Kinetic Modeling of Glycerol Hydrogenolysis: A Short Review
Abstract
1. Introduction
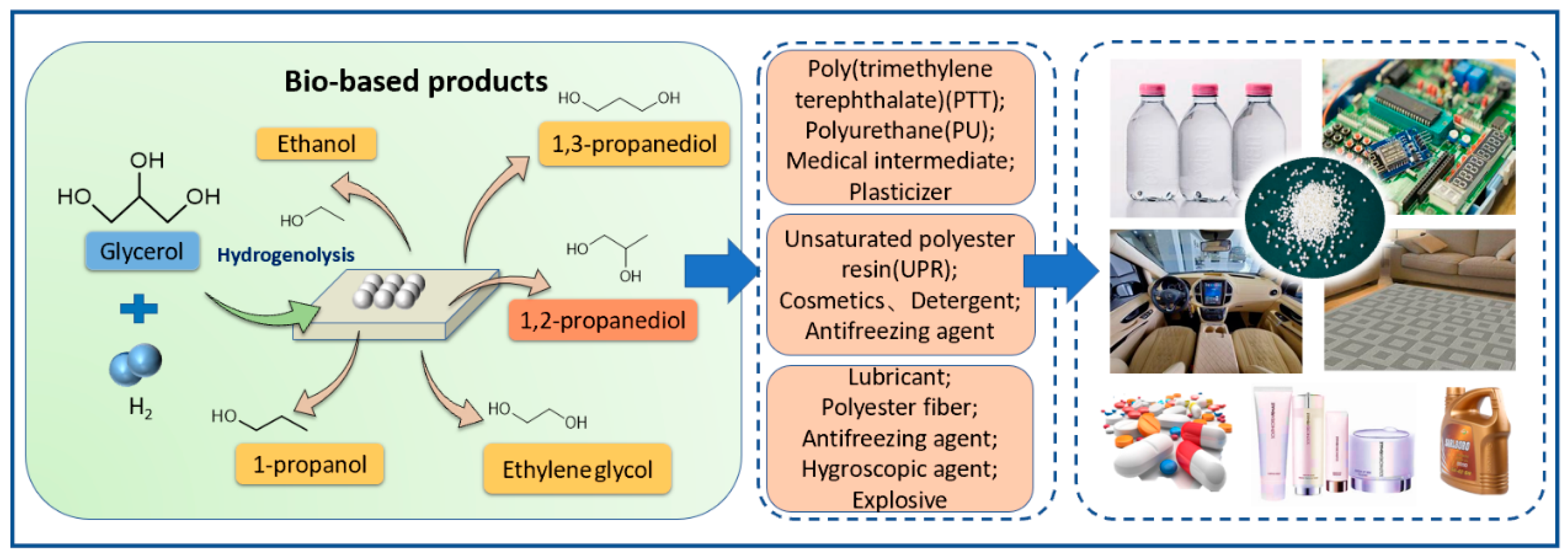
1.1. Glycerol Hydrogenolysis: Key Technology for Downstream Renewables
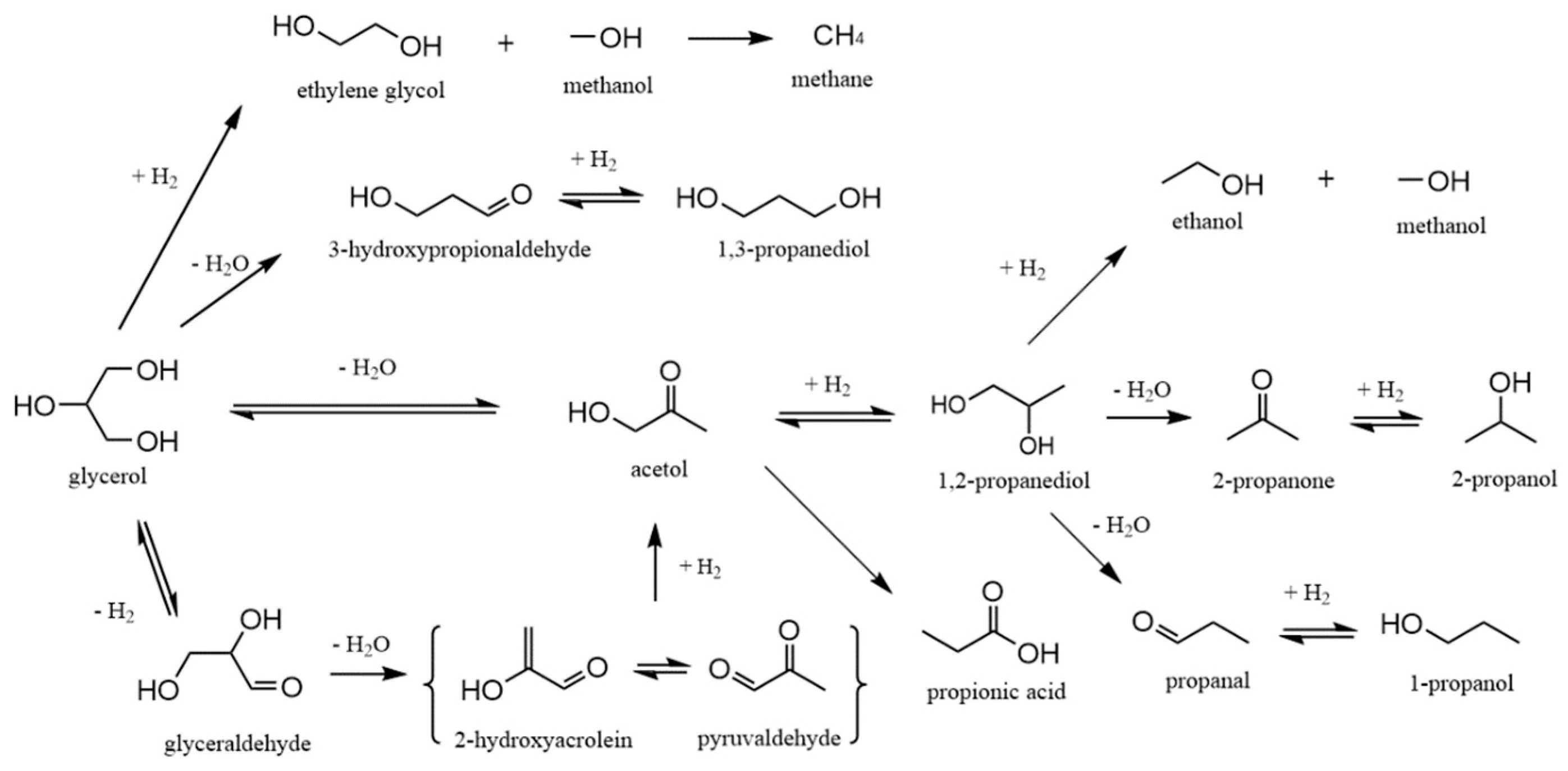
1.2. Conversion Routes of Glycerol Hydrogenolysis
1.3. Kinetic Modeling: Opportunities and Challenges
2. Kinetic Modeling on Cu-Based Catalysts
2.1. Power Law Models
2.2. LHHW Models
2.3. Horiuti/Temkin Model
3. Kinetic Modeling on Ru-Based Catalysts
4. Kinetic Modeling on Other Metal Catalysts
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| r | Reaction Rate |
| Ki | equilibrium constant of component i |
| KH | adsorption equilibrium constant of hydrogen atom |
| KALK | apparent equilibrium constant for the formation of bound 2,3-dihydroxypropanoxide from glycerol |
| ki | rate coefficient of i |
| kP-PA | reaction constant of hydrohydrolysis of 1,2-propanediol to 1-propanol |
| PH | hydrogen atom pressure |
| G | glycerol |
| H2 | hydrogen |
| P | 1,2-propanediol |
| EG | ethylene glycol |
| A | acetol |
| W | water |
| iG | ethylene glycol and 1,2-propanediol |
| CT$ | total concentration of active sites |
| Ci | concentration of component i |
| COH- | base concentration |
| θi* | the surface coverages of surface species i |
| θ* | the fractional coverage of free sites |
| SIg | the respective selectivity factors |
| H | Henry’s constant |
| rG | total reaction rate of glycerol consumption |
| rG-P | reaction rate of hydrohydrolysis of glycerol to 1,2-propanediol |
| rG-EG | reaction rate of hydrohydrolysis of glycerol to ethylene glycol |
| rG-A | reaction rate of glycerol to acetol |
| rA-P | reaction rate of acetol to 1,2-propanediol |
| riG | degradation rate of EG and P |
References
- Jin, X.; Fang, T.; Wang, J.; Liu, M.; Pan, S.; Subramaniam, B.; Shen, J.; Yang, C.; Chaudhari, R.V. Nanostructured Metal Catalysts for Selective Hydrogenation and Oxidation of Cellulosic Biomass to Chemicals. Chem. Rec. 2018, 19, 1952–1994. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yin, B.; Zhang, W.; Yu, X.; Du, Y.; Zhao, S.; Zhang, G.; Liu, M.; Yan, H.; Abbotsi-Dogbey, M.; et al. Catalytic Transfer Hydrogenolysis of Glycerol over Heterogeneous Catalysts: A Short Review on Mechanistic Studies. Chem. Rec. 2021, 21, 1792–1810. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Morales, I.; Vila, F.; Mariscal, R.; Jiménez-López, A. Hydrogenolysis of glycerol to obtain 1,2-propanediol on Ce-promoted Ni/SBA-15 catalysts. Appl. Catal. B Environ. 2012, 117, 253–259. [Google Scholar] [CrossRef]
