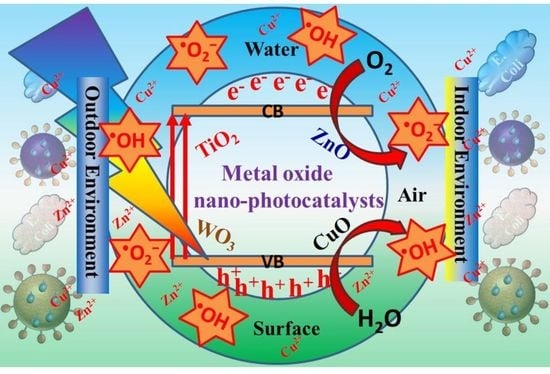
Recent Advances on Metal Oxide Based Nano-Photocatalysts as Potential Antibacterial and Antiviral Agents
Abstract
:1. Introduction
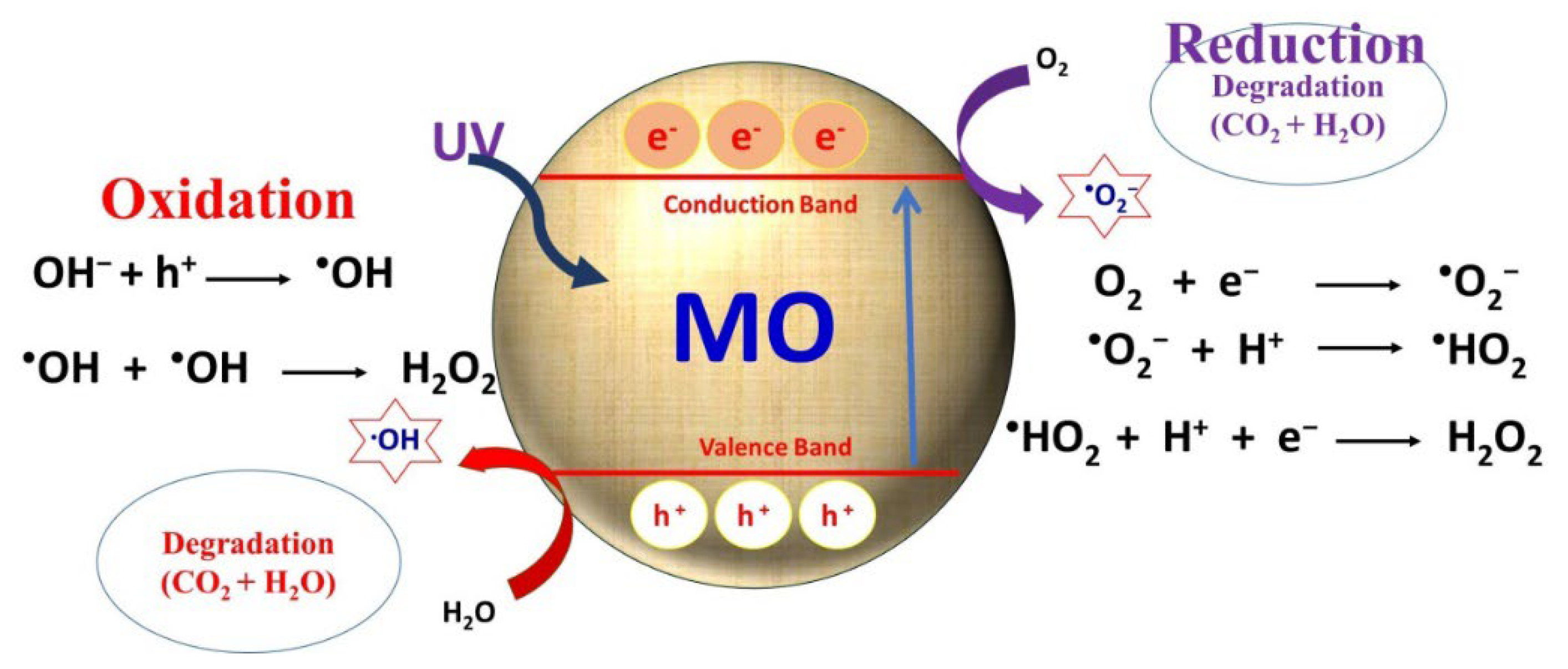
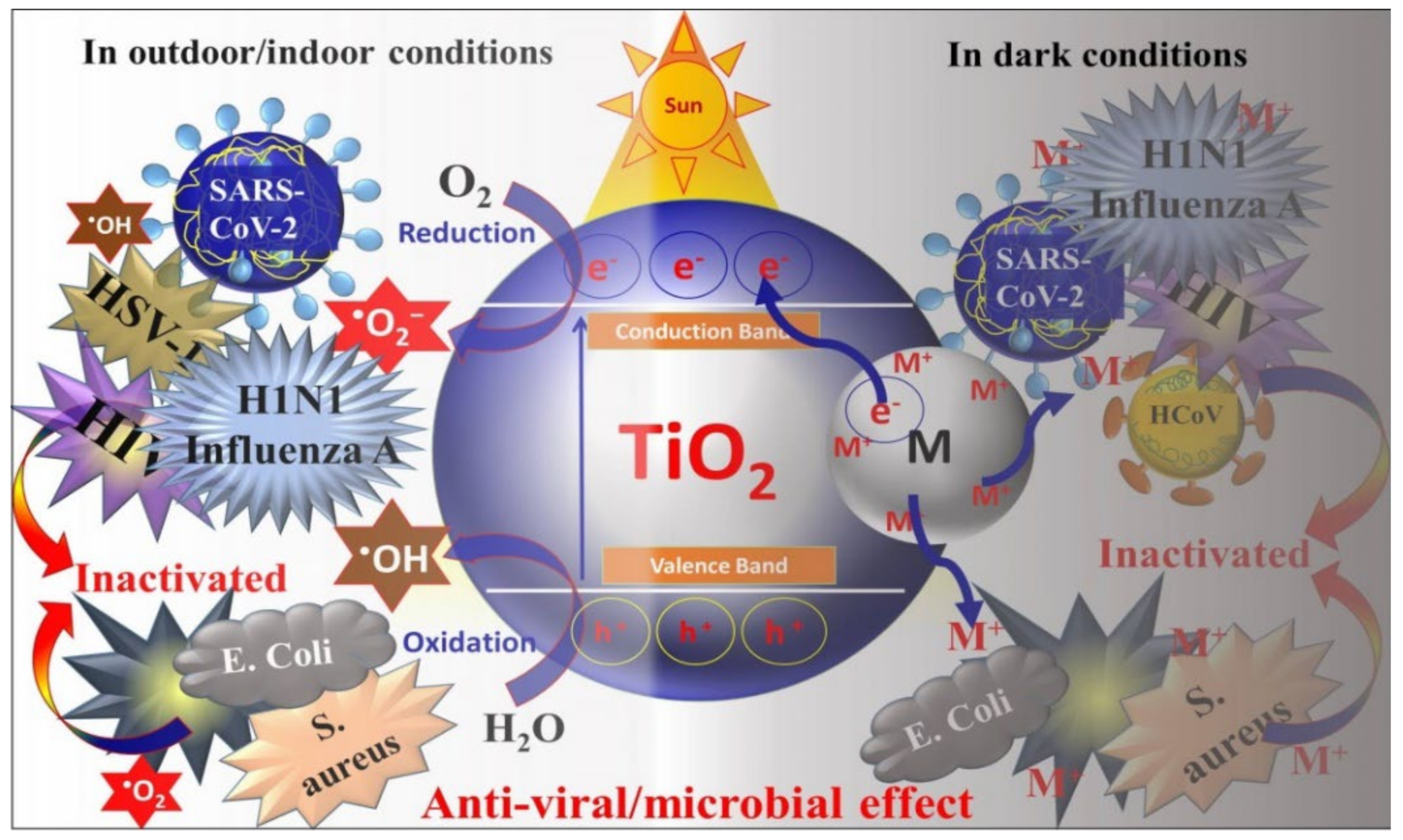
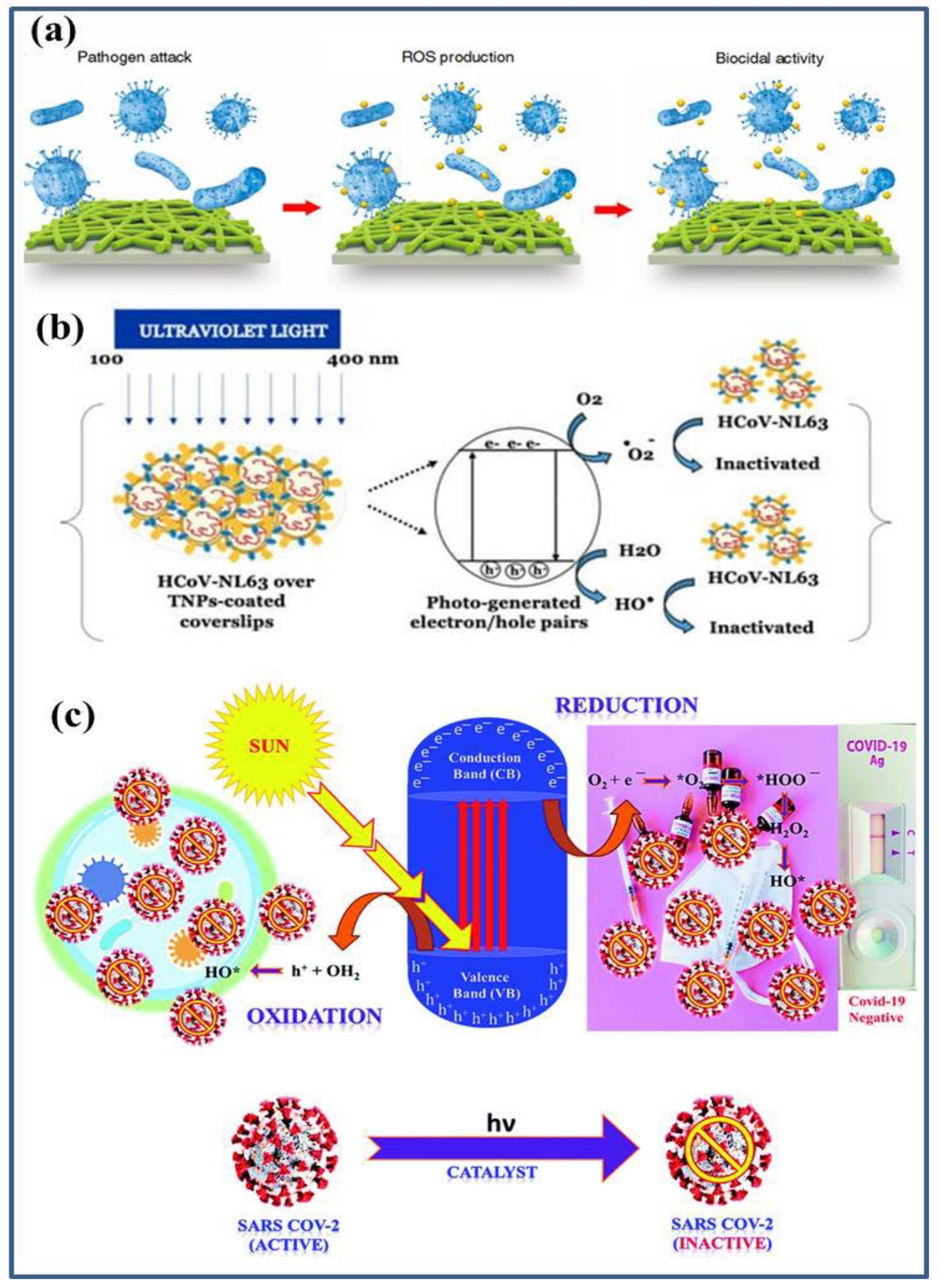
2. Metal Oxide Based Nano-Photocatalysts: Antibacterial and Antiviral Mechanisms
3. Recent Advances on Metal Oxide Based Nano-Photocatalysts as Potential Antibacterial and Antiviral Agents
3.1. TiO2 Based Nano-Photocatalysts as Potential Antibacterial and Antiviral Agents
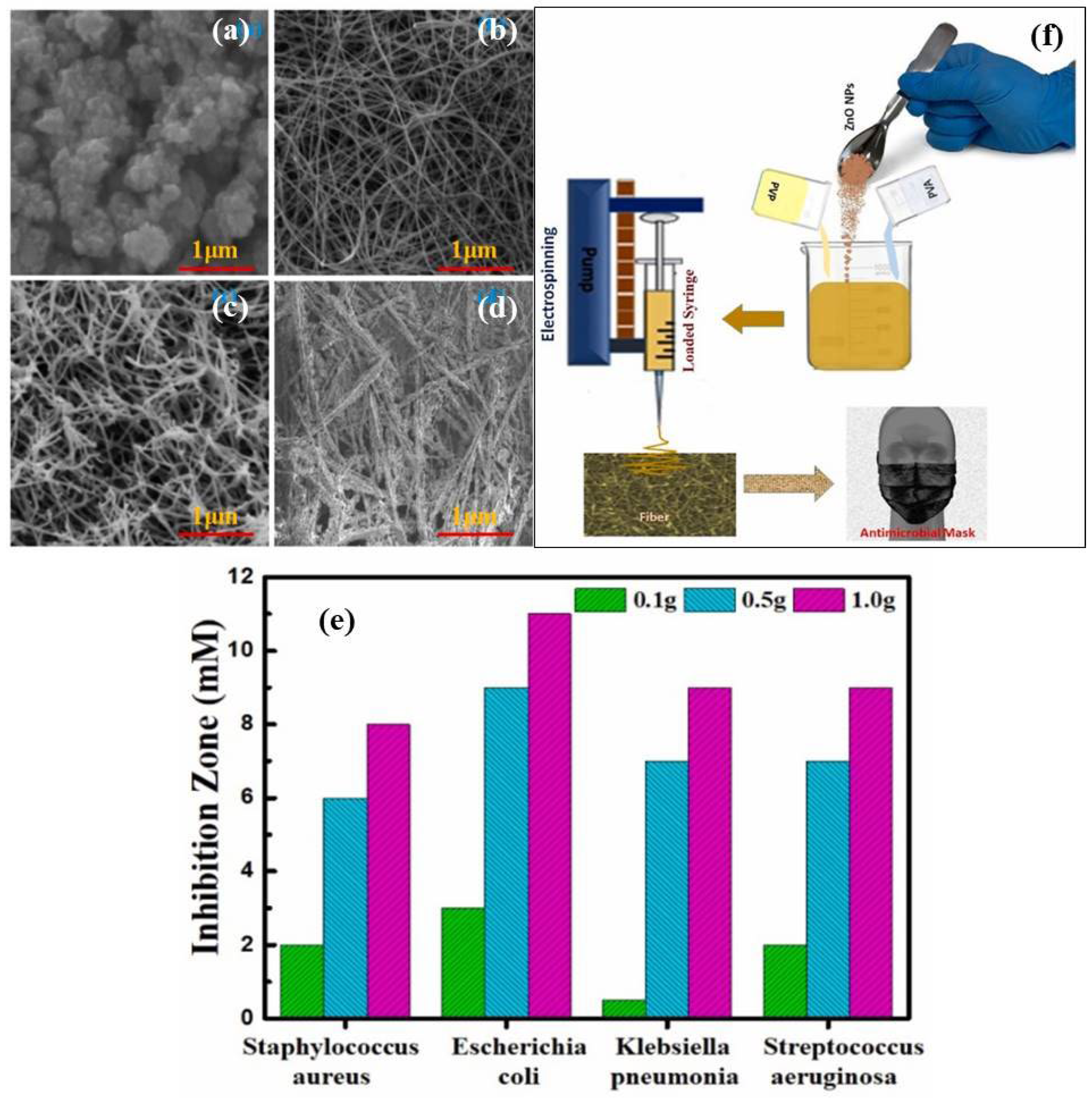
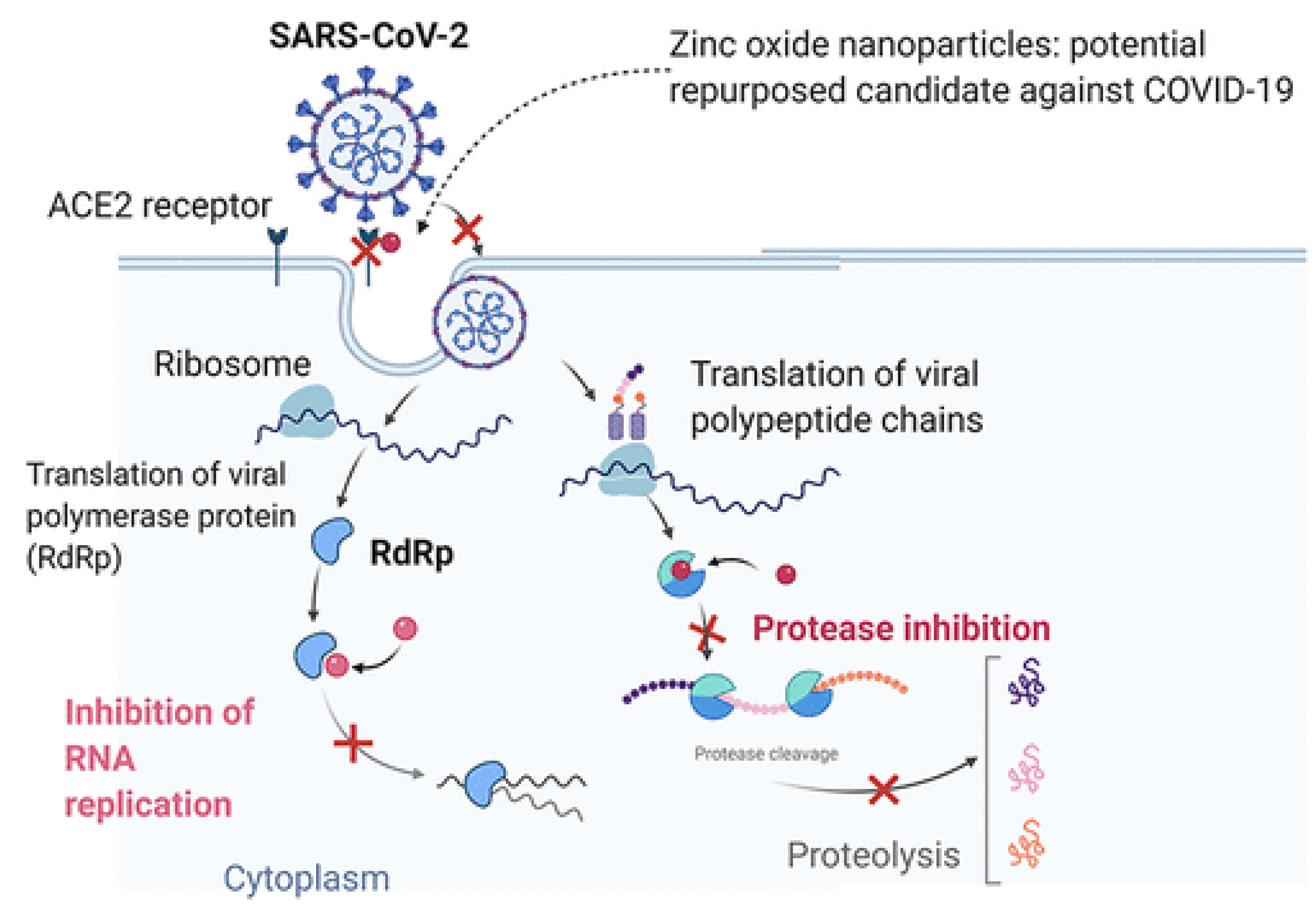
3.2. ZnO Based Nano-Photocatalysts as Potential Antibacterial and Antiviral Agents
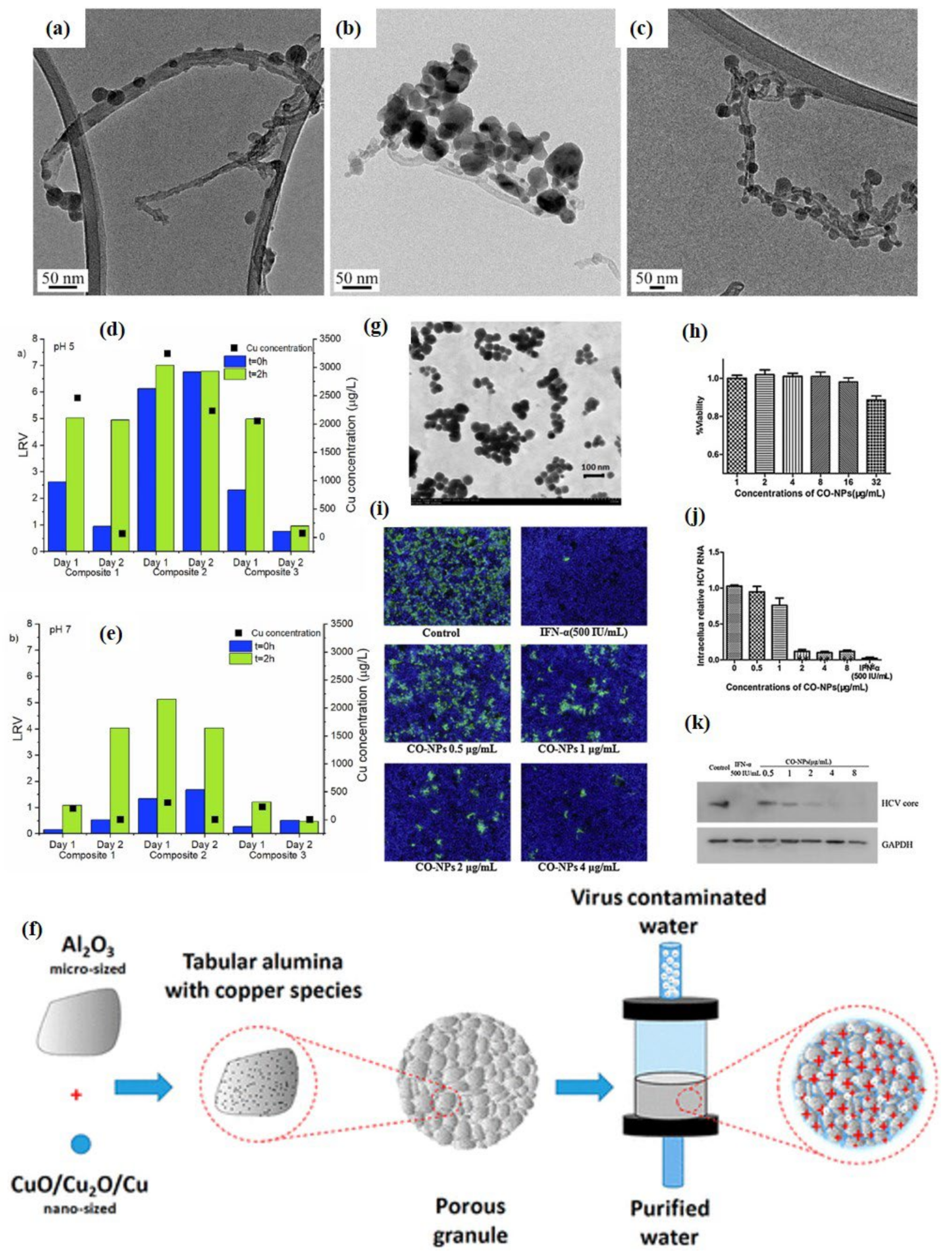
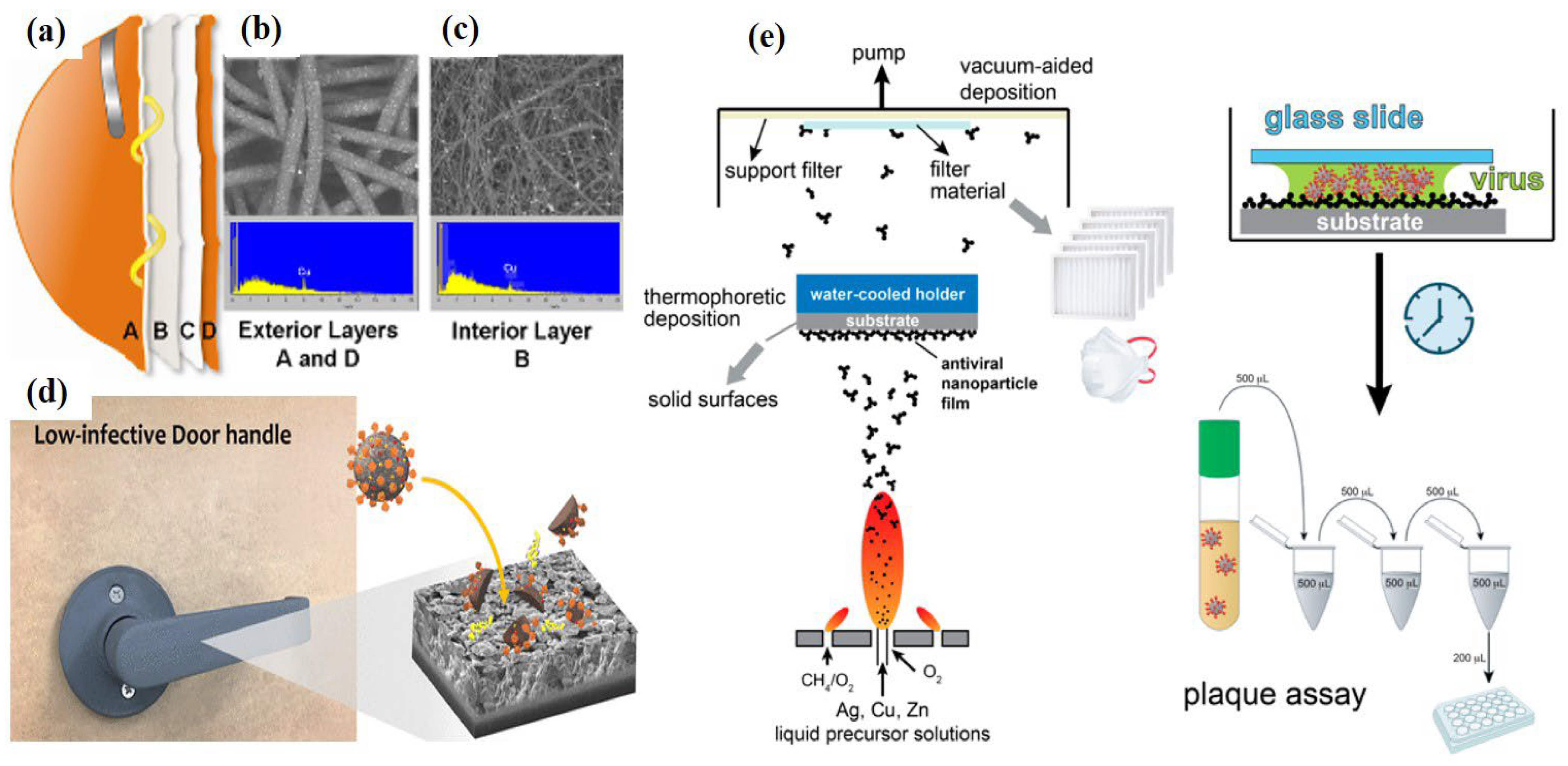
3.3. CuO and Other Metal Oxides Based Nano-Photocatalysts as Potential Antimicrobial and Antiviral Agents
4. Challenges, Future Prospects and Summary
5. Challenges
- Due to the wide bandgap that it possesses, natural TiO2 can only be excited by the near-UV photons that are present in the solar spectrum (390 nm). However, visible light accounts for approximately 42% of solar radiation [132], despite the fact that UV makes up only 4% of solar light. Because of its wide bandgap, it is not useful for applications involving the environment. As a result, boosting the photocatalytic activity of TiO2 is a difficult problem;
- It is common knowledge that photocatalytic nanomaterials could generate ROS, which can then destroy the structural components of viruses. However, the light source has a significant impact on their performance, which may result in an increase in the cost of their application;
- It is important to tailor the development and choice of antiviral nanomaterials to their intended uses. The high flow rate of air purifiers makes it easier for viruses to gain momentum when antiviral materials are used. Because of this, nanomaterials’ electrostatic effects on their surfaces should be amplified to improve their adsorption capacities toward viruses, and their physical structures should be strengthened to break viruses;
- The biodistribution of metal oxide NPs is influenced by their interactions with proteins, which take place through a process known as opsonization. As a result, the NP’s properties are altered [133];
- When metal NPs are utilized for in vivo applications, it is imperative that the potentially harmful effects of these particles be taken into consideration. Nanotoxicity can be explained by two factors: (i) the potential release of toxic ions from metallic nanoparticles, and (ii) the oxidative stress caused by the inherent properties of the nanoparticles themselves (morphology, surface charge, size, and chemical surface composition) [134].
Funding
Acknowledgments
Conflicts of Interest
References
- Prakash, J.; Cho, J.; Mishra, Y.K. Photocatalytic TiO2 nanomaterials as potential antimicrobial and antiviral agents: Scope against blocking the, SARS-CoV-2 spread. Micro Nano Eng. 2022, 14, 100100. [Google Scholar] [CrossRef]
- Soni, V.; Khosla, A.; Singh, P.; Nguyen, V.-H.; Van Le, Q.; Selvasembian, R.; Hussain, C.M.; Thakur, S.; Raizada, P. Current perspective in metal oxide based photocatalysts for virus disinfection: A review. J. Environ. Manag. 2022, 308, 114617. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Wallace, G.G.; Schroeder, A.; Stellacci, F.; Conde, J. Nanotechnology-based disinfectants and sensors for, SARS-CoV-2. Nat. Nanotechnol. 2020, 15, 618–621. [Google Scholar] [CrossRef]
- Aghalari, Z.; Dahms, H.-U.; Sillanpää, M. Investigating the effectiveness of nanotechnologies in environmental health with an emphasis on environmental health journals. Life Sci. Soc. Policy 2021, 17, 8. [Google Scholar] [CrossRef]
- Soliman, A.M.; Khalil, M.; Ali, I.M. Novel and Facile Method for Photocatalytic Disinfection and Removal of Organic Material from Water Using Immobilized Copper Oxide Nano Rods. J. Water Process Eng. 2021, 41, 102086. [Google Scholar] [CrossRef]
- Chakraborty, A.; Samriti Ruzimuradov, O.; Gupta, R.K.; Cho, J.; Prakash, J. TiO2 nanoflower photocatalysts: Synthesis, modifications and applications in wastewater treatment for removal of emerging organic pollutants. Environ. Res. 2022, 212, 113550. [Google Scholar] [CrossRef]
