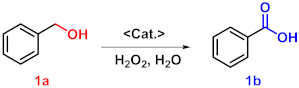
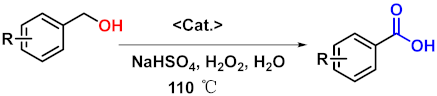Abstract
Benzoic acid has found a wide range of applications in the chemical industry. The selective oxidation of benzyl alcohol is one of the main routes to produce benzoic acid. In this work, tris(4-bromobiphenyl)phosphine was chosen as a building block to synthesize PAF-181 with a high specific surface area and high yield via a Yamamoto–Ullmann reductive coupling reaction. Subsequently, the WO4@PAF-181 catalyst was successfully prepared via methylation and ion exchange, in which PAF-181 acts as a carrier while WO42− serves as the active catalytic site. The synergistic effect between functional carriers and active sites endows WO4@PAF-181 with distinctive catalytic property for efficient selective oxidation of benzyl alcohol to benzoic acid. Importantly, the catalyst can be conveniently recovered and reused by simple filtration, still maintaining its high catalytic activity.
1. Introduction
Benzoic acid, as the simplest organic aromatic acid, finds extensive applications in the fields of medicine, food, and the chemical industry [1,2]. It also serves as a pivotal precursor in the organic synthesis industry [3]. Recent research indicates that electrocatalysis [4], photocatalysis [5], and chemical catalysis [6,7,8,9,10,11,12,13,14,15,16,17] have been extensively investigated for the purpose of selectively oxidizing benzyl alcohol. Particularly, there has been significant scholarly interest in exploring the selective oxidation of benzyl alcohol through chemical catalysis. Oxidizing agents such as KMnO4 [6], CrO3 [7], and iodosobenzene [8] have been employed in existing research, but often lead to substantial production of pollutants. To address these concerns, alternative oxidants including O2 [9,10], H2O2 [11,12,13], potassium peroxymonosulfate (Oxone) [14,15], and tert-butyl hydroperoxide (t-BuOOH) [16,17] have been utilized for the efficient synthesis of benzoic acid. Moreover, various transition metals have been investigated as catalysts for the selective oxidation of alcohols to carboxylic acids. Notably, noble metal catalysts such as gold, palladium, and iridium have exhibited remarkable catalytic activity in this regard [18,19,20,21].
Recently, porous organic polymers (POPs) have gained significant attention and emerged as promising catalyst supports in the field of organic catalysis due to their exceptional characteristics, including high specific surface area, excellent stability, and facile post-modification capabilities [22,23]. In 2017, Huang successfully synthesized a series of imidazole-containing crosslinked POPs (poly(divinylbenzene-co-N-vinylimidazole), PDVB-VI-n) via free-radical polymerization. Subsequently, these POPs were employed as supports for immobilizing gold nanoparticles (Au NPs), which were prepared through coordination of AuCl3 and NaBH4 reduction, exhibiting good activity for the heterogeneous catalysis of the aerobic oxidation of benzyl alcohol [1]. Furthermore, in 2019, Loh installed Au NPs and Ag NPs onto the surface of thiol-functionalized block copolymer particles, achieving the first interfacial gold- and silver-catalyzed aerobic oxidation of benzyl alcohol to benzoic acid [24]. However, it should be noted that metal NPs are susceptible to agglomeration and deactivation during the reaction [25]. Additionally, the preparation of POPs through free-radical polymerization presents challenges in terms of process control, resulting in relatively random product structures. Moreover, the use of metal catalysts may lead to contamination of the final product [26,27,28].
Porous aromatic frameworks (PAFs) represent a novel category of POPs. These frameworks consist of structural units derived from aromatic rings, which are interconnected by robust carbon–carbon covalent bonds formed via straightforward organic coupling reactions [29]. The pore structure of PAFs can be tailored through the topological design of monomers, and the aromatic monomers can also be easily pre-modified and post-modified to achieve desired functionality [30]. Furthermore, PAFs exhibit not only high specific surface area but also exceptional chemical and thermal stability under harsh conditions, such as elevated temperatures, strong acids and alkalis, and various organic solvents. These remarkable properties render PAFs promising candidates as supports for heterogeneous catalysts [31]. Given the advantages of PAFs and the significance of benzoic acid, our objective is to develop cost-effective, recyclable, and environmentally benign catalysts by strategically designing the framework structure to enable the green and sustainable synthesis of benzoic acid.
Herein, we intend to develop the strategy to immobilize inexpensive metal ions into PAFs to prepare heterogeneous catalysts that enhance their selective catalytic efficiency for the oxidation of benzyl alcohol to benzoic acid. PAF-181 is designed and synthesized through Ullmann coupling of tris(4-bromobiphenyl)phosphine [29] as a heterogeneous catalyst platform on the basis of the following considerations: (i) the employment of long-sized biphenyl units easily creates larger pore volumes, providing enough space for the introduction of the subsequent active centers and the accommodation of organic substrates; (ii) the electron-donating nature of the phosphine center (P III) enables facile coordination with transition metals; (iii) phosphine can be oxidized to phosphonium cations (P V), thereby constructing a cationic framework for convenient modification [32,33]. Given the enhanced stability of high-valence P in ambient conditions, we employed methyl iodide to oxidize the P center in PAF-181 to phosphonium cations by a methylation reaction, leading to the successful synthesis of I@PAF-181. Subsequently, we conducted an anion ion exchange using low-cost sodium tungstate to replace I− in I@PAF-181 [34]. Finally, the target catalyst WO4@PAF-181 was successfully obtained, which was further used to catalyze the selective green oxidation of benzyl alcohol (Scheme 1).
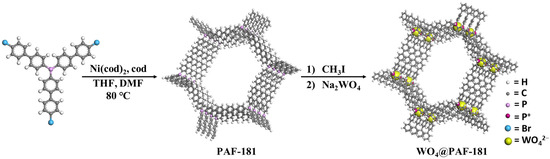
Scheme 1.
The synthetic route of PAFs and their theoretical model.
2. Results
2.1. Structural Characterization
The structure of the materials was systematically investigated through a series of characterization techniques. Fourier transform infrared (FT-IR) spectroscopy (Figure 1a) was employed to characterize the structure and composition of PAF-181 and its building blocks. It was observed that the C–Br stretching vibration absorption peak at 518 cm−1 (curve I) in the fingerprint region vanished in curve II, proving the successful synthesis of PAF-181. Following the methylation reaction, I@PAF-181 exhibited a saturated C–H stretching vibration absorption peak at 2800–3000 cm−1 (curve III), indicating the successful installation of methyl groups. The comparison between WO4@PAF-181 and I@PAF-181 revealed that WO4@PAF-181 displayed a stretching vibration absorption peak of W–O at 841 cm−1 in curve IV, signifying the effective immobilization of WO42− anions within the porous framework.