- Xia, Q.; Jin, X.; Zhang, G.; Liu, M.; Wang, J.; Li, Y.; Fang, T.; Ding, J.; Zhang, D.; Meng, K.; et al. Catalytic Deoxygenation of Xylitol to Renewable Chemicals: Advances on Catalyst Design and Mechanistic Studies. Chem. Rec. 2020, 21, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Balaraju, M.; Rekha, V.; Devi, B.P.; Prasad, R.; Prasad, P.S.; Lingaiah, N. Surface and structural properties of titania-supported Ru catalysts for hydrogenolysis of glycerol. Appl. Catal. A Gen. 2010, 384, 107–114. [Google Scholar] [CrossRef]
- Okolie, J.A. Insights on production mechanism and industrial applications of renewable propylene glycol. iScience 2022, 25, 104903. [Google Scholar] [CrossRef]
- D’Hondt, E.; Van de Vyver, S.; Sels, B.F.; Jacobs, P.A. Catalytic glycerol conversion into 1,2-propanediol in absence of added hydrogen. Chem. Commun. 2008, 45, 6011–6012. [Google Scholar] [CrossRef]
- Wawrzetz, A.; Peng, B.; Hrabar, A.; Jentys, A.; Lemonidou, A.; Lercher, J. Towards understanding the bifunctional hydrodeoxygenation and aqueous phase reforming of glycerol. J. Catal. 2010, 269, 411–420. [Google Scholar] [CrossRef]
- Miyazawa, T.; Koso, S.; Kunimori, K.; Tomishige, K. Glycerol hydrogenolysis to 1,2-propanediol catalyzed by a heat-resistant ion-exchange resin combined with Ru/C. Appl. Catal. A Gen. 2007, 329, 30–35. [Google Scholar] [CrossRef]
- Feng, J.; Fu, H.; Wang, J.; Li, R.; Chen, H.; Li, X. Hydrogenolysis of glycerol to glycols over ruthenium catalysts: Effect of support and catalyst reduction temperature. Catal. Commun. 2008, 9, 1458–1464. [Google Scholar] [CrossRef]
- Yin, A.-Y.; Guo, X.-Y.; Dai, W.-L.; Fan, K.-N. The synthesis of propylene glycol and ethylene glycol from glycerol using Raney Ni as a versatile catalyst. Green Chem. 2009, 11, 1514–1516. [Google Scholar] [CrossRef]
- Perosa, A.; Tundo, P. Selective Hydrogenolysis of Glycerol with Raney Nickel. Ind. Eng. Chem. Res. 2005, 44, 8535–8537. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H. Selective hydrogenolysis of glycerol to propylene glycol on Cu-ZnO catalysts. Catal. Lett. 2007, 117, 62–67. [Google Scholar] [CrossRef]
- Rekha, V.; Raju, N.; Sumana, C.; Paul Douglas, S.; Lingaiah, N. Selective Hydrogenolysis of Glycerol Over Cu-ZrO2-MgO Catalysts. Catal. Lett. 2016, 146, 1487–1496. [Google Scholar] [CrossRef]
- Dasari, M.A.; Kiatsimkul, P.-P.; Sutterlin, W.R.; Suppes, G.J. Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl. Catal. A Gen. 2005, 281, 225–231. [Google Scholar] [CrossRef]
- Feng, J.; Yuan, M.; Chen, H.; Li, X. Studies and applications of catalytic hydrogenolysis of glycerol. Prog. Chem. 2007, 19, 651–658. [Google Scholar]
- Fang, W.; Yao, X.; Yang, J.; Cui, F. Research Progress of Catalysts in Hydrogenolysis of Bioglycerol to 1,3-Propanediol. J. Mol. Catal. 2018, 32, 581–593. [Google Scholar]
