Abstract
A novel metal oxide catalyst, PdOx/CuO-ST, was prepared by calcining a Pd-embedded CuBTC precursor and compared with a PdOx/CuO-SG catalyst synthesized via a sol–gel method. Characterization results indicated that in both catalysts, Pd species were incorporated into the CuO lattice, forming synergistic interactions that lowered the reduction temperature of CuO. The PdOx/CuO-ST catalyst exhibited superior catalytic activity in the oxidative carbonylation of phenol to diphenyl carbonate when calcined at low temperature, which was attributed to well-dispersed Cu atoms and enhanced Pd–Cu integration. However, high-temperature calcination led to catalyst sintering and the formation of surface-adsorbed oxygen species, which reacted with PdO on CuO to generate inactive PdxCuyO phases, thereby reducing the active Pd2+ content and degrading catalytic performance. Under optimized reaction conditions (100 °C, 7 h, Pd/phenol molar ratio = 1/425, and 6.6 MPa), the PdOx/CuO-ST catalyst achieved a maximum phenol conversion of 79.5% and a diphenyl carbonate selectivity of 84.5%. Stability tests revealed that although the catalyst structure remained intact, deactivation occurred due to Pd leaching and the reduction in active PdO to metallic Pd0.
1. Introduction
Diphenyl carbonate (DPC) is an important chemical intermediate in the synthesis of polycarbonates (PCs) [1]. PCs are widely used in automotive manufacturing, electronic appliances, optical lenses, and medical devices due to their excellent toughness, impact resistance, and comprehensive performance. The rapid development of these industries has led to an increase in demand for PCs, consequently driving a significant rise in the demand for DPC. Therefore, developing an efficient and environmentally friendly synthesis processes for DPC is of great importance and holds broad market prospects.
Currently, the industrial production of DPC primarily relies on the phosgene route. However, phosgene is highly toxic and corrosive, which not only increases production costs but also poses significant threats to operators’ health and environmental pollution risks. To overcome the drawbacks of the phosgene method, researchers have explored several non-phosgene synthesis routes. Among these, a process route that involves synthesizing ethylene carbonate from carbon dioxide and ethylene oxide, followed by transesterification to produce dimethyl carbonate, and finally transesterification with phenol to prepare DPC has gained considerable attention [2,3,4]. However, this route involves multiple transesterification and purification steps, making the process complex and increasing both production costs and operational difficulties. Thus, achieving the one-step synthesis of DPC has become a research hotspot. Common one-step synthesis methods for DPC include the carbonylation and oxidative carbonylation of phenol. Direct synthesis of DPC from phenol and CO2 faces significant challenges due to the high thermodynamic stability of CO2 [5,6]. In contrast, the oxidative carbonylation of phenol using phenol, CO, and O2 as raw materials is a more promising approach. This method overcomes the disadvantages of phenol carbonylation and offers advantages such as low raw material cost and lower toxicity [7].
The oxidative carbonylation of phenol (OCP) is a complex multi-step electron transfer reaction [3], typically requiring palladium-based catalysts and the synergistic action of various co-catalysts to proceed efficiently [4,5,6,7,8,9,10,11,12]. During the reaction, the Pd(II) active center is reduced to catalytically inactive Pd(0) [8]. The role of the co-catalyst(s) is to re-oxidize Pd(0) back to Pd(II), enabling the Pd-catalyzed OCP reaction to function as a true catalytic cycle [3]. Heterogeneous catalysts are generally preferred for this reaction to facilitate separation and recovery processes. The supports in these heterogeneous catalysts not only carry the active sites but often assist in promoting the reaction. Xue et al. [13] prepared a Pd-Cu-O/SiO2 catalyst using a water-in-oil microemulsion method for the OCP reaction. They found that the Cu promoter interacted with Pd to form a CuPdO2 complex oxide, which facilitated the regeneration of deactivated Pd(0) by enabling electron transfer to Cu(II), resulting in higher catalytic activity. Yuan et al. [14] and Fu et al. [15] prepared Pd catalysts supported on CeO2 nanotubes (Pd/CeO2-NT). Because the CeO2-NT support possessed excellent reducibility and oxygen storage capacity, the catalytic performance was significantly superior to that of Pd catalysts supported on zero-dimensional CeO2 nanoparticles, achieving a maximum phenol conversion of 67.7% and a DPC selectivity of 93.3%. Zhou et al. [16] prepared a Pd catalyst supported on a Ce-Zr solid solution (Pd/CexZr1−xO2). Doping with Zr increased the surface Ce3+ concentration and oxygen content, which benefited the re-oxidation of Pd species, leading to excellent catalytic performance with a DPC yield reaching 54.0%. Yang et al. [17] discovered that a Pd catalyst supported on Pb-doped manganese oxide octahedral molecular sieves (Pd@Pb-OMS-2) significantly enhanced the activity for the OCP reaction without requiring additional homogeneous co-catalysts. The Mn(IV)/Mn(III) redox couple in the support promoted the re-oxidation of Pd, thus replacing the role of traditional transition metal co-catalysts. They further introduced Ni and Cu as second metals into Pd@Pb-OMS-2 [18]. The results indicated that the second metal not only improved the catalytic activity but also enhanced the long-term stability of the reaction.
Although the aforementioned supported Pd catalysts exhibit high activity in the OCP reaction, their stability is generally poor. Yin et al. [12] investigated the deactivation mechanisms of various supported Pd catalysts and identified Pd leaching, reduction and agglomeration of Pd(II), and coke deposition as the main causes of catalyst deactivation in the OCP reaction. To improve stability, Peng et al. [19] prepared a Pd catalyst supported on a SiO2-based composite oxide aerogel. The three-dimensional network structure and large specific surface area of the aerogel facilitated high dispersion of Pd(II) and strong metal–support interaction (SMSI), thereby improving reaction stability to some extent. Liu et al. [20] used F127 as a stabilizer and chitosan as a carbon and nitrogen source to synthesize N-doped carbon material, which was then used to support Pd, resulting in a Pd/NCF catalyst. Compared to the Pd/NC catalyst prepared without F127, Pd/NCF showed higher stability in the OCP reaction. F127 enhanced the hydrogen bonding with chitosan, anchoring the nitrogen in free amino groups, increasing the nitrogen content of the carbon material, providing ample deposition sites for Pd nanoparticles, and promoting metal dispersion. Meanwhile, the enhanced SMSI suppressed the leaching and agglomeration of Pd nanoparticles, and pyridinic nitrogen helped stabilize Pd2+ and reduce its loss. However, the stability of this catalyst remained unsatisfactory, as its activity decreased significantly upon the third reuse.
