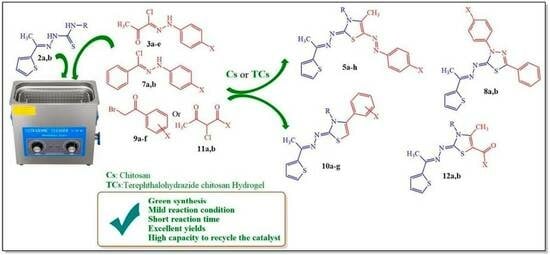
Green Synthesis and Molecular Docking Study of Some New Thiazoles Using Terephthalohydrazide Chitosan Hydrogel as Ecofriendly Biopolymeric Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the TCs Hydrogel
2.1.1. Fourier Transform Infrared (FTIR) Analyses
2.1.2. X-ray Diffraction (XRD) Analyses
2.1.3. Scanning Electron Microscopy (SEM) Analyses
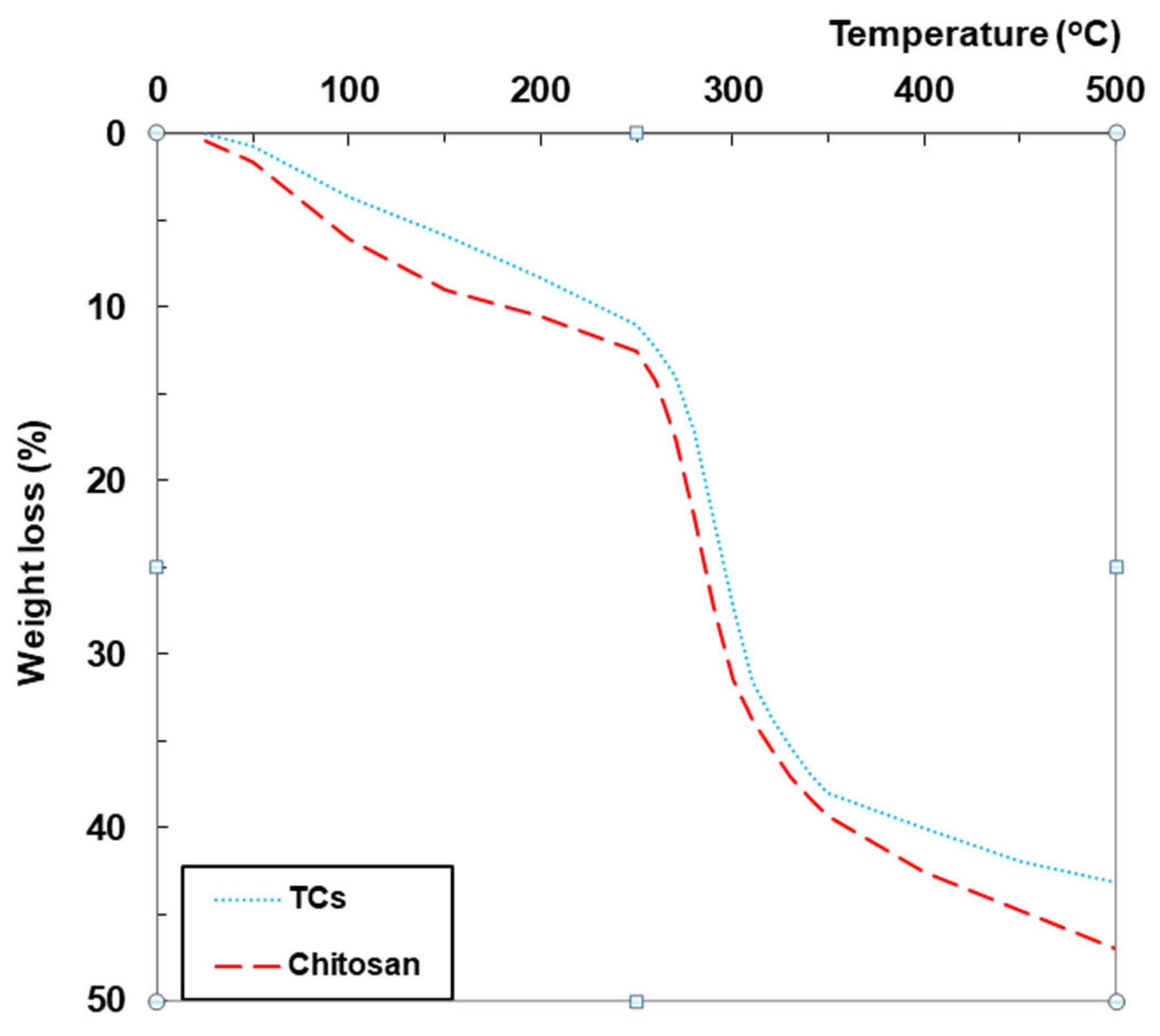
2.1.4. Thermogravimetric (TG) Analyses
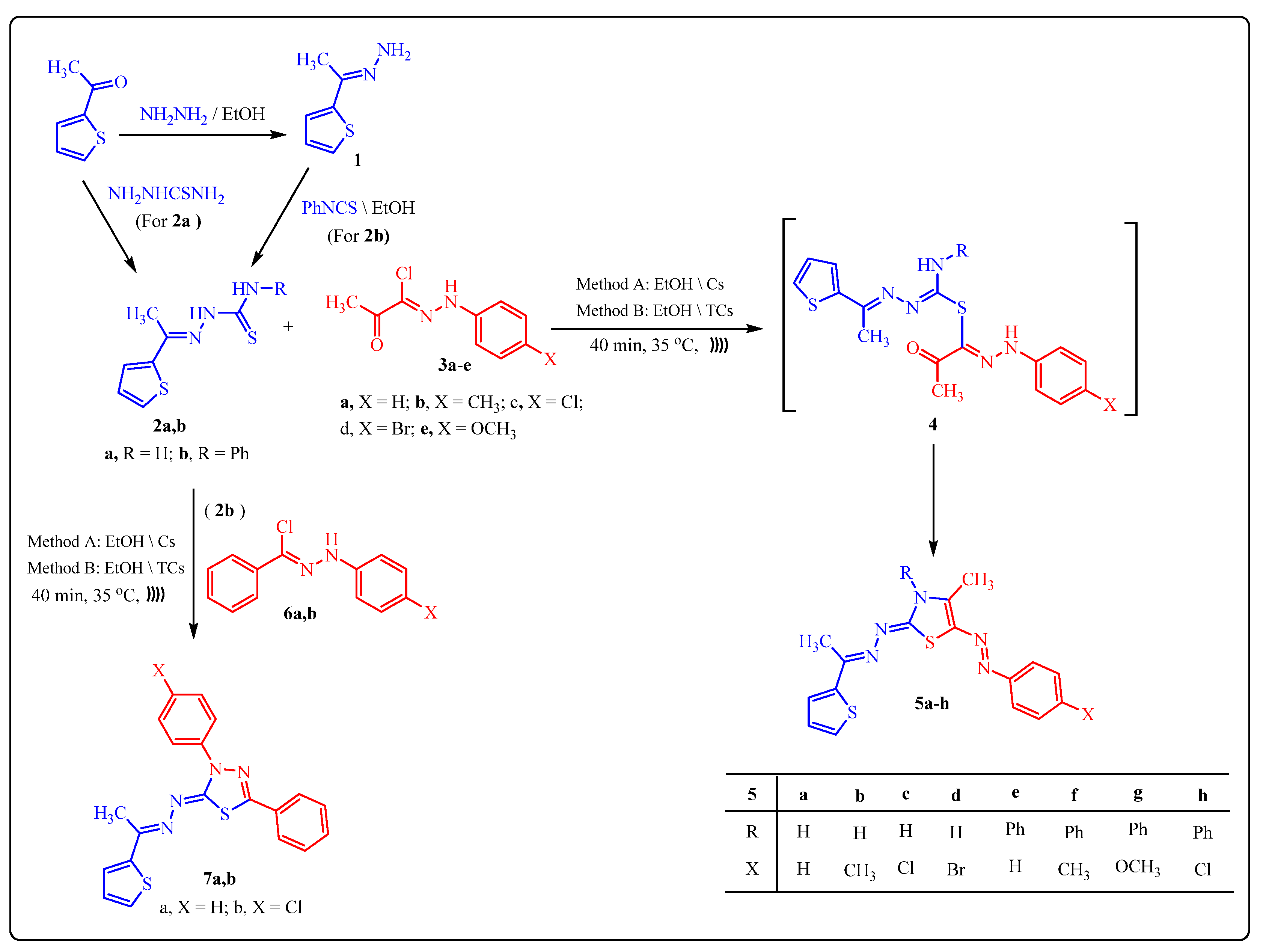
2.2. Synthesis of Thiazole and Thiadiazole Derivatives Using TCs as Basic Heterogeneous Catalyst
- -
- -
- -
- -
- The effect of temperature on the reaction was examined; the outcomes are shown in Table 3 (entries 3, 10–13). As indicated in Table 3, employing USI led to a sequential elevation of reaction temperatures from 25 °C to 30 °C, 35 °C, and 40 °C. This caused a corresponding escalation in product yields, with enhancements of 77%, 83%, and 88%, respectively. The optimal temperature was finally determined to be 35 ° C (Table 3, entry 3).
| Entry | TCs (mol%) | Solvent | Time (min) | Temperature (°C) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 2 | EtOH | 40 | 35 | 79 |
| 2 | 5 | EtOH | 40 | 35 | 83 |
| 3 a | 10 | EtOH | 40 | 35 | 88 |
| 4 | 20 | EtOH | 40 | 35 | 88 |
| 5 | 10 | DMSO | 40 | 35 | 81 |
| 6 | 10 | Dioxane | 40 | 35 | 85 |
| 7 | 10 | EtOH | 20 | 35 | 81 |
| 8 | 10 | EtOH | 30 | 35 | 85 |
| 9 | 10 | EtOH | 50 | 35 | 88 |
| 10 | 10 | EtOH | 40 | 25 | 77 |
| 11 | 10 | EtOH | 40 | 30 | 83 |
| 12 | 10 | EtOH | 40 | 35 | 88 |
| 13 | 10 | EtOH | 40 | 40 | 88 |
2.3. Molecular Docking Studies
SAR Analysis for Synthesized Thiazole Derivatives
2.4. ADMET Property Studies: The Absorption, Distribution, Metabolism, and Excretion (ADME) Aspects of the Medications Were Calculated Using the SwissADME, Which Predicts Physically and Pharmaceutically Significant Components of Organic Compounds [84]
In Silico ADMET Study
3. Materials and Methods
3.1. Chemicals and Methods
3.2. Measurements
3.3. Synthesis of Thiosemicarbazone Derivative 2b and Thiazole Derivatives 5a–h, 9a–g, 11a,b and Thiadiazoles 7a,b
3.3.1. Synthesis of N-Phenyl-2-(1-(thiophen-2-yl)ethylidene)hydrazine-1-carbothioamide (2b)
3.3.2. General Procedure for Synthesis of Thiazoles 5a–h, 9a–g, 11a,b and Thiadiazoles 7a,b
- Method A
- Method B
3.4. Docking Study
3.5. Protein Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Casti, F.; Basoccu, F.; Mocci, R.; Luca, L.D.; Porcheddu, A.; Cuccu, F. Appealing renewable materials in green chemistry. Molecules 2022, 27, 1988. [Google Scholar] [CrossRef] [PubMed]
- Macquarrie, D.J.; Hardy, J.J.E. Applications of Functionalized Chitosan in Catalysis. Ind. Eng. Chem. Res. 2005, 44, 8499–8520. [Google Scholar] [CrossRef]
- Guibal, E. Heterogeneous catalysis on chitosan-based materials: A review. Prog. Polym. Sci. 2005, 30, 71–109. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Toffey, A.; Samaranayake, G.; Frazier, C.E.; Glasser, W.G. Chitin derivatives. I. Kinetics of the heat-induced conversion of chitosan to chitin. J. Appl. Polym. Sci. 1996, 60, 75–85. [Google Scholar] [CrossRef]
- Sahu, P.K.; Sahu, P.K.; Gupta, S.K.; Agarwal, D.D. Chitosan: An efficient, reusable, and biodegradable catalyst for green synthesis of heterocycles. Ind. Eng. Chem. Res. 2014, 53, 2085–2091. [Google Scholar] [CrossRef]
- Ma, J.; Sahai, Y. Chitosan biopolymer for fuel cell applications. Carbohydr. Polym. 2013, 92, 955–975. [Google Scholar] [CrossRef]
