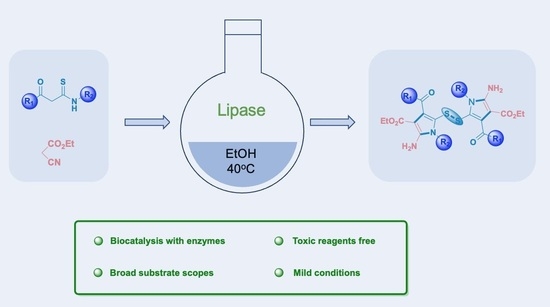
Efficient Synthesis of Pyrrole Disulfides Catalyzed by Lipase in Ethanol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Lipase Source
2.2. Effect of Solvents
2.3. Effect of Temperature
2.4. Effect of Lipase Dosage
2.5. Substrate Scope
2.6. Mechanistic Speculation
3. Materials and Methods
3.1. Materials
3.2. General Procedure for Synthesis of 1
3.3. General Procedure for Lipase-Catalyzed Synthesis of 3
3.4. Data of Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pali, P.; Yadav, D.; Sahoo, S.C.; Shankar Singh, M. Metal-Free One-Pot Annulative Coupling of 2-Hydroxybenzaldehydes with β-Ketothioamides: Access to Diverse 2-Arylimino-2H-Chromenes. J. Org. Chem. 2022, 87, 12342–12351. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Wen, L.-R.; Niu, X.-D.; Guo, W.-S.; Li, M. InCl3 catalyzed synthesis of thiophene derivatives via vinyl azides and β-ketothioamides. Tetrahedron 2023, 138, 133412. [Google Scholar] [CrossRef]
- Khan, S.; Ansari, M.A.; Singh, M.S. Access to Functionalized Thiazolothiadiazoles via the Chemoselective Cascade Heteroannulation of Thioamides with Hypervalent Iodine Reagents. Org. Lett. 2023, 25, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Shukla, G.; Srivastava, A.; Singh, M.S. Metal- and Catalyst-Free, Formal [4 + 1] Annulation via Tandem C═O/C═S Functionalization: One-Pot Access to 3,5-Disubstituted/Annulated Isothiazoles. Org. Lett. 2016, 18, 2451–2454. [Google Scholar] [CrossRef]
- Lu, H.; Tan, C.-Y.; Zhang, H.-X.; Zhang, J.-L.; Liu, J.-Y.; Li, H.-Y.; Xu, P.-F. Participation of β-Ketothioamides in N-Heterocyclic Carbene-Catalyzed [3 + 3] Spiroannulation: Asymmetric Synthesis of Functionalized Spiro-piperidinone Derivatives. J. Org. Chem. 2018, 83, 15245–15255. [Google Scholar] [CrossRef]
- Zeng, Q.; Huang, X.; Liu, M.; Yu, Z.; Xiao, Y. Synthesis of Trifluoromethylated 4H-Pyran and 4H-Thiopyran via Divergent Reaction of β-CF3-1,3-Enynes with β-Ketothioamides. Org. Lett. 2022, 24, 8186–8191. [Google Scholar] [CrossRef] [PubMed]
- Man, N.-N.; Wang, J.-Q.; Zhang, L.-M.; Wen, L.-R.; Li, M. Chemo-, Regio-, and Stereoselective Construction of Core Skeleton of Furo[2,3-b]pyrrole via Multicomponent Bicyclization Reaction. J. Org. Chem. 2017, 82, 5566–5573. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-S.; Xin, X.; Zhao, K.-L.; Wen, L.-R.; Li, M. Acid/base-controlled chemodivergent synthesis of two differently functionalized tetrahydroimidazo[1,2-a]pyridines. RSC Adv. 2015, 5, 70429–70432. [Google Scholar] [CrossRef]
- Guo, W.-S.; Wen, L.-R.; Li, M. β-Ketothioamides: Efficient reagents in the synthesis of heterocycles. Org. Biomol. Chem. 2015, 13, 1942–1953. [Google Scholar] [CrossRef]
- Jagodziński, T.S. Thioamides as Useful Synthons in the Synthesis of Heterocycles. Chem. Rev. 2003, 103, 197–228. [Google Scholar] [CrossRef]
