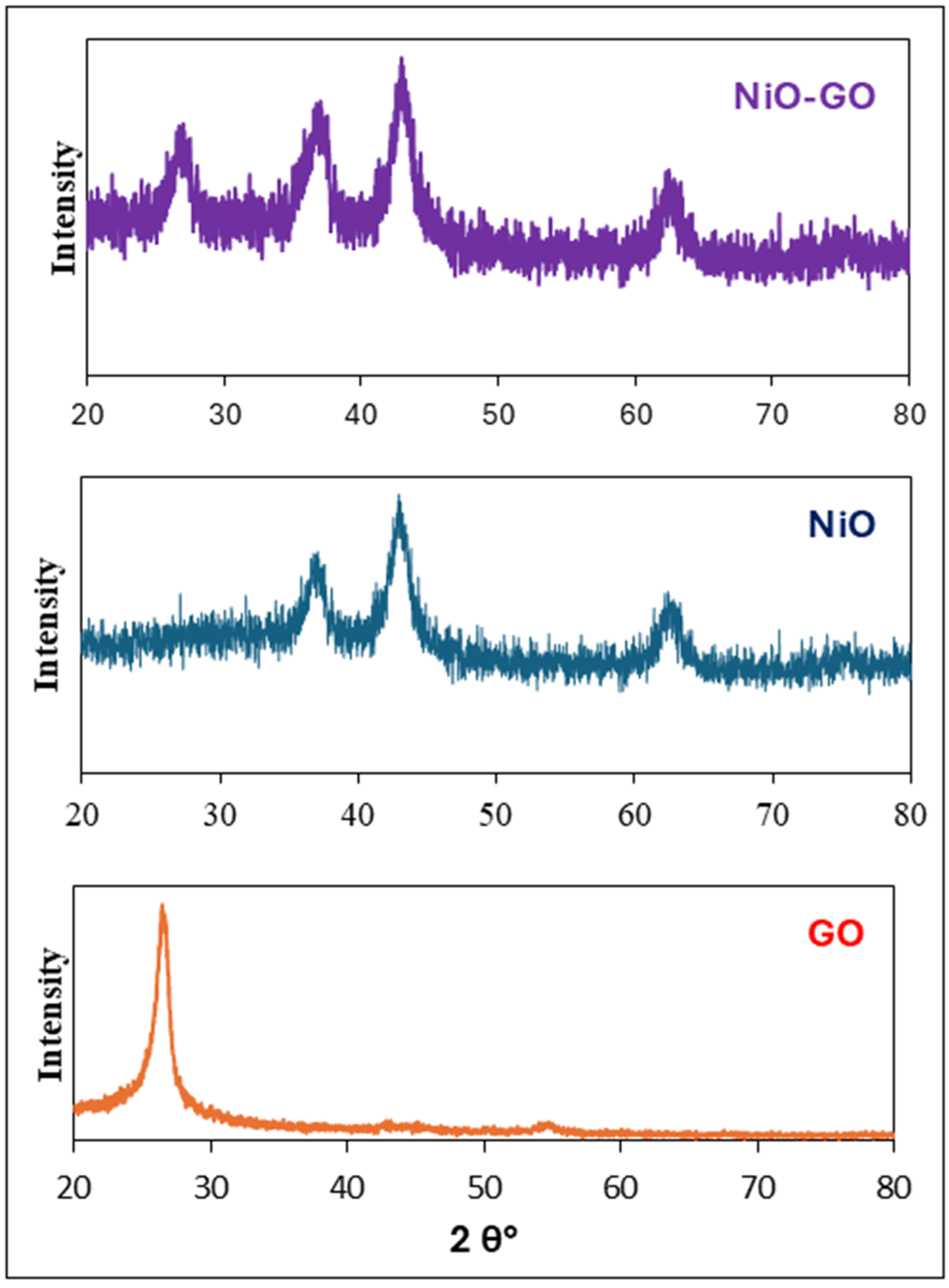
2.1. Characterization
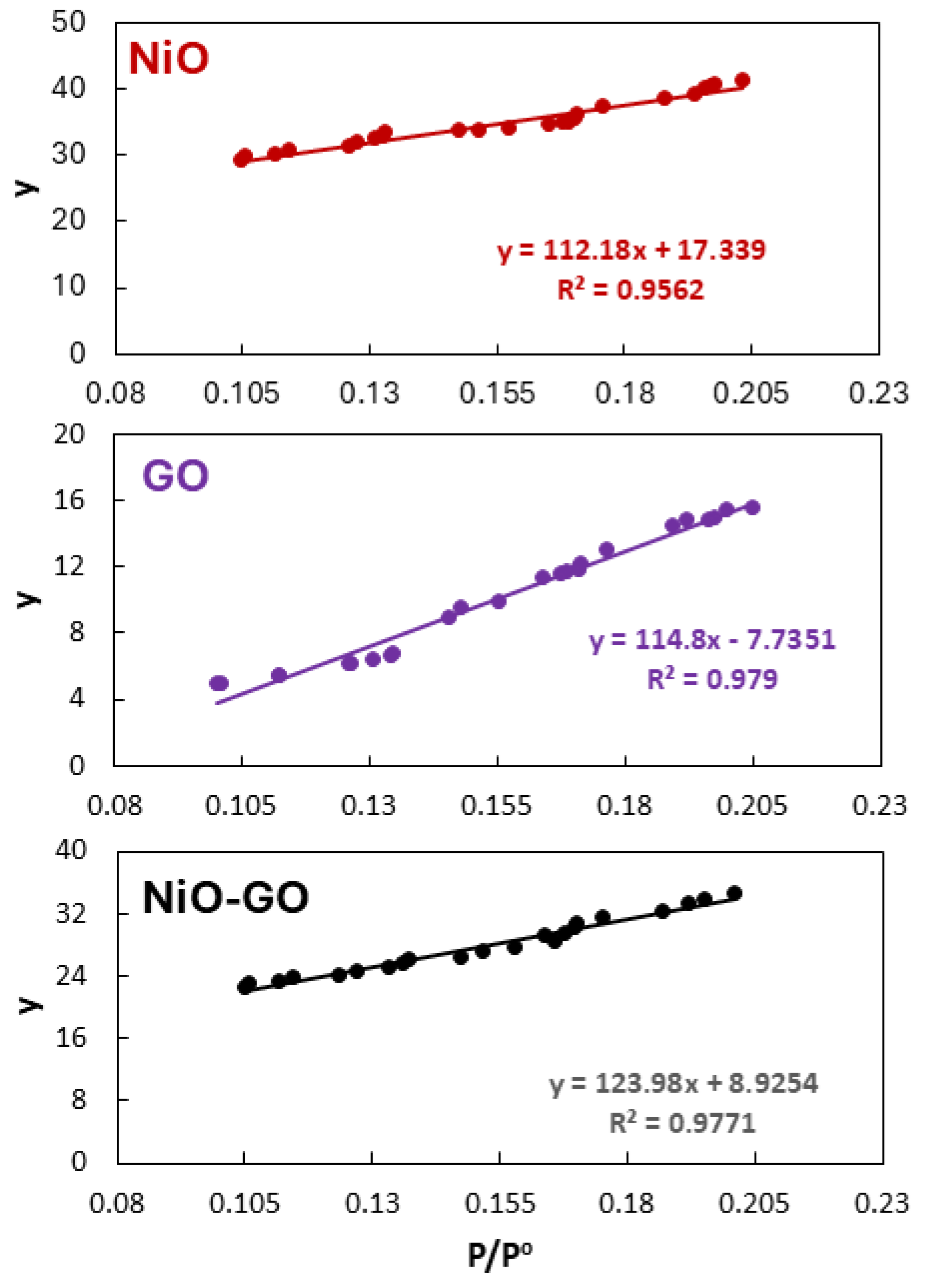
| Adsorbent | Mass (g) | Surface Area (m2/g) |
|---|---|---|
| NiO | 0.362 | 74.8 |
| GO | 0.198 | 164.3 |
| NiO-GO | 0.179 | 146.4 |
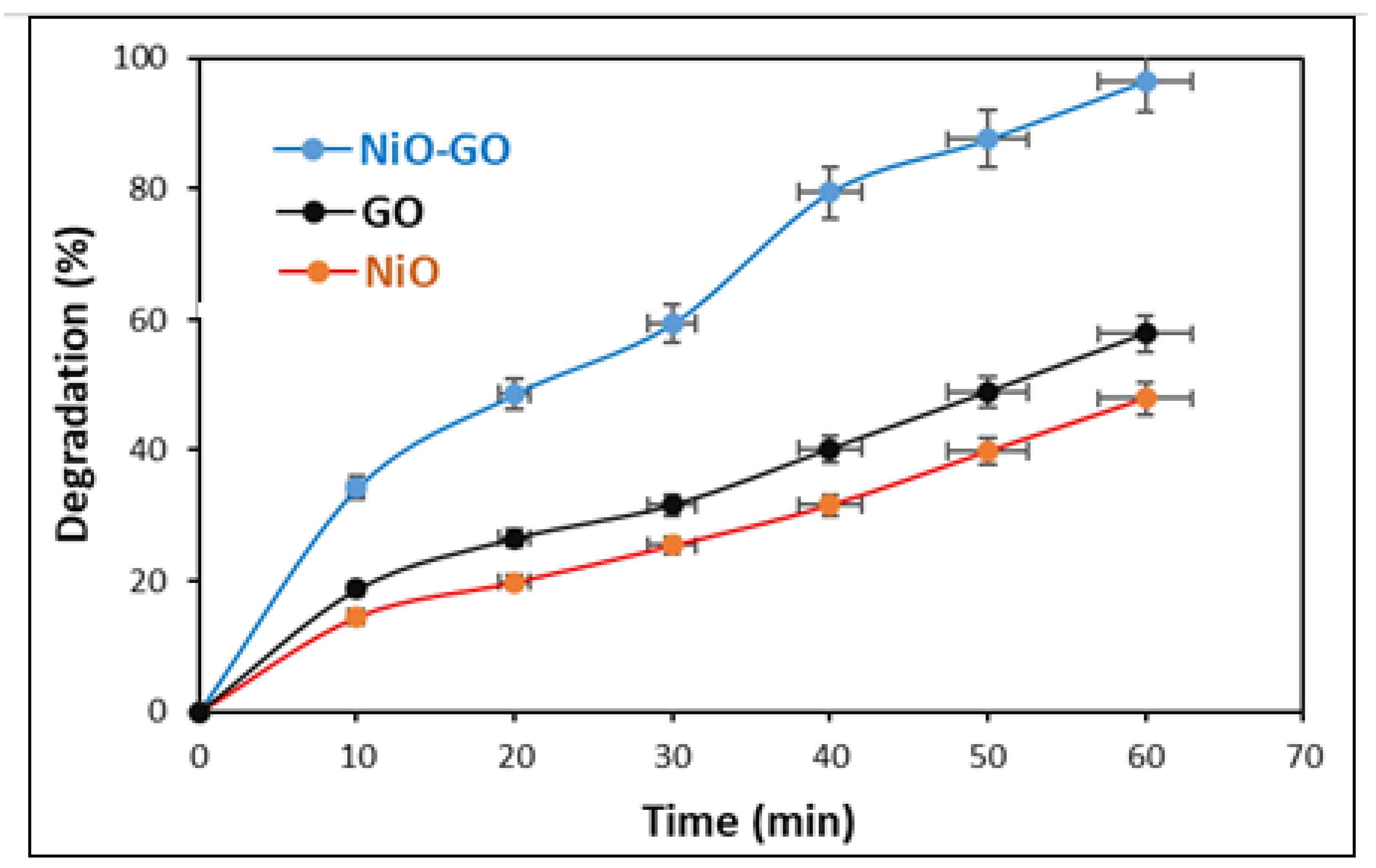
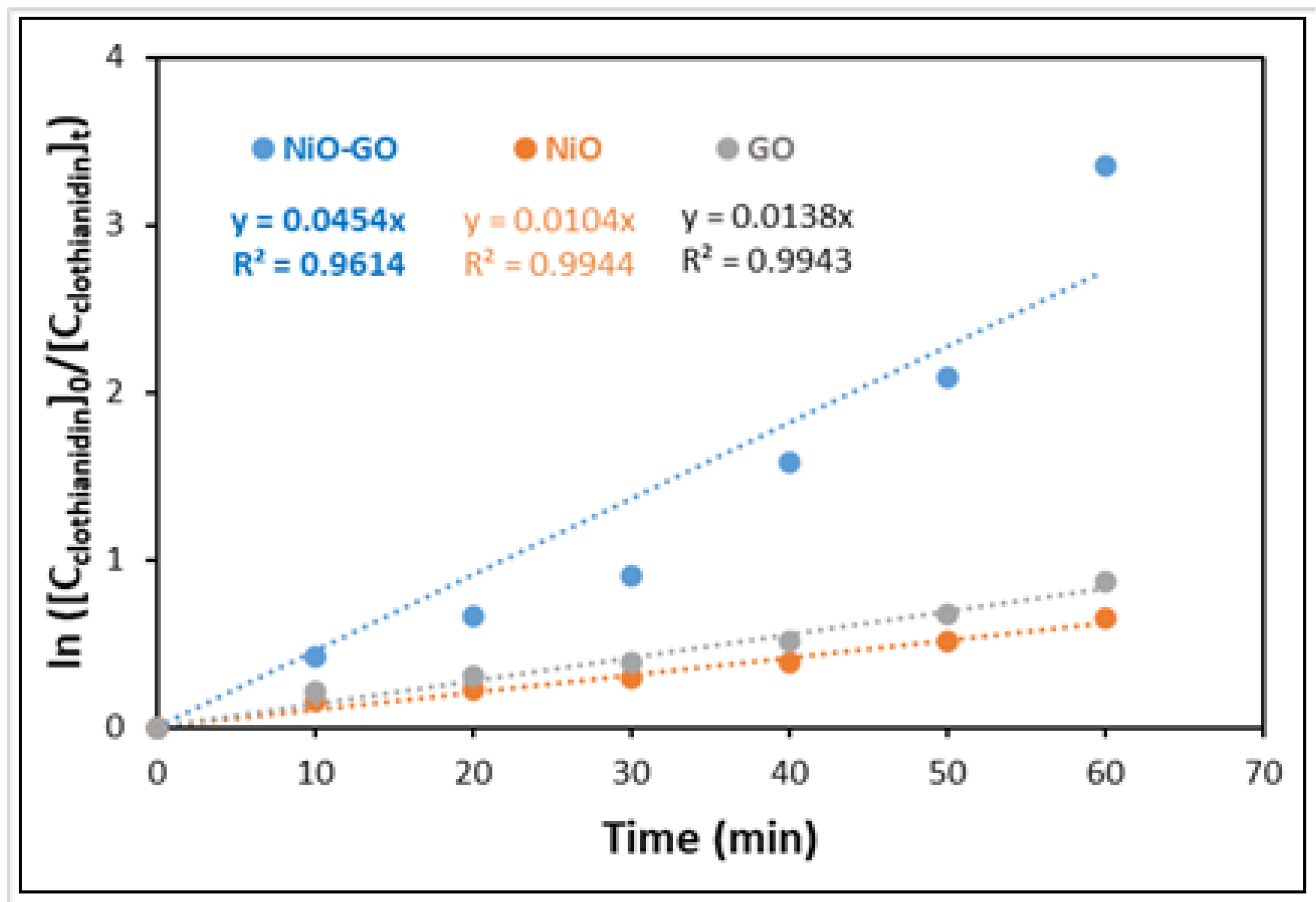
2.2. Evaluation of Photocatalytic Activity
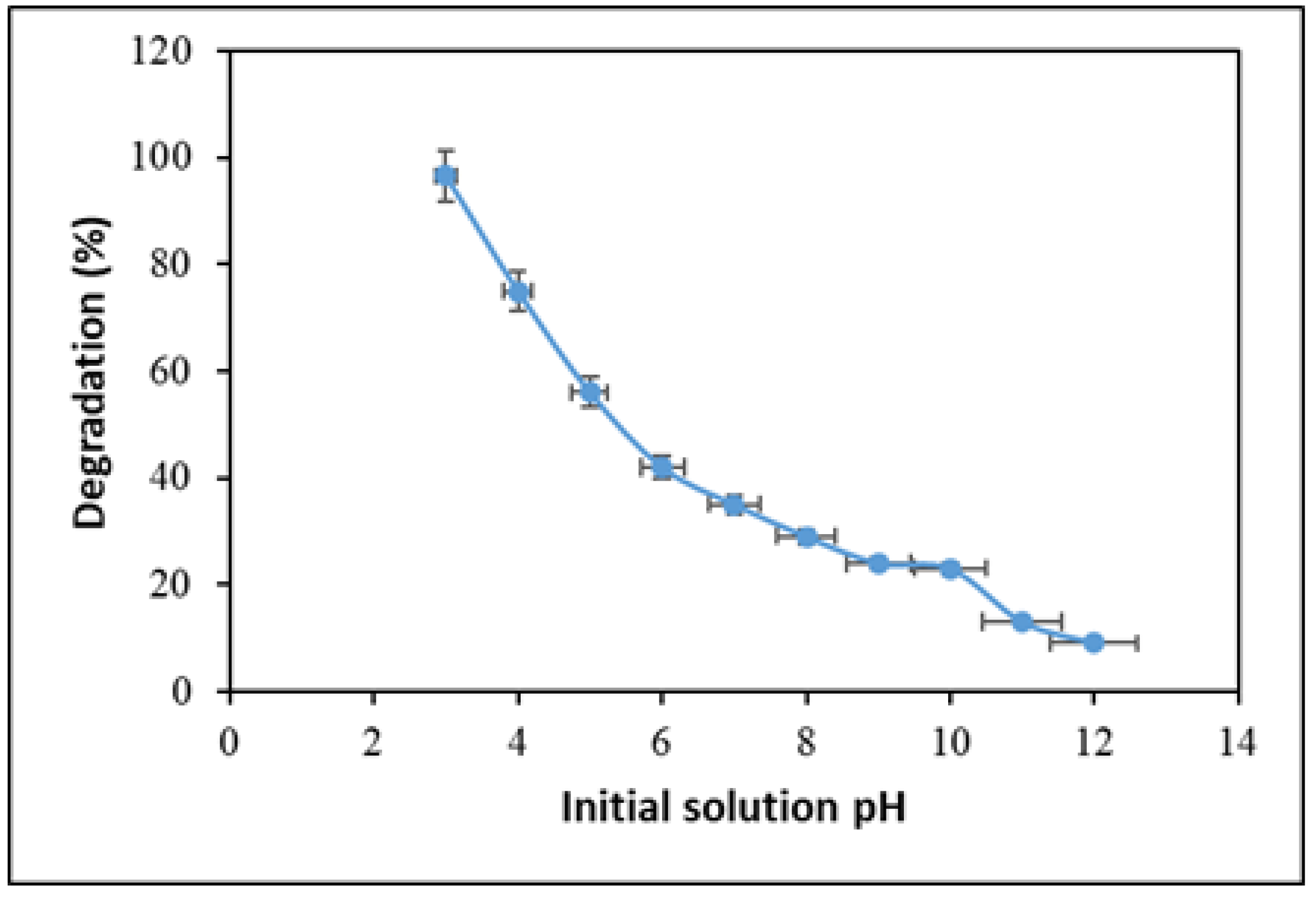
2.3. Effect of pH
2.4. Effect of Photocatalyst Loading
2.5. Effect of Initial Concentration
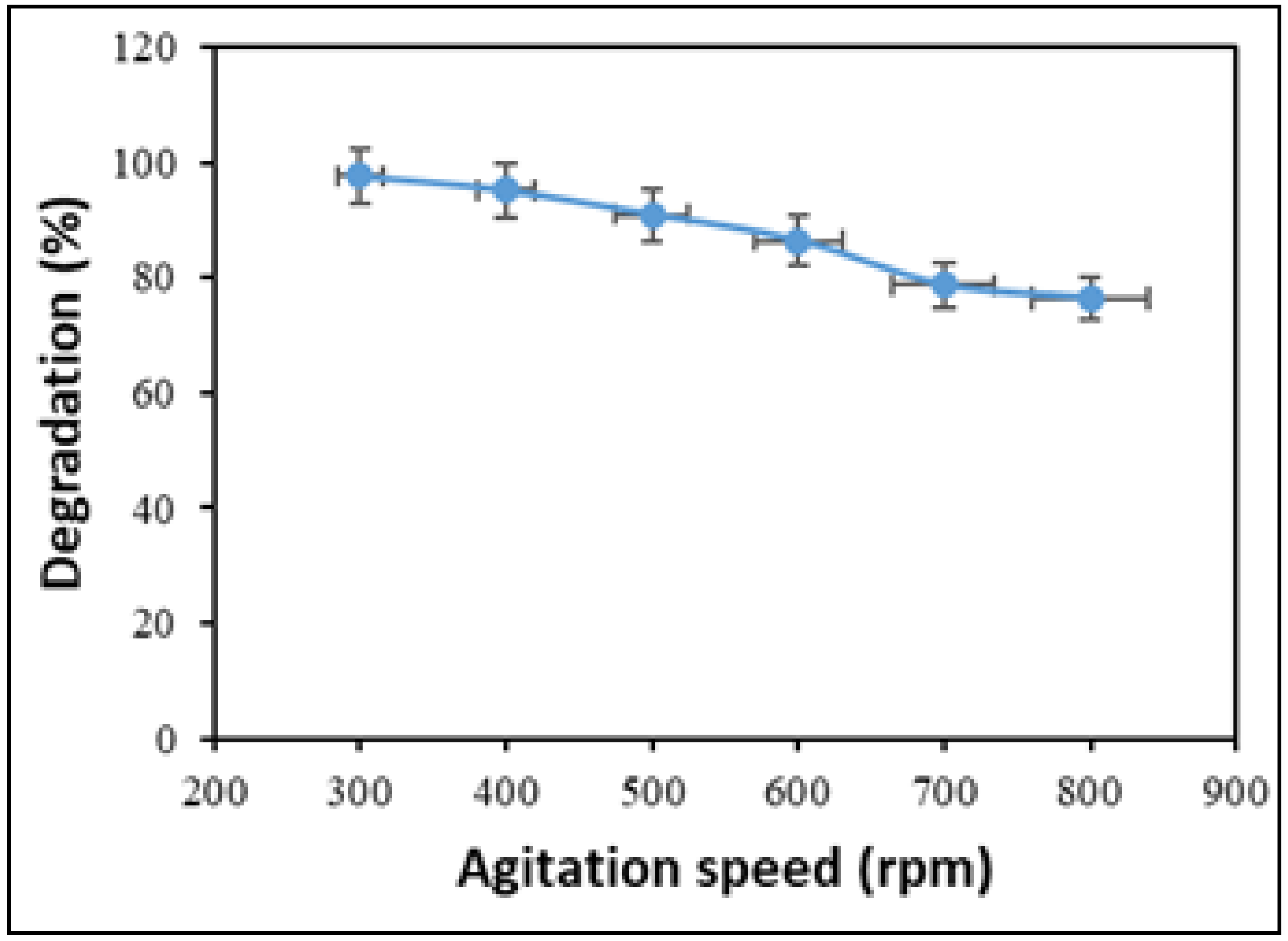
2.6. Effect of Agitation Speed
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instruments
3.3. Collection and Preparation of Pea Peels
3.4. Preparation of NiO Nanoparticles
3.5. Preparation of Graphene Oxide (GO)
3.6. Preparation of NiO-GO Nanocomposite
3.7. Photocatalytic Degradation Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, B.; Wang, X.; Khurshid, A.; Saleem, S.F. Environmental Governance, Green Finance, and Mitigation Technologies: Pathways to Carbon Neutrality in European Industrial Economies. Int. J. Environ. Sci. Technol. 2025, 22, 14899–14912. [Google Scholar] [CrossRef]
- Wang, X.; Su, H.; Liu, X. The Impact of Green Technological Innovation on Industrial Structural Optimization Under Dual-Carbon Targets: The Role of the Moderating Effect of Carbon Emission Efficiency. Sustainability 2025, 17, 6313. [Google Scholar] [CrossRef]
- Fang, Q.; Sun, Q.; Ge, J.; Wang, H.; Qi, J. Multidimensional Engineering of Nanoconfined Catalysis: Frontiers in Carbon-Based Energy Conversion and Utilization. Catalysts 2025, 15, 477. [Google Scholar] [CrossRef]
- Lu, S.; Shen, C.; Liu, M.; Zhang, J.; Fang, M.; Wang, F.; Li, Q.; Li, C.; Zhang, J. Research and Application Analysis on the Pollution Control of Tail Gas Emissions from CO2 Capture Absorber. Results Eng. 2025, 25, 103677. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Y.-J.; Cheng, Y.-J.; Qin, Z.-H.; Wang, G.-D.; Bai, Y.; Lin, Y.; Wang, H.; Sui, Y.; Hou, L.; et al. Capture of CO2 from N2 and CH4 over a Wide Temperature Range on a Robust MOF with Brønsted Acidic and Lewis Basic Dual Functional Sites. J. Mater. Chem. A Mater. 2025, 13, 10581–10589. [Google Scholar] [CrossRef]
- Su, H.; Zio, E.; Zhang, J.; Li, Z.; Wang, H.; Zhang, F.; Chi, L.; Fan, L.; Wang, W. A Systematic Method for the Analysis of Energy Supply Reliability in Complex Integrated Energy Systems Considering Uncertainties of Renewable Energies, Demands and Operations. J. Clean. Prod. 2020, 267, 122117. [Google Scholar] [CrossRef]
- Su, H.; Zio, E.; Zhang, J. A Method for the Risk Analysis of Energy Supply in Integrated Energy Systems. In Proceedings of the 30th European Safety and Reliability Conference and 15th Probabilistic Safety Assessment and Management Conference, Venice, Italy, 1–5 November 2020; Research Publishing Services: Singapore, 2020; pp. 1829–1835. [Google Scholar]
- Sha, X.; Zhu, Y.; Sha, X.; Guan, Z.; Wang, S. ZHPO-LightXBoost an Integrated Prediction Model Based on Small Samples for Pesticide Residues in Crops. Environ. Model. Softw. 2025, 188, 106440. [Google Scholar] [CrossRef]
