Constructing Interconnected Hollow Mesopore Sn-Si Mixed Oxide Microspheres by Aerosol-Assisted Alkali Treatment with Enhanced Catalytic Performance in Baeyer-Villiger Oxidation
Abstract
:1. Introduction
2. Results and Discussion
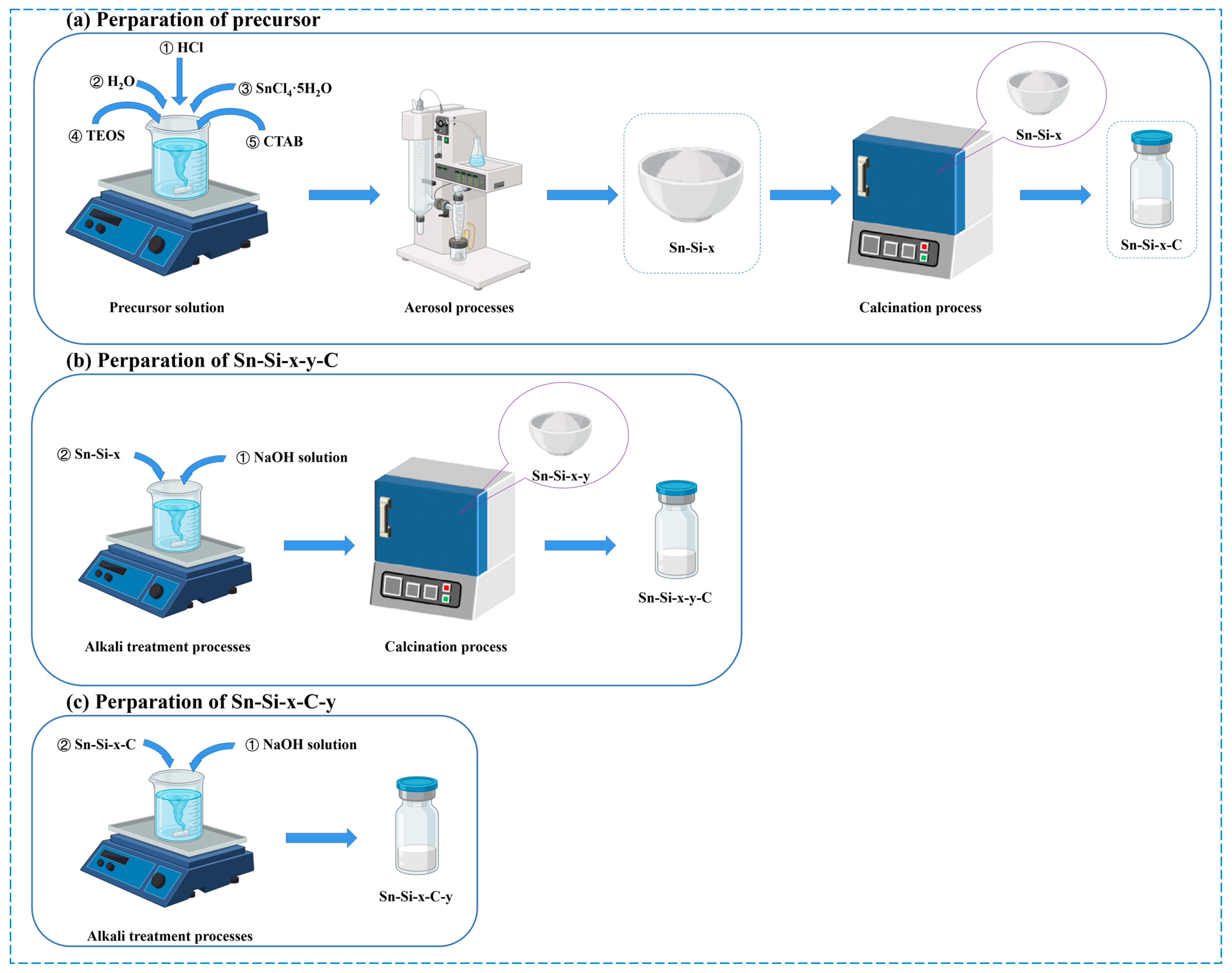
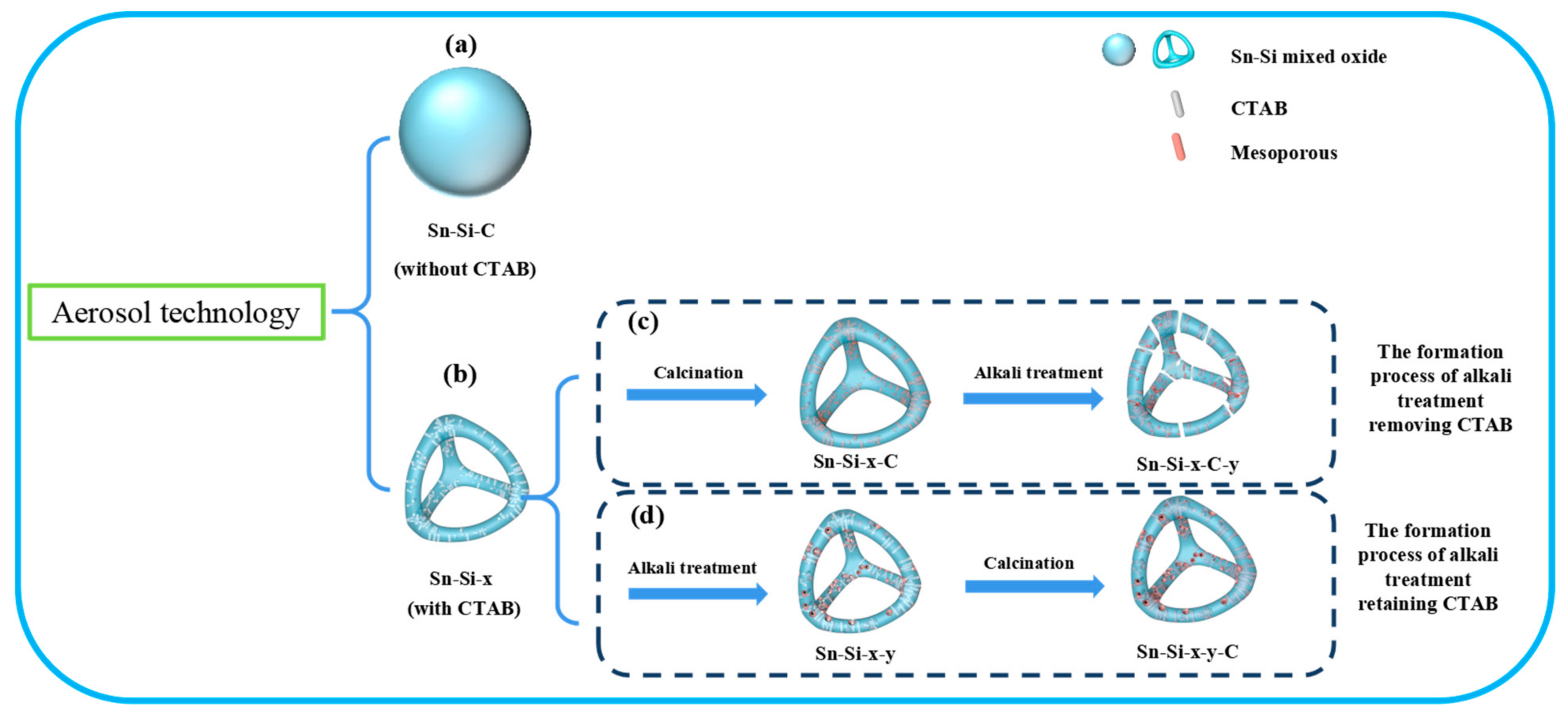
2.1. The Synthesis
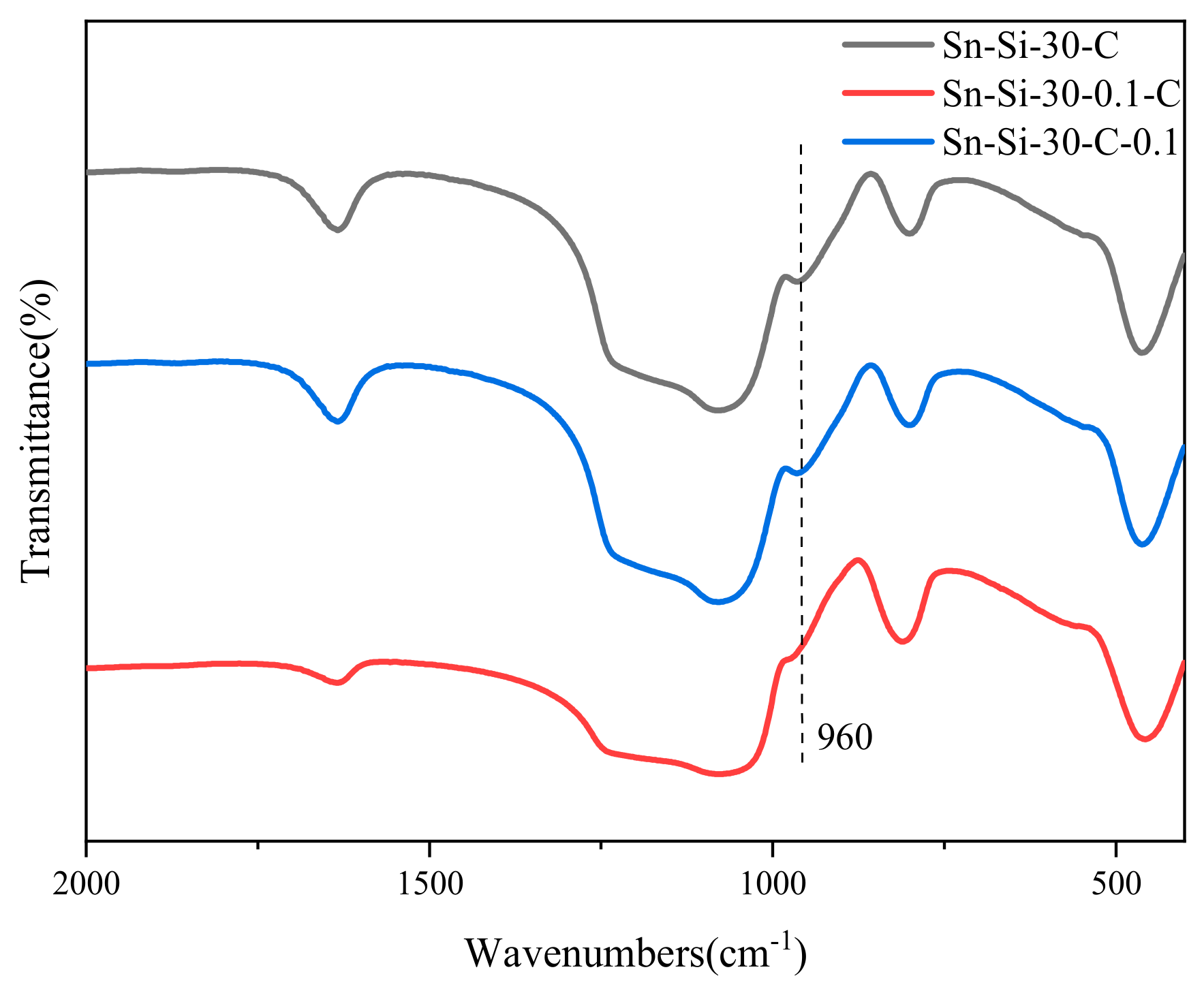
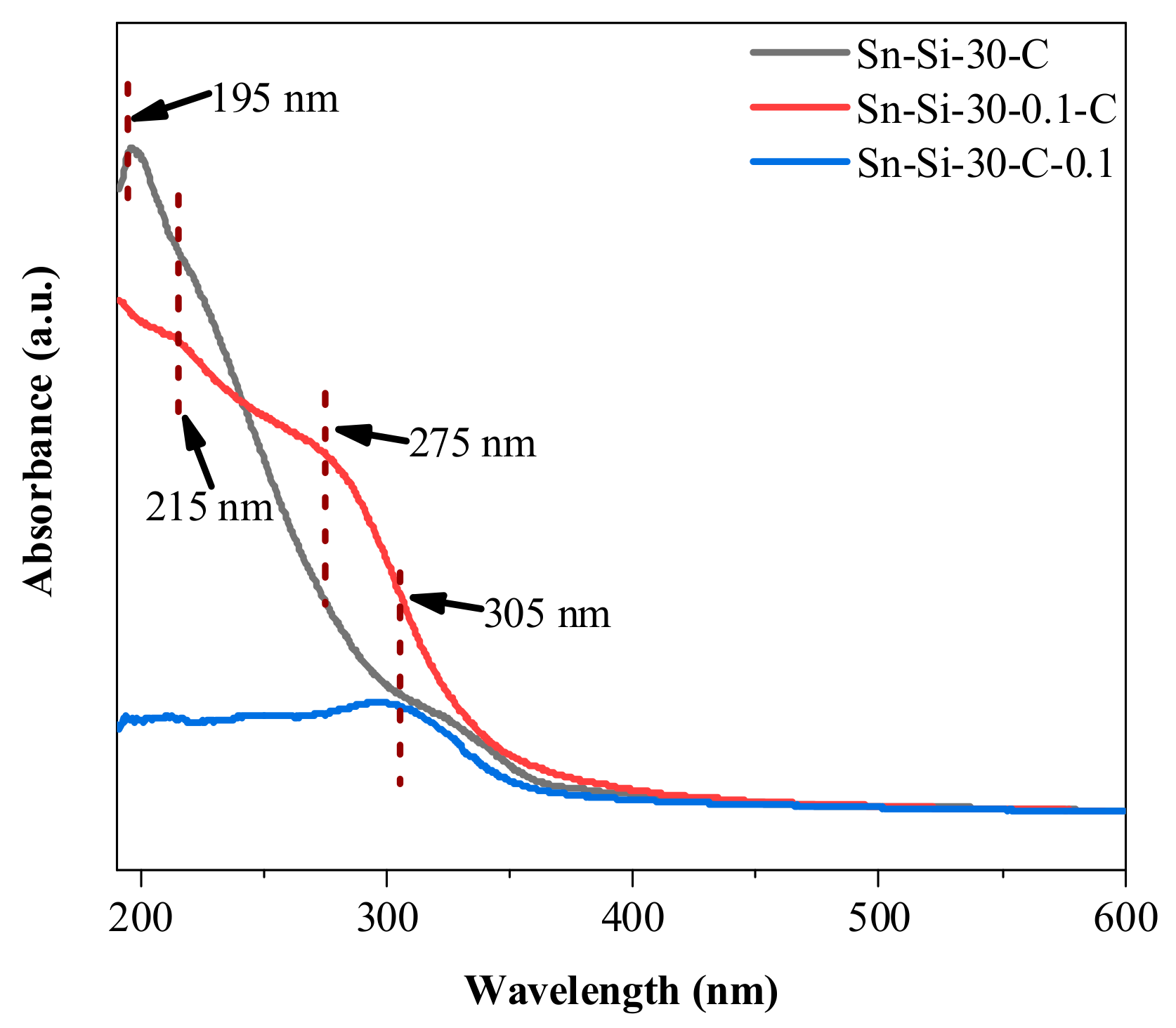
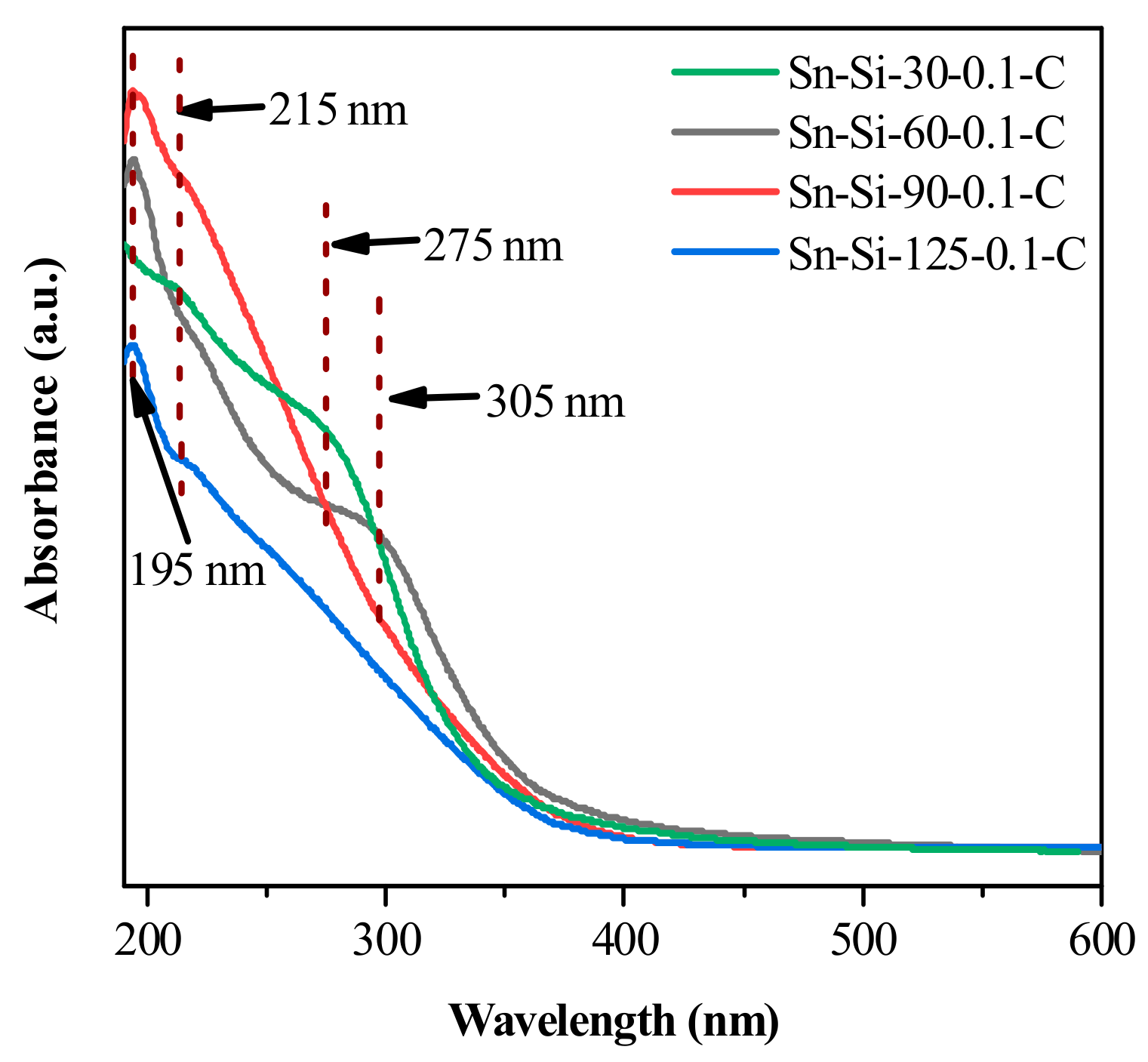
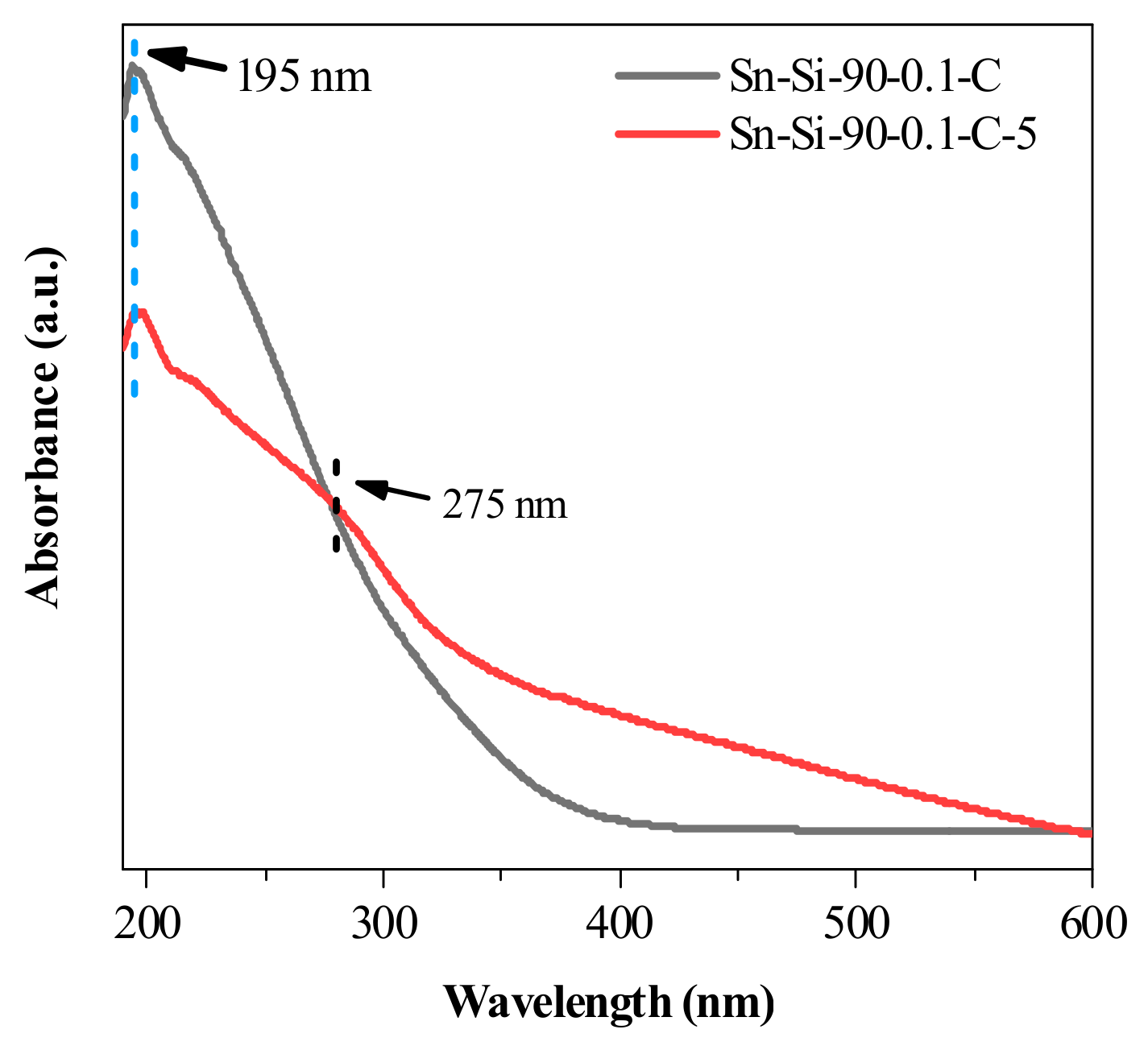
2.2. Mechanistic Investigation on the Role of CTAB
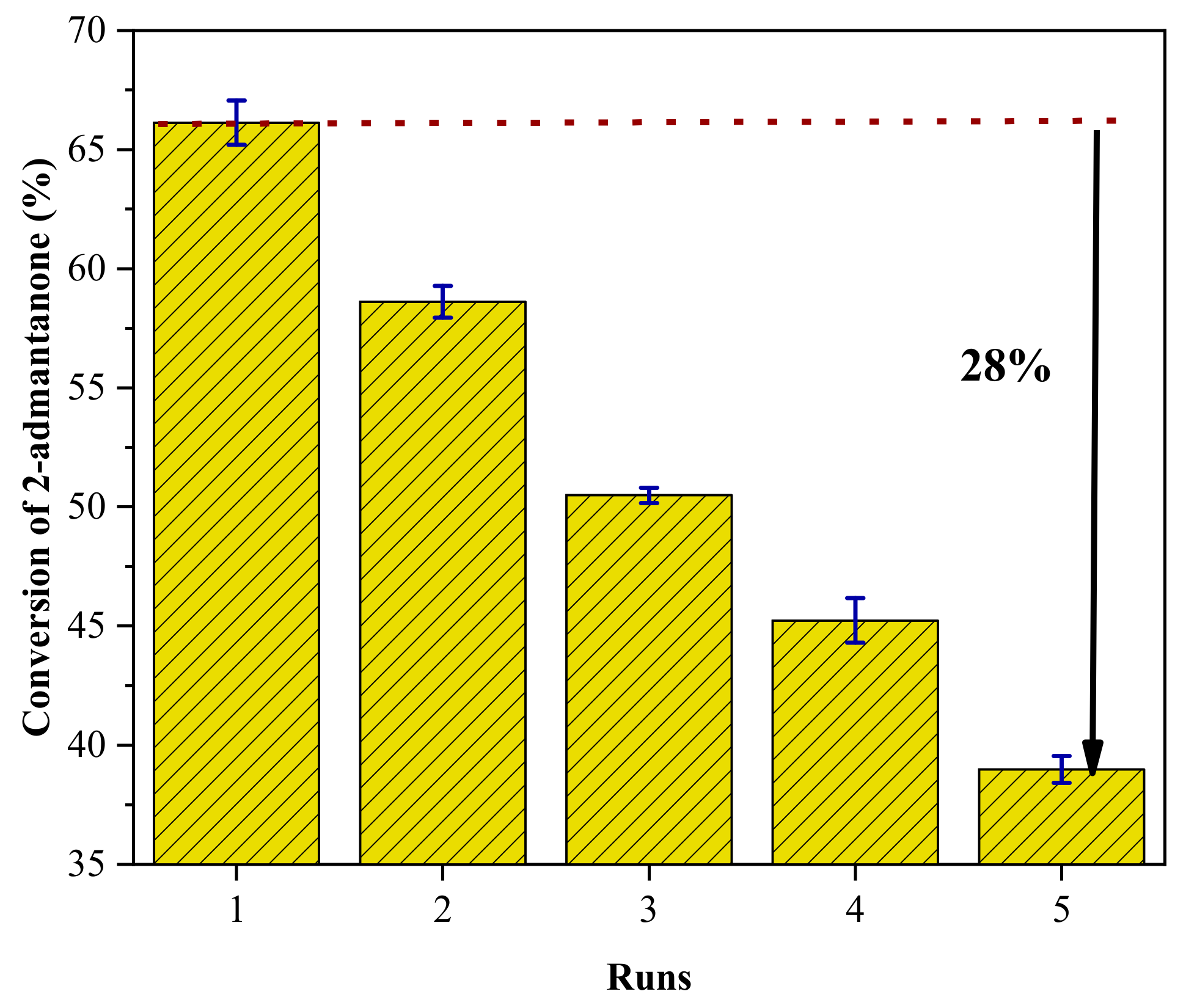
2.3. Catalytic Performance Evaluation
3. Materials and Methods
3.1. Chemicals
3.2. Synthetic Procedures
3.3. Catalyst Characterization
3.4. Performance Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Seo, E.-J.; Kim, M.-J.; Park, S.-Y.; Park, S.; Oh, D.-K.; Bornscheuer, U.; Park, J.-B. Enzyme access tunnel engineering in Baeyer-Villiger monooxygenases to improve oxidative stability and biocatalyst performance. Adv. Synth. Catal. 2022, 364, 555–564. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, J.; Yang, B.; Wu, H.; Wu, P. Effective Baeyer–Villiger oxidation of ketones over germanosilicates. Catal. Commun. 2014, 55, 83–86. [Google Scholar] [CrossRef]
- Strukul, G. How green was my ester. Nature 2001, 412, 388–389. [Google Scholar] [CrossRef]
- Sicard, R.; Chen, L.S.; Marsaioli, A.J.; Reymond, J.-L. A Fluorescence-Based Assay for Baeyer–Villiger Monooxygenases, Hydroxylases and Lactonases. Adv. Synth. Catal. 2005, 347, 1041–1050. [Google Scholar] [CrossRef]
- Michelin, R.A.; Sgarbossa, P.; Scarso, A.; Strukul, G. The Baeyer–Villiger oxidation of ketones: A paradigm for the role of soft Lewis acidity in homogeneous catalysis. Coord. Chem. Rev. 2010, 254, 646–660. [Google Scholar] [CrossRef]