- Elmehbad, N.Y.; Mohamed, N.A. Terephthalohydrazido cross-linked chitosan hydrogels: Synthesis, characterization and applications. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 969–982. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Abd El-Ghany, N.A. Novel aminohydrazide cross-linked chitosan filled with multi-walled carbon nanotubes as antimicrobial agents. Int. J. Biol. Macromol. 2018, 115, 651–662. [Google Scholar] [CrossRef]
- Ablouh, E.; Hanani, Z.; Eladlani, N.; Rhazi, M.; Taourirte, M. Chitosan microspheres/sodium alginate hybrid beads: An efficient green adsorbent for heavy metals removal from aqueous solutions. Sustain. Environ. Res. 2019, 29, 5. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Abd El-Ghany, N.A. Synthesis, characterization, and antimicrobial activity of chitosan hydrazide derivative. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 410–415. [Google Scholar] [CrossRef]
- Kumar, S.; Singhal, N.; Singh, R.K.; Gupta, P.; Singh, R.; Jain, S.L. Dual catalysis with magnetic chitosan: Direct synthesis of cyclic carbonates from olefins with carbon dioxide using isobutyraldehyde as the sacrificial reductant. Dalton Trans. 2015, 44, 11860–11866. [Google Scholar] [CrossRef] [PubMed]
- Dekamin, M.G.; Azimoshan, M.; Ramezani, L. Chitosan: A highly efficient renewable and recoverable bio-polymer catalyst for the expeditious synthesis of α-amino nitriles and imines under mild conditions. Green Chem. 2013, 15, 811–820. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Nadagouda, M.N.; Varma, R.S. Ruthenium on chitosan: A recyclable heterogeneous catalyst for aqueous hydration of nitriles to amides. Green Chem. 2014, 16, 2122. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Cheng, W.; Zhang, J.; Li, X.; Zhang, S.; She, Y. Chitosan functionalized ionic liquid as a recyclable biopolymer-supported catalyst for cycloaddition of CO2. Green Chem. 2012, 14, 654–660. [Google Scholar] [CrossRef]
- Alshabanah, L.A.; Al-Mutabagani, L.A.; Gomha, S.M.; Ahmed, H.A. Three-component synthesis of some new coumarin derivatives as anti-cancer agents. Front. Chem. 2022, 9, 762248. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and anticancer activities of thiazoles, 1,3-thiazines, and thiazolidine using chitosan-grafted-poly(vinylpyridine) as basic catalyst. Heterocycles 2015, 91, 1227–1243. [Google Scholar]
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and anticancer activity of arylazothiazoles and 1,3,4-thiadiazoles using chitosan-grafted-poly(4-vinylpyridine) as a novel copolymer basic catalyst. Chem. Heterocycl. Compd. 2015, 51, 1030–1038. [Google Scholar] [CrossRef]
- Alshabanah, L.A.; Gomha, S.M.; Al-Mutabagani, L.A.; Abolibda, T.Z.; Abd El-Ghany, N.A.; El-Enany, W.A.M.A.; El-Ziaty, A.K.; Ali, R.S.; Mohamed, N.A. Cross-linked chitosan/multi- walled carbon nanotubes composite as ecofriendly biocatalyst for synthesis of some novel benzil bis-thiazoles. Polymers 2021, 13, 1728. [Google Scholar] [CrossRef]
- Xu, H.; Liao, W.M.; Li, H.F. A mild and efficient ultrasound-assisted synthesis of diaryl ethers without any catalyst. Ultrason. Sonochem. 2007, 14, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Fokin, V.V.; Garella, D.; Binello, A.; Boffa, L.; Barge, A. Ultrasound-promoted copper-catalyzed azide-alkyne cycloaddition. J. Comb. Chem. 2010, 12, 13–15. [Google Scholar] [CrossRef]
- Gomha, S.M.; Khalil, K.D. A Convenient ultrasound-promoted synthesis and cytotoxic activity of some new thiazole derivatives bearing a coumarin nucleus. Molecules 2012, 17, 9335–9347. [Google Scholar] [CrossRef] [PubMed]
- Pizzuti, L.; Martins, P.L.G.; Ribeiro, B.A.; Quina, F.H.; Pinto, E.; Flores, A.F.C.; Venzke, D.; Pereira, C.M.P. Efficient sonochemical synthesis of novel 3,5-diaryl-4,5-dihydro-1H-pyrazole-1-carboximidamides. Ultrason. Sonochem. 2010, 17, 34–37. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- PLoS Medicine Editors. Antimicrobial resistance: Is the world UNprepared? PLoS Med. 2016, 13, e1002130. [Google Scholar]
