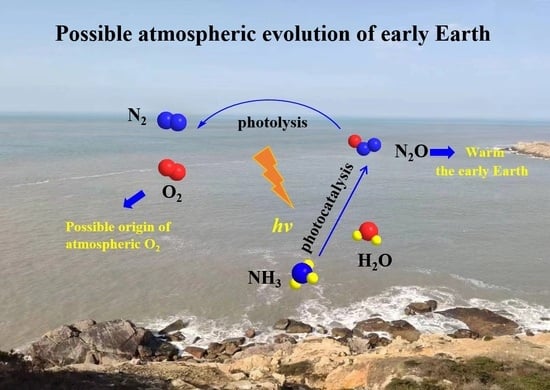
Photocatalysis: A Possible Vital Contributor to the Evolution of the Prebiotic Atmosphere and the Warming of the Early Earth
Abstract
1. Introduction
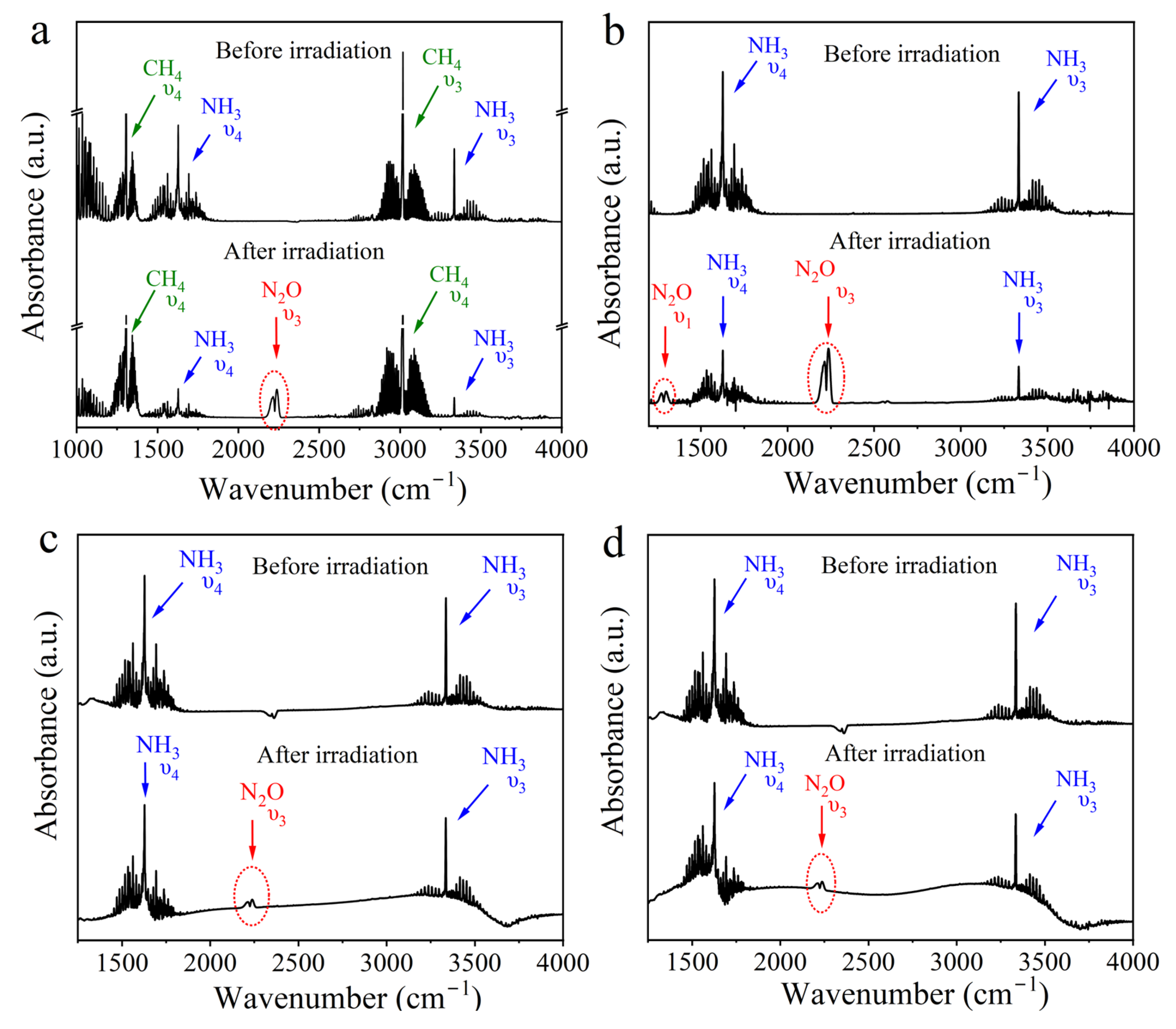
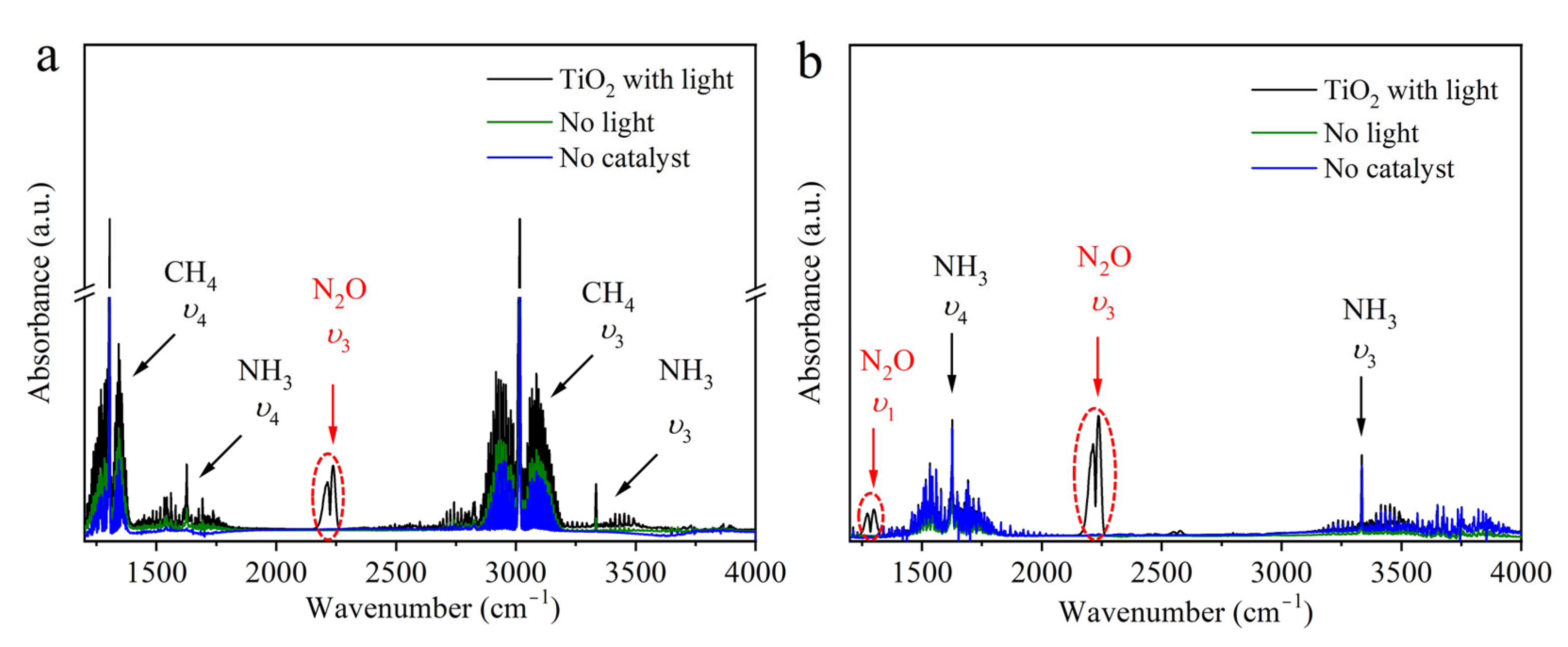
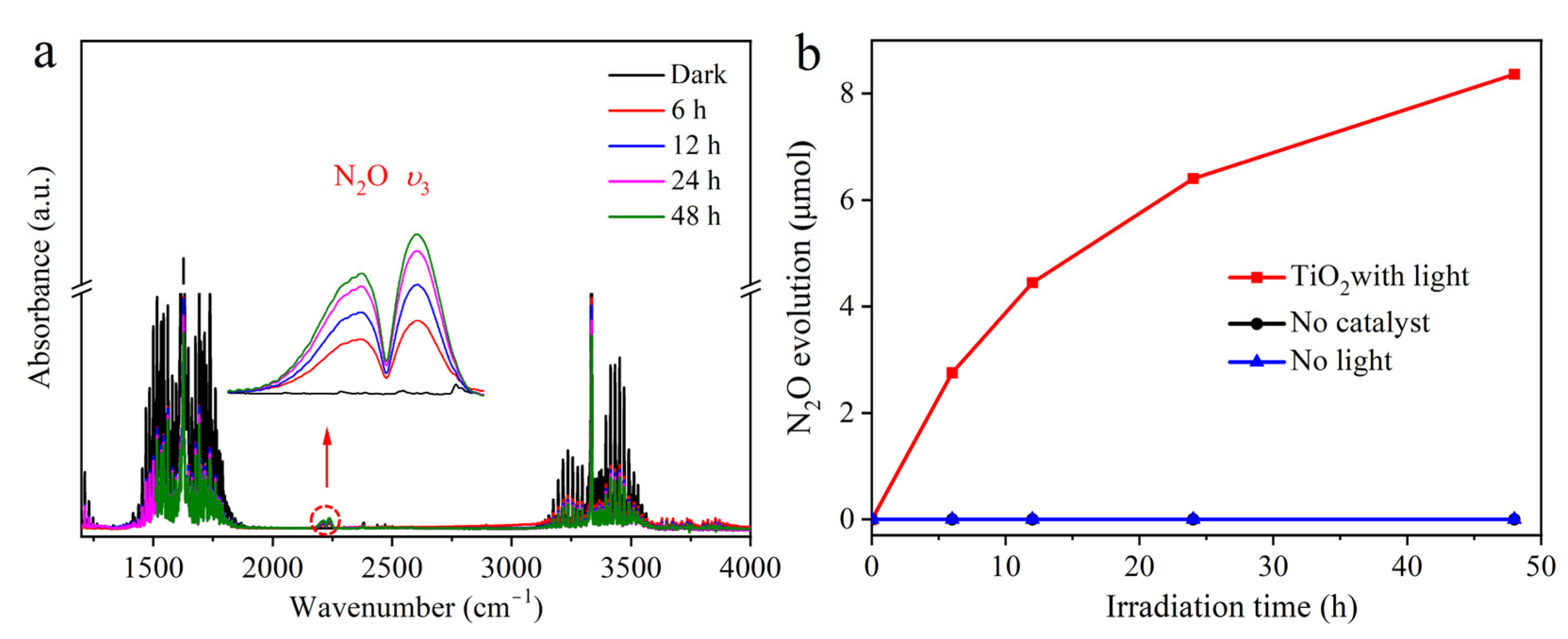
2. Results and Discussion
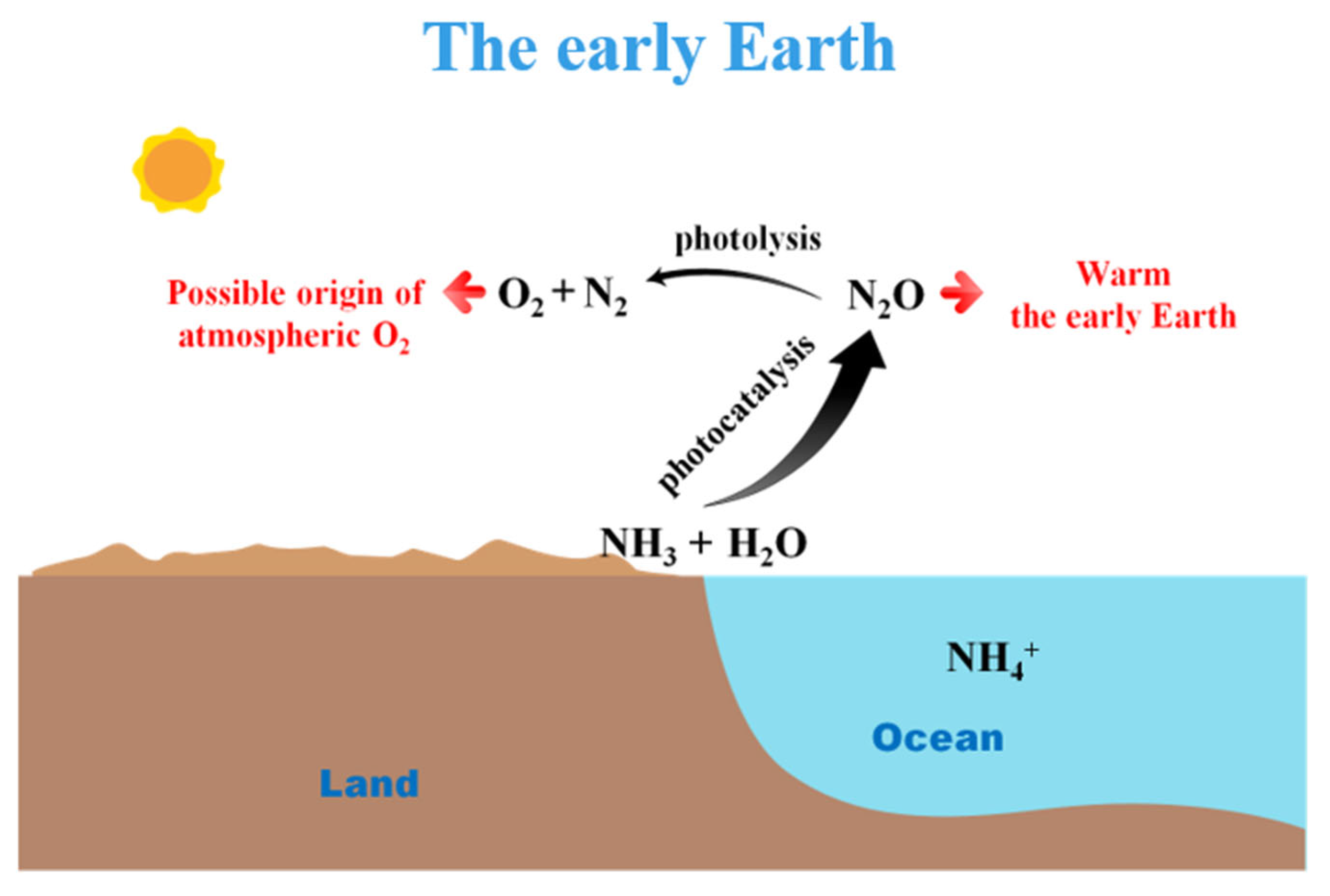
2.1. The Faint Young Sun Paradox
2.2. The Possible Origin of Atmospheric O2
3. Materials and Methods
3.1. Chemicals
3.2. Characterization
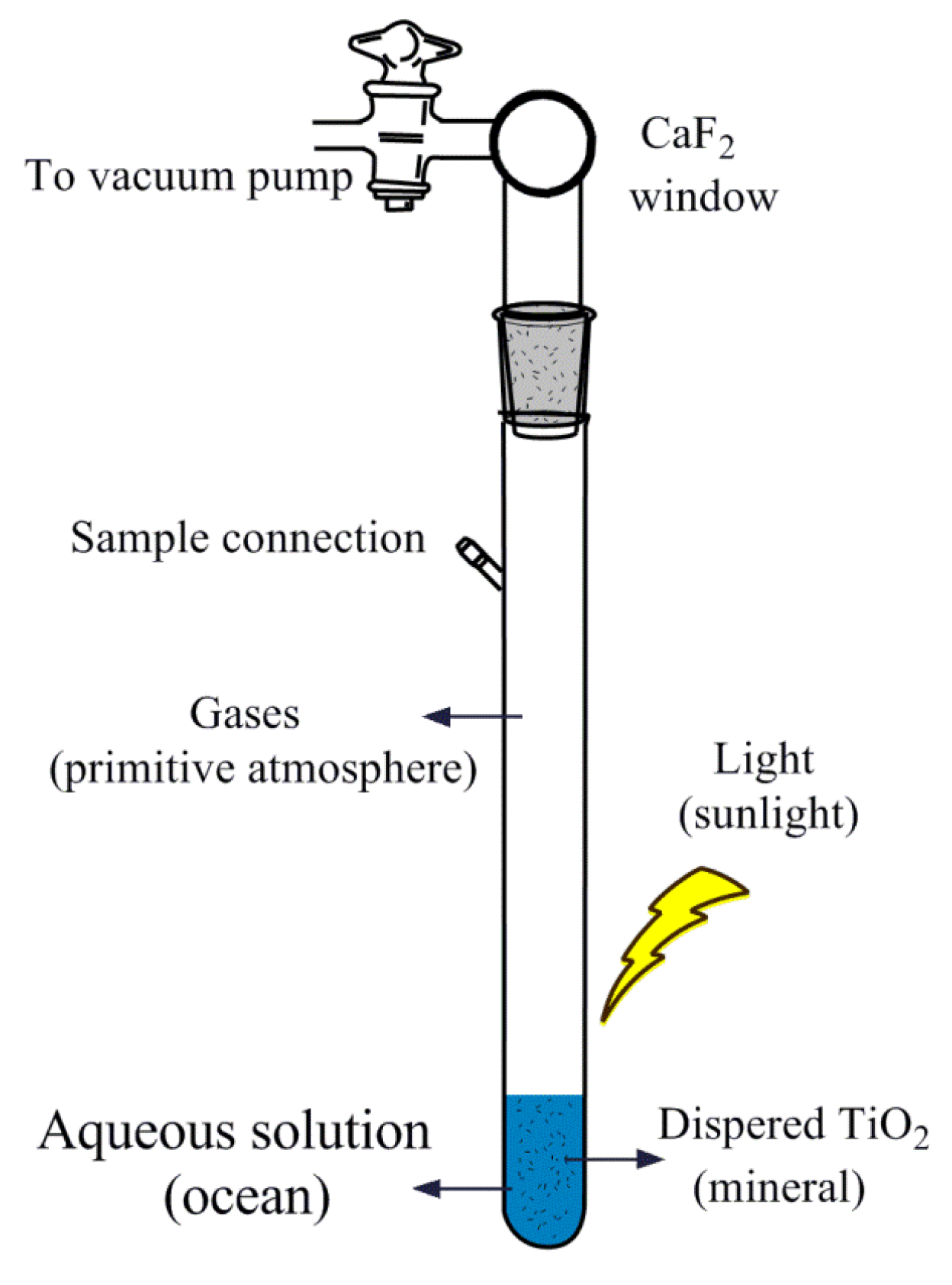
3.3. Photocatalytic Reaction
3.4. Isotope Ratio Mass Spectrometer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goldblatt, C.; Zahnle, K.J. Faint young Sun paradox remains. Nature 2011, 474, E1. [Google Scholar] [CrossRef]
- Sagan, C.; Mullen, G. Earth and Mars: Evolution of atmospheres and surface temperatures. Science 1972, 177, 52–56. [Google Scholar] [CrossRef]
- Peck, W.H.; Valley, J.W.; Wilde, S.A.; Graham, C.M. Oxygen isotope ratios and rare earth elements in 3.3 to 4.4 Ga zircons: Ion microprobe evidence for high delta O18 continental crust and oceans in the Early Archean. Geochim. Cosmochim. Acta 2001, 65, 4215–4229. [Google Scholar] [CrossRef]
- Kiehl, J.T.; Dickinson, R.E. A Study of the Radiative Effects of Enhanced Atmospheric CO2 and CH4 on Early Earth Surface Temperatures. J. Geophys. Res. Atmos. 1987, 92, 2991–2998. [Google Scholar] [CrossRef]
- Byrne, B.; Goldblatt, C. Diminished greenhouse warming from Archean methane due to solar absorption lines. Clim. Past 2015, 11, 559–570. [Google Scholar] [CrossRef]
- Rosing, M.T.; Bird, D.K.; Sleep, N.H.; Bjerrum, C.J. No climate paradox under the faint early Sun. Nature 2010, 464, 744–747. [Google Scholar] [CrossRef]