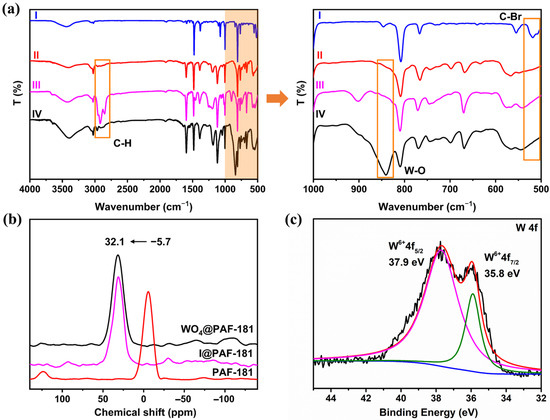
Figure 1.
FT-IR and 31P CP/MAS solid-state NMR spectra of the PAFs and XPS fine spectra of W 4f for WO4@PAF-181: (a) FT-IR spectra of tris(4-bromobiphenyl)phosphine (I, blue), PAF-181 (II, red), I@PAF-181 (III, magenta), and WO4@PAF-181 (IV, black); (b) 31P solid-state NMR of PAF-181 (red), I@PAF-181 (magenta), and WO4@PAF-181 (black); (c) peak fitting of W 4f spectrum for WO4@PAF-181.
Powder X-ray diffraction (PXRD) was performed to investigate the crystallinity of PAF-181, I@PAF-181, and WO4@PAF-181 (see Figure S1a). The results show that the three PAFs all exhibit an amorphous nature. Notably, the absence of diffraction peaks corresponding to Na2WO4 or tungsten oxide in WO4@PAF-181 suggests a homogeneous dispersion of W species within the material’s pores, predominantly in the ionic form of WO42−.
The 13C cross-polarization/magic angle spinning (CP/MAS) solid-state nuclear magnetic resonance (NMR) spectrum of PAF-181 (see Figure S2) indicated the presence of aromatic carbon signal peaks at 139 and 126 ppm. In the spectra of I@PAF-181 and WO4@PAF-181, an additional peak of aliphatic carbon was observed at 30 ppm, which can be attributed to the methyl carbon bonded to phosphorus, thereby confirming the methylation of P(III). However, it should be noted that the methyl carbon peak in the WO4@PAF-181 NMR spectrum appeared relatively weak due to the signal-to-noise ratio of solid-state NMR and the influence of WO42− on the carbon spectrum. Furthermore, the 31P CP/MAS solid-state NMR analysis of PAF-181 (Figure 1b) demonstrated a signal peak for P(III) at −5.7 ppm, while the P(V) signal peaks for I@PAF-181 and WO4@PAF-181 were observed at 32.1 ppm. The aforementioned characterization results provide evidence for the successful synthesis of the desired products.
The morphological characteristics of the materials were examined using scanning electron microscopy (SEM). As shown in Figure S3, the three PAFs are all composed of small particles partially bonded together, albeit with slight morphological variations. PAF-181 displays numerous irregular small particles and partially spherical-like particles of varying sizes adhered together, resulting in a relatively rough particle surface. After methylation, the particles in I@PAF-181 demonstrate enhanced uniformity and a smoother surface. WO4@PAF-181 showcases more approximately spherical particles that are uniformly distributed throughout the image. The transmission electron microscope (TEM) mapping images (see Figure S4) reveal that the elements of PAF-181 (C, P), I@PAF-181 (C, P, I), and WO4@PAF-181 (C, P, O, W) are uniformly distributed throughout the frameworks. Furthermore, the absence of I in the WO4@PAF-181 sample proves a relatively complete exchange of I− for WO42− ions. X-ray photoelectron spectroscopy (XPS) (Figure 1c and Figure S5) also confirms the successful loading of WO42− onto the WO4@PAF-181 framework. The binding energies of 35.8 eV and 37.9 eV are attributed to the W6+4f7/2 and W6+4f5/2 orbital characteristics of WO42−, respectively. An inductively coupled plasma atomic emission spectroscopy (ICP-AES) test demonstrates that the P content in PAF-181 is 5.8 wt%, which closely aligns with the theoretical content of 6.3 wt%. Additionally, the P and W contents in WO4@PAF-181 are 4.1 wt% and 14.1 wt% (see Table S1), respectively, which are also in agreement with the theoretical contents (P: 4.9 wt%, W: 14.6 wt%).
The thermal stability of the materials was assessed using a thermogravimetric analyzer (TGA) in an air atmosphere. It was observed that PAF-181 (Figure 2a) exhibited favorable thermal stability, with significant framework collapse occurring at approximately 456 °C. However, a new inflection point emerged around 533 °C, potentially attributable to the oxidation of the P element on the framework to form P2O5. Following the methylation, the removal of free I− in I@PAF-181 resulted in a weight loss of nearly 20% within the temperature range of 200–400 °C [35]. After ion exchange, WO4@PAF-181 still retained high thermal stability, with framework collapse occurring around 456 °C. Furthermore, a minor weight loss in the low-temperature region (<200 °C) was observed, which can be attributed to the desorption of water molecules absorbed by the ionic material [35]. The W content determined from the combustion residual mass fraction of WO3 (17.5 wt%) was found to be 13.8 wt%, consistent with the ICP-AES analysis result.
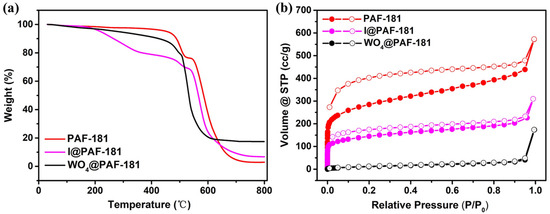
Figure 2.
Thermogravimetric and N2 physical sorption characterization of the PAFs: (a) TGA plots of PAF-181, I@PAF-181, and WO4@PAF-181; (b) N2 adsorption–desorption isotherms at 77 K for PAF-181, I@PAF-181, and WO4@PAF-181.