- Das, R.; Vecitis, C.D.; Schulze, A.; Cao, B.; Ismail, A.F.; Lu, X.; Chen, J.; Ramakrishna, S. Recent Advances in Nanomaterials for Water Protection and Monitoring. Chem. Soc. Rev. 2017, 46, 6946–7020. [Google Scholar] [CrossRef]
- Xu, C.; Chen, L.; You, L.; Xu, Z.; Ren, L.-F.; Yew-Hoong Gin, K.; He, Y.; Kai, W. Occurrence, Impact Variables and Potential Risk of PPCPs and Pesticides in a Drinking Water Reservoir and Related Drinking Water Treatment Plants in the Yangtze Estuary. Environ. Sci. Process Impacts 2018, 20, 1030–1045. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Wang, J.; Pan, H.; Dou, M.; Teng, Y.; Fu, X.; Liu, Z.; Huang, X.; Wang, M. Bagasse-Based Porous Flower-like MoS2/Carbon Composites for Efficient Microwave Absorption. Carbon Lett. 2025, 35, 145–160. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the Status and Global Strategy for Neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Uneme, H. Chemistry of Clothianidin and Related Compounds. J. Agric. Food Chem. 2011, 59, 2932–2937. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R. Neonicotinoids—From Zero to Hero in Insecticide Chemistry. Pest. Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Yanai, S.; Takada, T.; Yoneda, N.; Omotehara, T.; Kubota, N.; Minami, K.; Yamamoto, A.; Mantani, Y.; Yokoyama, T.; et al. NOAEL-Dose of a Neonicotinoid Pesticide, Clothianidin, Acutely Induce Anxiety-Related Behavior with Human-Audible Vocalizations in Male Mice in a Novel Environment. Toxicol. Lett. 2018, 282, 57–63. [Google Scholar] [CrossRef]
- Mori, T.; Wang, J.; Tanaka, Y.; Nagai, K.; Kawagishi, H.; Hirai, H. Bioremediation of the Neonicotinoid Insecticide Clothianidin by the White-Rot Fungus Phanerochaete sordida. J. Hazard. Mater. 2017, 321, 586–590. [Google Scholar] [CrossRef]
- Goulson, D. REVIEW: An Overview of the Environmental Risks Posed by Neonicotinoid Insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Kim, B.M.; Park, J.-S.; Choi, J.-H.; Abd El-Aty, A.M.; Na, T.W.; Shim, J.-H. Residual Determination of Clothianidin and Its Metabolites in Three Minor Crops via Tandem Mass Spectrometry. Food Chem. 2012, 131, 1546–1551. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Hyne, R.V. Detection and Analysis of Neonicotinoids in River Waters—Development of a Passive Sampler for Three Commonly Used Insecticides. Chemosphere 2014, 99, 143–151. [Google Scholar] [CrossRef]
- de Perre, C.; Murphy, T.M.; Lydy, M.J. Fate and Effects of Clothianidin in Fields Using Conservation Practices. Environ. Toxicol. Chem. 2014, 34, 258–265. [Google Scholar] [CrossRef]
- Yang, K.; Li, C.; Zhu, Q.; Wang, H.; Qi, J. Rich Oxygen Vacancies in Bimetallic MnCo2O4.5 Spheres for Enhancing Lean Methane Catalytic Oxidation. Nanomaterials 2025, 15, 524. [Google Scholar] [CrossRef]
- Hou, B.; Zhao, Y.; Sun, W.; Lu, S.; Chen, S. Glycine Based Modification of Activated Carbons for VOCs Adsorption. Chem. Eng. J. Adv. 2021, 7, 100126. [Google Scholar] [CrossRef]
