Recent Advances in Palladium-Catalyzed Enantioselective Cyclization for the Construction of Atropisomers
Abstract
1. Introduction
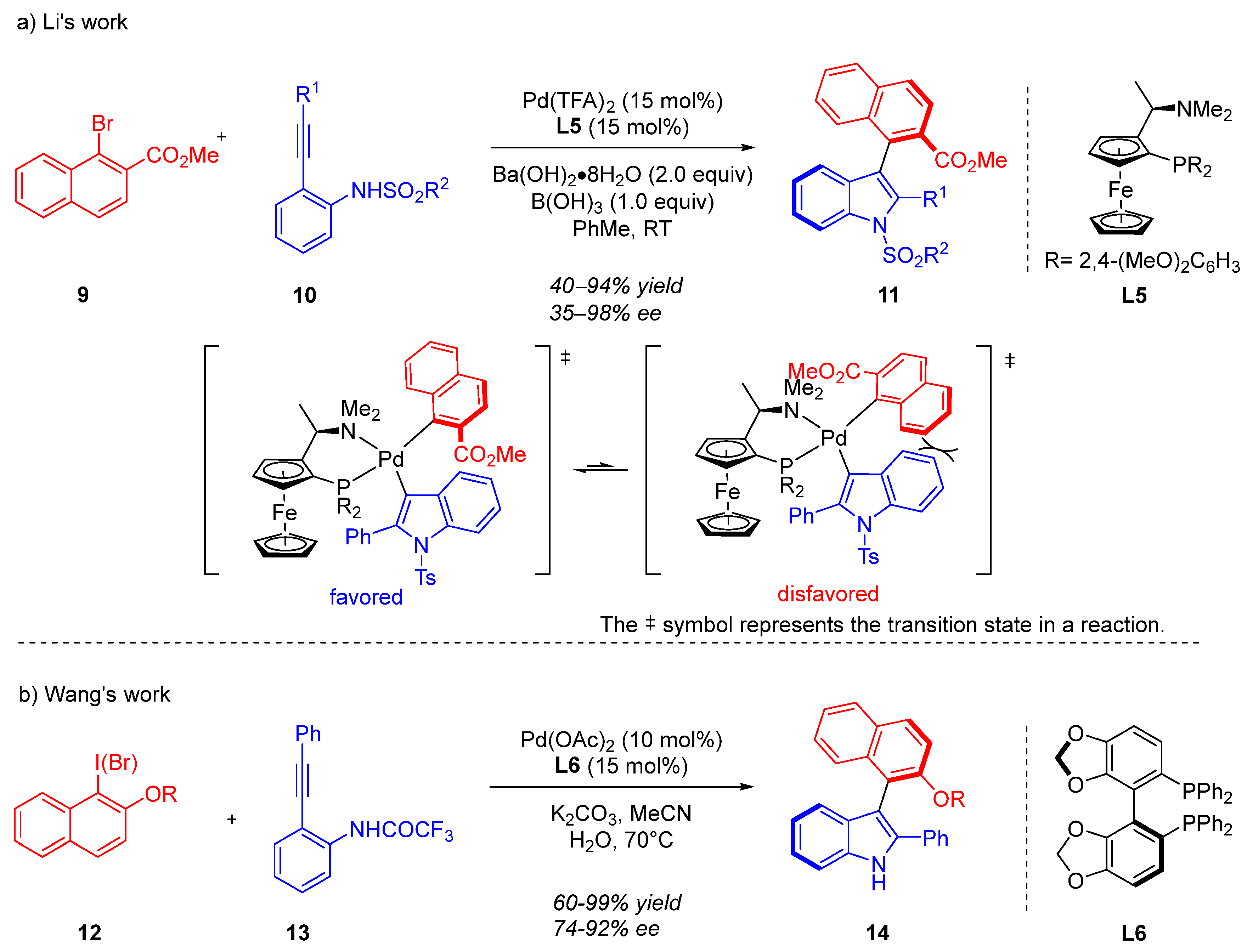
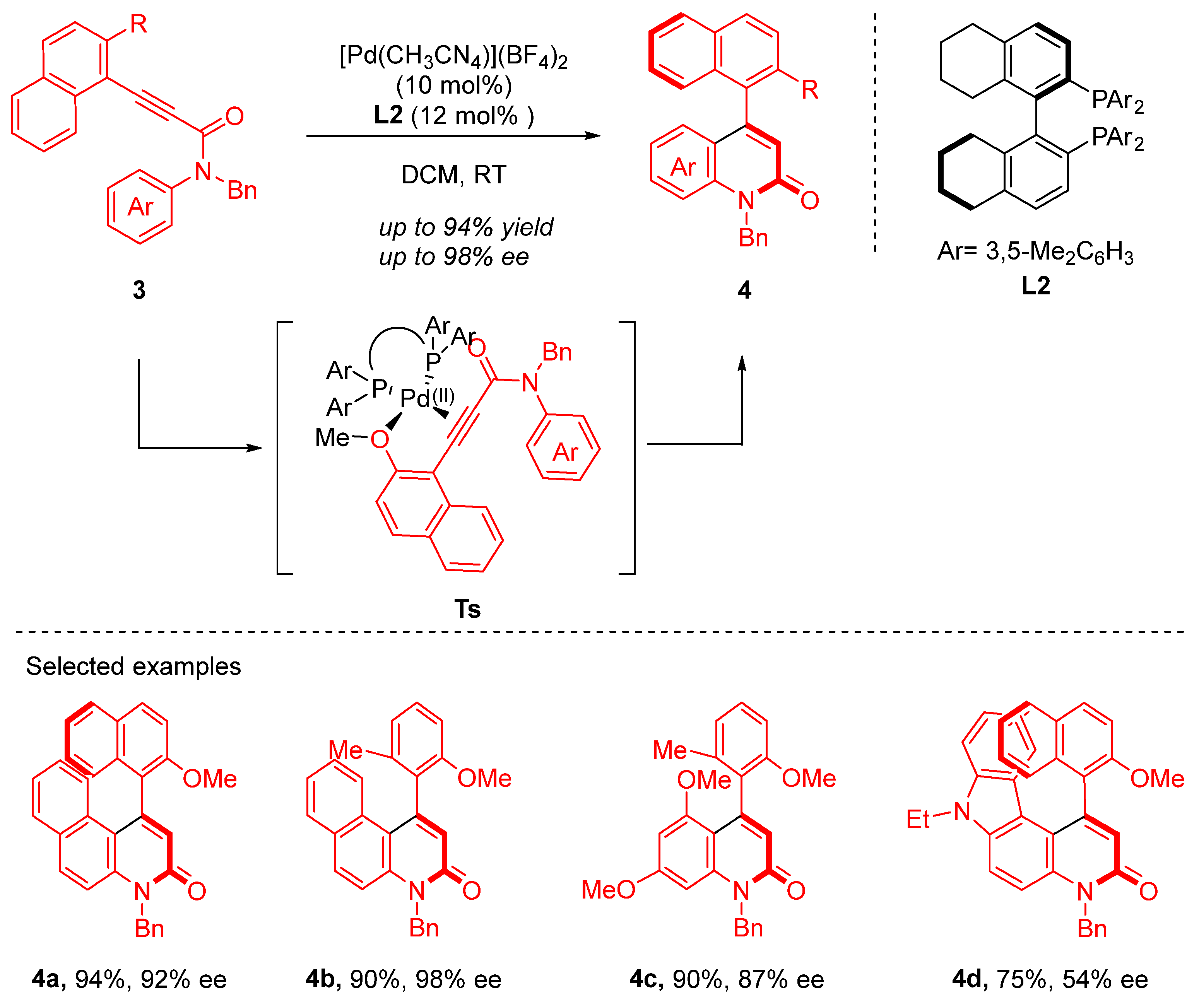
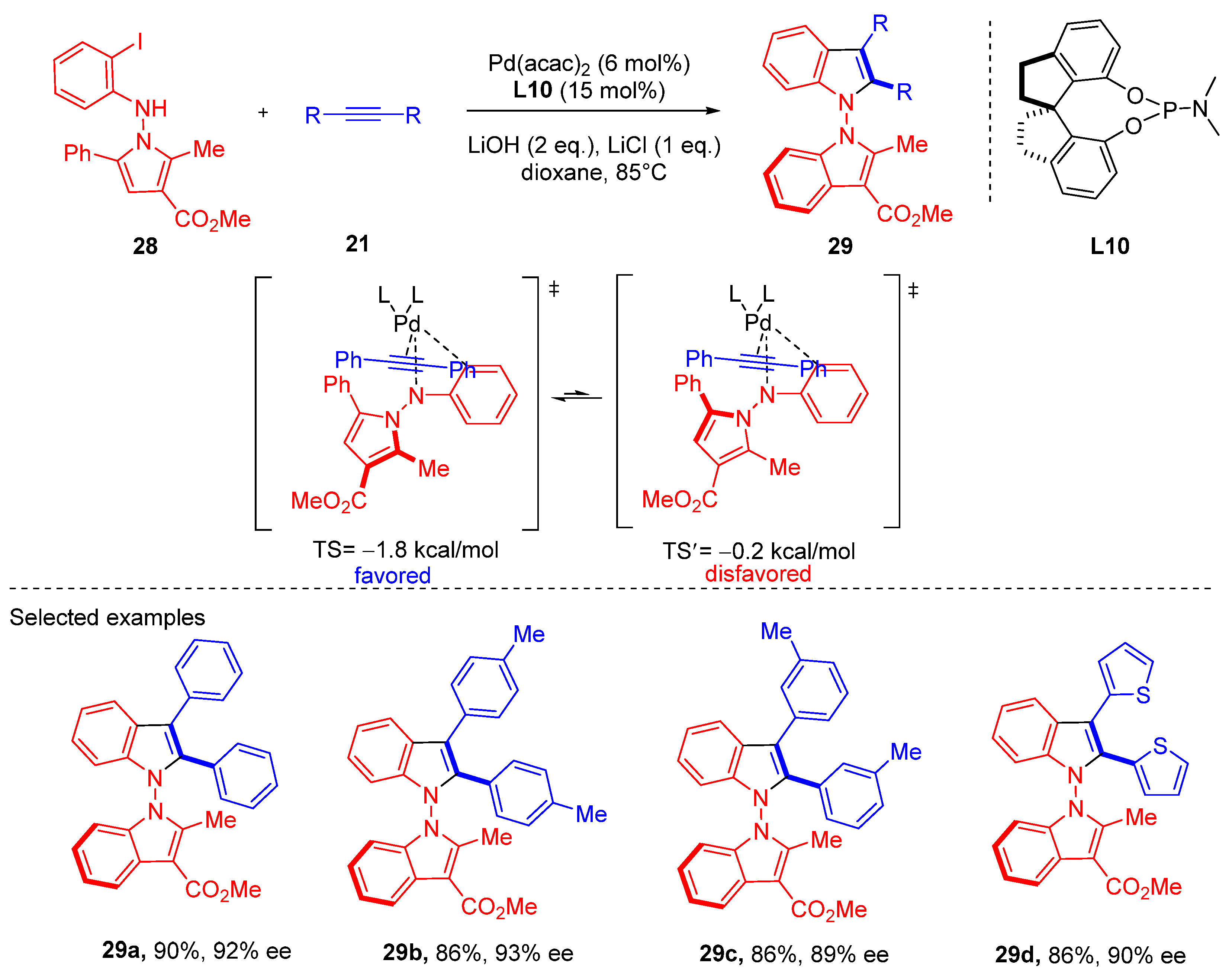
2. Heteropalladation of Triple Bonds Initiates Cyclization
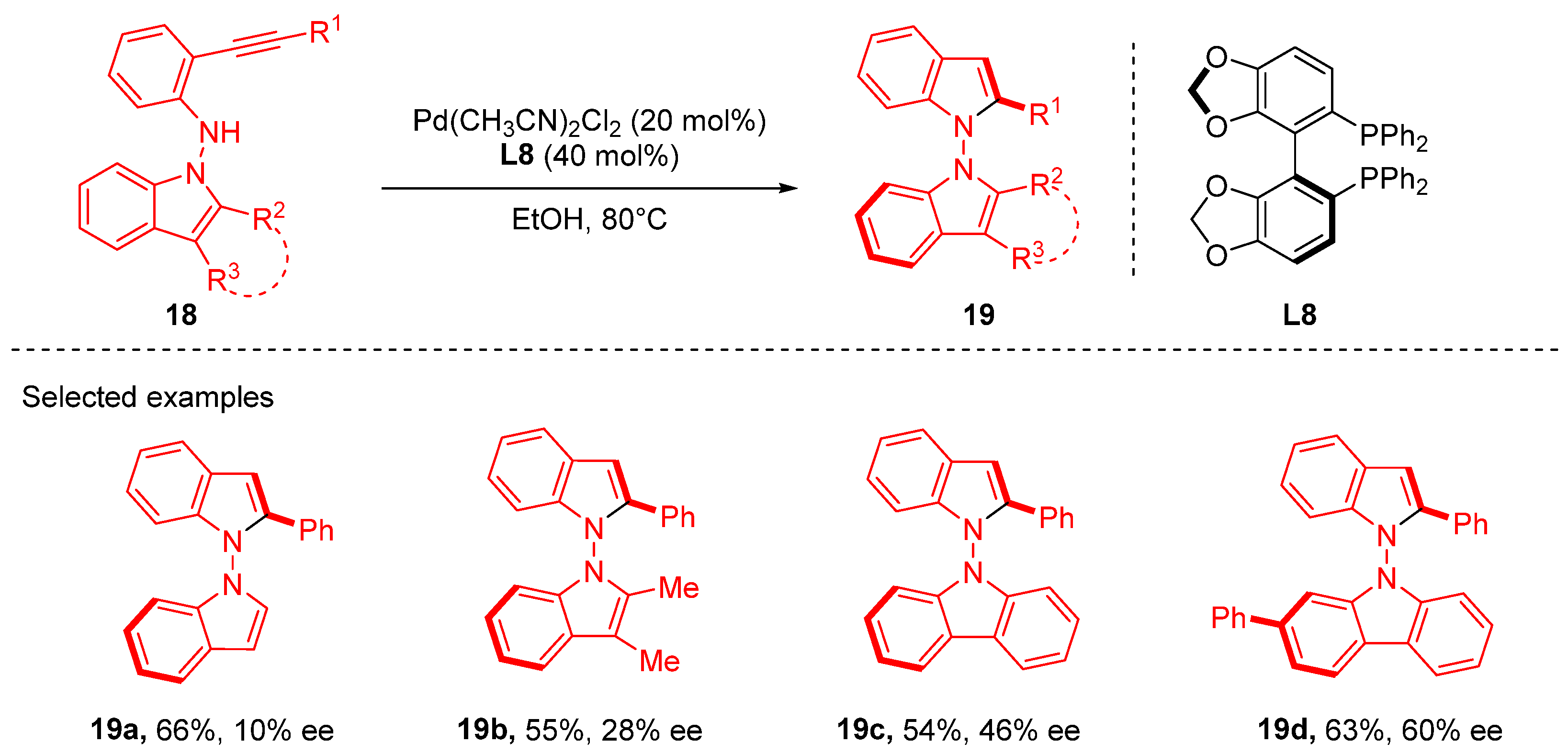
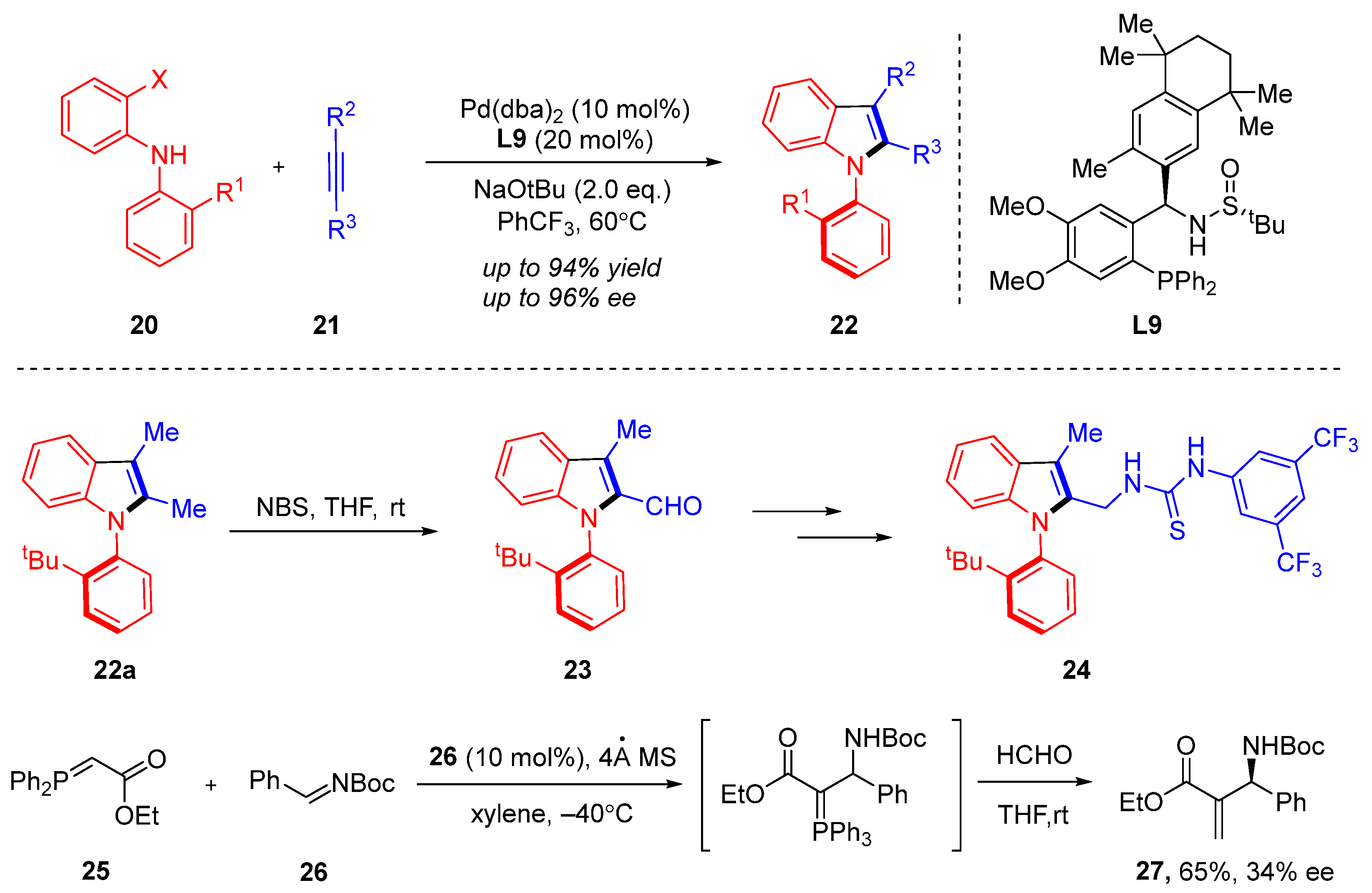
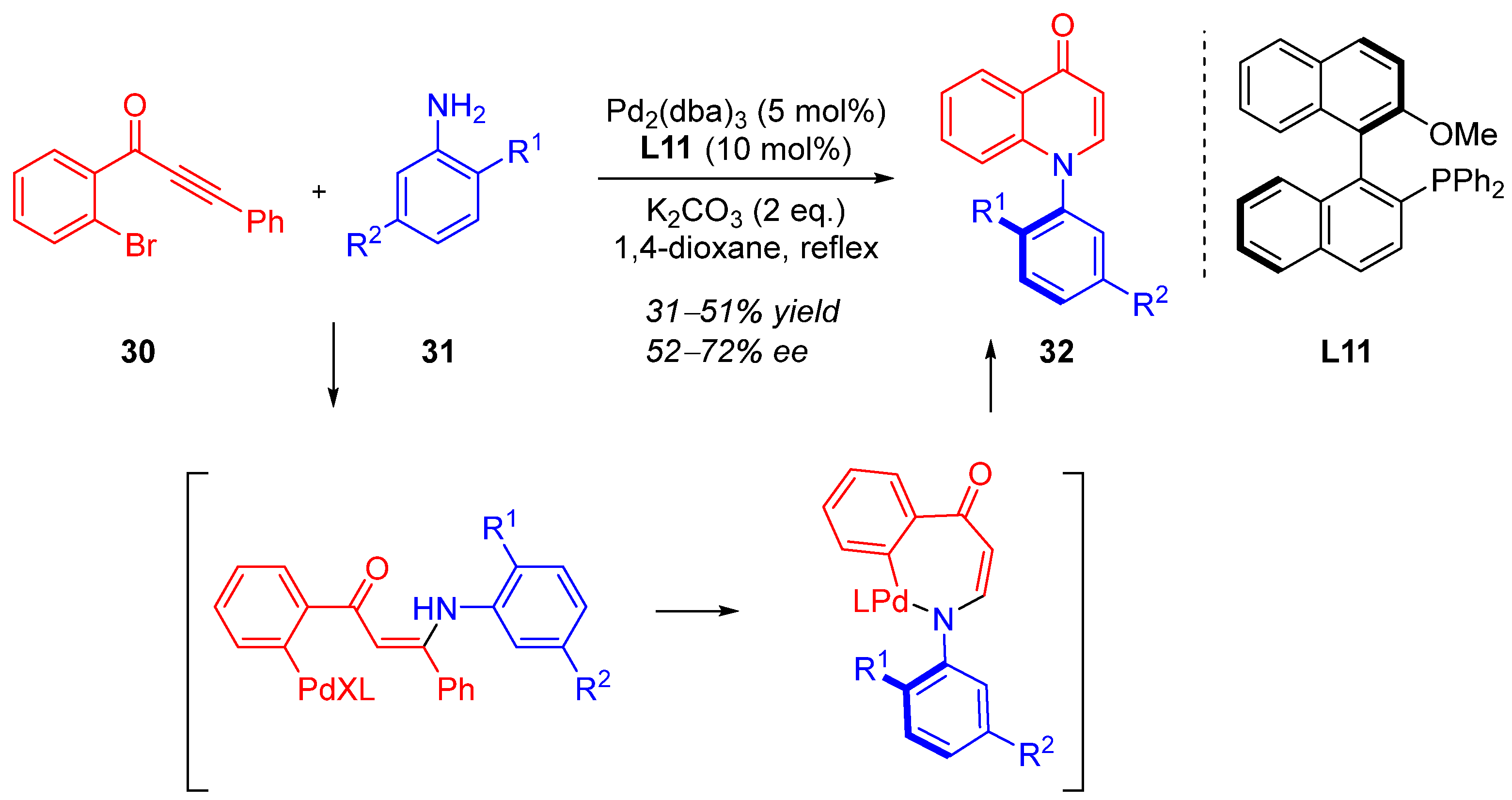
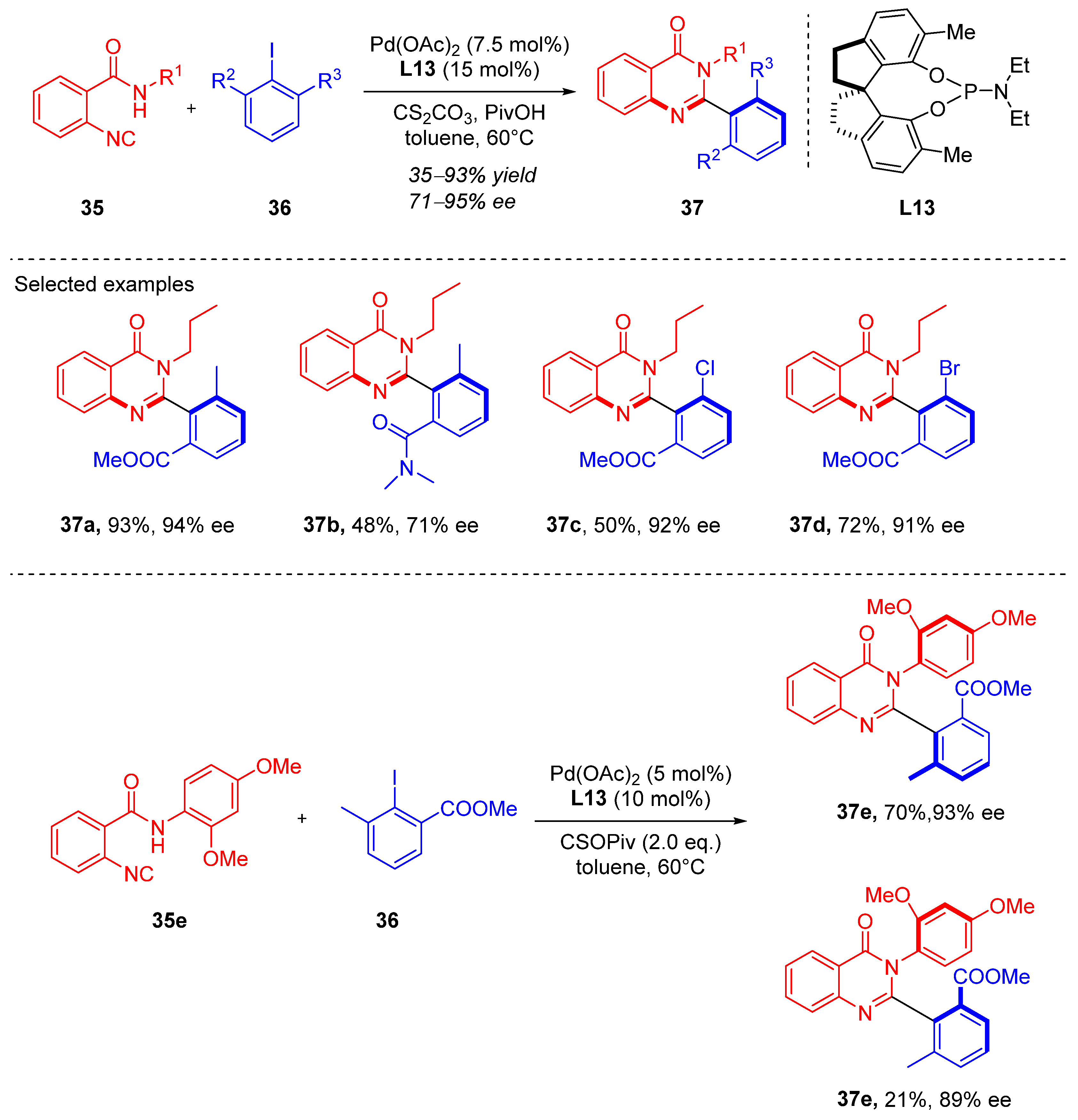
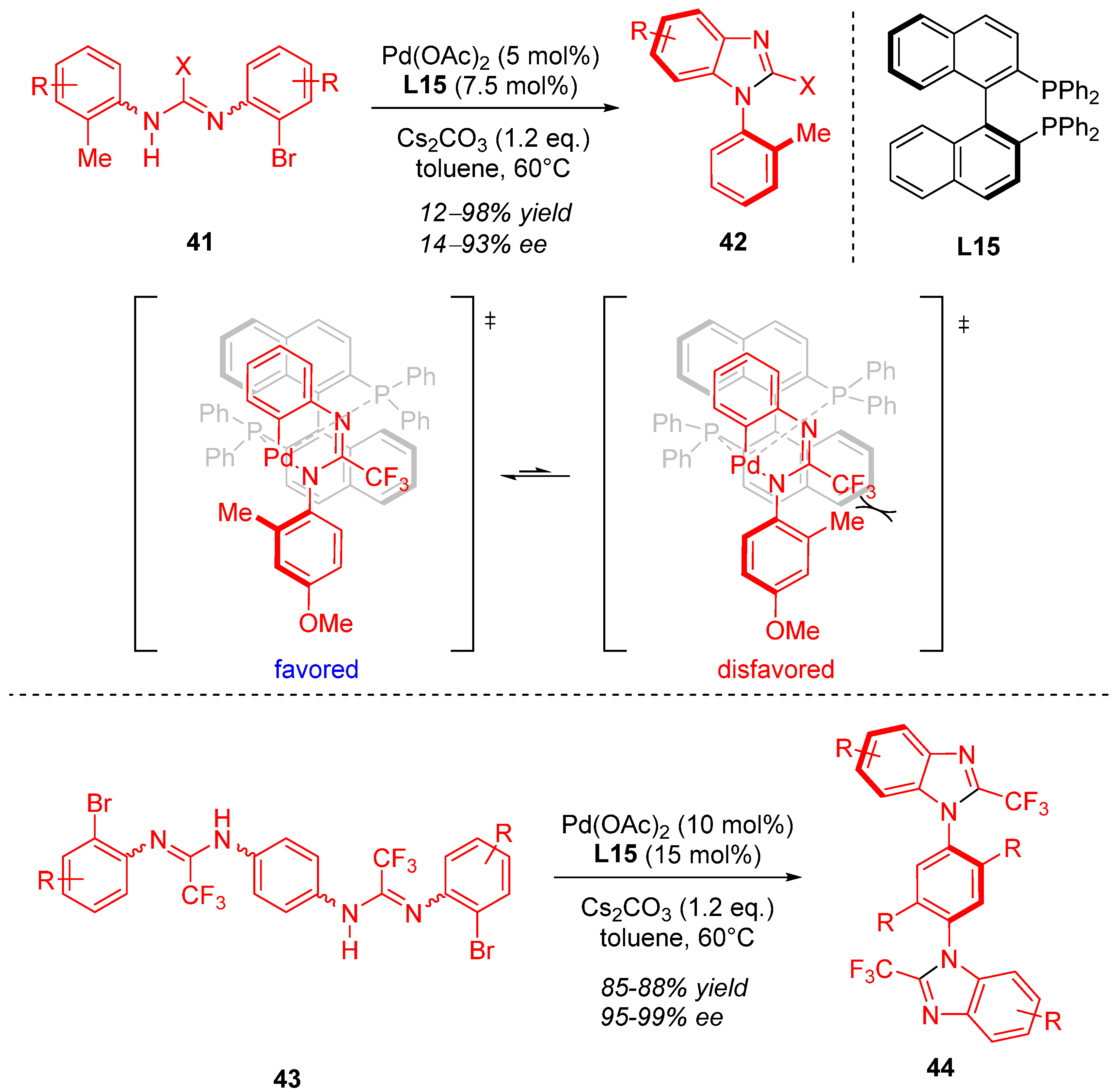
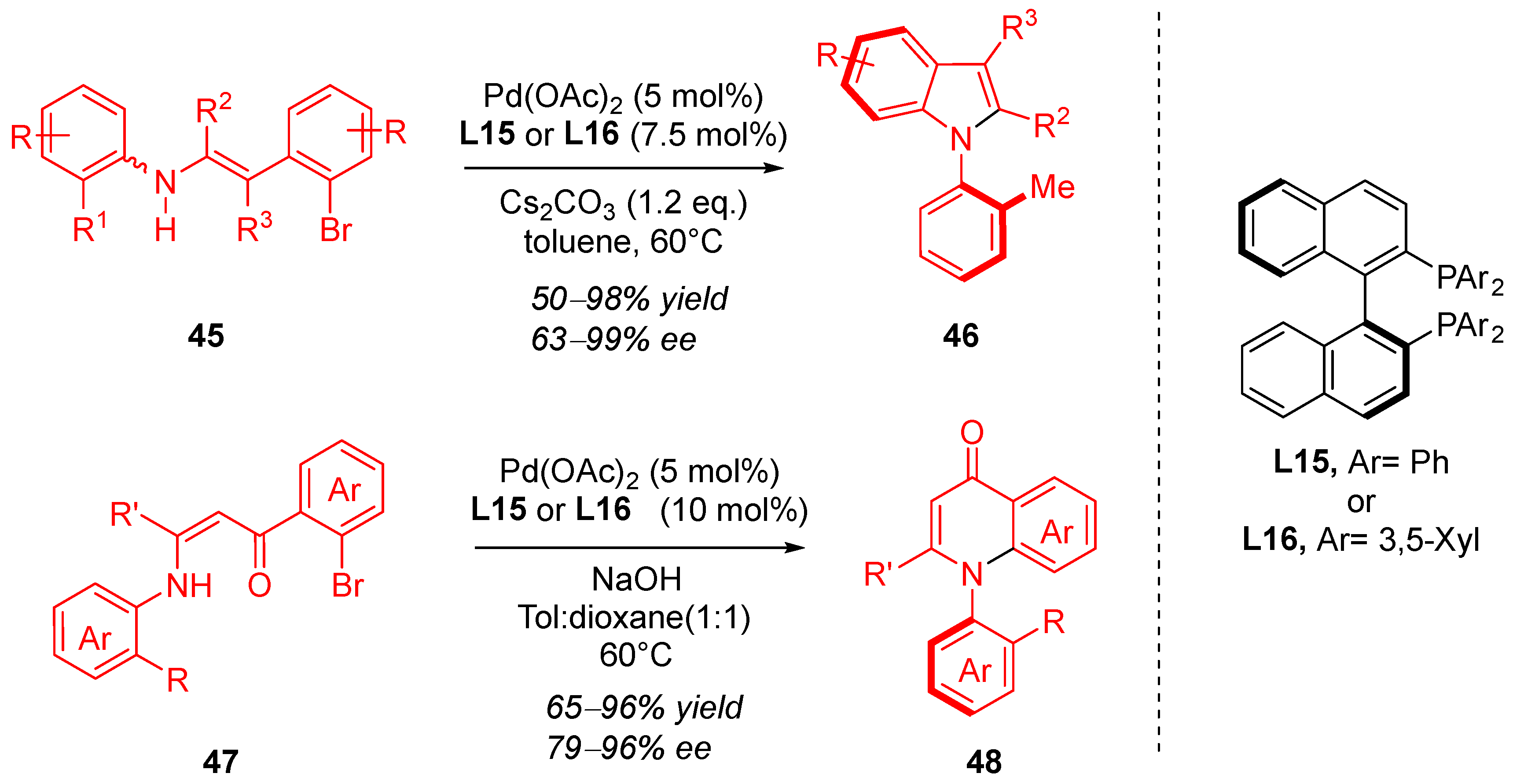
3. Cyclization via C–N Coupling
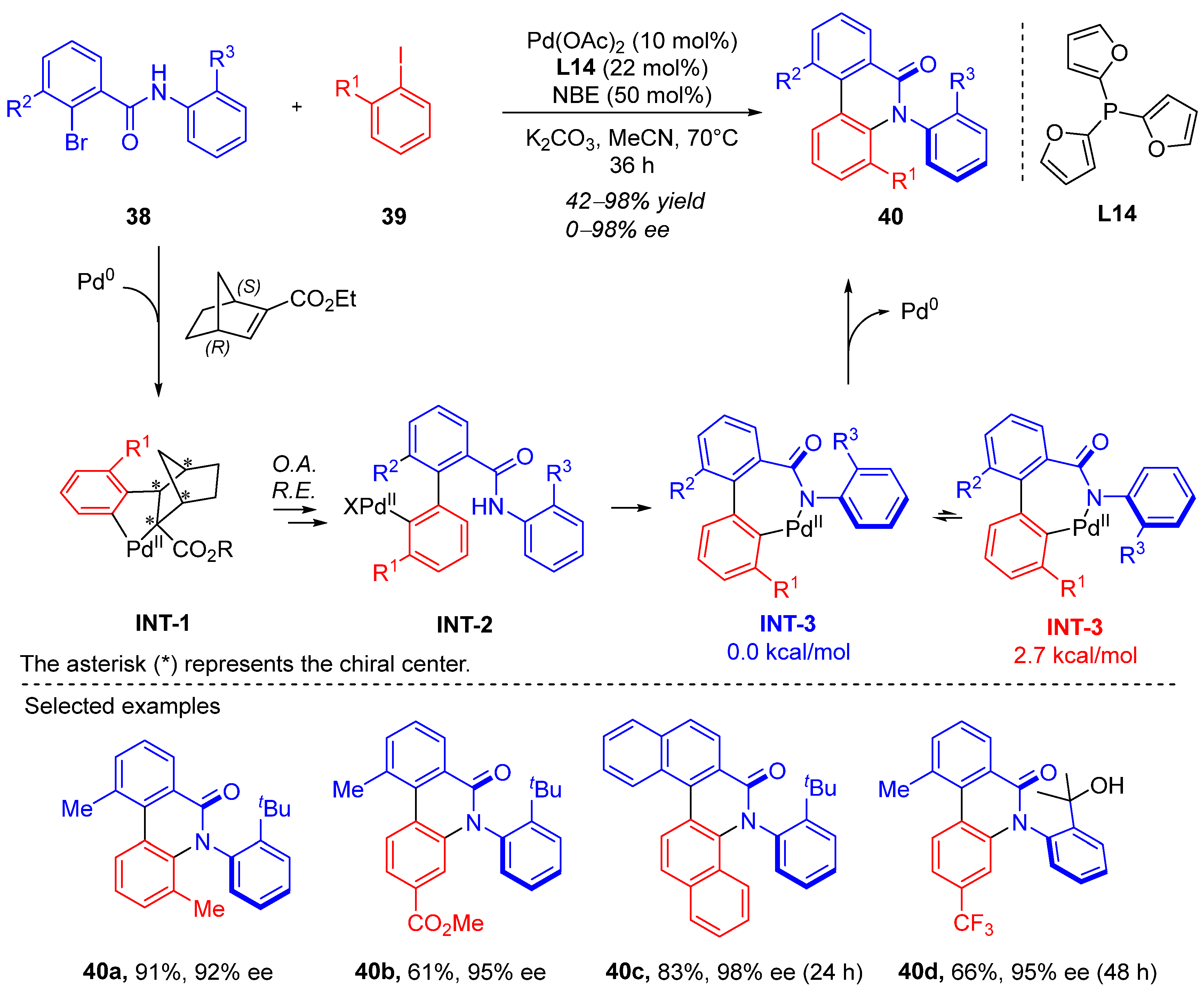
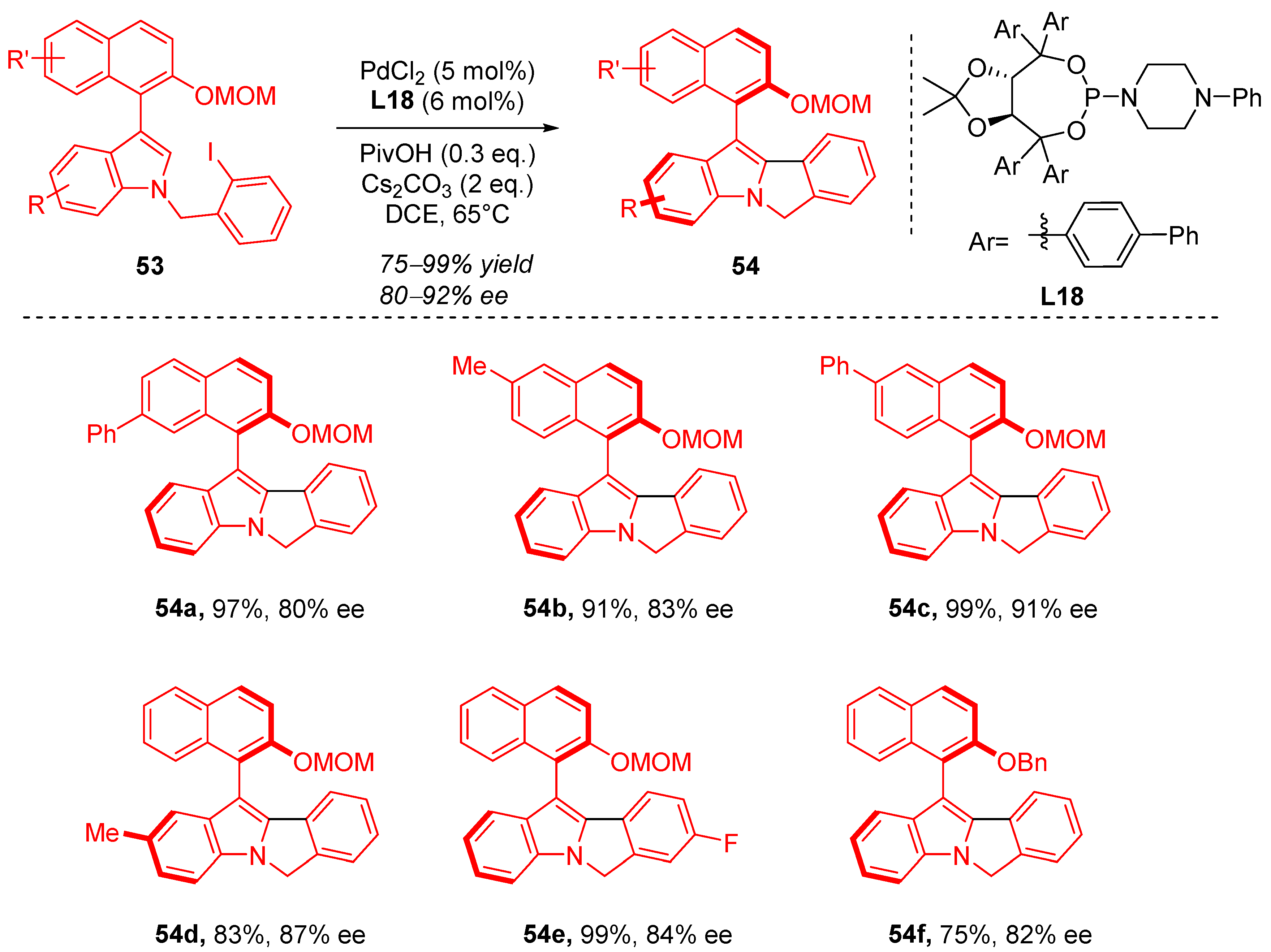
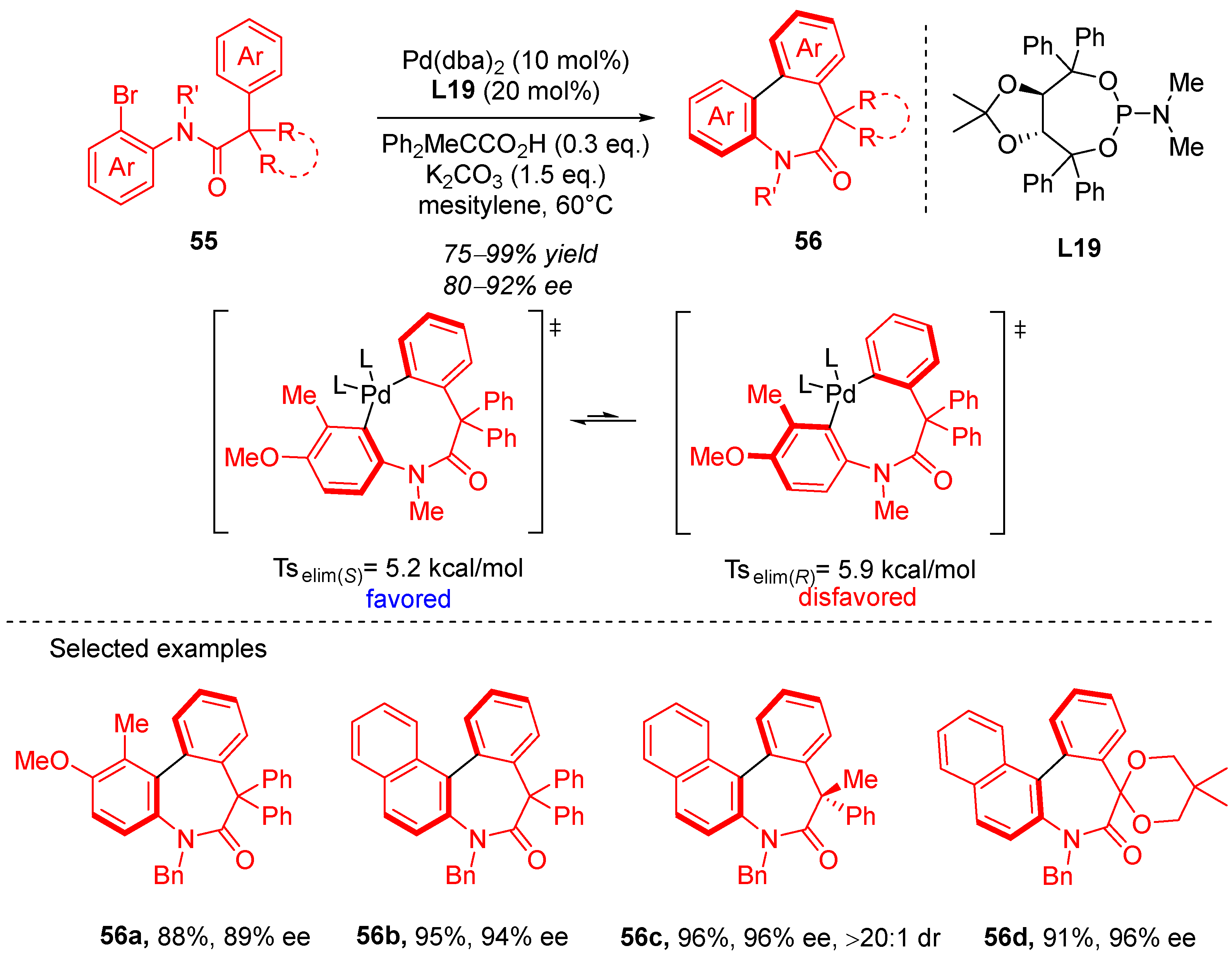
4. Cyclization via C–H Activation
5. Cyclization via Carbonylation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liao, G.; Zhou, T.; Yao, Q.-J.; Shi, B.-F. Recent advances in the synthesis of axially chiral biaryls via transition metal-catalysed asymmetric C–H functionalization. Chem. Commun. 2019, 55, 8514–8523. [Google Scholar]
- Bao, X.; Rodriguez, J.; Bonne, D. Enantioselective Synthesis of Atropisomers with Multiple Stereogenic Axes. Angew. Chem. Int. Ed. 2020, 59, 12623–12634. [Google Scholar] [CrossRef]
- Carmona, J.A.; Rodríguez-Franco, C.; Fernández, R.; Hornillos, V.; Lassaletta, J.M. Atroposelective transformation of axially chiral(hetero)biaryls. From desymmetrization to modern resolution strategies. Chem. Soc. Rev. 2021, 50, 2968–2983. [Google Scholar] [PubMed]
- Cheng, J.K.; Xiang, S.-H.; Li, S.; Ye, L.; Tan, B. Recent Advances in Catalytic Asymmetric Construction of Atropisomers. Chem. Rev. 2021, 121, 4805–4902. [Google Scholar]
- Liu, C.-X.; Zhang, W.-W.; Yin, S.-Y.; Gu, Q.; You, S.-L. Synthesis of Atropisomers by Transition-Metal-Catalyzed Asymmetric C–H Functionalization Reactions. J. Am. Chem. Soc. 2021, 143, 14025–14040. [Google Scholar]
- Mei, G.-J.; Koay, W.L.; Guan, C.-Y.; Lu, Y. Atropisomers beyond the C–C axial chirality: Advances in catalytic asymmetric synthesis. Chem 2022, 8, 1855–1893. [Google Scholar]
- Kitagawa, O. Structural Chemistry of C–N Axially Chiral Compounds. J. Org. Chem. 2024, 89, 11089–11099. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.A.; Hutskalova, V.; Sparr, C. Atroposelective catalysis. Nat. Rev. Chem. 2024, 8, 497–517. [Google Scholar]
