Interfacial Engineering of CN-B/Ti3C2 MXene Heterojunction for Synergistic Solar-Driven CO2 Reduction
Abstract
1. Introduction
2. Results and Discussion
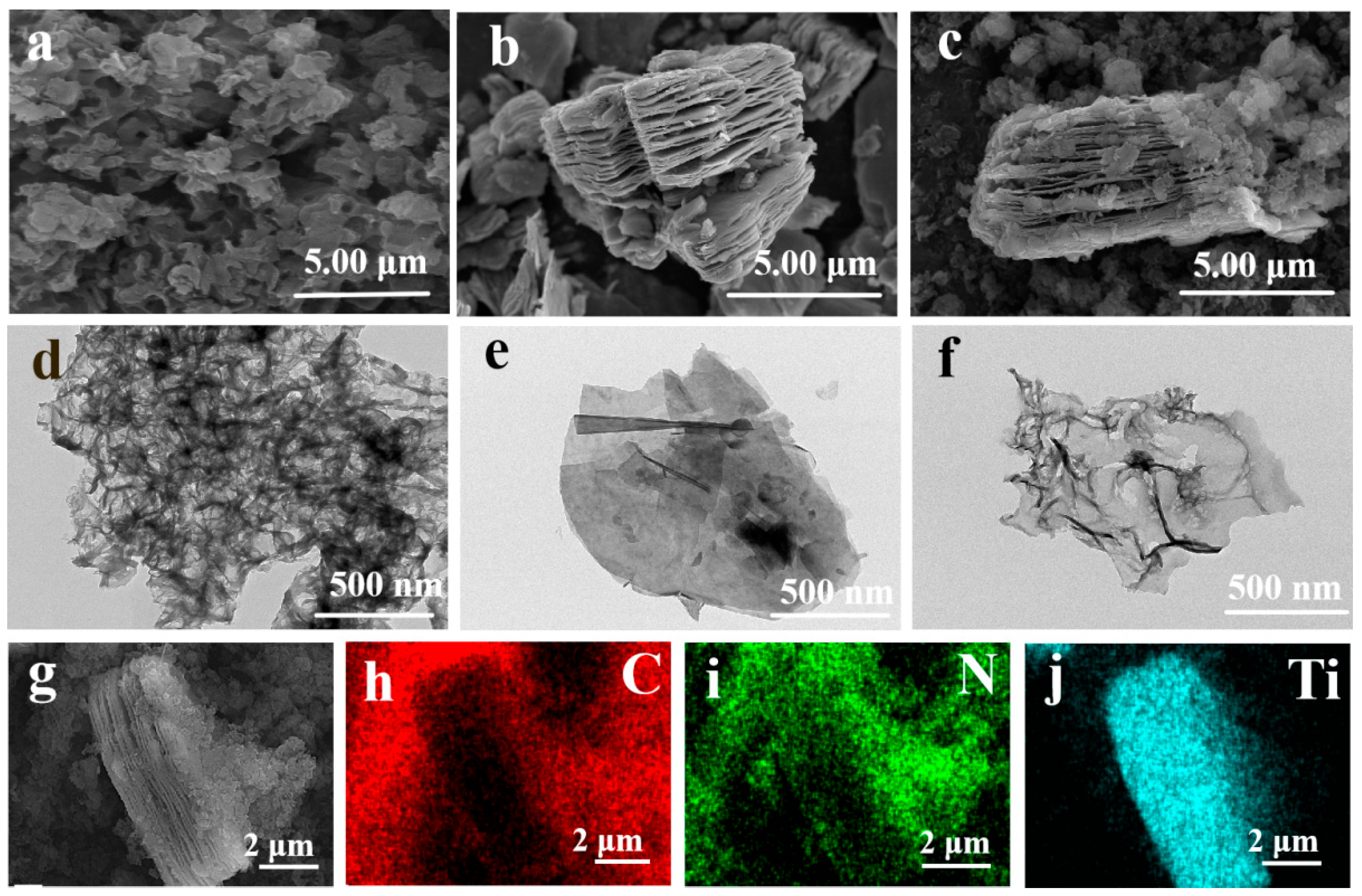
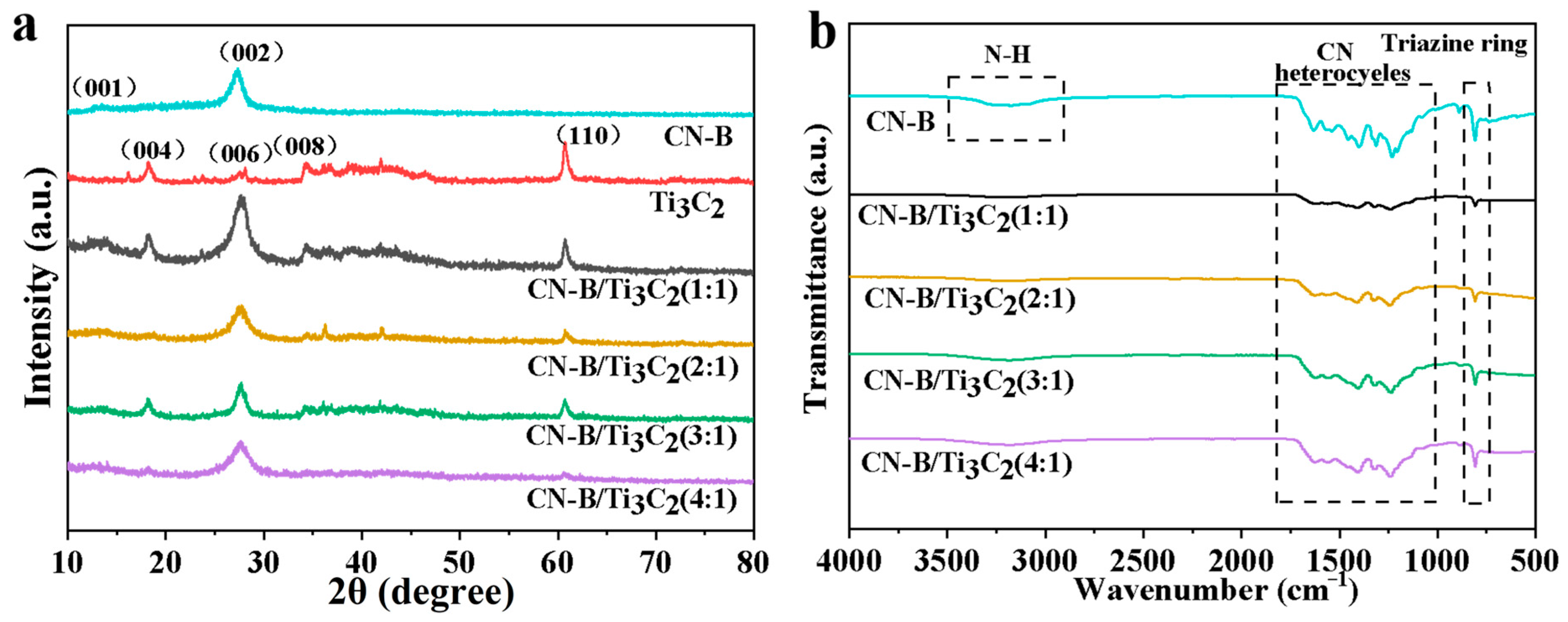
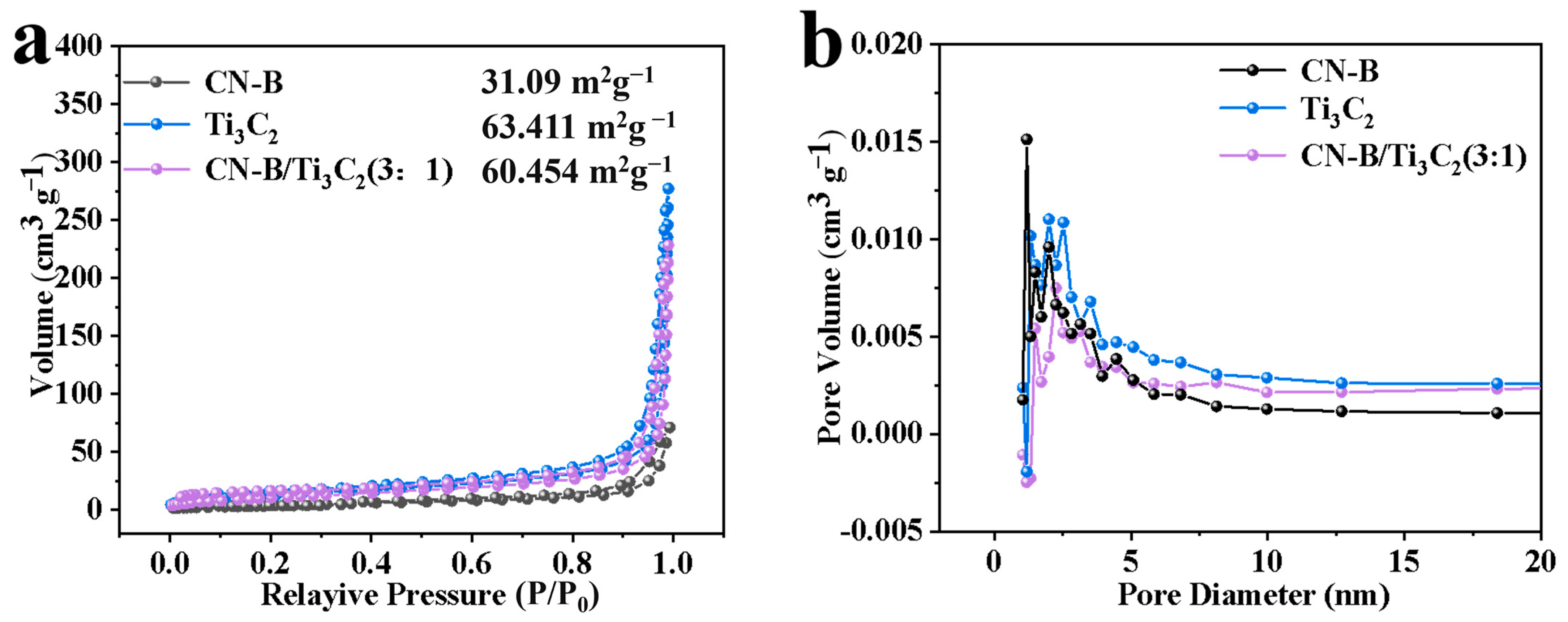
2.1. Photocatalyst Characterization
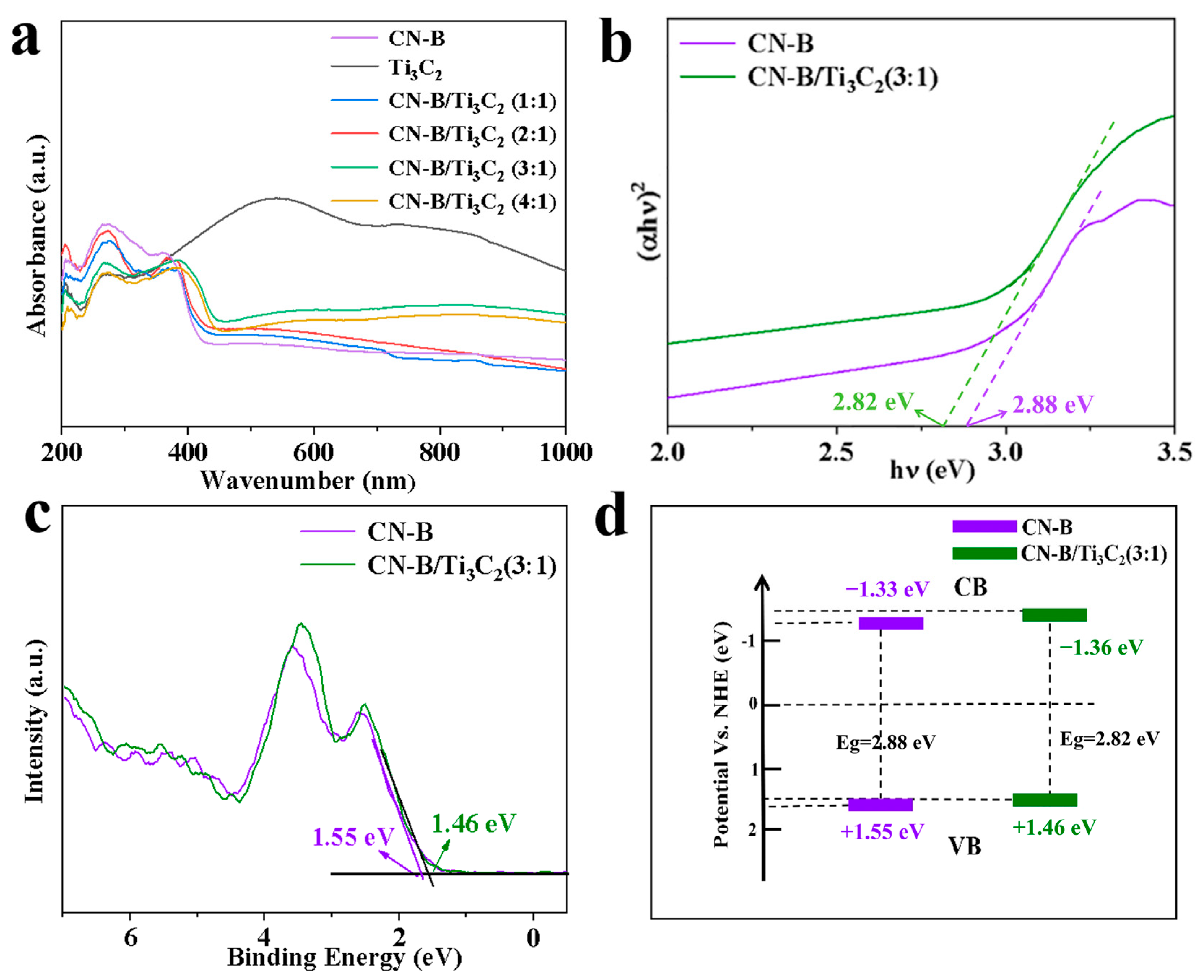
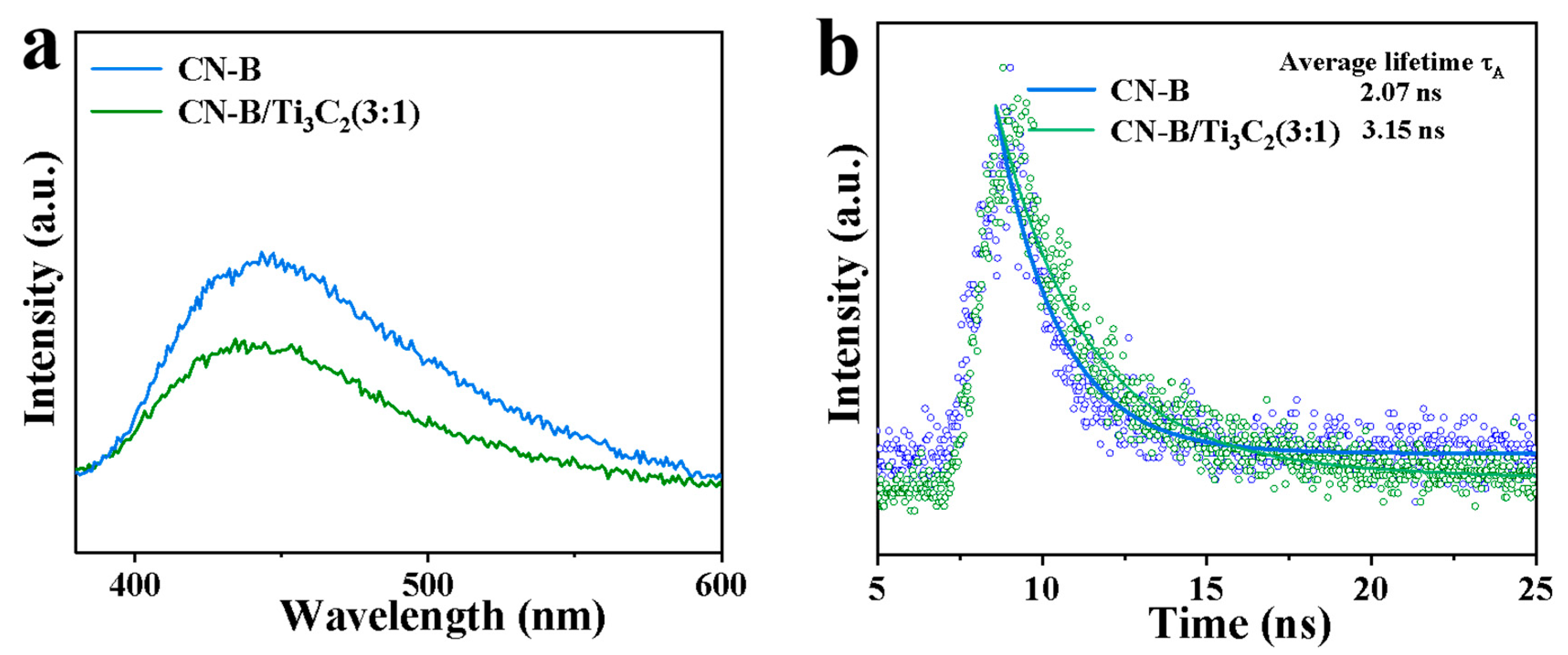
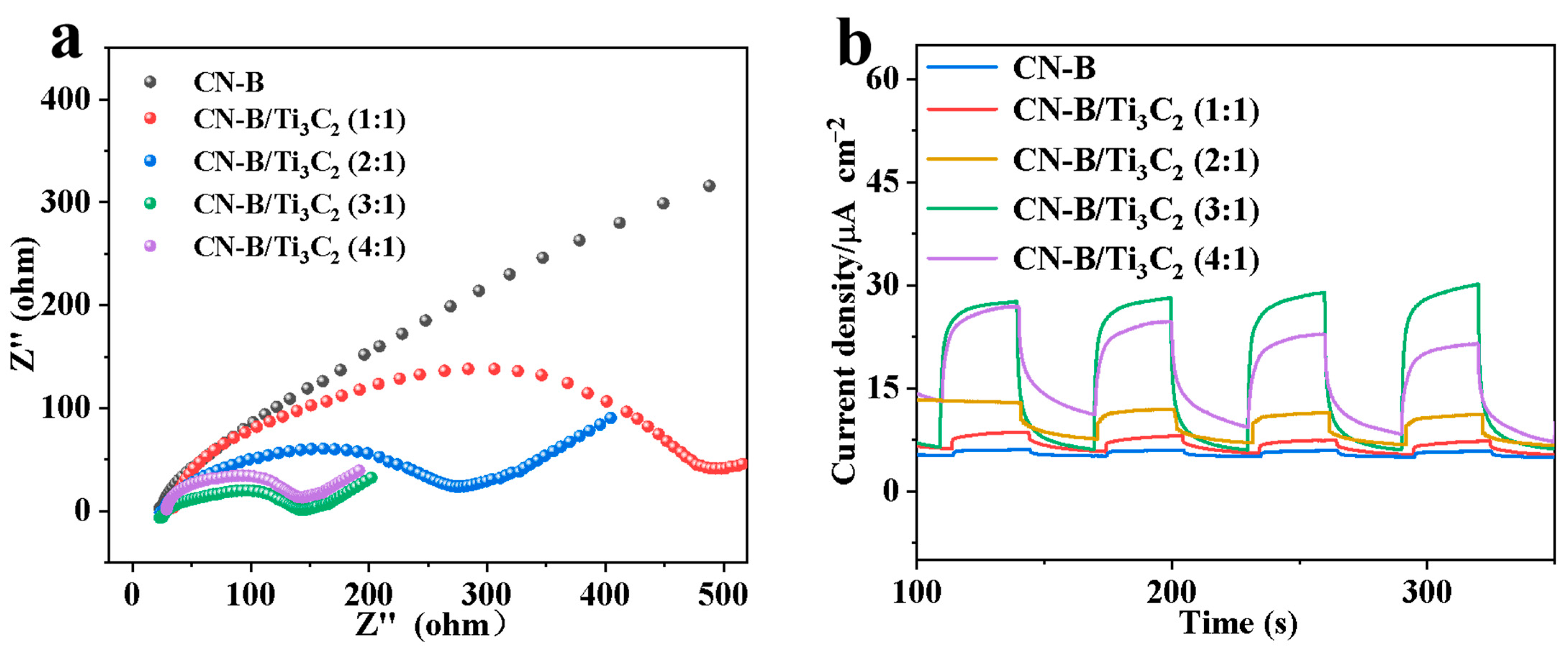
2.2. Surface Composition and Photoelectric Analysis
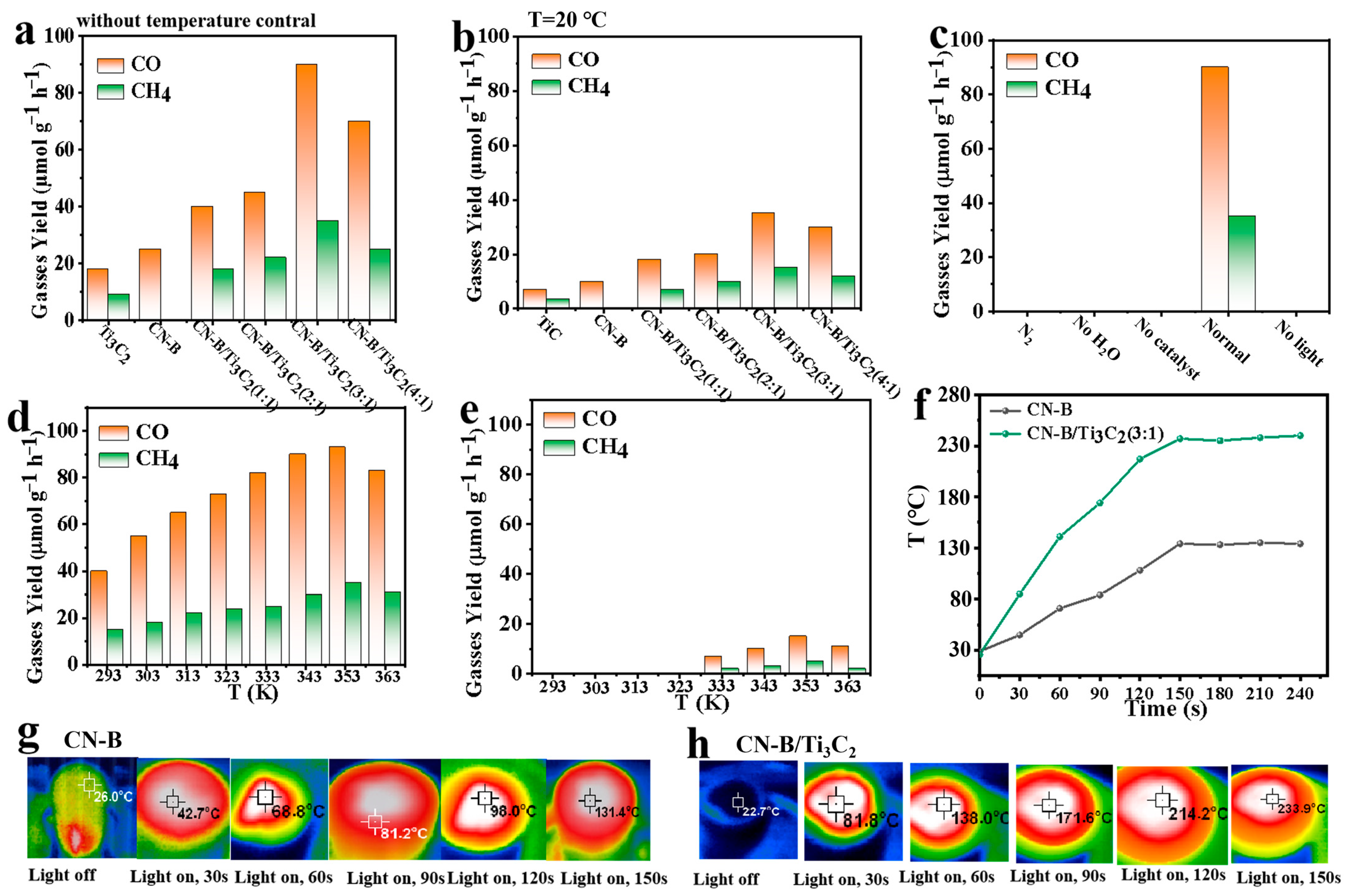
2.3. Photocatalytic Activity
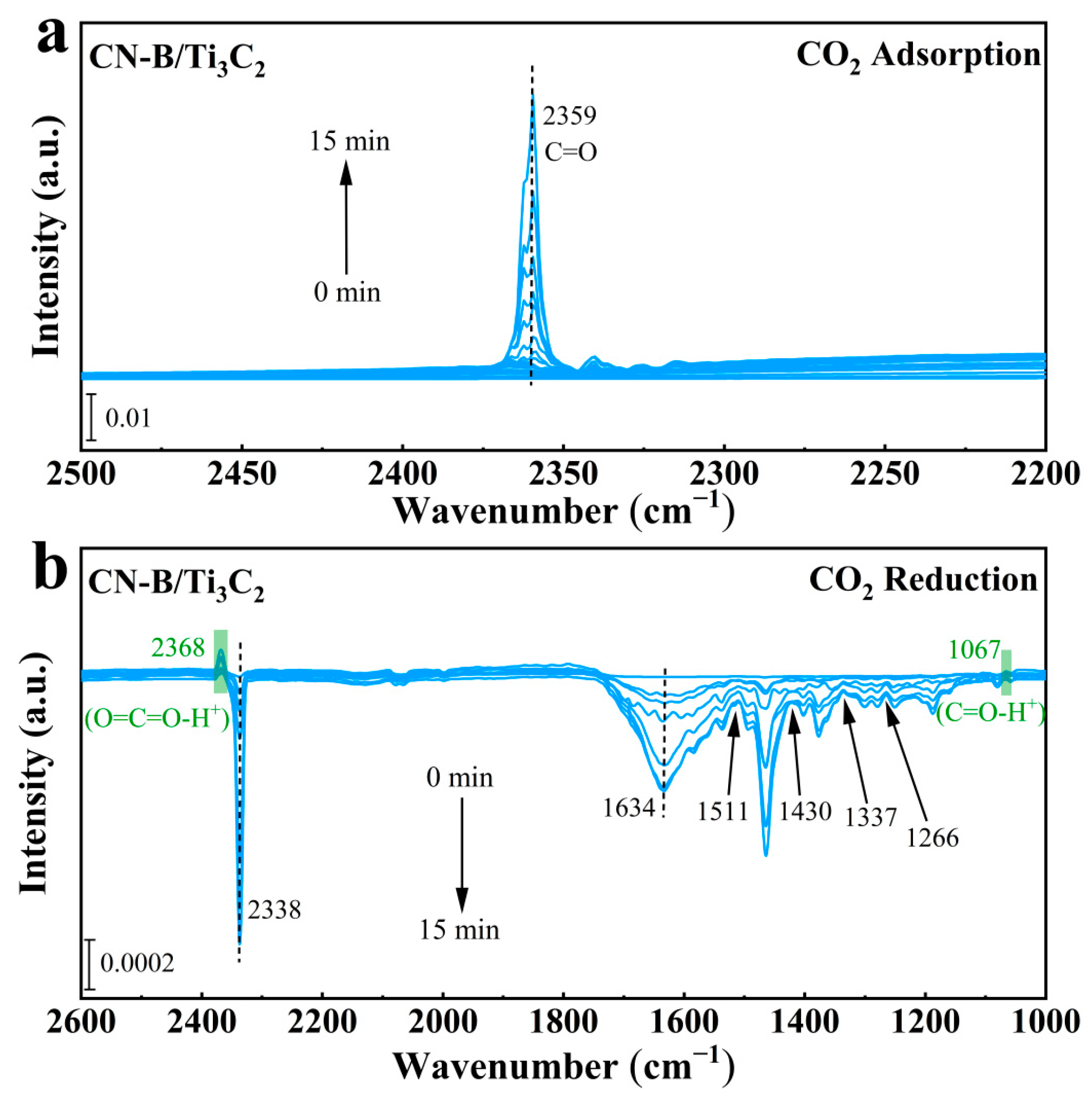
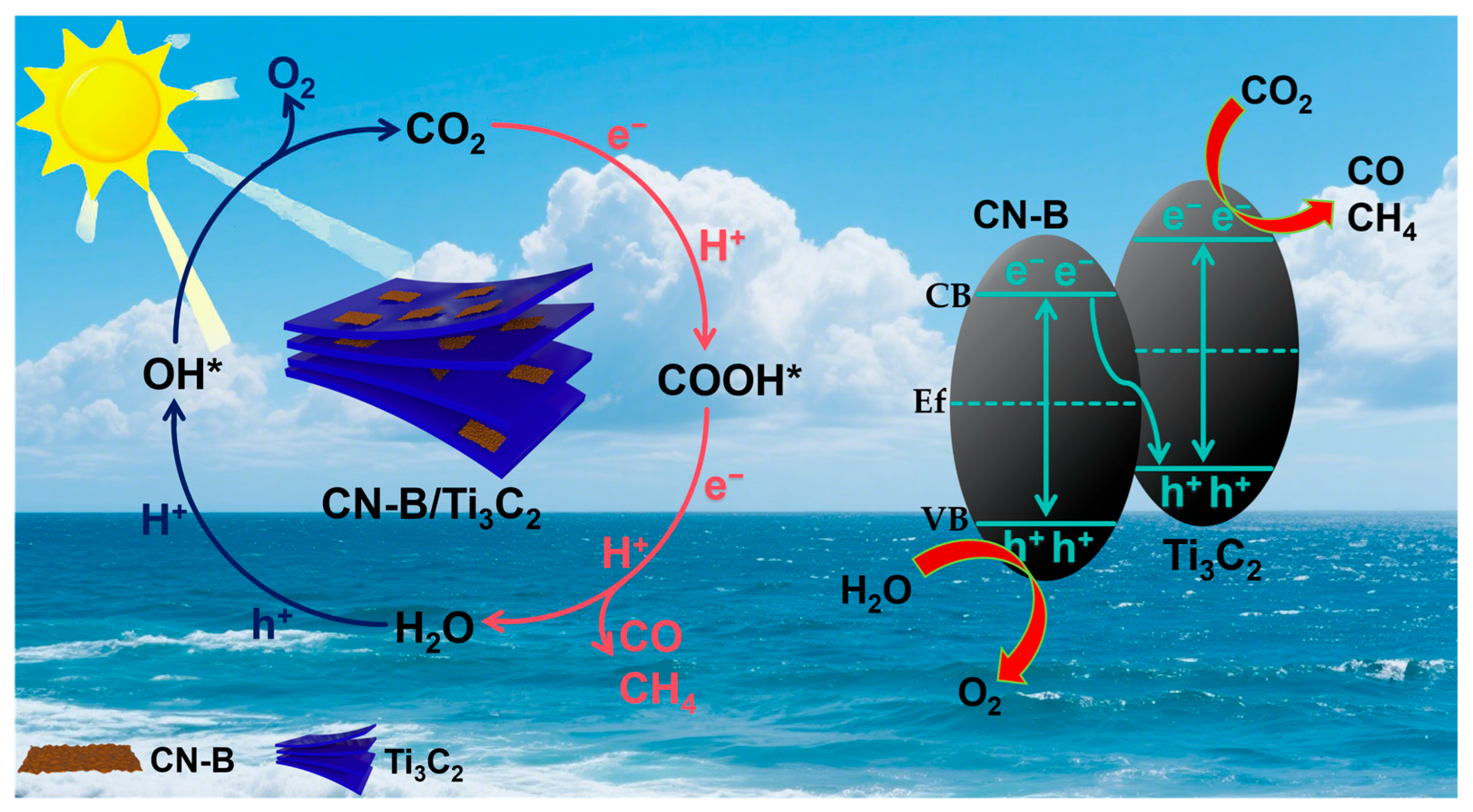
2.4. Mechanism of CO2 Photoreduction
3. Experimental Section
3.1. Methods
3.1.1. Materials and Chemicals
3.1.2. Synthesis of CN-B
3.1.3. Synthesis of Ti3C2 MXene
3.1.4. Synthesis of CN-B/Ti3C2 Composites
3.2. Characterizations
3.3. CO2 Photoreduction Experiments
3.4. Photo-Electrochemical Measurements
3.5. Calculation of AQYs Parameters for Photoreduction of Carbon Dioxide by CN-B/Ti3C2 (3:1) at Different Wavelengths
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abram, N.; Purich, A.; England, M.; McCormack, F.; Strugnell, J.; Bergstrom, D.; Vance, T.; Stål, T.; Wienecke, B.; Heil, P.; et al. Emerging evidence of abrupt changes in the Antarctic environment. Nature 2025, 644, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Tang, Y.; Cheng, P.; Song, Y.; Li, X.; Lou, J.; Iqbal, B.; Zhao, X.; Hameed, R.; Li, G.; et al. Effects of degradable and non-degradable microplastics and oxytetracycline co-exposure on soil N2O and CO2 emissions. Appl. Soil Ecol. 2024, 197, 105331. [Google Scholar] [CrossRef]
- Zhu, Z.; Xing, X.; Qi, Q.; Li, H.; Han, D.; Song, X.; Tang, X.; Ng, Y.; Huo, P. Regulation CN reduction of CO2 products selectivity by adjusting the number of V sites and mechanism exploration. Fuel 2025, 388, 134509. [Google Scholar] [CrossRef]