- Du, H.; Zhang, A.; Zhang, Q.; Sun, Y.; Zhu, H.; Wang, H.; Tan, Z.; Zhang, X.; Chen, G. Fabrication of Recoverable Bi2O2S/Bi5O7I/ZA Hydrogel Beads for Enhanced Photocatalytic Hg0 Removal in the Presence of H2O2. Sep. Purif. Technol. 2025, 359, 130597. [Google Scholar] [CrossRef]
- Jia, M.; Yang, Z.; Xu, H.; Song, P.; Xiong, W.; Cao, J.; Zhang, Y.; Xiang, Y.; Hu, J.; Zhou, C.; et al. Integrating N and F Co-Doped TiO2 Nanotubes with ZIF-8 as Photoelectrode for Enhanced Photo-Electrocatalytic Degradation of Sulfamethazine. Chem. Eng. J. 2020, 388, 124388. [Google Scholar] [CrossRef]
- Siddique, M.; Khan, N.M.; Saeed, M.; Ali, S.; Shah, Z. Green Synthesis of Cobalt Oxide Nanoparticles Using Citrus Medica Leaves Extract: Characterization and Photo-Catalytic Activity. Z. Für Phys. Chem. 2021, 235, 663–681. [Google Scholar] [CrossRef]
- Pervaiz, S.; Saeed, M.; Habila, M.A.; Jamal, M.A.; Haq, A.U.; Khan, I.; Javed, M. Synthesis and Photocatalytic Performance of G-C3N4-CuO-ZnO for Efficient Degradation of Crystal Violet (CV) and Methyl Orange (MO) Dyes under Sunlight Irradiation. Prog. React. Kinet. Mech. 2025, 50, e010. [Google Scholar] [CrossRef]
- Saeed, M.; Asghar, H.; Khan, I.; Akram, N.; Usman, M. Synthesis of TiO2-g-C3N4 for Efficient Photocatalytic Degradation of Congo Red Dye. Catal. Today 2025, 447, 115154. [Google Scholar] [CrossRef]
- You, Z.; Lu, D.; Kondamareddy, K.K.; Gu, W.; Su, Y.; Pan, J.; Yang, J.; Cheng, P.; Ho, W. Organic-Inorganic PVDF/Zn0.5Cd0.5S/Ti-NT Photocatalytic Membrane with Synergistic Effect of Heterojunction and Its Derived Multi-Element Excitation NO Purification. Sep. Purif. Technol. 2025, 361, 131293. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, A.; Liu, C.; Cheng, J.; Hu, M. Helical Polyether-Immobilized Chiral Aza-Bis(Oxazolines): Synthesis and Synergistic Effect on the Enantioselectivity of Zn-Catalyzed Henry Reaction. Eur. Polym. J. 2023, 194, 112160. [Google Scholar] [CrossRef]
- Guo, L.; Chen, W.; Wang, C.; Dong, B. Application of Electrochemically Assisted Synthesis of MOFs-Derived Phosphides as Catalyst for CH4-CO2 Reforming. Int. J. Electrochem. Sci. 2023, 18, 26–32. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Cheng, G.; Zhang, K.; Liu, X. Performance and Mechanism of Tetracycline Removal by Peroxymonosulfate-Assisted Double Z-Scheme LaFeO3/g-C3N4/ZnO Heterojunction under Visible Light Drive. Environ. Technol. Innov. 2025, 39, 104302. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, A.J.; Arshad, M.; Javed, M.S.; Ahmad, A.; Shah, S.S.A.; Khan, M.R.; Akram, S.; Zulfiqar; Ali, S.; et al. Charge Storage in Binder-Free 2D-Hexagonal CoMoO4 Nanosheets as a Redox Active Material for Pseudocapacitors. Ceram. Int. 2021, 47, 8659–8667. [Google Scholar] [CrossRef]
- Hussain, S.; Yang, X.; Aslam, M.K.; Shaheen, A.; Javed, M.S.; Aslam, N.; Aslam, B.; Liu, G.; Qiao, G. Robust TiN Nanoparticles Polysulfide Anchor for Li–S Storage and Diffusion Pathways Using First Principle Calculations. Chem. Eng. J. 2020, 391, 123595. [Google Scholar] [CrossRef]