- Wu, C.; Jin, Y.; Zhang, X.; Gao, R.; Dou, X. Development of Configurationally Labile Biaryl Reagents for Atropisomer Synthesis. Eur. J. Org. Chem. 2024, 27, e202400402. [Google Scholar]
- Xiang, S.-H.; Ding, W.-Y.; Wang, Y.-B.; Tan, B. Catalytic atroposelective synthesis. Nat. Catal. 2024, 7, 483–498. [Google Scholar]
- Christie, G.H.; Kenner, J. LXXI.—The molecular configurations of polynuclear aromatic compounds. Part I. The resolution of γ-6:6’-dinitro- and 4:6:4’:6’-tetranitro-diphenic acids into optically active components. J. Chem. Soc. Trans. 1922, 121, 614–620. [Google Scholar]
- Clayden, J.; Moran, W.J.; Edwards, P.J.; LaPlante, S.R. The Challenge of Atropisomerism in Drug Discovery. Angew. Chem. Int. Ed. 2009, 48, 6398–6401. [Google Scholar]
- Kozlowski, M.C.; Morgan, B.J.; Linton, E.C. Total synthesis of chiral biaryl natural products by asymmetric biaryl coupling. Chem. Soc. Rev. 2009, 38, 3193–3207. [Google Scholar]
- LaPlante, S.R.; Edwards, P.J.; Fader, L.D.; Jakalian, A.; Hucke, O. Revealing Atropisomer Axial Chirality in Drug Discovery. ChemMedChem 2011, 6, 505–513. [Google Scholar] [PubMed]
- Smyth, J.E.; Butler, N.M.; Keller, P.A. A twist of nature—The significance of atropisomers in biological systems. Nat. Prod. Rep. 2015, 32, 1562–1583. [Google Scholar] [CrossRef]
- Basilaia, M.; Chen, M.H.; Secka, J.; Gustafson, J.L. Atropisomerism in the Pharmaceutically Relevant Realm. Acc. Chem. Res. 2022, 55, 2904–2919. [Google Scholar] [PubMed]
- Perreault, S.; Chandrasekhar, J.; Patel, L. Atropisomerism in Drug Discovery: A Medicinal Chemistry Perspective Inspired by Atropisomeric Class I PI3K Inhibitors. Acc. Chem. Res. 2022, 55, 2581–2593. [Google Scholar]
- Miyashita, A.; Yasuda, A.; Takaya, H.; Toriumi, K.; Ito, T.; Souchi, T.; Noyori, R. Synthesis of 2,2’-bis(diphenylphosphino)-1,1’-binaphthyl (BINAP), an atropisomeric chiral bis(triaryl)phosphine, and its use in the rhodium(I)-catalyzed asymmetric hydrogenation of .alpha.-(acylamino)acrylic acids. J. Am. Chem. Soc. 1980, 102, 7932–7934. [Google Scholar]
- Parmar, D.; Sugiono, E.; Raja, S.; Rueping, M. Complete Field Guide to Asymmetric BINOL-Phosphate Derived Brønsted Acid and Metal Catalysis: History and Classification by Mode of Activation; Brønsted Acidity, Hydrogen Bonding, Ion Pairing, and Metal Phosphates. Chem. Rev. 2014, 114, 9047–9153. [Google Scholar]
- Bringmann, G.; Price Mortimer, A.J.; Keller, P.A.; Gresser, M.J.; Garner, J.; Breuning, M. Atroposelective Synthesis of Axially ChiralBiaryl Compounds. Angew. Chem. Int. Ed. 2005, 44, 5384–5427. [Google Scholar]
