Hydrogenation of Xylose to Xylitol in the Presence of Bimetallic Nanoparticles Ni3Fe Catalyst in the Presence of Choline Chloride
Abstract
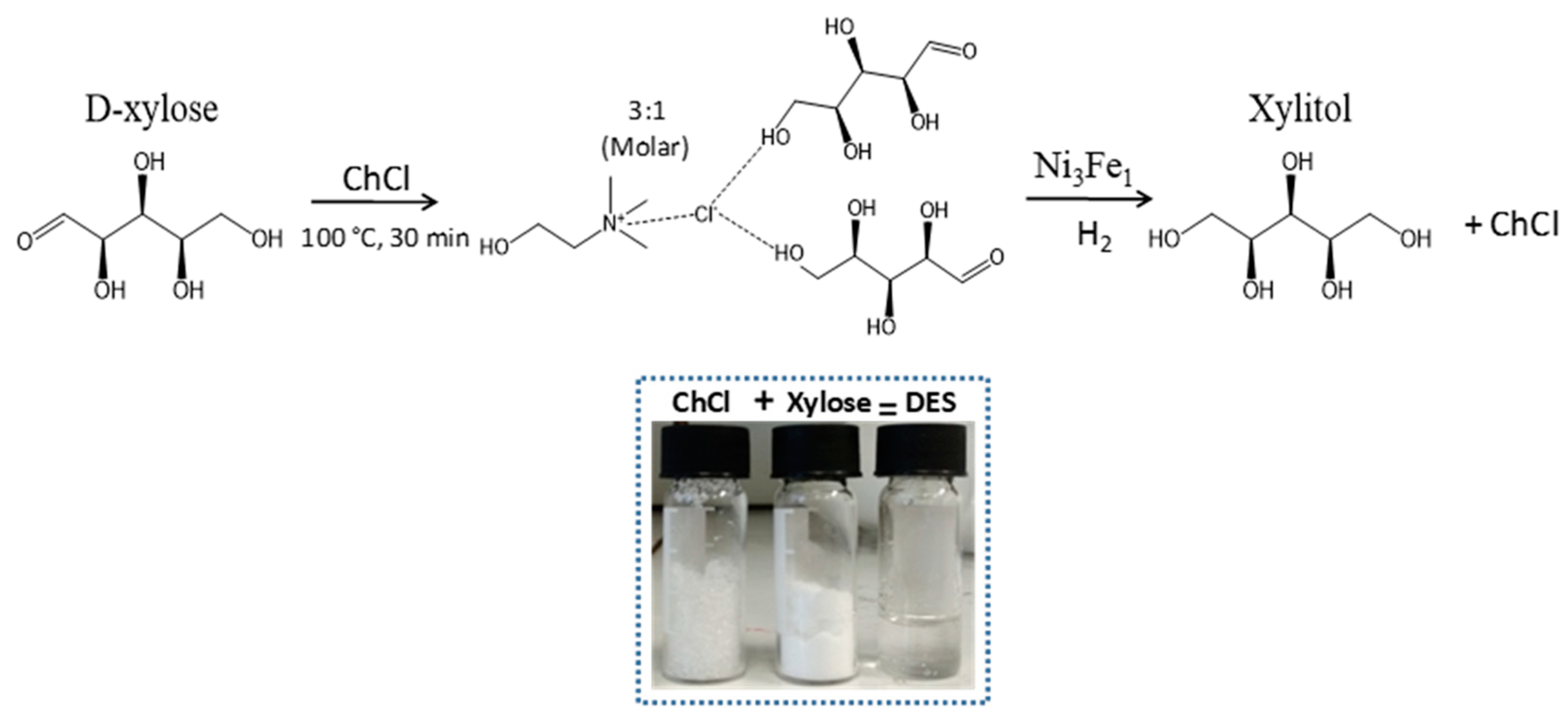
:1. Introduction
