In Situ Preparation of 0D/2D Zn-Ag-In-S Quantum Dots/RGO Heterojunctions for Efficient Photocatalytic Hydrogen Production
Abstract
:1. Introduction
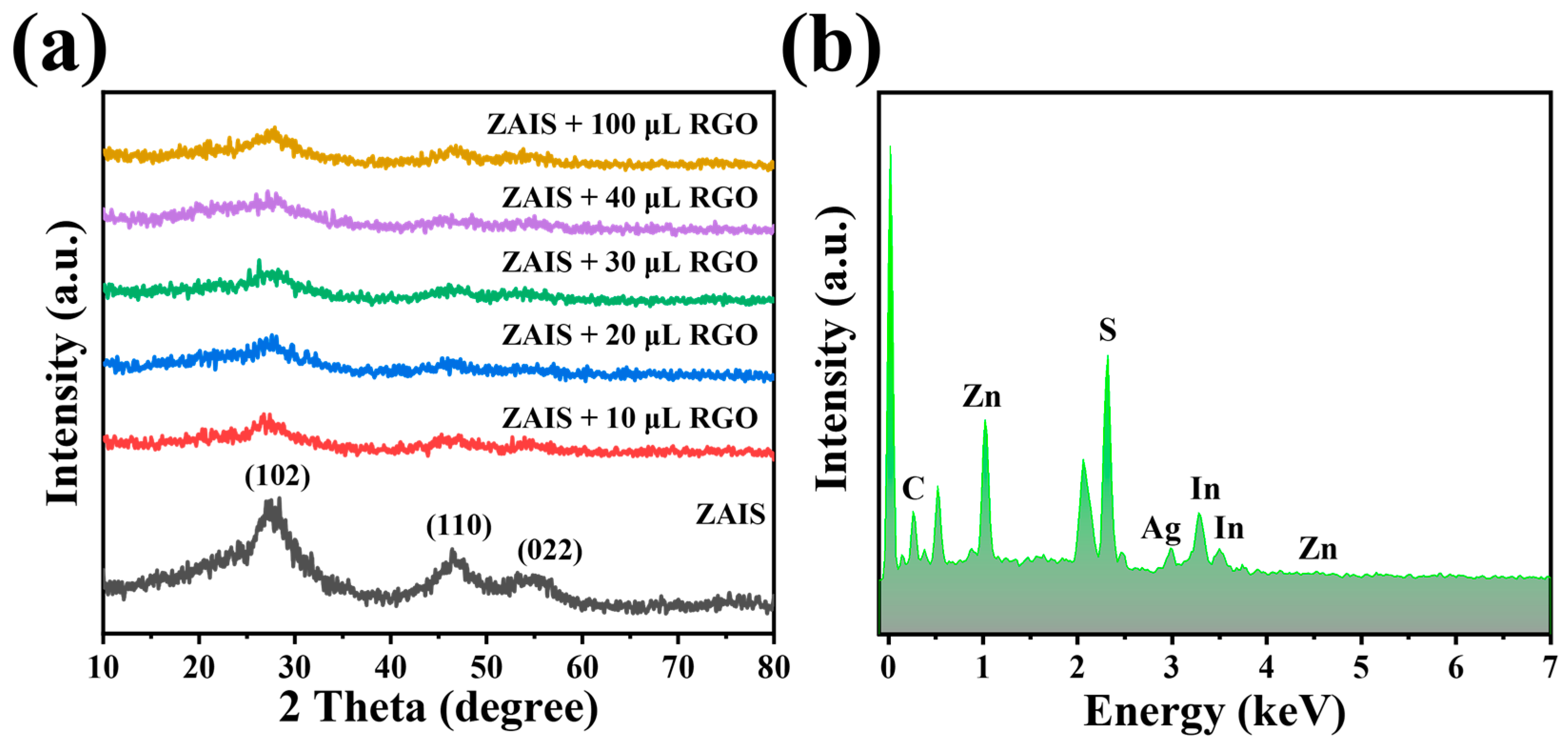
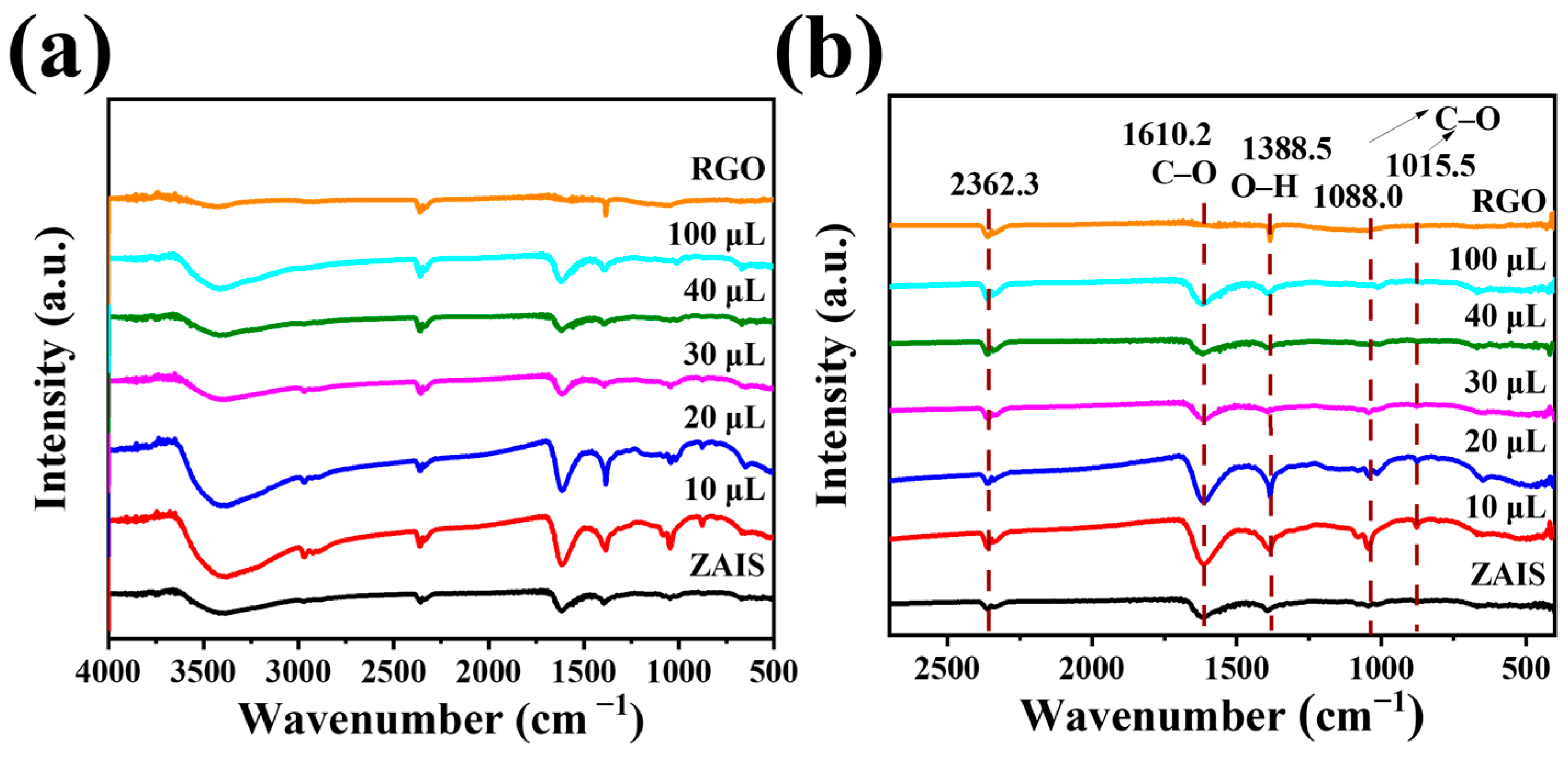
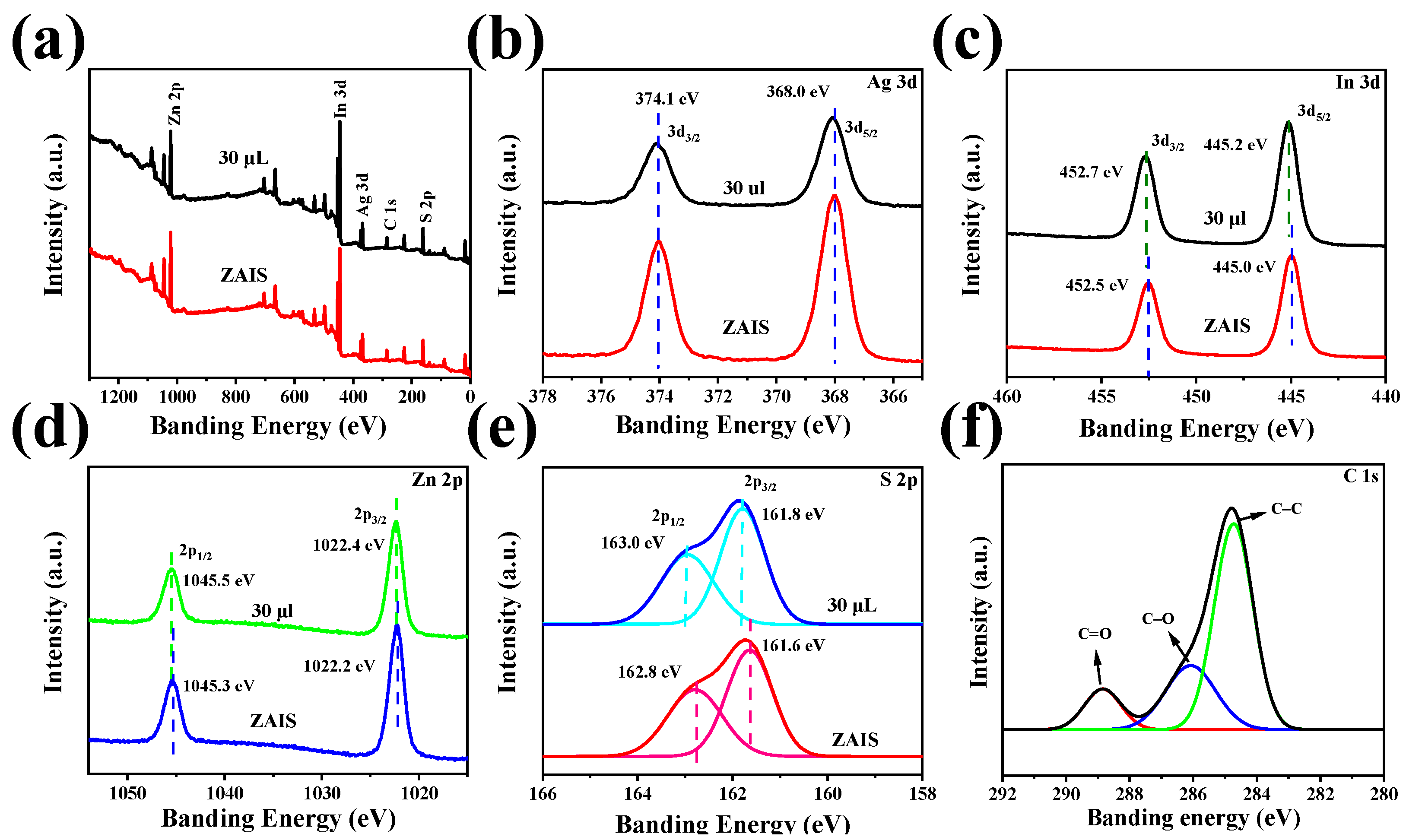
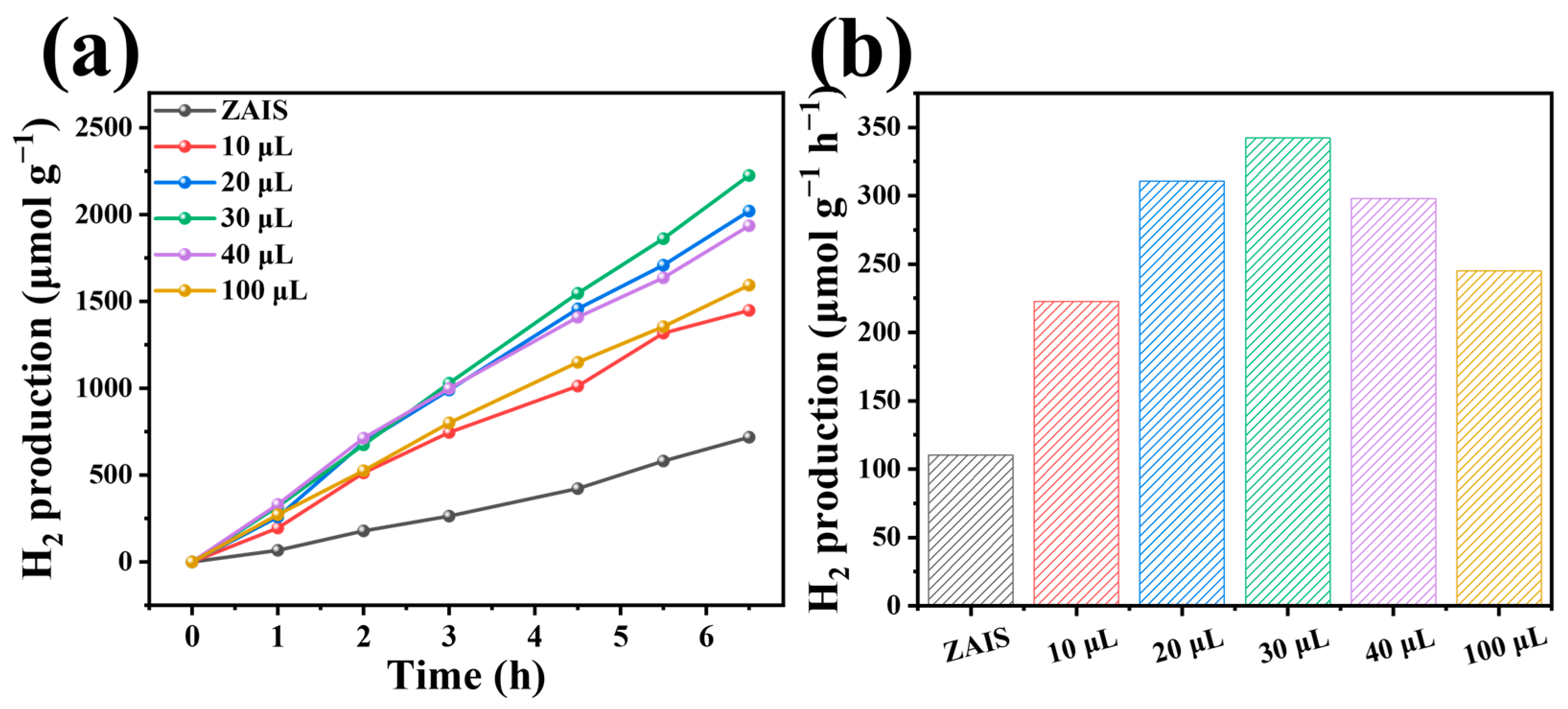
2. Results and Discussion
3. Experimental
3.1. Materials
3.2. Preparation of Photocatalysts
3.3. Characterizations
3.4. Photocatalytic Performance Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Onneken, C.; Morack, T.; Soika, J.; Sokolova, O.; Niemeyer, N.; Mück-Lichtenfeld, C.; Daniliuc, C.G.; Neugebauer, J.; Gilmour, R. Light-enabled deracemization of cyclopropanes by Al-salen photocatalysis. Nature 2023, 621, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Yigao, Y.; Linan, Z.; Hossein, R.; Junwei, L.B.; Jingyi, Z.; Aaron, B.; Lin, Y.; Minghe, L.; Minhan, L.; Suman, K.; et al. Earth-abundant photocatalyst for H2 generation from NH3 with light-emitting diode illumination. Science 2022, 378, 889–893. [Google Scholar] [CrossRef]
- Bellotti, P.; Huang, H.-M.; Faber, T.; Glorius, F. Photocatalytic late-stage C–H functionalization. Chem. Rev. 2023, 123, 4237–4352. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Martirez, J.M.P.; Finzel, J.; Zhang, C.; Swearer, D.F.; Tian, S.; Robatjazi, H.; Lou, M.; Dong, L.; Henderson, L.; et al. Light-driven methane dry reforming with single atomic site antenna-reactor plasmonic photocatalysts. Nat. Energy 2020, 5, 61–70. [Google Scholar] [CrossRef]
- Li, C.; Kong, X.Y.; Lyu, M.; Tay, X.T.; Đokić, M.; Chin, K.F.; Yang, C.T.; Lee, E.K.X.; Zhang, J.; Tham, C.Y.; et al. Upcycling of non-biodegradable plastics by base metal photocatalysis. Chem 2023, 9, 2683–2700. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.; Li, J.; Jin, Z.; Li, Y.; Tsubaki, N. Amorphous/crystalline heterojunction interface driving the spatial separation of charge carriers for efficient photocatalytic hydrogen evolution. Mater. Today Phys. 2022, 27, 100767. [Google Scholar] [CrossRef]