- Samriti, M.; Chen, Z.; Sun, S.; Prakash, J. Design and engineering of graphene nanostructures as independent solar-driven photocatalysts for emerging applications in the field of energy and environment. Mol. Syst. Des. Eng. 2022, 7, 213–238. [Google Scholar] [CrossRef]
- Prakash, J.; Sun, S.; Swart, H.C.; Gupta, R.K. Noble metals-TiO2 nanocomposites: From fundamental mechanisms to photocatalysis, surface enhanced, Raman scattering and antibacterial applications. Appl. Mater. Today 2018, 11, 82–135. [Google Scholar] [CrossRef]
- Gupta, T.; Cho, J.; Prakash, J. Hydrothermal synthesis of TiO2 nanorods: Formation chemistry, growth mechanism, and tailoring of surface properties for photocatalytic activities. Mater. Today Chem. 2021, 20, 100428. [Google Scholar] [CrossRef]
- Prakash, J.; Kumar, P.; Harris, R.A.; Swart, C.; Neethling, J.H.; van Vuuren, A.J.; Swart, H.C. Synthesis, characterization and multifunctional properties of plasmonic Ag–TiO2 nanocomposites. Nanotechnology 2016, 27, 355707. [Google Scholar] [CrossRef]
- Singh, N.; Prakash, J.; Gupta, R.K. Design and engineering of high-performance photocatalytic systems based on metal oxide–Graphene—Noble metal nanocomposites. Mol. Syst. Des. Eng. 2017, 2, 422–439. [Google Scholar] [CrossRef]
- Rajput, V.; Gupta, R.K.; Prakash, J. Engineering metal oxide semiconductor nanostructures for enhanced charge transfer: Fundamentals and emerging SERS applications. J. Mater. Chem. C 2022, 10, 73–95. [Google Scholar]
- Ahmadi, Y.; Bhardwaj, N.; Kim, K.-H.; Kumar, S. Recent advances in photocatalytic removal of airborne pathogens in air. Sci. Total Environ. 2021, 794, 148477. [Google Scholar] [CrossRef]
- Channegowda, M. Functionalized Photocatalytic Nanocoatings for Inactivating COVID-19 Virus Residing on Surfaces of Public and Healthcare Facilities. Coronaviruses 2021, 2, 3–11. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Karishma, S.; Kiruthika, A.R. Photocatalytic disinfection of micro-organisms: Mechanisms and applications. Environ. Technol. Innov. 2021, 24, 101909. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Feng, X.; Li, H. Thermal-Sprayed Photocatalytic Coatings for Biocidal Applications: A Review. J. Therm. Spray Technol. 2021, 30, 1–24. [Google Scholar] [CrossRef]
- Kumar, P.; Mathpal, M.C.; Prakash, J.; Viljoen, B.C.; Roos, W.; Swart, H. Band gap tailoring of cauliflower-shaped CuO nanostructures by Zn doping for antibacterial applications. J. Alloys Compd. 2020, 832, 154968. [Google Scholar] [CrossRef]
- da Silva, B.L.; Caetano, B.L.; Chiari-Andréo, B.G.; Pietro, R.C.L.R.; Chiavacci, L.A. Increased antibacterial activity of ZnO nanoparticles: Influence of size and surface modification. Colloids Surf. B. Biointerfaces 2019, 177, 440–447. [Google Scholar] [CrossRef]
- Matsuura, R.; Lo, C.-W.; Wada, S.; Somei, J.; Ochiai, H.; Murakami, T.; Saito, N.; Ogawa, T.; Shinjo, A.; Benno, Y.; et al. SARS-CoV-2 Disinfection of Air and Surface Contamination by TiO2 Photocatalyst-Mediated Damage to Viral Morphology, RNA, and Protein. Viruses 2021, 13, 942. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Liaqat, F.; Khazi, M.I.; Awan, A.S.; Eltem, R.; Li, J. 15-Antimicrobial studies of metal oxide nanomaterials. In Metal Oxide-Carbon Hybrid Materials; Chaudhry, M.A., Hussain, R., Butt, F.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 407–435. [Google Scholar]
- Kumar, V.; Prakash, J.; Singh, J.P.; Chae, K.H.; Swart, C.; Ntwaeaborwa, O.M.; Swart, H.C.; Dutta, V. Role of silver doping on the defects related photoluminescence and antibacterial behaviour of zinc oxide nanoparticles. Colloids Surf. B Biointerfaces 2017, 159, 191–199. [Google Scholar] [CrossRef]
- Zacarías, S.M.; Satuf, M.L.; Vaccari, M.C.; Alfano, O.M. Photocatalytic inactivation of bacterial spores using TiO2 films with silver deposits. Chem. Eng. J. 2015, 266, 133–140. [Google Scholar] [CrossRef]
- Park, G.W.; Cho, M.; Cates, E.L.; Lee, D.; Oh, B.-T.; Vinjé, J.; Kim, J.-H. Fluorinated TiO2 as an ambient light-activated virucidal surface coating material for the control of human norovirus. J. Photochem. Photobiol. B Biol. 2014, 140, 315–320. [Google Scholar] [CrossRef]
- Si, Y.; Zhang, Z.; Wu, W.; Fu, Q.; Huang, K.; Nitin, N.; Ding, B.; Sun, G. Daylight-driven rechargeable antibacterial and antiviral nanofibrous membranes for bioprotective applications. Sci. Adv. 2018, 4, eaar5931. [Google Scholar] [CrossRef]