2. Results/Discussion
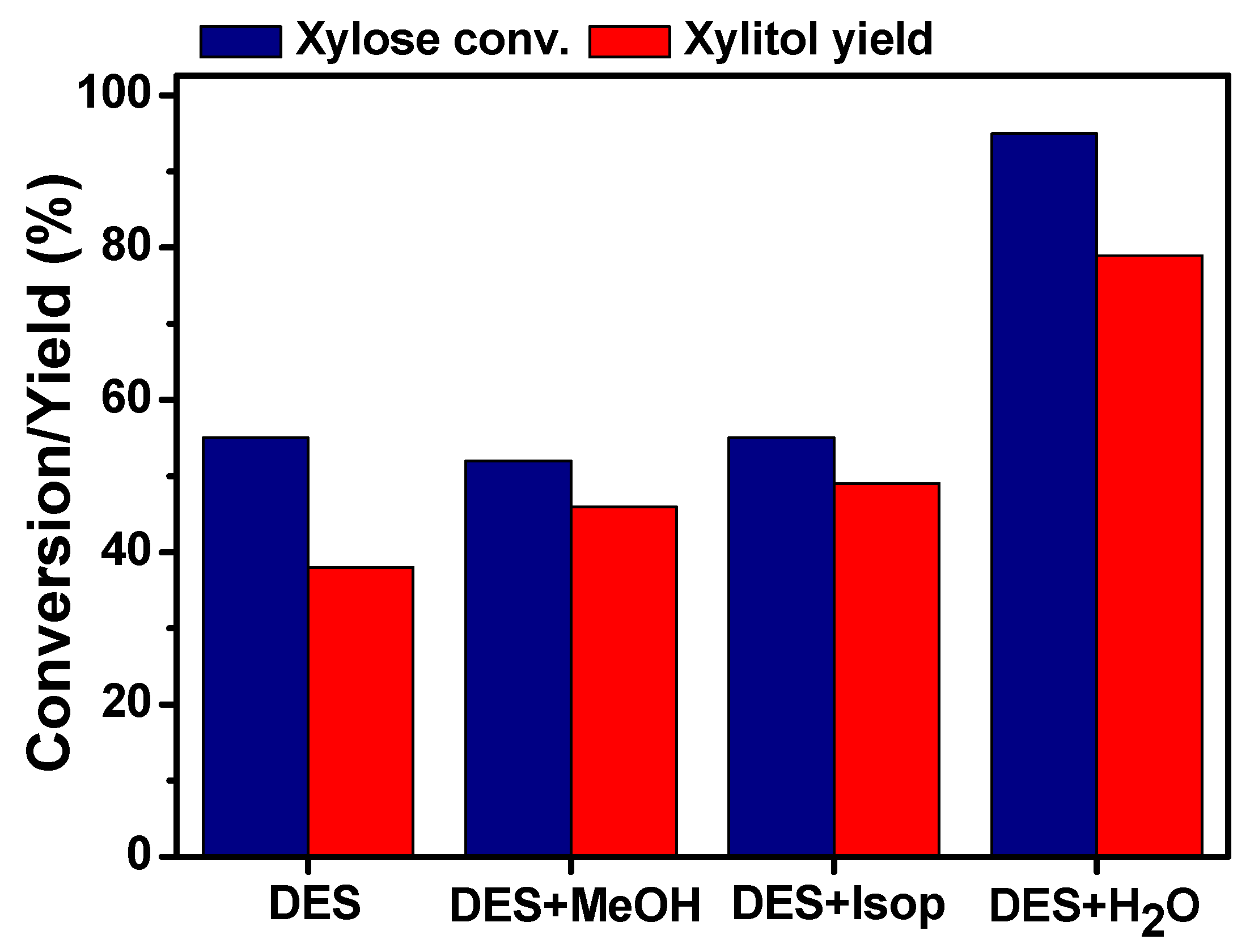
2.1. Effect of Reaction Media
