Mesoporous Carbon-Based Materials: A Review of Synthesis, Modification, and Applications
Abstract
1. Introduction
2. Synthesis of Mesoporous Carbons
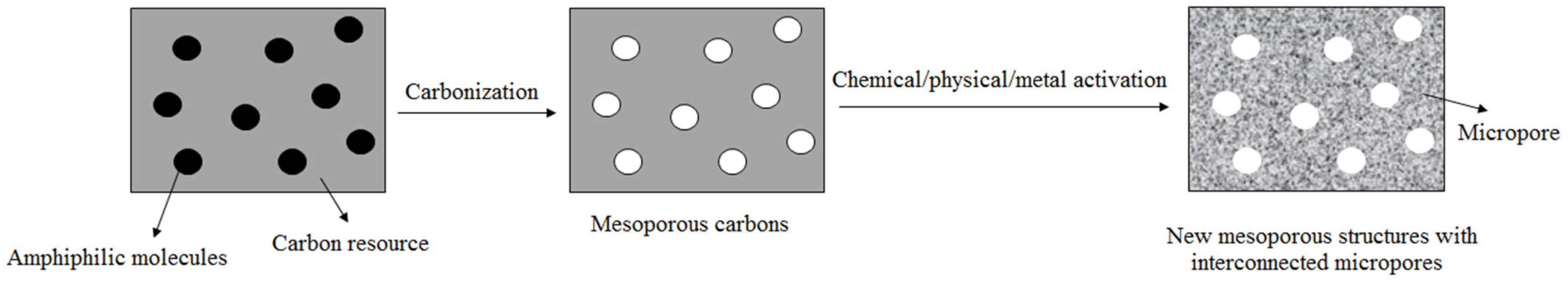
2.1. Activation Methods
2.2. Catalytic Activation Using Metal Ions
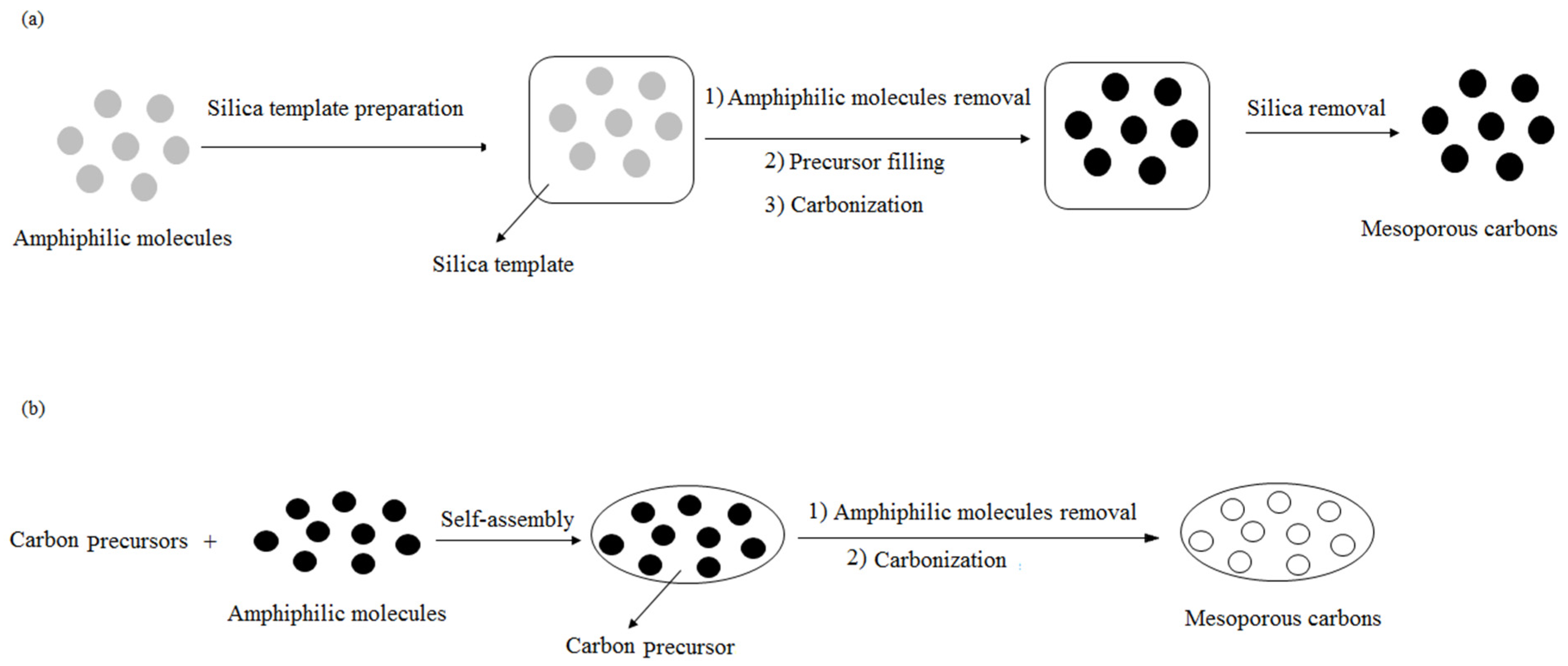
2.3. Template Methods
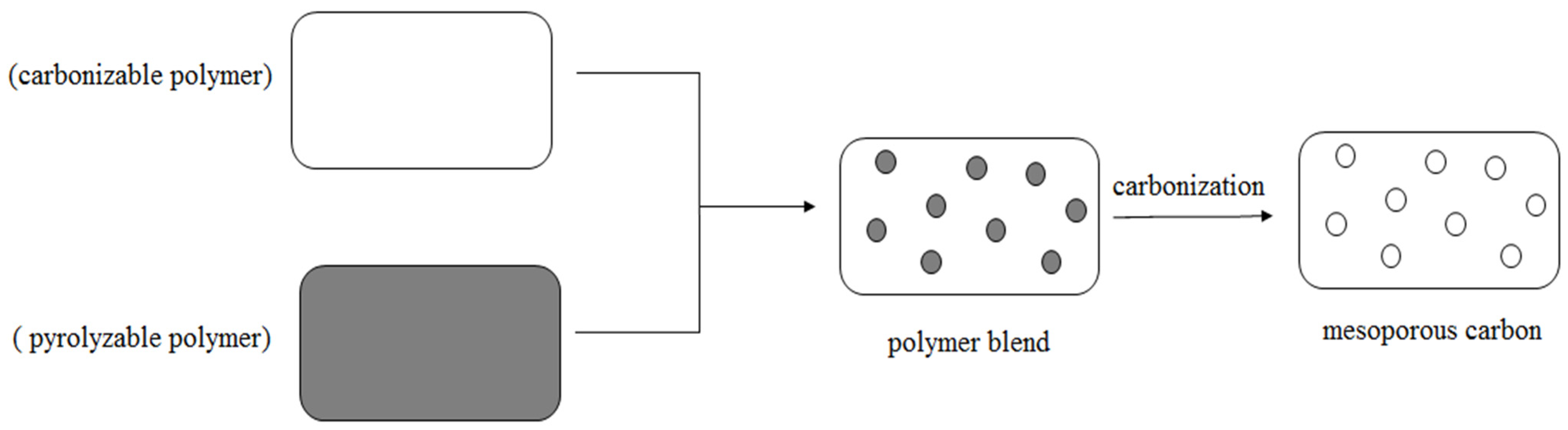
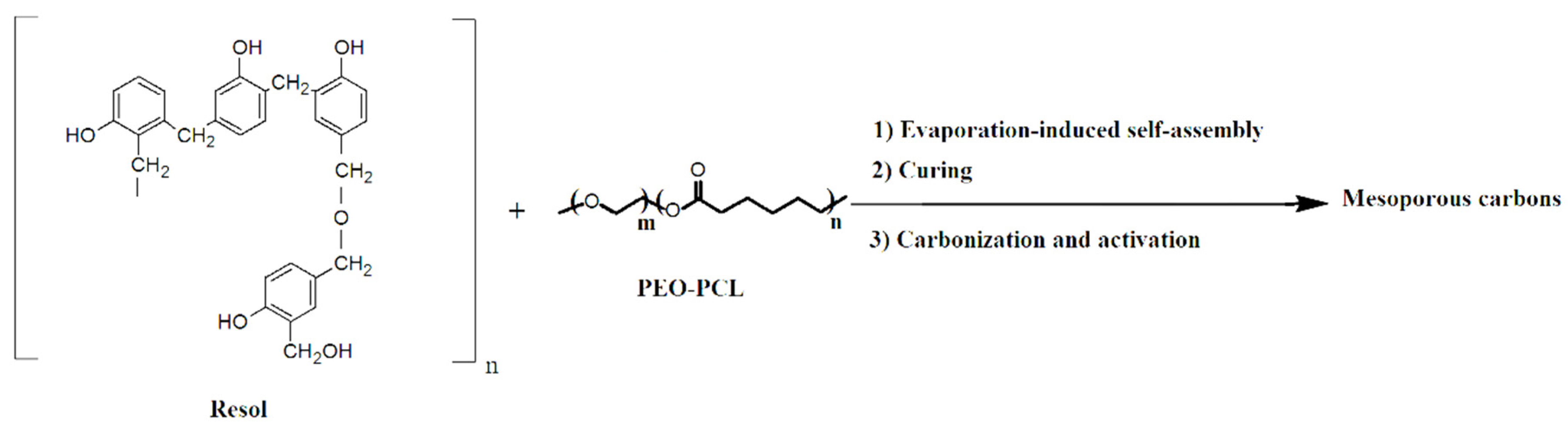
2.4. Carbonized Method
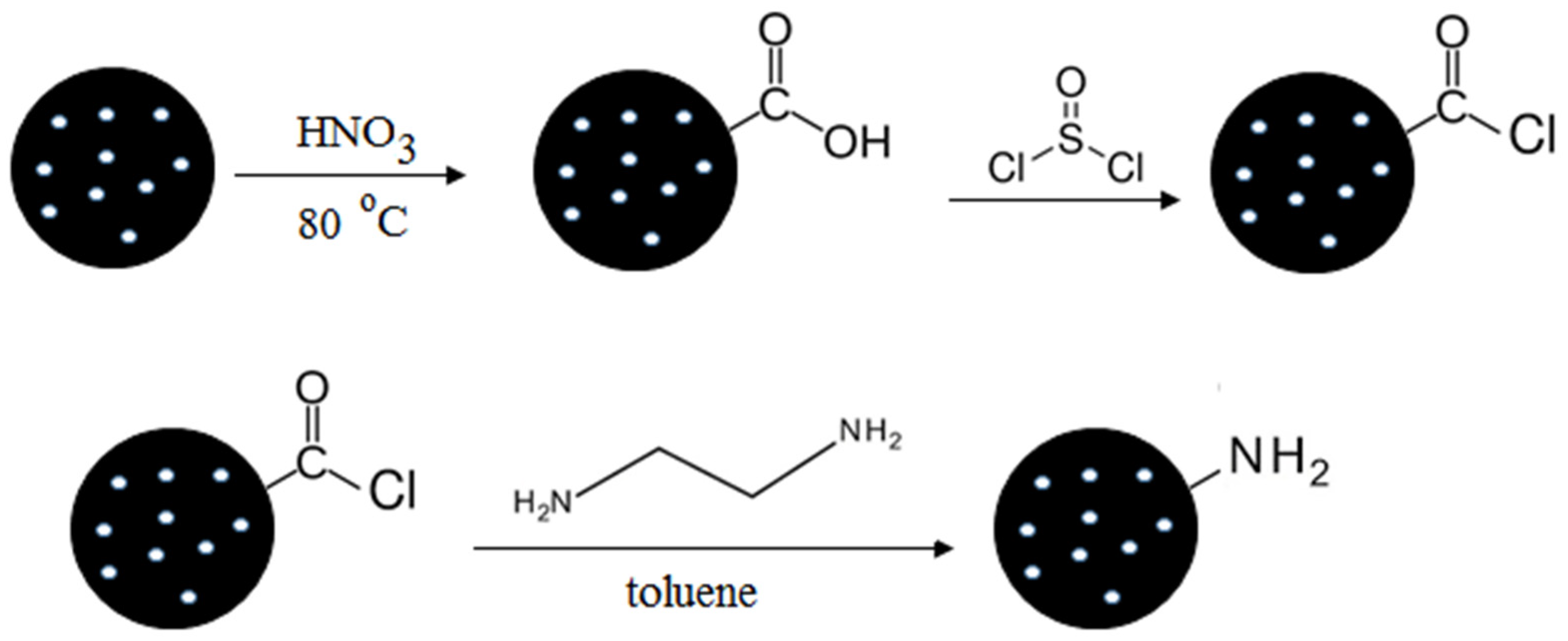
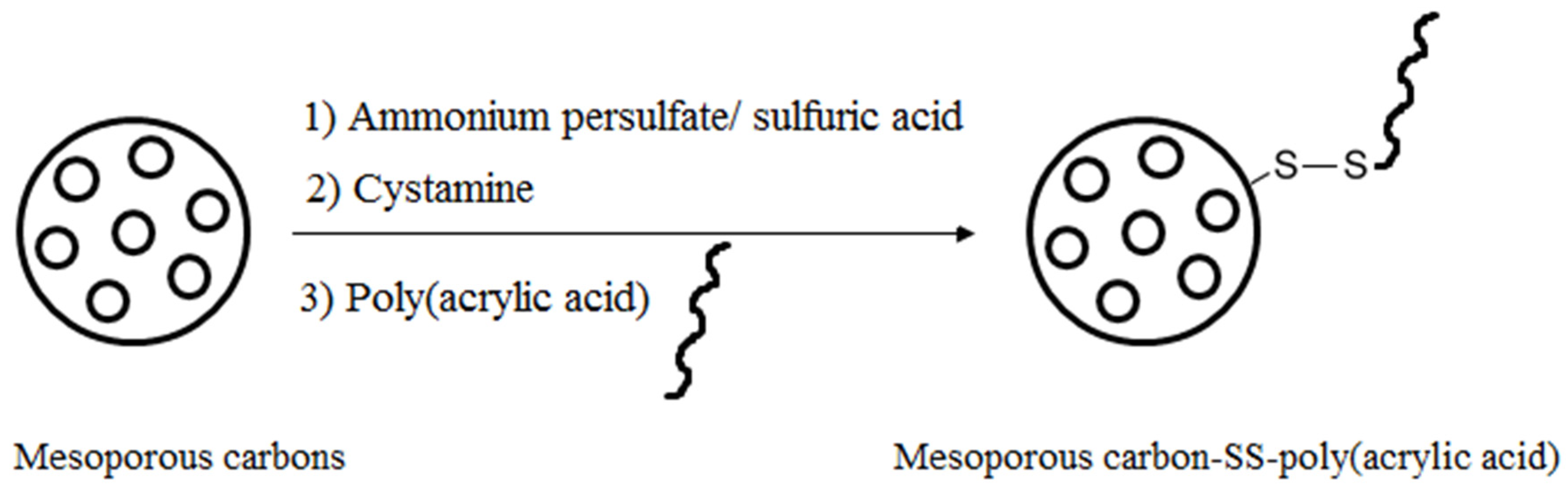
3. Modification of Mesoporous Carbon Materials
4. Applications of Mesoporous Carbon Materials
4.1. Adsorption
4.2. Supercapacitors
4.3. Lithium-Ion Batteries
4.4. Fuel Cells
4.5. Biosensors
4.6. Catalysis
4.7. Enzyme Immobilization
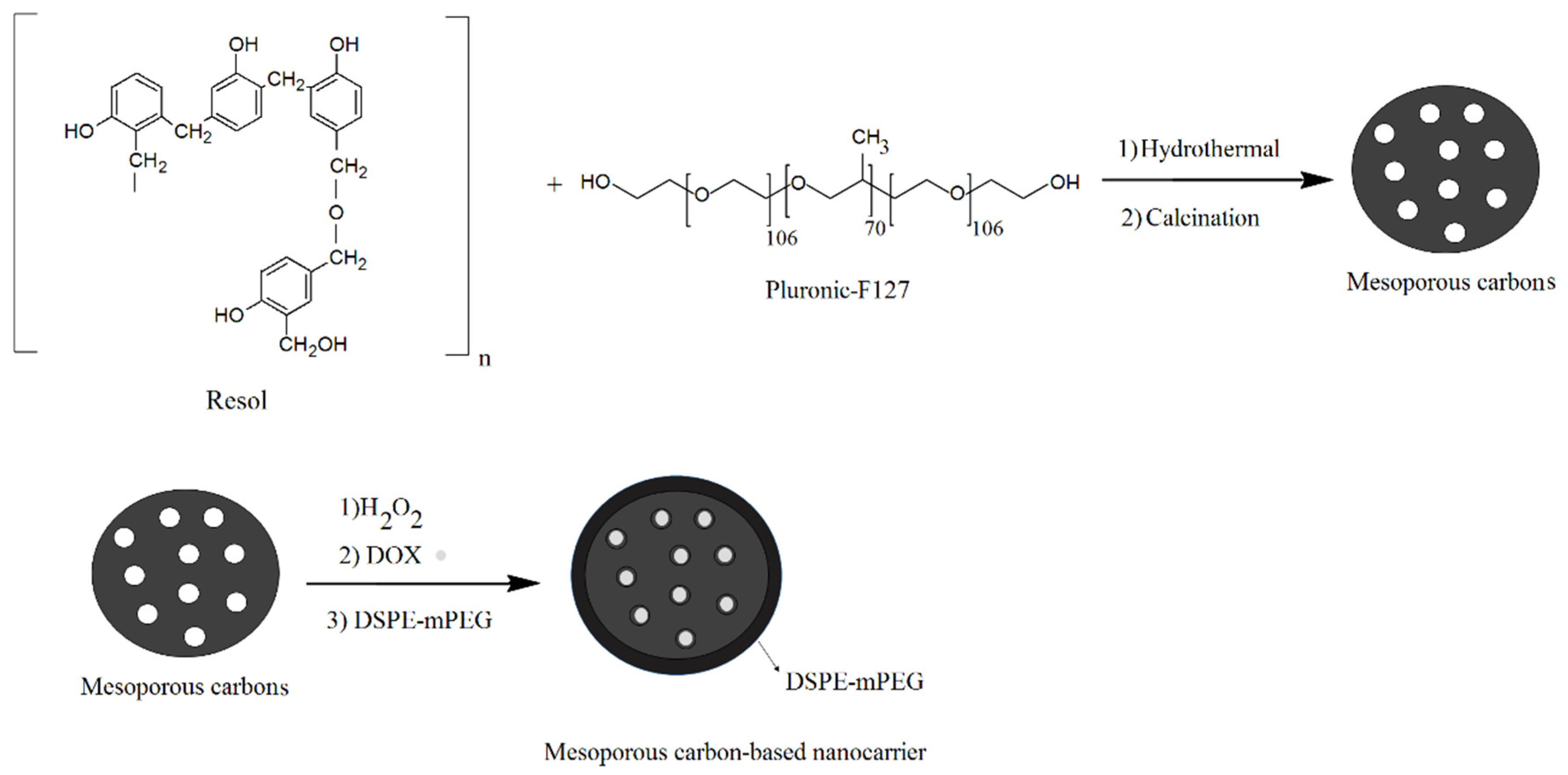
4.8. Drug Delivery
4.9. Other Applications
5. Conclusions and Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Notarianni, M.; Liu, J.; Vernon, K.; Motta, N. Synthesis and applications of carbon nanomaterials for energy generation and storage. Beilstein J. Nanotechnol. 2016, 7, 149–196. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Li, F.; Cheng, H.M. Carbon nanotubes and graphene for flexible electrochemical energy storage: From materials to devices. Adv. Mater. 2016, 28, 4306–4337. [Google Scholar] [CrossRef] [PubMed]
- Nishi, M.; Chen, S.Y.; Takagi, H. Energy efficient and intermittently variable ammonia synthesis over mesoporous carbon-supported Cs-Ru nanocatalysts. Catalysts 2019, 9, 406. [Google Scholar] [CrossRef]
- Wan, K.; Yu, Z.P.; Liang, Z.X. Polyaniline-derived ordered mesoporous carbon as an efficient electrocatalyst for oxygen reduction reaction. Catalysts 2015, 5, 1034–1045. [Google Scholar] [CrossRef]
- Banham, D.; Feng, F.; Fürstenhaupt, T.; Pei, K.; Ye, S.; Birss, V. Novel mesoporous carbon supports for PEMFC catalysts. Catalysts 2015, 5, 1046–1067. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.; Xie, L.; Zhao, D.; Kong, B. Interfacial assembly of functional mesoporous carbon-based materials into films for batteries and electrocatalysis. Adv. Mater. Interfaces 2022, 9, 2101998. [Google Scholar] [CrossRef]
- Dou, S.; Tian, Q.; Liu, T.; Xu, J.; Jing, L.; Zeng, C.; Yuan, Q.; Xu, Y.; Jia, Z.; Cai, Q.; et al. Stress-regulation design of mesoporous carbon spheres anodes with radial pore channels toward ultrastable potassium-ion batteries. Small Sci. 2022, 2, 2200045. [Google Scholar] [CrossRef]
- Berestok, T.; Diestel, C.; Ortlieb, N.; Glunz, S.W.; Fischer, A. A monolithic silicon-mesoporous carbon photosupercapacitor with high overall photoconversion efficiency. Adv. Mater. Technol. 2022, 7, 2200237. [Google Scholar] [CrossRef]
- Zhang, M.; He, L.; Shi, T.; Zha, R. Nanocasting and direct synthesis strategies for mesoporous carbons as supercapacitor electrodes. Chem. Mater. 2018, 30, 7391–7412. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Li, W. Preparation and application of carbon-based hollow structured nanomaterials. Charact. Appl. Nanomater. 2022, 5, 83–98. [Google Scholar] [CrossRef]
- Li, B.; Xiong, H.; Xiao, Y. Progress on synthesis and applications of porous carbon materials. Int. J. Electrochem. Sci. 2020, 15, 1363–1377. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhang, S.; Yang, C.; Zhang, J.Y.; Su, Y.; Zheng, G.; Fang, X. Controllable states and porosity of Cu-carbon for CO2 electroreduction to hydrocarbons. Small 2022, 18, 2202238. [Google Scholar] [CrossRef]
- Kamitaka, Y.; Takeshita, T.; Morimoto, Y. MgO-templated mesoporous carbon as a catalyst support for polymer electrolyte fuel cells. Catalysts 2018, 8, 230. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kurtyka, K.; Majumder, M.; Yang, X.; Ta, H.Q.; Bachmatiuk, A.; Liu, L.; Trzebicka, B.; Rummeli, M.H. Recent advances in boron-and nitrogen-doped carbon-based materials and their various applications. Adv. Mater. Interfaces 2022, 9, 2101964. [Google Scholar] [CrossRef]
- Wang, Q.; Su, J.; Chen, H.; Wang, D.; Tian, X.; Zhang, Y.; Feng, X.; Wang, S.; Li, J.; Jin, H. Highly conductive nitrogen-doped sp2/sp3 hybrid carbon as a conductor-free charge storage host. Adv. Funct. Mater. 2022, 32, 2209201. [Google Scholar] [CrossRef]
- Baskar, A.V.; Singh, G.; Ruban, A.M.; Davidraj, J.M.; Bahadur, R.; Sooriyakumar, P.; Kumar, P.; Karakoti, A.; Yi, J.; Vinu, A. Recent progress in synthesis and application of biomass-based hybrid electrodes for rechargeable batteries. Adv. Funct. Mater. 2022; 2208349, in press. [Google Scholar]
- Sathish, C.I.; Kothandam, G.; Selvarajan, P.; Lei, Z.; Lee, J.; Qu, J.; Al-Muhtaseb, A.A.H.; Yu, X.; Breese, M.B.; Zheng, R.; et al. Ordered mesoporous boron carbon nitrides with tunable mesopore nanoarchitectonics for energy storage and CO2 adsorption properties. Adv. Sci. 2022, 9, 2105603. [Google Scholar] [CrossRef]
- Yogeswari, B.; Khan, I.; Kumar, M.S.; Vijayanandam, N.; Devarani, P.A.; Anandaram, H.; Chaturvedi, A.; Misganaw, W. Role of carbon-based nanomaterials in enhancing the performance of energy storage devices: Design small and store big. J. Nanomater. 2022, 2022, 4949916. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Yu, R.; Wu, J. Unraveling the reaction mechanisms of electrode materials for sodium-ion and potassium-ion batteries by in situ transmission electron microscopy. Interdiscip. Mater. 2022, 1, 196–212. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Tsujimura, S.; Morishita, T. Templated mesoporous carbons: Synthesis and applications. Carbon 2016, 107, 448–473. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, L.; Hu, L.L.; Chang, Y.Q.; He, R.H.; Chen, M.L.; Shu, Y.; Wang, J.H. Mesoporous carbon nanoparticles capped with polyacrylic acid as drug carrier for bi-trigger continuous drug release. J. Mater. Chem. B 2016, 4, 5178–5184. [Google Scholar] [CrossRef]
- Liang, C.; Hong, K.; Guiochon, G.A.; Mays, J.W.; Dai, S. Synthesis of a large-scale highly ordered porous carbon film by self-assembly of block copolymers. Angew. Chem. Int. Ed. 2004, 43, 5785–5789. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Dai, S. Synthesis of mesoporous carbon materials via enhanced hydrogen-bonding interaction. J. Am. Chem. Soc. 2006, 128, 5316–5317. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Joo, S.H.; Ryoo, R.; Kruk, M.; Jaroniec, M.; Liu, Z.; Ohsuna, T.; Terasaki, O. Synthesis of new, nanoporous carbon with hexagonally ordered mesostructure. J. Am. Chem. Soc. 2000, 122, 10712–10713. [Google Scholar] [CrossRef]