- Pavliuk, M.V.; Wrede, S.; Liu, A.; Brnovic, A.; Wang, S.; Axelsson, M.; Tian, H. Preparation, characterization, evaluation and mechanistic study of organic polymer nano-photocatalysts for solar fuel production. Chem. Soc. Rev. 2022, 51, 6909–6935. [Google Scholar] [CrossRef]
- Sun, P.; Xing, Z.; Li, Z.; Zhou, W. Recent advances in quantum dots photocatalysts. Chem. Eng. J. 2023, 458, 141399. [Google Scholar] [CrossRef]
- Hao, J.; Liu, H.; Wang, K.; Sun, X.W.; Delville, J.-P.; Delville, M.-H. Hole scavenging and electron–hole pair photoproduction rate: Two mandatory key factors to control single-tip Au-CdSe/CdS nanoheterodimers. ACS Nano 2021, 15, 15328–15341. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Mao, B.; Wu, Z.; Yan, W.; Zhang, D.; Li, Q.; Huang, H.; Kang, Z.; Shi, W. Carbon-dot-mediated highly efficient visible-driven photocatalytic hydrogen evolution coupled with organic osxidation. Adv. Funct. Mater. 2023, 2305318. [Google Scholar] [CrossRef]
- Kowalik, P.; Bujak, P.; Penkala, M.; Tomaszewski, W.; Lisowski, W.; Sobczak, J.W.; Siepietowska, D.; Maroń, A.M.; Polak, J.; Bartoszek, M.; et al. Ag-In-Zn-S quaternary nanocrystals prepared from InCl2 precursor: Photophysical and spectroscopic properties and application as visible light photocatalysts of aromatic aldehyde photoreduction. Chem. Mater. 2023, 35, 6447–6462. [Google Scholar] [CrossRef]
- Gong, G.; Liu, Y.; Mao, B.; Tan, L.; Yang, Y.; Shi, W. Ag doping of Zn-In-S quantum dots for photocatalytic hydrogen evolution: Simultaneous bandgap narrowing and carrier lifetime elongation. Appl. Catal. B 2017, 216, 11–19. [Google Scholar] [CrossRef]
