Synthesis of g-C3N4@ZnIn2S4 Heterostructures with Extremely High Photocatalytic Hydrogen Production and Reusability
Abstract
1. Introduction
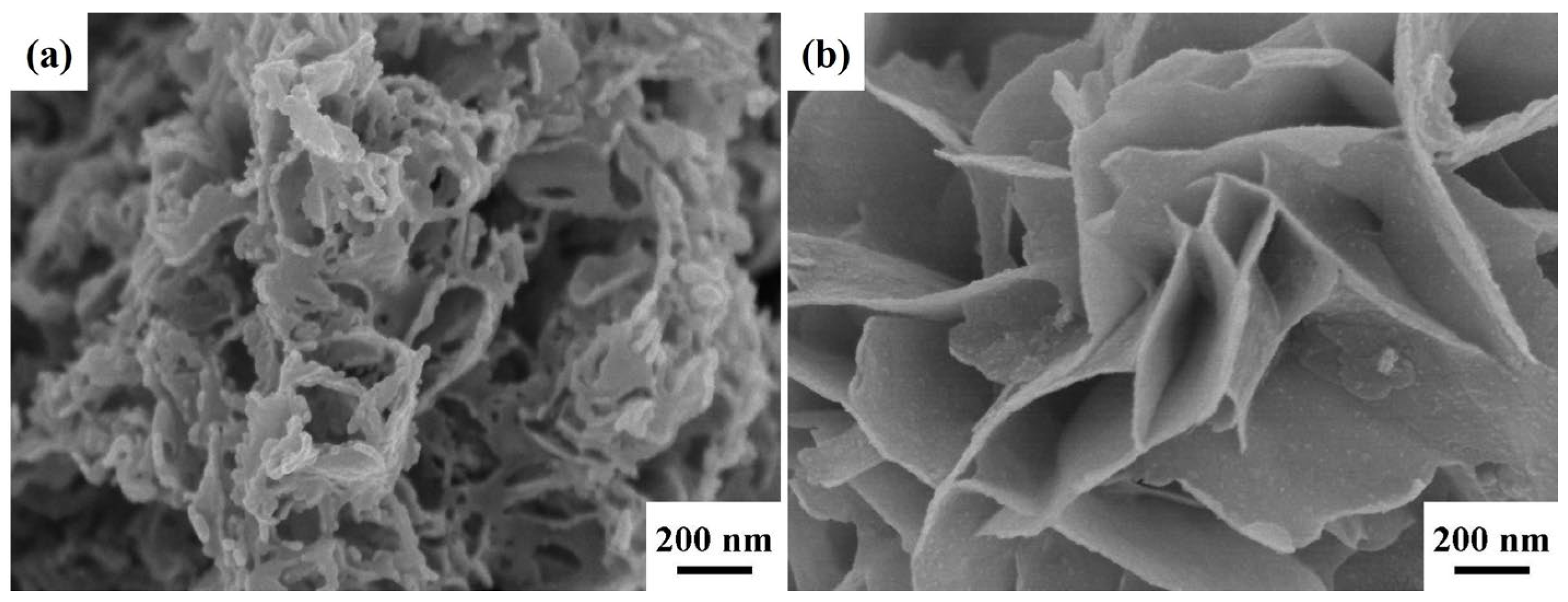
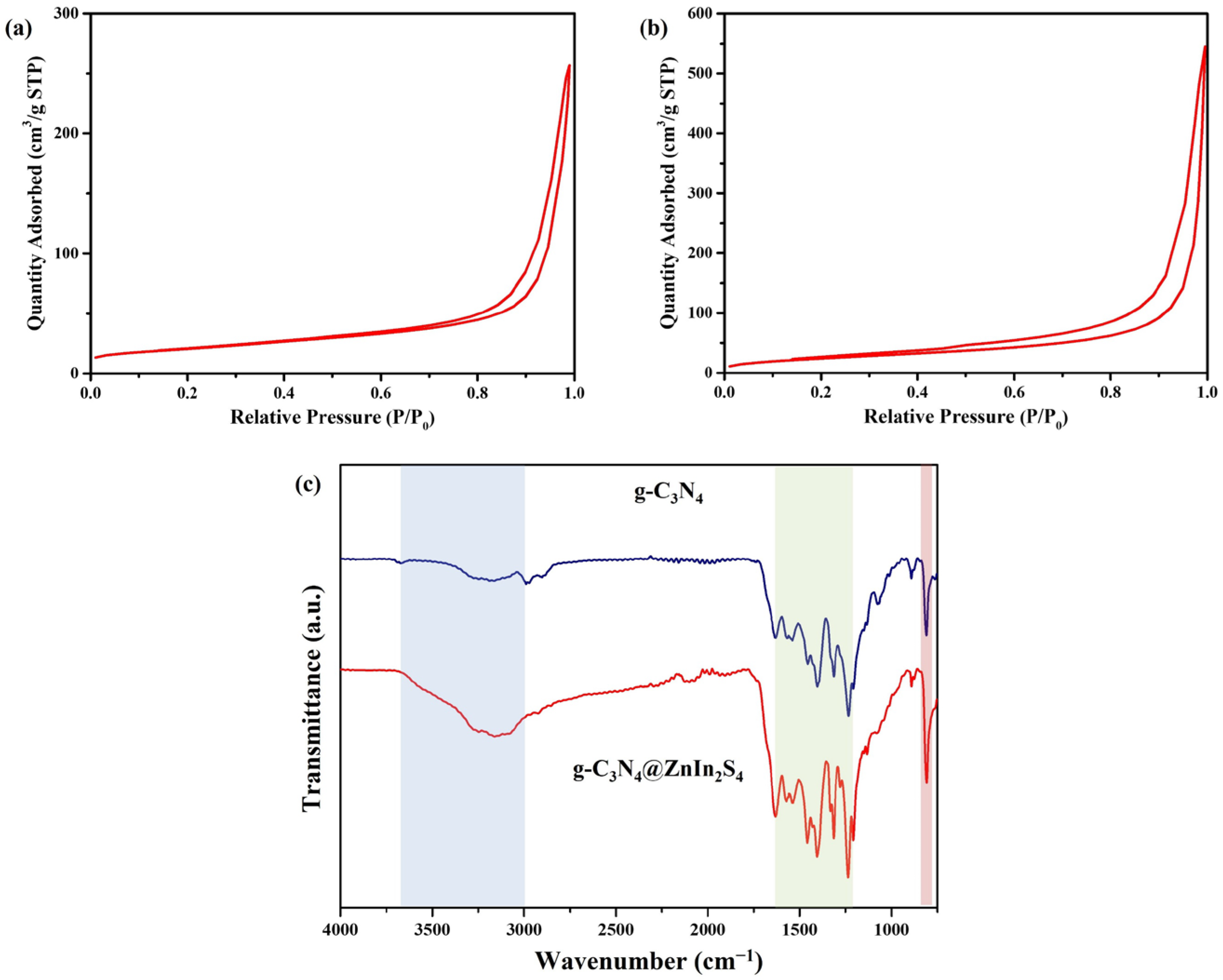
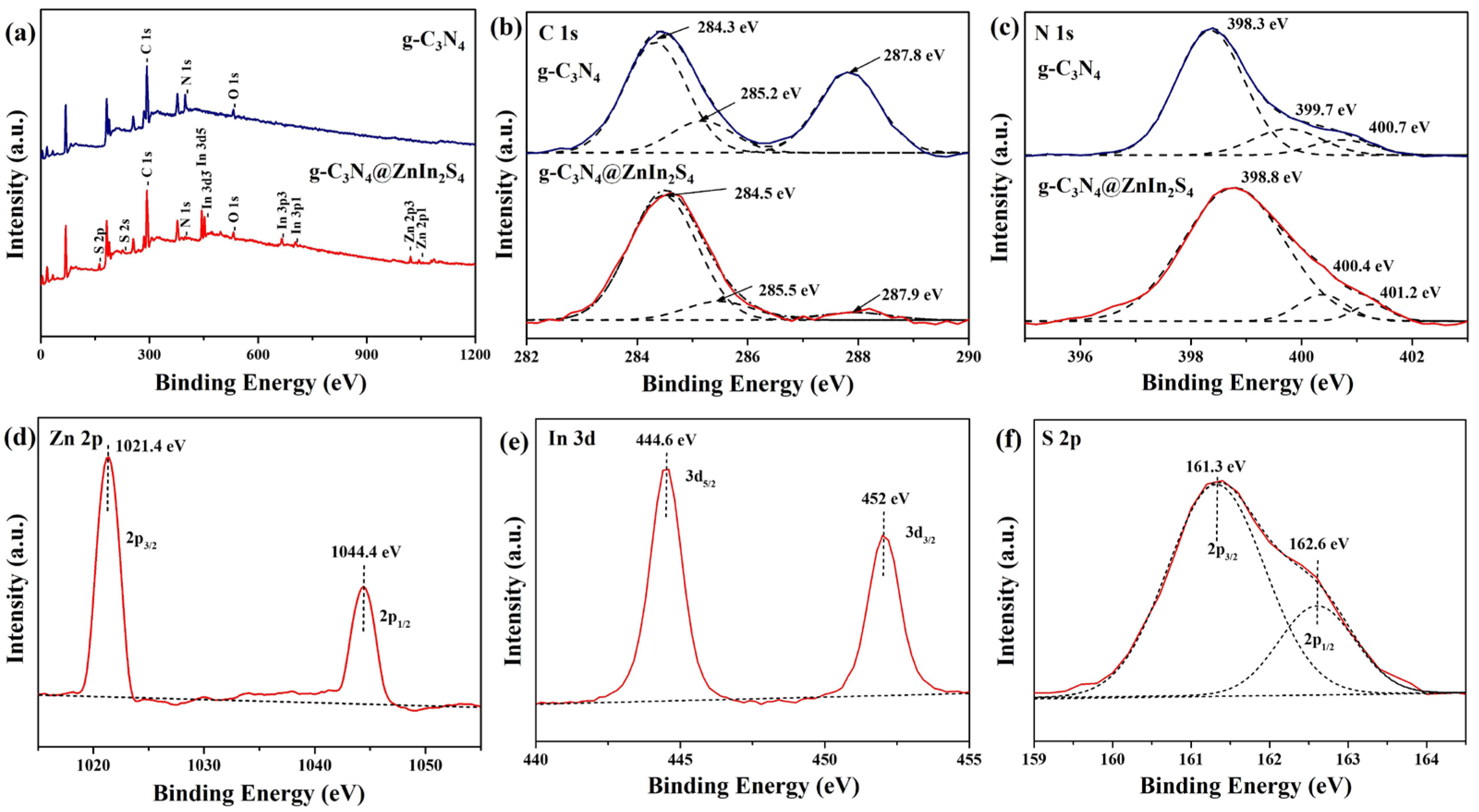
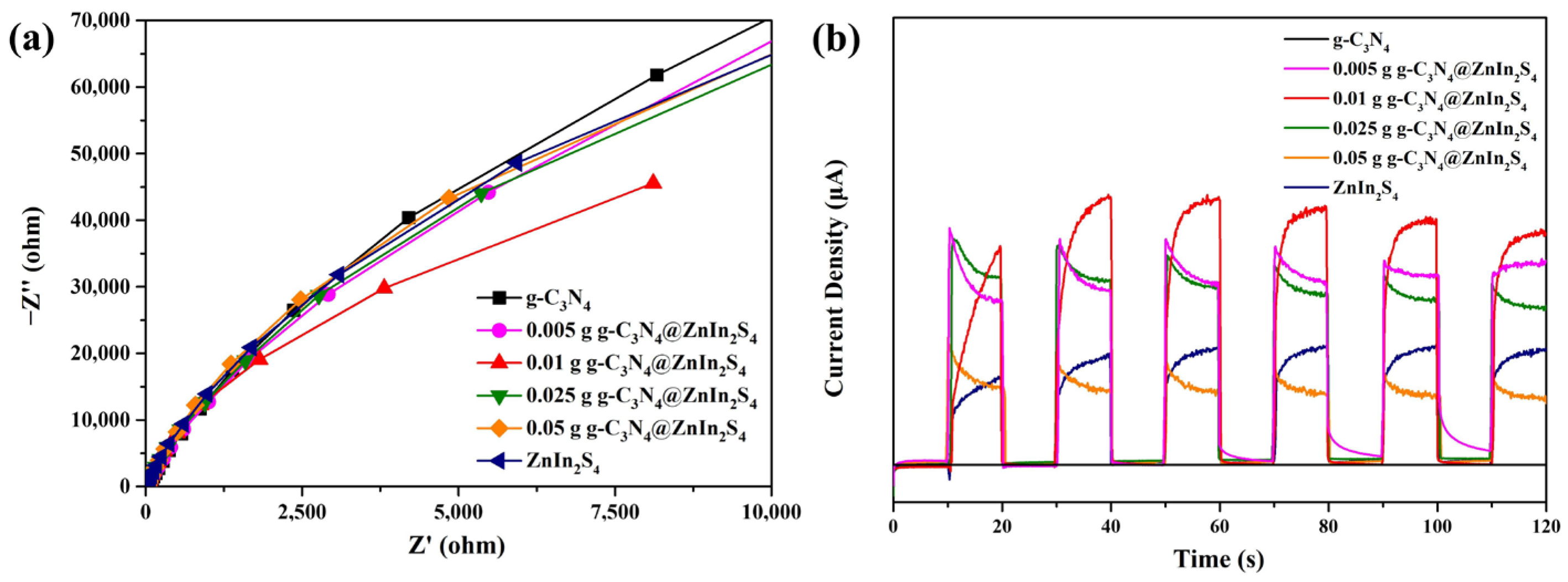
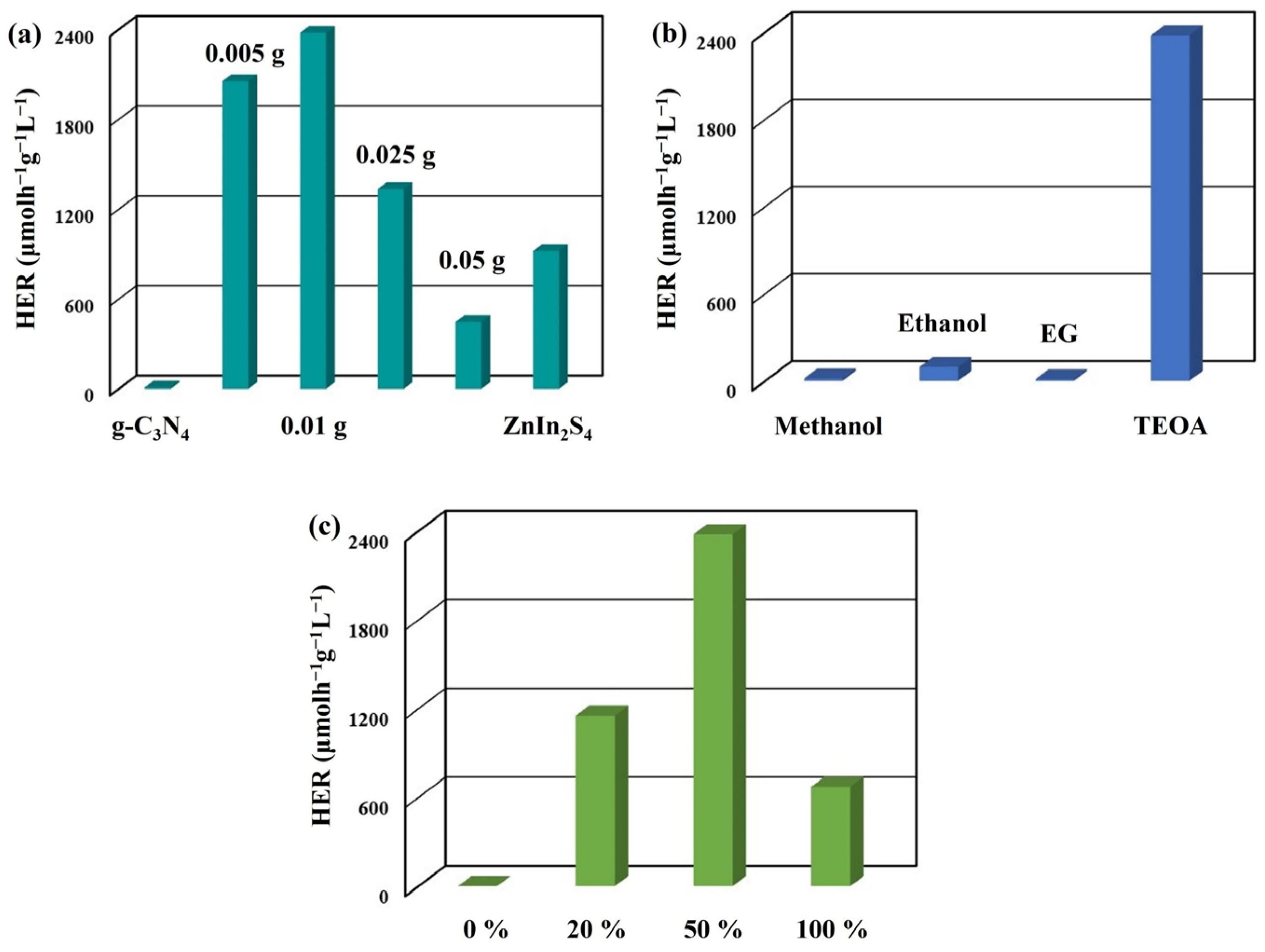
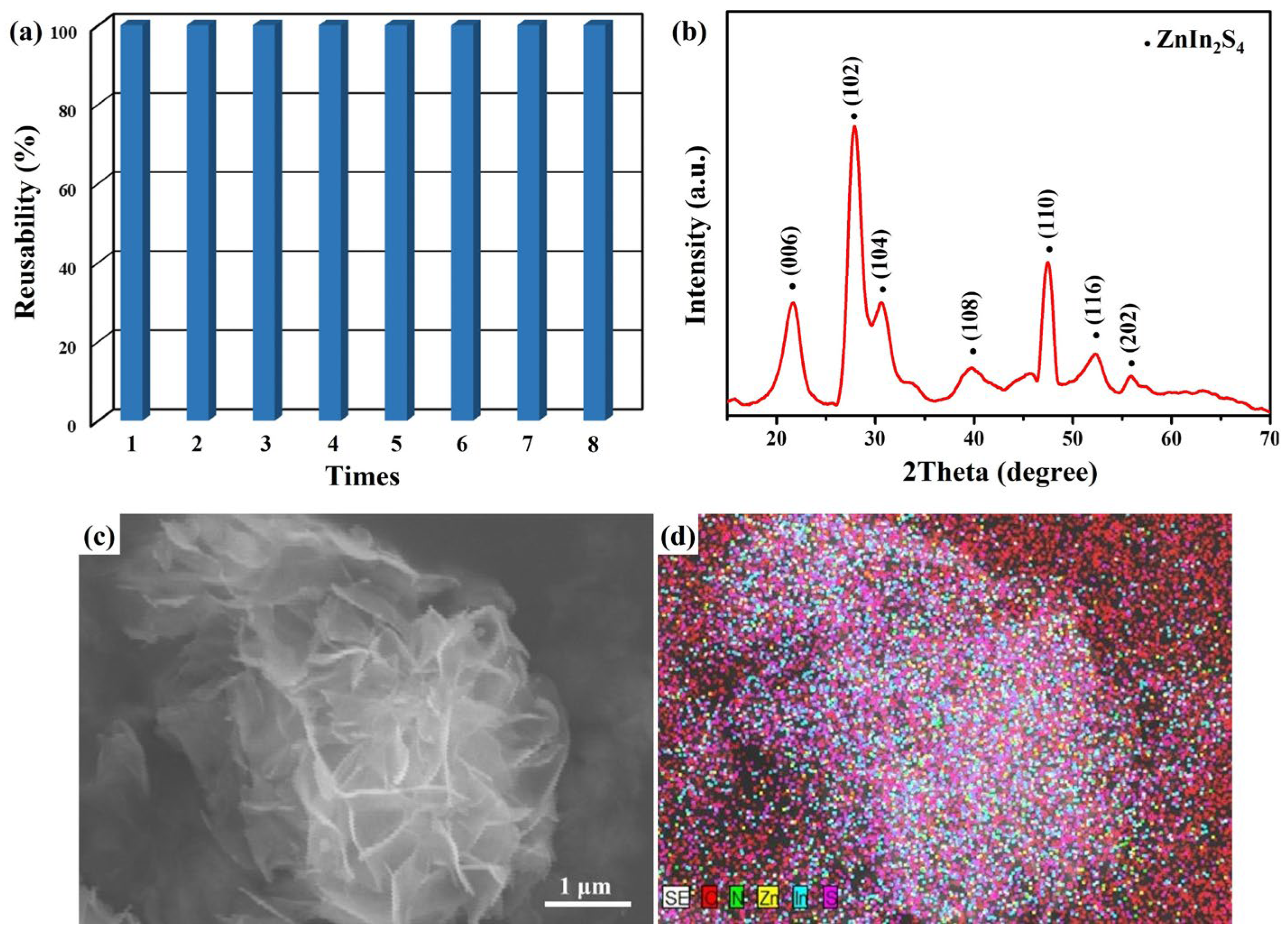
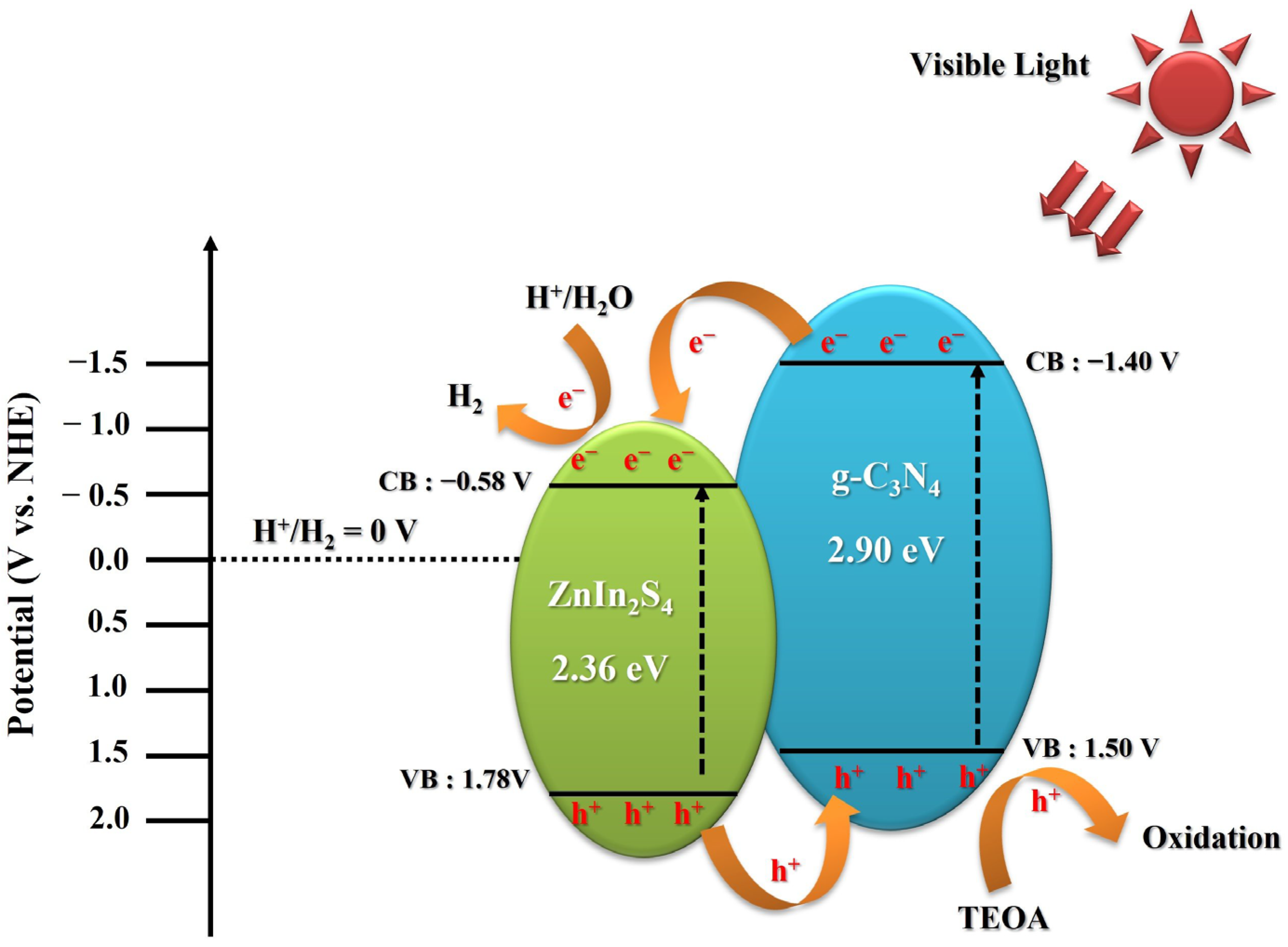
2. Results and Discussion
3. Material and Methods
3.1. Chemicals
3.2. Synthesis of g-C3N4
3.3. Synthesis of g-C3N4@ZnIn2S4 Heterostructures
3.4. Characterization
3.5. Photocatalytic Hydrogen Production Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acar, C.; Dincer, I.; Naterer, G.F. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energ. Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.-L.; Sa, B.; Ahuja, R. Review of two-dimensional materials for photocatalytic water splitting from a theoretical perspective. Catal. Sci. Technol. 2017, 7, 545–559. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic Water Splitting—The Untamed Dream: A Review of Recent Advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sust. Energ. Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Xu, J.; Wang, G.; Fan, J.; Liu, B.; Cao, S.; Yu, J. g-C3N4 modified TiO2 nanosheets with enhanced photoelectric conversion efficiency in dye-sensitized solar cells. J. Power Sources 2015, 274, 77–84. [Google Scholar] [CrossRef]
- Xu, J.; Brenner, T.J.K.; Chabanne, L.; Neher, D.; Antonietti, M.; Shalom, M. Liquid-Based Growth of Polymeric Carbon Nitride Layers and Their Use in a Mesostructured Polymer Solar Cell with Voc Exceeding 1 V. J. Am. Chem. Soc. 2014, 136, 13486–13489. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, P.; Ma, R.; Luo, C.; Wen, T.; Zhao, G.; Cheng, W.; Wang, X. Recent developments in fabrication and structure regulation of visible-light-driven g-C3N4-based photocatalysts towards water purification: A critical review. Catal. Today 2019, 335, 65–77. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Antonietti, M. Polymeric Graphitic Carbon Nitride as a Heterogeneous Organocatalyst: From Photochemistry to Multipurpose Catalysis to Sustainable Chemistry. Angew. Chem. Int. Ed. 2012, 51, 68–89. [Google Scholar] [CrossRef]