Metal–organic frameworks (MOFs) are a class of crystalline porous materials characterized by high surface areas, abundant active sites, and ease of chemical modification. They show broad application in catalysis, separation, gas storage, and molecular recognition [21]. Furthermore, MOF-derived catalysts have also demonstrated excellent performance in various reactions. Gao et al. [22] used Mn/Co-MOFs to derive MnCo spinel oxide catalysts with large specific surface area, abundant octahedral metal ions, and weak metal–oxygen bonds, which exhibited high activity, good reusability, and excellent thermal stability in the catalytic oxidation of toluene. Sayidov et al. [23] employed a MOF-derived titanomagnetite catalyst for the low-temperature reverse water-gas shift reaction, achieving 97% CO selectivity and CO2 conversion close to equilibrium, offering a new route for CO2 hydrogenation to produce chemicals and fuels. He et al. [24] used Ce-UiO-66 to derive a CeO2 support for loading Ru, achieving high conversion and methane selectivity in the CO2 methanation reaction. Phan et al. [25] found that a TiO2 catalyst derived from Ti-MOF (MIL-125) could efficiently convert plastic waste and exhibited excellent impurity removal capabilities in aqueous environments. These results demonstrate the potential of MOF-derived catalysts in the energy and environmental fields and offer important references for subsequent studies.
CuBTC is a typical Cu-based MOF widely used in catalysis [26,27]. Its extremely high specific surface area and highly ordered framework structure can provide a confined space that inhabits the growth and agglomeration of guest species, favoring the high dispersion of active components. Moreover, the Cu2+ within its structure can serve as co-catalysts for the Pd-catalyzed OCP reaction. Yang et al. [28] prepared a MOF-supported Pd catalyst (Pd/CuBTC) using a two-step method of solvothermal synthesis followed by thermal reflux and applied it to the OCP reaction. The results showed that the Pd species had good dispersion on the CuBTC support, with an average particle size of 2.3 nm. Under optimized reaction conditions (100 °C for 8 h), this catalyst achieved a phenol conversion of 53.8% and a selectivity for DPC of 71.3%. However, the catalyst exhibited poor recyclability, mainly attributed to the leaching and possible aggregation of Pd species during the reaction.
In view of this, this study synthesized CuBTC and then converted it into CuO through calcination. Using this CuO as a support, palladium oxide (PdOx) was loaded onto its surface to prepare a PdOx/CuO catalyst. This approach not only has the potential to retain the high specific surface area and structural orderliness of CuBTC but also strengthens the interaction between the metal and the support. It helps improve the anchoring ability of Pd species and their resistance to redox deactivation, thereby prolonging the catalyst’s lifetime.
2. Results and Discussions
2.1. Characterization of Pd/CuBTC and PdOx/CuO
The structural properties of CuBTC and Pd/CuBTC were initially characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM), as shown in Figure 1. The as-synthesized CuBTC (Figure 1A,B) exhibited well-defined octahedral crystals with particle sizes ranging from 15 to 25 μm. After Pd loading, the octahedral architecture of the MOF remained largely intact, with the primary observable change being a roughened surface (Figure 1C), indicating good structural integrity and stability of this Cu-MOF. However, thermogravimetric (TG) analysis of CuBTC before and after Pd loading (Figure S1) revealed that both the onset and conclusion temperatures of the MOF decomposition stage shifted significantly earlier for Pd/CuBTC, suggesting that the incorporation of Pd influenced the framework structure.
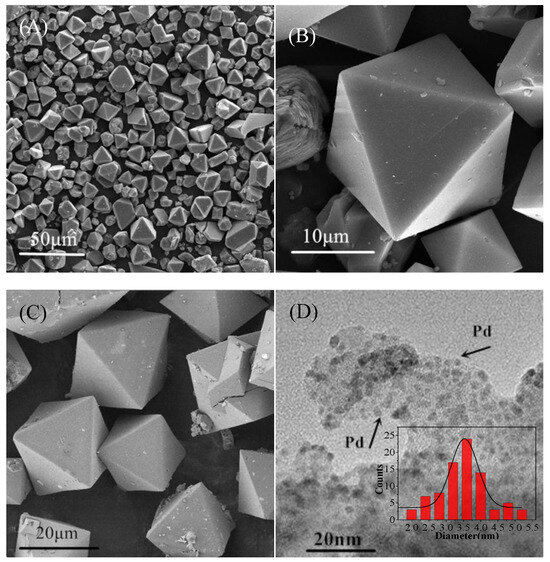
Figure 1.
SEM images of CuBTC (A,B), Pd/CuBTC (C);TEM images of: Pd/CuBTC (D); size distribution of Pd nanoparticles (inset).
TEM images of Pd/CuBTC (Figure 1D) showed uniformly dispersed Pd nanoparticles adhered to the framework surface, with an average size of 3.5 ± 0.5 nm. Furthermore, N2 adsorption–desorption isotherms and pore size distribution analysis of Pd/CuBTC (Figure S2) indicated that a considerable number of Pd nanoparticles had accessed and were accommodated within the MOF pore channels. The ingress of Pd nanoparticles likely plugged smaller pores while leaving larger ones accessible, resulting in a decrease in the specific surface area from 1395.0 m2/g to 466.0 m2/g. Energy-dispersive X-ray spectroscopy (EDS) mapping (Figure S3) of Pd/CuBTC clearly demonstrated a uniform three-dimensional distribution of ultrafine Pd nanoparticles throughout the internal cavities of CuBTC, a benefit attributed to the innate porous structure of the MOF. This observation aligns with literature reports that the crystalline porous structures of MOFs can significantly enhance the distribution of metal nanoparticles (MNPs) with narrow size distributions and prevent their aggregation [29,30].
X-ray diffraction (XRD) was used for phase identification and crystalline structure analysis. The XRD patterns in Figure S4 show that the major diffraction peaks of the as-synthesized CuBTC are consistent with the simulated pattern (calculated from crystallographic data), except for an additional peak at 5.7°, which is associated with variations in the degree of hydration [31]. This confirms the successful synthesis of a pure, highly crystalline CuBTC material. Meanwhile, the absence of obvious Pd diffraction peaks in Pd/CuBTC confirms the highly dispersed state of Pd [32], consistent with the TEM results.