- Li, X.; Zhang, L.; Gao, D.; Shi, W.; Fan, Y. Progress on the production of 1,3-propanediol by fermentation. Chem. Ind. Eng. Prog. 2017, 36, 1395–1403. [Google Scholar]
- Li, M.; Ma, J.; Xu, D.; Ge, Q. Advance in Cu-based catalysts for hydrogenation of dimethyl oxalate to ethylene glycol. Nat. Gas Chem. Ind. 2013, 38, 72–77. [Google Scholar]
- Anyuan, Y.; Liangfeng, C.; Weilin, D.; Minghua, Q.; Kangnian, W. Advances in Studies of Preparation of Ethylene Glycol by Catalytic Hydrogenation of Oxalate Esters. Chem. World 2008, 49, 369–373. [Google Scholar]
- Jamil, F.; Aslam, M.; Al-Muhtaseb, A.H.; Bokhari, A.; Rafiq, S.; Khan, Z.; Inayat, A.; Ahmed, A.; Hossain, S.; Khurram, M.S.; et al. Greener and sustainable production of bioethylene from bioethanol: Current status, opportunities and perspectives. Rev. Chem. Eng. 2020, 38, 185–207. [Google Scholar] [CrossRef]
- Mizugaki, T.; Kaneda, K. Development of High Performance Heterogeneous Catalysts for Selective Cleavage of C-O and C-C Bonds of Biomass-Derived Oxygenates. Chem. Rec. 2018, 19, 1179–1198. [Google Scholar] [CrossRef] [PubMed]
- Rekha, V.; Sumana, C.; Douglas, S.P.; Lingaiah, N. Understanding the role of Co in Co-ZnO mixed oxide catalysts for the selective hydrogenolysis of glycerol. Appl. Catal. A Gen. 2015, 491, 155–162. [Google Scholar] [CrossRef]
- Tomishige, K.; Tamura, M.; Nakagawa, Y. Role of Re Species and Acid Cocatalyst on Ir-ReOx/SiO2 in the C-O Hydrogenolysis of Biomass-Derived Substrates. Chem. Rec. 2014, 14, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Gabrysch, T.; Muhler, M.; Peng, B. The kinetics of glycerol hydrodeoxygenation to 1,2-propanediol over Cu/ZrO2 in the aqueous phase. Appl. Catal. A Gen. 2019, 576, 47–53. [Google Scholar] [CrossRef]
- Mondal, S.; Malviya, H.; Biswas, P. Kinetic modelling for the hydrogenolysis of bio-glycerol in the presence of a highly selective Cu-Ni-Al2O3 catalyst in a slurry reactor. React. Chem. Eng. 2019, 4, 595–609. [Google Scholar] [CrossRef]
- Mondal, S.; Biswas, P. Conversion of bio-glycerol to propylene glycol over basic oxides (MgO, La2O3, MgO-La2O3, CaO, and BaO2) supported Cu-Zn bimetallic catalyst: A reaction kinetic study. Environ. Technol. Innov. 2022, 27, 102367. [Google Scholar] [CrossRef]
- Liu, Y.; Rempel, G.L.; Ng, F.T.T. Kinetic Study of Pd-Promoting Effect on Cu/ZnO/Al2O3 Catalyst for Glycerol Hydrogenolysis to Produce 1,2-Propanediol at Low Hydrogen Pressure. Biomass 2022, 2, 27–45. [Google Scholar] [CrossRef]