- Zhang, D.; Mao, B.; Li, D.; Liu, Y.; Li, F.; Dong, W.; Jiang, T.; Shi, W. 0D/2D Z-scheme heterojunctions of Zn-AgIn5S8 QDs/α-Fe2O3 nanosheets for efficient visible-light-driven hydrogen production. Chem. Eng. J. 2021, 417, 128275. [Google Scholar] [CrossRef]
- Hu, J.; Shen, X.; Liu, A.; Lu, Z.; Xie, J.; Hao, A.; Jiang, X.; Wang, J.; Cao, Y. Highly dispersed Cu2O quantum dots (about 2 nm) constructed by a simple functional group anchoring strategy boost the photocatalytic water splitting ability by 72 times. J. Mater. Chem. A 2023, 11, 1290–1300. [Google Scholar] [CrossRef]
- Shen, H.; Wang, Y.-Z.; Liu, G.; Li, L.; Xia, R.; Luo, B.; Wang, J.; Suo, D.; Shi, W.; Yong, Y.-C. A whole-cell inorganic-biohybrid system integrated by reduced graphene oxide for boosting solar hydrogen production. ACS Catal. 2020, 10, 13290–13295. [Google Scholar] [CrossRef]
- Xin, H.; Sun, L.; Zhao, Y.; Lv, Y.; Luo, Q.; Guo, S.; Li, D.; Mu, C.; Huang, B.; Ma, F. Size-controllable Rh2P nanoparticles on reduced graphene oxide toward highly hydrogen production. Chem. Eng. J. 2023, 466, 143277. [Google Scholar] [CrossRef]
- Yuan, C.; Lv, H.; Zhang, Y.; Fei, Q.; Xiao, D.; Yin, H.; Lu, Z.; Zhang, Y. Three-dimensional nanoporous heterojunction of CdS/np-rGO for highly efficient photocatalytic hydrogen evolution under visible light. Carbon 2023, 206, 237–245. [Google Scholar] [CrossRef]
- Raja, A.; Son, N.; Swaminathan, M.; Kang, M. Effective graphene incorporation of strontium niobate–doped titanium oxide for photocatalytic hydrogen production. J. Cleaner Prod. 2023, 423, 138809. [Google Scholar] [CrossRef]
- Samajdar, S.; Bera, S.; Das, P.S.; Finch, H.; Dhanak, V.R.; Chakraborty, S.; Maiyalagan, T.; Annapurna, K.; Ghosh, S. Exploration of 1D-2D LaFeO3/RGO S-scheme heterojunction for photocatalytic water splitting. Int. J. Hydrogen Energy 2023, 48, 17838–17851. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Liu, P.; Li, Y.; Liu, X.; Li, G.; Wong, P.K.; An, T.; Zhao, H. Cross-linked ZnIn2S4/rGO composite photocatalyst for sunlight-driven photocatalytic degradation of 4-nitrophenol. Appl. Catal. B 2015, 168–169, 266–273. [Google Scholar] [CrossRef]