SEM images of PdOx/CuO-ST revealed that the majority of CuO particles retained the stereochemical architecture of the parent MOF, albeit with most corners lost. The shrunken surface and interior texture filled with substantial cavities (Figure 2E) may result from the escape and diffusion of CO2 or H2O generated during the decomposition of organic ligands upon calcination. In contrast to the visible palladium species anchored on the MOF template, no distinct Pd nanoparticles were observable on the CuO derived from the annealed Cu-organic framework (Figure 2F,H). However, the measured lattice fringe spacing of 0.30 nm in the high-resolution TEM image corresponds well to the (100) plane of PdO, demonstrating that Pd species may exist within the CuO in a different form. Notably, the bulk of the PdOx/CuO-SG sample appeared disorganized and was primarily formed by the agglomeration of CuO particles.

Figure 2.
SEM images of PdOx/CuO-ST (A,B), PdOx/CuO-SG (C,D), TEM images of PdOx/CuO-ST (E,F,G), PdOx/CuO-SG (H). G is an enlarged view of the yellow-squared area in F.
Nitrogen physisorption tests on the two catalysts further revealed that the specific surface areas of PdOx/CuO-ST and PdOx/CuO-SG are 7.4 m2/g and 5.4 m2/g, respectively. Critically, the specific surface area of PdOx/CuO-ST is substantially lower than that of its precursor, Pd/CuBTC (1395.0 m2/g). This significant reduction stems from two primary factors: (1) the collapse of the MOF during high-temperature calcination, which eliminates pore channels through organic ligand decomposition, and (2) the agglomeration of CuO particles into disordered structures, which disrupts the high porosity inherent to the original MOF.
XRD patterns in Figure 3 were used to determine the chemical state of Pd species in PdOx/CuO. A pure CuO sample was purposefully synthesized for comparison using a method similar to PdOx/CuO-ST, except without embedding Pd nanoparticles onto CuBTC prior to calcination. All diffraction peaks could be indexed to the monoclinic phase of copper oxide (JCPDS card no. 48-1548). No impurity peaks were detected, indicating the complete thermal conversion of the Cu-MOF precursor into CuO. Moreover, no peaks attributable to palladium or its oxides were observed, likely due to the high dispersion of Pd species on the CuO support [33]. The average crystallite sizes and textural properties of CuO and PdOx/CuO are summarized in Table 1. The results indicate that the crystallite size of PdOx/CuO is smaller than that of pure CuO, suggesting that Pd species inhibit the growth of CuO crystals. Furthermore, the main diffraction peaks corresponding to the (002) and (111) crystal planes for PdOx/CuO shifted to lower angles compared to pure CuO. Since the ionic radius of Pd2+ (0.86 Å) is larger than that of Cu2+ (0.73 Å), lattice expansion and a consequent shift of diffraction peaks to lower angles would occur if Pd2+ substitutes for Cu2+ in the lattice [34]. This indicates that Pd was incorporated into the CuO lattice, distorting its structural integrity.
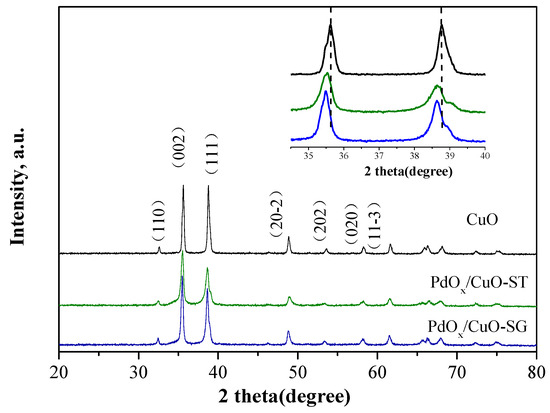
Figure 3.
XRD patterns of PdOx/CuO-ST, PdOx/CuO-SG and pure CuO.

Table 1.
The average crystallite sizes and internal lattice parameters of CuO and PdOx/CuO.
To further elucidate the distribution of Pd species within the MOF-derived CuO support, high-angle annular dark-field (HAADF) imaging was performed on PdOx/CuO-ST (Figure 4). The results clearly confirm the successful doping of Pd2+ ions into the entire CuO matrix, exhibiting a homogeneous and non-agglomerated state.

Figure 4.
HAADF images of PdOx/CuO-ST.
2.2. Catalytic Activity Evaluation
The effects of calcination temperature and time on the activity of the PdOx/CuO-ST catalyst for the oxidative carbonylation of phenol were investigated, and the results are shown in Figure 5. Based on the TG curve of Pd/CuBTC (Figure S1), a calcination temperature above 300 °C was selected to ensure complete formation of the CuO substrate.
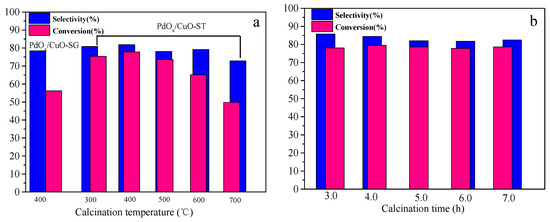
Figure 5.
Catalytic performance of PdOx/CuO-SG and PdOx/CuO-ST for OCP reaction; (a) Catalysts calcined for 6 h at different temperature; (b) Catalysts calcined at 400 °C for different times. Reaction condition: 7 h, 100 °C, O2 0.6 MPa, CO 6.6 MPa, Pd/Phenol = 1/425 (molar ratio). Co-catalysts amount: Pd/Cu(OAc)2/TBAB/H2BQ = 1/5/40/40 in molar ratio.
The activity of the PdOx/CuO-ST catalyst showed only slight variations when the calcination temperature was between 300 and 500 °C. However, phenol conversion decreased drastically when the temperature exceeded 500 °C. In contrast, the calcination time had an insignificant effect on the catalytic performance. The optimal activity for PdOx/CuO-ST was achieved under calcination conditions of 400 °C for 4 h, yielding a phenol conversion of 79.5% and a DPC selectivity of 84.5%.
For comparison, the PdOx/CuO-SG catalyst was also evaluated, demonstrating inferior catalytic performance (56.2% phenol conversion, 78.4% DPC selectivity) compared to PdOx/CuO-ST.
To better demonstrate the advantages of the PdOx/CuO-ST catalyst, a comparison of its activity with other catalysts used in oxidative carbonylation of phenol, as reported in the literature, was carried out. As can be seen from the results presented in Table S1, relatively high phenol conversion and DPC selectivity were achieved by this catalyst. Moreover, when the overall DPC yield was taken into consideration, the best performance was demonstrated by it. Thus, great potential is held by this catalyst, and it is worthy of further study.