- Solomon, S.L.; Oliver, K.B. Antibiotic resistance threats in the United States: Stepping back from the brink. Am. Fam. Physician 2014, 89, 938–941. [Google Scholar] [PubMed]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution. Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar]
- Khan, S.; Iqbal, S.; Shah, M.; Rehman, W.; Hussain, R.; Rasheed, L.; Alrbyawi, H.; Dera, A.A.; Alahmdi, M.I.; Pashameah, R.A.; et al. Synthesis, in vitro anti-microbial analysis and molecular docking study of aliphatic hydrazide-based benzene sulphonamide derivatives as potent inhibitors of α-glucosidase and urease. Molecules 2022, 27, 7129. [Google Scholar] [CrossRef]
- Guo, M.; Gao, Y.; Xue, Y.; Liu, Y.; Zeng, X.; Cheng, Y.; Ma, J.; Wang, H.; Sun, J.; Wang, Z.; et al. Bacteriophage cocktails protect dairy cows against mastitis caused by drug resistant Escherichia coli infection. Front. Cell. Infect. Microbiol. 2021, 11, 690377. [Google Scholar] [CrossRef]
- Faazil, S.; Malik, M.S.; Ahmed, S.A.; Jamal, Q.M.S.; Basha, S.T.; Al-Rooqi, M.M.; Obaid, R.J.; Qurban, J.; Shaikh, I.N.; Asghar, B.H.; et al. New quinoline-thiolactone conjugates as potential antitubercular and antibacterial agents. J. Mol. Str. 2023, 1271, 134099. [Google Scholar] [CrossRef]
- Raimondi, M.V.; Presentato, A.; Li Petri, G.; Buttacavoli, M.; Ribaudo, A.; De Caro, V.; Alduina, R.; Cancemi, P. New synthetic nitro-pyrrolomycins as promising antibacterial and anticancer agents. Antibiotics 2020, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, M.V.; Listro, R.; Cusimano, M.G.; La Franca, M.; Faddetta, T.; Gallo, G.; Schillaci, D.; Collina, S.; Leonchiks, A.; Barone, G. Pyrrolomycins as antimicrobial agents. Microwave-assisted organic synthesis and insights into their antimicrobial mechanism of action. Bioorg. Med. Chem. 2019, 27, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Spano, V.; Rocca, R.; Barreca, M.; Giallombardo, D.; Montalbano, A.; Carbone, A.; Raimondi, M.V.; Gaudio, E.; Bortolozzi, R.; Bai, R.; et al. Pyrrolo[2′,3′:3,4]cyclohepta[1,2-d][1,2]oxazoles, a New Class of Antimitotic Agents Active against Multiple Malignant Cell Types. J. Med. Chem. 2020, 63, 12023–12042. [Google Scholar] [CrossRef]
- Cascioferro, S.; Maggio, B.; Raffa, D.; Raimondi, M.V.; Cusimano, M.G.; Schillaci, D.; Manachini, B.; Leonchiks, A.; Daidone, G. A new class of phenylhydrazinylidene derivatives as inhibitors of Staphylococcus aureus biofilm formation. Med. Chem. Res. 2016, 25, 870–878. [Google Scholar] [CrossRef]
- Whatmore, J.L.; Swann, E.; Barraja, P.; Newsome, J.J.; Bunderson, M.; Beall, H.D.; Tooke, J.E.; Moody, C.J. Comparative study of isoflavone, quinoxaline and oxindole families of anti-angiogenic agents. Angiogenesis 2002, 5, 45–51. [Google Scholar] [CrossRef]
- Jampilek, J. Heterocycles in Medicinal Chemistry. Molecules 2019, 24, 3839. [Google Scholar] [CrossRef] [PubMed]
- Gomtsyan, A. Heterocycles in drugs and drug discovery. Chem. Heterocycl. Comp. 2012, 48, 7–10. [Google Scholar] [CrossRef]
- Abdallah, A.E.; Mabrouk, R.R.; Al Ward, M.M.S.; Eissa, S.I.; Elkaeed, E.B.; Mehany, A.B.M.; Abo-Saif, M.A.; El-Feky, O.A.; Alesawy, M.S.; El-Zahabi, M.A. Synthesis, biological evaluation, and molecular docking of new series of antitumor and apoptosis inducers designed as VEGFR-2 inhibitors. J. Enz. Inhib. Med. Chem. 2022, 37, 573–591. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Ammar, Y.A.; Elhagali, G.A.M.; Eyada, H.A.; Aboul-Magd, D.S.; Ragab, A. In vitro antimicrobial evaluation, single-point resistance study, and radiosterilization of novel pyrazole incorporating thiazol-4-one/thiophene derivatives as dual DNA gyrase and DHFR inhibitors against MDR pathogens. ACS Omega 2022, 7, 4970–4990. [Google Scholar] [CrossRef]