- Kurmach, M.M.; Samotoi, A.O.; Sotnik, S.O.; Terebilenko, A.V.; Yaremov, P.S.; Shvets, O.V.; Shcherban, N.D. Baeyer–Villiger oxidation of cyclic ketones with H2O2 over mesoporous Sn- and Zr-BEA zeolites. Appl. Nanosci. 2023, 13, 6919–6928. [Google Scholar] [CrossRef]
- Karcz, R.; Olszowka, J.E.; Napruszewska, B.D.; Kryściak-Czerwenka, J.; Serwicka, E.M.; Klimek, A.; Bahranowski, K. Combined H2O2/nitrile/bicarbonate system for catalytic Baeyer-Villiger oxidation of cyclohexanone to ε-caprolactone over MgAl hydrotalcite catalysts. Catal. Commun. 2019, 132, 105821. [Google Scholar] [CrossRef]
- Ilovaisky, A.I.; Merkulova, V.M.; Vil’, V.A.; Chernoburova, E.I.; Shchetinina, M.A.; Loguzov, S.D.; Dmitrenok, A.S.; Zavarzin, I.V.; Terent’ev, A.O. Regioselective Baeyer–Villiger Oxidation of Steroidal Ketones to Lactones Using BF3/H2O2. Eur. J. Org. Chem. 2020, 2020, 402–405. [Google Scholar] [CrossRef]
- Pembere, A.M.S.; Louis, H.; Wu, H. Mechanism and dynamics of Baeyer–Villiger oxidation of furfural to maleic anhydride in presence of H2O2 and Au clusters. J. Mol. Model. 2023, 29, 359. [Google Scholar] [CrossRef]
- Yang, B.; Cui, T. Preparation of extra-large micropore Sn-zeolites and their catalytic performance in Baeyer–Villiger oxidations. J. Chem. Res. 2021, 45, 269–274. [Google Scholar] [CrossRef]
- Burek, B.O.; Bormann, S.; Hollmann, F.; Bloh, J.Z.; Holtmann, D. Hydrogen peroxide driven biocatalysis. Green Chem. 2019, 21, 3232–3249. [Google Scholar] [CrossRef]