2.3. Analysis of the Impact of Calcination Temperature on PdOx/CuO Catalysts
The influence of calcination temperature on the catalytic behavior of PdOx/CuO-ST is significant. X-ray photoelectron spectroscopy (XPS) and H2 temperature-programmed reduction (TPR) were employed to investigate the underlying reasons.
Figure 6 shows the XPS spectra of O1s and Pd3d for the PdOx/CuO-SG and PdOx/CuO-ST catalysts. The O1s spectrum can be deconvoluted into two components: the Oα species at a lower binding energy (~530 eV), ascribed to lattice oxygen, and the Oβ species at a higher binding energy (531–532 eV), assigned to surface-adsorbed oxygen [35,36]. The proportion of Oα species, quantified from the peak area, is listed in Table 2. The distribution of oxygen species was influenced by the calcination temperature, and the trend in the Oα ratio for PdOx/CuO-ST correlated with its catalytic activity. Previous studies report that oxygen species, particularly lattice oxygen, participate in the regeneration of reduced Pd during the catalytic redox cycle, as surface-adsorbed oxygen is under-coordinated and less readily reduced than lattice oxygen [5]. Therefore, lattice oxygen likely plays a key role in enhancing the activity of the PdOx/CuO catalyst.
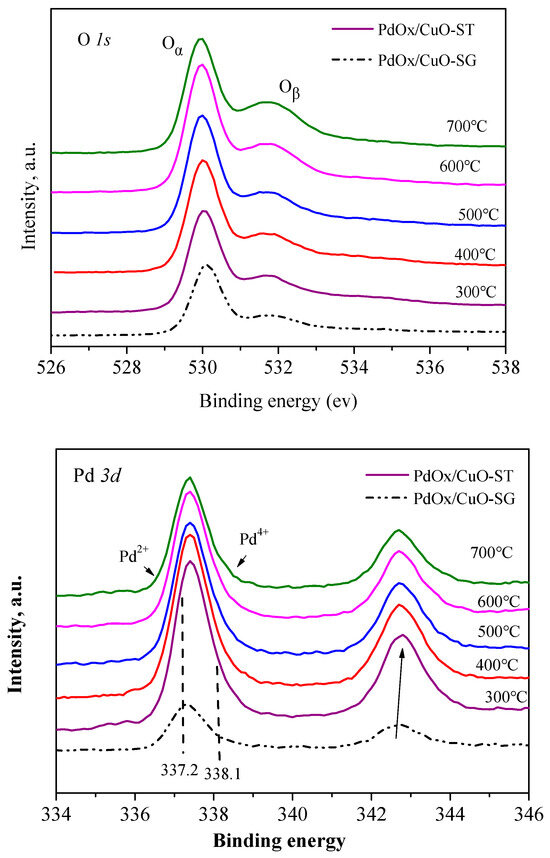
Figure 6.
XPS spectra of O 1s and Pd 3d for PdOx/CuO-SG and PdOx/CuO-ST under different calcination temperature.

Table 2.
Content and Proportion Distribution of O and Pd Species on PdOx/CuO Surface.
The Pd 3d5/2 peaks could be resolved into two components: one at approximately 337.2 eV, characteristic of Pd2+ in PdO, and another at 338.1 eV, assigned to Pd4+ in PdO2 [37]. The relative amount of PdO is presented in Table 2. For PdOx/CuO-ST, the Pd2+/Pd4+ ratio changed little with calcination temperature, but the total surface Pd content decreased as the temperature increased. This suggests that the calcination temperature alters the surface Pd content, thereby affecting catalytic activity. The surface Pd content decreased rapidly, and catalytic performance declined significantly when the calcination temperature exceeded 500 °C. Additionally, the Pd atomic content on the PdOx/CuO-SG surface was only 0.98%, less than half of that on PdOx/CuO-ST (2.27%). This can be attributed to the more dispersed Cu atoms in PdOx/CuO-ST derived from the regular MOF structure, facilitating the combination of Pd with exposed Cu sites.
Interestingly, the Pd atomic content on the surface of PdOx/CuO-SG (0.98%) was lower than that of PdOx/CuO-ST calcined at 700 °C (1.46%), yet the former exhibited superior catalytic performance (PdOx/CuO-ST at 700 °C achieved 49.8% phenol conversion and 72.9% DPC selectivity). Higher proportions of Oα and Pd2+ species are responsible for this better performance. The lower Pd 3d binding energies for PdOx/CuO-SG indicate a higher Pd2+ content compared to PdOx/CuO-ST. Furthermore, a higher Oα/Oβ ratio is favorable for activity. Guimarães et al. concluded that PdO2 forms at the PdO interface where adsorbed oxygen from co-catalysts is readily incorporated [38]. Thus, it is speculated that more Pd2+ associated with Oβ promotes Pd4+ formation, leading to weaker activity for PdOx/CuO-ST derived from pyrolysis at 700 °C. Consequently, the higher Oα content in PdOx/CuO-SG implies a weaker interaction between surface oxygen and PdO, which benefits the reaction since Pd2+ is the active species for the oxidative carbonylation of phenol.
The redox properties of the catalysts were characterized by H2-TPR (Figure 7 and Figure 8, Table S2). Pure CuO shows a reduction peak around 250 °C, which can be deconvoluted into two peaks (α and β) corresponding to the reduction of Cu2+ to Cu0 [39]. The reduction temperature of CuO decreased upon Pd addition due to the H2 spillover effect [40]. The reduction signal for PdOx/CuO-ST was stronger than for PdOx/CuO-SG, primarily due to a more stable interaction between Pd and the well-dispersed Cu atoms originating from the regular MOF template. A new reduction peak (γ) appeared after Pd loading, assigned to the reduction of a PdxCuyO species [39]. The intensity of this peak increased with calcination temperature. However, the poor catalytic performance of PdOx/CuO-ST above 500 °C indicates the detrimental effect of excessive PdxCuyO formation on the oxidative carbonylation reaction.
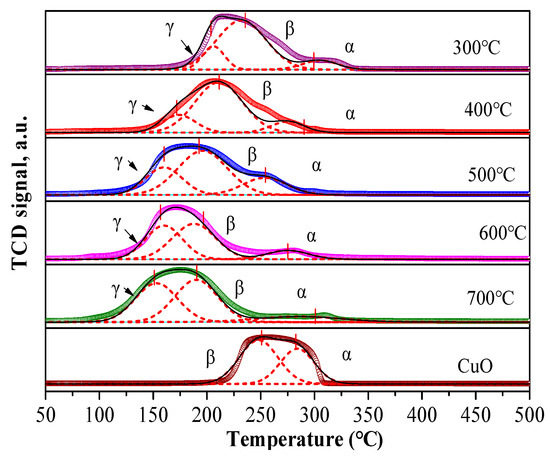
Figure 7.