- DeVree, B.T.; Steiner, L.M.; Głazowska, S.; Ruhnow, F.; Herburger, K.; Persson, S.; Mravec, J. Current and Future Advances in Fluorescence-Based Visualization of Plant Cell Wall Components and Cell Wall Biosynthetic Machineries. Biotechnol. Biofuels 2021, 14, 78. [Google Scholar] [CrossRef]
- Eray, E.; Candelario, V.M.; Boffa, V.; Safafar, H.; Østedgaard-Munck, D.N.; Zahrtmann, N.; Kadrispahic, H.; Jørgensen, M.K. A Roadmap for the Development and Applications of Silicon Carbide Membranes for Liquid Filtration: Recent Advancements, Challenges, and Perspectives. Chem. Eng. J. 2021, 414, 128826. [Google Scholar] [CrossRef]
- Feng, G.; Jiang, F.; Hu, Z.; Jiang, W.; Liu, J.; Zhang, Q.; Wu, Q.; Hu, Q.; Miao, L.; Cheng, S. A Novel Porous Egg-White (EW)/Titania Composite Photocatalytic Material for Efficient Photodegradation Applications. RSC Adv. 2020, 10, 8525–8529. [Google Scholar] [CrossRef]
- Pérez-Molina, Á.; Morales-Torres, S.; Maldonado-Hódar, F.; Pastrana-Martínez, L. Functionalized Graphene Derivatives and TiO2 for High Visible Light Photodegradation of Azo Dyes. Nanomaterials 2020, 10, 1106. [Google Scholar] [CrossRef]
- Liu, W.M.; Li, J.; Zhang, H.Y. Reduced Graphene Oxide Modified Zinc Oxide Composites Synergistic Photocatalytic Activity under Visible Light Irradiation. Optik 2020, 207, 163778. [Google Scholar] [CrossRef]
- Ashfaq, M.; Ali, A.; Abbood, N.K.; Panchal, S.; Akram, N.; Saeed, M.; Doshi, O.P.; Ali, F.; Muhammad, S.; Sameeh, M.Y.; et al. Enhanced Photocatalytic Activity of the Bi2O3-NiO Heterojunction for the Degradation of Methyl Orange under Irradiation of Sunlight. Water 2023, 15, 3182. [Google Scholar] [CrossRef]
- Saeed, M.; Albalawi, K.; Khan, I.; Akram, N.; Abd El-Rahim, I.H.A.; Alhag, S.K.; Ezzat Ahmed, A. Faiza Synthesis of P-n NiO-ZnO Heterojunction for Photodegradation of Crystal Violet Dye. Alex. Eng. J. 2023, 65, 561–574. [Google Scholar] [CrossRef]
- Zhao, W.; Yan, A.; Su, Z.; Huang, F.; Wang, Q.; Li, S.; Lu, S.; Wang, C.; Zhang, T.; Zhang, J.; et al. Multiobjective-Optimization MoS2/Cd-ZnIn2S4 /CdS Composites Prepared by In Situ Structure-Tailored Technique for High-Efficiency Hydrogen Generation. Small Struct. 2024, 5, 2300569. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, B.; Liang, J.; Liu, G.; Guo, Z.; Hao, H.; Li, W.; Shen, W. Defect-Engineered RGO−CoNi2S4 with Enhanced Electrochemical Performance for Asymmetric Supercapacitor. Trans. Nonferrous Met. Soc. China 2025, 35, 563–578. [Google Scholar] [CrossRef]
- Pham, C.V.; Repp, S.; Thomann, R.; Krueger, M.; Weber, S.; Erdem, E. Charge Transfer and Surface Defect Healing within ZnO Nanoparticle Decorated Graphene Hybrid Materials. Nanoscale 2016, 8, 9682–9687. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Bacsa, R.R.; Benyounes, A.; Axet, R.; Serp, P.; Silva, C.G.; Silva, A.M.T.; Faria, J.L. Synergistic Effect between Carbon Nanomaterials and ZnO for Photocatalytic Water Decontamination. J. Catal. 2015, 331, 172–180. [Google Scholar] [CrossRef]