- Gong, Y.; Li, M.; Li, H.; Wang, Y. Graphitic carbon nitride polymers: Promising catalysts or catalyst supports for heterogeneous oxidation and hydrogenation. Green Chem. 2015, 17, 715–736. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Antonietti, M. Graphitic carbon nitride “reloaded”: Emerging applications beyond (photo)catalysis. Chem. Soc. Rev. 2016, 45, 2308–2326. [Google Scholar] [CrossRef]
- Wang, X.L.; Yang, H.G. Facile fabrication of high-yield graphitic carbon nitride with a large surface area using bifunctional urea for enhanced photocatalytic performance. Appl. Catal. B Environ. 2017, 205, 624–630. [Google Scholar] [CrossRef]
- Abu-Sari, S.M.; Daud, W.M.A.W.; Patah, M.F.A.; Ang, B.C. A review on synthesis, modification method, and challenges of light-driven H2 evolution using g-C3N4-based photocatalyst. Adv. Colloid Interface Sci. 2022, 307, 102722. [Google Scholar] [CrossRef]
- Sun, S.; Liang, S. Recent advances in functional mesoporous graphitic carbon nitride (mpg-C3N4) polymers. Nanoscale 2017, 9, 10544–10578. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, D.; Li, Z.; Nan, Y.; Ding, F.; Shen, Y.; Jiang, Z. Thylakoid-Inspired Multishell g-C3N4 Nanocapsules with Enhanced Visible-Light Harvesting and Electron Transfer Properties for High-Efficiency Photocatalysis. ACS Nano 2017, 11, 1103–1112. [Google Scholar] [CrossRef]
- Xia, X.; Xie, C.; Xu, B.; Ji, X.; Gao, G.; Yang, P. Role of B-doping in g-C3N4 nanosheets for enhanced photocatalytic NO removal and H2 generation. J. Ind. Eng. Chem. 2022, 105, 303–312. [Google Scholar] [CrossRef]
- Patnaik, S.; Sahoo, D.P.; Parida, K. Recent advances in anion doped g-C3N4 photocatalysts: A review. Carbon 2021, 172, 682–711. [Google Scholar] [CrossRef]
- Huang, Y.; Li, D.; Fang, Z.; Chen, R.; Luo, B.; Shi, W. Controlling carbon self-doping site of g-C3N4 for highly enhanced visible-light-driven hydrogen evolution. Appl. Catal. B Environ. 2019, 254, 128–134. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.; Xiong, T.; Ni, Z.; Zhang, W.; Sun, Y.; Ho, W.-K. In Situ Construction of g-C3N4/g-C3N4 Metal-Free Heterojunction for Enhanced Visible-Light Photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11392–11401. [Google Scholar] [CrossRef]