- Airapetian, V.S.; Glocer, A.; Gronoff, G.; Hébrard, E.; Danchi, W. Prebiotic chemistry and atmospheric warming of early Earth by an active young Sun. Nat. Geosci. 2016, 9, 452–455. [Google Scholar] [CrossRef]
- Lan, X.; Zhu, L.; Yuan, Q. Long-Term Variation of Greenhouse Gas N2O Observed by MLS during 2005–2020. Remote Sens. 2022, 14, 955. [Google Scholar] [CrossRef]
- Rotmans, J.; Elzen, M.G.J.D. A model-based approach to the calculation of global warming potentials (GWP). Int. J. Clim. 1992, 12, 865–874. [Google Scholar] [CrossRef]
- Nna-Mvondo, D.; Navarro-González, R.; Raulin, F.; Coll, P. Nitrogen Fixation By Corona Discharge On The Early Precambrian Earth. Space Life Sci. 2005, 35, 401–409. [Google Scholar] [CrossRef]
- Paytan, A. Earth history—Sulfate clues for the early history of atmospheric oxygen. Science 2000, 288, 626–627. [Google Scholar] [CrossRef]
- Planavsky, N.J.; Asael, D.; Hofmann, A.; Reinhard, C.T.; Lalonde, S.V.; Knudsen, A.; Wang, X.L.; Ossa, F.O.; Pecoits, E.; Smith, A.J.B.; et al. Rouxel Evidence for oxygenic photosynthesis half a billiion years before the Great Oxidation Event. Nat. Geosci. 2014, 7, 283–286. [Google Scholar] [CrossRef]
- Sumner, D.Y.; Hawes, I.; Mackey, T.J.; Jungblut, A.D.; Doran, P.T. Antarctic microbial mats: A modern analog for Archean lacustrine oxygen oases. Geology 2015, 43, 887–890. [Google Scholar] [CrossRef]
- Olson, S.L.; Kump, L.R.; Kasting, J.F. Quantifying the areal extent and dissolved oxygen concentrations of Archean oxygen oases. Chem. Geol. 2013, 362, 35–43. [Google Scholar] [CrossRef]
- Dismukes, G.C.; Klimov, V.V.; Baranov, S.V.; Kozlov, Y.N.; DasGupta, J.; Tyryshkin, A. The origin of atmospheric oxygen on Earth: The innovation of oxygenic photosynthesis. Proc. Natl. Acad. Sci. USA 2001, 98, 2170–2175. [Google Scholar] [CrossRef]