- Sadhukhan, S.; Bhattacharyya, A.; Rana, D.; Ghosh, T.K.; Orasugh, J.T.; Khatua, S.; Acharya, K.; Chattopadhyay, D. Synthesis of RGO/NiO Nanocomposites Adopting a Green Approach and Its Photocatalytic and Antibacterial Properties. Mater. Chem. Phys. 2020, 247, 122906. [Google Scholar] [CrossRef]
- Al-Rawashdeh, N.A.F.; Allabadi, O.; Aljarrah, M.T. Photocatalytic Activity of Graphene Oxide/Zinc Oxide Nanocomposites with Embedded Metal Nanoparticles for the Degradation of Organic Dyes. ACS Omega 2020, 5, 28046–28055. [Google Scholar] [CrossRef] [PubMed]
- Pu, Q.; Zou, J.; Wang, J.; Lu, S.; Ning, P.; Huang, L.; Wang, Q. Systematic Study of Dynamic CO2 Adsorption on Activated Carbons Derived from Different Biomass. J. Alloys Compd. 2021, 887, 161406. [Google Scholar] [CrossRef]
- Ramu, A.G.; Umar, A.; Ibrahim, A.A.; Algadi, H.; Ibrahim, Y.S.A.; Wang, Y.; Hanafiah, M.M.; Shanmugam, P.; Choi, D. Synthesis of Porous 2D Layered Nickel Oxide-Reduced Graphene Oxide (NiO-RGO) Hybrid Composite for the Efficient Electrochemical Detection of Epinephrine in Biological Fluid. Environ. Res. 2021, 200, 111366. [Google Scholar] [CrossRef]
- Lamba, P.; Singh, P.; Singh, P.; Kumar, A.; Singh, P.; Bharti; Kumar, Y.; Gupta, M. Simple and Rapid Eco-Friendly Synthesis of NiO/RGO Nanocomposites Using Guava Leaf Extract and Their Physicochemical Characterization. Mater. Today Proc. 2022, 68, 2705–2714. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Dideikin, A.T.; Kirilenko, D.A.; Baidakova, M.V.; Shnitov, V.V.; Roth, F.; Konyakhin, S.V.; Besedina, N.A.; Pavlov, S.I.; Kuricyn, R.A.; et al. Facile Reduction of Graphene Oxide Suspensions and Films Using Glass Wafers. Sci. Rep. 2018, 8, 14154. [Google Scholar] [CrossRef]
- Song, R.; Zhang, H.; Lv, J. NiO Nanoparticle-Decorated Graphene Oxide Nanosheets Modified Glassy Carbon Electrode for Sensitive Electrochemical Detection of Pethidine. Int. J. Electrochem. Sci. 2023, 18, 100221. [Google Scholar] [CrossRef]
- Gao, X.; Dai, Y.; Zhang, C.; Zhang, Y.; Zong, W.; Zhang, W.; Chen, R.; Zhu, J.; Hu, X.; Wang, M.; et al. When It’s Heavier: Interfacial and Solvation Chemistry of Isotopes in Aqueous Electrolytes for Zn-ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202300608. [Google Scholar] [CrossRef]
- Khairnar, S.D.; Shrivastava, V.S. Facile Synthesis of Nickel Oxide Nanoparticles for the Degradation of Methylene Blue and Rhodamine B Dye: A Comparative Study. J. Taibah Univ. Sci. 2019, 13, 1108–1118. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, J.; Zhu, G.; Xie, F.; Tang, X.; Luo, X. Highly Efficient Preparation of Crystalline Yttrium Carbonate in Sodium Carbonate System: Formation and Growth Mechanism. J. Rare Earths 2025, 43, 1492–1501. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, J.; Zhu, G.; Xu, H.; Tang, X.; Luo, X. Flotation Behavior and Mechanism of Smithsonite under the System of Bidentate Ligand Sulfide Sodium Thiocyanate. Sep. Purif. Technol. 2024, 334, 126086. [Google Scholar] [CrossRef]
- Emran, M.Y.; Khalifa, H.; Gomaa, H.; Shenashen, M.A.; Akhtar, N.; Mekawy, M.; Faheem, A.; El-Safty, S.A. Hierarchical C-N Doped NiO with Dual-Head Echinop Flowers for Ultrasensitive Monitoring of Epinephrine in Human Blood Serum. Microchim. Acta 2017, 184, 4553–4562. [Google Scholar] [CrossRef]