H2-TPR profiles of PdOx/CuO-ST prepared at different temperature. The dashed lines in the figure represent the results of peak deconvolution fitting.
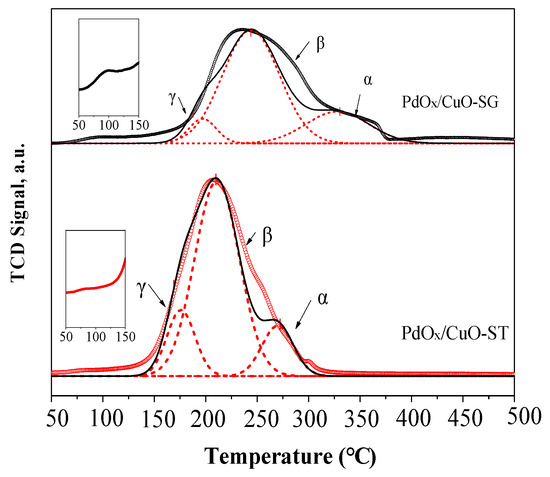
Figure 8.
H2-TPR profiles of PdOx/CuO-ST and PdOx/CuO-SG. The dashed lines in the figure represent the results of peak deconvolution fitting.
A reduction peak observed around 98 °C, corresponding to the reduction of PdO [41], was evident for PdOx/CuO-SG but not for PdOx/CuO-ST. Conversely, the γ peak (PdxCuyO) was more pronounced in PdOx/CuO-ST. These results indicate that PdxCuyO forms more readily in the PdOx/CuO-ST catalyst, confirming that Pd atoms interact more strongly with the MOF-derived CuO.
2.4. Catalyst Stability
The stability of the PdOx/CuO-ST catalyst was investigated. The recovered catalyst (denoted Re-PdOx/CuO-ST) was reused in the reaction. The catalytic performance results are shown in Figure 9. The activity decreased significantly after the first use. SEM, TEM, XPS, and ICP analyses were conducted to study the deactivation.
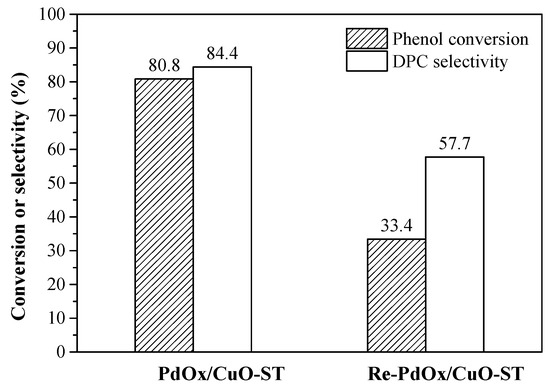
Figure 9.
Reusable performance of PdOx/CuO-ST catalysts. Reaction conditions: 7 h, 100 °C, O2 0.6 Mpa, CO 6.6 Mpa, phenol 25.5 mmol, 20 mL CH2Cl2, Pd 0.057 mmol, 4.0 g 4A molecular sieve, TBAB/H2BQ/Cu(OAc)2/Pd = 40/40/5/1 in molar ratio.
SEM images of the fresh and recovered catalyst (Figure 10) show that most of the Re-PdOx/CuO-ST particles retained an octahedral morphology, indicating that the catalyst maintained its structural integrity during the reaction.
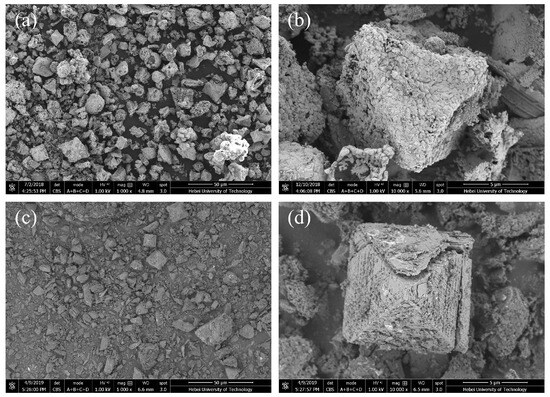
Figure 10.
SEM images of PdOx/CuO-ST before (a,b) and after reaction (c,d).
XPS analysis of the fresh and spent catalysts revealed changes in the states of O and Pd (Figure 11). The proportion of lattice oxygen (Oα) decreased significantly after reaction, while adsorbed oxygen (Oβ) changed only slightly, consistent with the direct involvement of lattice oxygen in the redox cycle. A new peak appeared in the Pd 3d5/2 region at 335.0–335.5 eV for the spent catalyst, corresponding to Pd0 species [42,43], and the proportion of Pd2+ species decreased significantly, indicating the reduction of active PdO to Pd0 during the reaction.
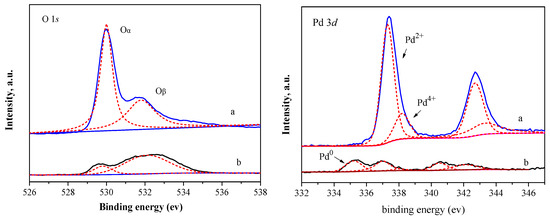
Figure 11.
XPS spectra of O 1s and Pd 3d of PdOx/CuO-ST before (a) and after (b) reaction. The dashed lines in the figure represent the results of peak deconvolution fitting.
ICP results showed that the Pd content in the catalyst decreased from 5.02% to 2.78% after reaction, confirming that Pd leaching is another significant cause of deactivation.
3. Materials and Methods
3.1. Catalyst Preparation
CuBTC was synthesized by solvothermal method according to the reported literature [18]. The detailed procedure was conducted as follows: 1.05 g trimesic (H3BTC, 5.0 mmol) acid and 2.18 g cupric nitrate trihydrate (Cu(NO3)2·3H2O, 9.0 mmol) was dissolved in 30 mL ethanol and 30 mL deionized water, respectively. And then the solutions were mixed and stirred for 30 min at ambient temperature. Subsuquently, the mixture was transferred to a 100 mL Teflon autoclave and crystallized for 12 h at 120 °C. After that, the CuBTC product was filtered and washed with ethanol and deionized water. Finally, the blue solid powder was obtained by further dry under vacuum overnight at 150 °C.