- Lu, Z.; Chang, Y.C.; Yin, Q.-Z.; Ng, C.Y.; Jackson, W.M. Evidence for direct molecular oxygen production in CO2 photodissociation. Science 2014, 346, 61–64. [Google Scholar] [CrossRef]
- Wang, X.D.; Gao, X.F.; Xuan, C.J.; Tian, S.X. Dissociative electron attachment to CO2 produces molecular oxygen. Nat. Chem. 2016, 8, 258–263. [Google Scholar] [CrossRef]
- Yamashita, T.A. Vannice N2O decomposition over manganese oxides. J. Catal. 1996, 161, 254–262. [Google Scholar] [CrossRef]
- Tsuji, M.; Kawahara, M.; Noda, K.; Senda, M.; Sako, H.; Kamo, N.; Kawahara, T.; Kamarudin, K.S. Photochemical removal of NO2 by using 172-nm Xe2 excimer lamp in N2 or air at atmospheric pressure. J. Hazard. Mater. 2009, 162, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Yu, S.; Xie, T.; Chen, W.; Wang, S.; Tan, Y.; Wang, T.; Yuan, K.; Yang, X.; Wang, X. Photodissociation Dynamics of Nitrous Oxide near 145 nm: The O(1S0) and O(3PJ=2,1,0) Product Channels. J. Phys. Chem. A 2018, 122, 2663–2669. [Google Scholar] [CrossRef] [PubMed]
- Honma, K. Laser initiated reactions in N2O clusters studied by time-sliced ion velocity imaging technique. J. Chem. Phys. 2013, 139, 044307. [Google Scholar] [CrossRef] [PubMed]
- McCollom, T.M. Miller-Urey and Beyond: What Have We Learned About Prebiotic Organic Synthesis Reactions in the Past 60 Years? Annu. Rev. Earth Planet. Sci. 2013, 41, 207–229. [Google Scholar] [CrossRef]
- Ning, S.; Ou, H.; Li, Y.; Lv, C.; Wang, S.; Wang, D.; Ye, J. Co0−Coδ+ Interface Double-Site-Mediated C-C Coupling for the Photothermal Conversion of CO2 into Light Olefins. Angew. Chem. Int. Ed. 2023, 62, e202302253. [Google Scholar] [CrossRef]
- Ning, S.; Xu, H.; Qi, Y.; Song, L.; Zhang, Q.; Ouyang, S.; Ye, J. Microstructure Induced Thermodynamic and Kinetic Modulation to Enhance CO2 Photothermal Reduction: A Case of Atomic-Scale Dispersed Co-N Species Anchored Co@C Hybrid. ACS Catal. 2020, 10, 4726–4736. [Google Scholar] [CrossRef]