- Ngo, Y.-L.T.; Hur, S.H. Low-Temperature NO2 Gas Sensor Fabricated with NiO and Reduced Graphene Oxide Hybrid Structure. Mater. Res. Bull. 2016, 84, 168–176. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Manjusha, V.; Shainy, F. Magnetically Retrievable Cysteine Modified Graphene Oxide@nickelferrite@titanium Dioxide Photocatalyst for the Effective Degradation of Chlorpyrifos from Aqueous Solutions. Environ. Technol. Innov. 2021, 23, 101633. [Google Scholar] [CrossRef]
- Anjum, F.; Asiri, A.M.; Khan, M.A.; Khan, M.I.; Khan, S.B.; Akhtar, K.; Bakhsh, E.M.; Alamry, K.A.; Alfifi, S.Y.; Chakraborty, S. Photo-Degradation, Thermodynamic and Kinetic Study of Carcinogenic Dyes via Zinc Oxide/Graphene Oxide Nanocomposites. J. Mater. Res. Technol. 2021, 15, 3171–3191. [Google Scholar] [CrossRef]
- Maruthupandy, M.; Muneeswaran, T.; Vennila, T.; Vaishali, C.V.; Anand, M.; Cho, W.-S.; Quero, F. Photocatalytic Efficiency of Graphene/Nickel Oxide Nanocomposites towards the Degradation of Anionic and Cationic Dye Molecules under Visible Light. J. Photochem. Photobiol. A Chem. 2022, 427, 113819. [Google Scholar] [CrossRef]
- Alsalmah, H.A.; Aadil, M.; Zulfiqar, S. Hydrothermal–Ultrasonication-Assisted Fabrication of Ce-Doped ZnO/g-C3N4 Heterojunctions for Enhanced Visible-Light Degradation of Dye and Drug Pollutants. Ceram. Int. 2025, 51, 46808–46818. [Google Scholar] [CrossRef]
- Mishra, S.R.; Gadore, V.; Singh, K.R.B.; Pandey, S.S.; Ahmaruzzaman, M. Developing In2S3 upon Modified MgTiO3 Anchored on Nitrogen-Doped CNT for Sustainable Sensing and Removal of Toxic Insecticide Clothianidin. Environ. Res. 2024, 259, 119435. [Google Scholar] [CrossRef]
- Duan, P.; Qi, Y.; Feng, S.; Peng, X.; Wang, W.; Yue, Y.; Shang, Y.; Li, Y.; Gao, B.; Xu, X. Enhanced Degradation of Clothianidin in Peroxymonosulfate/Catalyst System via Core-Shell FeMn @ N-C and Phosphate Surrounding. Appl. Catal. B 2020, 267, 118717. [Google Scholar] [CrossRef]
- de la Flor, M.P.; Camarillo, R.; Martínez, F.; Jiménez, C.; Quiles, R.; Rincón, J. Synthesis and Characterization of Bimetallic TiO2/CNT/Pd-Cu for Efficient Remediation of Endocrine Disruptors under Solar Light. J. Environ. Chem. Eng. 2022, 10, 107245. [Google Scholar] [CrossRef]
- Mohanta, D.; Ahmaruzzaman, M. A Novel Au-SnO2-RGO Ternary Nanoheterojunction Catalyst for UV-LED Induced Photocatalytic Degradation of Clothianidin: Identification of Reactive Intermediates, Degradation Pathway and in-Depth Mechanistic Insight. J. Hazard. Mater. 2020, 397, 122685. [Google Scholar] [CrossRef] [PubMed]
- de la Flor, M.P.; Camarillo, R.; Martínez, F.; Jiménez, C.; Quiles, R.; Rincón, J. Synthesis and Characterization of TiO2/CNT/Pd: An Effective Sunlight Photocatalyst for Neonicotinoids Degradation. J. Environ. Chem. Eng. 2021, 9, 106278. [Google Scholar] [CrossRef]