- Yavuz, C.; Ela, S.E. Fabrication of g-C3N4-reinforced CdS nanosphere-decorated TiO2 nanotablet composite material for photocatalytic hydrogen production and dye-sensitized solar cell application. J. Alloys Compd. 2023, 936, 168209. [Google Scholar] [CrossRef]
- Faisal, M.; Jalalah, M.; Harraz, F.A.; El-Toni, A.M.; Khan, A.; Al-Assiri, M.S. Au nanoparticles-doped g-C3N4 nanocomposites for enhanced photocatalytic performance under visible light illumination. Ceram. Int. 2020, 46, 22090–22101. [Google Scholar] [CrossRef]
- Zhao, C.; Liao, Z.; Liu, W.; Liu, F.; Ye, J.; Liang, J.; Li, Y. Carbon quantum dots modified tubular g-C3N4 with enhanced photocatalytic activity for carbamazepine elimination: Mechanisms, degradation pathway and DFT calculation. J. Hazard Mater. 2020, 381, 120957. [Google Scholar] [CrossRef]
- Asadzadeh-Khaneghah, S.; Habibi-Yangjeh, A. g-C3N4/carbon dot-based nanocomposites serve as efficacious photocatalysts for environmental purification and energy generation: A review. J. Clean. Prod. 2020, 276, 124319. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, X. A Review on Quantum Dots Modified g-C3N4-Based Photocatalysts with Improved Photocatalytic Activity. Catalysts 2020, 10, 142. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, H.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A mini-review on ZnIn2S4-Based photocatalysts for energy and environmental application. Green Energy Environ. 2022, 7, 176–204. [Google Scholar] [CrossRef]
- Mao, S.; Shi, J.-W.; Sun, G.; Ma, D.; He, C.; Pu, Z.; Song, K.; Cheng, Y. Au nanodots@thiol-UiO66@ZnIn2S4 nanosheets with significantly enhanced visible-light photocatalytic H2 evolution: The effect of different Au positions on the transfer of electron-hole pairs. Appl. Catal. B Environ. 2021, 282, 119550. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, Z.; Cheng, X.; Shi, W. Research Progress of ZnIn2S4-Based Catalysts for Photocatalytic Overall Water Splitting. Catalysts 2023, 13, 967. [Google Scholar] [CrossRef]