- Lv, T.; Wu, M.; Guo, M.; Liu, Q.; Jia, L. Self-assembly photocatalytic reduction synthesis of graphene-encapusulated LaNiO3 nanoreactor with high efficiency and stability for photocatalytic water splitting to hydrogen. Chem. Eng. J. 2019, 356, 580–591. [Google Scholar] [CrossRef]
- Iwase, A.; Ng, Y.H.; Amal, R.; Kudo, A. Solar hydrogen evolution using a CuGaS2 photocathode improved by incorporating reduced graphene oxide. J. Mater. Chem. A 2015, 3, 8566–8570. [Google Scholar] [CrossRef]
- Xie, C.; Lu, X.; Deng, F.; Luo, X.; Gao, J.; Dionysiou, D.D. Unique surface structure of nano-sized CuInS2 anchored on rGO thin film and its superior photocatalytic activity in real wastewater treatment. Chem. Eng. J. 2018, 338, 591–598. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, B.; Gong, G.; Li, D.; Liu, Y.; Cao, W.; Xing, L.; Zeng, J.; Shi, W.; Yuan, S. In-situ growth of Zn-AgIn5S8 quantum dots on g-C3N4 towards 0D/2D heterostructured photocatalysts with enhanced hydrogen production. Int. J. Hydrogen Energy 2019, 44, 15882–15891. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, J.; Zhu, B.; Fedin, M.V.; Cheng, B.; Yu, J.; Zhang, L. ZnO/COF S-scheme heterojunction for improved photocatalytic H2O2 production performance. Chem. Eng. J. 2022, 444, 136584. [Google Scholar] [CrossRef]
- Koczoń, P.; Hołaj-Krzak, J.T.; Palani, B.K.; Bolewski, T.; Dąbrowski, J.; Bartyzel, B.J.; Gruczyńska-Sękowska, E. The analytical possibilities of FT-IR spectroscopy powered by vibrating molecules. Int. J. Mol. Sci. 2023, 24, 1013. [Google Scholar] [CrossRef]
- Yang, L.; Guo, J.; Yu, Y.; An, Q.; Wang, L.; Li, S.; Huang, X.; Mu, S.; Qi, S. Hydrogen bonds of sodium alginate/Antarctic krill protein composite material. Carbohydr. Polym. 2016, 142, 275–281. [Google Scholar] [CrossRef]