- Jo, S.C.; Hong, J.W.; Choi, I.H.; Kim, M.J.; Kim, B.G.; Lee, Y.J.; Choi, H.Y.; Kim, D.; Kim, T.; Baeg, K.J.; et al. Multimodal capturing of polysulfides by phosphorus-doped carbon composites for flexible high-energy-density lithium–sulfur batteries. Small 2022, 18, 2200326. [Google Scholar] [CrossRef] [PubMed]
- Che, S.; Lund, K.; Tatsumi, T.; Iijima, S.; Joo, S.H.; Ryoo, R.; Terasaki, O. Direct observation of 3D mesoporous structure by scanning electron microscopy (SEM): SBA-15 silica and CMK-5 carbon. Angew. Chem. Int. Ed. 2003, 42, 2182–2185. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yoon, S.; Oh, S.M.; Shin, C.H.; Hyeon, T. Development of a new mesoporous carbon using an HMS aluminosilicate template. Adv. Mater. 2000, 12, 359–362. [Google Scholar] [CrossRef]
- Oh, S.; Kim, K. Synthesis of a new mesoporous carbon and its application to electrochemical double-layer capacitors. Chem. Commun. 1991, 21, 2177–2178. [Google Scholar]
- Chang, H.; Joo, S.H.; Pak, C. Synthesis and characterization of mesoporous carbon for fuel cell applications. J. Mater. Chem. 2007, 17, 3078–3088. [Google Scholar] [CrossRef]
- Chung, L.H.; Lin, Z.Q.; He, J. Metal-free carbon-based nanomaterials: Fuel cell applications as electrocatalysts. In Functional Nanomaterials: Synthesis, Properties, and Applications; Wiley Online Library: New York, NY, USA, 2022; pp. 73–139. [Google Scholar]
- Liu, M.; Li, W.; Ruan, S.; Fei, Y. N-doped hierarchical mesoporous carbon from mesophase pitch and polypyrrole for supercapacitors. Energy Fuels 2020, 34, 5044–5051. [Google Scholar] [CrossRef]
- Su, X.L.; Li, S.H.; Jiang, S.; Peng, Z.K.; Guan, X.X.; Zheng, X.C. Superior capacitive behavior of porous activated carbon tubes derived from biomass waste-cotonier strobili fibers. Adv. Powder Technol. 2018, 29, 2097–2107. [Google Scholar] [CrossRef]
- Ortíz-de-Lira, A.; Reynel-Ávila, H.E.; Díaz-Muñoz, L.L.; Mendoza-Castillo, D.I.; Aminabhavi, T.M.; Badawi, M.; Bonilla-Petriciolet, A. Sustainable downstream separation of itaconic acid using carbon-based adsorbents. Adsorpt. Sci. Technol. 2022, 2022, 7333005. [Google Scholar] [CrossRef]
- Xue, H.; Wang, T.; Gong, H.; Guo, H.; Fan, X.; Song, L.; Xia, W.; Feng, Y.; He, J. Co3O4 nanoparticle-decorated n-doped mesoporous carbon nanofibers as an efficient catalyst for oxygen reduction reaction. Catalysts 2017, 7, 189. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.; Chung, D.Y.; Yoo, J.M.; Lee, H.S.; Kim, M.J.; Mun, B.S.; Kwon, S.G.; Sung, Y.E.; Hyeon, T. Design principle of Fe–N–C electrocatalysts: How to optimize multimodal porous structures? J. Am. Chem. Soc. 2019, 141, 2035–2045. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Kaneti, Y.V.; Hou, D.; Yamauchi, Y.; Mai, Y. Self-assembly of block copolymers towards mesoporous materials for energy storage and conversion systems. Chem. Soc. Rev. 2020, 49, 4681–4736. [Google Scholar] [CrossRef]
- Panja, T.; Bhattacharjya, D.; Yu, J.S. Nitrogen and phosphorus co-doped cubic ordered mesoporous carbon as a supercapacitor electrode material with extraordinary cyclic stability. J. Mater. Chem. A 2015, 3, 18001–18009. [Google Scholar] [CrossRef]
- Aram, E.; Mehdipour-Ataei, S. Preparation of thermally stable, low dielectric constant, pyridine-based polyimide and related nanofoams. J. Appl. Polym. Sci. 2013, 128, 4387–4394. [Google Scholar] [CrossRef]
- Mehdipour-Ataei, S.; Aram, E. New polyimide and nanoporous structures with low dielectric constant. Adv. Polym. Technol. 2014, 33, 21407–21418. [Google Scholar] [CrossRef]
- Aram, E.; Mehdipour-Ataei, S. A review on the micro-and nanoporous polymeric foams: Preparation and properties. Int. J. Polym. Mater. 2016, 65, 358–375. [Google Scholar] [CrossRef]
- Mui, E.L.; Ko, D.C.; McKay, G. Production of active carbons from waste tyres—A review. Carbon 2004, 42, 2789–2805. [Google Scholar] [CrossRef]
- Yang, T.; Lua, A.C. Characteristics of activated carbons prepared from pistachio-nut shells by physical activation. J. Colloid Interface Sci. 2003, 267, 408–417. [Google Scholar] [CrossRef]
- Bergna, D.; Hu, T.; Prokkola, H.; Romar, H.; Lassi, U. Effect of some process parameters on the main properties of activated carbon produced from peat in a lab-scale process. Waste Biomass Valoriz. 2020, 11, 2837–2848. [Google Scholar] [CrossRef]
- Castro-Gutiérrez, J.; Canevesi, R.L.S.; Emo, M.; Izquierdo, M.T.; Celzard, A.; Fierro, V. CO2 outperforms KOH as an activator for high-rate supercapacitors in aqueous electrolyte. Renew. Sustain. Energy Rev. 2022, 167, 112716. [Google Scholar] [CrossRef]
- Guo, Y.; Rockstraw, D.A. Physical and chemical properties of carbons synthesized from xylan, cellulose, and kraft lignin by H3PO4 activation. Carbon 2006, 44, 1464–1475. [Google Scholar] [CrossRef]
- Xing, Z.; Qi, Y.; Tian, Z.; Xu, J.; Yuan, Y.; Bommier, C.; Lu, J.; Tong, W.; Jiang, D.; Ji, X. Identify the removable substructure in carbon activation. Chem. Mater. 2017, 29, 7288–7295. [Google Scholar] [CrossRef]
- Smith, M.R.; Bittner, E.W.; Shi, W.; Johnson, J.K.; Bockrath, B.C. Chemical activation of single-walled carbon nanotubes for hydrogen adsorption. J. Phys. Chem. B 2003, 107, 3752–3760. [Google Scholar] [CrossRef]
- Kyotani, T. Control of pore structure in carbon. Carbon 2000, 38, 269–286. [Google Scholar] [CrossRef]
- Javed, H.; Pani, S.; Antony, J.; Sakthivel, M.; Drillet, J.F. Synthesis of mesoporous carbon spheres via a soft-template route for catalyst supports in PEMFC cathodes. Soft Matter 2021, 17, 7743–7754. [Google Scholar] [CrossRef]
- Xin, W.; Song, Y. Mesoporous carbons: Recent advances in synthesis and typical applications. RSC Adv. 2015, 5, 83239–83285. [Google Scholar] [CrossRef]
- Liang, C.; Li, Z.; Dai, S. Mesoporous carbon materials: Synthesis and modification. Angew. Chem. Int. Ed. Engl. 2008, 47, 3696–3717. [Google Scholar] [CrossRef]