- Tu, X.; Lu, J.; Li, M.; Su, Y.; Yin, G.; He, D. Hierarchically ZnIn2S4 nanosheet-constructed microwire arrays: Template-free synthesis and excellent photocatalytic performances. Nanoscale 2018, 10, 4735–4744. [Google Scholar] [CrossRef]
- Shang, L.; Zhou, C.; Bian, T.; Yu, H.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Facile synthesis of hierarchical ZnIn2S4 submicrospheres composed of ultrathin mesoporous nanosheets as a highly efficient visible-light-driven photocatalyst for H2 production. J. Mater. Chem. A 2013, 1, 4552–4558. [Google Scholar] [CrossRef]
- Janani, R.; Preethi, V.R.; Singh, S.; Rani, A.; Chang, C.-T. Hierarchical Ternary Sulfides as Effective Photocatalyst for Hydrogen Generation Through Water Splitting: A Review on the Performance of ZnIn2S4. Catalysts 2021, 11, 277. [Google Scholar] [CrossRef]
- Chen, K.; Shi, Y.; Shu, P.; Luo, Z.; Shi, W.; Guo, F. Construction of core–shell FeS2@ZnIn2S4 hollow hierarchical structure S-scheme heterojunction for boosted photothermal-assisted photocatalytic H2 production. Chem. Eng. J. 2023, 454, 140053. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, K.; Wang, L.; Bai, B.; Liu, H.; Wang, Q. Aminated flower-like ZnIn2S4 coupled with benzoic acid modified g-C3N4 nanosheets via covalent bonds for ameliorated photocatalytic hydrogen generation. Appl. Catal. B 2020, 268, 118462. [Google Scholar] [CrossRef]
- Jiao, X.; Chen, Z.; Li, X.; Sun, Y.; Gao, S.; Yan, W.; Wang, C.; Zhang, Q.; Lin, Y.; Luo, Y.; et al. Defect-Mediated Electron–Hole Separation in One-Unit-Cell ZnIn2S4 Layers for Boosted Solar-Driven CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 7586–7594. [Google Scholar] [CrossRef]
- Ye, X.; Zhu, T.; Hui, Z.; Wang, X.; Wei, J.; Chen, S. Revealing the transfer mechanisms of photogenerated charge carriers over g-C3N4/ZnIn2S4 composite: A model study for photocatalytic oxidation of aromatic alcohols with visible light. J. Catal. 2021, 401, 149–159. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, K.; Feng, Z.; Bao, Y.; Dong, B. Hierarchical Sheet-on-Sheet ZnIn2S4/g-C3N4 Heterostructure with Highly Efficient Photocatalytic H2 production Based on Photoinduced Interfacial Charge Transfer. Sci. Rep. 2016, 6, 19221. [Google Scholar] [CrossRef]