- Žabar, R.; Komel, T.; Fabjan, J.; Kralj, M.B.; Trebše, P. Photocatalytic Degradation with Immobilised TiO2 of Three Selected Neonicotinoid Insecticides: Imidacloprid, Thiamethoxam and Clothianidin. Chemosphere 2012, 89, 293–301. [Google Scholar] [CrossRef]
- Radwan, I.M.; Wang, C.; Kim, J.-H.; Wei, H.; Wang, D. Sorptive Removal of Neonicotinoid Pesticides by Nanobiochars: Efficiency, Kinetics, and Reusability. J. Hazard. Mater. 2025, 493, 138354. [Google Scholar] [CrossRef]
- Satheeshkumar, A.; Duraimurugan, R.; Velu, P.; Muthukumar, B.; Santhosh, S.; Devanesan, S.; AlSalhi, M.S.; Sathishkumar, K.; Rahman, P.K.; Rajasekar, A. Biodegradation Pathway and Mechanism of New Emerging Pollutants Neonicotinoids. J. Environ. Sci. 2026, 160, 197–205. [Google Scholar] [CrossRef]
- Rahimi, B.; Jafari, N.; Abdolahnejad, A.; Farrokhzadeh, H.; Ebrahimi, A. Application of Efficient Photocatalytic Process Using a Novel BiVO/TiO2-NaY Zeolite Composite for Removal of Acid Orange 10 Dye in Aqueous Solutions: Modeling by Response Surface Methodology (RSM). J. Environ. Chem. Eng. 2019, 7, 103253. [Google Scholar] [CrossRef]
- Bruckmann, F.S.; Schnorr, C.; Oviedo, L.R.; Knani, S.; Silva, L.F.O.; Silva, W.L.; Dotto, G.L.; Bohn Rhoden, C.R. Adsorption and Photocatalytic Degradation of Pesticides into Nanocomposites: A Review. Molecules 2022, 27, 6261. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, J.; Xiao, C.; Si, Z.; Tan, X. Solar Photocatalytic Degradation of Methylene Blue in Carbon-Doped TiO2 Nanoparticles Suspension. Sol. Energy 2008, 82, 706–713. [Google Scholar] [CrossRef]
- Pardeshi, S.K.; Patil, A.B. A Simple Route for Photocatalytic Degradation of Phenol in Aqueous Zinc Oxide Suspension Using Solar Energy. Sol. Energy 2008, 82, 700–705. [Google Scholar] [CrossRef]
- Hanafi, M.F.; Sapawe, N. Effect of Initial Concentration on the Photocatalytic Degradation of Remazol Brilliant Blue Dye Using Nickel Catalyst. Mater. Today Proc. 2020, 31, 318–320. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Ben Smida, Y.; Onwudiwe, D.C. Visible Light-Driven Photocatalytic Reduction of Monovalent Silver Using a Composite of Ni3Bi2S2 and O-Doped GC3N4. Results Eng. 2022, 15, 100540. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Gobile, N.; Oyewo, O.A.; Makgato, S.S. Photocatalytic Reduction of Hexavalent Chromium Using Zn2SnO4–ZnO Modified g-C3N4 Composite. Results Eng. 2023, 20, 101521. [Google Scholar] [CrossRef]
- Alwared, A.I.; Sulaiman, F.A.; Raad, H.; Al-Musawi, T.J.; Mohammed, N.A. Ability of FeNi3/SiO2/TiO2 Nanocomposite to Degrade Amoxicillin in Wastewater Samples in Solar Light-Driven Processes. S. Afr. J. Bot. 2023, 153, 195–202. [Google Scholar] [CrossRef]
- Ghasemi, A.H.; Zoqi, M.J.; Zanganeh Ranjbar, P. Enhanced Photocatalytic Degradation of Methylene Blue Using a Novel Counter-Rotating Disc Reactor. Front. Chem. 2024, 12, 1335180. [Google Scholar] [CrossRef]
- Haleem, A.; Shafiq, A.; Chen, S.-Q.; Nazar, M. A Comprehensive Review on Adsorption, Photocatalytic and Chemical Degradation of Dyes and Nitro-Compounds over Different Kinds of Porous and Composite Materials. Molecules 2023, 28, 1081. [Google Scholar] [CrossRef]