The catalytic performance of POPs is heavily influenced by their pore structure [36]. To further analyze the pore structures of PAF-181, I@PAF-181, and WO4@PAF-181, N2 physical sorption at 77 K was conducted and their pore characteristics (see Table S2) were obtained based on the corresponding isotherms (Figure 2b). PAF-181 and I@PAF-181 exhibit a type I isotherm with steep adsorption at low pressures of P/P0 ≤ 0.01, which is a typical characteristic of surface coverage in porous materials, indicating the presence of micropores in the framework. The hysteresis loop observed between the adsorption and desorption branches of the isotherms across the entire relative pressure range suggests the existence of inner framework mesopores with uneven sizes or outer particle–particle large pores [37]. Methylation results in a significant reduction in the adsorption capacity of I@PAF-181 at low pressure due to the occupation of pores by -CH3 and I−. In general, both PAFs can be classified as microporous–mesoporous composite materials. However, the adsorption capacity of WO4@PAF-181 decreases sharply at low pressure (P/P0 = 0.01, 3.8 cc/g), but increases exponentially as pressure rises. This overall trend indicates a type III isotherm, suggesting that larger WO42− ions occupy most of the micropores after ion exchange, leading to a significant increase in the ratio of mesopores and macropores. This result is consistent with the pore volume information obtained from the adsorption–desorption isotherms.
According to Brunauer–Emmett–Teller (BET) theory, surface areas of 1029, 579, and 45 m2 g−1 are obtained for PAF-181, I@PAF-181, and WO4@PAF-181, respectively. The surface area decreases significantly as the size of the modified functional group increases. The pore size distributions calculated by the quenched solid density functional theory (QSDFT) with the lowest fitting-error principle show the main pore sizes of 1.27 nm and 1.19 nm for PAF-181 and I@PAF-181, respectively (see Figure S6). And their mean pore diameters are 3.44 nm and 3.54 nm, respectively. The exchange of WO42− results in a significant increase in the mean pore diameter (24.1 nm) for WO4@PAF-181, despite the main pore size of 1.83 nm (micropore range). Consequently, there is a substantial reduction in the proportion of micropores, while mesopores dominate. Nonetheless, a significant quantity of preserved mesopores is sufficient to offer space for subsequent chemical reactions.
The water wetting properties of the materials were measured using a water contact angle meter (see Figure S7). The water contact angle of PAF-181 was measured to be 130°, indicating its hydrophobic nature. Following the methylation reaction, the water contact angle decreased to 93°, suggesting a significant enhancement in the hydrophilicity of I@PAF-181. After ion exchange, the contact angle of WO4@PAF-181 was found to be 88°, signifying its hydrophilic nature. The inherent hydrophilicity of WO4@PAF-181 facilitates its effective dispersion in aqueous media, thereby enhancing its interaction with water-soluble reactants.
2.2. Catalytic Performance
After conducting a comprehensive investigation on the structural characterization of WO4@PAF-181, the catalytic behavior towards the oxidation of benzyl alcohol by H2O2 was explored [38,39,40]. The optimization of the reaction conditions was investigated. The corresponding results are presented in Table 1. It is observed that the presence of acid significantly influences the reaction outcome. In the absence of acid (Table 1, entry 1), the reaction yield is notably low (13%). Consequently, various common acids such as tartaric acid, p-toluenesulfonic acid (TsOH), and NaHSO4 (Table 1, entries 2, 3, 11) were screened. The yield of benzoic acid gradually increased with the acidity level of the employed acid. This finding is consistent with the relevant literature [41], which indicates that a benzaldehyde intermediate is formed during the oxidation process of benzyl alcohol to benzoic acid. The resultant benzaldehyde undergoes a hydration balance in an acidic environment and will be partially converted to acetal, which can generate benzoic acid via α-H elimination. Therefore, the enhanced acidity of the system is advantageous for promoting benzoic acid formation, which also aligns with the experimental yields. It is important to note that the utilization of 1 mol% NaHSO4 (99%) leads to higher yields compared to the use of 0.5 mol% (53%) (Table 1, entries 4 and 11). The absence of a catalyst resulted in a low yield (26%, Table 1, entry 5), underscoring the essential role of a catalyst in achieving a high yield of benzoic acid. Furthermore, comparative experiments were conducted employing various catalysts (Table 1, entries 6, 7, 8, and 11). Entries 6 and 7 demonstrate that PAF-181 and I@PAF-181 exhibit limited catalytic activity, while WO42− demonstrates good catalytic activity (Table 1, entry 8). And entry 11 reveals that WO4@PAF-181 exhibits superior catalytic activity (99% yield) compared to PAF-181, I@PAF-181, and WO42−. The limited activity of PAF-181 and I@PAF-181 may be ascribed to the acceleration of the decomposition of H2O2, resulting in lower yields compared to the catalytic system containing WO42− [42]. Additionally, due to its poor solubility in water, benzyl alcohol tends to accumulate on the surface and pores of WO4@PAF-181 through hydrophobic interaction, thereby increasing its contact probability with catalytic active sites. The synergistic effect between the support material and the catalytically active sites enhances the conversion efficiency of benzyl alcohol. Furthermore, to exclude the potential oxidation impact of WO42−, a control experiment was conducted without the addition of H2O2 and no product was observed (Table 1, entry 9), indicating that H2O2 served as the oxygen source for the reaction. It is noteworthy that the addition of 4 mmol H2O2 results in a higher yield compared to the addition of 3 mmol H2O2 (Table 1, entries 10 and 11). Subsequently, the optimal reaction temperature was also screened (Table 1, entries 11–13), revealing that an increase in temperature correlated positively with an increase in the yield of the desired product, with 110 °C being the preferred temperature. Based on these experimental results, the optimal conditions for this reaction were established (Table 1, entry 11), and the corresponding GC spectrum is shown in Figure S8. Importantly, when compared to the previously reported heterogeneous catalyst Au/NPC (benzoic acid yield: 60%) supported by noble metal NPs [43], it was observed that the WO4@PAF-181 composite exhibited superior yields under identical conditions. These outcomes undoubtedly validate the exceptional catalytic activity and promising potential of WO4@PAF-181 for environmentally friendly benzoic acid synthesis.

Table 1.
The control experiments for the oxidation reaction of benzyl alcohol catalyzed by WO4@PAF-181 a.