- Mulina, O.M.; Doronin, M.M.; He, L.-N.; Terent’ev, A.O. Disulfides as versatile starting reagents: Effective sulfonylation of alkenes with disulfides under electrochemical conditions. Org. Chem. Front. 2023, 10, 3559–3566. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.; Yoshimura, A.; Sun, C.; Wang, T.; Chen, Y.; Chen, Z.; Littlejohn, A.; Xiang, Y.; Hundekar, P.; et al. Vanadium disulfide flakes with nanolayered titanium disulfide coating as cathode materials in lithium-ion batteries. Nat. Commun. 2019, 10, 1764. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S. Lithium titanium disulfide cathodes. Nat. Energy 2021, 6, 214. [Google Scholar] [CrossRef]
- Fass, D.; Thorpe, C. Chemistry and Enzymology of Disulfide Cross-Linking in Proteins. Chem. Rev. 2018, 118, 1169–1198. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, X.-L.; Chiu, J.; Bowley, S.; Wu, Y.; Hogg, P.J.; Fang, C. Extracellular Protein Disulfide Isomerase Cleaves Allosteric Disulfides in Histidine-Rich Glycoprotein to Regulate Thrombus Formation. Blood 2020, 136 (Suppl. S1), 11–12. [Google Scholar] [CrossRef]
- Arafa, W.A.A.; Hussein, M.F. Design, Sonosynthesis, Quantum-Chemical Calculations, and Evaluation of New Mono- and Bis-pyridine Dicarbonitriles as Antiproliferative Agents. Chin. J. Chem. 2020, 38, 501–508. [Google Scholar] [CrossRef]
- Abdelhamid, A.A.; Salama, K.S.M.; Elsayed, A.M.; Gad, M.A.; Ali Ali El-Remaily, M.A.E.A. Synthesis and Toxicological Effect of Some New Pyrrole Derivatives as Prospective Insecticidal Agents against the Cotton Leafworm, Spodoptera littoralis (Boisduval). ACS Omega 2022, 7, 3990–4000. [Google Scholar] [CrossRef]
- Meng, X.; Guo, W.; Nan, G.; Li, M. Synthesis of pyrrole disulfides via umpolung of β-ketothioamides. Org. Biomol. Chem. 2022, 20, 7609–7612. [Google Scholar] [CrossRef]
- Dunham, N.P.; Arnold, F.H. Nature’s Machinery, Repurposed: Expanding the Repertoire of Iron-Dependent Oxygenases. ACS Catal. 2020, 10, 12239–12255. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, F.; Xu, Y.; Wang, C.; Xu, Y.; Wu, J.; Li, Z.; Wang, Z.; Wang, L. Vitreoscilla hemoglobin: A natural carbene transfer catalyst for diastereo- and enantioselective synthesis of nitrile-substituted cyclopropanes. Green Chem. 2023, 25, 6853–6858. [Google Scholar] [CrossRef]
- Xu, J.; Peng, Y.; Wang, Z.; Hu, Y.; Fan, J.; Zheng, H.; Lin, X.; Wu, Q. Exploiting Cofactor Versatility to Convert a FAD-Dependent Baeyer–Villiger Monooxygenase into a Ketoreductase. Angew. Chem. Int. Ed. 2019, 58, 14499–14503. [Google Scholar] [CrossRef] [PubMed]
- López-Iglesias, M.; González-Martínez, D.; Gotor, V.; Busto, E.; Kroutil, W.; Gotor-Fernández, V. Biocatalytic Transamination for the Asymmetric Synthesis of Pyridylalkylamines. Structural and Activity Features in the Reactivity of Transaminases. ACS Catal. 2016, 6, 4003–4009. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Xu, H.; Feng, L.; Shen, X.-F.; Wang, C.; Huo, X.-K.; Tian, X.-G.; Ning, J.; Zhang, B.-J.; Sun, C.-P.; et al. Oxidative coupling of coumarins catalyzed by laccase. Int. J. Biol. Macromol. 2019, 135, 1028–1033. [Google Scholar] [CrossRef]