- Ten Brink, G.J.; Arends, I.W.C.E.; Sheldon, R.A. The Baeyer–Villiger Reaction: New Developments toward Greener Procedures. Chem. Rev. 2004, 104, 4105–4124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wen, S.; Lei, Z. Heterogeneous Baeyer–Villiger oxidation of ketones using hydrogen peroxide as oxidant catalyzed by aminomethyl polystyrene resin-supported tin complex. React. Funct. Polym. 2006, 66, 1278–1283. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Zhang, Z.; Li, C.; Yang, Z.; Lei, Z. The Influence of Pore Structures and Degree of Crosslinking on Catalytic Properties of Aminomethyl Polystyrene Resins Supported Dendritic Sn Complexes. J. Macromol. Sci. A 2008, 45, 672–679. [Google Scholar] [CrossRef]
- Corma, A.; Navarro, M.T.; Nemeth, L.; Renz, M. Sn-MCM-41—A heterogeneous selective catalyst for the Baeyer–Villiger oxidation with hydrogen peroxide. Chem. Commun. 2001, 21, 2190–2191. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Yang, Z.; Hu, Z.; Lei, Z. Baeyer–Villiger oxidation of ketones with hydrogen peroxide catalyzed by cellulose-supported dendritic Sn complexes. Catal. Commun. 2007, 8, 1202–1208. [Google Scholar] [CrossRef]
- Hara, T.; Hatakeyama, M.; Kim, A.; Ichikuni, N.; Shimazu, S. Preparation of clay-supported Sn catalysts and application to Baeyer–Villiger oxidation. Green Chem. 2012, 14, 771–777. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Y.; Li, Y.; Xu, X.; Li, D.; Lin, K. Mesoporous silica beads containing active and stable tin species for the Baeyer-Villiger oxidations of cyclic ketones. Micropor. Mesopor. Mat. 2017, 253, 40–48. [Google Scholar] [CrossRef]
- Rajalakshmi, R.; Maheswari, R.; Ramanathan, A. Characterization and activity of novel tin incorporated ordered cubic mesoporous silicate, Sn-KIT-6. Mater. Res. Bull. 2016, 75, 224–229. [Google Scholar] [CrossRef]