- Ozin, G. Accelerated optochemical engineering solutions to CO2 photocatalysis for a sustainable future. Matter 2022, 5, 2594. [Google Scholar] [CrossRef]
- Yang, J.; Ma, B.; Zhu, Y. A novel NDI/PTA organic heterojunction photocatalyst with built-in electric field for efficient hydrogen production. Appl. Catal. B Environ. Energy 2026, 381, 125890. [Google Scholar] [CrossRef]
- Bi, X.; Li, L.; Luo, L.; Liu, X.; Li, J.; You, T. A ratiometric fluorescence aptasensor based on photoinduced electron transfer from CdTe QDs to WS2 NTs for the sensitive detection of zearalenone in cereal crops. Food Chem. 2022, 385, 132657. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Gao, X.; Lv, X.; Zhao, Y.; Yin, X.; Liu, Y.; Fang, J.; Yu, X.; Ma, X.; Ma, T.; et al. Decade Milestone Advancement of Defect-Engineered g-C3N4 for Solar Catalytic Applications. Nano-Micro Lett. 2024, 16, 70. [Google Scholar] [CrossRef]
- Li, B.; Li, N.; Guo, Z.; Wang, C.; Zhu, Z.; Tang, X.; Andrew Penyikie, G.; Wang, X.; Huo, P. Understanding the effect of multiple configurations in polymeric carbon nitride for efficient solar-driven hydrogen peroxide photosynthesis. Chem. Eng. J. 2024, 491, 152022. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Liu, P.; Zhao, K.; Ye, S.; Liang, G. Rapid, ultrasensitive and non-enzyme electrochemiluminescence detection of hydrogen peroxide in food based on the ssDNA/g-C3N4 nanosheets hybrid. Food Chem. 2021, 357, 129753. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Z.; Fang, R.; Yan, Y.; Ran, J.; Zhang, L. A State-of-the-art review on action mechanism of photothermal catalytic reduction of CO2 in full solar spectrum. Chem. Eng. J. 2022, 429, 132322. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, F.; Zhong, L.; Yu, D. Photoelectric/Photothermal Synergy Activation in Floating Aerogel Cathode Breaks Neutral Triphase Reaction Barriers for High-Efficiency Full-Spectrum-Responsive Seawater Batteries. Angew. Chem. Int. Ed. 2025, 64, e202508644. [Google Scholar] [CrossRef]