- Shen, L.; Li, Y.; Zhao, W.; Wang, K.; Ci, X.; Wu, Y.; Liu, G.; Liu, C.; Fang, Z. Tuning F-doped degree of rGO: Restraining corrosion-promotion activity of EP/rGO nanocomposite coating. J. Mater. Sci. Technol. 2020, 44, 121–132. [Google Scholar] [CrossRef]
- Siddique, S.; Zain ul, A.; Waseem, M.; Naseem, T.; Bibi, A.; Hafeez, M.; Din, S.U.; Haq, S.; Qureshi, S. Photo-catalytic and anti-microbial activities of rGO/CuO nanocomposite. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1359–1372. [Google Scholar] [CrossRef]
- Durai, M.; Ahn, Y.-H. Photocatalytic H2 generation under blue and white LEDs by Fe2O3 /KTLO/rGO S-scheme composite photocatalyst. J. Alloys Compd. 2023, 965, 171457. [Google Scholar] [CrossRef]
- Hossen, M.A.; Khatun, F.; Ikreedeegh, R.R.; Muhammad, A.D.; Abd Aziz, A.; Leong, K.H.; Sim, L.C.; Lihua, W.; Monir, M.U. Enhanced photocatalytic CO2 reduction to CH4 using novel ternary photocatalyst RGO/Au-TNTAs. Energies 2023, 16, 5404. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, M.; Liu, Q.; Zhang, Y.; Wang, H.; Yang, G.; Huo, P. Reduced graphene oxide-modified Z-scheme g-C3N4/CdS photocatalyst with a staggered structure for the enhanced photoreduction of CO2. Sustain. Energy Fuels 2022, 6, 3768–3777. [Google Scholar] [CrossRef]
- Wang, T.; Yue, D.; Li, X.; Zhao, Y. Lead-free double perovskite Cs2AgBiBr6/RGO composite for efficient visible light photocatalytic H2 evolution. Appl. Catal. B 2020, 268, 118399. [Google Scholar] [CrossRef]
- Peng, X.; Ye, L.; Ding, Y.; Yi, L.; Zhang, C.; Wen, Z. Nanohybrid photocatalysts with ZnIn2S4 nanosheets encapsulated UiO-66 octahedral nanoparticles for visible-light-driven hydrogen generation. Appl. Catal. B 2020, 260, 118152. [Google Scholar] [CrossRef]
- Du, C.; Yan, B.; Yang, G. Self-integrated effects of 2D ZnIn2S4 and amorphous Mo2C nanoparticles composite for promoting solar hydrogen generation. Nano Energy 2020, 76, 105031. [Google Scholar] [CrossRef]