For the loading of palladium onto CuBTC, typically, 0.034 g (0.19 mmol) palladium chloride was dispersed in 20 mL acetonitrile under ultrasound condition for 3 h. Afterwards, 1.0 g CuBTC material was added into the above solution, followed by heating reflux for 24 h at 70 °C. Finally, the solvent was removed by rotary evaporation and the resulting solid was dried under vacuum at 100 °C for 6 h. The sample was denoted as Pd/CuBTC.The PdOx/CuO-ST catalyst was obtained by further pyrolysis of Pd/CuBTC under air at 400 °C for 6 h.
For comparison, a PdOx/CuO-SG catalyst was prepared by sol–gel method with citric acid as gel. 1.26 g (6 mmol) of citric acid and 2.18 g (9.0 mmol) of Cu(NO3)2·3H2O were dissolved in 30 mL of deionized water, and stirred continuously in water bath at 80 °C until became viscous glue. And then dried at 120 °C for 20 h. The obtained solid was loaded with Pd, the loading process was similar with Pd/CuBTC. Finally, the product was calcinated in the air at 400 °C for 6 h.
All the aforementioned reagents were supplied by InnoChem (Beijing, China) and used without further purification.
3.2. Catalyst Characterization
X-ray Diffraction (XRD) patterns were recorded on a D8 Advance (Bruker, Billerica, MA, USA) diffractometer using Cu Kα radiation operated at 40 kV and 200 mA, with 2θ from 10° to 80°. The thermogravimetric analysis (TGA) was carried out by using a SDT-Q600 instrument (TA, Newcastle, DE, USA) under air flow at 10 °C min−1 from room temperature to 600 °C. Scanning electron microscope (SEM), along with energy dispersive spectrometer (EDS) was performed using Nova Nano SEM 450 (FEI, Hillsboro, OR, USA). Transmission electron microscopy (TEM) images and HAADF photos of the catalysts were taken on a Talos F200s (FEI, Hillsboro, OR, USA) microscope operated at 200 kV. The BET surface area and the textural properties were determined by N2 adsorption/desorption measurements at liquid nitrogen temperature using a ASAP2020 Apparatus (Micromeritics, Norcross, GA, USA). X-ray Photoelectron Spectroscopy (XPS) analysis was carried out on a Escalab 250Xi instrument (Thermo Fisher Scientific, Waltham, MA, USA), employing Al-Kα radiation. The binding energy (BE) for the samples was calibrated by setting the measured BE of C 1s to 284.6 eV. The Pd content in the catalyst was determined by an Optima 7300 v (Perkin Elmer, Waltham, MA, USA). Temperature- programmed reduction by hydrogen (H2-TPR) was carried out in the 10% H2/Ar gas mixture on an AutoChem II-2920 apparatus (Micromeritics, Norcross, GA, USA). The flow rate of the gas was 50 mL/min and the heating rate was 10 °C/min.
3.3. Catalyst Activity Evaluation
The catalyst activity was evaluated in 100 mL stainless steel autoclave lined with Teflon. The catalyst, cocatalyst (Cu(OAc)2, tetrabutylammonium bromide and hydroquinone), desiccant (4A molecular sieve), solvent (CH2Cl2) and phenol were added to the vessel, sealed and filled with O2 and CO. After that, the vessel was heated to the predetermined temperature for reaction. After the reaction was completed, the mixture was cooled to room temperature and the catalyst and 4A molecular sieves were removed by filtration.
Quantitative analysis of the product was performed on an e2695 liquid chromatograph (Waters, Milford, MA, USA) with a Symmetry C18 column (5 μL, 4.6 × 250 mm), CH3OH/H2O (65/35, V/V) was used as the mobile phase and the detection wavelength was 254 nm. Flow rate 0.6 was mL/min, injection volume was 20 μL and column temperature was kept at 35 °C. External standard method was used to quantify diphenyl carbonate and phenol in the reaction solution.
4. Conclusions
In this study, a novel PdOx/CuO catalyst was successfully fabricated through the calcination of a Pd-embedded CuBTC precursor. Comprehensive characterization techniques confirmed that the resulting catalyst inherited the high surface area and porous architecture of the MOF template, which facilitated the highly dispersed incorporation of Pd species into the CuO lattice.
The catalytic performance of PdOx/CuO-ST in the oxidative carbonylation of phenol was significantly influenced by the calcination temperature. An optimal temperature of 400 °C yielded a catalyst with the highest activity, achieving 79.5% phenol conversion and 84.5% DPC selectivity. This high performance is attributed to the well-dispersed Pd species and the strong metal–support interaction fostered by the MOF-derived CuO support, which enhanced the catalyst’s redox properties.
However, calcination at excessively high temperatures (>500 °C) promoted the formation of less active PdxCuyO species and led to a decrease in surface Pd content, resulting in a notable decline in activity. Furthermore, the catalyst underwent significant deactivation after the first reaction cycle. This deactivation was primarily caused by the reduction of active PdO to inert Pd0 and the leaching of Pd species, as confirmed by XPS and ICP analyses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15111039/s1, Figure S1: TG curves of CuBTC and Pd/CuBTC; Figure S2: N2 adsorption–desorption isotherms and pore size distribution of CuBTC and Pd/CuBTC, Figure S3: SEM-EDS images of Pd/CuBTC, Figure S4: XRD patterns of CuBTC and Pd/CuBTC, Figure S5: XPS spectra of O 1s of PdOx/CuO-ST catalysts calcinated at (a) 300 °C, (b) 400 °C, (c) 500 °C, (d) 600 °C, (e) 700 °C and (f) PdOx/CuO-SG catalyst, Figure S6: XPS spectra of Pd 3d of PdOx/CuO-ST catalysts calcinated at (a) 300 °C, (b) 400 °C, (c) 500 °C, (d) 600 °C, (e) 700 °C and (f) PdOx/CuO-SG catalyst, Table S1: Activity comparison of different catalysts for the oxidative carbonylation of phenol, Table S2: H2-TPR results of PdOx/CuO-ST prepared at different temperature and PdOx/CuO-SG.
Author Contributions
Y.F.: data curation, methodology, formal analysis, investigation, writing—original draft, visualization. Z.F.: data curation, methodology. H.L.: investigation, methodology. Z.W.: conceptualization, writing—review and editing. W.X.: writing—review and editing, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, grant number 21776057.
Data Availability Statement
The original contributions presented in this study are included in the article materials; further inquiries can be directed to the corresponding author.