- Kumar, A.; Soni, V.; Singh, P.; Khan, A.A.P.; Nazim, M.; Mohapatra, S.; Saini, V.; Raizada, P.; Hussain, C.M.; Shaban, M.; et al. Green aspects of photocatalysts during corona pandemic: A promising role for the deactivation of COVID-19 virus. RSC Adv. 2022, 12, 13609–13627. [Google Scholar] [CrossRef]
- Khaiboullina, S.; Uppal, T.; Dhabarde, N.; Subramanian, V.R.; Verma, S.C. Inactivation of Human Coronavirus by Titania Nanoparticle Coatings and UVC Radiation: Throwing Light on SARS-CoV-2. Viruses 2021, 13, 19. [Google Scholar] [CrossRef]
- Tong, Y.; Shi, G.; Hu, G.; Hu, X.; Han, L.; Xie, X.; Xu, Y.; Zhang, R.; Sun, J.; Zhong, J. Photo-catalyzed TiO2 inactivates pathogenic viruses by attacking viral genome. Chem. Eng. J. 2021, 414, 128788. [Google Scholar] [CrossRef]
- Bono, N.; Ponti, F.; Punta, C.; Candiani, G. Effect of UV Irradiation and TiO2-Photocatalysis on Airborne Bacteria and Viruses: An Overview. Materials 2021, 14, 1075. [Google Scholar] [CrossRef]
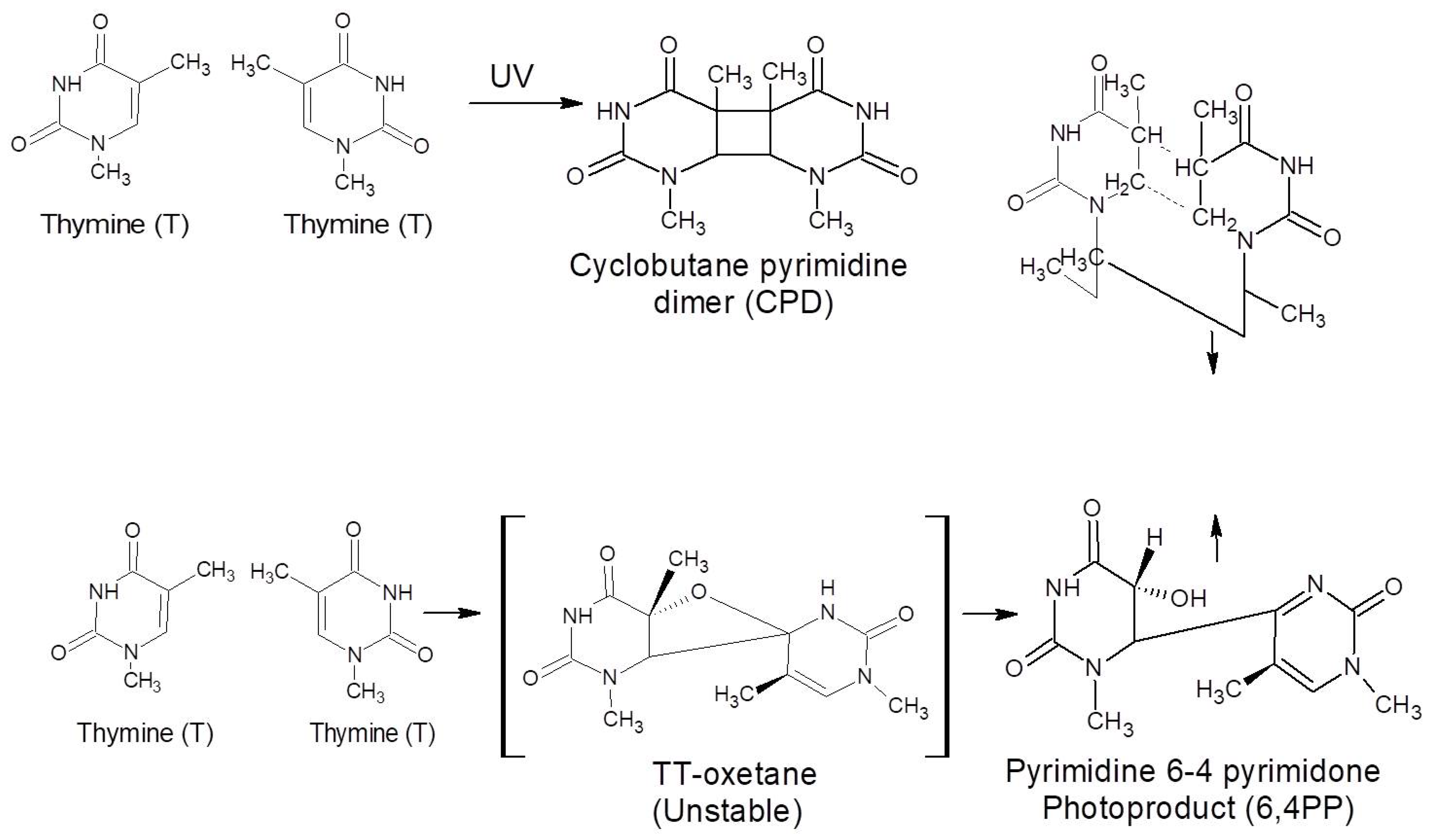
- Mullenders, L.H.F. Solar UV damage to cellular DNA: From mechanisms to biological effects. Photochem. Photobiol. Sci. 2018, 17, 1842–1852. [Google Scholar] [CrossRef]
- Prakash, J.; Samriti, K.A.; Dai, H.; Janegitz, B.C.; Krishnan, V.; Swart, H.C.; Sun, S. Novel rare earth metal–doped one-dimensional TiO2 nanostructures: Fundamentals and multifunctional applications. Mater. Today Sustain. 2021, 13, 100066. [Google Scholar] [CrossRef]
- Cai, T.; Liu, Y.; Wang, L.; Zhang, S.; Ma, J.; Dong, W.; Zeng, Y.; Yuan, J.; Liu, C.; Luo, S. “Dark Deposition” of Ag Nanoparticles on TiO2: Improvement of Electron Storage Capacity To Boost “Memory Catalysis” Activity. ACS Appl. Mater. Interfaces 2018, 10, 25350–25359. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J. Mechanistic Insights into Graphene Oxide Driven Photocatalysis as Co-Catalyst and Sole Catalyst in Degradation of Organic Dye Pollutants. Photochem 2022, 2, 651–671. [Google Scholar] [CrossRef]
- Verma, S.; Mal, D.S.; de Oliveira, P.R.; Janegitz, B.C.; Prakash, J.; Gupta, R.K. A facile synthesis of novel polyaniline/graphene nanocomposite thin films for enzyme-free electrochemical sensing of hydrogen peroxide. Mol. Syst. Des. Eng. 2022, 7, 158–170. [Google Scholar] [CrossRef]
- Sharma, P.; Kherb, J.; Prakash, J.; Kaushal, R. A novel and facile green synthesis of SiO2 nanoparticles for removal of toxic water pollutants. Appl. Nanosci. 2021. [Google Scholar] [CrossRef]
- Prakash, J.; Harris, R.A.; Swart, H.C. Embedded plasmonic nanostructures: Synthesis, fundamental aspects and their surface enhanced Raman scattering applications. Int. Rev. Phys. Chem. 2016, 35, 353–398. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. A review on TiO2-based Z-scheme photocatalysts. Chin. J. Catal. 2017, 38, 1936–1955. [Google Scholar] [CrossRef]
- Yu, J.; Wang, W.; Cheng, B.; Su, B.-L. Enhancement of Photocatalytic Activity of Mesporous TiO2 Powders by Hydrothermal Surface Fluorination Treatment. J. Phys. Chem. C 2009, 113, 6743–6750. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.; Ho, W.; Jiang, Z.; Zhang, L. Effects of F- Doping on the Photocatalytic Activity and Microstructures of Nanocrystalline TiO2 Powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Li, J.; Ren, D.; Wu, Z.; Huang, C.; Yang, H.; Chen, Y.; Yu, H. Visible-light-mediated antifungal bamboo based on Fe-doped TiO2 thin films. RSC Adv. 2017, 7, 55131–55140. [Google Scholar] [CrossRef]
- Kumar, P.; Mathpal, M.C.; Prakash, J.; Jagannath, G.; Roos, W.D.; Swart, H.C. Plasmonic and nonlinear optical behavior of nanostructures in glass matrix for photonics application. Mater. Res. Bull. 2020, 125, 110799. [Google Scholar] [CrossRef]
- Prakash, J.; Tripathi, A.; Gautam, S.; Chae, K.H.; Song, J.; Rigato, V.; Tripathi, J.; Asokan, K. Phenomenological understanding of dewetting and embedding of noble metal nanoparticles in thin films induced by ion irradiation. Mater. Chem. Phys. 2014, 147, 920–924. [Google Scholar] [CrossRef]
- Kumar, P.; Mathpal, M.C.; Jagannath, G.; Prakash, J.; Maze, J.-R.; Roos, W.D.; Swart, H.C. Optical limiting applications of resonating plasmonic Au nanoparticles in a dielectric glass medium. Nanotechnology 2021, 32, 345709. [Google Scholar] [CrossRef]
- Li, J.; Ma, R.; Wu, Z.; He, S.; Chen, Y.; Bai, R.; Wang, J. Visible-Light-Driven Ag-Modified TiO2 Thin Films Anchored on Bamboo Material with Antifungal Memory Activity against Aspergillus niger. J. Fungi 2021, 7, 592. [Google Scholar] [CrossRef]