- Ning, S.; Sun, Y.; Ouyang, S.; Qi, Y.; Ye, J. Solar light-induced injection of hot electrons and photocarriers for synergistically enhanced photothermocatalysis over Cu-Co/SrTiO3 catalyst towards boosting CO hydrogenation into C2–C4 hydrocarbons. Appl. Catal. B Environ. 2022, 310, 121063. [Google Scholar] [CrossRef]
- Cnossen, I.; Sanz-Forcada, J.; Favata, F.; Witasse, O.; Zegers, T.; Arnold, N.F. Habitat of early life: Solar X-ray and UV radiation at Earth’s surface 4–3.5 billion years ago. J. Geophys. Res. 2007, 112, E02008. [Google Scholar] [CrossRef]
- Cockell, C.S. The ultraviolet history of the terrestrial planets—implications for biological evolution. Planet. Space Sci. 2000, 48, 203–214. [Google Scholar] [CrossRef]
- Avice, G.; Marty, B.; Burgess, R. The origin and degassing history of the Earth’s atmosphere revealed by Archean xenon. Nat. Commun. 2017, 8, 15455. [Google Scholar] [CrossRef] [PubMed]
- Reiche, H.; Bard, A.J. Heterogeneous photosynthetic production of amino acids from methane-ammonia-water at platinum/titanium dioxide. implications in chemical evolution. Chem. Informationsdienst 1979, 10, 3127–3128. [Google Scholar] [CrossRef]
- Doane, T.A. A survey of photogeochemistry. Geochem. Trans. 2017, 18, 1. [Google Scholar] [PubMed]
- Ferris, J.P.; Hill, A.R.; Liu, R.H.; Orgel, L.E. Synthesis of long prebiotic oligomers on mineral surfaces. Nature 1996, 381, 59–61. [Google Scholar] [CrossRef]
- Hazen, R.M.; Papineau, D.; Leeker, W.B.; Downs, R.T.; Ferry, J.M.; McCoy, T.J.; Sverjensky, D.A.; Yang, H.X. Mineral evolution. Am. Mineral. 2008, 93, 1693–1720. [Google Scholar] [CrossRef]