- Roman, G. Thiophene-containing compounds with antimicrobial activity. Arch. Pharm Res. 2022, 355, 2100462. [Google Scholar] [CrossRef] [PubMed]
- Mabkhot, Y.N.; Kaal, N.A.; Alterary, S.; Mubarak, M.S.; Alsayari, A.; Bin Muhsinah, A. New thiophene derivatives as antimicrobial agents. J. Heterocycl. Chem. 2019, 56, 2845–2953. [Google Scholar] [CrossRef]
- Wang, M.; Ma, J.; Wang, H.; Hu, F.; Sun, B.; Tan, T.; Li, M.; Huang, G. Brønsted acid-promoted ring-opening and annulation of thioamides and 2H-azirines to synthesize 2,4,5-trisubstituted thiazoles. Org. Biomolecul. Chem. 2023, 21, 5532–5536. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.X.; Wang, Y.T.; Zhang, S.N.; Li, Y.; Chen, X.B.; Wang, S.Q.; Liu, H.M. Application and synthesis of thiazole ring in clinically approved drugs. Eur. J. Med. Chem. 2023, 250, 115172. [Google Scholar] [CrossRef] [PubMed]
- Abouzied, A.S.; Al-Humaidi, J.Y.; Bazaid, A.S.; Qanash, H.; Binsaleh, N.K.; Alamri, A.; Ibrahim, S.M.; Gomha, S.M. Synthesis, Molecular Docking Study, and Cytotoxicity Evaluation of Some Novel 1,3,4-Thiadiazole as well as 1,3-Thiazole Derivatives Bearing a Pyridine Moiety. Molecules 2022, 27, 6368. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, A.M.; Gomha, S.M.; Zaki, M.E.A.; Abolibda, T.Z.; Kheder, N.A. A Green Synthesis, DFT calculations, and molecular docking study of some new indeno[2,1-b]quinoxalines containing thiazole moiety. J. Mol. Str. 2023, 1292, 136044. [Google Scholar] [CrossRef]
- Anthwal, T.; Paliwal, S.; Nain, S. Diverse Biological Activities of 1,3,4-Thiadiazole Scaffold. Chemistry 2022, 4, 1654–1671. [Google Scholar] [CrossRef]
- Krasavin, M. Novel 5-Nitrofuran-Tagged Imidazo-Fused Azines and Azoles Amenable by the Groebke-Blackburn-Bienaymé Multicomponent Reaction: Activity Profile against ESKAPE Pathogens and Mycobacteria. Biomedicines 2022, 10, 2203. [Google Scholar]
- Lukin, A.; Komarova, K.; Vinogradova, L.; Rogacheva, E.; Kraeva, L.; Krasavin, M. Synthesis and Antibacterial Evaluation of Ciprofloxacin Congeners with Spirocyclic Amine Periphery. Int. J. Nol. Sci. 2023, 24, 954. [Google Scholar] [CrossRef]
- Shetnev, A.; Tarasenko, M.; Kotlyarova, V.; Baykov, S.; Geyl, K.; Kasatkina, S.; Sibinčić, N.; Sharoyko, V.; Rogacheva, E.V.; Kraeva, L.A. External oxidant-free and transition metal-free synthesis of 5-amino-1,2,4-thiadiazoles as promising antibacterials against ESKAPE pathogen strains. Mol. Divers. 2023, 27, 651–666. [Google Scholar] [CrossRef]
- Hu, J.; Su, Z.; Dong, B.; Wang, D.; Liu, X.; Meng, H.; Guo, Q.; Zhou, H. Characterization of a Bacillus subtilis S-16 Thiazole-Synthesis-Related Gene thiS Knockout and Antimicrobial Activity Analysis. Curr. Issues Mol. Biol. 2023, 45, 4600–4611. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Shi, J.; Chen, J.; Hu, D.; Song, B. Design, Synthesis, Antibacterial Activity, and Mechanisms of Novel 1,3,4-Thiadiazole Derivatives Containing an Amide Moiety. J. Agric. Food Chem. 2021, 69, 8660–8670. [Google Scholar] [CrossRef]
- King, D.E.; Malone, R.; Lilley, S.H. New classification and update on the quinolone antibiotics. Am. Fam. Physician 2000, 61, 2741–2748. [Google Scholar] [PubMed]
- Abu-Melha, S.; Edrees, M.M.; Riyadh, S.M.; Abdelaziz, M.R.; Elfiky, A.A.; Gomha, S.M. Clean grinding technique: A facile synthesis and in silico antiviral activity of hydrazones, pyrazoles, and pyrazines bearing thiazole moiety against SARS-CoV-2 main protease (Mpro). Molecules 2020, 25, 4565. [Google Scholar] [CrossRef]