- He, H.; Ren, Y.; Zhu, Y.; Peng, R.; Lan, S.; Zhou, J.; Yang, B.; Si, Y.; Li, N. Continuous Flow Photothermal Catalytic CO2 Reduction: Materials, Mechanisms, and System Design. ACS Catal. 2025, 15, 10480–10520. [Google Scholar] [CrossRef]
- Kuang, L.; Chen, Z.; Yan, Y.; Guo, F.; Shi, W. Research progress of g-C3N4-based materials for photothermal-assisted photocatalysis. Int. J. Hydrogen Energy 2024, 87, 20–49. [Google Scholar] [CrossRef]
- Lei, D.; Su, D.; Maier, S. New insights into plasmonic hot-electron dynamics. Light Sci. Appl. 2024, 13, 243. [Google Scholar]
- Chen, Y.; Lin, X.; Li, W.; Sun, H.; Jia, S.; Zhou, Y.; Hao, Y.; Liu, Z.; Yin, S.; Guo, C.; et al. Harnessing Photo-to-Thermal Conversion in Sulfur-Vulcanized Mxene for High-Efficiency Solar-to-Carbon-Fuel Synthesis. Adv. Funct. Mater. 2024, 34, 2400121. [Google Scholar] [CrossRef]
- Karmakar, S.; Barman, S.; Rahimi, F.; Rambabu, D.; Nath, S.; Maji, T. Confining charge-transfer complex in a metal-organic framework for photocatalytic CO2 reduction in water. Nat. Commun. 2023, 14, 4508. [Google Scholar] [CrossRef]
- Han, M.; Yin, X.; Wu, H.; Hou, Z.; Song, C.; Li, X.; Zhang, L.; Cheng, L. Ti3C2 MXenes with Modified Surface for High-Performance Electromagnetic Absorption and Shielding in the X-Band. ACS Appl. Mater. Interfaces 2016, 8, 21011. [Google Scholar] [CrossRef]
- Du, X.; Du, W.; Sun, J.; Jiang, D. Self-powered photoelectrochemical sensor for chlorpyrifos detection in fruit and vegetables based on metal-ligand charge transfer effect by Ti3C2 based Schottky junction. Food Chem. 2022, 385, 132731. [Google Scholar] [CrossRef] [PubMed]