- Byrne, B.; Goldblatt, C. Radiative forcings for 28 potential Archean greenhouse gases. Clim. Past. 2014, 10, 2011–2053. [Google Scholar] [CrossRef][Green Version]
- Chyba, C.F. Atmospheric science—Rethinking Earth’s early atmosphere. Science 2005, 308, 962–963. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ore, C.M.D.; Cruikshank, D.P.; Protopapa, S.; Scipioni, F.; McKinnon, W.B.; Cook, J.C.; Grundy, W.M.; Schmitt, B.; Stern, S.A.; Moore, J.M.; et al. Detection of ammonia on Pluto’s surface in a region of geologically recent tectonism. Sci. Adv. 2019, 5, eaav5731. [Google Scholar] [CrossRef]
- Healy, M.G.; Ibrahim, T.G.; Lanigan, G.J.; Serrenho, A.J.; Fenton, O. Nitrate removal rate, efficiency and pollution swapping potential of different organic carbon media in laboratory denitrification bioreactors. Ecol. Eng. 2012, 40, 198–209. [Google Scholar] [CrossRef]
- Stüeken, E.E.; Kipp, M.A.; Koehler, M.C.; Buick, R. The evolution of Earth’s biogeochemical nitrogen cycle. Earth-Sci. Rev. 2016, 160, 220–239. [Google Scholar] [CrossRef]
- Stueken, E.E. Nitrogen in Ancient Mud: A Biosignature? Astrobiology 2016, 16, 730–735. [Google Scholar] [CrossRef]
- Stüeken, E.; Buick, R.; Schauer, A. Nitrogen isotope evidence for alkaline lakes on late Archean continents. Earth Planet. Sci. Lett. 2015, 411, 1–10. [Google Scholar] [CrossRef]
- Rapf, R.J.; Vaida, V. Sunlight as an energetic driver in the synthesis of molecules necessary for life. Phys. Chem 2016, 18, 20067–20084. [Google Scholar] [CrossRef]
- Buick, R. Did the Proterozoic ‘Canfield Ocean’ cause a laughing gas greenhouse? Geobiology 2010, 5, 97–100. [Google Scholar] [CrossRef]
- Roberson, A.L.; Roadt, J.; Halevy, I.; Kasting, J.F. Greenhouse warming by nitrous oxide and methane in the Proterozoic Eon. Geobiology 2011, 9, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L. A Production of Amino Acids Under Possible Primitive Earth Conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef] [PubMed]