- Tanaka, K. Transition-Metal-Catalyzed Enantioselective [2+2+2] Cycloadditions for the Synthesis of Axially Chiral Biaryls. Chem.-Asian J. 2009, 4, 508–518. [Google Scholar] [PubMed]
- Kumarasamy, E.; Raghunathan, R.; Sibi, M.P.; Sivaguru, J. Nonbiaryl and Heterobiaryl Atropisomers: Molecular Templates withPromise for Atropselective Chemical Transformations. Chem. Rev. 2015, 115, 11239–11300. [Google Scholar] [PubMed]
- Wencel-Delord, J.; Panossian, A.; Leroux, F.R.; Colobert, F. Recent advances and new concepts for the synthesis of axiallystereoenriched biaryls. Chem. Soc. Rev. 2015, 44, 3418–3430. [Google Scholar] [CrossRef]
- Yang, H.; Yang, X.; Tang, W. Transition-metal catalyzedasymmetric carbon-carbon cross-coupling with chiral ligands. Tetrahedron 2016, 72, 6143–6174. [Google Scholar]
- Link, A.; Sparr, C. Stereoselective arene formation. Chem. Soc. Rev. 2018, 47, 3804–3815. [Google Scholar] [PubMed]
- Wang, Y.-B.; Tan, B. Construction of Axially Chiral Compoundsvia Asymmetric Organocatalysis. Acc. Chem. Res. 2018, 51, 534–547. [Google Scholar]
- Liao, G.; Zhang, T.; Lin, Z.-K.; Shi, B.-F. Transition Metal-Catalyzed Enantioselective C−H Functionalization via Chiral Tran-sient Directing Group Strategy. Angew. Chem., Int. Ed. 2020, 59, 19773–19786. [Google Scholar] [CrossRef]
- Li, T.-Z.; Liu, S.-J.; Tan, W.; Shi, F. Catalytic AsymmetricConstruction of Axially Chiral Indole-Based Frameworks: An Emerging Area. Chem. Eur. J. 2020, 26, 15779–15792. [Google Scholar]
- Wang, J.; Zhao, C.; Wang, J. Recent Progress toward theConstruction of Axially Chiral Molecules Catalyzed by an N-heterocyclic Carbene. ACS Catal. 2021, 11, 12520–12531. [Google Scholar] [CrossRef]
- Song, R.; Xie, Y.; Jin, Z.; Chi, Y.R. Carbene-CatalyzedAsymmetric Construction of Atropisomers. Angew. Chem. Int. Ed. 2021, 60, 26026–26037. [Google Scholar]
- Zhang, X.; Zhao, K.; Gu, Z. Transition Metal-Catalyzed Biaryl Atropisomer Synthesis via a Torsional Strain Promoted Ring-Opening Reaction. Acc. Chem. Res. 2022, 55, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Mino, T.; Akita, K.; Sakamoto, M.; Fujita, T. Development of Chiral (S)-Prolinol-Derived Ligands for Palladium-Catalyzed Asymmetric Allylic Alkylation: Effect of a Siloxymethyl Group on the Pyrrolidine Backbone. J. Org. Chem. 2004, 69, 6679–6687. [Google Scholar] [CrossRef] [PubMed]