- Ma, R.; Li, J.; Han, S.; Wu, Z.; Bao, Y.; He, S.; Chen, Y. Solar-driven WO3·H2O/TiO2 heterojunction films immobilized onto bamboo biotemplate: Relationship between physical color, crystal structure, crystal morphology, and energy storage ability. Surf. Interfaces 2022, 31, 102028. [Google Scholar] [CrossRef]
- Tatsuma, T.; Takeda, S.; Saitoh, S.; Ohko, Y.; Fujishima, A. Bactericidal effect of an energy storage TiO2–WO3 photocatalyst in dark. Electrochem. Commun. 2003, 5, 793–796. [Google Scholar] [CrossRef]
- Singh, J.P.; Chen, C.L.; Dong, C.L.; Prakash, J.; Kabiraj, D.; Kanjilal, D.; Pong, W.F.; Asokan, K. Role of surface and subsurface defects in MgO thin film: XANES and magnetic investigations. Superlattices Microstruct. 2015, 77, 313–324. [Google Scholar] [CrossRef]
- Pathak, T.K.; Kumar, V.; Prakash, J.; Purohit, L.P.; Swart, H.C.; Kroon, R.E. Fabrication and characterization of nitrogen doped p-ZnO on n-Si heterojunctions. Sens. Actuators A. Phys. 2016, 247, 475–481. [Google Scholar] [CrossRef]
- Patel, S.P.; Chawla, A.K.; Chandra, R.; Prakash, J.; Kulriya, P.K.; Pivin, J.C.; Kanjilal, D.; Kumar, L. Structural phase transformation in ZnS nanocrystalline thin films by swift heavy ion irradiation. Solid State Commun. 2010, 150, 1158–1161. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, G.; Prakash, J.; Zheng, Y.; Sun, S. Rational Design of Novel Catalysts with Atomic Layer Deposition for the Reduction of Carbon Dioxide. Adv. Energy Mater. 2019, 9, 1900889. [Google Scholar] [CrossRef]
- Prakash, J.; Tripathi, A.; Khan, S.A.; Pivin, J.C.; Singh, F.; Tripathi, J.; Kumar, S.; Avasthi, D.K. Ion beam induced interface mixing of Ni on PTFE bilayer system studied by quadrupole mass analysis and electron spectroscopy for chemical analysis. Vacuum 2010, 84, 1275–1279. [Google Scholar] [CrossRef]
- Prakash, J.; Swart, H.C.; Zhang, G.; Sun, S. Emerging applications of atomic layer deposition for the rational design of novel nanostructures for surface-enhanced Raman scattering. J. Mater. Chem. C 2019, 7, 1447–1471. [Google Scholar] [CrossRef]
- Prakash, J. Fundamentals and applications of recyclable SERS substrates. Int. Rev. Phys. Chem. 2019, 38, 201–242. [Google Scholar] [CrossRef]
- Singh, N.; Prakash, J.; Misra, M.; Sharma, A.; Gupta, R.K. Dual Functional Ta-Doped Electrospun TiO2 Nanofibers with Enhanced Photocatalysis and SERS Detection for Organic Compounds. ACS Appl. Mater. Interfaces 2017, 9, 28495–28507. [Google Scholar] [CrossRef]
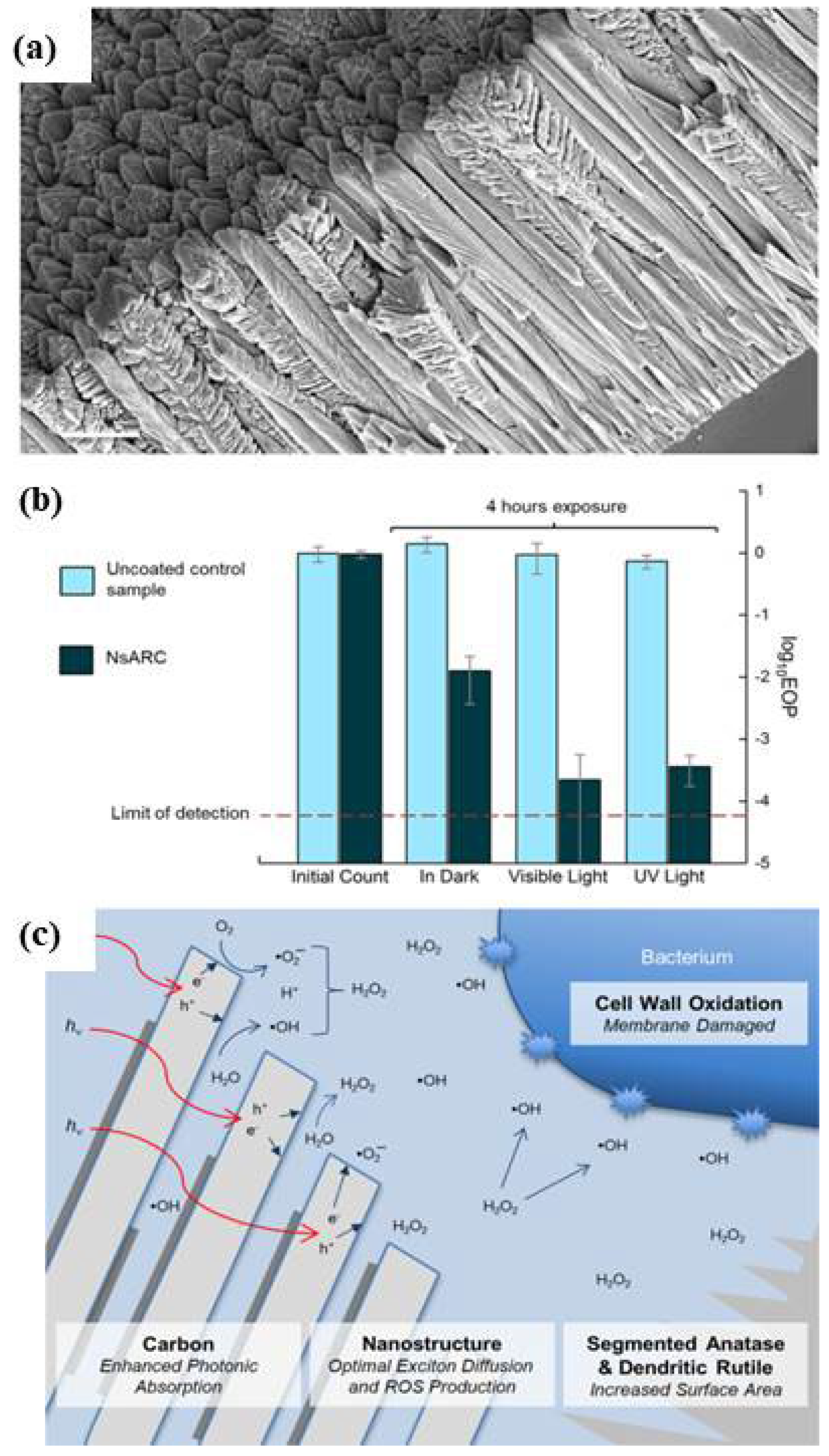
- Krumdieck, S.P.; Boichot, R.; Gorthy, R.; Land, J.G.; Lay, S.; Gardecka, A.J.; Polson, M.I.J.; Wasa, A.; Aitken, J.E.; Heinemann, J.A.; et al. Nanostructured TiO2 anatase-rutile-carbon solid coating with visible light antimicrobial activity. Sci. Rep. 2019, 9, 1883. [Google Scholar] [CrossRef]
- Wanag, A.; Rokicka, P.; Kusiak-Nejman, E.; Kapica-Kozar, J.; Wrobel, R.J.; Markowska-Szczupak, A.; Morawski, A.W. Antibacterial properties of TiO2 modified with reduced graphene oxide. Ecotoxicol. Environ. Saf. 2018, 147, 788–793. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, M.; Ye, S.; Xu, Y.; Zhou, S.; Cai, Q.; Xie, G.; Huang, L.; Zheng, L.; Li, Y. Antibacterial activity and mechanism of the graphene oxide (rGO)- modified TiO2 catalyst against Enterobacter hormaechei. Int. Biodeterior. Biodegrad. 2021, 162, 105260. [Google Scholar] [CrossRef]
- Rokicka-Konieczna, P.; Wanag, A.; Sienkiewicz, A.; Kusiak-Nejman, E.; Morawski, A.W. Antibacterial effect of TiO2 nanoparticles modified with APTES. Catal. Commun. 2020, 134, 105862. [Google Scholar] [CrossRef]
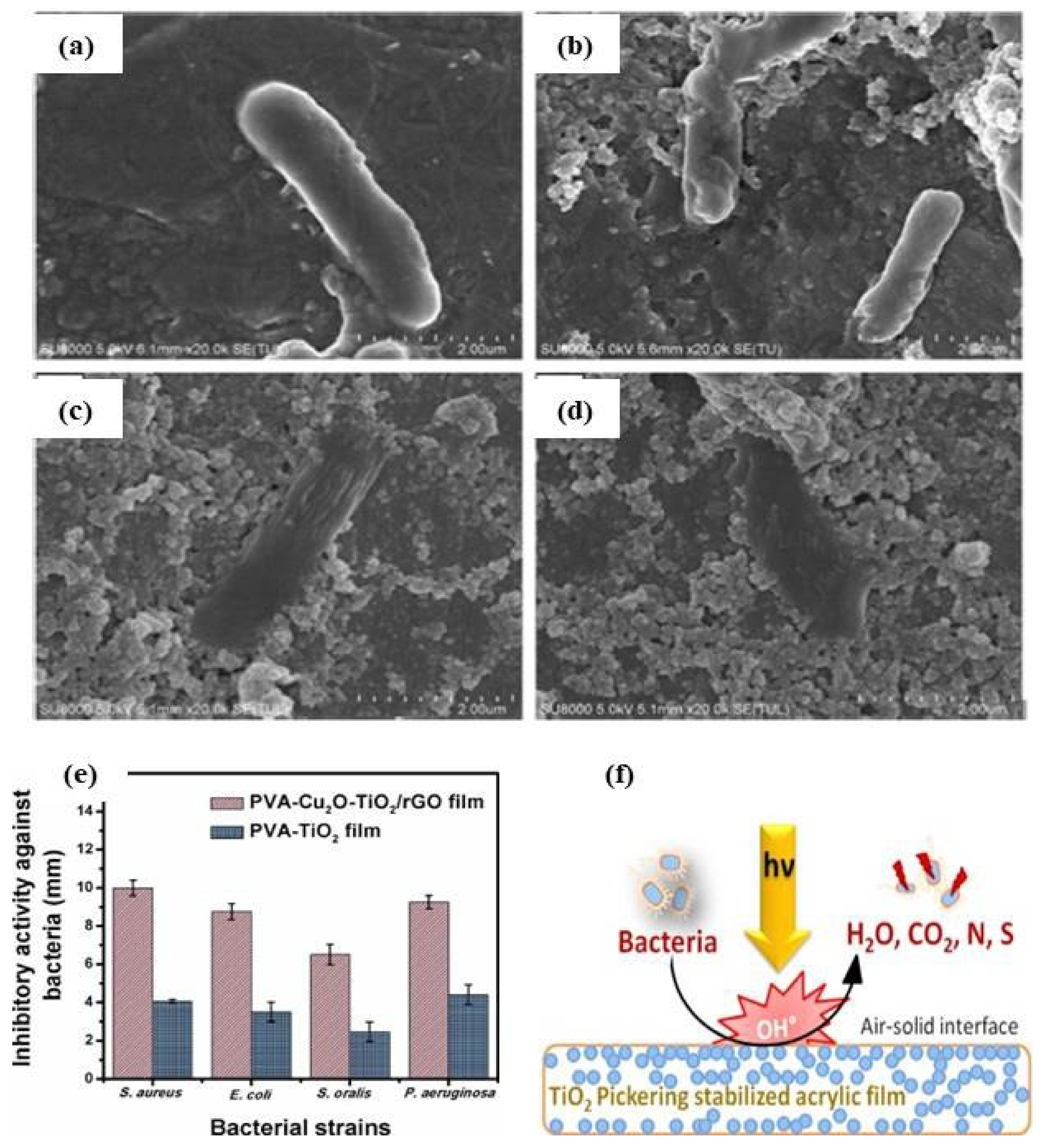
- Dhanasekar, M.; Jenefer, V.; Nambiar, R.B.; Babu, S.G.; Selvam, S.P.; Neppolian, B.; Bhat, S.V. Ambient light antimicrobial activity of reduced graphene oxide supported metal doped TiO2 nanoparticles and their PVA based polymer nanocomposite films. Mater. Res. Bull. 2018, 97, 238–243. [Google Scholar] [CrossRef]
- Bonnefond, A.; González, E.; Asua, J.M.; Leiza, J.R.; Kiwi, J.; Pulgarin, C.; Rtimi, S. New evidence for hybrid acrylic/TiO2 films inducing bacterial inactivation under low intensity simulated sunlight. Colloids Surf. B. Biointerfaces 2015, 135, 1–7. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Ksari, Y.; Prakash, J.; Giovanelli, L.; Valmalette, J.-C.; Themlin, J.-M. Nitrogen-doping processes of graphene by a versatile plasma-based method. Carbon 2014, 73, 216–224. [Google Scholar] [CrossRef]