- Ferus, M.; Pietrucci, F.; Saitta, A.M.; Knížek, A.; Kubelík, P.; Ivanek, O.; Shestivska, V.; Civiš, S. Formation of nucleobases in a Miller–Urey reducing atmosphere. Proc. Natl. Acad. Sci. USA 2017, 114, 4306–4311. [Google Scholar] [CrossRef]
- Liu, Y.M.; Cheng, Z.; He, B.; Gu, C.; Xiao, T.; Zhou, Z.; Guo, J.; Liu, H.; He, B.; Ye, B.; et al. Pothole-rich ultrathin WO3 nanosheets triggering N≡N bond activation of nitrogen for direct nitrate photosynthesis. Angew. Chem. Int. Ed. 2018, 58, 731–735. [Google Scholar] [CrossRef]
- Plank, T.; Langmuir, C.H. The chemical composition of subducting sediment and its consequences for the crust and mantle. Chem. Geol. 1998, 145, 325–394. [Google Scholar] [CrossRef]
- Civiš, S.; Knížek, A.; Ivanek, O.; Kubelík, P.; Zukalová, M.; Kavan, L.; Ferus, M. The origin of methane and biomolecules from a CO2 cycle on terrestrial planets. Nat. Astron. 2017, 1, 721–726. [Google Scholar] [CrossRef]
- Montzka, S.A.; Dlugokencky, E.J.; Butler, J.H. Non-CO2 greenhouse gases and climate change. Nature 2011, 476, 43–50. [Google Scholar] [CrossRef]
- Riding, R.; Fralick, P.; Liang, L. Identification of an Archean marine oxygen oasis. Precambrian Res. 2014, 251, 232–237. [Google Scholar] [CrossRef]
- Mloszewska, A.M.; Cole, D.B.; Planavsky, N.J.; Kappler, A.; Whitford, D.S.; Owttrim, G.W.; Konhauser, K.O. UV radiation limited the expansion of cyanobacteria in early marine photic environments. Nat. Commun. 2018, 9, 3088. [Google Scholar] [CrossRef]
- Sheridan, P.P.; Freeman, K.H.; Brenchley, J.E. Estimated minimal divergence times of the major bacterial and archaeal phyla. Geomicrobiol. J. 2003, 20, 1–14. [Google Scholar] [CrossRef]