- Faraji, M.; Bafekry, A.; Fadlallah, M.; Molaei, F.; Hieu, N.; Qian, P.; Ghergherehchi, M.; Gogova, D. Surface modification of titanium carbide MXene monolayers (Ti2C and Ti3C2) via chalcogenide and halogenide atoms. Phys. Chem. Chem. Phys. 2021, 23, 15319. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, Q.; Wu, Z. Construction of Few-Layer Ti3C2 MXene and Boron-Doped g-C3N4 for Enhanced Photocatalytic CO2 Reduction. ACS Sustain. Chem. Eng. 2021, 9, 8425–8434. [Google Scholar] [CrossRef]
- Liu, X.; Mu, Y.; Shi, L.; Yu, J.; Tao, R.; Chu, Z.; Liu, S.; Fan, X.; Liu, K. Enhanced photocatalytic overall water splitting via Al-doped SrTiO3/Ti3C2 Mxene Schottky heterojunction. Int. J. Hydrogen Energy 2025, 170, 151173. [Google Scholar] [CrossRef]
- Xiao, W.; Yu, H.; Xu, C.; Pu, Z.; Cheng, X.; Yu, F.; Liu, C.; Zhang, Q.; Zou, Z. 2D/2D Ti3C2 MXene/HTiNbO5 nanosheets Schottky heterojunction for boosting photothermal-assisted solar-driven photodegradation of tetracycline hydrochloride. J. Mater. Sci. Technol. 2024, 180, 193–206. [Google Scholar] [CrossRef]
- Xing, W.; Lin, H.; Shao, W.; Jian, N.; Liu, T.; Wen, S.; Han, J.; Wu, G. Heteroatom- and bonded tailored Schottky barrier in Ti3C2 MXene/carbon nitride heterojunctions toward efficient photocatalysis. Chem. Eng. J. 2025, 520, 166126. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, Y.; Ye, L.; Gong, T.; Lu, X.; Yan, N.; Fu, Y. Establishing Dual-Interface Built-InElectric Fieldswithin Janus Heterostructures for Cooperative Photoredox Catalysis. J. Am. Chem. Soc. 2025, 147, 18637–18650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ali, A.; Osman, H.; Samad, S.; Abdulrahman, A.; Zhang, K.; Mahariq, I.; Kutliev, U.; Abduvokhidov, A. Engineering a Multifunctional Cu2O/BiVO4@Ti3C2 MXene Z-Scheme heterojunction for photocatalytic environmental Detoxification, H2 Generation, and CO2 reduction to CO and CH4: Experimental modeling and mechanistic insights. Sep. Purif. Technol. 2025, 369, 133110. [Google Scholar] [CrossRef]