- Khalil, K.D.; Riyadh, S.M.; Gomha, S.M.; Ali, I. Synthesis, characterization and application of copper oxide chitosan nanocomposite for green regioselective synthesis of [1,2,3]triazoles. Int. J. Biol. Macromol. 2019, 130, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.R.; Gomha, S.M.; Taher, E.A.; Muhammad, Z.A.; El-Seedi, H.R.; Gaber, H.M.; Ahmed, M.M. One-pot synthesis of novel thiazoles as potential anti- cancer agents. Drug Des. Devel. Ther. 2020, 14, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Riyadh, S.M.; Alharbi, R.A.K.; Zaki, M.E.A.; Abolibda, T.Z.; Farag, B. Green route synthesis and molecular docking of azines using cellulose sulfuric acid under microwave irradiation. Crystals 2023, 13, 260. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M. Synthesis of triazolo[4,3-b][1,2,4,5]tetrazines and triazolo[3,4-b][1,3,4]thiadiazines using chitosan as ecofriendly catalyst under microwave irradiation. Arkivoc 2009, xi, 58–68. [Google Scholar] [CrossRef]
- Abu-Melha, S.; Gomha, S.M.; Abouzied, A.S.; Edrees, M.M.; Abo Dena, A.S.; Muhammad, Z.A. Microwave-assisted one pot three-component synthesis of novel bioactive thiazolyl-pyridazinediones as potential antimicrobial agents against antibiotic-resistant bacteria. Molecules 2021, 26, 4260. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdalla, M.A.; Abdelaziz, M.; Serag, N. Eco-friendly one-pot synthesis and antiviral evaluation of pyrazolyl pyrazolines of medicinal interest. Turk. J. Chem. 2016, 40, 484–498. [Google Scholar] [CrossRef]
- Gomha, S.M.; Edrees, M.M.; Muhammad, Z.A.; El-Reedy, M.A.A. 5-(Thiophen-2-yl)-1,3,4-thiadiazole derivatives: Synthesis, molecular docking and in-vitro cytotoxicity evaluation as potential anti-cancer agents. Drug Des. Dev. Ther. 2018, 12, 1511–1523. [Google Scholar] [CrossRef]
- Aljohani, G.F.; Abolibda, T.Z.; Alhilal, M.; Al-Humaidi, J.Y.; Alhilal, S.; Ahmed, H.A.; Gomha, S.M. Novel thiadiazole-thiazole hybrids: Synthesis, molecular docking, and cytotoxicity evaluation against liver cancer cell lines. J. Taibah Uni. Sci. 2022, 16, 1005–1015. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Gomha, S.M.; Abdelaziz, M.R.; Serag, N. Synthesis and antiviral evaluation of some novel thiazoles and 1,3-thiazines substituted with pyrazole moiety against rabies virus. Turk. J. Chem. 2016, 40, 441–453. [Google Scholar] [CrossRef]
- Alghamdi, A.; Abouzied, A.S.; Alamri, A.; Anwar, S.; Ansari, M.; Khadra, M.; Zaki, Y.H.; Gomha, S.M. Synthesis, Molecular Docking and Dynamic Simulation Tar-geting Main Protease (Mpro) of New Thiazole Clubbed Pyridine Scaffolds as Potential CoV-19 Inhibitors. Curr. Issues Mol. Biol. 2023, 45, 93. [Google Scholar] [CrossRef]
- Gomha, S.M.; Muhammad, Z.A.; Abdel-aziz, M.R.; Abdel-aziz, H.M.; Gaber, H.M.; Elaasser, M.M. One pot synthesis of new thiadiazolyl-pyridines as anticancer and antioxidant agents. J. Heterocycl. Chem. 2018, 55, 530–536. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdelhady, H.A.; Hassain, D.Z.H.; Abdelmonsef, A.H.; El-Naggar, M.; Elaasser, M.M.; Mahmoud, H.K. Thiazole based thiosemicarbazones: Synthesis, cytotoxicity evaluation and molecular docking study. Drug Des. Dev. Ther. 2021, 15, 659–677. [Google Scholar] [CrossRef] [PubMed]
- Khalil, K.D.; Ahmed, H.A.; Bashal, A.H.; Bräse, S.; Nayl, A.; Gomha, S.M. Efficient, Recyclable, heterogeneous base nanocatalyst for thiazoles with chitosan capped calcium oxide nanocomposite. Polymer 2022, 14, 3347. [Google Scholar] [CrossRef]