- Rajalakshmi, R.; Srinivasan, V.V.; Pachamuthu, M.P.; Maheswari, R. Characterizations of tin (SnO2) doped KIT-5 by direct synthesis. Mater. Chem. Phys. 2015, 154, 164–169. [Google Scholar] [CrossRef]
- Ho, S.-T.; Dinh, Q.-K.; Tran, T.-H.; Nguyen, H.-P.; Nguyen, T.-D. One-step synthesis of ordered Sn-substituted SBA-16 mesoporous materials using prepared silica source of rice husk and their selectively catalytic activity. Can. J. Chem. Eng. 2013, 91, 34–46. [Google Scholar] [CrossRef]
- Pachamuthu, M.P.; Shanthi, K.; Luque, R.; Ramanathan, A. SnTUD-1: A solid acid catalyst for three component coupling reactions at room temperature. Green Chem. 2013, 15, 2158–2166. [Google Scholar] [CrossRef]
- Chen, T.; Wang, B.; Li, Y.; Liu, L.; Qiu, S. Hydrothermal synthesis of tin containing mesoporous silicas and their catalytic performance over Baeyer–Villiger oxidation of cyclohexanone to ε-caprolactone: Comparison of Sn/MCM-41 and Sn/SBA-15. J. Porous. Mat. 2015, 22, 949–957. [Google Scholar] [CrossRef]
- Jiang, N.; Koo, J.-B.; Han, S.-C.; Park, S.-E. Lewis-type catalytic activity of direct incorporated Zr- and Sn-SBA-16 catalysts. Res. Chem. Intermediat. 2008, 34, 507–517. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Okuyama, K. Progress in developing spray-drying methods for the production of controlled morphology particles: From the nanometer to submicrometer size ranges. Adv. Powder Technol. 2011, 22, 1–19. [Google Scholar] [CrossRef]
- Lwin, S.; Wachs, I.E. Olefin Metathesis by Supported Metal Oxide Catalysts. ACS Catal. 2014, 4, 2505–2520. [Google Scholar] [CrossRef]
- Pega, S.; Boissière, C.; Grosso, D.; Azaïs, T.; Chaumonnot, A.; Sanchez, C. Direct Aerosol Synthesis of Large-Pore Amorphous Mesostructured Aluminosilicates with Superior Acid-Catalytic Properties. Angew. Chem. Int. Ed. 2009, 121, 2822–2825. [Google Scholar] [CrossRef]