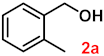
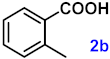
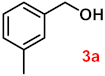
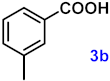
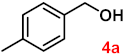
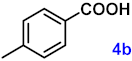
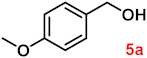
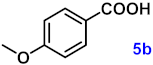
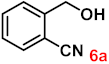
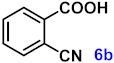
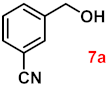
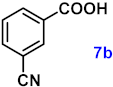
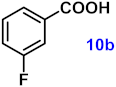
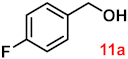
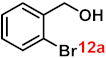
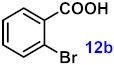
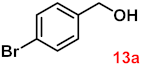
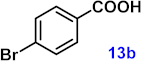
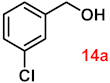
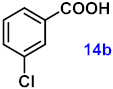
To investigate the applicability of the above catalytic reaction conditions to diverse substrates, a series of catalytic oxidation reactions involving substituted benzyl alcohols were examined, and the isolated yields of the corresponding benzoic acid derivatives were determined. The experimental results in Table 2 demonstrate that the presence of electron-donating groups (such as –CH3 and –OCH3) in the substituted benzyl alcohols result in enhanced catalytic activity of the catalyst, leading to the production of the desired aromatic acid with a yield exceeding 93%. However, when the benzyl alcohols contain substituents with halogen atoms (e.g., –F, –Cl, –Br) or electron-withdrawing groups (e.g., –CN), a slight decrease in yield (<7%) was observed. Nevertheless, a high yield (ranging from 89% to 93%) of the target product was still obtained. Overall, the impact of different substituents of the substrates on the reaction yield was found to be negligible.

Table 2.
Oxidation reactions of substituted benzyl alcohols catalyzed by WO4@PAF-181 a.
Based on the relevant literature [44,45,46] and our experimental results, a plausible mechanism for the catalytic oxidation of benzyl alcohol by WO4@PAF-181 is proposed (Figure 3). Initially, the W=O double bond of WO42− reacts with H2O2 to form sodium peroxytungstate (I) [44]. Subsequently, benzyl alcohol enriched in the WO4@PAF-181 matrix undergoes a reaction with sodium peroxytungstate (I) to yield intermediate (II), which next generates benzaldehyde through the α-H elimination [45]. Further oxidation of the C=O double bond of benzaldehyde occurs via H2O2, resulting in the formation of intermediate (III). Finally, benzoic acid is produced through α-H elimination [46]. Nevertheless, similar investigations have suggested that the oxidation reaction of peroxides may also involve a free-radical mechanism [47,48].
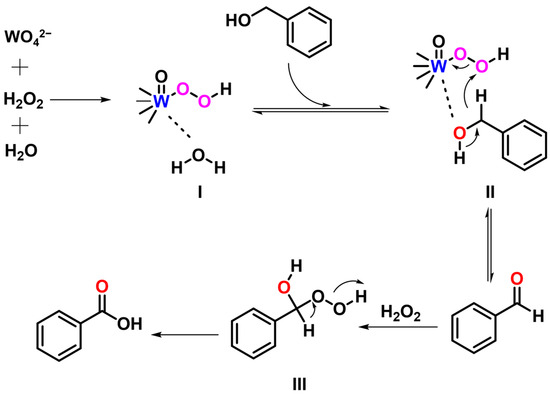
Figure 3.
A possible mechanism diagram of the reaction.
Recyclability serves as a distinctive characteristic of heterogeneous catalysts. To assess the recyclability of WO4@PAF-181, ten catalytic cycles of the oxidation of benzyl alcohol were conducted, with the results presented in Figure 4. Remarkably, the yield of benzoic acid remained notably high (>90%) even after the completion of the tenth cycle and the TOF of the reaction also remains almost unchanged, thus attesting to no substantial decrease in the catalytic efficiency. Furthermore, the retrieval and restoration of the catalyst can be readily achieved through a straightforward process involving filtration, ethanol washing, and subsequent vacuum drying at 100 °C for 12 h. These experiments demonstrate WO4@PAF-181 is an excellent heterogeneous catalyst with good recyclability and selectivity.
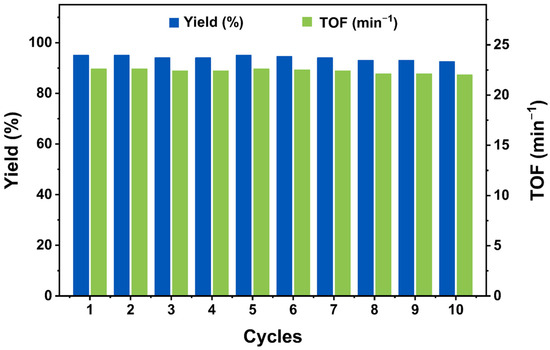
Figure 4.
Recycling tests for WO4@PAF-181 catalyst in the oxidation of benzyl alcohol (see the loop test for conditions). The blue column represents benzoic acid yield, and the green column represents TOF.
In addition, we also studied the kinetics of the reaction (see Figure S9). The benzaldehyde concentration in the system underwent a progression from a lower level to a higher level and subsequently returned to a lower level, whereas the rate of increase in benzoic acid concentration initially exhibited a rapid rise followed by deceleration. This phenomenon could potentially be attributed to the initial abundance of oxidant content during the early phase of the reaction, followed by a gradual decline in concentration during the later stage of the reaction as well as dynamic equilibrium between two-step reactions.
Subsequently, the catalyst recovered after the tenth cycle was characterized using PXRD and FT-IR (see Figures S1b and S10) and it was found that the material framework remained unchanged. The SEM images (see Figure S11) also demonstrated that the recovered WO4@PAF-181 maintained its original morphology. Furthermore, the contents of P and W in the recovered WO4@PAF-181 were analyzed using ICP-AES and determined to be 4.0 wt% and 13.5 wt%, respectively (see Table S1), which are consistent with the initial WO4@PAF-181, indicating almost no leaching of metallic species. Moreover, we have conducted a hot catalyst filtration (after 3.5 h, ca. 51.6% benzyl alcohol conversion). Following the implementation of hot filtration on the catalyst, an extended heating period failed to exhibit discernible transformation of benzyl alcohol and benzaldehyde within the filtrate (Figures S12 and S13). Subsequent analysis via ICP-AES further confirmed the absence of tungsten (W) in the filtrate (Table S1). The results indicate that tungsten did not leach and WO4@PAF-181 is indeed a heterogeneous catalyst in nature. All of the above investigations validate that the catalyst did not experience structural alterations, thus confirming the exceptional catalytic activity and robust stability of WO4@PAF-181.
In the presence of various catalysts, the oxidation reactions of benzyl alcohol were compared, and the data summary is shown in Table 3.

Table 3.
Oxidation of benzyl alcohol catalyzed by different catalysts.