Acknowledgments
We sincerely thank all those who worked so hard on this study and provided characterization.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wu, T.-W.; Ohno, H.; Wang, A.; Guzman-Urbina, A.; Shinkai, Y.; Furuhashi, H.; Umezu, R.; Lin, S.-T.; Fukushima, Y. Comparative analysis of CO2-based alternative pathways for diphenyl carbonate production. Chem. Eng. J. 2025, 520, 166354. [Google Scholar] [CrossRef]
- Fukuoka, S.; Kawamura, M.; Komiya, K.; Tojo, M.; Hachiya, H.; Hasegawa, K.; Aminaka, M.; Okamoto, H.; Fukawad, I.; Konno, S. A novel non-phosgene polycarbonate production process using by-product CO2 as starting material. Green Chem. 2003, 5, 497–507. [Google Scholar] [CrossRef]
- Wang, D.; Shi, F.; Yang, G. Diphenyl carbonate: Recent progress on its catalytic synthesis by transesterification. Catalysts 2024, 14, 250. [Google Scholar] [CrossRef]
- Tan, L.; Huang, Y.-F.; Mao, W.; Hu, X.-Y.; Huang, Y.-Q.; Chen, H.; Luo, X. Anatase-layered TiO2 for selective transesterification for diphenyl carbonate A study of the active site and deactivation mechanism. Ind. Eng. Chem. Res. 2024, 63, 16347–16355. [Google Scholar] [CrossRef]
- Akiyama, T.; Kita, Y.; Chen, P.; Tamura, M. Direct synthesis of diphenyl carbonate from CO2 and phenol with CeO2 and 2-cyanopyridine. Mol. Catal. 2025, 574, 114886. [Google Scholar] [CrossRef]
- Xing, M.; Hui, T.; Zhang, R.; Zheng, T.; Liu, Z.; Liu, H.; Xu, C.; Meng, X. Diphenyl carbonate synthesis from CO2 over a ZnCeZrOX ternary solid solution: Synergistic catalysis using oxygen vacancies and Lewis acid sites. Catal. Sci. Technol. 2025, 15, 4872–4884. [Google Scholar] [CrossRef]
- Goyal, M.; Nagahata, R.; Sugiyama, J.-I.; Asai, M.; Ueda, M.; Takeuchi, K. Effect of inorganic redox cocatalyst on Pd-catalyzed oxidative carbonylation of phenol for direct synthesis of diphenyl carbonate. Catal. Lett. 1998, 54, 29–31. [Google Scholar] [CrossRef]
- Linsen, K.J.L.; Libens, J.; Jacobs, P.A. A new heterogeneous catalyst for the oxidative carbonylation of phenol to diphenyl carbonate. Chem. Commun. 2002, 34, 2728–2729. [Google Scholar] [CrossRef] [PubMed]
- Ronchin, L.; Vavasori, A.; Amadio, E.; Cavinato, G.; Toniolo, L. Oxidative carbonylation of phenols catalyzed by homogeneous and heterogeneous Pd precursors. J. Mol. Catal. A Chem. 2009, 298, 23–30. [Google Scholar] [CrossRef]
- Gong, J.L.; Ma, X.B.; Wang, S.P. Phosgene-free approaches to catalytic synthesis of diphenyl carbonate and its intermediates. Appl. Catal. A Gen. 2007, 316, 1–21. [Google Scholar] [CrossRef]
- Vavasori, A.; Toniolo, L. Multistep electron-transfer catalytic system for the oxidative carbonylation of phenol to diphenyl carbonate. J. Mol. Catal. A Chem. 1999, 139, 109–119. [Google Scholar] [CrossRef]
- Yin, C.F.; Zhou, J.; Chen, Q.M.; Han, J.; Wu, Y.; Yang, X. Deactivation causes of supported palladium catalysts for the oxidative carbonylation of phenol. J. Mol. Catal. A Chem. 2016, 424, 377–383. [Google Scholar] [CrossRef]
- Xue, W.; Zhang, J.C.; Wang, Y.J.; Zhao, Q.; Zhao, X. Effect of promoter copper on the oxidative carbonylation of phenol over the ultrafine embedded catalyst Pd-Cu-O/SiO2. J. Mol. Catal. A Chem. 2005, 232, 77–81. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Z.M.; An, H.L.; Xue, W.; Wang, Y. Oxidative carbonylation of phenol with a Pd-O/CeO2-nanotube catalyst. Chin. J. Catal. 2015, 36, 1142–1152. [Google Scholar] [CrossRef]
- Fu, Z.J.; Wang, Z.M.; Wang, H.J.; Li, F.; Xue, W.; Wang, Y. Pd catalyst supported on CeO2 nanotubes with enhanced structural stability toward oxidative carbonylation of phenol. RSC Adv. 2019, 9, 11356–11364. [Google Scholar] [CrossRef]
- Zhou, L.C.; Feng, G.; Liu, X.J.; Wang, Z.; Li, F.; Xue, W.; Wang, Y. Effect of Zr-doping on Pd/CexZr1−xO2 catalysts for oxidative carbonylation of phenol. Chin. J. Chem. Eng. 2020, 28, 2592–2599. [Google Scholar] [CrossRef]
- Yang, X.J.; Han, J.Y.; Du, Z.P.; Yuan, H.; Jin, F.; Wu, Y.X. Effects of Pb dopant on structure and activity of Pd/K-OMS-2 catalysts for heterogeneous oxidative carbonylation of phenol. Catal. Commun. 2010, 11, 643–646. [Google Scholar] [CrossRef]
- Yang, X.J.; Bai, H.; Qian, M.M.; Wu, C.J.; Cao, S.; Yan, Z.G.; Tian, Q.F.; Du, Z.P. Enhanced activity of Pd-Cu(Ni) bimetallic catalysts for oxidative carbonylation of phenol. Mater. Chem. Phys. 2019, 234, 48–54. [Google Scholar] [CrossRef]
- Peng, M.; Hong, C.; Cai, N.; Hu, Y.; Yuan, H. Effect of metal doping on multi-step electron transfer and oxygen species of silicon-based nanocomposite aerogel supported Pd catalysts in oxidative carbonylation of phenol. Mol. Catal. 2020, 482, 110684. [Google Scholar] [CrossRef]
- Liu, X.J.; Zhao, R.H.; Zhao, H.; Wang, Z.M.; Li, F.; Xue, W.; Wang, Y.J. Enhanced stability of nitrogen-doped carbon-supported palladium catalyst for oxidative carbonylation of phenol. Chin. J. Chem. Eng. 2024, 65, 19–28. [Google Scholar] [CrossRef]
- Cai, G.R.; Yan, P.; Zhang, L.L.; Zhou, H.-C.; Jiang, H.-L. Metal-organic framework-based hierarchically porous materials: Synthesis and applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Bi, F.K.; Zhou, Z.X.; Zhang, Y.F.; Wei, J.F.; Lv, X.T.; Liu, B.L.; Huang, Y.D.; Zhang, X.D. A bimetallic MOF-derived MnCo spinel oxide catalyst to enhance toluene catalytic degradation. Chem. Commun. 2024, 60, 7455–7458. [Google Scholar] [CrossRef]