- Komba, N.; Zhang, G.; Wei, Q.; Yang, X.; Prakash, J.; Chenitz, R.; Rosei, F.; Sun, S. Iron (II) phthalocyanine/N-doped graphene: A highly efficient non-precious metal catalyst for oxygen reduction. Int. J. Hydrog. Energy 2019, 44, 18103–18114. [Google Scholar] [CrossRef]
- Prakash, J.; Pivin, J.C.; Swart, H.C. Noble metal nanoparticles embedding into polymeric materials: From fundamentals to applications. Adv. Colloid Interface Sci. 2015, 226, 187–202. [Google Scholar] [CrossRef] [PubMed]
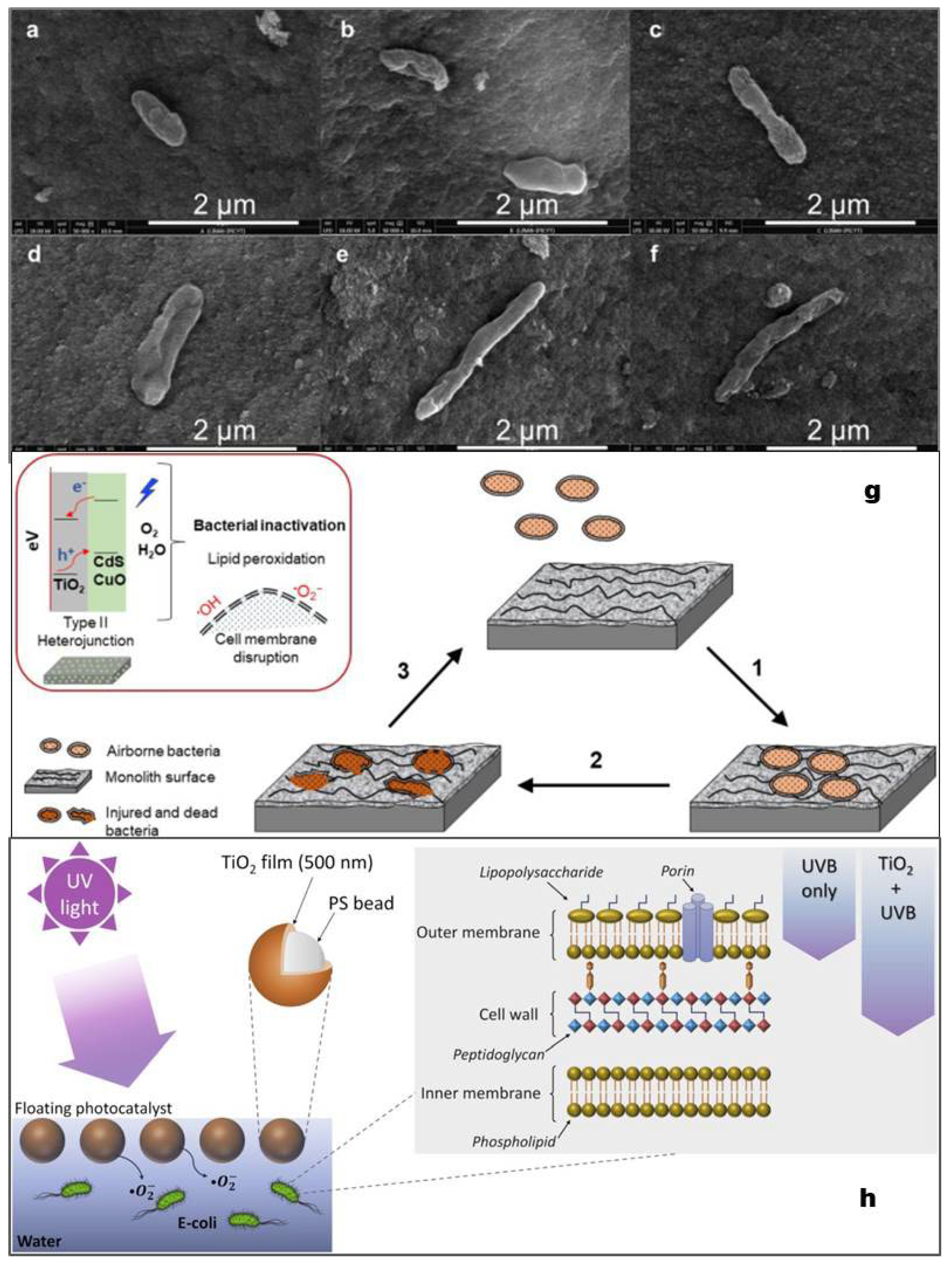
- Hernández-Gordillo, A.; Arriaga, S. Mesoporous TiO2 Monoliths Impregnated with CdS and CuO Nanoparticles for Airborne Bacteria Inactivation Under Visible Light. Catal. Lett. 2022, 152, 629–640. [Google Scholar] [CrossRef]
- Islam, M.A.; Ikeguchi, A.; Naide, T. Effectiveness of an air cleaner device in reducing aerosol numbers and airborne bacteria from an enclosed type dairy barn. Environ. Sci. Pollut. Res. 2022, 29, 53022–53035. [Google Scholar] [CrossRef]
- Lee, M.; Koziel, J.A.; Macedo, N.R.; Li, P.; Chen, B.; Jenks, W.S.; Zimmerman, J.; Paris, R.V. Mitigation of Particulate Matter and Airborne Pathogens in Swine Barn Emissions with Filtration and UV-A Photocatalysis. Catalysts 2021, 11, 1302. [Google Scholar] [CrossRef]
- Valdez-Castillo, M.; Saucedo-Lucero, J.O.; Arriaga, S. Photocatalytic inactivation of airborne microorganisms in continuous flow using perlite-supported ZnO and TiO2. Chem. Eng. J. 2019, 374, 914–923. [Google Scholar] [CrossRef]
- Urbonavicius, M.; Varnagiris, S.; Sakalauskaite, S.; Demikyte, E.; Tuckute, S.; Lelis, M. Application of Floating TiO2 Photocatalyst for Methylene Blue Decomposition and Salmonella typhimurium Inactivation. Catalysts 2021, 11, 794. [Google Scholar] [CrossRef]
- Varnagiris, S.; Urbonavicius, M.; Sakalauskaite, S.; Daugelavicius, R.; Pranevicius, L.; Lelis, M.; Milcius, D. Floating TiO2 photocatalyst for efficient inactivation of E. coli and decomposition of methylene blue solution. Sci. Total Environ. 2020, 720, 137600. [Google Scholar] [CrossRef]
- Sboui, M.; Lachheb, H.; Bouattour, S.; Gruttadauria, M.; La Parola, V.; Liotta, L.F.; Boufi, S. TiO2/Ag2O immobilized on cellulose paper: A new floating system for enhanced photocatalytic and antibacterial activities. Environ. Res. 2021, 198, 111257. [Google Scholar] [CrossRef]
- Mathur, G. COVID Killing Air Purifier Based on UV & Titanium Dioxide Based Photocatalysis System. SAE Int. J. Adv. Curr. Pract. Mobil. 2021, 4, 143–150. [Google Scholar]
- Nakano, R.; Yamaguchi, A.; Sunada, K.; Nagai, T.; Nakano, A.; Suzuki, Y.; Yano, H.; Ishiguro, H.; Miyauchi, M. Inactivation of various variant types of SARS-CoV-2 by indoor-light-sensitive TiO2-based photocatalyst. Sci. Rep. 2022, 12, 5804. [Google Scholar] [CrossRef]
- Uppal, T.; Reganti, S.; Martin, E.; Verma, S.C. Surface Inactivation of Human Coronavirus by MACOMATM UVA-TiO2 Coupled Photocatalytic Disinfection System. Catalysts 2022, 12, 690. [Google Scholar] [CrossRef]
- Zan, L.; Fa, W.; Peng, T.; Gong, Z.-K. Photocatalysis effect of nanometer TiO2 and TiO2-coated ceramic plate on Hepatitis B virus. J. Photochem. Photobiol. B Biol. 2007, 86, 165–169. [Google Scholar] [CrossRef]
- Bhardwaj, S.K.; Singh, H.; Deep, A.; Khatri, M.; Bhaumik, J.; Kim, K.-H.; Bhardwaj, N. UVC-based photoinactivation as an efficient tool to control the transmission of coronaviruses. Sci. Total Environ. 2021, 792, 148548. [Google Scholar] [CrossRef]
- Micochova, P.; Chadha, A.; Hesseloj, T.; Fraternali, F.; Ramsden, J.; Gupta, R. Rapid inactivation of SARS-CoV-2 by titanium dioxide surface coating. Wellcome Open Res. 2021, 6, 56. [Google Scholar] [CrossRef]
- Kupferschmidt, K.; Wadman, M. Delta variant triggers new phase in the pandemic. Science 2021, 372, 1375–1376. [Google Scholar] [CrossRef]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A review on ZnO: Fundamental properties and applications. Mater. Today Proc. 2020, 49, 3028–3035. [Google Scholar] [CrossRef]
- Yamada, H.; Suzuki, K.; Koizumi, S. Gene expression profile in human cells exposed to zinc. J. Toxicol. Sci. 2007, 32, 193–196. [Google Scholar] [CrossRef]