- Bhatt, H.; Goswami, T.; Yadav, D.K.; Ghorai, N.; Shukla, A.; Kaur, G.; Kaur, A.; Ghosh, H.N. Ultrafast Hot Electron Transfer and Trap-State Mediated Charge Carrier Separation toward Enhanced Photocatalytic Activity in g-C3N4/ZnIn2S4 Heterostructure. J. Phys. Chem. Lett. 2021, 12, 11865–11872. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, J.; Wang, Z.; Wang, L.; Zhao, Z.; Li, S.; Li, G.; Cai, Z. Facile construction of g-C3N4/ZnIn2S4 nanocomposites for enhance Cr(VI) photocatalytic reduction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 276, 121184. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Guo, J.-Y.; Chen, C.-M.; Di, H.-W.; Hsu, C.-C. Construction of CuO/In2S3/ZnO heterostructure arrays for enhanced photocatalytic efficiency. Nanoscale 2017, 9, 13235–13244. [Google Scholar] [CrossRef]
- Hao, C.-C.; Chen, F.-Y.; Shi, W.-L.; Tang, Y.-B.; Guo, F.; Bian, K.; Xie, L. Construction of core-shell g-C3N4@ZnIn2S4 hierarchical hollow heterostructure for enhanced photocatalytic activity under visible light irradiation. Mater. Chem. Phys. 2022, 285, 126137. [Google Scholar] [CrossRef]
- Liu, H.; Jin, Z.; Xu, Z.; Zhang, Z.; Ao, D. Fabrication of ZnIn2S4–g-C3N4 sheet-on-sheet nanocomposites for efficient visible-light photocatalytic H2-evolution and degradation of organic pollutants. RSC Adv. 2015, 5, 97951–97961. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Lu, J.; Feng, Y.; Meng, F.; Ma, C.; Yan, Y.; Meng, M. Synergy between van der waals heterojunction and vacancy in ZnIn2S4/g-C3N4 2D/2D photocatalysts for enhanced photocatalytic hydrogen evolution. Appl. Catal. B 2020, 277, 119254. [Google Scholar] [CrossRef]
- Kong, L.; Ji, Y.; Dang, Z.; Yan, J.; Li, P.; Li, Y.; Liu, S. g-C3N4 Loading Black Phosphorus Quantum Dot for Efficient and Stable Photocatalytic H2 Generation under Visible Light. Adv. Funct. Mater. 2018, 28, 1800668. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chiao, Y.-C.; Hsu, P.