3. Materials and Methods
3.1. Synthesis of tris(4-bromobiphenyl)phosphine
To a 500 mL two-necked flask was added 4,4′-dibromobiphenyl (14.88 g, 48 mmol) and anhydrous THF (300 mL) under an N2 atmosphere. The mixture was stirred at −78 °C for 20 min and n-BuLi (2.5 M, 19.4 mL, 48 mmol) was slowly added dropwise. After stirring for 2 h, a large pale-yellow precipitate appeared. Subsequently, PCl3 (1.4 mL, 16 mmol) was added dropwise. The mixture was stirred at −78 °C for 1 h and slowly warmed to room temperature overnight. The reaction was quenched with saturated NaCl (100 mL), extracted with THF (100 mL × 3), dried over anhydrous Na2SO4, and the solvent was removed by rotatory evaporation to obtain the crude product. The crude product was purified using flash column chromatography using CH2Cl2 and petroleum ether (v:v = 1:4) as the eluent. The target product tris(4-bromobiphenyl)phosphine was obtained as a white solid. (3.5 g, yield: 30%). 1H NMR (500 MHz, CDCl3) δ 7.57–7.55 (m, 12H), 7.46–7.42 (m, 12H); 13C NMR (126 MHz, CDCl3) δ 140.47, 139.33, 136.39, 136.30, 134.41, 134.25, 132.01, 128.66, 127.13, 127.07, 121.98; 31P NMR (202 MHz, CDCl3) δ –7.38.
3.2. Synthesis of PAF-181
To a 100 mL flask was added Ni(cod)2 (400 mg, 1.4 mmol), 2,2′-bipyridine (224 mg, 1.4 mmol), anhydrous DMF (6 mL), anhydrous THF (6 mL), and 1,5-cyclooctadiene (0.18 mL, 1.4 mmol) under an N2 atmosphere. The mixture was stirred at 80 °C for 1 h, during which the mixture turned dark purple.
To a 25 mL one-neck flask was added tris(4-bromobiphenyl)phosphine (290 mg, 0.4 mmol), and anhydrous DMF (6 mL). Then, the mixture was preheated at 80 °C. After the white solid dissolved completely, the resulting solution was added into the above-mentioned mixture. The mixture was stirred at 80 °C for 24 h. Cooled to room temperature, the precipitate was filtered, washed with HCl (6 M, 200 mL × 3), H2O (100 mL × 3), extracted with THF Soxhlet for 48 h, and dried under vacuum at 80 °C for 24 h (186 mg, yield: 95%).
3.3. Synthesis of WO4@PAF-181
To a 25 mL double-necked flask was added PAF-181 (100 mg), and anhydrous CH2Cl2 (20 mL) under an N2 atmosphere. The mixture was soaked in an ice-water bath for 20 min. CH3I (0.2 mL) was added dropwise and then the reaction was stirred at room temperature for 24 h. Filtered and dried under vacuum at 80 °C for 24 h, the desired product (I@PAF-181) was obtained as a light-yellow solid powder (yield: 99%).
An I@PAF-181 (100 mg) and Na2WO4 solution (0.3 M, 20 mL) was added into a 50 mL beaker and the mixture was stirred at room temperature for 48 h. After filtration, the precipitate was washed with H2O (50 mL × 3) and EtOH (50 mL × 3) and dried under vacuum at 80 °C for 24 h. WO4@PAF-181 was obtained as a white solid powder (yield: 99%).
3.4. Catalytic Reaction
WO4@PAF-181 (13 mg, WO42−: 1 mol%) was added as a catalyst for the reaction in a 10 mL pressure-resistant tube. Subsequently, a magnetic stir bar, benzyl alcohol (104 µL, 1.0 mmol), 30% H2O2 (408 µL, 4.0 mmol, 4.0 eq.), NaHSO4 (12 mg, 1 mol%, 0.01 eq.), and H2O (4 mL) were added. The pressure-resistant tube was placed in an oil bath at 110 °C, and the reaction was stirred for 7 h. Cooled to room temperature, the organic phase was extracted with ethyl acetate (10 mL × 3), dried over anhydrous Na2SO4, and the solvent was removed using rotatory evaporation. Pure benzoic acid was obtained after recrystallization using ethyl acetate and petroleum ether.
3.5. Leaching Experiment
To a 25 mL single-neck flask, WO4@PAF-181 (26 mg, WO42−: 1 mol%), NaHSO4 (24 mg, 1 mol%, 0.01 eq), benzyl alcohol (208 µL, 2.0 mmol), 30% H2O2 (816 µL, 8 mmol, 4.0 eq), H2O (8 mL), and a magnetic stirrer was added. The mixture was sealed firmly with a rubber stopper and soaked in an oil bath at 110 °C. After stirring for 3.5 h, 0.2 mL of the filtrate was injected for GC analysis (see Figure S13). Then, a 30 mL glass sand core funnel was preheated in a 120 °C oven and the filtrate was quickly filtered into another 25 mL single-neck flask with a magnetic stirrer that had been previously placed in an oil bath at 110 °C. After further heating and stirring for 3.5 h, the mixture was cooled. A volume of 0.2 mL of the filtrate was taken for GC analysis. A few drops of a mixed solution of hydrogen fluoride and nitric acid (v:v = 3:1) was added to the remaining filtrate to ensure the complete leak of the W species and full dissolution. The mixture was diluted to 25 mL in a plastic volumetric flask for the ICP-AES test (see Table S1) [57].
3.6. Loop Test
WO4@PAF-181 (26 mg, WO42−: 1 mol%) was added into a 25 mL pressure-resistant tube, and then a magnetic stirring bar, benzyl alcohol (204 µL, 2 mmol), 30% H2O2 (816 µL, 8.0 mmol, 4.0 eq.), NaHSO4 (24 mg, 1 mol%, 0.01 eq.), and H2O (8 mL) were added subsequently. The pressure-resistant tube was placed in an oil bath at 110 °C, and the reaction stirred for 7 h. Cooled down to room temperature, the precipitate was filtrated, washed with EtOH (30 mL × 3), and dried under vacuum at 80 °C for 12 h. It was noted there may be a very small amount of loss during the recovery, and therefore, the amount of recovered catalyst was reduced proportionally with respect to the amount of raw material. The above experiment was conducted 10 times.