- Al-Ghamdi, R.F.; Fahmi, M.M.; Mohamed, N.A. Thermal stability and degradation behavior of novel wholly aromatic azopolyamide-hydrazides. Polym. Degrad. Stab. 2006, 91, 1530–1544. [Google Scholar] [CrossRef]
- Mohamed, N.A. Biologically active maleimido aromatic 1,3,4-oxadiazole derivatives evaluated thermo-gravimetrically as stabilizers for rigid PVC. J. Therm. Anal. Calorim. 2018, 131, 2535–2546. [Google Scholar] [CrossRef]
- Dessingiou, J.; Khedkar, J.K.; Rao, C.P. Chemosensing ability of hydroxynaphthylidene derivatives of hydrazine towards Cu2+: Experimental and computational studies. J. Chem. Sci. 2014, 126, 1135–1141. [Google Scholar] [CrossRef]
- Gomha, S.M.; Edrees, M.M.; Altalbawy, F.M.A. Synthesis and characterization of some new bis-pyrazolyl-thiazoles incorporating the thiophene moiety as potent anti-tumor agents. Inter. J. Mol. Sci. 2016, 17, 1499. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Abdel-Aziz, H.A. Enaminones as building blocks in heterocyclic preparations: Synthesis of novel pyrazoles, pyrazolo[3,4-d]pyridazines, yrazolo[1,5-a]pyrimidines, pyrido[2,3-d]pyrimidines linked to imidazo[2,1-b]thiazole system. Heterocycles 2012, 85, 2291–2303. [Google Scholar] [CrossRef]
- Shawali, A.S.; Harb, N.M.S.; Badahdah, K.O. A study of tautomerism in diazonium coupling products of 4-hydroxycoumarin. J. Heterocycl. Chem. 1985, 22, 1397–1403. [Google Scholar] [CrossRef]
- Gomha, S.M. A facile one-pot synthesis of 6,7,8,9-tetrahydrobenzo[4,5]thieno[2,3-d]-1,2,4- triazolo[4,5-a]pyrimidin-5-ones. Monatsh. Chem. 2009, 140, 213–220. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; El-Hashash, M.A.; Elkaeed, E.B. Eco-friendly sequential one-pot synthesis, molecular docking, and anticancer evaluation of arylidene-hydrazinyl-thiazole derivatives as CDK2 inhibitors. Bioorg. Chem. 2021, 108, 104615. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, F.; Bizzarri, B.; Bolasco, A.; Secci, D.; Chimenti, P.; Granese, A.; Carradori, S.; D’Ascenzio, M.; Rivanera, D. Synthesis and biological evaluation of novel 2,4-disubstituted-1,3-thiazoles as anti-Candida spp. agents. Eur. J. Med. Chem. 2011, 46, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Alqurashi, R.M.; Farghaly, T.A.; Sabour, R.; Shaabana, M.R. Design, Synthesis, antimicrobial screening and molecular modeling of novel 6, 7 dimethylquinoxalin-2 (1H)-one and thiazole derivatives targeting DNA gyrase enzyme. Bioorg. Chem. 2023, 134, 106433. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Tandon, N.; Singh, I.; Tandon, R. Structure-activity relationship (SAR) and antibacterial activity of pyrrolidine based hybrids: A review. J. Mol. Str. 2023, 1283, 135175. [Google Scholar] [CrossRef]
- Yıldırım, M.; Çelikel, D.; Dürüst, Y.; Knight, D.W.; Kariuki, B.M. A rapid and efficient protocol for the synthesis of novel nitrothiazolo [3, 2-c] pyrimidines via microwave-mediated Mannich cyclisation. Tetrahedron 2014, 70, 2122–2128. [Google Scholar] [CrossRef]
- Asgaonkar, K.; Tanksali, S.; Abhang, K.; Sagar, A. Development of optimized pyrimido-thiazole scaffold derivatives as anticancer and multitargeting tyrosine kinase inhibitors using computational studies. J. Indian Chem. Soc. 2023, 100, 100803. [Google Scholar] [CrossRef]
- Alam, R.; Wahi, D.; Singh, R.; Sinha, D.; Tandon, V.; Grover, A. Design, synthesis, cytotoxicity, HuTopoIIα inhibitory activity and molecular docking studies of pyrazole derivatives as potential anticancer agents. Bioorg. Chem. 2016, 69, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Ragab, A.; Ammar, Y.A.; Ezzat, A.; Mahmoud, A.M.; Mohamed, M.B.I.; El-Tabl, A.S.; Farag, R.S. Synthesis, characterization, thermal properties, antimicrobial evaluation, ADMET study, and molecular docking simulation of new mono Cu (II) and Zn (II) complexes with 2-oxoindole derivatives. Comput. Biol. Med. 2022, 145, 105473. [Google Scholar] [CrossRef]