-C. Rapid Microwave-Assisted Synthesis of ZnIn2S4 Nanosheets for Highly Efficient Photocatalytic Hydrogen Production. Nanomaterials 2023, 13, 1957. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Yang, F.; Zhang, Y.; Yan, L.; Li, K.; Guo, H.; Yan, J.; Lin, J. Construction of Au/g-C3N4/ZnIn2S4 plasma photocatalyst heterojunction composite with 3D hierarchical microarchitecture for visible-light-driven hydrogen production. Int. J. Hydrogen Energy 2022, 47, 2900–2913. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Chen, S.; Xie, Y.; Ma, Y.; Luo, Y.; Huang, J.; Ling, Y.; Ye, J.; Liang, Y.; et al. In situ loading of ZnIn2S4 nanosheets onto S doped g-C3N4 nanosheets to construct type II heterojunctions for improving photocatalytic hydrogen production. J. Alloys Compd. 2022, 924, 166569. [Google Scholar] [CrossRef]
- Yu, K.; Li, Z.; Zhang, T.; He, T.; Fan, Z.; Huang, H.; Liao, F.; Liu, Y.; Kang, Z. Carbon dots driven directional charge migration on sulfur vacancy enriched ZnIn2S4 nanosheets for enhanced photocatalytic hydrogen evolution. Appl. Surf. Sci. 2023, 635, 157762. [Google Scholar] [CrossRef]
- Tateishi, I.; Furukawa, M.; Katsumata, H.; Kaneco, S. Improvement of Photocatalytic H2-Generation under Visible Light Irradiation by Controlling the Band Gap of ZnIn2S4 with Cu and In. Catalysts 2019, 9, 681. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, S.; Yao, S.; Li, H.; Liu, J.; Geng, Y.; Pu, X. Highly efficient visible/NIR photocatalytic activity and mechanism of Yb3+/Er3+ co-doped Bi4O5I2 up-conversion photocatalyst. Sep. Purif. Technol. 2020, 248, 117040. [Google Scholar] [CrossRef]
- Cui, L.; Ding, X.; Wang, Y.; Shi, H.; Huang, L.; Zuo, Y.; Kang, S. Facile preparation of Z-scheme WO3/g-C3N4 composite photocatalyst with enhanced photocatalytic performance under visible light. Appl. Surf. Sci. 2017, 391, 202–210. [Google Scholar] [CrossRef]
- Herrera-Beurnio, M.C.; López-Tenllado, F.J.; Hidalgo-Carrillo, J.; Martín-Gómez, J.; Estévez, R.; Castillo-Rodríguez, M.; de Miguel, G.; Urbano, F.J.; Marinas, A. Controlled photodeposition of Pt onto TiO2-g-C3N4 systems for photocatalytic hydrogen production. Catal. Today 2023, 413–415, 113967. [Google Scholar] [CrossRef]