- Yu, J.; Guo, M.; Muhammad, F.; Wang, A.; Yu, G.; Ma, H.; Zhu, G. Simple fabrication of an ordered nitrogen-doped mesoporous carbon with resorcinol–melamine–formaldehyde resin. Microporous Mesoporous Mater. 2014, 190, 117–127. [Google Scholar] [CrossRef]
- Xia, K.; Gao, Q.; Wu, C.; Song, S.; Ruan, M. Activation, characterization and hydrogen storage properties of the mesoporous carbon CMK-3. Carbon 2007, 45, 1989–1996. [Google Scholar] [CrossRef]
- Li, Z.; Del Cul, G.D.; Yan, W.; Liang, C.; Dai, S. Fluorinated carbon with ordered mesoporous structure. J. Am. Chem. Soc. 2004, 126, 12782–12783. [Google Scholar] [CrossRef]
- Li, Z.; Yan, W.; Dai, S. Surface functionalization of ordered mesoporous carbons a comparative study. Langmuir 2005, 21, 11999–12006. [Google Scholar] [CrossRef]
- Enterría, M.; Figueiredo, J.L. Nanostructured mesoporous carbons: Tuning texture and surface chemistry. Carbon 2016, 108, 79–102. [Google Scholar] [CrossRef]
- Wang, L.; Tang, Z.; Liu, X.; Niu, W.; Zhou, K.; Yang, H.; Zhou, W.; Li, L.; Chen, S. Ordered mesoporous carbons-supported gold nanoparticles as highly efficient electrocatalysts for oxygen reduction reaction. RSC Adv. 2015, 5, 103421–103427. [Google Scholar] [CrossRef]
- Stein, A.; Wang, Z.; Fierke, M.A. Functionalization of porous carbon materials with designed pore architecture. Adv. Mater. 2009, 21, 265–293. [Google Scholar] [CrossRef]
- Wang, J.; Han, W.Q. A review of heteroatom doped materials for advanced lithium–sulfur batteries. Adv. Funct. Mater. 2021, 32, 2107166. [Google Scholar] [CrossRef]
- Cui, F.; Deng, Q.; Zhao, H.; Jiang, Y.; Li, J. Ionic liquid promoted synthesis of nitrogen, phosphorus, and fluorine triple-doped mesoporous carbon as metal-free electrocatalyst for oxygen reduction reaction. Ionics 2022, 26, 4609–4619. [Google Scholar] [CrossRef]
- Yaumi, A.L.; Bakar, M.A.; Hameed, B.H. Reusable nitrogen-doped mesoporous carbon adsorbent for carbon dioxide adsorption in fixed-bed. Energy 2017, 138, 776–784. [Google Scholar] [CrossRef]
- Azam, K.; Raza, R.; Shezad, N.; Shabir, M.; Yang, W.; Ahmad, N.; Shafiq, I.; Akhter, P.; Razzaq, A.; Hussain, M. Development of recoverable magnetic mesoporous carbon adsorbent for removal of methyl blue and methyl orange from wastewater. J. Environ. Chem. Eng. 2020, 8, 104220. [Google Scholar] [CrossRef]
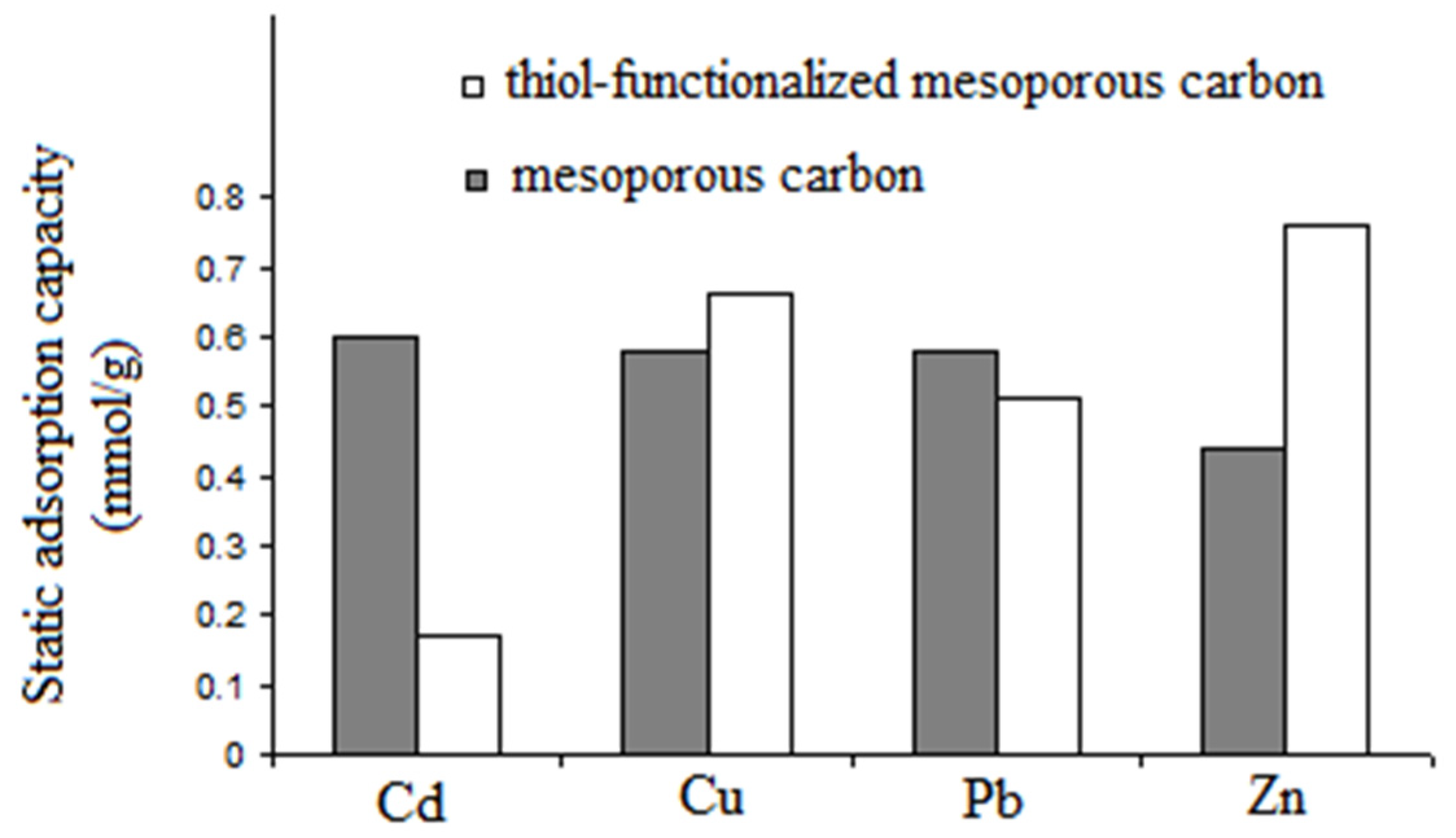
- Michalak-Zwierz, K.; Gdula, K.; Tyszczuk-Rotko, K.; Zawadzki, W.; Dąbrowski, A.; Barczak, M. Thiol-functionalized mesoporous carbons as adsorbents of heavy-metal ions. Adsorpt. Sci. Technol. 2015, 33, 663–668. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, P.; Li, M.; Shan, Z.; Wang, J.; Deng, Q.; Zeng, Z.; Deng, S. Facile preparation of biomass-derived mesoporous carbons for highly efficient and selective SO2 capture. Ind. Eng. Chem. Res. 2019, 58, 14929–14937. [Google Scholar] [CrossRef]
- Zhao, P.; Yin, Y.; Cheng, W.; Xu, X.; Yang, D.; Yuan, W. Development of facile synthesized mesoporous carbon composite adsorbent for efficient CO2 capture. J. CO2 Util. 2021, 50, 101612. [Google Scholar] [CrossRef]
- Nejad, N.F.; Shams, E.; Amini, M.K. Synthesis of magnetic ordered mesoporous carbon (Fe-OMC) adsorbent and its evaluation for fuel desulfurization. J. Magn. Magn. Mater. 2015, 390, 1–7. [Google Scholar] [CrossRef]
- Sriram, G.; Supriya, S.; Kurkuri, M.; Hegde, G. Efficient CO2 adsorption using mesoporous carbons from biowastes. Mater. Res. Express. 2019, 7, 015605. [Google Scholar] [CrossRef]
- Anbia, M.; Karami, S. Desulfurization of gasoline using novel mesoporous carbon adsorbents. J. Nanostruct. Chem. 2015, 5, 131–137. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, D. Mesoporous carbon originated from non-permanent porous MOFs for gas storage and CO2/CH4 separation. Sci. Rep. 2014, 4, 5711. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Al-Maswari, B.M.; Alqadami, A.A.; Alshehri, S.M. Nickel ferrite bearing nitrogen-doped mesoporous carbon as efficient adsorbent for the removal of highly toxic metal ion from aqueous medium. J. Chem. Eng. 2017, 330, 1351–1360. [Google Scholar] [CrossRef]
- Zu, L.; Zhang, W.; Qu, L.; Liu, L.; Li, W.; Yu, A.; Zhao, D. Mesoporous materials for electrochemical energy storage and conversion. Adv. Energy Mater. 2020, 10, 2002152. [Google Scholar] [CrossRef]
- Serrano, J.M.; Spiering, G.A.; Xu, Z.; Croft, Z.; Guo, D.; Cao, K.; Moore, R.B.; Liu, G. Humidity-Controlled Preparation of Flexible Porous Carbon Fibers from Block Copolymers. ACS Appl. Polym. Mater. 2022, 4, 4980–4992. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Wang, S.; Li, S.M.; Shan, S.L.; Wang, H.; Lu, J.X. Synthesis of Ag nanoparticles/ordered mesoporous carbon as a highly efficient catalyst for the electroreduction of benzyl bromide. RSC Adv. 2020, 10, 756–762. [Google Scholar] [CrossRef]
- Li, M.; Ding, J.; Xue, J. Mesoporous carbon decorated graphene as an efficient electrode material for supercapacitors. J. Mater. Chem. 2013, 1, 7469–7476. [Google Scholar] [CrossRef]
- Xiang, D.; Yin, L.; Wang, C.; Zhang, L. High electrochemical performance of RuO2–Fe2O3 nanoparticles embedded ordered mesoporous carbon as a supercapacitor electrode material. Energy 2016, 106, 103–111. [Google Scholar] [CrossRef]
- Lin, X.X.; Tan, B.; Peng, L.; Wu, Z.F.; Xie, Z.L. Ionothermal synthesis of microporous and mesoporous carbon aerogels from fructose as electrode materials for supercapacitors. J. Mater. Chem. 2016, 4, 4497–4505. [Google Scholar] [CrossRef]
- Khan, I.A.; Badshah, A.; Khan, I.; Zhao, D.; Nadeem, M.A. Soft-template carbonization approach of MOF-5 to mesoporous carbon nanospheres as excellent electrode materials for supercapacitor. Microporous Mesoporous Mat. 2017, 253, 169–176. [Google Scholar] [CrossRef]
- Lufrano, F.; Staiti, P. Mesoporous carbon materials as electrodes for electrochemical supercapacitors. Int. J. Electrochem. Sci. 2010, 5, 903–916. [Google Scholar]
- Tian, X.; Zhu, S.; Peng, J.; Zuo, Y.; Wang, G.; Guo, X.; Zhao, N.; Ma, Y.; Ma, L. Synthesis of micro-and meso-porous carbon derived from cellulose as an electrode material for supercapacitors. Electrochim. Acta 2017, 241, 170–178. [Google Scholar] [CrossRef]