- Gutnov, A. Palladium-Catalyzed Asymmetric Conjugate Addition of Aryl–Metal Species. Eur. J. Org. Chem. 2008, 2008, 4547–4554. [Google Scholar] [CrossRef]
- Chen, Q.-A.; Ye, Z.-S.; Duan, Y.; Zhou, Y.-G. Homogeneous palladium-catalyzed asymmetric hydrogenation. Chem. Soc. Rev. 2013, 42, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.Y.; Stoltz, B.M. The Construction of All-Carbon Quaternary Stereocenters by Use of Pd-Catalyzed Asymmetric Allylic Alkylation Reactions in Total Synthesis. Eur. J. Org. Chem. 2013, 2013, 2745–2759. [Google Scholar] [CrossRef]
- Shockley, S.E.; Holder, J.C.; Stoltz, B.M. Palladium-Catalyzed Asymmetric Conjugate Addition of Arylboronic Acids to α,β-Unsaturated Cyclic Electrophiles. Org. Process Res. Dev. 2015, 19, 974–981. [Google Scholar] [CrossRef]
- Pullarkat, S.A. Recent Progress in Palladium-Catalyzed Asymmetric Hydrophos phination. Synthesis 2016, 48, 493–503. [Google Scholar] [CrossRef]
- Nascimento de Oliveira, M.; Arseniyadis, S.; Cossy, J. Palladium-Catalyzed Asymmetric Allylic Alkylation of 4-Substituted Isoxazolidin-5-ones: Straightforward Access to β2,2-Amino Acids. Chem. Eur. J. 2018, 24, 4810–4814. [Google Scholar] [CrossRef]
- James, J.; Jackson, M.; Guiry, P.J. Palladium-Catalyzed Decarboxylative Asymmetric Allylic Alkylation: Development, Mechanistic Understanding and Recent Advances. Adv. Syn. Catal. 2019, 361, 3016–3049. [Google Scholar] [CrossRef]
- Wang, Z. Palladium-catalyzed asymmetric dearomative cyclization in natural product synthesis. Org. Biomol. Chem. 2020, 18, 4354–4370. [Google Scholar] [CrossRef]
- Yang, K.; Song, M.; Liu, H.; Ge, H. Palladium-catalyzed direct asymmetric C–H bond functionalization enabled by the directing group strategy. Chem. Sci. 2020, 11, 12616–12632. [Google Scholar] [PubMed]
- Pàmies, O.; Margalef, J.; Cañellas, S.; James, J.; Judge, E.; Guiry, P.J.; Moberg, C.; Bäckvall, J.-E.; Pfaltz, A.; Pericàs, M.A.; et al. Recent Advances in Enantioselective Pd-Catalyzed Allylic Substitution: From Design to Applications. Chem. Rev. 2021, 121, 4373–4505. [Google Scholar] [PubMed]
- Peng, J.-B.; Liu, X.-L.; Li, L.; Wu, X.-F. Palladium-catalyzed enantioselective carbonylation reactions. Sci. China Chem. 2022, 65, 441–461. [Google Scholar]
- Yin, X.; Li, S.; Guo, K.; Song, L.; Wang, X. Palladium-Catalyzed Enantioselective Hydrofunctionalization of Alkenes: Recent Advances. Eur. J. Org. Chem. 2023, 26, e202300783. [Google Scholar] [CrossRef]