- Sayidov, O.; Garzon-Tovar, L.; Patarroyo, J.; Bekmukhamedov, G.; Stewart, J.A.; Vandegehuchte, B.D.; Montroussier, N.; Ruiz-Martinez, J.; Gascon, J. An efficient titanomaghemite MOF-derived catalyst for reverse water-gas shift. Catal. Sci. Technol. 2025, 15, 2908–2918. [Google Scholar] [CrossRef]
- He, Y.C.; Zheng, X.; Mao, D.S.; Meng, T.; Mao, H.F.; Yu, J. Promoting catalytic CO2 methanation using Ru catalyst supported on Ce-MOF-derived CeO2. Renew. Energy 2025, 245, 122834. [Google Scholar] [CrossRef]
- Phan, D.-P.; Seo, P.W.; Pham, D.V.; Bhatti, A.H.; Yun, D.; Ro, I.; Park, S.; Kang, K.H. Aquathermolysis of polyolefin plastic wastes over a MOF-derived TiO2 anatase catalyst: Enhancing C–N bond cleavage and coke suppression. Fuel 2025, 381, 133337. [Google Scholar] [CrossRef]
- Świrk Da Costa, K.; Delahay, G.; Zaki, A.; Adil, K.; Cadiau, A. Facile modifications of HKUST-1 by V, Nb and Mn for low-temperature selective catalytic reduction of nitrogen oxides by NH3. Catal. Today 2022, 384–386, 25–32. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhang, J.L.; Song, L.X.; Xu, W.Y.; Guo, Z.R.; Yang, X.M.; Wu, X.X.; Chen, X. Au-HKUST-1 composite nanocapsules: Synthesis with a coordination replication strategy and catalysis on CO oxidation. ACS Appl. Mater. Interfaces 2016, 8, 22745–22750. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.M.; Fu, Z.J.; Wang, X.F.; Xue, W. Oxidative carbonylation of phenol to diphenyl carbonate catalyzed by Pd//HKUST-1. J. Chem. Eng. Chin. Univ. 2023, 37, 581–590. [Google Scholar] [CrossRef]
- Chen, L.; Chen, H.; Luque, R.; Li, Y. Metal−organic framework encapsulated Pd nanoparticles: Towards advanced heterogeneous catalysts. Chem. Sci. 2014, 5, 3708–3714. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Garcia, H. Catalysis by metal nanoparticles embedded on metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 5262–5284. [Google Scholar] [CrossRef]
- Schlichte, K.; Kratzke, T.; Kaskel, S. Improved synthesis, thermal stability and catalytic properties of the metal-organic framework compound Cu3(BTC)2. Micropor. Mesopor. Mater. 2004, 73, 81–88. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Liu, Y.; Yang, Z.; Feng, X.; Lu, X.; Huo, F. Well-dispersed and size-controlled supported metal oxide nanoparticles derived from MOF composites and further application in catalysis. Small 2015, 11, 3130–3134. [Google Scholar] [CrossRef]
- Li, G.; Li, L.; Yuan, Y.; Shi, J.; Yuan, Y.; Li, Y.; Zhao, W.; Shi, J. Highly efficient mesoporous Pd/CeO2 catalyst for low temperature CO oxidation especially under moisture condition. Appl. Catal. B Environ. 2014, 158–159, 341–347. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, Z.; Chen, C.; Wen, T.; Zhao, X.; Xie, L. Highly sensitive H2S gas sensors based on Pd-doped CuO nanoflowers with low operating temperature. Sens. Actuators B Chem. 2017, 253, 809–817. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, L.; Sun, N.; Wang, H.; Zhong, L.; He, C.; Wei, W.; Sun, Y. Hollow MnOx-CeO2 mixed oxides as highly efficient catalysts in NO oxidation. Chem. Eng. J. 2017, 322, 46–55. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, M.; Shen, G.; Liu, H.; Chen, Y.; Wang, Q. Catalytic removal of benzene over CeO2-MnOx composite oxides with rod-like morphology supporting PdO. J. Nanopart. Res. 2014, 16, 2367–2375. [Google Scholar] [CrossRef]
- Ivanova, A.S.; Slavinskaya, E.M.; Gulyaev, R.V.; Zaikovskii, V.I.; Stonkus, O.A.; Danilova, I.G.; Plyasova, L.M.; Polukhina, I.A.; Boronin, A.I. Metal-support interactions in Pt/Al2O3 and Pd/Al2O3 catalysts for CO oxidation. Appl. Catal. B Environ. 2010, 97, 57–71. [Google Scholar] [CrossRef]
- Guimarães, A.L.; Dieguez, L.C.; Schmal, M. The effect of precursors salts on surface state of Pd/Al2O3 and Pd/CeO2/Al2O3 catalysts. An. Acad. Bras. Cienc. 2004, 76, 825–832. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, Y. Effects of large CuO contents on the performance of Pd/Al2O3-CuO catalysts in ethanol oxidation reaction. Chem. Phys. Lett. 2019, 722, 26–31. [Google Scholar] [CrossRef]
- Han, L.; Zhang, L.; Zhao, G.; Chen, Y.; Zhang, Q.; Chai, R.; Liu, Y.; Lu, Y. Copper-fiber-structured Pd-Au-CuOx: Preparation and catalytic performance in the vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol. Chemcatchem 2016, 8, 1065–1073. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, Z.; Shan, W.; Shen, W.; Wang, J. Pd/CeO2-TiO2 catalyst for CO oxidation at low temperature: A TPR study with H2 and CO as reducing agents. J. Catal. 2004, 225, 267–277. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, Y.; Zhao, B.; Wang, T.; Zhang, K.; Yuen, M.M.F.; Fu, X.-Z.; Sun, R.; Wong, C.-P. Urchin-like Pd@CuO-Pd yolk-shell nanostructures: Synthesis, characterization and electrocatalysis. J. Mater. Chem. A 2015, 3, 13653–13661. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Z.; An, X.; Zhang, Y. Designed synthesis CuO hollow microboxes coated with Pd nanosheets and SnO2 nanoparticles as a highly efficient Rochow reaction catalyst. Appl. Surf. Sci. 2017, 426, 714–724. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).