- Umar, A.; Rahman, M.; Vaseem, M.; Hahn, Y.-B. Ultra-sensitive cholesterol biosensor based on low-temperature grown ZnO nanoparticles. Electrochem. Commun. 2009, 11, 118–121. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Sandiya, K.; Santhiya, S.; Pradeep, R.S.; Kumar, N.M.; Suriyanarayanan, N.; Thirumarimurugan, M. Biosynthesis, characterization, and antibacterial activity of zinc oxide nanoparticles derived from Bauhinia tomentosa leaf extract. J. Nanostruct. Chem. 2018, 8, 293–299. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Loconsole, D.; Sallustio, A.; Picca, R.A.; Felici, R.; Chironna, M.; Cioffi, N. On the Efficacy of ZnO Nanostructures against SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 3040. [Google Scholar] [CrossRef]
- Raj, N.B.; PavithraGowda, N.; Pooja, O.; Purushotham, B.; Kumar, M.A.; Sukrutha, S.; Ravikumar, C.; Nagaswarupa, H.; Murthy, H.A.; Boppana, S.B. Harnessing ZnO nanoparticles for antimicrobial and photocatalytic activities. J. Photochem. Photobiol. 2021, 6, 100021. [Google Scholar] [CrossRef]
- Milionis, A.; Tripathy, A.; Donati, M.; Sharma, C.S.; Pan, F.; Maniura-Weber, K.; Ren, Q.; Poulikakos, D. Water-based scalable methods for self-cleaning antibacterial ZnO-nanostructured surfaces. Ind. Eng. Chem. Res. 2020, 59, 14323–14333. [Google Scholar] [CrossRef]
- Prakash, J.; Kumar, V.; Erasmus, L.J.B.; Duvenhage, M.M.; Sathiyan, G.; Bellucci, S.; Sun, S.; Swart, H.C. Phosphor Polymer Nanocomposite: ZnO:Tb3+ Embedded Polystyrene Nanocomposite Thin Films for Solid-State Lighting Applications. ACS Appl. Nano Mater. 2018, 1, 977–988. [Google Scholar] [CrossRef]
- Kumar, R.S.; Dananjaya, S.H.S.; De Zoysa, M.; Yang, M. Enhanced antifungal activity of Ni-doped ZnO nanostructures under dark conditions. RSC Adv. 2016, 6, 108468–108476. [Google Scholar] [CrossRef]
- Iqbal, G.; Faisal, S.; Khan, S.; Shams, D.F.; Nadhman, A. Photo-inactivation and efflux pump inhibition of methicillin resistant Staphylococcus aureus using thiolated cobalt doped ZnO nanoparticles. J. Photochem. Photobiol. B Biol. 2019, 192, 141–146. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Sivaraj, D. Enhanced antibacterial activity of Cr doped ZnO nanorods synthesized using microwave processing. RSC Adv. 2015, 5, 68461–68469. [Google Scholar] [CrossRef]
- Naskar, A.; Lee, S.; Kim, K.-S. Antibacterial potential of Ni-doped zinc oxide nanostructure: Comparatively more effective against Gram-negative bacteria including multi-drug resistant strains. RSC Adv. 2020, 10, 1232–1242. [Google Scholar] [CrossRef]
- Geetha, K.; Sivasangari, D.; Kim, H.-S.; Murugadoss, G.; Kathalingam, A. Electrospun nanofibrous ZnO/PVA/PVP composite films for efficient antimicrobial face masks. Ceram. Int. 2022, 48, 29197–29204. [Google Scholar] [CrossRef]
- Attia, G.H.; Moemen, Y.S.; Youns, M.; Ibrahim, A.M.; Abdou, R.; El Raey, M.A. Antiviral zinc oxide nanoparticles mediated by hesperidin and in silico comparison study between antiviral phenolics as anti-SARS-CoV-2. Colloids Surf. B Biointerfaces 2021, 203, 111724. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Abdel-Bar, H.M.; Elmowafy, E.; El-Khouly, A.; Mansour, M.; Awad, G.A. Investigating the internalization and COVID-19 antiviral computational analysis of optimized nanoscale zinc oxide. ACS Omega 2021, 6, 6848–6860. [Google Scholar] [CrossRef] [PubMed]
- El-Megharbel, S.M.; Alsawat, M.; Al-Salmi, F.A.; Hamza, R.Z. Utilizing of (zinc oxide nano-spray) for disinfection against “SARS-CoV-2” and testing its biological effectiveness on some biochemical parameters during (COVID-19 pandemic)—“ZnO nanoparticles have antiviral activity against (SARS-CoV-2)”. Coatings 2021, 11, 388. [Google Scholar] [CrossRef]
- Ishida, T. Anti-viral vaccine activity of Zinc (II) for viral prevention, entry, replication, and spreading during pathogenesis process. Curr. Trends. Biomed. Eng. Biosci. 2019, 19, MS.ID.556012. [Google Scholar] [CrossRef]
- Antoine, T.E.; Mishra, Y.K.; Trigilio, J.; Tiwari, V.; Adelung, R.; Shukla, D. Prophylactic, therapeutic and neutralizing effects of zinc oxide tetrapod structures against herpes simplex virus type-2 infection. Antivir. Res. 2012, 96, 363–375. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Rahman, A.U.; Tajuddin, H.A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc in human health: Effect of zinc on immune cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef]
- Te Velthuis, A.J.; van den Worm, S.H.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef]