- Debecker, D.P.; Stoyanova, M.; Colbeau-Justin, F.; Rodemerck, U.; Boissière, C.; Gaigneaux, E.M.; Sanchez, C. One-Pot Aerosol Route to MoO3-SiO2-Al2O3 Catalysts with Ordered Super Microporosity and High Olefin Metathesis Activity. Angew. Chem. Int. Ed. 2012, 51, 2129–2131. [Google Scholar] [CrossRef]
- Godard, N.; Vivian, A.; Fusaro, L.; Cannavicci, L.; Aprile, C.; Debecker, D.P. High-Yield Synthesis of Ethyl Lactate with Mesoporous Tin Silicate Catalysts Prepared by an Aerosol-Assisted Sol–Gel Process. ChemCatChem 2017, 9, 2211–2218. [Google Scholar] [CrossRef]
- Garcia Marquez, A.; Horcajada, P.; Grosso, D.; Ferey, G.; Serre, C.; Sanchez, C.; Boissiere, C. Green scalable aerosol synthesis of porous metal–organic frameworks. Chem. Commun. 2013, 49, 3848–3850. [Google Scholar] [CrossRef]
- Meng, Q.; Liu, J.; Liu, L.; Xiong, G. Aerosol-assisted hydrothermal synthesis of hierarchical Sn-Beta nanoaggregates in fluoride media. Micropor. Mesopor. Mat. 2021, 320, 111090. [Google Scholar] [CrossRef]
- Iskandar, F.; Gradon, L.; Okuyama, K. Control of the morphology of nanostructured particles prepared by the spray drying of a nanoparticle sol. J. Collold Interf. Sci. 2003, 265, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Dong, H.; Xu, H. The effect of Lewis acidity of tin loading siliceous MCM-41 on glucose conversion into 5-hydroxymethylfurfural. Renew. Energy 2023, 218, 119305. [Google Scholar] [CrossRef]
- Candela-Noguera, V.; Alfonso, M.; Amorós, P.; Aznar, E.; Marcos, M.D.; Martínez-Máñez, R. In-depth study of factors affecting the formation of MCM-41-type mesoporous silica nanoparticles. Micropor. Mesopor. Mat. 2024, 363, 112840. [Google Scholar] [CrossRef]