- Pandhare, N.; Pudi, S.M.; Mondal, S.; Pareta, K.; Kumar, M.; Biswas, P. Development of Kinetic Model for Hydrogenolysis of Glycerol over Cu/MgO Catalyst in a Slurry Reactor. Ind. Eng. Chem. Res. 2017, 57, 101–110. [Google Scholar] [CrossRef]
- Sharma, R.V.; Kumar, P.; Dalai, A.K. Selective hydrogenolysis of glycerol to propylene glycol by using Cu:Zn:Cr:Zr mixed metal oxides catalyst. Appl. Catal. A Gen. 2014, 477, 147–156. [Google Scholar] [CrossRef]
- Zhou, Z.; Xun, L.; Tianying, Z.; Wenbin, H.; Cheng, Z.; Weikang, Y. Kinetics of Hydrogenolysis of Glycerol to Propylene Glycol over Cu-ZnO-Al2O3 Catalysts. Chin. J. Chem. Eng. 2010, 18, 384–390. [Google Scholar] [CrossRef]
- Meena, M.L.; Pandey, D.K.; Malviya, H.; Biswas, P. Kinetic Model for the Manufacturing of 1,2-Propanediol (1,2-PDO) via Hydrogenolysis of Bio-glycerol Over Layered Double Hydroxide (LDH) Derived Cu0.45Zn0.15Mg5.4Al2O9 Catalyst in an Autoclave Reactor. Catal. Lett. 2021, 152, 2155–2163. [Google Scholar] [CrossRef]
- Vasiliadou, E.; Lemonidou, A. Kinetic study of liquid-phase glycerol hydrogenolysis over Cu/SiO2 catalyst. Chem. Eng. J. 2013, 231, 103–112. [Google Scholar] [CrossRef]
- Yfanti, V.; Ipsakis, D.; Lemonidou, A. Kinetic model of glycerol hydrodeoxygenation under inert conditions over copper catalyst. In Proceedings of the 11th Panhellenic Scientific Conference on Chemical Engineering (PSCCE), Thessaloniki, Greece, 25–27 May 2017. [Google Scholar]
- Montassier, C.; Ménézo, J.; Hoang, L.; Renaud, C.; Barbier, J. Aqueous polyol conversions on ruthenium and on sulfur-modified ruthenium. J. Mol. Catal. 1991, 70, 99–110. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, S.; Liu, H. Selective Hydrogenolysis of Glycerol to Propylene Glycol on Supported Pd Catalysts: Promoting Effects of ZnO and Mechanistic Assessment of Active PdZn Alloy Surfaces. ACS Catal. 2017, 7, 4265–4275. [Google Scholar] [CrossRef]
- Roy, D.; Subramaniam, B.; Chaudhari, R.V. Aqueous phase hydrogenolysis of glycerol to 1,2-propanediol without external hydrogen addition. Catal. Today 2010, 156, 31–37. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y.; Song, W.; Shen, W. Glycerol Hydrogenolysis over Co Catalysts Derived from a Layered Double Hydroxide Precursor. Catal. Lett. 2011, 141, 1458–1463. [Google Scholar] [CrossRef]
- Rekha, V.; Raju, N.; Sumana, C.; Lingaiah, N. Continuous Hydrogenolysis of Glycerol to 1,2-Propanediol Over Bi-metallic Ni-Ag Supported on gamma-Al2O3 Catalysts. Catal. Lett. 2017, 147, 1441–1452. [Google Scholar] [CrossRef]
- Feng, J.; Xiong, W.; Jia, Y.; Wang, J.; Liu, D.; Chen, H.; Li, X. Hydrogenolysis of Glycerol to 1,2-Propanediol over Ru/TiO2 Catalyst. Chin. J. Catal. 2013, 32, 1545–1549. [Google Scholar] [CrossRef]