- Moghimifar, Z.; Yazdani, F.; Tabar-Heydar, K.; Sadeghi, M. Photocatalytic Hydrogen Evolution Under Visible Light Using MoS2/g-C3N4 Nano-Photocatalysts. Catal. Lett. 2023. [Google Scholar] [CrossRef]
- Wang, M.; Shen, S.; Li, L.; Tang, Z.; Yang, J. Effects of sacrificial reagents on photocatalytic hydrogen evolution over different photocatalysts. J. Mater. Sci. 2017, 52, 5155–5164. [Google Scholar] [CrossRef]
- Kumaravel, V.; Imam, M.D.; Badreldin, A.; Chava, R.K.; Do, J.Y.; Kang, M.; Abdel-Wahab, A. Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef]
- Li, T.; Vijeta, A.; Casadevall, C.; Gentleman, A.S.; Euser, T.; Reisner, E. Bridging Plastic Recycling and Organic Catalysis: Photocatalytic Deconstruction of Polystyrene via a C–H Oxidation Pathway. ACS Catal. 2022, 12, 8155–8163. [Google Scholar] [CrossRef]
- Mukonza, S.S.; Chaukura, N.; Mishra, A.K. Photocatalytic Activity and Reusability of F, Sm3+ Co-Doped TiO2/MWCNTs Hybrid Heterostructure for Efficient Photocatalytic Degradation of Brilliant Black Bis-Azo Dye. Catalysts 2023, 13, 86. [Google Scholar] [CrossRef]
- Xie, A.; Jin, M.; Zhu, J.; Zhou, Q.; Fu, L.; Wu, W. Photocatalytic Technologies for Transformation and Degradation of Microplastics in the Environment: Current Achievements and Future Prospects. Catalysts 2023, 13, 846. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Z. TiOF2/g-C3N4 composite for visible-light driven photocatalysis. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126471. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, X.; Yang, C.; Wei, C.; Hu, Y. Cu atoms on UiO-66-NH2/ZnIn2S4 nanosheets enhance photocatalytic performance for recovering hydrogen energy from organic wastewater treatment. Appl. Catal. B 2023, 330, 122572. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Tasi, C.-L.; Ko, F.-H. Construction of ZnIn2S4/ZnO heterostructures with enhanced photocatalytic decomposition and hydrogen evolution under blue LED irradiation. Int. J. Hydrogen Energy 2021, 46, 10281–10292. [Google Scholar] [CrossRef]