- Busto, E.; Gotor-Fernández, V.; Gotor, V. Hydrolases: Catalytically promiscuous enzymes for non-conventional reactions in organic synthesis. Chem. Soc. Rev. 2010, 39, 4504–4523. [Google Scholar] [CrossRef]
- Ding, X.; Dong, C.-L.; Guan, Z.; He, Y.-H. Concurrent Asymmetric Reactions Combining Photocatalysis and Enzyme Catalysis: Direct Enantioselective Synthesis of 2,2-Disubstituted Indol-3-ones from 2-Arylindoles. Angew. Chem. Int. Ed. 2019, 58, 118–124. [Google Scholar] [CrossRef]
- Li, X.; Hu, X.; Qiao, Y.; Lu, T.; Bai, Y.; Xiong, J.; Li, X.; Gou, Q.; Ge, J. Enzyme-bimetallic hybrid catalyst for one-pot chemoenzymatic reactions. Chem. Eng. J. 2023, 452, 139356. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Gao, X.; Jiang, W.; Li, Z.; Yao, Q.; Yang, F.; Wang, F.; Liu, J. Kinetic model of the enzymatic Michael addition for synthesis of mitomycin analogs catalyzed by immobilized lipase from T. laibacchii. Mol. Catal. 2019, 466, 146–156. [Google Scholar] [CrossRef]
- Evitt, A.S.; Bornscheuer, U.T. Lipase CAL-B does not catalyze a promiscuous decarboxylative aldol addition or Knoevenagel reaction. Green Chem. 2011, 13, 1141–1142. [Google Scholar] [CrossRef]
- Mustafa, A.; Ramadan, R.; Niikura, F.; Inayat, A.; Hafez, H. Highly selective synthesis of glyceryl monostearate via lipase catalyzed esterification of triple pressed stearic acid and glycerin. Sustain. Energy Technol. Assess. 2023, 57, 103200. [Google Scholar] [CrossRef]
- Wu, L.-L.; Xiang, Y.; Yang, D.-C.; Guan, Z.; He, Y.-H. Biocatalytic asymmetric Mannich reaction of ketimines using wheat germ lipase. Catal. Sci. Technol. 2016, 6, 3963–3970. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Jiang, W.; Yao, Q.; Li, Z.; Gao, X.; Liu, T.; Yang, F.; Wang, F.; Liu, J. Lipase-Catalyzed Oxidation of Cyclohexanone To Form ε-Caprolactone and Kinetic Modeling. ACS Sustain. Chem. Eng. 2019, 7, 13294–13306. [Google Scholar] [CrossRef]
- Xu, Y.; Li, F.; Ma, J.; Li, J.; Xie, H.; Wang, C.; Chen, P.; Wang, L. Lipase-Catalyzed Phospha-Michael Addition Reactions under Mild Conditions. Molecules 2022, 27, 7798. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, Y.; Wang, C.; Wang, C.; Xie, H.; Xu, Y.; Chen, P.; Wang, L. Efficient synthesis of substituted pyrazoles Via [3+2] cycloaddition catalyzed by lipase in ionic liquid. Process Biochem. 2023, 124, 253–258. [Google Scholar] [CrossRef]
- Li, F.; Wang, C.; Xu, Y.; Gao, X.; Xu, Y.; Xie, H.; Chen, P.; Wang, L. Lipase-Catalyzed Synthesis of Anthrone Functionalized Benzylic Amines via a Multicomponent Reaction in Supercritical Carbon Dioxide. ChemistrySelect 2022, 7, e202104517. [Google Scholar] [CrossRef]