- Komiya, T.; Hirata, T.; Kitajima, K.; Yamamoto, S.; Shibuya, T.; Sawaki, Y.; Ishikawa, T.; Shu, D.; Li, Y.; Han, J. Evolution of the composition of seawater through geologic time, and its influence on the evolution of life. Gondwana Res. 2008, 14, 159–174. [Google Scholar] [CrossRef]
- Och, L.M.; Shields-Zhou, G.A. The Neoproterozoic oxygenation event: Environmental perturbations and biogeochemical cycling. Earth Sci. Rev. 2012, 110, 26–57. [Google Scholar] [CrossRef]
- Kannan, S.; Swamy, C.S. Catalytic Decomposition of Nitrous-Oxide on in-Situ Generated Thermally Calcined Hydrotalcites. Appl. Catal. B 1994, 3, 109–116. [Google Scholar] [CrossRef]
- Johnston, D.; Poulton, S.; Goldberg, T.; Sergeev, V.; Podkovyrov, V.; Vorob’Eva, N.; Bekker, A.; Knoll, A. Late Ediacaran redox stability and metazoan evolution. Earth Planet. Sci. Lett. 2012, 335–336, 25–35. [Google Scholar] [CrossRef]
- Selwyn, G.S.; Johnston, H.S. Ultraviolet absorption spectrum of nitrous oxide as a function of temperature and isotopic substitution. J. Chem. Phys. 1981, 74, 3791–3803. [Google Scholar] [CrossRef][Green Version]
- Harding, D.J.; Neugebohren, J.; Grütter, M.; Schmidt-May, A.F.; Auerbach, D.J.; Kitsopoulos, T.N.; Wodtke, A.M. Single-field slice-imaging with a movable repeller: Photodissociation of N2O from a hot nozzle. J. Chem. Phys. 2014, 141, 054201. [Google Scholar] [CrossRef]
- Angulo-Brown, F.; Rosales, M.A.; Barranco-Jiménez, M.A. The Faint Young Sun Paradox: A Simplified Thermodynamic Approach. Adv. Astron. 2012, 2012, 478957. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.K.; Chen, J.J.; Zhang, X.; Huang, Y.X.; Li, W.W.; Yu, H.Q. Self-induced synthesis of phase-junction TiO2 with a tailored rutile to anatase ratio below phase transition temperature. Sci. Rep. 2016, 6, 20491. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, W.; Qian, X.Y.; Liu, J.B.; Wu, J.M. UV and visible light photocatalytic activity of Au/TiO2 nanoforests with Anatase/Rutile phase junctions and controlled Au locations. Sci. Rep. 2017, 7, 41253. [Google Scholar] [CrossRef] [PubMed]
- Batalovic, K.; Bundaleski, N.; Radakovic, J.; Abazovic, N.; Mitric, M.; Silva, R.A.; Savic, M.; Belosevic-Cavor, J.; Rakocevic, Z.; Rangel, C.M. Modification of N-doped TiO2 photocatalysts using noble metals (Pt, Pd)—A combined XPS and DFT study. Phys. Chem. Chem. Phys. 2017, 19, 7062–7071. [Google Scholar] [CrossRef] [PubMed]


| Sample | Ampl 44/28 | δ18OV-SMOW‰ | AT% 18O/16O |
|---|---|---|---|
| 18O-labeled H2O | 486 | 7209.5 | 1.4826 |
| N2O | 126 | 1018.3 | 0.4051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Xu, F.; Shi, W.; Wang, Q.; Huang, C. Photocatalysis: A Possible Vital Contributor to the Evolution of the Prebiotic Atmosphere and the Warming of the Early Earth. Catalysts 2023, 13, 1310. https://doi.org/10.3390/catal13091310
Cheng C, Xu F, Shi W, Wang Q, Huang C. Photocatalysis: A Possible Vital Contributor to the Evolution of the Prebiotic Atmosphere and the Warming of the Early Earth. Catalysts. 2023; 13(9):1310. https://doi.org/10.3390/catal13091310
Chicago/Turabian StyleCheng, Chuchu, Fangjie Xu, Wenwen Shi, Qiaoyun Wang, and Caijin Huang. 2023. "Photocatalysis: A Possible Vital Contributor to the Evolution of the Prebiotic Atmosphere and the Warming of the Early Earth" Catalysts 13, no. 9: 1310. https://doi.org/10.3390/catal13091310
APA StyleCheng, C., Xu, F., Shi, W., Wang, Q., & Huang, C. (2023). Photocatalysis: A Possible Vital Contributor to the Evolution of the Prebiotic Atmosphere and the Warming of the Early Earth. Catalysts, 13(9), 1310. https://doi.org/10.3390/catal13091310