- Feng, J.; Xi, L.-L.; Lu, C.-J.; Liu, R.-R. Transition-metal-catalyzed enantioselective C–N cross-coupling. Chem. Soc. Rev. 2024, 53, 9560–9581. [Google Scholar]
- Sadeghi, M. Cyclization Through Dual C(sp3)−H Functionalization. Adv. Syn. Catal. 2024, 366, 3542–3563. [Google Scholar]
- Xu, B.; Wang, Q.; Fang, C.; Zhang, Z.-M.; Zhang, J. Recent advances in Pd-catalyzed asymmetric cyclization reactions. Chem. Soc. Rev. 2024, 53, 883–971. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, H.; Liu, S.; Yu, R.; Ma, Y.; Zhang, R.; Fang, Y.; Lan, Y.; Shen, R. Access to indenofluorene skeletons via Pd-catalyzed sequential reaction involving cyclization of indenone–allenyne intermediates. Org. Chem. Front. 2024, 11, 5884–5889. [Google Scholar]
- Qin, W.; Liu, Y.; Yan, H. Enantioselective Synthesis of Atropisomers via Vinylidene ortho-Quinone Methides (VQMs). Acc. Chem. Res. 2022, 55, 2780–2795. [Google Scholar]
- Kitagawa, O. Chiral Pd-Catalyzed Enantioselective Syntheses of Various N–C Axially Chiral Compounds and Their Synthetic Applications. Acc. Chem. Res. 2021, 54, 719–730. [Google Scholar] [CrossRef]
- Takahashi, I.; Suzuki, Y.; Kitagawa, O. Asymmetric Synthesis of Atropisomeric Compounds with an N–C Chiral Axis. Org. Prep. Proced. Int. 2014, 46, 1–23. [Google Scholar] [CrossRef]
- Colobert, F.; Shi, B.-F. C–N atropopure compounds: New directions. Chem Catal. 2021, 1, 483–485. [Google Scholar] [CrossRef]
- Shi, B.-F.; Colobert, F. Perfect control of C–N atropisomeric axis for creating high-added-value compounds. Chem Catal. 2021, 1, 485–487. [Google Scholar] [CrossRef]
- Ototake, N.; Morimoto, Y.; Mokuya, A.; Fukaya, H.; Shida, Y.; Kitagawa, O. Catalytic Enantioselective Synthesis of Atropisomeric Indoles with an N—C Chiral Axis. Chem. Eur. J. 2010, 16, 6752–6755. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, T.; Shibata, Y.; Noguchi, K.; Tanaka, K. Palladium-Catalyzed Enantioselective Intramolecular Hydroarylation of Alkynes To Form Axially Chiral 4-Aryl 2-Quinolinones. Angew. Chem. Int. Ed. 2011, 50, 3963–3967. [Google Scholar] [CrossRef]
- Peng, C.; Kusakabe, T.; Kikkawa, S.; Mochida, T.; Azumaya, I.; Dhage, Y.D.; Takahashi, K.; Sasai, H.; Kato, K. Asymmetric Cyclizative Dimerization of (ortho-Alkynyl Phenyl) (Methoxymethyl) Sulfides with Palladium(II) Bisoxazoline Catalyst. Chem. Eur. J. 2019, 25, 733–737. [Google Scholar] [CrossRef]