- Cheng, W.; Jiang, Y.; Xu, X.; Wang, Y.; Lin, K.; Pescarmona, P.P. Easily recoverable titanosilicate zeolite beads with hierarchical porosity: Preparation and application as oxidation catalysts. J. Catal. 2016, 333, 139–148. [Google Scholar] [CrossRef]
- Trong On, D.; Nguyen, S.V.; Hulea, V.; Dumitriu, E.; Kaliaguine, S. Mono- and bifunctional MFI, BEA and MCM-41 titanium-molecular sieves. Part 1. Synthesis and characterization. Micropor. Mesopor. Mat. 2003, 57, 169–180. [Google Scholar] [CrossRef]
- Boronat, M.; Concepción, P.; Corma, A.; Navarro, M.T.; Renz, M.; Valencia, S. Reactivity in the confined spaces of zeolites: The interplay between spectroscopy and theory to develop structure–activity relationships for catalysis. Phys. Chem. Chem. Phys. 2009, 11, 2876–2884. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, X.; Liu, H.; Qiu, J.; Han, W.; Yeung, K.L. Factors affecting the formation of Sn-Beta zeolites by steam-assisted conversion method. Mater. Chem. Phys. 2013, 141, 519–529. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, L.; Su, Y.; Yang, X.; He, H. Effect of silica source on the synthesis, property and catalytic performance of Sn-Beta zeolite. Mater. Chem. Phys. 2021, 272, 124995. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, H.; Jiang, J.; Guan, Y.; Wu, P. Sn-Beta zeolite hydrothermally synthesized via interzeolite transformation as efficient Lewis acid catalyst. J. Catal. 2017, 352, 1–12. [Google Scholar] [CrossRef]