- Jia, W.L.; Li, W.J.; Yuan, H.Y.; Wu, X.; Liu, Y.; Dai, S.; Cheng, Q.; Liu, P.F.; Yang, H.G. Surface Cu+ modified ZnIn2S4 for promoted visible-light photocatalytic hydrogen evolution. J. Energy Chem. 2022, 74, 341–348. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, D.; Irfan, R.M.; Tang, S.; Chen, X.; Du, P. Highly efficient and selective photocatalytic dehydrogenation of benzyl alcohol for simultaneous hydrogen and benzaldehyde production over Ni-decorated Zn0.5Cd0.5S solid solution. J. Energy Chem. 2019, 30, 71–77. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Yan, J.; Liu, S.F. Heterointerface engineering of ZnO/CdS heterostructures through ZnS Layers for photocatalytic water splitting. Chemistry 2022, 28, e202202662. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, M.; Qiu, L.; Sheng, J.; Yang, W.; Yu, Y. Sulfur vacancies-induced “Electron Bridge” in Ni4Mo/Sv-ZnxCd1−xS regulates electron transfer for efficient H2-releasing photocatalysis. J. Energy Chem. 2023, 79, 64–71. [Google Scholar] [CrossRef]
- Zhang, R.; Wan, W.; Li, D.; Dong, F.; Zhou, Y. Three-dimensional MoS2/reduced graphene oxide aerogel as a macroscopic visible-light photocatalyst. Chin. J. Catal. 2017, 38, 313–320. [Google Scholar] [CrossRef]
- Dhandapani, P.; AlSalhi, M.S.; Karthick, R.; Chen, F.; Devanesan, S.; Kim, W.; Rajasekar, A.; Ahmed, M.; Aljaafreh, M.J.; Muhammad, A. Biological mediated synthesis of RGO-ZnO composites with enhanced photocatalytic and antibacterial activity. J. Hazard. Mater. 2021, 409, 124661. [Google Scholar] [CrossRef] [PubMed]