- Ge, H.; Tian, H.; Zhou, Y.; Wu, S.; Liu, D.; Fu, X.; Song, X.-M.; Shi, X.; Wang, X.; Li, N. Influence of Surface States on the Evaluation of the Flat Band Potential of TiO2. ACS Appl. Mater. Interfaces 2014, 6, 2401–2406. [Google Scholar] [CrossRef]
- Bhattacharya, C.; Lee, H.C.; Bard, A.J. Rapid Screening by Scanning Electrochemical Microscopy (SECM) of Dopants for Bi2WO6 Improved Photocatalytic Water Oxidation with Zn Doping. J. Phys. Chem. C 2013, 117, 9633–9640. [Google Scholar] [CrossRef]
- Guo, H.; Wang, G.; Li, H.; Xia, C.; Dong, B.; Cao, L. Direct Z-scheme high-entropy metal phosphides/ZnIn2S4 heterojunction for efficient photocatalytic hydrogen evolution. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131915. [Google Scholar] [CrossRef]
- Liang, X.; Wang, G.; Huo, T.; Dong, X.; Wang, G.; Ma, H.; Liang, H.; Zhang, X. Band structure modification of g-C3N4 for efficient heterojunction construction and enhanced photocatalytic capability under visible light irradiation. Catal. Commun. 2019, 123, 44–48. [Google Scholar] [CrossRef]
- Yue, X.; Yi, S.; Wang, R.; Zhang, Z.; Qiu, S. Cadmium Sulfide and Nickel Synergetic Co-catalysts Supported on Graphitic Carbon Nitride for Visible-Light-Driven Photocatalytic Hydrogen Evolution. Sci. Rep. 2016, 6, 22268. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Chiao, Y.-C.; Chang, C.-J. Synthesis of g-C3N4@ZnIn2S4 Heterostructures with Extremely High Photocatalytic Hydrogen Production and Reusability. Catalysts 2023, 13, 1187. https://doi.org/10.3390/catal13081187
Chang Y-C, Chiao Y-C, Chang C-J. Synthesis of g-C3N4@ZnIn2S4 Heterostructures with Extremely High Photocatalytic Hydrogen Production and Reusability. Catalysts. 2023; 13(8):1187. https://doi.org/10.3390/catal13081187
Chicago/Turabian StyleChang, Yu-Cheng, Yung-Chang Chiao, and Chi-Jung Chang. 2023. "Synthesis of g-C3N4@ZnIn2S4 Heterostructures with Extremely High Photocatalytic Hydrogen Production and Reusability" Catalysts 13, no. 8: 1187. https://doi.org/10.3390/catal13081187
APA StyleChang, Y.-C., Chiao, Y.-C., & Chang, C.-J. (2023). Synthesis of g-C3N4@ZnIn2S4 Heterostructures with Extremely High Photocatalytic Hydrogen Production and Reusability. Catalysts, 13(8), 1187. https://doi.org/10.3390/catal13081187