- Mukhtar, S.S.; Morsy, N.M.; Hassan, A.S.; Hafez, T.S.; Hassaneen, H.M.; Saleh, F.M. A Review of Chalcones: Synthesis, Reactions, and Biological Importance. Egypt. J. Chem. 2022, 65, 379–395. [Google Scholar] [CrossRef]
- Qiu, X.; Janson, C.A.; Smith, W.W.; Green, S.M.; McDevitt, P.; Johanson, K.; Carter, P.; Hibbs, M.; Lewis, C.; Chalker, A.; et al. Crystal structure of Staphylococcus aureus tyrosyl-tRNA synthetase in complex with a class of potent and specific inhibitors. Protein Sci. 2001, 10, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Labute, P. Protonate3D: Assignment of ionization states and hydrogen coordinates to macromolecular structures. Proteins 2009, 75, 187–205. [Google Scholar] [CrossRef]
- Kattan, S.W.; Nafie, M.S.; Elmgeed, G.A.; Alelwani, W.; Badar, M.; Tantawy, M.A. Molecular docking, anti-proliferative activity and induction of apoptosis in human liver cancer cells treated with androstane derivatives: Implication of PI3K/AKT/mTOR pathway. J. Steroid Biochem. Mol. Biolog. 2020, 198, 105604. [Google Scholar] [CrossRef] [PubMed]
- Nafie, M.S.; Tantawy, M.A.; Elmgeed, G.A. Screening of different drug design tools to predict the mode of action of steroidal derivatives as anti-cancer agents. Steroids 2019, 152, 108485. [Google Scholar] [CrossRef] [PubMed]





| 1st Step | 2nd Step | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Samples | T(°C) at 5% Mass Loss | T(°C) at 10% Mass Loss | Tstart-Tend (°C) | Mass Loss% | Tpeak (°C) | Tstart-Tend (°C) | Mass Loss% | Tpeak (°C) | Residue% |
| Chitosan | 90.32 | 185.26 | 25–150 | 9.00 | 57.14 | 250–500 | 34.53 | 300.56 | 52.94 |
| TCs | 130.45 | 235.37 | 25–150 | 5.88 | 59.61 | 250–500 | 32.22 | 482.43 | 56.78 |
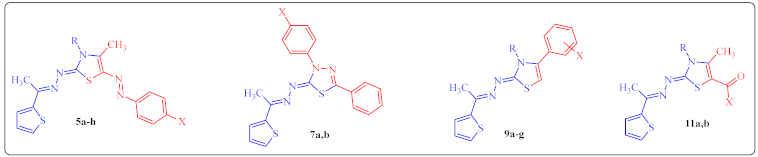 | ||||||
|---|---|---|---|---|---|---|
| Compd. No. | R | X | (%) YieldUsing Cs | (%) YieldUsing TCs | M.p.(°C) a | Reference |
| 5a | H | H | 77 | 88 | 186–188 | [72] |
| 5b | H | Me | 76 | 86 | 172–174 | [72] |
| 5c | H | Cl | 79 | 87 | 216–218 | [72] |
| 5d | H | Br | 75 | 88 | 203–205 | [72] |
| 5e | Ph | H | 80 | 87 | 216–218 | - |
| 5f | Ph | Me | 81 | 86 | 207–209 | - |
| 5g | Ph | OMe | 78 | 85 | 173–175 | - |
| 5h | Ph | Cl | 80 | 88 | 238–240 | - |
| 7a | - | H | 69 | 80 | 160–162 | - |
| 7b | - | Cl | 72 | 84 | 183–185 | - |
| 9a | H | H | 82 | 89 | 261–263 | [76] |
| 9b | H | MeO | 78 | 88 | 250–252 | [77] |
| 9c | H | NO2 | 80 | 87 | 246–248 | [76] |
| 9d | Ph | H | 82 | 91 | 212–214 | - |
| 9e | Ph | Me | 84 | 90 | 201–203 | - |
| 9f | Ph | Cl | 83 | 92 | 229–231 | - |
| 9g | Ph | Br | 82 | 93 | 215–217 | - |
| 11a | H | Me | 77 | 87 | 220–222 | [76] |
| 11b | Ph | OEt | 79 | 90 | 231–233 | - |
| State of Catalyst | New Catalyst | Recycled Catalyst | ||||
|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | ||
| Product 5a (% Yield) | 88 | 85 | 82 | 79 | 51 | 27 |
| Compd. No. | Docking Score (kcal/mol) | No. of Hydrogen Bonding | No. of Arene Interaction | RMSD kcal·mol−1Å−1 |
|---|---|---|---|---|
| 5e | −7.36 | - | - | 0.84 |
| 5f | −7.49 | - | - | 1.08 |
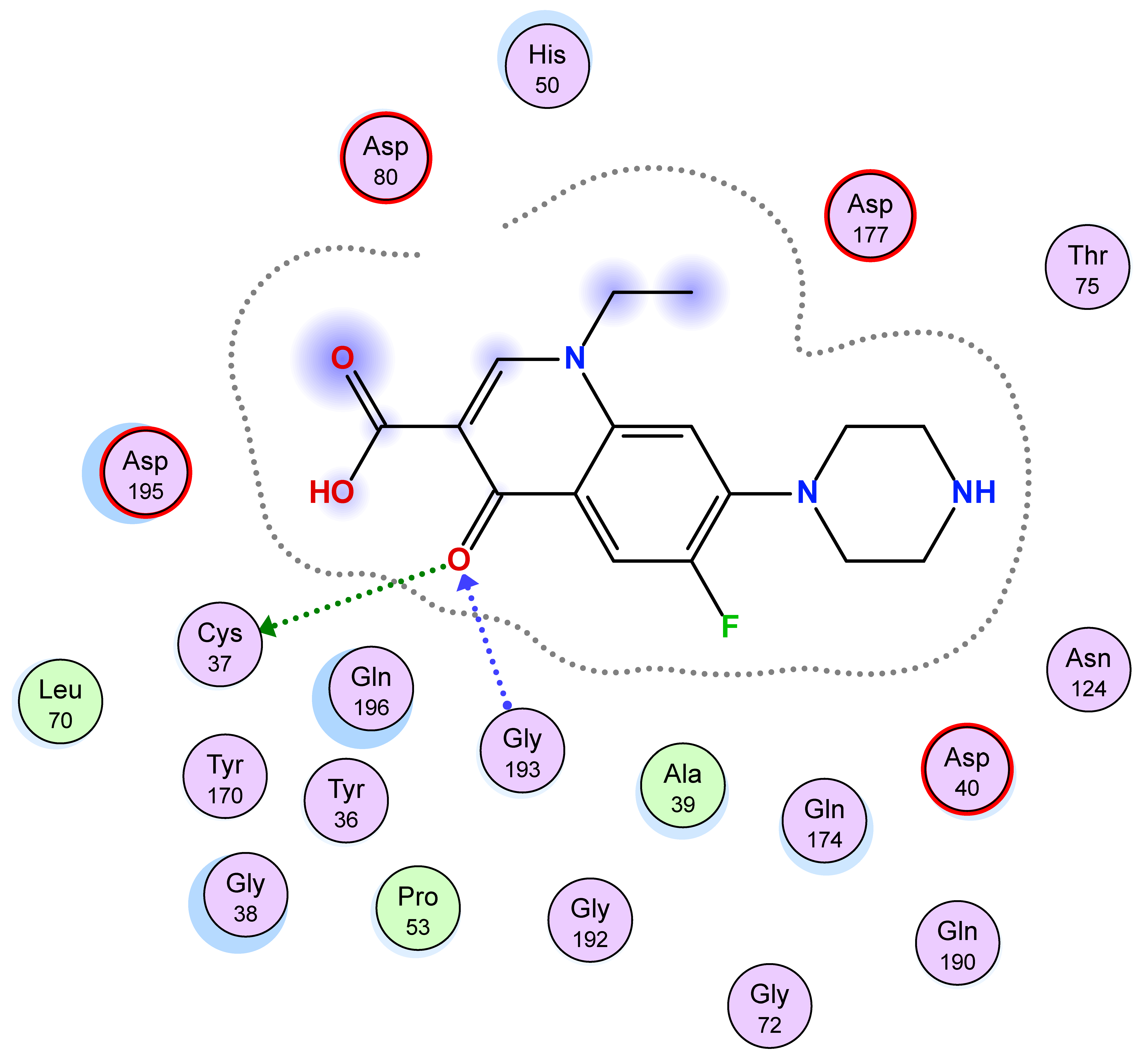
| 5g | −7.78 | 1 (Asp40) 1 (Thr175) | 1 (pi-H) [Asp40] | 1.08 |
| 5h | −7.85 | - | - | 1.43 |
| 7a | −7.26 | - | 1 (pi-H) [His50] 1 (pi-H) [Asp80] | 1.00 |
| 7b | −7.60 | 1 (Gly49) | - | 1.03 |
| 9a | −6.48 | 1 (Cly49) 1 (Vall91) | - | 1.54 |
| 9b | −7.15 | 2 (Cys37) 1 (Vall91) | 1 (H-pi) [Tyr36] | 1.73 |
| 9c | −6.76 | - | - | 0.80 |
| 9d | −7.23 | 1 (Vall91) | - | 2.24 |
| 9e | −7.25 | - | - | 2.06 |
| 9f | −7.3 | 1 (Asp177) | - | 0.87 |