- Kuate, L.; Chen, Z.; Lu, J.; Wen, H.; Guo, F.; Shi, W. Photothermal-Assisted Photocatalytic Degradation of Tetracycline in Seawater Based on the Black g-C3N4 Nanosheets with Cyano Group Defects. Catalysts 2023, 13, 1147. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Li, H.; Lin, S.; Ding, W.; Zhu, X.; Sheng, Z.; Wang, H.; Zhu, X.; Sun, Y. 2D/2D 1T-MoS2/Ti3C2 MXene Heterostructure withExcellent Supercapacitor Performance. Adv. Funct. Mater. 2020, 30, 1910302. [Google Scholar]
- Saini, B.; Harikrishna, K.; Laishram, D.; Krishnapriya, R.; Singhal, R.; Sharma, R. Roleof ZnO in ZnO Nanoflake/Ti3C2 MXene Compositesin Photocatalyticand Electrocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2022, 5, 9319–9333. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Xie, T.; Feng, S.; Xue, L. Boosting visible-light-driven photocatalytic activity of BiPO4 via constructing Schottky junction with Ti3C2 MXene. Mater. Des. 2020, 192, 108772. [Google Scholar] [CrossRef]
- Shi, H.; Shao, R.; Lei, S.; Zhou, M.; Yuan, X.; Chen, Z.; Wei, S.; Zhong, S.; Zhao, Y.; Zhao, L.; et al. Breaking Janus shells in hollow heterostructured photocatalysts with spatially separated redox sites for enhanced synergy of CO2 methanation and H2O oxidation. Appl. Catal. B Environ. Energy 2025, 373, 125363. [Google Scholar] [CrossRef]
- Luo, L.; Huang, Y.; Cheng, K.; Alhassan, A.; Alqahtani, M.; Tang, L.; Wu, J. MXene-GaN van der Waals metal-semiconductor junctions for high performance multiple quantum well photodetectors. Light-Sci. Appl. 2021, 10, 177. [Google Scholar] [CrossRef]
- Pacheco-Peña, V.; Hallam, T.; Healy, N. MXene supported surface plasmons on telecommunications optical fibers. Light-Sci. Appl. 2022, 11, 22. [Google Scholar] [CrossRef]
- Jiang, L.; Zheng, Y.; Zhao, W.; Li, D.; Liu, M.; Pan, W.; Lan, M.; Huang, M.; Zhou, Y.; Jin, S. Ti3C2/AuNR heterojunction for near-infrared II plasma enhanced photothermal and photodynamic synergistic antibacterial therapy. Chem. Eng. J. 2025, 518, 164804. [Google Scholar] [CrossRef]