- He, Y.-P.; Wu, H.; Wang, Q.; Zhu, J. Palladium-Catalyzed Enantioselective Cacchi Reaction: Asymmetric Synthesis of Axially Chiral 2,3-Disubstituted Indoles. Angew. Chem. Int. Ed. 2020, 59, 2105–2109. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Qi, Z.; Li, X. Construction of Atropisomeric 3-Arylindoles via Enantioselective Cacchi Reaction. Org. Lett. 2021, 23, 5901–5905. [Google Scholar] [CrossRef]
- Wang, C.-S.; Wei, L.; Fu, C.; Wang, X.-H.; Wang, C.-J. Asymmetric Synthesis of Axially Chiral Naphthyl-C3-indoles via a Palladium-Catalyzed Cacchi Reaction. Org. Lett. 2021, 23, 7401–7406. [Google Scholar] [CrossRef]
- Yang, W.-C.; Chen, X.-B.; Song, K.-L.; Wu, B.; Gan, W.-E.; Zheng, Z.-J.; Cao, J.; Xu, L.-W. Pd-Catalyzed Enantioselective Tandem C–C Bond Activation/Cacchi Reaction between Cyclobutanones and o-Ethynylanilines. Org. Lett. 2021, 23, 1309–1314. [Google Scholar] [CrossRef]
- Hutskalova, V.; Sparr, C. Control over Stereogenic N–N Axes by Pd-Catalyzed 5-endo-Hydroaminocyclizations. Synth. 2023, 55, 1770–1782. [Google Scholar]
- Larock, R.C.; Yum, E.K. Synthesis of indoles via palladium-catalyzed heteroannulation of internal alkynes. J. Am. Chem. Soc. 1991, 113, 6689–6690. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, B.; Yang, J.; Zhang, J. Pd-Catalyzed Asymmetric Larock Indole Synthesis to Access Axially Chiral N-Arylindoles. J. Am. Chem. Soc. 2024, 146, 5493–5501. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, D.; Wang, F.; Yu, S.; Huang, G.; Li, X. Pd-catalyzed asymmetric Larock reaction for the atroposelective synthesis of N—N chiral indoles. Sci. Adv. 2024, 10, eado4489. [Google Scholar] [CrossRef]
- Zhou, F.; Cai, Q. Recent advances in copper-catalyzed asymmetric coupling reactions. Beilstein J. Org. Chem. 2015, 11, 2600–2615. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-J.; Xu, Q.; Feng, J.; Liu, R.-R. The Asymmetric Buchwald–Hartwig Amination Reaction. Angew. Chem. Int. Ed. 2023, 62, e202216863. [Google Scholar] [CrossRef]
- Wanting, C.; Xiongwei, Z.; Jiale, X.; Changshu, W.; Yang, G. Progress in Asymmetric Catalytic Synthesis of C—N Axis Chiral Compounds. Chinese J. Org. Chem. 2024, 44, 349–377. [Google Scholar]
- Takahashi, I.; Morita, F.; Kusagaya, S.; Fukaya, H.; Kitagawa, O. Catalytic enantioselective synthesis of atropisomeric 2-aryl-4-quinolinone derivatives with an N–C chiral axis. Tetrahedron: Asymmetry 2012, 23, 1657–1662. [Google Scholar] [CrossRef]