- Klibanov, A.M. Improving enzymes by using them in organic solvents. Nature 2001, 409, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Haki, G.D.; Rakshit, S.K. Developments in industrially important thermostable enzymes: A review. Bioresour. Technol. 2003, 89, 17–34. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Geronimo, I.; Denning, C.A.; Heidary, D.K.; Glazer, E.C.; Payne, C.M. Molecular Determinants of Substrate Affinity and Enzyme Activity of a Cytochrome P450(BM3) Variant. Biophys. J. 2018, 115, 1251–1263. [Google Scholar] [CrossRef]
- Suo, H.; Xu, L.; Xu, C.; Qiu, X.; Chen, H.; Huang, H.; Hu, Y. Graphene Oxide Nanosheets Shielding of Lipase Immobilized on Magnetic Composites for the Improvement of Enzyme Stability. ACS Sustain. Chem. Eng. 2019, 7, 4486–4494. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef]
- Feng, X.-W.; Li, C.; Wang, N.; Li, K.; Zhang, W.-W.; Wang, Z.; Yu, X.-Q. Lipase-catalysed decarboxylative aldol reaction and decarboxylative Knoevenagel reaction. Green Chem. 2009, 11, 1933–1936. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, C.; Xie, H.; Xu, Y.; Wang, C.; Du, C.; Wang, Z.; Wang, L. Green Synthesis of Spirooxindoles via Lipase-Catalyzed One-Pot Tandem Reaction in Aqueous Media. Catalysts 2023, 13, 143. [Google Scholar] [CrossRef]
- Feng, X.; Wang, J.-J.; Xun, Z.; Huang, Z.-B.; Shi, D.-Q. Multicomponent Strategy to Indeno[2,1-c]pyridine and Hydroisoquinoline Derivatives through Cleavage of Carbon–Carbon Bond. J. Org. Chem. 2015, 80, 1025–1033. [Google Scholar] [CrossRef]




 | ||
| Entry | Enzyme b | Yield (%) |
| 1 | PPL | 88 |
| 2 | Cal-B | 68 |
| 3 | Novozym 435 | 77 |
| 4 | CSL | 40 |
| 5 | CRL | 48 |
| 6 | MML | 55 |
| 7 | BSA c | N.D. d |
| 8 | PPL e | N.D. |
| 9 | PPL f | N.D. |
| 10 | None | N.D. |
| 11 | Na2CO3 g | 80 |
| Entry | Solvent | Yield (%) |
|---|---|---|
| 1 | Water | 73 |
| 2 | N, N-dimethylformamide | 68 |
| 3 | Dimethyl sulfoxide | 57 |
| 4 | Ethanol | 88 |
| 5 | Acetonitrile | 89 |
| 6 | Ethyl Acetate | 80 |
| 7 | Dichloromethane | 37 |
| 8 | Toluene | 36 |
| 9 | n-Hexane | 27 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, F.; Xu, Y.; Li, F.; Ma, J.; Wang, Z.; Zhang, H.; Wang, L. Efficient Synthesis of Pyrrole Disulfides Catalyzed by Lipase in Ethanol. Catalysts 2023, 13, 1493. https://doi.org/10.3390/catal13121493
Wen F, Xu Y, Li F, Ma J, Wang Z, Zhang H, Wang L. Efficient Synthesis of Pyrrole Disulfides Catalyzed by Lipase in Ethanol. Catalysts. 2023; 13(12):1493. https://doi.org/10.3390/catal13121493
Chicago/Turabian StyleWen, Feiyang, Yuelin Xu, Fengxi Li, Jinglin Ma, Zhi Wang, Hong Zhang, and Lei Wang. 2023. "Efficient Synthesis of Pyrrole Disulfides Catalyzed by Lipase in Ethanol" Catalysts 13, no. 12: 1493. https://doi.org/10.3390/catal13121493
APA StyleWen, F., Xu, Y., Li, F., Ma, J., Wang, Z., Zhang, H., & Wang, L. (2023). Efficient Synthesis of Pyrrole Disulfides Catalyzed by Lipase in Ethanol. Catalysts, 13(12), 1493. https://doi.org/10.3390/catal13121493