- Kalsi, T.; Mitra, H.; Roy, T.K.; Godara, S.K.; Kumar, P. Comprehensive Analysis of Band Gap and Nanotwinning in Cd1–xMgxS QDs. Cryst. Growth Des. 2020, 20, 6699–6706. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Momeni, M.; Mirhosseini, M.; Nazari, Z.; Kazempour, A.; Hakimiyan, M. Antibacterial and photocatalytic activity of CuO nanostructure films with different morphology. J. Mater. Sci. Mater. Electron. 2016, 27, 8131–8137. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Kumar, S.S.; Manikandan, M.; Saravanan, M. Photocatalytic properties and antimicrobial efficacy of Fe doped, CuO nanoparticles against the pathogenic bacteria and fungi. Microb. Pathog. 2018, 122, 84–89. [Google Scholar] [CrossRef]
- Giti, R.; Zomorodian, K.; Firouzmandi, M.; Zareshahrabadi, Z.; Rahmannasab, S. Antimicrobial activity of thermocycled polymethyl methacrylate resin reinforced with titanium dioxide and copper oxide nanoparticles. Int. J. Dent. 2021, 2021, 6690806. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227. [Google Scholar] [CrossRef]
- Soni, V.; Xia, C.; Cheng, C.K.; Nguyen, V.-H.; Nguyen, D.L.T.; Bajpai, A.; Kim, S.Y.; Van Le, Q.; Khan, A.A.P.; Singh, P. Advances and recent trends in cobalt-based cocatalysts for solar-to-fuel conversion. Appl. Mater. Today 2021, 24, 101074. [Google Scholar] [CrossRef]
- Bandala, E.R.; Kruger, B.R.; Cesarino, I.; Leao, A.L.; Wijesiri, B.; Goonetilleke, A. Impacts of COVID-19 pandemic on the wastewater pathway into surface water: A review. Sci. Total Environ. 2021, 774, 145586. [Google Scholar] [CrossRef]
- Ghotekar, S.; Pansambal, S.; Bilal, M.; Pingale, S.S.; Oza, R. Environmentally friendly synthesis of Cr2O3 nanoparticles: Characterization, applications and future perspective—A review. Case Stud. Chem. Environ. Eng. 2021, 3, 100089. [Google Scholar] [CrossRef]
- Tavakoli, A.; Hashemzadeh, M.S. Inhibition of herpes simplex virus type 1 by copper oxide nanoparticles. J. Virol. Methods 2020, 275, 113688. [Google Scholar] [CrossRef]
- Dulta, K.; Ağçeli, G.K.; Chauhan, P.; Jasrotia, R.; Ighalo, J.O. Multifunctional CuO nanoparticles with enhanced photocatalytic dye degradation and antibacterial activity. Sustain. Environ. Res. 2022, 32, 1–15. [Google Scholar] [CrossRef]
- Akhavan, O.; Azimirad, R.; Safa, S.; Hasani, E. CuO/Cu(OH)2 hierarchical nanostructures as bactericidal photocatalysts. J. Mater. Chem. 2011, 21, 9634–9640. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Cu and CuO nanoparticles immobilized by silica thin films as antibacterial materials and photocatalysts. Surf. Coat. Technol. 2010, 205, 219–223. [Google Scholar] [CrossRef]
- Gurianov, Y.; Nakonechny, F.; Albo, Y.; Nisnevitch, M. Antibacterial composites of cuprous oxide nanoparticles and polyethylene. Int. J. Mol. Sci. 2019, 20, 439. [Google Scholar] [CrossRef] [Green Version]
- Domagała, E. Stability evaluation of Cu2O/MWCNTs filters for virus removal from water. Water Res. 2020, 179, 115879. [Google Scholar] [CrossRef]
- Mazurkow, J.M.; Yüzbasi, N.S.; Domagala, K.W.; Pfeiffer, S.; Kata, D.; Graule, T. Nano-sized copper (oxide) on alumina granules for water filtration: Effect of copper oxidation state on virus removal performance. Environ. Sci. Technol. 2019, 54, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Etemadifar, Z.; Daneshkazemi, A.; Nateghi, M. Antimicrobial effect of copper oxide nanoparticles on some oral bacteria and candida species. J. Dent. Biomater. 2017, 4, 347. [Google Scholar] [PubMed]
- Hang, X.; Peng, H.; Song, H.; Qi, Z.; Miao, X.; Xu, W. Antiviral activity of cuprous oxide nanoparticles against Hepatitis C Virus in vitro. J. Virol. Methods 2015, 222, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A novel anti-influenza copper oxide containing respiratory face mask. PLoS ONE 2010, 5, e11295. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.H.; Chu, D.K.; Shiu, E.Y.; Chan, K.H.; McDevitt, J.J.; Hau, B.J.; Cowling, B.J. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020, 26, 676–680. [Google Scholar] [CrossRef]
- Hosseini, M.; Chin, A.W.; Behzadinasab, S.; Poon, L.L.; Ducker, W.A. Cupric oxide coating that rapidly reduces infection by SARS-CoV-2 via solids. ACS Appl. Mater. Interfaces 2021, 13, 5919–5928. [Google Scholar] [CrossRef]
- Merkl, P.; Long, S.; McInerney, G.M.; Sotiriou, G.A. Antiviral activity of silver, copper oxide and zinc oxide nanoparticle coatings against SARS-CoV-2. Nanomaterials 2021, 11, 1312. [Google Scholar] [CrossRef]