- Hirata, T.; Takahashi, I.; Suzuki, Y.; Yoshida, H.; Hasegawa, H.; Kitagawa, O. Catalytic Enantioselective Synthesis of N—C Axially Chiral Phenanthridin-6-one Derivatives. J. Org. Chem. 2016, 81, 318–323. [Google Scholar] [CrossRef]
- Teng, F.; Yu, T.; Peng, Y.; Hu, W.; Hu, H.; He, Y.; Luo, S.; Zhu, Q. Palladium-Catalyzed Atroposelective Coupling–Cyclization of 2-Isocyanobenzamides to Construct Axially Chiral 2-Aryl- and 2,3-Diarylquinazolinones. J. Am. Chem. Soc. 2021, 143, 2722–2728. [Google Scholar] [CrossRef]
- Liu, Z.-S.; Xie, P.-P.; Hua, Y.; Wu, C.; Ma, Y.; Chen, J.; Cheng, H.-G.; Hong, X.; Zhou, Q. An axial-to-axial chirality transfer strategy for atroposelective construction of C–N axial chirality. Chem 2021, 7, 1917–1932. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, X.-M.; Xu, Q.; Guo, C.-Q.; Wang, P.; Lu, C.-J.; Liu, R.-R. Enantioselective Synthesis of Atropisomeric Biaryls by Pd-Catalyzed Asymmetric Buchwald–Hartwig Amination. Angew. Chem. Int. Ed. 2021, 60, 21718–21722. [Google Scholar]
- Zhang, P.; Guo, C.-Q.; Yao, W.; Lu, C.-J.; Li, Y.; Paton, R.S.; Liu, R.-R. Pd-Catalyzed Asymmetric Amination of Enamines: Expedient Synthesis of Structurally Diverse N–C Atropisomers. ACS Catal. 2023, 13, 7680–7690. [Google Scholar]
- Zhang, P.; Xu, Q.; Wang, X.-M.; Feng, J.; Lu, C.-J.; Li, Y.; Liu, R.-R. Enantioselective Synthesis of N−N Bisindole Atropisomers. Angew. Chem. Int. Ed. 2022, 61, e202212101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.-Y.; Chen, P.; Xia, J.-B. Synthesis of Planar Chiral Ferrocenes by Transition-Metal-Catalyzed Enantioselective C−H Activation. ChemCatChem 2016, 8, 68–73. [Google Scholar] [CrossRef]
- Shi, S.; Nawaz, K.S.; Zaman, M.K.; Sun, Z. Advances in Enantioselective C–H Activation/Mizoroki-Heck Reaction and Suzuki Reaction. Catalysts 2018, 8, 90. [Google Scholar] [CrossRef]
- Loup, J.; Dhawa, U.; Pesciaioli, F.; Wencel-Delord, J.; Ackermann, L. Enantioselective C−H Activation with Earth-Abundant 3d Transition Metals. Angew. Chem. Int. Ed. 2019, 58, 12803–12818. [Google Scholar]
- Vyhivskyi, O.; Kudashev, A.; Miyakoshi, T.; Baudoin, O. Chiral Catalysts for Pd0-Catalyzed Enantioselective C−H Activation. Chem. Eur. J. 2021, 27, 1231–1257. [Google Scholar] [CrossRef]
- Zhan, B.-B.; Jin, L.; Shi, B.-F. Palladium-catalyzed enantioselective C–H functionalization via C–H palladation. Trends Chem. 2022, 4, 220–235. [Google Scholar] [CrossRef]