- Rajkhowa, T.; Marin, G.B.; Thybaut, J.W. A comprehensive kinetic model for Cu catalyzed liquid phase glycerol hydrogenolysis. Appl. Catal. B Environ. 2017, 205, 469–480. [Google Scholar] [CrossRef]
- Kukushkin, R.; Bulavchenko, O.; Kaichev, V.; Yakovlev, V. Influence of Mo on catalytic activity of Ni-based catalysts in hydrodeoxygenation of esters. Appl. Catal. B Environ. 2015, 163, 531–538. [Google Scholar] [CrossRef]
- Lahr, D.G.; Shanks, B.H. Kinetic Analysis of the Hydrogenolysis of Lower Polyhydric Alcohols: Glycerol to Glycols. Ind. Eng. Chem. Res. 2003, 42, 5467–5472. [Google Scholar] [CrossRef]
- Feng, J.; Xu, B.; Jiang, W.D.; Xiong, W.; Wang, J.B. Hydrogenolysis of Glycerol on Supported Ru-Co Bimetallic Catalysts. In Proceedings of the 2nd International Symposium on Chemical Engineering and Material Properties (ISCEMP 2012), Taiyuan, China, 22–24 June 2012. [Google Scholar]
- Ma, L.; He, D. Hydrogenolysis of Glycerol to Propanediols Over Highly Active Ru–Re Bimetallic Catalysts. Top. Catal. 2009, 52, 834–844. [Google Scholar] [CrossRef]
- Cai, F.; Jin, F.; Hao, J.; Xiao, G. Selective hydrogenolysis of glycerol to 1,2-propanediol on Nb-modified Pd-Zr-Al catalysts. Catal. Commun. 2019, 131, 105801. [Google Scholar] [CrossRef]
- Chang, F.-W.; Kuo, M.-S.; Tsay, M.-T.; Hsieh, M.-C. Effect of calcination temperature on catalyst reducibility and hydrogenation reactivity in rice husk ash–alumina supported nickel systems. J. Chem. Technol. Biotechnol. 2004, 79, 691–699. [Google Scholar] [CrossRef]
- Xi, Y.; Holladay, J.E.; Frye, J.G.; Oberg, A.A.; Jackson, J.E.; Miller, D.J. A Kinetic and Mass Transfer Model for Glycerol Hydrogenolysis in a Trickle-Bed Reactor. Org. Process Res. Dev. 2010, 14, 1304–1312. [Google Scholar] [CrossRef]
- Furikado, I.; Miyazawa, T.; Koso, S.; Shimao, A.; Kunimori, K.; Tomishige, K. Catalytic performance of Rh/SiO2 in glycerol reaction under hydrogen. Green Chem. 2007, 9, 582–588. [Google Scholar] [CrossRef]
- Ahmed, T.S.; Abdelaziz, O.Y.; Roberts, G.W. Hydrogenolysis of Glycerol over gamma-Al2O3-Supported Iridium Catalyst. Period. Polytech. Chem. Eng. 2017, 61, 295–300. [Google Scholar] [CrossRef]
- Yfanti, V.-L.; Ipsakis, D.; Lemonidou, A.A. Kinetic study of liquid phase glycerol hydrodeoxygenation under inert conditions over a Cu-based catalyst. React. Chem. Eng. 2018, 3, 559–571. [Google Scholar] [CrossRef]
- Torres, A.; Roy, D.; Subramaniam, B.; Chaudhari, R.V. Kinetic Modeling of Aqueous-Phase Glycerol Hydrogenolysis in a Batch Slurry Reactor. Ind. Eng. Chem. Res. 2010, 49, 10826–10835. [Google Scholar] [CrossRef]