4. Conclusions
In conclusion, considering the extensive utilization of benzoic acid in various domains of daily life and industrial production, a highly efficient and environmentally friendly heterogeneous catalyst was developed for the synthesis of benzoic acid. PAF-181, possessing abundant phosphorus centers, was successfully synthesized via a Yamamoto–Ullmann coupling reaction using tris(4-bromobiphenyl)phosphine as the reactive monomer. Subsequently, WO4@PAF-181 was prepared through a methylation reaction and ion exchange. The catalytic performance of WO4@PAF-181 on the selective oxidation of benzyl alcohol was investigated, employing H2O2 as the oxygen source and water as the reaction solvent. Remarkably high yields (89–96%) of benzoic acid and its derivatives were obtained. Furthermore, WO4@PAF-181 exhibited superior catalytic activity compared to PAF-181, Na2WO4, and most other catalysts, thereby supporting the effectiveness of harnessing the synergistic effect between functionalized supports and active sites in constructing efficient heterogeneous catalysts. As a heterogeneous catalyst, WO4@PAF-181 demonstrated excellent stability and recyclability, maintaining its high catalytic activity and selectivity after ten consecutive cycles. This study also highlights the significant potential applications of PAFs in organic synthesis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13091309/s1. Figure S1. Experimental PXRD patterns of PAF-181, I@PAF-181, WO4@PAF-181 (a), Na2WO4·2H2O, fresh WO4@PAF-181, and recycled WO4@PAF-181 (b); Figure S2. 13C CP/MAS solid-state NMR spectra of PAF-181, I@PAF-181, and WO4@PAF-181; Figure S3. SEM images of PAF-181, I@PAF-181, and WO4@PAF-181; Figure S4. TEM-EDX mapping images of PAF-181, I@PAF-181, and WO4@PAF-181; Figure S5. XPS survey scan of WO4@PAF-181; Figure S6. Pore size distributions of PAF-181, I@PAF-181, and WO4@PAF-181; Figure S7. The water contact angles of PAF-181, I@PAF-181, and WO4@PAF-181; Figure S8. GC spectrum of the standard mixed solution of ethyl acetate in equimolar amounts of benzyl alcohol, benzaldehyde, benzoic acid, and biphenyl (internal standard) (a), GC spectrum for the optimal reaction conditions (b); Figure S9. Plot of unconverted amount of benzyl alcohol and yield of benzaldehyde and benzoic acid vs. time during benzyl alcohol oxidation under optimal reaction conditions; Figure S10. FT-IR spectra of fresh WO4@PAF-184 (I) and recycled WO4@PAF-184 (II); Figure S11. SEM images of fresh WO4@PAF-181 and recycled WO4@PAF-181; Figure S12. Plot of benzyl alcohol conversion vs. time for the oxidation of benzyl alcohol, under the reaction conditions of entry 11, Table 1, in the presence of WO4@PAF-181 (blue curve) and with filtering off the catalyst WO4@PAF-181 after 3.5 h reaction time (orange curve); Figure S13. GC spectra of the filtrate at 3.5 h with WO4@PAF-181 (a) and at 7 h without WO4@PAF-181 (b) during the hot filtration test; Table S1. ICP-AES tests results for the P and W elements of PAF-181, fresh WO4@PAF-181, recycled WO4@PAF-181 (red font), and the filtrate after filtering off the catalyst WO4@PAF-181 during the hot filtration test; Table S2. Characteristics of pores in PAF-181, I@PAF-181, and WO4@PAF-181 (calculated from their N2 adsorption–desorption isotherms at 77 K using quenched solid density functional theory).
Author Contributions
Conceptualization, B.Y., S.W. and B.W.; methodology, B.Y.; validation, B.Y., Z.C. and B.W.; formal analysis, B.Y.; investigation, B.Y.; data curation, B.Y.; writing—original draft preparation, B.Y., Z.C., S.W. and B.W.; writing—review and editing, Y.T.; visualization, B.Y.; supervision, B.W.; project administration, B.W.; funding acquisition, B.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department of Science and Technology of Jilin Province, grant number 20220402028GH.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors appreciate the support from Analytical and Testing Center of NENU for structural characterization.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, H.; Shi, Y.; Haruta, M.; Huang, J. Aerobic oxidation of benzyl alcohol in water catalyzed by gold nanoparticles supported on imidazole containing crosslinked polymer. Appl. Catal. A Gen. 2017, 536, 27–34. [Google Scholar] [CrossRef]
- Mao, X.; Yang, Q.; Chen, D.; Yu, B.; He, J. Benzoic acid used as food and feed additives can regulate gut functions. BioMed Res. Int. 2019, 2019, 5721585. [Google Scholar] [CrossRef] [PubMed]
- Oktay, Y.; Leyla, G.; Ecem, A.; Saban, M.; Emel, A.; Ozer, K. Benzoic acid formation and its relationship with microbial properties in traditional Turkish cheese varieties. Food Biosci. 2021, 41, 101040. [Google Scholar] [CrossRef]
- Xu, M.; Geng, J.; Xu, H.; Zhang, S.; Zhang, H. In situ construction of NiCo2O4 nanosheets on nickel foam for efficient electrocatalytic oxidation of benzyl alcohol. Inorg. Chem. Front. 2023, 10, 2053–2059. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Ma, Y.; Li, Z.; Du, J.; Han, Z. Three-dimensional silver-containing polyoxotungstate frameworks for photocatalytic aerobic oxidation of benzyl alcohol. Inorg. Chem. 2022, 61, 20596–20607. [Google Scholar] [CrossRef] [PubMed]
- Branko, J. Surfactant assisted permanganate oxidation of aromatic compounds. Can. J. Chem. 1989, 67, 1381–1383. [Google Scholar] [CrossRef]
- Zhao, M.Z.; Li, J.; Song, Z.G.; Desmond, R.; Tschaen, D.M.; Grabowski, E.J.J.; Reider, R.J. A novel chromium trioxide catalyzed oxidation of primary alcohols to the carboxylic acids. Tetrahedron Lett. 1998, 39, 5323–5326. [Google Scholar] [CrossRef]
- Tohma, H.; Takizawa, S.; Maegawa, T.; Prof, Y.K. Facile and clean oxidation of alcohols in water using hypervalent iodine (III) reagents. Angew. Chem. Int. Ed. 2000, 39, 1306–1308. [Google Scholar] [CrossRef]