- Smeets, V.; Boissière, C.; Sanchez, C.; Gaigneaux, E.M.; Peeters, E.; Sels, B.F.; Dusselier, M.; Debecker, D.P. Aerosol Route to TiO2–SiO2 Catalysts with Tailored Pore Architecture and High Epoxidation Activity. Chem. Mater. 2019, 31, 1610–1619. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Ji, P.; Wu, H.; Guan, Y.; Wu, P. Hydrothermal synthesis of Sn-Beta zeolites in F−-free medium. Inorg. Chem. Front. 2018, 5, 2763–2771. [Google Scholar] [CrossRef]
- Yuan, E.-H.; Li, M.; Zhou, J.-F.; Niu, Y.; Song, Y.-H.; Zhang, K.; Yang, M.-H.; Jiang, J.; Zhang, B.; Xiao, F.-S.; et al. Ultrafast crystallization of mesoporous Sn-MFI single crystals achieved by addition of the cationic polyelectrolyte in starting gels. Micropor. Mesopor. Mat. 2022, 337, 111922. [Google Scholar] [CrossRef]
- Xiong, G.; Yang, H.; Liu, L.; Liu, J. Post-synthesis of Sn-beta zeolite by aerosol method. RSC Adv. 2023, 13, 4835–4842. [Google Scholar] [CrossRef] [PubMed]
- Concepción, P.; Pérez, Y.; Hernández-Garrido, J.C.; Fajardo, M.; Calvino, J.J.; Corma, A. The promotional effect of Sn-beta zeolites on platinum for the selective hydrogenation of α,β-unsaturated aldehydes. Phys. Chem. Chem. Phys. 2013, 15, 12048–12055. [Google Scholar] [CrossRef]
- Vivian, A.; Soumoy, L.; Fusaro, L.; Fiorilli, S.; Debecker, D.P.; Aprile, C. Surface-functionalized mesoporous gallosilicate catalysts for the efficient and sustainable upgrading of glycerol to solketal. Green Chem. 2021, 23, 354–366. [Google Scholar] [CrossRef]

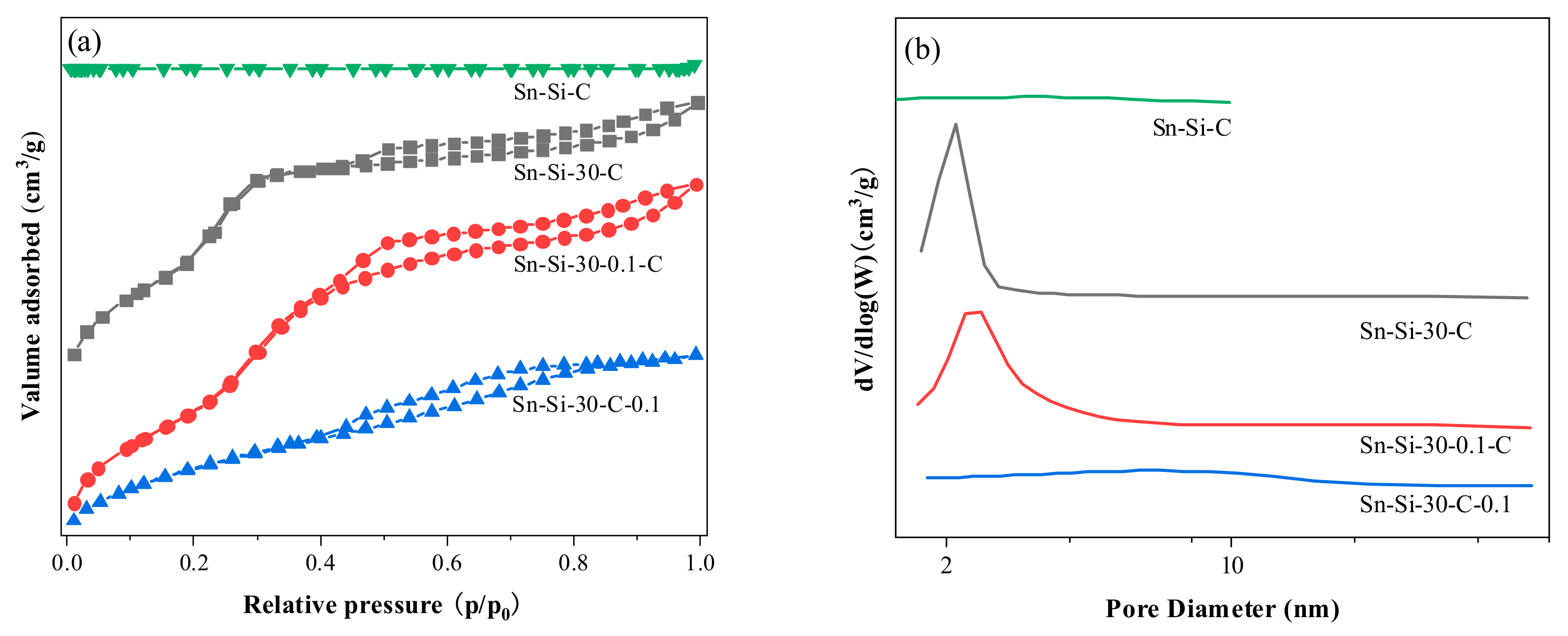

| Entry | Sample | Si/Sn a | SBET (m2/g) b | Vtotal (cm3/g) c | Vmeso (cm3/g) d | Vmeso/Vtotal (%) |
|---|---|---|---|---|---|---|
| 1 | Sn-Si-C | 30 | -- | -- | -- | -- |
| 2 | Sn-Si-30-C | 29 | 1236 | 0.76 | 0.63 | 84 |
| 3 | Sn-Si-30-0.1-C | 51 | 1187 | 0.87 | 0.79 | 90 |
| 4 | Sn-Si-30-C-0.1 | 83 | 549 | 0.47 | 0.38 | 80 |