- Ezhilarasi, A.A.; Vijaya, J.J.; Kaviyarasu, K.; Zhang, X.; Kennedy, L.J. Green Synthesis of Nickel Oxide Nanoparticles Using Solanum Trilobatum Extract for Cytotoxicity, Antibacterial and Photocatalytic Studies. Surf. Interfaces 2020, 20, 100553. [Google Scholar] [CrossRef]
- Arun, L.; Karthikeyan, C.; Philip, D.; Unni, C. Optical, Magnetic, Electrical, and Chemo-Catalytic Properties of Bio-Synthesized CuO/NiO Nanocomposites. J. Phys. Chem. Solids 2020, 136, 109155. [Google Scholar] [CrossRef]
- Firisa, S.G.; Muleta, G.G.; Yimer, A.A. Synthesis of Nickel Oxide Nanoparticles and Copper-Doped Nickel Oxide Nanocomposites Using Phytolacca dodecandra L’Herit Leaf Extract and Evaluation of Its Antioxidant and Photocatalytic Activities. ACS Omega 2022, 7, 44720–44732. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.M.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H.; et al. Metal Nanoparticles Fabricated by Green Chemistry Using Natural Extracts: Biosynthesis, Mechanisms, and Applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef]


| No | Catalyst/Adsorbent | Conditions | Efficiency | Reference |
|---|---|---|---|---|
| 1 | In2S3/MgTiO3/TiO2@N-CNT | Conc. 10 mg/L, Catalyst 0.3 g, 23 W LED/H2O2 | 98% in 18 min | [62] |
| 2 | FeMn@N–C | Conc. 5 mg/L, Catalyst 0.1 g, Visible light/PMS | 99% in 90 min | [63] |
| 3 | TiO2/CNT/Pd–Cu | Conc. 3 mg/L, Catalyst 0.1 g, 450 W Xe arc lamp/H2O2 | 100% in 180 min | [64] |
| 4 | Au–SnO2-rGO | Conc. 1 mg/L, Catalyst 0.003 g, 30 W UV-LED lamp | 97% in 120 min | [65] |
| 5 | TiO2/CNT/Pd | Conc. 5 mg/L, Catalyst 0.1 g, 450 W Xe arc lamp/H2O2 | 85% in 180 min | [66] |
| 6 | TiO2 on glass | Conce. 100 mg/L, Catalyst 10 mg, UVA (315–400 nm) | 14% in 120 min | [67] |
| 7 | Biochars | Conc. 100 ng/L, Sorbent 0.5 g/L | 100% | [68] |
| 8 | Stutzerimonas sp. SA1 and Pseudomonas sp. SA3 | Conc. 100 mg/L, Bacteria culture 3.2 × 102 | 87% | [69] |
| 9 | NiO-GO | Conc. 20 mg/L, Catalyst 0.05 g, Natural sunlight | 96% in 60 min | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haq, A.u.; Hussein, R.K.; Panchal, S.; Saeed, M.; Abubakar, H.M.; Alrub, S.A. Solar-Driven Photocatalytic Degradation of Clothianidin Using Green NiO-GO Composite. Catalysts 2025, 15, 1078. https://doi.org/10.3390/catal15111078
Haq Au, Hussein RK, Panchal S, Saeed M, Abubakar HM, Alrub SA. Solar-Driven Photocatalytic Degradation of Clothianidin Using Green NiO-GO Composite. Catalysts. 2025; 15(11):1078. https://doi.org/10.3390/catal15111078
Chicago/Turabian StyleHaq, Atta ul, Rageh K. Hussein, Sandeep Panchal, Muhammad Saeed, Hafiz Muhammad Abubakar, and Sharif Abu Alrub. 2025. "Solar-Driven Photocatalytic Degradation of Clothianidin Using Green NiO-GO Composite" Catalysts 15, no. 11: 1078. https://doi.org/10.3390/catal15111078
APA StyleHaq, A. u., Hussein, R. K., Panchal, S., Saeed, M., Abubakar, H. M., & Alrub, S. A. (2025). Solar-Driven Photocatalytic Degradation of Clothianidin Using Green NiO-GO Composite. Catalysts, 15(11), 1078. https://doi.org/10.3390/catal15111078