- Könning, D.; Olbrisch, T.; Sypaseuth, F.D.; Tzschuckea, C.C.; Christmann, M. Oxidation of allylic and benzylic alcohols to aldehydes and carboxylic acids. Chem. Commun. 2014, 50, 5014–5016. [Google Scholar] [CrossRef]
- Gao, W.; Li, S.; Pal, M.; Liu, Y.; Wan, X.; Li, W.; Wang, S.; Wang, C.; Zheng, G.; Zhao, D. Capping agent-free highly dispersed noble metal nanoparticles supported in ordered mesoporous carbon with short channels and their catalytic applications. RSC Adv. 2016, 6, 61064–61072. [Google Scholar] [CrossRef]
- Pai, Z.P.; Kochubey, D.I.; Berdnikova, P.V.; Kanazhevskiy, V.V.; Prikhodʹko, I.Y.; Chesalov, Y.A. Structure and properties of tungsten peroxopolyoxo complexes-promising catalysts for organics oxidation. I. structure of peroxocomplexes studied during the stepwise synthesis of tetra(diperoxotungsten)phosphate-tetra-n-butyl ammonium. J. Mol. Catal. A Chem. 2010, 332, 122–127. [Google Scholar] [CrossRef]
- Sloboda-Rozner, D.; Witte, P.; Alsters, P.L.; Neumann, R. Aqueous biphasic oxidation: A water-soluble polyoxometalate catalyst for selective oxidation of various functional groups with hydrogen peroxide. Adv. Synth. Catal. 2004, 346, 339–345. [Google Scholar] [CrossRef]
- Saisaha, P.; Buettner, L.; Meer, M.V.; Hage, R.; Feringa, B.L.; Browne, W.R.; Boer, J.W. Selective catalytic oxidation of alcohols, aldehydes, alkanes and alkenes employing manganese catalysts and hydrogen peroxide. Adv. Synth. Catal. 2013, 355, 2591–2603. [Google Scholar] [CrossRef]
- Seth, S.; Jhulki, S.; Moorthy, J.N. Catalytic and chemoselective oxidation of activated alcohols and direct conversion of diols to lactones with in situ-generated bis-IBX catalyst. Eur. J. Org. Chem. 2013, 2013, 2445–2452. [Google Scholar] [CrossRef]
- Mirza, M.; Tavana, M.M.; Boukherroub, R. Oxone/iron (II) sulfate/graphite oxide as a highly effective system for oxidation of alcohols under ultrasonic irradiation. Tetrahedron Lett. 2014, 55, 342–345. [Google Scholar] [CrossRef]
- Gowda, R.R.; Chakraborty, D. Ceric ammonium nitrate catalyzed oxidation of aldehydes and alcohols. Chin. J. Chem. 2011, 29, 2379–2384. [Google Scholar] [CrossRef]
- Das, R.; Chakraborty, D. Cu (II) bromide catalyzed oxidation of aldehydes and alcohols. Appl. Organometal. Chem. 2011, 25, 437–442. [Google Scholar] [CrossRef]
- da Silva, M.J.; da Silva, G.R.N.; Sampaio, V.F.C.; Vilanculo, C.B.; Fernandes, S.A.; Teixeira, M.G. One-pot synthesis of benzaldehyde derivatives in PdCl2-catalyzed reactions with H2O2 in alcoholic solutions. Chem. Pap. 2021, 75, 1545–1554. [Google Scholar] [CrossRef]
- Masuda, S.; Takano, S.; Yamazoe, S.; Tsukuda, T. Synthesis of active, robust and cationic Au25 cluster catalysts on double metal hydroxide by long-term oxidative aging of Au25(SR)18. Nanoscale 2022, 14, 3031–3039. [Google Scholar] [CrossRef]
- Tiburcio, E.; Greco, R.; Mon, M.; Ballesteros-Soberanas, J.; Ferrando-Soria, J.; López-Haro, M.; Hernández-Garrido, J.C.; Oliver-Meseguer, J.; Marini, C.; Boronat, M.; et al. Soluble/MOF-supported palladium single atoms catalyze the ligand-, additive-, and solvent-free aerobic oxidation of benzyl alcohols to benzoic acids. J. Am. Chem. Soc. 2021, 143, 2581–2592. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, C.; Wu, S.; Zhang, W.; Xue, W.; Zeng, Z. Synthesis of benzaldehyde and benzoic acid by selective oxidation of benzyl alcohol with iron (III) tosylate and hydrogen peroxide: A solvent-controlled reaction. Catal. Lett. 2018, 148, 3082–3092. [Google Scholar] [CrossRef]
- Sun, Q.; Dai, Z.; Meng, X.; Xiao, F. Porous polymer catalysts with hierarchical structures. Chem. Soc. Rev. 2015, 44, 6018–6034. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Xiao, F. Porous polymeric catalysts constructed from vinylated functionalities. Acc. Mater. Res. 2022, 3, 772–781. [Google Scholar] [CrossRef]
- Ferreira, G.A.; Loh, W. Planet–satellite nanostructures based on block copolymer-surfactant nanoparticles surface-decorated with gold and silver: A new strategy for interfacial catalysis. Adv. Mater. Interfaces 2019, 6, 1900348. [Google Scholar] [CrossRef]
- Tao, R.; Ma, X.; Wei, X.; Jin, Y.; Qiu, L.; Zhang, W. Porous organic polymer material supported palladium nanoparticles. J. Mater. Chem. A 2020, 8, 17360–17391. [Google Scholar] [CrossRef]
- Yamashina, M.; Sei, Y.; Akita, M.; Yoshizawa, M. Safe storage of radical initiators within a polyaromatic nanocapsule. Nat. Commun. 2014, 5, 4662. [Google Scholar] [CrossRef]
- Boonpangrak, S.; Whitcombe, M.J.; Prachayasittikul, V.; Mosbach, K.; Ye, L. Preparation of molecularly imprinted polymers using nitroxide-mediated living radical polymerization. Biosens. Bioelectron. 2006, 22, 349–354. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, K.; Liu, M.; Yun, J.; Dong, J.; Chen, Z.; Xie, Z.; Pan, X. Photocatalytic metal-free radical hydrosilylation for polymer functionalization. Chin. J. Chem. 2023, 41, 2275–2281. [Google Scholar] [CrossRef]
- Ben, T.; Ren, H.; Ma, S.Q.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, G. Porous aromatic frameworks (PAFs). Chem. Rev. 2020, 120, 8934–8986. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Q.; Zhao, R.; Wang, H.; Diao, W.; Cui, F.; Li, S.; Wang, H.; Zhu, G. Salen-based porous aromatic frameworks with multi-active sites as anode materials for lithium-ion batteries. J. Colloid Interface Sci. 2023, 648, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Das, A.; Maji, B. Phosphorus containing porous organic polymers: Synthetic techniques and applications in organic synthesis and catalysis. Org. Biomol. Chem. 2021, 19, 4174–4192. [Google Scholar] [CrossRef]