- Pandey, D.K.; Pandhare, N.N.; Biswas, P. Production of propylene glycol (propane-1,2-diol) in vapor phase over Cu-Ni/-Al2O3 catalyst in a down flow tubular reactor: Effect of catalyst calcination temperature and kinetic study. React. Kinet. Mech. Catal. 2019, 127, 523–542. [Google Scholar] [CrossRef]
- Zhang, Z.; Jackson, A.J.E.; Miller, D.J. Kinetics of Aqueous-Phase Hydrogenation of Lactic Acid to Propylene Glycol. Ind. Eng. Chem. Res. 2002, 41, 691–696. [Google Scholar] [CrossRef]
- Yan, H.; Yao, S.; Liang, W.; Feng, X.; Jin, X.; Liu, Y.; Chen, X.; Yang, C. Selective oxidation of glycerol to carboxylic acids on Pt(111) in base-free medium: A periodic density functional theory investigation. Appl. Surf. Sci. 2019, 497, 143661. [Google Scholar] [CrossRef]
- Yan, H.; Yao, S.; Yin, B.; Liang, W.; Jin, X.; Feng, X.; Liu, Y.; Chen, X.; Yang, C. Synergistic effects of bimetallic PtRu/MCM-41 nanocatalysts for glycerol oxidation in base-free medium: Structure and electronic coupling dependent activity. Appl. Catal. B Environ. 2019, 259, 118070. [Google Scholar] [CrossRef]
- Yan, H.; Qin, H.; Feng, X.; Jin, X.; Liang, W.; Sheng, N.; Zhu, C.; Wang, H.; Yin, B.; Liu, Y.; et al. Synergistic Pt/MgO/SBA-15 nanocatalysts for glycerol oxidation in base-free medium: Catalyst design and mechanistic study. J. Catal. 2019, 370, 434–446. [Google Scholar] [CrossRef]
| Cat. (Supporter) | Promoter | Kinetic | Batch | Continuous | Ref. |
|---|---|---|---|---|---|
| Cu (Al2O3, Cr2O3, MgO, SiO2, ZnO, ZrO2) | Ni, Pd | √ | √ | √ | [23,24,25,26,27,28,29,30,31,32,33,34] |
| Co (C, ZnO, Al2O3) | Re, Pd | √ | √ | [14,35,36,37] | |
| Ni (C, Al2O3) | Cu, Ce, Ag | √ | √ | √ | [3,38,39,40] |
| Ru (C, Al2O3, TiO2) | Re, Co | √ | √ | √ | [5,41,42,43,44,45,46] |
| Pt (C, Al2O3) | - | √ | [8,9,46] | ||
| Pd (Cr2O3, ZrO2, ZnO) | Cu, Re, Co | √ | √ | √ | [36,47,48] |
| Rh (Al2O3, SiO2) | - | √ | [49] | ||
| Ir (Al2O3) | - | √ | [50] |
| No. | Catalyst | Condition | X (%) | SP (%) | Kinetic Model | Ref. | |
|---|---|---|---|---|---|---|---|
| Ea (kJ·mol−1) | Expression | ||||||
| 1 | Cu0.45Zn0.15Mg5.4Al2O9 | Temperature: 190–250 °C H2 pressure: 3.5–5 MPa Glycerol concentration: 20 wt% Catalyst loading: 5.4 wt% Time: 12 h | 100 | 93.7 | G-P: 35.1 | [30] | |
| 2 | Cu/MgO | Temperature: 190–230 °C H2 pressure: 3–6 MPa Glycerol concentration: 20–60 wt% Catalyst loading: 35 wt% Time: 2–12 h | 96 | 89 | G-P: 84.9 | [27] | |
| 3 | Cu/SiO2 | Temperature: 180–240 °C H2 pressure: 2–8 MPa Glycerol concentration: 28–50 wt% Catalyst loading: 18 wt% | - | 95 | G-P: 94.3 | [31] | |
| No. | Catalyst | Condition | X (%) | SP (%) | Kinetic Model | Ref. | |
|---|---|---|---|---|---|---|---|
| Ea (kJ·mol−1) | Expression | ||||||