- Shen, W.; Qi, Q.; Hu, B.; Zhu, Z.; Huo, P.; Jiang, J.; Tang, X. Studying bimetals copper-indium for enhancing PCN photocatalytic CO2 reduction activity and selectivity mechanism. J. Ind. Eng. Chem. 2025, 145, 384–394. [Google Scholar] [CrossRef]
- Jing, X.; Mi, X.; Lu, W.; Lu, N.; Du, S.; Wang, G.; Zhang, Z. Edge effect-enhanced CO2 adsorption and photo-reduction over g-C3N4 nanosheet. Chin. J. Catal. 2024, 67, 112–123. [Google Scholar] [CrossRef]
- Otgonbayar, Z.; Oh, W. Synthesis of 2D/2D structural Ti3C2 MXene/g-C3N4 via the Schottky junction with metal oxides: Photocatalytic CO2 reduction with a cationic scavenger. Appl. Mater. Today 2023, 32, 101814. [Google Scholar] [CrossRef]
- Li, J.; Peng, H.; Luo, B.; Cao, J.; Ma, L.; Jing, D. The enhanced photocatalytic and photothermal effects of Ti3C2 Mxene quantum dot/macrosco picporous graphitic carbon nitride heterojunction for Hydrogen Production. J. Colloid Interface Sci. 2023, 641, 309–318. [Google Scholar] [CrossRef]
- He, F.; Zhu, B.; Cheng, B.; Yu, J.; Ho, W.; Macyk, W. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal. B Environ. 2020, 272, 119006. [Google Scholar] [CrossRef]
- Saeed, A.; Chen, W.; Shah, A.; Zhang, Y.; Mehmood, I.; Liu, Y. Enhancement of photocatalytic CO2 reduction for novel Cd0.2Zn0.8S@Ti3C2 (MXenes) nanocomposites. J. CO2 Util. 2021, 47, 101501. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, J.; Sun, Y.; Jia, S.; Liu, Z.; Tang, X.; Hu, B.; Zhang, Y.; Yan, Y.; Zhu, Z. Research on Cu-Site Modification of g-C3N4/CeO2-like Z-Scheme Heterojunction for Enhancing CO2 Reduction and Mechanism Insight. Catalysts 2024, 14, 546. [Google Scholar] [CrossRef]
- Deng, X.; Ke, Y.; Ding, J.; Zhou, Y.; Huang, H.; Liang, Q.; Kang, Z. Construction of ZnO@CDs@Co3O4 sandwich heterostructure with multi-interfacial electron-transfer toward enhanced photocatalytic CO2 reduction. Chin. Chem. Lett. 2024, 35, 109064. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, H.; Liu, Q.; Yu, J.; Zhu, J.; Li, Y.; Li, R.; Wang, J. 2D/2D heterojunction of Ti3C2/porous few-layer g-C3N4 nanosheets for high-efficiency extraction of uranium (VI). Sep. Purif. Technol. 2023, 312, 123442. [Google Scholar] [CrossRef]