| 9g | −7.44 | 1 (Asp177) | - | 0.83 |
| 11a | −6.21 | 1 (Asp80) 1 (Lys84) | - | 1.25 |
| 11b | −7.13 | 1 (Cys37) | - | 1.09 |
| Norfloxacin | −7.10 | 1 (Cys37) 1 (Gly193) | - | 0.83 |
| Compd. No. | 3D Ligand Interaction | 2D Ligand Interaction |
|---|---|---|
| 5e | 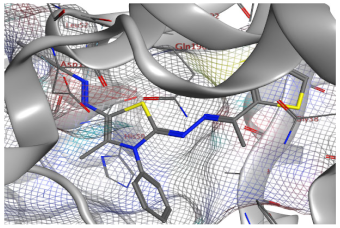 | 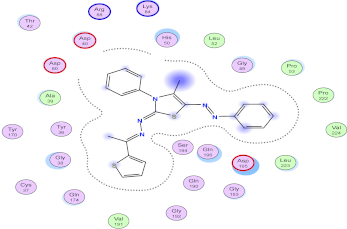 |
| 5f | 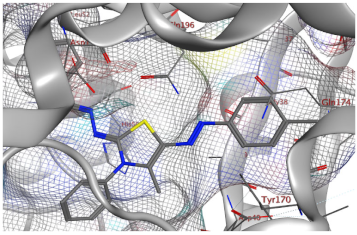 | 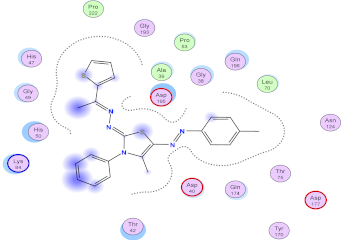 |
| 5g | 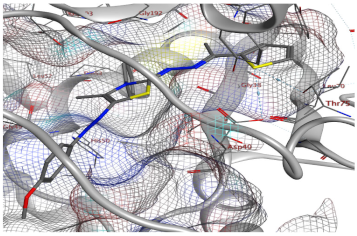 | 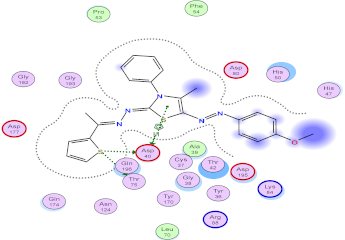 |
| 5h |  | 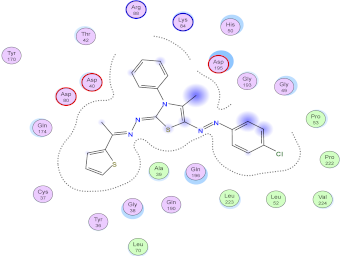 |
| 7a | 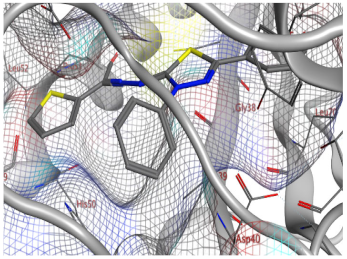 | 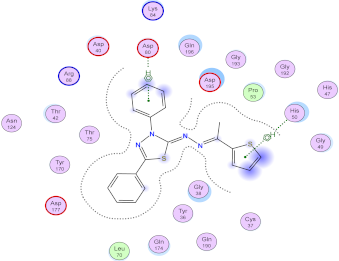 |
| 7b | 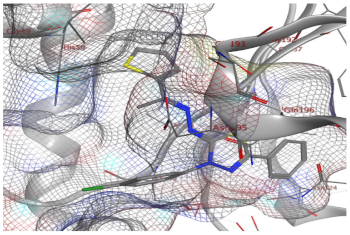 |  |
| 9a | 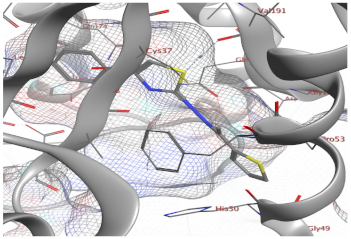 |  |
| 9b | 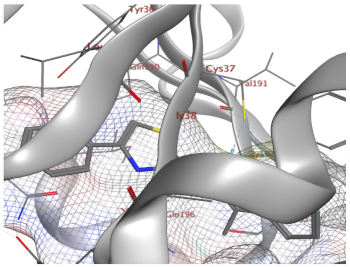 | 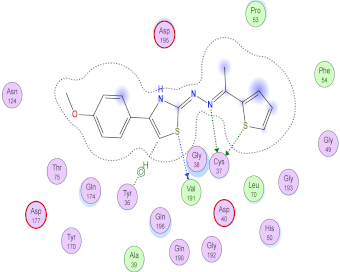 |
| 9c | 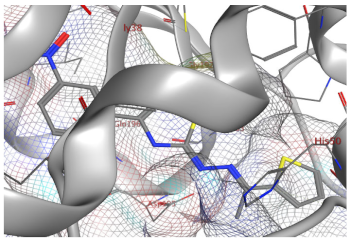 | 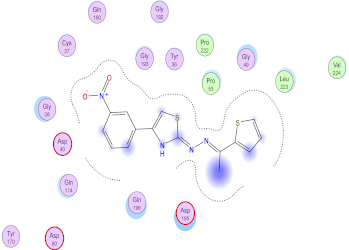 |
| 9d |  | 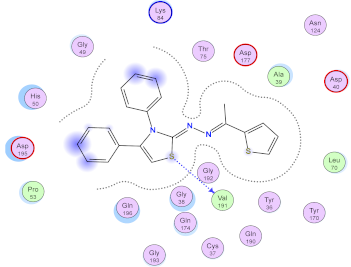 |
| 9e | 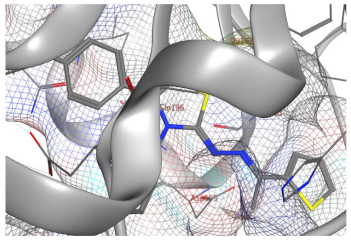 |  |
| 9f |  |  |
| 9g |  | 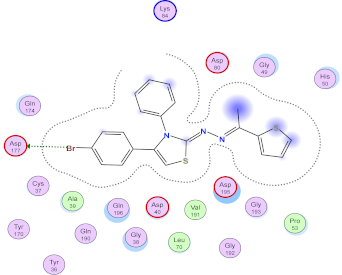 |
| 11a | 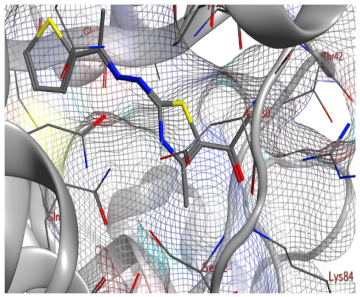 |  |
| 11b | 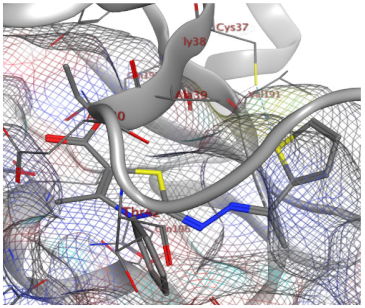 | 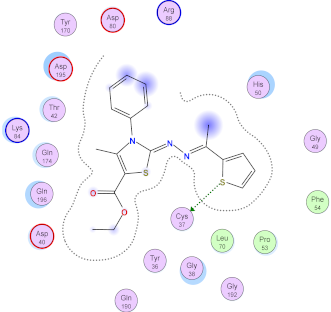 |
| Test Items | 5h | 7b | 9b | 9g | 11b | Norfloxacin |
|---|---|---|---|---|---|---|
| Molecular properties | ||||||
| PSA (°A2) | 110.85 | 99.02 | 106.22 | 86.13 | 112.43 | 74.57 |
| M. Wt. | 451.99 | 410.94 | 329.44 | 454.41 | 385.50 | 319.33 |
| HBA | 4 | 3 | 3 | 2 | 4 | 5 |
| HBD | 0 | 0 | 1 | 0 | 0 | 2 |
| NRB | 5 | 4 | 4 | 4 | 6 | 3 |
| Pharmacokinetics | ||||||
| GI absorption | Low | High | High | Low | High | High |
| BBB permeant | No | No | No | No | No | No |
| P-gp substrate | No | No | No | No | No | Yes |
| Skin permeation (Log Kp) cm/s | −3.99 | −4.21 | −5.46 | −4.47 | −5.14 | −8.98 |
| Drug likeness and Medicinal Chemistry | ||||||
| PAINS | 1 | 0 | 0 | 0 | 0 | 0 |
| Synthetic accessibility | 4.06 | 3.73 | 3.23 | 3.61 | 3.79 | 2.46 |
| Bioavailability Score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| Lipinski Rule (violation) | Yes (1) | Yes (1) | Yes (0) | Yes (1) | Yes (0) | Yes (0) |
| Veber Rule (violation) | Yes | Yes | Yes | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Humaidi, J.Y.; Gomha, S.M.; El-Ghany, N.A.A.; Farag, B.; Zaki, M.E.A.; Abolibda, T.Z.; Mohamed, N.A. Green Synthesis and Molecular Docking Study of Some New Thiazoles Using Terephthalohydrazide Chitosan Hydrogel as Ecofriendly Biopolymeric Catalyst. Catalysts 2023, 13, 1311. https://doi.org/10.3390/catal13091311
Al-Humaidi JY, Gomha SM, El-Ghany NAA, Farag B, Zaki MEA, Abolibda TZ, Mohamed NA. Green Synthesis and Molecular Docking Study of Some New Thiazoles Using Terephthalohydrazide Chitosan Hydrogel as Ecofriendly Biopolymeric Catalyst. Catalysts. 2023; 13(9):1311. https://doi.org/10.3390/catal13091311
Chicago/Turabian StyleAl-Humaidi, Jehan Y., Sobhi M. Gomha, Nahed A. Abd El-Ghany, Basant Farag, Magdi E. A. Zaki, Tariq Z. Abolibda, and Nadia A. Mohamed. 2023. "Green Synthesis and Molecular Docking Study of Some New Thiazoles Using Terephthalohydrazide Chitosan Hydrogel as Ecofriendly Biopolymeric Catalyst" Catalysts 13, no. 9: 1311. https://doi.org/10.3390/catal13091311
APA StyleAl-Humaidi, J. Y., Gomha, S. M., El-Ghany, N. A. A., Farag, B., Zaki, M. E. A., Abolibda, T. Z., & Mohamed, N. A. (2023). Green Synthesis and Molecular Docking Study of Some New Thiazoles Using Terephthalohydrazide Chitosan Hydrogel as Ecofriendly Biopolymeric Catalyst. Catalysts, 13(9), 1311. https://doi.org/10.3390/catal13091311