| 1 | Cu0.45Zn0.15Mg5.4Al2O9 | Temperature: 210 °C H2 pressure: 3.5–5 MPa Glycerol concentration: 20 wt% Catalyst loading: 8 wt% Time: 12 h | 100 | 93.7 | G-P: 35.1 | [30] | |
| 2 | Cu/MgO | Temperature: 190–230 °C H2 pressure: 3–6 MPa Glycerol concentration: 20–60 wt% Catalyst loading: 35 wt% Time: 2–12 h | 96 | 89 | G-P: 84.9 | [27] | |
| 3 | Cu/ZnO-ZrO2-Cr2O3 | Temperature: 220–250 °C H2 pressure: 1–4 MPa Glycerol concentration: 60–100 wt% Catalyst loading: 3 wt% Time: 10 h | 100 | 97 | G-P: 131.9 | [28] | |
| 4 | Cu/ZnO-Al2O3 | Temperature: 220–240 °C H2 pressure: 3–5 MPa Glycerol concentration: 60–100 wt% Catalyst loading: 37 wt% | 81.5 | 93.4 | G-A: 86.6 | [29] | |
| A-P: 57.8 | |||||||
| 5 | Cu/ZnO-Al2O3 | Temperature: 200–270 °C N2 pressure: 3 MPa Glycerol concentration: 1–5 wt% Catalyst loading: 49 wt% Time: 0–1.25 h | 95.6 | 79.4 | G-A: 87 | [32] | |
| A-P: 68.4 | |||||||
| 6 | Cu/ZnO-Al2O3 | Temperature: 200–270 °C N2 pressure: 10 MPa Glycerol concentration: 1.3–5 wt% Catalyst loading: 49 wt% Time: 0–1.25 h | 95.6 | 79.4 | G-A: 87 | [33] | |
| A-P: 68.4 | |||||||
| 7 | Cu-based | Temperature: 190–240 °C H2 pressure: 6.5–8 MPa Glycerol concentration: 99.5 wt% Space times (W/FG0): 25–340 kg·s·mol−1 | 75 | 90 | G-A: 84 | [34] | |
| A-P: 59 | |||||||
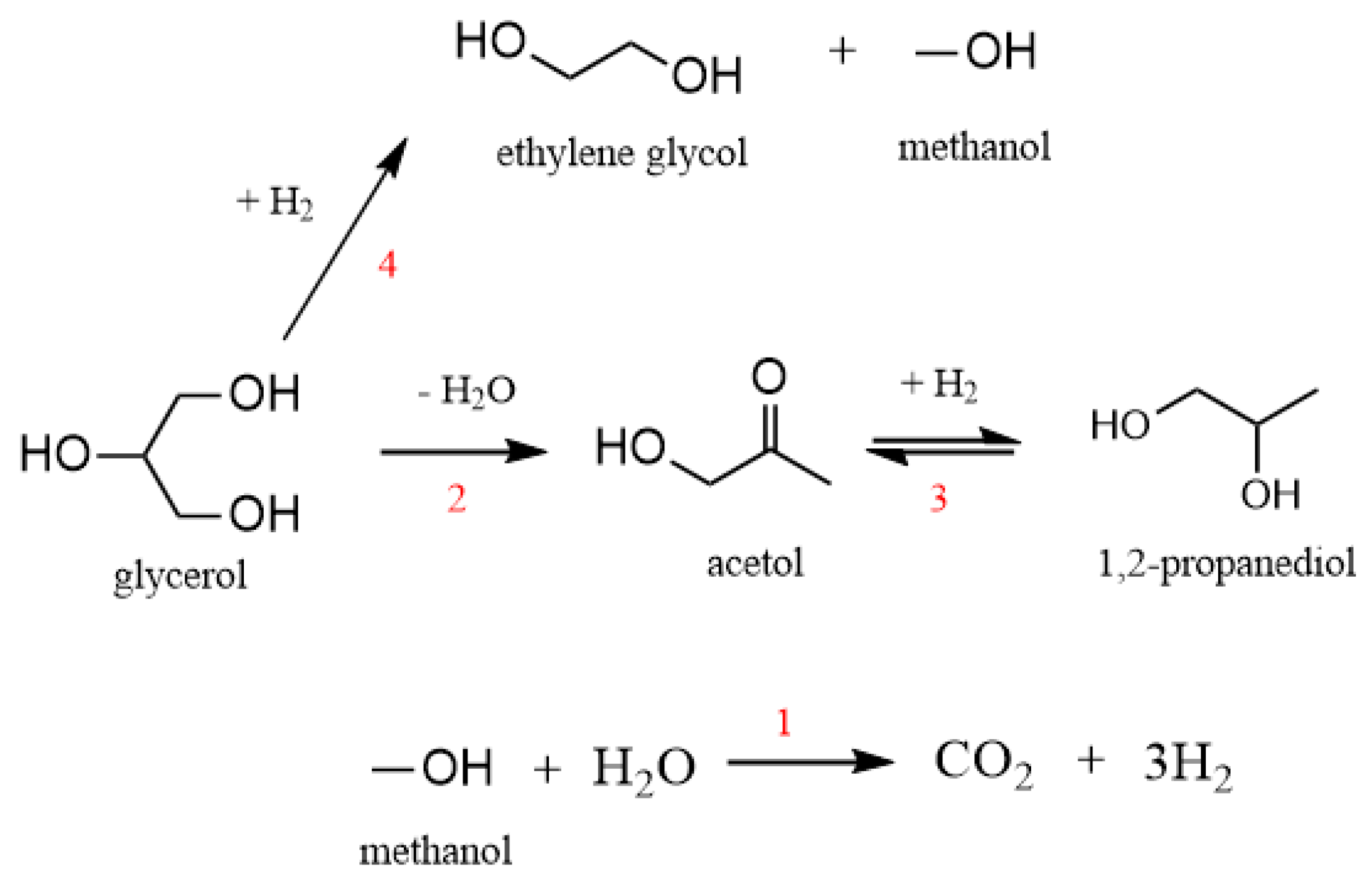
| Dual-Site Mechanism [30] | Single-Site Mechanism for P and EG Formation [27] | With Acetol as the Intermediate [34] | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st step. Adsorption on site | G + 2θ ↔ θ·G·θ H2 + 2θ ↔ 2H·θ | KG KH2 | 1st step. Adsorption on site | G + θ ↔ G·θ H + θ ↔ H·θ | KG KH | 1st step. Adsorption on site | G + θ ↔ G·θ H2 + 2θ ↔ 2H·θ | KG KH2 |
| 2nd step. Surface reaction | θ·G·θ + 2H·θ ↔ P·θ + 3θ + W | rG-P, kG-P | 2nd step. Surface reaction | G·θ + H·θ ↔ P·θ + W·θ G·θ + H·θ ↔ E·θ + θ | rG-P, kG-P rG-EG, kG-EG | 2nd step. Surface reaction | G·θ + θ ↔ A·θ + W·θ A·θ + H·θ ↔ AH·θ + θ AH·θ + H·θ ↔ P·θ + θ | rG-A, kG-A KAH rA-P, kA-P |
| 3rd step. Desorption | P·θ ↔ P + θ | KP | 3rd step. Desorption | P·θ ↔ p + θ E·θ ↔ E + θ | KP KEG | 3rd step. Desorption | A·θ ↔ A + θ P·θ ↔ p + θ W·θ ↔ W + θ | KA KP |
| Dual-Site Mechanism [29] | Single-Site Mechanism Considering Water Adsorption [28] | APR of Methanol for In-Situ H2 [32,33] | ||||||
| 1st step. Adsorption on site | G + θ ↔ G·θ H2 + 2$ ↔ 2H·$ | KG KH2 | 1st step. Adsorption on site | G + θ ↔ G·θ H2 + 2θ ↔ 2H·θ | KG KH2 | 1st step. H2 formation | W + θ ↔ W·θ W·θ +θ ↔ OH−·θ + H·θ CH3OH + θ ↔ CH3OH·θ CH3OH·θ + θ → CH3O·θ + H·θ CH3O·θ + θ → CH2O·θ + H·θ CO·θ + OH−·θ → H·θ + CO2 2H·θ ↔ H2 + 2θ | KW KOH KCH3OH rCH3OH, kCH3OH KH2 |