- Li, J.G.; Ho, Y.F.; Ahmed, M.M.; Liang, H.C.; Kuo, S.W. Mesoporous carbons templated by PEO-PCL block copolymers as electrode materials for supercapacitors. Chem.-A Eur. J. 2019, 25, 10456–10463. [Google Scholar] [CrossRef]
- Dong, W.; Wang, Z.; Zhang, Q.; Ravi, M.; Yu, M.; Tan, Y.; Liu, Y.; Kong, L.; Kang, L.; Ran, F. Polymer/block copolymer blending system as the compatible precursor system for fabrication of mesoporous carbon nanofibers for supercapacitors. J. Power Sources 2019, 419, 137–147. [Google Scholar] [CrossRef]
- Mohammadi, N.; Pourreza, K.; Adeh, N.B.; Omidvar, M. Defective mesoporous carbon/MnO2 nanocomposite as an advanced electrode material for supercapacitor application. J. Alloys Compd. 2021, 883, 160874. [Google Scholar] [CrossRef]
- Niu, S.Z.; Lu, W.; Yang, Q.H.; Kang, F.Y. A one-step hard-templating method for the preparation of a hierarchical microporous-mesoporous carbon for lithium-sulfur batteries. New Carbon Mater. 2017, 32, 289–296. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Chen, Z.; Liu, H.K.; Guo, Z. Large-scale synthesis of ordered mesoporous carbon fiber and its application as cathode material for lithium—Sulfur batteries. Carbon 2015, 81, 782–787. [Google Scholar] [CrossRef]
- Jin, R.; Wang, G.; Gao, S.; Kang, H.; Chen, S. NiS1.03@ NiMoS4 nanocrystals encapsulated into the mesoporous carbon microspheres for high performance lithium ion batteries. J. Electroanal. Chem. 2021, 895, 115502. [Google Scholar] [CrossRef]
- Zhu, B.; Guo, G.; Wu, G.; Zhang, Y.; Dong, A.; Hu, J.; Yang, D. Preparation of dual layers N-doped carbon@ mesoporous carbon@ Fe3O4 nanoparticle superlattice and its application in lithium-ion battery. J. Alloys Compd. 2019, 775, 776–783. [Google Scholar] [CrossRef]
- Shi, Z.; Jin, G.; Wang, J.; Zhang, J. Free-standing, welded mesoporous carbon nanofibers as anode for high-rate performance Li-ion batteries. J. Electroanal. Chem. 2017, 795, 26–31. [Google Scholar] [CrossRef]
- Li, P.; Liu, Y.; Liu, J.; Li, Z.; Wu, G.; Wu, M. Facile synthesis of ZnO/mesoporous carbon nanocomposites as high-performance anode for lithium-ion battery. Chem. Eng. J. 2015, 271, 173–179. [Google Scholar] [CrossRef]
- Raza, A.; Ghani, F.; Lim, J.; Nah, I.W.; Kim, H.S. Eco-friendly prepared mesoporous carbon encapsulated SnO2 nanoparticles for high-reversible lithium-ion battery anodes. Microporous Mesoporous Mat. 2021, 314, 110853. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, T.; Cong, Y.; Zhang, Y.; Li, X.; Dong, Z.; Li, Y.; Yuan, G.; Zhang, J.; Cui, Z. Improved rate performance and cycling stability of graphitized mesoporous carbon as anode materials for lithium-ion batteries. J. Mater. Sci. 2019, 54, 648–658. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, X.; Chen, J.; Wang, S. Foamed mesoporous carbon/silicon composite nanofiber anode for lithium ion batteries. J. Power Sources 2015, 281, 285–292. [Google Scholar] [CrossRef]
- Oroujzadeh, M.; Mehdipour-Ataei, S. Evaluation of properties and performance of poly (ether sulfone ketone) membranes in proton exchange membrane fuel cells. Polym. Adv. Technol. 2022, 33, 2896–2907. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent advances in functionalized micro and mesoporous carbon materials: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar] [CrossRef]
- Joo, S.H.; Pak, C.; You, D.J.; Lee, S.A.; Lee, H.I.; Kim, J.M.; Chang, H.; Seung, D. Ordered mesoporous carbons (OMC) as supports of electrocatalysts for direct methanol fuel cells (DMFC): Effect of carbon precursors of OMC on DMFC performances. Electrochim. Acta 2006, 52, 1618–1626. [Google Scholar] [CrossRef]
- Shu, C.; Tan, Q.; Deng, C.; Du, W.; Gan, Z.; Liu, Y.; Fan, C.; Jin, H.; Tang, W.; Yang, X.D.; et al. Hierarchically mesoporous carbon spheres coated with a single atomic Fe–N–C layer for balancing activity and mass transfer in fuel cells. Carbon Energy 2022, 4, 1–11. [Google Scholar] [CrossRef]
- Lilloja, J.; Kibena-Põldsepp, E.; Sarapuu, A.; Käärik, M.; Kozlova, J.; Paiste, P.; Kikas, A.; Treshchalov, A.; Leis, J.; Tamm, A.; et al. Transition metal and nitrogen-doped mesoporous carbons as cathode catalysts for anion-exchange membrane fuel cells. Appl. Catal. B 2022, 306, 121113. [Google Scholar] [CrossRef]
- Duraisamy, V.; Venkateshwaran, S.; Thangamuthu, R.; Kumar, S.M.S. Hard template derived N, S dual heteroatom doped ordered mesoporous carbon as an efficient electrocatalyst for oxygen reduction reaction. Int. J. Hydrog. Energy 2022, 47, 40327–40339. [Google Scholar] [CrossRef]
- Yarlagadda, V.; Ramaswamy, N.; Kukreja, R.S.; Kumaraguru, S. Ordered mesoporous carbon supported fuel cell cathode catalyst for improved oxygen transport. J. Power Sources 2022, 532, 231349. [Google Scholar] [CrossRef]
- Viva, F.A.; Bruno, M.M.; Franceschini, E.A.; Thomas, Y.R.; Sanchez, G.R.; Solorza-Feria, O.; Corti, H.R. Mesoporous carbon as Pt support for PEM fuel cell. Int. J. Hydrog. Energy 2014, 39, 8821–8826. [Google Scholar] [CrossRef]
- Kuppan, B.; Selvam, P. Platinum-supported mesoporous carbon (Pt/CMK-3) as anodic catalyst for direct methanol fuel cell applications: The effect of preparation and deposition methods. Prog. Nat. Sci. Mater. Int. 2012, 22, 616–623. [Google Scholar] [CrossRef][Green Version]
- Singh, D.K.; Ganesan, V.; Yadav, D.K.; Yadav, M. Metal (Mn, Fe, Co, Ni, Cu, and Zn) phthalocyanine-immobilized mesoporous carbon nitride materials as durable electrode modifiers for the oxygen reduction reaction. Langmuir 2020, 36, 12202–12212. [Google Scholar] [CrossRef]
- Chen, Q.; Pu, W.; Hou, H.; Hu, J.; Liu, B.; Li, J.; Cheng, K.; Huang, L.; Yuan, X.; Yang, C.; et al. Activated microporous-mesoporous carbon derived from chestnut shell as a sustainable anode material for high performance microbial fuel cells. Bioresour. Technol. 2018, 249, 567–573. [Google Scholar] [CrossRef]
- Xie, M.; Chu, T.; Wang, X.; Li, B.; Yang, D.; Ming, P.; Zhang, C. Effect of mesoporous carbon on oxygen reduction reaction activity as cathode catalyst support for proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2022, 47, 28074–28085. [Google Scholar] [CrossRef]