- Bayne, J.M.; Fasano, V.; Szkop, K.M.; Ingleson, M.J.; Stephan, D.W. Phosphorous(V) Lewis acids: Water/base tolerant P3-trimethylated trications. Chem. Commun. 2018, 54, 12467–12470. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ding, M.; Ding, J.; Kang, J.; Yan, Z.; Zhao, P.; Zhou, X.; Jin, Y.; Chen, S.; Xia, C. Targeted synthesis of a high-stability cationic porous aromatic framework for highly efficient remediation of 99TcO4−. Chem. Eng. J. 2022, 435, 134785. [Google Scholar] [CrossRef]
- Tian, Y.; Song, J.; Zhu, Y.; Zhao, H.; Muhammad, F.; Ma, T.; Chen, M.; Zhu, G. Understanding the desulphurization process in an ionic porous aromatic framework. Chem. Sci. 2019, 10, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.; Abbassi, A.; Ghazanfarian, J.; Jalilian, S. Investigation of microstructure effects on performance of hierarchically structured porous catalyst using a novel pore network model. Chem. Eng. J. 2020, 388, 124261. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, C.; Zhu, X.; Dong, L.; Sun, X. Mass and heat transfer of pressure swing adsorption oxygen production process with small adsorbent particles. Processes 2023, 11, 2485. [Google Scholar] [CrossRef]
- Hyodo, M.; Iwano, H.; Kasakado, T.; Fukuyama, T.; Ryu, I. Using high-power UV-LED to accelerate a decatungstate-anion-catalyzed reaction: A model study for the quick oxidation of benzyl alcohol to benzoic acid using molecular oxygen. Micromachines 2021, 12, 1307. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Roshani, M.; Heravi, M.M.; Safaie, S. The catalytic performance of preyssler’s anion, [NaP5W30O110]14−, in the oxidation of benzyl alcohols. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 2833–2841. [Google Scholar] [CrossRef]
- Neves, P.; Lysenko, A.B.; Gomes, A.C.; Pillinger, M.; Gonçalves, I.S.; Valente, A.A. Behavior of triazolylmolybdenum (VI) oxide hybrids as oxidation catalysts with hydrogen peroxide. Catal. Lett. 2017, 147, 1133–1143. [Google Scholar] [CrossRef]
- Su, H.; Yang, C. Selective oxidation of benzyl alcohol catalyzed by (TEAH)nH3-nPW12O40 and its reaction mechanism. Chin. J. Catal. 2014, 35, 1224–1234. [Google Scholar] [CrossRef]
- Aguinaco, A.; Pocostales, J.P.; García-Araya, J.F.; Beltran, F.J. Decomposition of hydrogen peroxide in the presence of activated carbons with different characteristics. J. Chem. Technol. Biotechnol. 2011, 86, 595–600. [Google Scholar] [CrossRef]
- Mao, D.; Jia, M.; Qiu, J.; Zhang, X.F.; Yao, J. N-doped porous carbon supported Au nanoparticles for benzyl alcohol oxidation. Catal. Lett. 2020, 150, 74–81. [Google Scholar] [CrossRef]
- Jacobson, S.E.; Muccigrosso, D.A.; Mares, F. Oxidation of alcohols by molybdenum and tungsten peroxo complexes. J. Org. Chem. 1979, 44, 921–924. [Google Scholar] [CrossRef]
- Carlo, V.; Marco, R. Oxidative cleavage of 1,2-diols to carboylic acids by hydrogen peroxide. J. Org. Chem. 1986, 51, 1599–1602. [Google Scholar] [CrossRef]
- Noyori, R.; Aokib, M.; Sato, K. Green oxidation with aqueous hydrogen peroxide. Chem. Commun. 2003, 34, 1977–1986. [Google Scholar] [CrossRef]
- Correia, L.M.M.; Soliman, M.M.A.; Granadeiro, C.M.; Balula, S.S.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; Alegria, E.C.B.A. Vanadium C-scorpionate supported on mesoporous aptes-functionalized SBA-15 as catalyst for the peroxidative oxidation of benzyl alcohol. Microporous Mesoporous Mater. 2021, 320, 111111. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Nizova, G.V.; Süss-Fink, G.; Stanislas, S.; Kitaygorodskiyc, A.; Kulikovad, V.S. Oxidations by the reagent “O2–H2O2–vanadium derivative–pyrazine-2-carboxylic acid”. Part 12.1 Main features, kinetics and mechanism of alkane hydroperoxidation. J. Chem. Soc. Perkin Trans. 2001, 2, 1351–1371. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Chen, X.; Chen, Y.; Zheng, X. Preparation, characterization and catalytic properties of MCM-48 supported tungstophosphoric acid mesoporous materials for green synthesis of benzoic acid. J. Solid State Chem. 2014, 211, 51–57. [Google Scholar] [CrossRef]
- Huang, X.; Wang, X.; Tan, M.; Zou, X.; Ding, W.; Lu, X. Selective oxidation of alcohols on P123-stabilized Au-Ag alloy nanoparticles in aqueous solution with molecular oxygen. Appl. Catal. A Gen. 2013, 467, 407–413. [Google Scholar] [CrossRef]
- Rautiainena, S.; Simakova, O.; Guo, H.; Leinoc, A.; Kordás, K.; Murzin, D.; Leskelä, M.; Repo, T. Solvent controlled catalysis: Synthesis of aldehyde, acid or ester by selective oxidation of benzyl alcohol with gold nanoparticles on alumina. Appl. Catal. A Gen. 2014, 485, 202–206. [Google Scholar] [CrossRef]
- Ghadamgahi, S.; Williamson, B.; Golovko, V. Activity of catalysts derived from Au101 immobilized on activated carbon. Catal. Lett. 2016, 146, 1027–1032. [Google Scholar] [CrossRef]
- Wang, C.; Jiao, L.; Ji, P. Interfacial synthesis of Co-based ultrathin nanosheets with NaF crystal as template and inducer: Fluorine doped Co(OH)2, porous fluorine doped Co3O4, and porous Co3O4. Appl. Surf. Sci. 2022, 589, 152923. [Google Scholar] [CrossRef]
- Sarkheil, M.; Lashanizadegan, M.; Ghiasi, M. High catalytic activity of magnetic Fe3O4@SiO2-Schiff base-Co (II) nanocatalyst for aerobic oxidation of alkenes and alcohols and DFT study. J. Mol. Struct. 2019, 1179, 278–288. [Google Scholar] [CrossRef]
- Feng, D.; Dong, Y.; Zhang, L.; Ge, X.; Zhang, W.; Dai, S.; Qiao, Z. Holey lamellar high entropy oxide as ultra-highly active heterogeneous catalyst for solvent-free aerobic oxidation of benzyl alcohol. Angew. Chem. Int. Ed. 2020, 59, 19503–19509. [Google Scholar] [CrossRef]
- Gao, W.; Song, Y.; Jiao, W.; Liu, Y. A catalyst-free and highly efficient approach to ozonation of benzyl alcohol to benzoic acid in a rotating packed bed. J. Taiwan Inst. Chem. Eng. 2019, 103, 1–6. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Wallau, M.; Arends, I.W.C.E.; Schuchardt, U. Heterogeneous catalysts for liquid-phase oxidations: Philosophers’ stones or trojan horses? Acc. Chem. Res. 1998, 31, 485–493. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).