- Delumeau, L.V.; Asgarimoghaddam, H.; Alkie, T.; Jones, A.J.B.; Lum, S.; Mistry, K.; Musselman, K.P. Effectiveness of antiviral metal and metal oxide thin-film coatings against human coronavirus 229E. APL Mater. 2021, 9, 111114. [Google Scholar] [CrossRef]
- Farah, J.; Ibadurrohman, M.; Slamet. Synthesis of CuO-TiO2 nano-composite for Escherichia coli disinfection and toluene degradation. AIP Conf. Proc. 2020, 2237, 020050. [Google Scholar]
- Kanako, Y.; Yui, S.; Momo, I.; Takakiyo, T.; Toshikazu, S.; Jin-ichi, S. Photocatalytic Antibacterial Activity of TiO2, TiO2+CuO, and WO3 +CuO -Evaluation of Codoping Effect. Technol. Innov. Pharm. Res. 2021, 1, 130–139. [Google Scholar]
- Uema, M.; Yonemitsu, K.; Momose, Y.; Ishii, Y.; Tateda, K.; Inoue, T.; Asakura, H. Effect of the Photocatalyst under Visible Light Irradiation in SARS-CoV-2 Stability on an Abiotic Surface. Biocontrol Sci. 2021, 26, 119–125. [Google Scholar] [CrossRef]
- Lin, H.; Li, T.; Janani, B.J.; Fakhri, A. Fabrication of Cu2MoS4 decorated WO3 nano heterojunction embedded on chitosan: Robust photocatalytic efficiency, antibacterial performance, and bacteria detection by peroxidase activity. J. Photochem. Photobiol. B Biol. 2022, 226, 112354. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Czech, B.; Wasilewska, A.; Boguszewska-Czubara, A.; Yubuta, K.; Wagata, H.; Daminova, S.S.; Kadirova, Z.C.; Vargas, R. Detoxifying SARS-CoV-2 antiviral drugs from model and real wastewaters by industrial waste-derived multiphase photocatalysts. J. Hazard. Mater. 2022, 429, 128300. [Google Scholar] [CrossRef]
- Czech, B.; Krzyszczak, A.; Boguszewska-Czubara, A.; Opielak, G.; Jośko, I.; Hojamberdiev, M. Revealing the toxicity of lopinavir- and ritonavir-containing water and wastewater treated by photo-induced processes to Danio rerio and Allivibrio fischeri. Sci. Total Environ. 2022, 824, 153967. [Google Scholar] [CrossRef]
- Teymoorian, T.; Teymourian, T.; Kowsari, E.; Ramakrishna, S. Direct and indirect effects of SARS-CoV-2 on wastewater treatment. J. Water Process Eng. 2021, 42, 102193. [Google Scholar] [CrossRef]
- Yang, J.Z.Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Huang, F.L.Y.; Leung, E.L.; Liu, X.; Liu, K.; Wang, Q.; Lan, Y.; Li, X.; Yu, H.; Cui, L.; Luo, H.; et al. A review of therapeutic agents and Chinese herbal medicines against SARS-COV-2 (COVID-19). Pharm. Res. 2020, 158, 104929. [Google Scholar] [CrossRef]
- Elgohary, E.A.; Mohamed, Y.M.A.; El Nazer, H.A.; Baaloudj, O.; Alyami, M.S.S.; El Jery, A.; Assadi, A.A.; Amrane, A. A Review of the Use of Semiconductors as Catalysts in the Photocatalytic Inactivation of Microorganisms. Catalysts 2021, 11, 1498. [Google Scholar] [CrossRef]
- Sanvicens, N.; Marco, M.P. Multifunctional nanoparticles–properties and prospects for their use in human medicine. Trends Biotechnol. 2008, 26, 425–433. [Google Scholar] [CrossRef]
- Seabra, A.B.; Durán, N. Nanotoxicology of metal oxide nanoparticles. Metals 2015, 5, 934–975. [Google Scholar] [CrossRef] [Green Version]
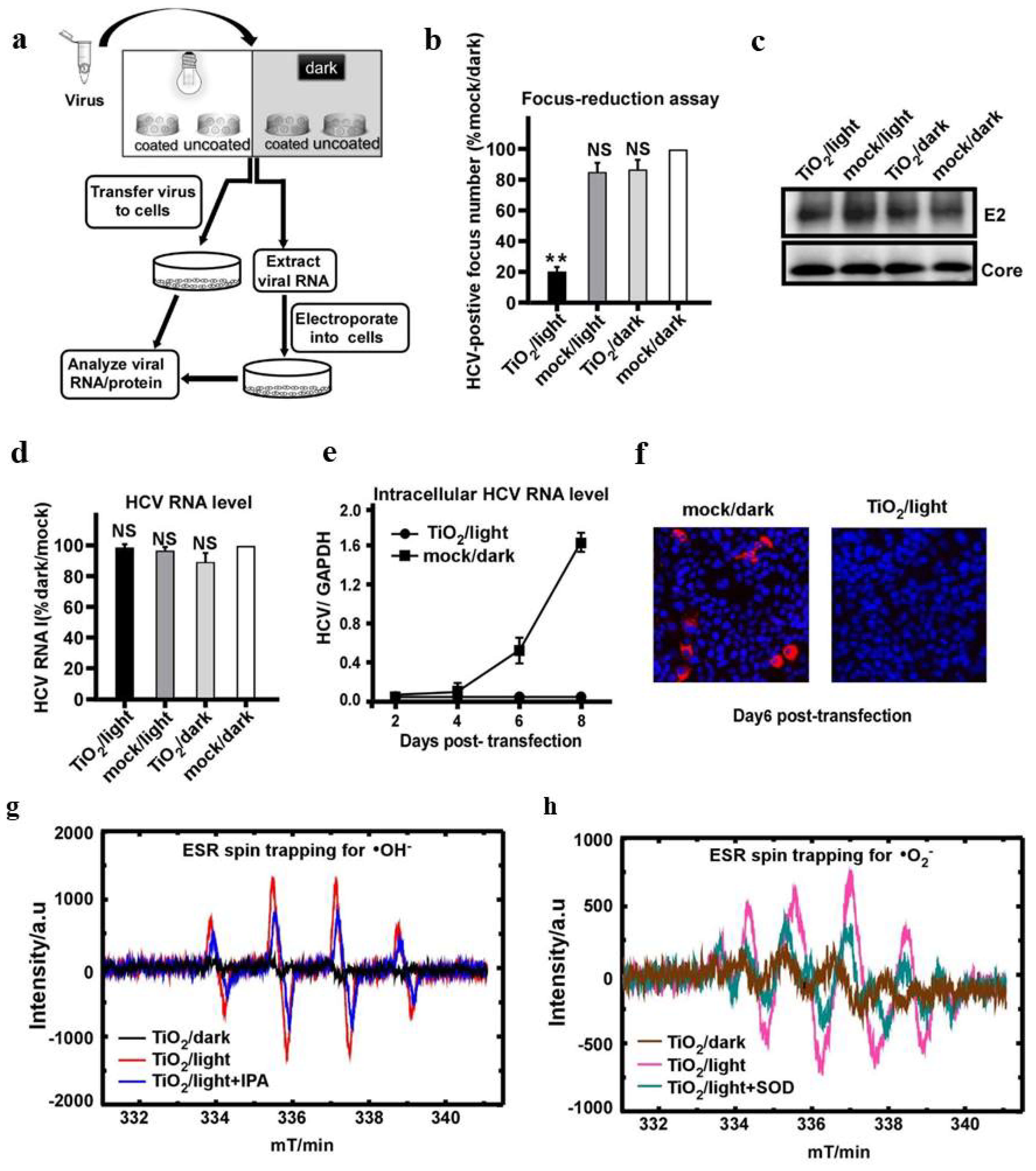


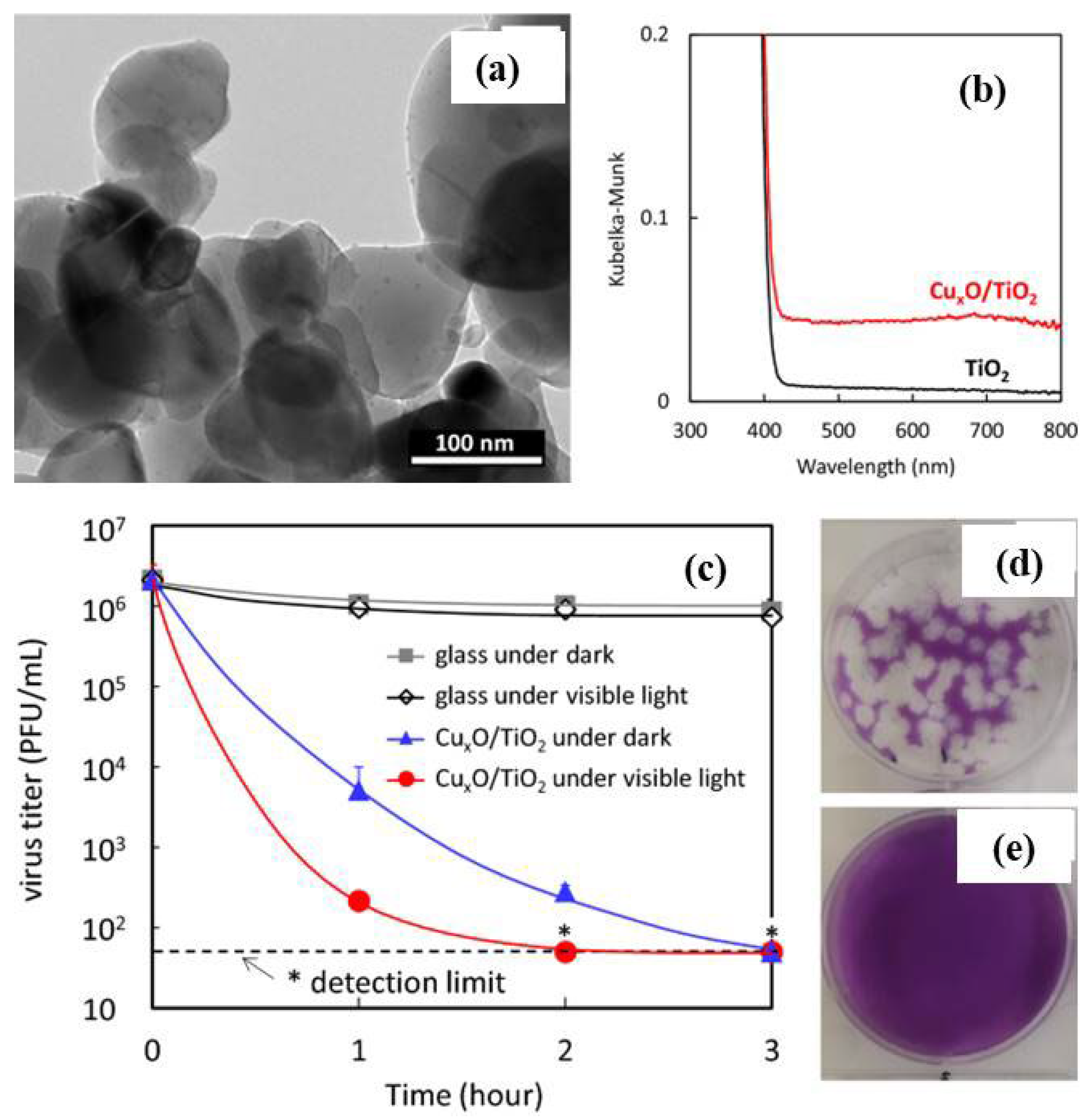
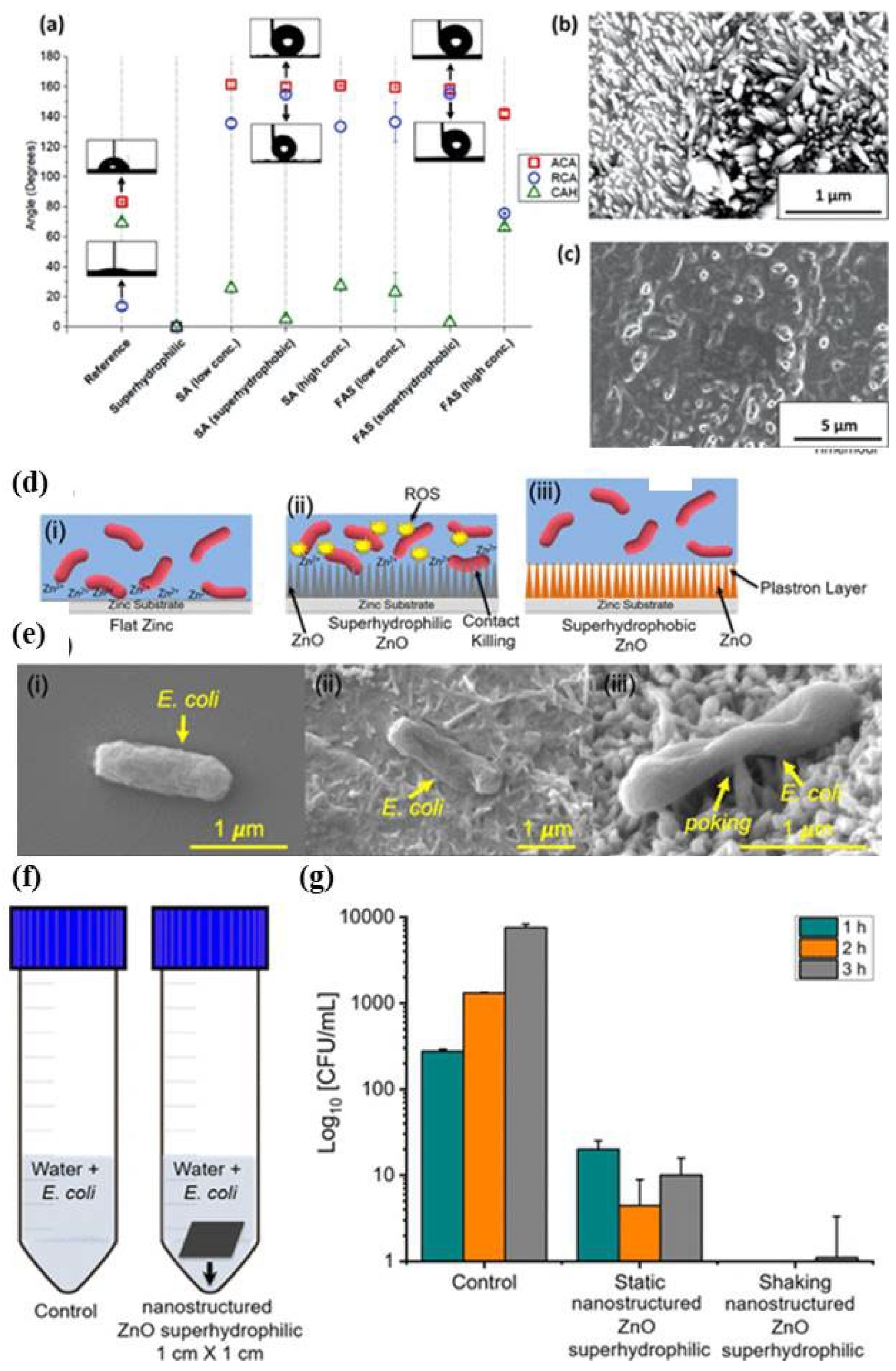
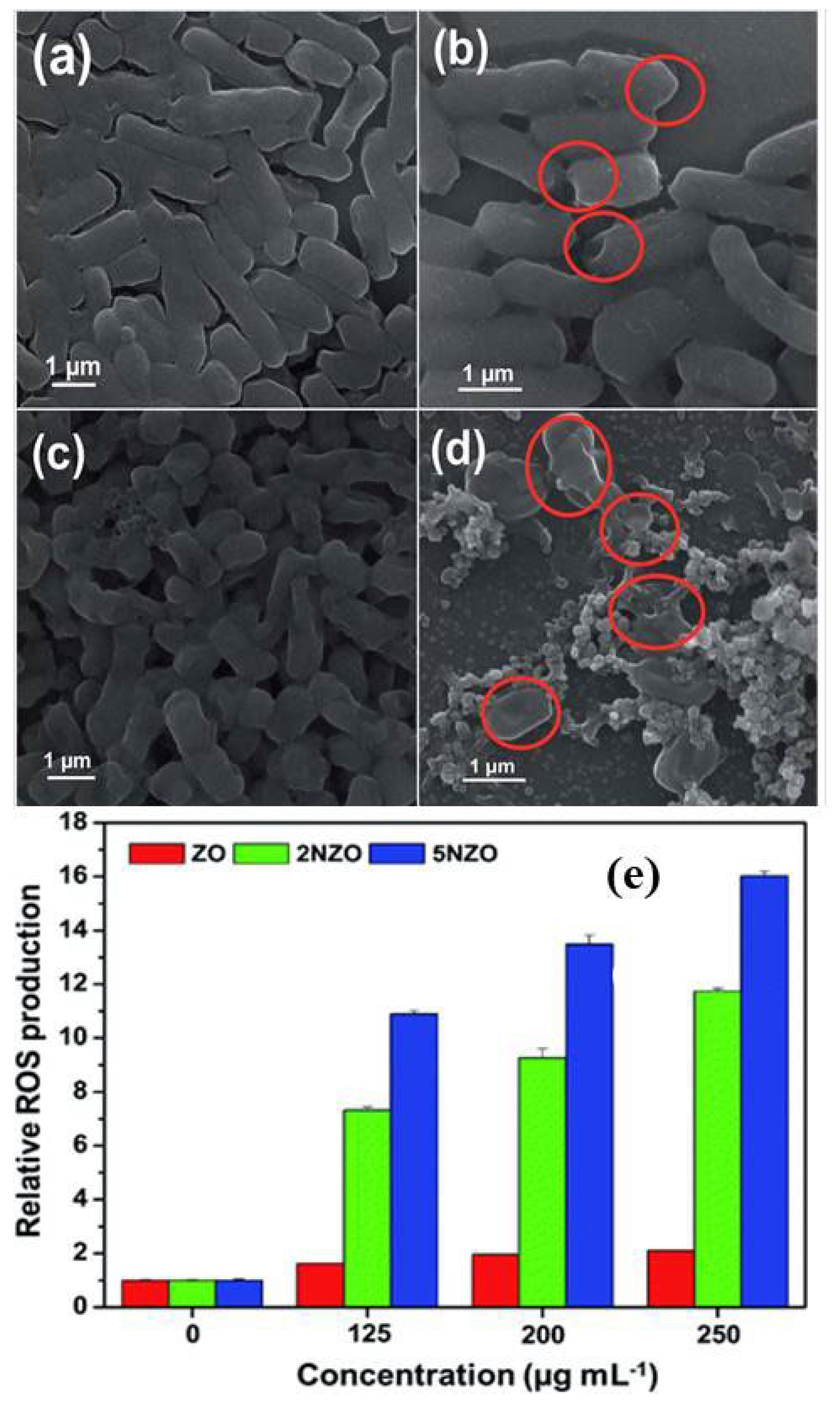
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakash, J.; Krishna, S.B.N.; Kumar, P.; Kumar, V.; Ghosh, K.S.; Swart, H.C.; Bellucci, S.; Cho, J. Recent Advances on Metal Oxide Based Nano-Photocatalysts as Potential Antibacterial and Antiviral Agents. Catalysts 2022, 12, 1047. https://doi.org/10.3390/catal12091047
Prakash J, Krishna SBN, Kumar P, Kumar V, Ghosh KS, Swart HC, Bellucci S, Cho J. Recent Advances on Metal Oxide Based Nano-Photocatalysts as Potential Antibacterial and Antiviral Agents. Catalysts. 2022; 12(9):1047. https://doi.org/10.3390/catal12091047
Chicago/Turabian StylePrakash, Jai, Suresh Babu Naidu Krishna, Promod Kumar, Vinod Kumar, Kalyan S. Ghosh, Hendrik C. Swart, Stefano Bellucci, and Junghyun Cho. 2022. "Recent Advances on Metal Oxide Based Nano-Photocatalysts as Potential Antibacterial and Antiviral Agents" Catalysts 12, no. 9: 1047. https://doi.org/10.3390/catal12091047
APA StylePrakash, J., Krishna, S. B. N., Kumar, P., Kumar, V., Ghosh, K. S., Swart, H. C., Bellucci, S., & Cho, J. (2022). Recent Advances on Metal Oxide Based Nano-Photocatalysts as Potential Antibacterial and Antiviral Agents. Catalysts, 12(9), 1047. https://doi.org/10.3390/catal12091047