| 2nd step. Surface reaction | G·θ → A·θ + H2O 2H·$ + A·θ → P·θ + 2$ | rG-A, kG-A rA-P, kA-P | 2nd step. Surface reaction | G·θ + H·θ ↔ P·θ + W·θ | rG-P, kG-P | 2nd step. acetol formation | G + θ ↔ G·θ G·θ + θ → A·θ + W·θ A·θ ↔ A + θ | KG rG-A, kG-A KA |
| 3rd step. Desorption | A·θ ↔ A + θ P·θ ↔ P + θ | KA KP | 3rd step. Desorption | P·θ ↔ P + θ W·θ ↔ W + θ | KP KW | 3rd step. 1,2-propanediol formation | A·θ + H·θ ↔ AH·θ + θ AH·θ + H·θ → P·θ + θ P·θ↔ P + θ | KAH rA-P, kA-P KP |
| 4th step. ethylene glycol formation | G·θ + H·θ → G·H + θ GH·θ + H·θ → E·θ+ CH3OH·θ E·θ ↔ E + θ | rGH, kGH KEG | ||||||
| No. | Catalyst | Condition | X (%) | SP (%) | Kinetic Model | Kinetic Equation | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Ea (kJ·mol−1) | Expression | |||||||
| 1 | Co/ZnO | Temperature: 160–220 °C H2 pressure: 2–6 MPa Glycerol concentration: 10–40 wt% Catalyst loading: 20–70 wt%, Time: 8 h, pH: 10 | 70 | 80 | PL | G-P: 31.08 | [35] | |
| 2 | RuRe/C | Temperature: 200–230 °C H2 pressure: 2.4–9.6 MPa Glycerol concentration: 10 wt% Catalyst loading: 1 wt% Ru, 1 wt% Re Time: 1–6 h | 57.7 | 36.6 | PL | G-P: 54.2 | [41] | |
| 3 | Ru/C | H2 pressure: 7 MPa Glycerol concentration: 10–15 wt% Catalyst loading: 5 wt% pH: 11.7 (CaO) and 8.0 (CaCO3) | - | 19 | LH | - | [43] | |
| 4 | CuNi/Al2O3 | Temperature: 220 °C H2 pressure: 0.75 MPa Glycerol concentration: 20 wt% Catalyst loading: 20 wt% Contact times: W/FAo = 101–811 kgcat·h·kmol−1 | 100 | 89.5 | Eley–Rideal | G-A: 55.14 | [53] | |
| A-P: 50.87 | ||||||||
| 5 | PdReCo/C | Temperature: 180–203 °C H2 pressure: 3.3–13.3 MPa Glycerol concentration: 40 wt% Catalyst loading: 2.5 wt% Co, 0.5 wt% Pd, and 2.4 wt% Re | 96 | - | Trickle-bed model | G-P: 86 | [48] | |
| 6 | Pd/m-ZrO2 + ZnO | Temperature: 220 °C H2 pressure: 6.0 MPa Catalyst loading: 1 wt% Glycerol concentration:10 wt% Time: 4 h | 40 | 94.1 | - | - | [36] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, J.; Xing, Z.; Zhang, X.; Luo, C.; Yan, W.; Jin, X. Kinetic Modeling of Glycerol Hydrogenolysis: A Short Review. Catalysts 2023, 13, 23. https://doi.org/10.3390/catal13010023
Liu Y, Liu J, Xing Z, Zhang X, Luo C, Yan W, Jin X. Kinetic Modeling of Glycerol Hydrogenolysis: A Short Review. Catalysts. 2023; 13(1):23. https://doi.org/10.3390/catal13010023
Chicago/Turabian StyleLiu, Yangzi, Jiayu Liu, Zhihao Xing, Xueqian Zhang, Chen Luo, Wenjuan Yan, and Xin Jin. 2023. "Kinetic Modeling of Glycerol Hydrogenolysis: A Short Review" Catalysts 13, no. 1: 23. https://doi.org/10.3390/catal13010023
APA StyleLiu, Y., Liu, J., Xing, Z., Zhang, X., Luo, C., Yan, W., & Jin, X. (2023). Kinetic Modeling of Glycerol Hydrogenolysis: A Short Review. Catalysts, 13(1), 23. https://doi.org/10.3390/catal13010023