 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Conversion (%) | Selectivity (%) | Yield (%) |
| 1 | Sn-Si-C | 7 | 99 | 7 |
| 2 | Sn-Si-30-C | 29 | 99 | 29 |
| 3 | Sn-Si-30-C-0.05 | 11 | 99 | 11 |
| 4 | Sn-Si-30-C-0.1 | 15 | 99 | 15 |
| 5 | Sn-Si-30-C-0.3 | 13 | 99 | 13 |
| 6 | Sn-Si-30-0.05-C | 32 | 99 | 32 |
| 7 | Sn-Si-30-0.1-C | 50 | 99 | 50 |
| 8 | Sn-Si-30-0.3-C | 46 | 99 | 46 |
| 9 | Sn-Si-30-0.5-C | 26 | 99 | 26 |
| 10 | Sn-Si-60-0.1-C | 53 | 99 | 53 |
| 11 | Sn-Si-90-0.1-C | 66 | 99 | 66 |
| 12 | Sn-Si-125-0.1-C | 27 | 99 | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Q.; Gao, X.; Li, D.; Liu, H. Constructing Interconnected Hollow Mesopore Sn-Si Mixed Oxide Microspheres by Aerosol-Assisted Alkali Treatment with Enhanced Catalytic Performance in Baeyer-Villiger Oxidation. Catalysts 2023, 13, 1494. https://doi.org/10.3390/catal13121494
Meng Q, Gao X, Li D, Liu H. Constructing Interconnected Hollow Mesopore Sn-Si Mixed Oxide Microspheres by Aerosol-Assisted Alkali Treatment with Enhanced Catalytic Performance in Baeyer-Villiger Oxidation. Catalysts. 2023; 13(12):1494. https://doi.org/10.3390/catal13121494
Chicago/Turabian StyleMeng, Qingrun, Xiaoxu Gao, Dezheng Li, and Huimin Liu. 2023. "Constructing Interconnected Hollow Mesopore Sn-Si Mixed Oxide Microspheres by Aerosol-Assisted Alkali Treatment with Enhanced Catalytic Performance in Baeyer-Villiger Oxidation" Catalysts 13, no. 12: 1494. https://doi.org/10.3390/catal13121494
APA StyleMeng, Q., Gao, X., Li, D., & Liu, H. (2023). Constructing Interconnected Hollow Mesopore Sn-Si Mixed Oxide Microspheres by Aerosol-Assisted Alkali Treatment with Enhanced Catalytic Performance in Baeyer-Villiger Oxidation. Catalysts, 13(12), 1494. https://doi.org/10.3390/catal13121494