- Yan, J.; Sun, Y.; Cai, J.; Cai, M.; Hu, B.; Yan, Y.; Zhang, Y.; Tang, X. Construction of ZnCdS Quantum-Dot-Modified CeO2 (0D–2D) Heterojunction for Enhancing Photocatalytic CO2 Reduction and Mechanism Insight. Catalysts 2024, 14, 599. [Google Scholar] [CrossRef]
- Chen, X.; Cai, M.; Huo, P.; Yan, Y.; Zhang, Y.; Li, P.; Zhu, Z. Sulfur-vulcanized CoFe2O4 with high-efficiency photo-to-thermal conversion for enhanced CO2 reduction and mechanistic insights into selectivity. Carbon Capture Sci. Technol. 2025, 14, 100377. [Google Scholar] [CrossRef]
- Qi, Q.; Shen, W.; Cai, M.; Cai, J.; Hu, B.; Han, D.; Tang, X.; Zhu, Z.; Huo, P. Construction of Cu-Modified g-C3N4 Nanosheets for Photoinduced CO2 Reduction to CO and Selectivity Mechanism Insight. ACS Appl. Nano Mater. 2024, 7, 24788–24797. [Google Scholar] [CrossRef]
- Yin, S.; Zhou, Y.; Liu, Z.; Wang, H.; Zhao, X.; Zhu, Z.; Yan, Y.; Huo, P. Elucidating protonation pathways in CO2 photoreduction using the kinetic isotope effect. Nat. Commun. 2024, 15, 437. [Google Scholar] [CrossRef]
- Ma, M.; Fang, Y.; Huang, Z.; Wu, S.; He, W.; Ge, S.; Zheng, Z.; Zhou, Y.; Fa, W.; Wang, X. Mechanistic Insights Into H2O Dissociation in Overall Photo-/Electro-Catalytic CO2 Reduction. Angew. Chem. Int. Ed. 2025, 64, e202425195. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, J.; Xiao, X.; Meng, H.; Wu, J.; Guo, C.; Zheng, M.; Wang, X.; Guo, S.; Jiang, B. Engineering of SnO2/TiO2 heterojunction compact interface with efficient charge transfer pathway for photocatalytic hydrogen evolution. Chin. Chem. Lett. 2023, 34, 107125. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, R.; Huang, J.; Jiang, Y.; Zhao, C.; Yang, X. In situ construction of protonated g-C3N4/Ti3C2 MXene Schottky heterojunctions for efficient photocatalytic hydrogen production. Chin. J. Catal. 2021, 42, 107–114. [Google Scholar] [CrossRef]