- Murillo Leo, I.; Soto, E.; Vaquero, F.; Mota, N.; Navarro, R.M.; Fierro, J.L.G. Influence of the reduction of graphene oxide (rGO) on the structure and photoactivity of CdS-rGO hybrid systems. Int. J. Hydrogen Energy 2017, 42, 13691–13703. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Chu, X.-S.; Liu, X.-Y.; Dang, Y.-Y. Regulation of bandgap and interfacial conductivity: Construction of carbon-doped three-dimensional porous h-BN/rGO hybrid for hydrogen evolution. Appl. Surf. Sci. 2021, 560, 150053. [Google Scholar] [CrossRef]
- Chen, Y.; Mou, Z.; Yin, S.; Huang, H.; Yang, P.; Wang, X.; Du, Y. Graphene enhanced photocatalytic hydrogen evolution performance of dye-sensitized TiO2 under visible light irradiation. Mater. Lett. 2013, 107, 31–34. [Google Scholar] [CrossRef]
- Pan, B.; Wang, Y.; Liang, Y.; Luo, S.; Su, W.; Wang, X. Nanocomposite of BiPO4 and reduced graphene oxide as an efficient photocatalyst for hydrogen evolution. Int. J. Hydrogen Energy 2014, 39, 13527–13533. [Google Scholar] [CrossRef]
- Gupta, B.; Melvin, A.A.; Matthews, T.; Dhara, S.; Dash, S.; Tyagi, A.K. Facile gamma radiolytic methodology for TiO2-rGO synthesis: Effect on photo-catalytic H2 evolution. Int. J. Hydrogen Energy 2015, 40, 5815–5823. [Google Scholar] [CrossRef]
- Li, D.; Zhao, H.; Li, L.; Mao, B.; Chen, M.; Shen, H.; Shi, W.; Jiang, D.; Lei, Y. Graphene-Sensitized Perovskite Oxide Monolayer Nanosheets for Efficient Photocatalytic Reaction. Adv. Funct. Mater. 2018, 28, 1806284. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.; Chen, Q.; Gu, X.; Dong, W.; Zhang, D.; Huang, H.; Mao, B.; Kang, Z.; Shi, W. Transient photovoltage study of the kinetics and synergy of electron/hole co-extraction in MoS2/Ag-In-Zn-S/carbon dot photocatalysts for promoted hydrogen production. Chem. Eng. J. 2022, 439, 135759. [Google Scholar] [CrossRef]

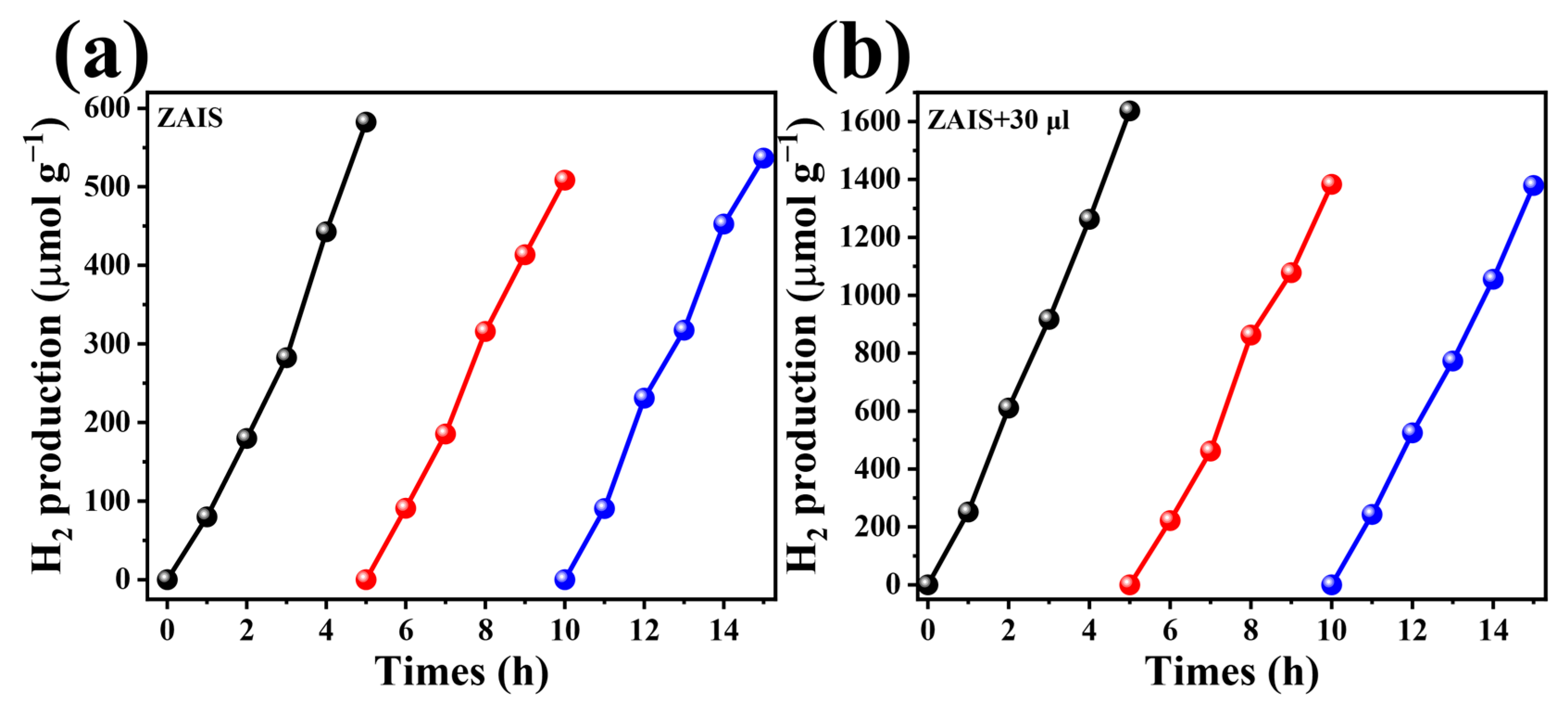

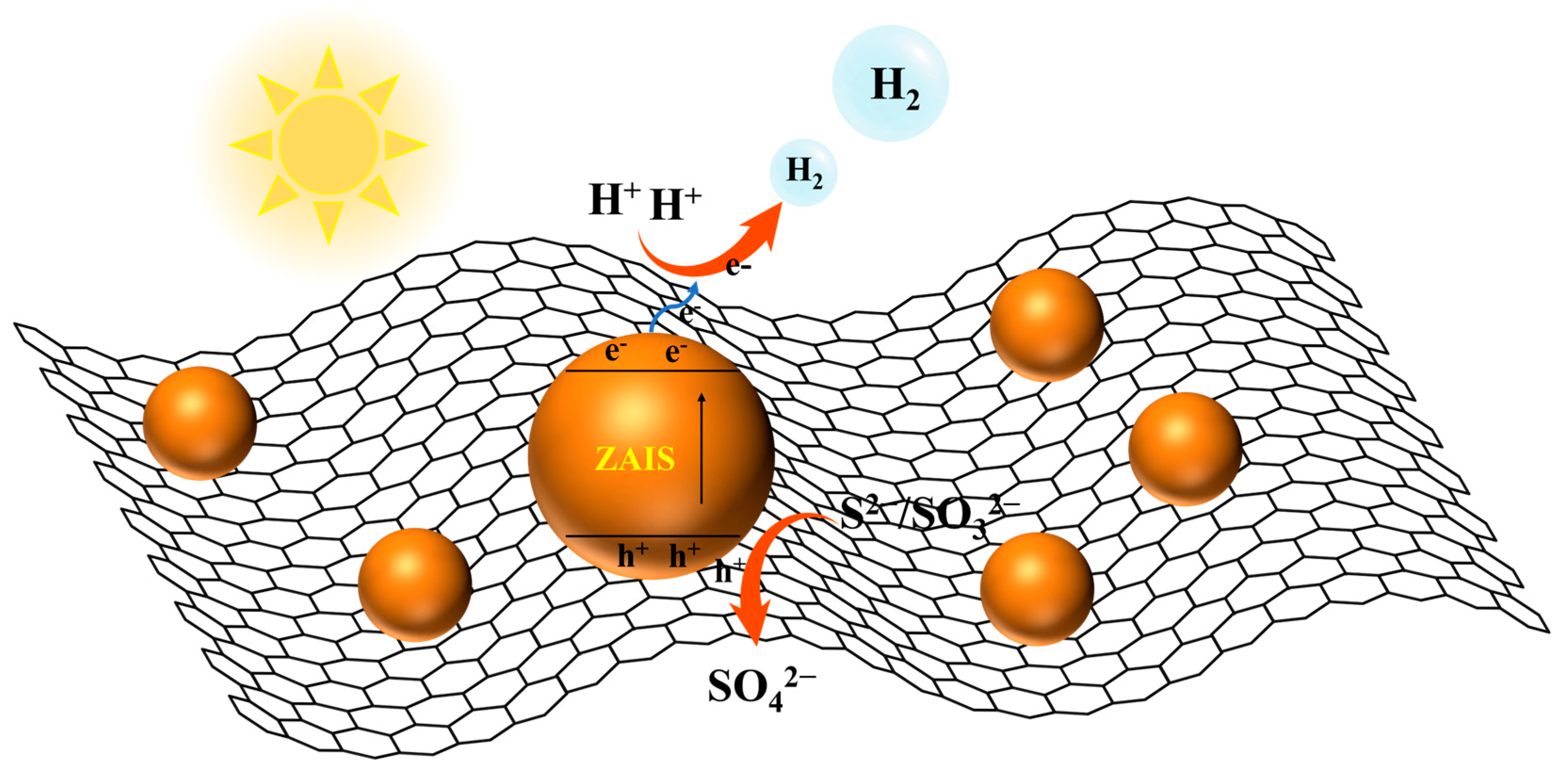
| Catalysts | Light Source | Reaction Conditions | H2 Evolution Rate (µmol g−1 h−1) | Ref. |
|---|---|---|---|---|
| ZAIS/RGO | λ ≥ 400 nm | 0.35 M Na2S + 0.25 M Na2SO3 | 342.34 | This work |