- Walcarius, A. Recent trends on electrochemical sensors based on ordered mesoporous carbon. Sensors 2017, 17, 1863. [Google Scholar] [CrossRef] [PubMed]
- Bo, X.; Bai, J.; Yang, L.; Guo, L. The nanocomposite of PtPd nanoparticles/onion-like mesoporous carbon vesicle for nonenzymatic amperometric sensing of glucose. Sens. Actuators B Chem. 2011, 157, 662–668. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, T.; Yan, X.; Gu, Y.; Liu, H.; Xu, Z.; Xu, H.; Li, X.; Zhang, Z.; Yang, M. Facile synthesis of 3D N-doped porous carbon nanosheets as highly active electrocatalysts toward the reduction of hydrogen peroxide. Nanoscale 2018, 10, 14923–14930. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ding, J.; Guo, L.P.; Shang, Q.K. Electrochemical behavior of L-cysteine and its detection at ordered mesoporous carbon-modified glassy carbon electrode. Anal. Chem. 2007, 79, 5328–5335. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, F.; Piao, J.; Chen, Y. Fabrication and application of a novel electrochemical biosensor based on a mesoporous carbon sphere@UiO-66-NH2/Lac complex enzyme for tetracycline detection. Analyst 2021, 146, 2825–2833. [Google Scholar] [CrossRef]
- Wang, L.; Shi, X.H.; Zhang, Y.F.; Liu, A.A.; Liu, S.L.; Wang, Z.G.; Pang, D.W. CdZnSeS quantum dots condensed with ordered mesoporous carbon for high-sensitive electrochemiluminescence detection of hydrogen peroxide in live cells. Electrochim. Acta 2020, 362, 137107. [Google Scholar] [CrossRef]
- Jia, N.; Wang, Z.; Yang, G.; Shen, H.; Zhu, L. Electrochemical properties of ordered mesoporous carbon and its electroanalytical application for selective determination of dopamine. Electrochem. Commun. 2007, 9, 233–238. [Google Scholar] [CrossRef]
- Song, S.; Gao, Q.; Xia, K.; Gao, L. Selective determination of dopamine in the presence of ascorbic acid at porous-carbon-modified glassy carbon electrodes. Electroanalysis 2008, 20, 1159–1166. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Balamurugan, T.S.T.; Chen, S.M.; Chen, T.W.; Lin, P.H. A novel, efficient electrochemical sensor for the detection of isoniazid based on the B/N doped mesoporous carbon modified electrode. Sens. Actuators B Chem. 2019, 283, 613–620. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Shang, J.K.; Ma, D.; Li, Y.X. Preparation of mesoporous carbon nitride materials using urea and formaldehyde as precursors and catalytic application as solid bases. Appl. Catal. A Gen. 2017, 538, 221–229. [Google Scholar] [CrossRef]
- Matos, I.; Bernardo, M.; Fonseca, I. Porous carbon: A versatile material for catalysis. Catal. Today 2017, 285, 194–203. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, N.; Yin, F.; Yang, T.; Wu, P.; Dang, Z.; Liu, M.; Wei, X. Biomass-derived heteroatoms-doped mesoporous carbon for efficient oxygen reduction in microbial fuel cells. Biosens. Bioelectron. 2017, 98, 350–356. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, B.; Douthwaite, M.; Liu, Q.; Zhang, C.; Wu, Q.; Shi, R.; Wu, P.; Zhao, F.; Hutchings, G. Macroporous–mesoporous carbon supported Ni catalysts for the conversion of cellulose to polyols. Green Chem. 2018, 20, 3634–3642. [Google Scholar] [CrossRef]
- Nabae, Y.; Nagata, S.; Ohnishi, K.; Liu, Y.; Sheng, L.; Wang, X.; Hayakawa, T. Block copolymer templated carbonization of nitrogen containing polymer to create fine and mesoporous carbon for oxygen reduction catalyst. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 464–470. [Google Scholar] [CrossRef]
- Morales-Acosta, D.; Rodríguez-Varela, F.J.; Benavides, R. Template-free synthesis of ordered mesoporous carbon: Application as support of highly active Pt nanoparticles for the oxidation of organic fuels. Int. J. Hydrog. Energy 2016, 41, 3387–3398. [Google Scholar] [CrossRef]
- Hwang, S.M.; Zhang, C.; Han, S.J.; Park, H.G.; Kim, Y.T.; Yang, S.; Jun, K.W.; Kim, S.K. Mesoporous carbon as an effective support for Fe catalyst for CO2 hydrogenation to liquid hydrocarbons. J. CO2 Util. 2020, 37, 65–73. [Google Scholar] [CrossRef]
- Xu, F.; Lu, Q.; Li, K.; Zhu, T.T.; Wang, W.K.; Hu, Z.H. Green synthesis of magnetic mesoporous carbon from waste-lignin and its application as an efficient heterogeneous Fenton catalyst. J. Clean. Prod. 2021, 285, 125363. [Google Scholar] [CrossRef]
- Qiu, Y.; Huo, J.; Jia, F.; Shanks, B.H.; Li, W. N-and S-doped mesoporous carbon as metal-free cathode catalysts for direct biorenewable alcohol fuel cells. J. Mater. Chem. A. 2016, 4, 83–95. [Google Scholar] [CrossRef]
- Parthiban, V.; Bhuvaneshwari, B.; Karthikeyan, J.; Murugan, P.; Sahu, A.K. Fluorine-enriched mesoporous carbon as efficient oxygen reduction catalyst: Understanding the defects in porous matrix and fuel cell applications. Nanoscale Adv. 2019, 1, 4926–4937. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, Q.; Guo, Y.; Sun, X.; Wang, X. Acetylcholinesterase biosensor based on the mesoporous carbon/ferroferric oxide modified electrode for detecting organophosphorus pesticides. RSC Adv. 2016, 6, 24698–24703. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, L.; Zeng, G.; Chen, J.; Cai, Y.; Zhang, Y.; Yang, G.; Liu, Y.; Zhang, C.; Tang, W. Mesoporous carbon nitride based biosensor for highly sensitive and selective analysis of phenol and catechol in compost bioremediation. Biosens. Bioelectron. 2014, 61, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Barathi, P.; Thirumalraj, B.; Chen, S.M.; Angaiah, S. A simple and flexible enzymatic glucose biosensor using chitosan entrapped mesoporous carbon nanocomposite. Microchem. J. 2019, 147, 848–856. [Google Scholar] [CrossRef]
- Pei, S.; Qu, S.; Zhang, Y. Direct electrochemistry and electrocatalysis of hemoglobin at mesoporous carbon modified electrode. Sensors 2010, 10, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Tian, C.; Zhu, D.; Yang, R. Ordered mesoporous carbon paste electrodes for electrochemical sensing and biosensing. Electroanalysis 2008, 20, 1128–1134. [Google Scholar] [CrossRef]
- Dăscălescu, D.; Apetrei, C. Development of a novel electrochemical biosensor based on organized mesoporous carbon and laccase for the detection of serotonin in food supplements. Chemosensors 2022, 10, 365. [Google Scholar] [CrossRef]