- Li, L.; Chen, F.; Zhao, B.; Yu, Y. Understanding of the structural evolution of catalysts and identification of active species during CO2 conversion. Chin. Chem. Lett. 2024, 35, 109240. [Google Scholar] [CrossRef]

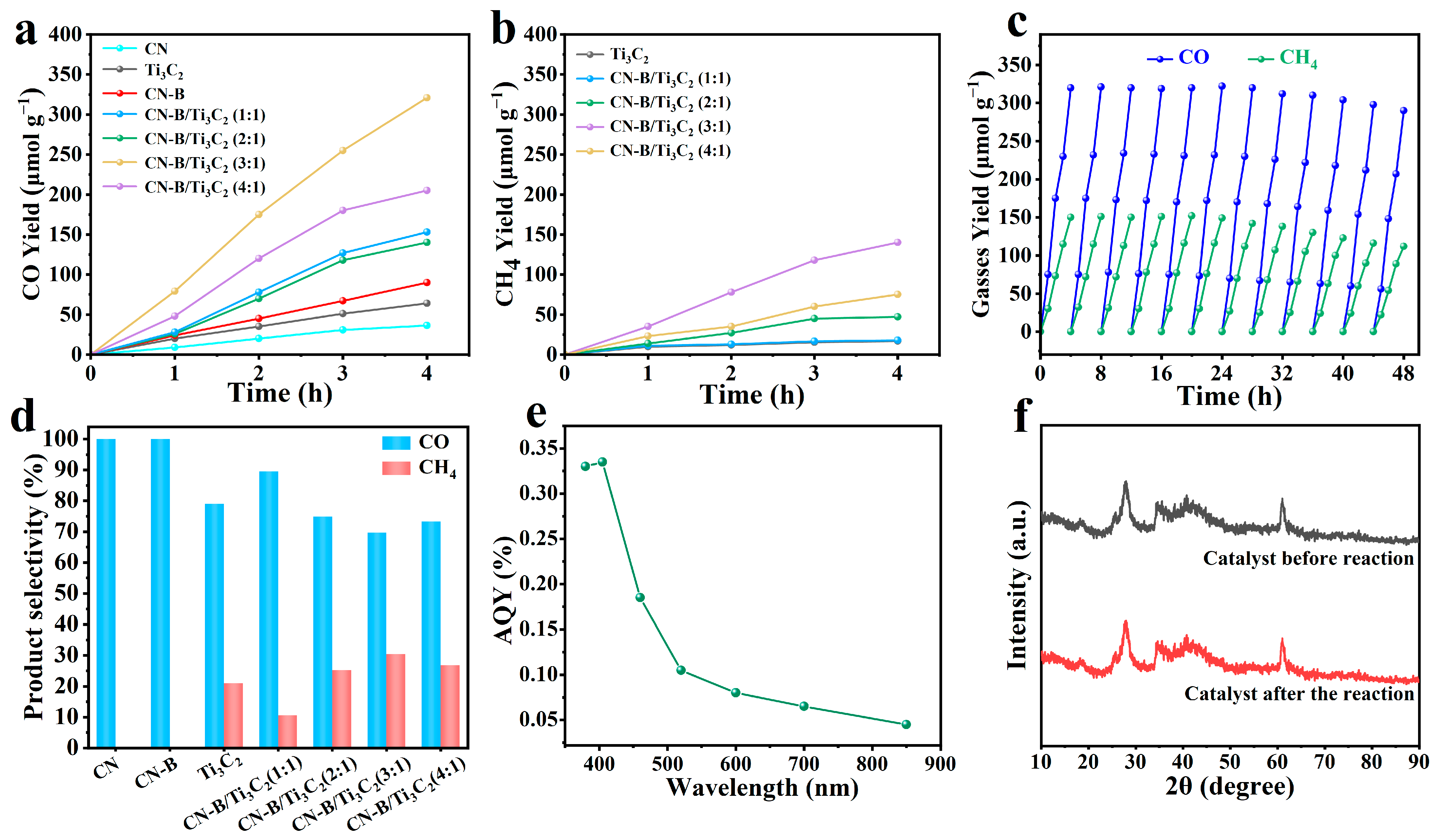
| Wavelength (nm) | CH4 Evolution (μmol g−1) | Light Intensity (mW cm−2) | Irradiation Time (h) | Irradiation Area (cm2) | AQY (%) |
|---|---|---|---|---|---|
| 380 | 0.447 | 20 | 1 | 4 | 0.33 |
| 405 | 0.4505 | 20 | 1 | 4 | 0.335 |
| 460 | 0.2485 | 20 | 1 | 4 | 0.185 |
| 520 | 0.142 | 20 | 1 | 4 | 0.105 |
| 600 | 0.1055 | 20 | 1 | 4 | 0.08 |
| 700 | 0.088 | 20 | 1 | 4 | 0.065 |
| 850 | 0.0625 | 20 | 1 | 4 | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, M.; Lv, S.; Li, Y.; Utomo, W.P.; Yan, Y.; Zhu, Z.; Zhao, J. Interfacial Engineering of CN-B/Ti3C2 MXene Heterojunction for Synergistic Solar-Driven CO2 Reduction. Catalysts 2025, 15, 1037. https://doi.org/10.3390/catal15111037
Cai M, Lv S, Li Y, Utomo WP, Yan Y, Zhu Z, Zhao J. Interfacial Engineering of CN-B/Ti3C2 MXene Heterojunction for Synergistic Solar-Driven CO2 Reduction. Catalysts. 2025; 15(11):1037. https://doi.org/10.3390/catal15111037
Chicago/Turabian StyleCai, Ming, Shaokun Lv, Yuanyuan Li, Wahyu Prasetyo Utomo, Yongsheng Yan, Zhi Zhu, and Jun Zhao. 2025. "Interfacial Engineering of CN-B/Ti3C2 MXene Heterojunction for Synergistic Solar-Driven CO2 Reduction" Catalysts 15, no. 11: 1037. https://doi.org/10.3390/catal15111037
APA StyleCai, M., Lv, S., Li, Y., Utomo, W. P., Yan, Y., Zhu, Z., & Zhao, J. (2025). Interfacial Engineering of CN-B/Ti3C2 MXene Heterojunction for Synergistic Solar-Driven CO2 Reduction. Catalysts, 15(11), 1037. https://doi.org/10.3390/catal15111037