| CdS-rGO/HI | λ ≥ 400 nm | 0.35 M Na2S + 0.25 M Na2SO3 | 76.6 | [42] |
| Cs2AgBiBr6/RGO | λ ≥ 400 nm | saturated HBr and H3PO2 solution | 48.9 | [33] |
| h-BN/rGO | 15 vol% TEOA | 157.63 | [43] | |
| MoS2/RGO | λ ≥ 400 nm | 0.1 M Na2S + 0.6 M Na2SO3 | 4.86 | [40] |
| Ru(dcbpy)3/TiO2/RGO/Pt | λ ≥ 400 nm | EDTA | 191.8 | [44] |
| BiPO4/RGO | 5 vol% ethanol | 306 | [45] | |
| TiO2-rGO | λ ≥ 400 nm | 25 vol% methanol | 35 | [46] |
| Samples | A1/% | τ1/ns | A2/% | τ2/ns | τave/ns |
|---|---|---|---|---|---|
| ZAIS | 2.90 | 8.93 | 97.10 | 294.36 | 294.10 |
| 30 μL | 10.17 | 39.78 | 89.83 | 421.79 | 417.76 |
| 100 μL | 2.67 | 9.01 | 97.33 | 331.02 | 330.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, B.; Yang, Y.; Khan, A.U.; Chen, Q.; Wang, X.; Ren, T.; Li, J.; Liu, Y.; Li, L.; Mao, B. In Situ Preparation of 0D/2D Zn-Ag-In-S Quantum Dots/RGO Heterojunctions for Efficient Photocatalytic Hydrogen Production. Catalysts 2023, 13, 1471. https://doi.org/10.3390/catal13121471
Deng B, Yang Y, Khan AU, Chen Q, Wang X, Ren T, Li J, Liu Y, Li L, Mao B. In Situ Preparation of 0D/2D Zn-Ag-In-S Quantum Dots/RGO Heterojunctions for Efficient Photocatalytic Hydrogen Production. Catalysts. 2023; 13(12):1471. https://doi.org/10.3390/catal13121471
Chicago/Turabian StyleDeng, Bangya, Yalin Yang, Afaq Ullah Khan, Qitao Chen, Xianjin Wang, Tong Ren, Jiaji Li, Yanhong Liu, Lixia Li, and Baodong Mao. 2023. "In Situ Preparation of 0D/2D Zn-Ag-In-S Quantum Dots/RGO Heterojunctions for Efficient Photocatalytic Hydrogen Production" Catalysts 13, no. 12: 1471. https://doi.org/10.3390/catal13121471
APA StyleDeng, B., Yang, Y., Khan, A. U., Chen, Q., Wang, X., Ren, T., Li, J., Liu, Y., Li, L., & Mao, B. (2023). In Situ Preparation of 0D/2D Zn-Ag-In-S Quantum Dots/RGO Heterojunctions for Efficient Photocatalytic Hydrogen Production. Catalysts, 13(12), 1471. https://doi.org/10.3390/catal13121471