- Yu, J.; Yu, D.; Zhao, T.; Zeng, B. Development of amperometric glucose biosensor through immobilizing enzyme in a Pt nanoparticles/mesoporous carbon matrix. Talanta 2008, 74, 1586–1591. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Z.; Zeng, G.; Tang, L.; Pang, Y.; Li, Z.; Liu, C.; Lei, X.; Wu, M.; Ren, P.; et al. Immobilization of laccase on magnetic bimodal mesoporous carbon and the application in the removal of phenolic compounds. Bioresour. Technol. 2012, 115, 21–26. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, D.; Bae, T.S.; Lee, Y.S. The electrochemical enzymatic glucose biosensor based on mesoporous carbon fibers activated by potassium carbonate. J. Ind. Eng. Chem. 2015, 25, 192–198. [Google Scholar] [CrossRef]
- Aram, E.; Moeni, M.; Abedizadeh, R.; Sabour, D.; Sadeghi-Abandansari, H.; Gardy, J.; Hassanpour, A. Smart and multi-functional magnetic nanoparticles for cancer treatment applications: Clinical challenges and future prospects. Nanomaterials 2022, 12, 3567. [Google Scholar] [CrossRef]
- Aram, E.; Mehdipour-Ataei, S. Carbon-based nanostructured composites for tissue engineering and drug delivery. Int. J. Polym. Mater. 2021, 70, 1167–1188. [Google Scholar] [CrossRef]
- Qian, G.; Zhang, L.; Li, X.; Shuai, C.; Wang, X. Construction of Fe3O4-loaded mesoporous carbon systems for controlled drug delivery. ACS Appl. Bio Mater. 2021, 4, 5304–5311. [Google Scholar] [CrossRef]
- Xu, G.; Liu, S.; Niu, H.; Lv, W. Functionalized mesoporous carbon nanoparticles for targeted chemo-photothermal therapy of cancer cells under near-infrared irradiation. RSC Adv. 2014, 4, 33986–33997. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, R.; Li, J.; Huang, C.; Sun, W.; Hou, Z.; Wang, P. A novel yolk–shell Fe3O4@ mesoporous carbon nanoparticle as an Effective tumor-targeting nanocarrier for improvement of chemotherapy and photothermal therapy. Int. J. Mol. Sci. 2022, 23, 1623. [Google Scholar] [CrossRef]
- Zhang, A.; Hai, L.; Wang, T.; Cheng, H.; Li, M.; He, X.; Wang, K. NIR-triggered drug delivery system based on phospholipid coated ordered mesoporous carbon for synergistic chemo-photothermal therapy of cancer cells. Chin. Chem. Lett. 2020, 31, 3158–3162. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Q.; Pan, H.; Jia, S.; Wu, H.; Shi, Y.; Wang, Z. Oxidation modification of chitosan-based mesoporous carbon by soft template method and the adsorption and release properties of hydroxycamptothecin. Sci. Rep. 2020, 10, 15772. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Ma, Z.; Wang, D.; Wang, L.; Zhan, J.; She, L.; Yang, F. Hydrophilic mesoporous carbon nanospheres with high drug-loading efficiency for doxorubicin delivery and cancer therapy. Int. J. Nanomed. 2016, 11, 1793. [Google Scholar]
- Wan, L.; Jiao, J.; Cui, Y.; Guo, J.; Han, N.; Di, D.; Chang, D.; Wang, P.; Jiang, T.; Wang, S. Hyaluronic acid modified mesoporous carbon nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanotechnology 2016, 27, 135102. [Google Scholar] [CrossRef]
- Tian, L.; Tao, L.; Li, H.; Zhao, S.; Zhang, Y.; Yang, S.; Xue, J.; Zhang, X. Hollow mesoporous carbon modified with cRGD peptide nanoplatform for targeted drug delivery and chemo-photothermal therapy of prostatic carcinoma. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 386–395. [Google Scholar] [CrossRef]
- Shou, W.; Guo, R.; Pan, H.; Gang, D.D. Ordered mesoporous carbon: Fabrication, characterization, and application as adsorbents. In Dekker Encyclopedia of Nanoscience and Nanotechnology; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–14. [Google Scholar]
- Tripathi, P.K.; Gan, L.; Liu, M.; Rao, N.N. Mesoporous carbon nanomaterials as environmental adsorbents. J. Nanosci. Nanotechnol. 2014, 14, 1823–1837. [Google Scholar] [CrossRef]
- Ezzeddine, Z.; Isabelle, B.G.; Pouilloux, Y.; Hamad, H. Removal of methylene blue by mesoporous CMK-3: Kinetics, isotherms and thermodynamics. J. Mol. Liq. 2016, 223, 763–770. [Google Scholar] [CrossRef]
- Guo, R.; Guo, J.; Yu, F.; Gang, D.D. Synthesis and surface functional group modifications of ordered mesoporous carbons for resorcinol removal. Microporous Mesoporous Mater. 2013, 175, 141–146. [Google Scholar] [CrossRef]
- Yang, S.J.; Jung, H.; Kim, T.; Park, C.R. Recent advances in hydrogen storage technologies based on nanoporous carbon materials. Prog. Nat. Sci. Mater. Int. 2012, 22, 631–638. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, L.; Xu, F.; Zhou, H.; Peng, X.; Sun, D.; Wang, J.; Du, Y. Nitrogen-doped porous carbons with high performance for hydrogen storage. Int. J. Hydrog. Energy 2016, 41, 8489–8497. [Google Scholar] [CrossRef]
- Felderhoff, M.; Weidenthaler, C.; von Helmolt, R.; Eberle, U. Hydrogen storage: The remaining scientific and technological challenges. Phys. Chem. Chem. Phys. 2007, 9, 2643–2653. [Google Scholar] [CrossRef]
- Yu, J.; Du, W.; Zhao, F.; Zeng, B. High sensitive simultaneous determination of catechol and hydroquinone at mesoporous carbon CMK-3 electrode in comparison with multi-walled carbon nanotubes and Vulcan XC-72 carbon electrodes. Electrochim. Acta 2009, 54, 984–988. [Google Scholar] [CrossRef]
- Ndamanisha, J.C.; Guo, L.P. Ordered mesoporous carbon for electrochemical sensing: A review. Anal. Chim. Acta 2012, 747, 19–28. [Google Scholar] [CrossRef]
- Wei, W.; Li, N.; Qin, G.T. Preparation of mesoporous carbon membranes for ultrafiltration with properties of environmental materials. Adv. Mater. Res. 2012, 600, 104–107. [Google Scholar]
- Qin, G.; He, J.; Wei, W. Ultrafiltration carbon membranes from organic sol-gel process. Chem. Eng. Commun. 2016, 203, 381–388. [Google Scholar] [CrossRef]
- Zhang, S.; Qian, X.; Zhang, L.; Peng, W.; Chen, Y. Composition–property relationships in multifunctional hollow mesoporous carbon nanosystems for PH-responsive magnetic resonance imaging and on-demand drug release. Nanoscale 2015, 7, 7632–7643. [Google Scholar] [CrossRef]
| Materials | Synthetic Method | Application | Purpose | Reference |
|---|---|---|---|---|