- He, C.; Hou, M.; Zhu, Z.; Gu, Z. Enantioselective Synthesis of Indole-Based Biaryl Atropisomers via Palladium-Catalyzed Dynamic Kinetic Intramolecular C–H Cyclization. ACS Catal. 2017, 7, 5316–5320. [Google Scholar] [CrossRef]
- Newton, C.G.; Braconi, E.; Kuziola, J.; Wodrich, M.D.; Cramer, N. Axially Chiral Dibenzazepinones by a Palladium(0)-Catalyzed Atropo-enantioselective C−H Arylation. Angew. Chem. Int. Ed. 2018, 57, 11040–11044. [Google Scholar]
- Ye, J.; Li, L.; You, Y.; Jiao, C.; Cui, Z.; Zhang, Y.; Jia, S.; Cong, H.; Liu, S.; Cheng, H.-G.; et al. Enantioselective Assembly of Ferrocenes with Axial and Planar Chiralities via Palladium/Chiral Norbornene Cooperative Catalysis. JACS Au 2023, 3, 384–390. [Google Scholar] [PubMed]
- Wang, X.; Xu, J.; Luo, Y.; Wang, Y.; Huang, J.; Zhu, Q.; Luo, S. DFT-Assisted Atroposelective Construction of Indole-Fused N-Heteroaromatic Frameworks through Palladium-Catalyzed C–H Imidoylation. ACS Catal. 2025, 15, 201–210. [Google Scholar]
- Chen, L.-P.; Chen, J.-F.; Zhang, Y.-J.; He, X.-Y.; Han, Y.-F.; Xiao, Y.-T.; Lv, G.-F.; Lu, X.; Teng, F.; Sun, Q.; et al. Atroposelective carbonylation of aryl iodides with amides: Facile synthesis of enantioenriched cyclic and acyclic amides. Org. Chem. Front. 2021, 8, 6067–6073. [Google Scholar]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Ren, W.; Zhang, J.; Zhao, S.; Zhou, D.; Chen, H.; Liu, T. Recent Advances in Palladium-Catalyzed Enantioselective Cyclization for the Construction of Atropisomers. Catalysts 2025, 15, 320. https://doi.org/10.3390/catal15040320
Wang X, Ren W, Zhang J, Zhao S, Zhou D, Chen H, Liu T. Recent Advances in Palladium-Catalyzed Enantioselective Cyclization for the Construction of Atropisomers. Catalysts. 2025; 15(4):320. https://doi.org/10.3390/catal15040320
Chicago/Turabian StyleWang, Xilong, Wei Ren, Jingyi Zhang, Shunwei Zhao, Duo Zhou, Hui Chen, and Tingting Liu. 2025. "Recent Advances in Palladium-Catalyzed Enantioselective Cyclization for the Construction of Atropisomers" Catalysts 15, no. 4: 320. https://doi.org/10.3390/catal15040320
APA StyleWang, X., Ren, W., Zhang, J., Zhao, S., Zhou, D., Chen, H., & Liu, T. (2025). Recent Advances in Palladium-Catalyzed Enantioselective Cyclization for the Construction of Atropisomers. Catalysts, 15(4), 320. https://doi.org/10.3390/catal15040320