| Mesoporous carbon nanoparticles with polyacrylic acid capping | Soft template | Drug delivery | Improvement the release of doxorubicin into HeLa cells | [21] |
| Nitrogen-doped mesoporous carbon | Carbonization and chemical activation | Adsorbent | Carbon dioxide adsorption in fixed bed | [61] |
| Magnetic mesoporous carbon | Chemical activation and sol-gel method | Adsorbent | Remove of methyl blue and methyl orange from wastewater | [62] |
| Thiol-functionalized mesoporous carbons | Hard template | Adsorbent | Adsorption of bivalent heavy-metal ions (Cd, Cu, Pb, Zn) from the aqueous solutions | [63] |
| Mesoporous carbons | Activation | Adsorbent | Elimination of sulfur dioxide from flue gases and natural gases | [64] |
| Amine-functionalized mesoporous carbon | Carbonization | Adsorbent | Adsorption of carbon dioxide from flue gas | [65] |
| Magnetic ordered mesoporous carbon | Soft template | Adsorbent | Adsorption of sulfur from oil | [66] |
| Mesoporous carbon | Pyrolysis | Adsorbent | Adsorption of carbon dioxide | [67] |
| Ordered mesoporous carbon | Soft template | Adsorbent | Desulfurization of gasoline | [68] |
| Mesoporous carbon | Carbonization | Adsorbent | Carbon dioxide/methane separation | [69] |
| Nickel ferrite bearing nitrogen-doped mesoporous carbon | Carbonization | Adsorbent | Remove of mercury (II) from aqueous solution | [70] |
| Nitrogen- and phosphorus-codoped ordered mesoporous carbon | Hard template | Supercapacitor | Supercapacitor electrode | [71,72,73] |
| Mesoporous carbon decorated graphene | Template | Supercapacitor | Supercapacitor electrode | [74] |
| Ruthenium oxide-iron oxide nanoparticles embedded ordered mesoporous carbon | Template | Supercapacitor | Supercapacitor electrode | [75] |
| Mesoporous carbon aerogels | Ionothermal carbonization | Supercapacitor | Supercapacitor electrode | [76] |
| Mesoporous carbon | Soft template | Supercapacitor | Supercapacitor electrode | [77] |
| Mesoporous carbon | Hard template | Supercapacitor | Electrodes for solid-state supercapacitors | [78] |
| Mesoporous carbon | Carbonization of cellulose aerogel | Supercapacitor | Supercapacitor electrode | [79] |
| Mesoporous carbon | Carbonization and activation | Electrochemical double-layer capacitors | Electrode materials for electric double-layer capacitor (EDLC) supercapacitors | [80] |
| Mesoporous carbon | Carbonization | Supercapacitor | Supercapacitor electrode | [81] |
| Mesoporous carbon/manganese dioxide nanocomposite | Hard template | Supercapacitor | Supercapacitor electrode | [82] |
| Microporous and mesoporous carbon | Hard template | Lithium-sulfur batteries | Cathode electrode | [83] |
| Ordered mesoporous carbon fiber sulfur composite | Carbonization | Lithium-sulfur batteries | Cathode electrode | [84] |
| Mesoporous carbons with metal sulfide and metal oxide | Hydrothermal | Lithium-ion batteries | Anode for lithium-ion batteries | [85] |
| Iron oxide nanoparticles with mesoporous carbon and nitrogen-doped carbon | Carbonization | Lithium-ion battery | Anode for lithium-ion batteries | [86] |
| Mesoporous carbon nanofibers | Carbonization | Lithium-ion battery | Anode of lithium-ion batteries | [87] |
| Mesoporous carbon/zinc oxide | Carbonization | Lithium-ion battery | Anode of lithium-ion batteries | [88] |
| Mesoporous carbon/Tin oxide nanoparticles | Hydrothermal | Lithium-ion battery | Anode of lithium-ion batteries | [89] |
| Graphitized mesoporous carbon | Hard template | Lithium-ion battery | Anode of lithium-ion batteries | [90] |
| Foamed mesoporous carbon/silicon composite | Foaming and carbonization | Lithium-ion battery | Anode of lithium-ion batteries | [91] |
| Ordered mesoporous carbons | Hard template | Fuel cell | Supports of electrocatalysts for direct methanol fuel cells | [92,93,94] |
| Iron-doped mesoporous carbon | Template method | Fuel cell | Catalyst for oxygen-reduction reaction | [95] |
| Transition metal (iron, cobalt, manganese) and nitrogen-doped mesoporous carbons | Pyrolysis | Fuel cell | Cathode catalysts for anion-exchange membrane fuel cells | [96] |
| Nitrogen- and sulfur-dual-heteroatom-doped ordered mesoporous carbon | Template | Fuel cell | Electrocatalyst for oxygen-reduction reaction | [97] |
| Mesoporous carbon | Carbonization | Fuel cell | Platinum support as anode and cathode catalyst | [98,99] |
| Mesoporous carbon | Hard template | Methanol fuel cell | Anodic catalyst | [100] |
| Mesoporous carbon nitride | Hard template | Fuel cell | Electrode modification for the oxygen-reduction reaction | [101] |
| Microporous/mesoporous carbon | Carbonization and activation | Microbial fuel cells | Anode material | [102] |
| Mesoporous carbon | Carbonization | Proton exchange membrane fuel cell | Cathode catalyst support for oxygen-reduction reaction | [103] |
| Platinum/palladium/mesoporous carbon | Carbonization | Biosensor | Non-enzymatic amperometric sensing of glucose | [104,105] |
| Nitrogen-doped porous carbon nanosheets | Pyrolysis | Biosensor | Electrocatalyst for reduction of hydrogen peroxide | [106] |
| Ordered mesoporous carbon | Hard template | Electrochemical sensors | Modification of glassy carbon electrode for detection of cysteine | [107] |
| Mesoporous carbon/zirconium-based metal organic frameworks/laccase | Carbonization | Biosensor | Modifier electrode for tetracycline detection | [108] |
| Quantum dots/ordered mesoporous carbon | Carbonization | Electrochemi-luminescence biosensor | Detection of hydrogen peroxide in live cells | [109] |
| Ordered mesoporous carbons | Hard template | Biosensor | Modification of glassy carbon for determination of dopamine | [110] |
| Mesoporous carbons | Hard template | Electrochemical sensor | Modification of glassy carbon electrode for detection of dopamine | [111] |
| Boron- and nitrogen-doped mesoporous carbons | Carbonization | Electrochemical sensor | Electrode modifier to detect isoniazid | [112] |
| Mesoporous carbon nitride | Hard template | Catalyst | Catalytic application as solid bases | [113,114] |
| Sulfur, phosphorus, boron and iron-doped mesoporous carbon | Carbonization | Catalyst | Oxygen-reduction catalyst in microbial fuel cells | [115] |
| Mesoporous/macroporous carbon | Hard template | Catalyst | Catalyst support for the conversion of cellulose to polyols | [116] |
| Ferrum- and nitrogen-doped mesoporous carbon | Soft template | Catalyst | Catalyst for oxygen reduction in fuel cells | [117] |
| Mesoporous carbon | Carbonization | Catalyst | Support of platinum nanoparticles for the oxidation of organic fuels | [118] |
| Mesoporous carbon | Hard template | Catalyst | Support of Fe catalyst for CO2 hydrogenation to liquid hydrocarbons | [119] |
| Magnetic mesoporous carbon | Thermal carbonization | Catalyst | Heterogeneous Fenton catalyst | [120] |
| Nitrogen- and sulfur-doped mesoporous carbon | Hard template | Catalyst | Cathode catalysts for direct biorenewable alcohol fuel cells | [121] |
| Fluorine-doped mesoporous carbon | Sol-gel | Catalyst | Oxygen-reduction catalyst | [122] |
| Mesoporous carbon/Ferro ferric oxide | Carbonization | Enzyme immobilization in biosensor | Modification of screen-printed carbon electrodes to detect organophosphorus pesticides | [123] |
| Mesoporous carbon nitride | Hard template | Enzyme immobilization in biosensor | Detection of phenol and catechol in compost bioremediation | [124] |
| Chitosan-entrapped mesoporous carbon nanocomposite | Template | Enzyme immobilization in biosensor | Modifier of electrode for enhanced amperometric sensing of glucose sensor | [125] |
| Mesoporous carbon | Organic-organic self-assembly | Enzyme immobilization in sensors | Support for redox protein immobilization | [126] |
| Ordered mesoporous carbon | Carbonization | Enzyme immobilization | Electrodes for biosensing | [127] |
| Mesoporous Carbon | Carbonization | Enzyme immobilization | Modifier of carbon screen-printed electrode and support for enzyme immobilization | [128] |
| Mesoporous carbon | Hard template | Enzyme immobilization in sensors | Modification of glassy carbon electrode for glucose biosensor | [129] |
| Magnetic mesoporous carbon | Hard template | Enzyme immobilization | Support for immobilization of laccase in phenol and p-chlorophenol removal | [130] |
| Mesoporous carbon | Carbonization | Enzyme immobilization | Fabrication of glucose-sensing electrodes | [131] |
| Magnetic mesoporous carbon | Carbonization | Drug delivery | Delivery of doxorubicin into cancer cells and improvement of cancer cell killing ability | [132,133,134] |
| Mesoporous carbon with polyethylenimine and folic acid | Hydrothermal | Drug delivery | Improvement the uptake of nanoparticles by HeLa cells | [135] |
| Magnetic mesoporous carbon | Carbonization | Drug delivery | Enhancement of tumor cell ablation capability | [136] |
| Phospholipid coated ordered mesoporous carbon | Carbonization | Drug delivery | Delivery of doxorubicin into MCF-7 cells | [137] |
| Nitrogen-doped mesoporous carbon nanoparticles | Soft template | Drug delivery | Improvement the adsorption capacity of hydroxycamptothecin | [138] |
| Polyethylene glycol-functionalized oxidized mesoporous carbon | Hydrothermal | Drug delivery | Inhibition the growth of cancer cells and improvement the therapeutic efficiency | [139] |
| Hyaluronic acid modified mesoporous carbon nanoparticles | Carbonization | Drug delivery | Delivery of doxorubicin and verapamil and improvement the therapeutic effect on HCT-116 tumor in BALB/c nude mice | [140] |
| Arginine-glycine-aspartic acid peptide-conjugated mesoporous carbon | Carbonization | Drug delivery | High doxorubicin loading and improvement the therapeutic efficacy toward PC3 cells | [141] |
| Adsorbents | Adsorption (%) |
|---|---|
| Magnetic mesoporous activated carbon | 98.5 |
| Mesoporous activated carbon | 66.9 |
| Activated carbon | 76 |
| Sample | Specific Capacitance (F g−1) | Performance (μF cm−2) |
|---|---|---|
| Mesoporous carbon | 132 | 12.05 |
| Activated carbon | 80.5 | 5.3 |
| Sample | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Discharge Capacity (mAh g−1) | Charge Capacity (mAh g−1) |
|---|---|---|---|---|
| SnO2 nanoparticles | 83.51 | 0.079 | 616.04 | 608.59 |
| SnO2/mesoporous carbon | 798.9 | 1.17 | 1129.54 | 1107.09 |
| Sample | Discharge Capacity in the 10th Cycle (mAh g−1) | Discharge Capacity in the 20th Cycle (mAh g−1) |
|---|---|---|
| Mesoporous polyacrylonitrile/silicon/aluminum acetylacetonate composite nanofibers | 1199 | 1045 |
| Nanoporous polyacrylonitrile/silicon composite nanofibers | 715 | 569 |
| Polyacrylonitrile nanofibers | 526 | 438 |
| Sample | Power Density (Wm−3) |
|---|---|
| Mesoporous carbon | 23.6 |
| Carbon cloth | 10.4 |
| Sample | Detection Limit (μM) |
|---|---|
| Mesoporous carbon-modified glass carbon electrode | 0.0045 |
| Mesoporous carbon (with carboxyl groups)-modified glass carbon electrode | 0.044 |
| Mesoporous carbon (with amino groups)-modified glass carbon electrode | 0.33 |
| Sample | Current Signals (μA M−1) | Detection Limit (mM) |
|---|---|---|
| Mesoporous carbon/glucose oxidase | 18.42 | 0.13 |
| Carbon nanotube/glucose oxidase | 2.92 | 0.072 |
| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Contact Angle | Drug Loading (mg/g) | Release after 12 h (%) |
|---|---|---|---|---|---|
| Mesoporous carbon | 804 | 0.87 | 133.4° | 676.97 | 83.40 |
| Oxidized mesoporous carbon | 322 | 0.64 | 58.2° | 647.20 | 81.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehdipour-Ataei, S.; Aram, E. Mesoporous Carbon-Based Materials: A Review of Synthesis, Modification, and Applications. Catalysts 2023, 13, 2. https://doi.org/10.3390/catal13010002
Mehdipour-Ataei S, Aram E. Mesoporous Carbon-Based Materials: A Review of Synthesis, Modification, and Applications. Catalysts. 2023; 13(1):2. https://doi.org/10.3390/catal13010002
Chicago/Turabian StyleMehdipour-Ataei, Shahram, and Elham Aram. 2023. "Mesoporous Carbon-Based Materials: A Review of Synthesis, Modification, and Applications" Catalysts 13, no. 1: 2. https://doi.org/10.3390/catal13010002
APA StyleMehdipour-Ataei, S., & Aram, E. (2023). Mesoporous Carbon-Based Materials: A Review of Synthesis, Modification, and Applications. Catalysts, 13(1), 2. https://doi.org/10.3390/catal13010002