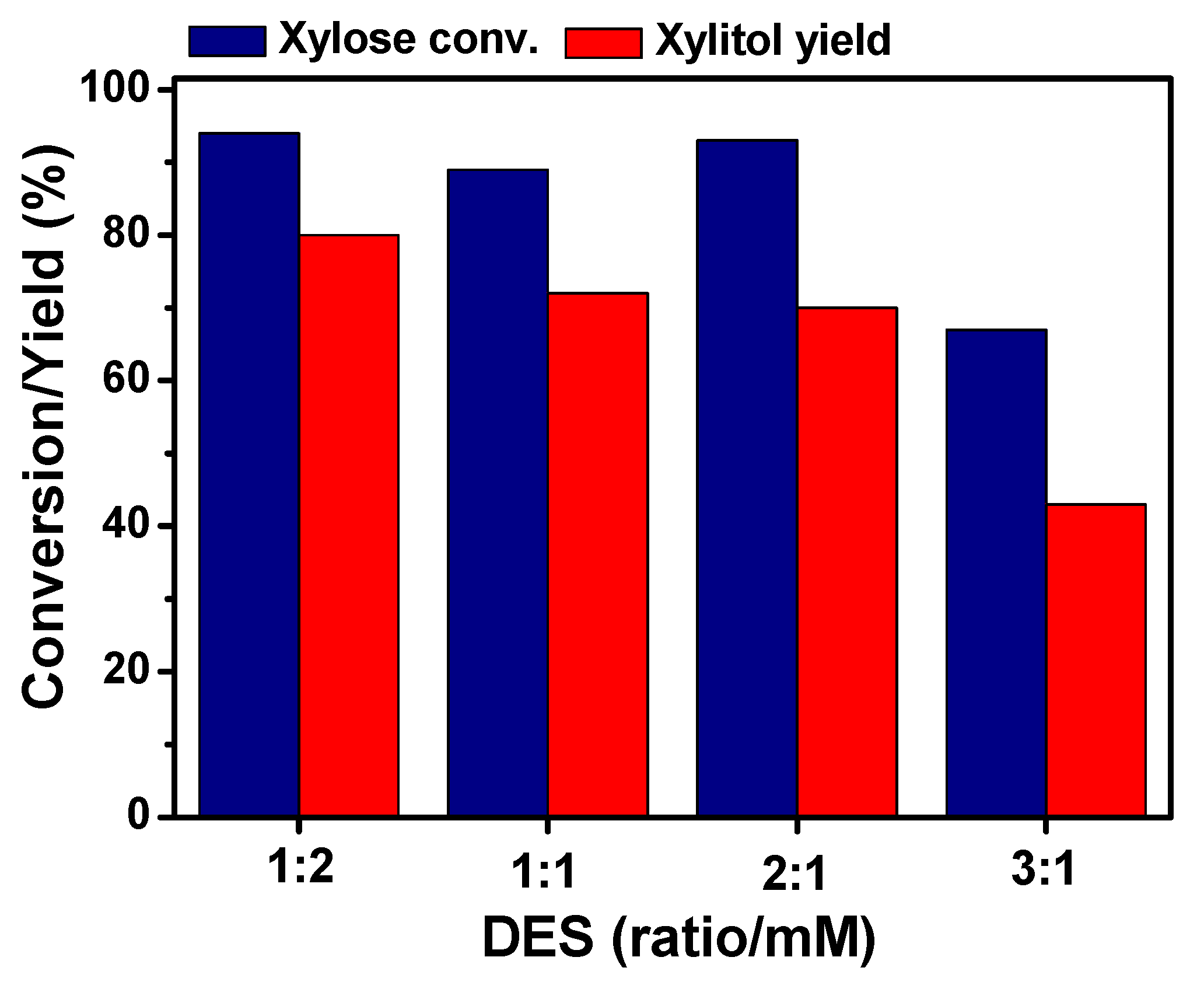
2.2. Effect of Molar Ratio D-Xylose/ChCl
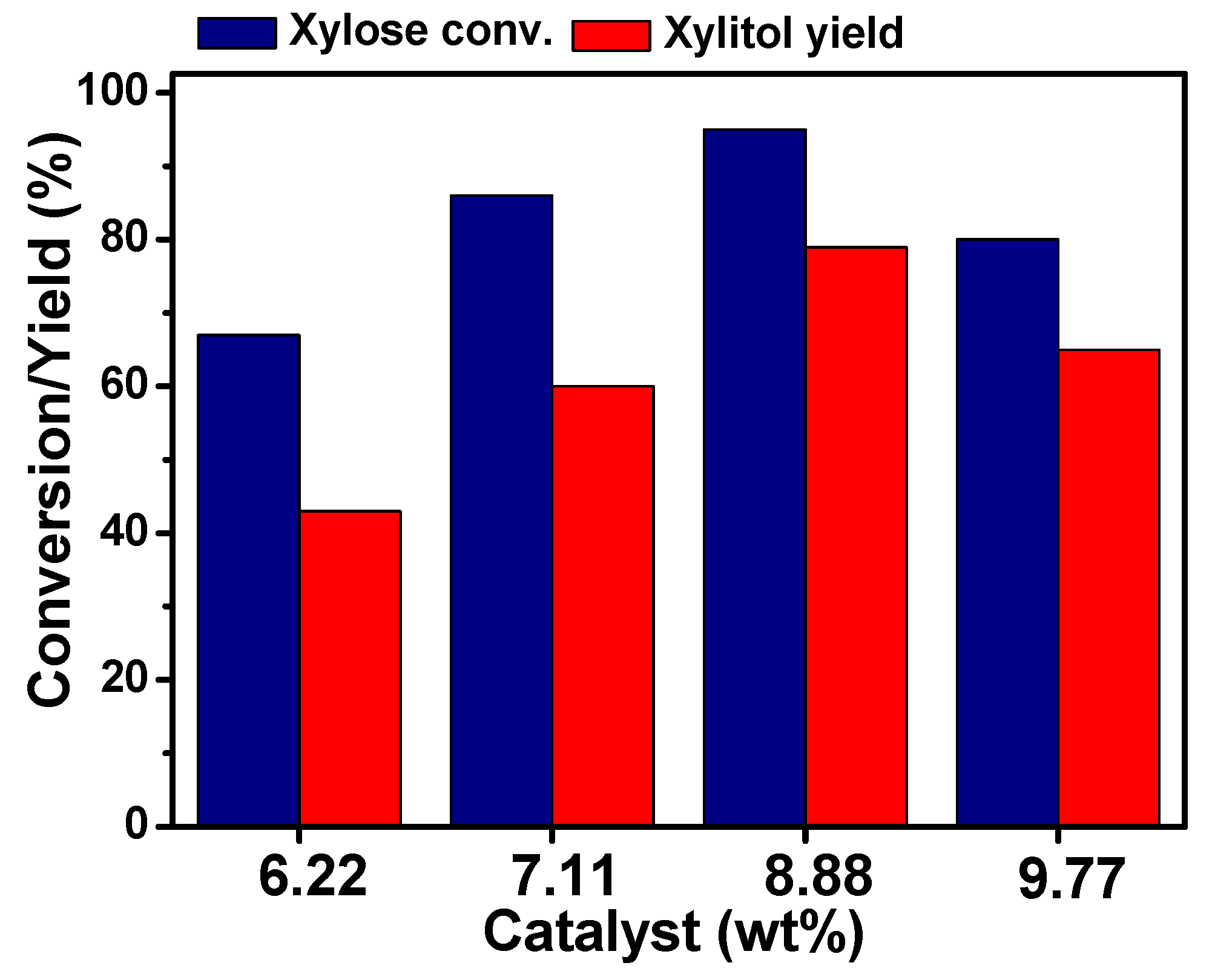
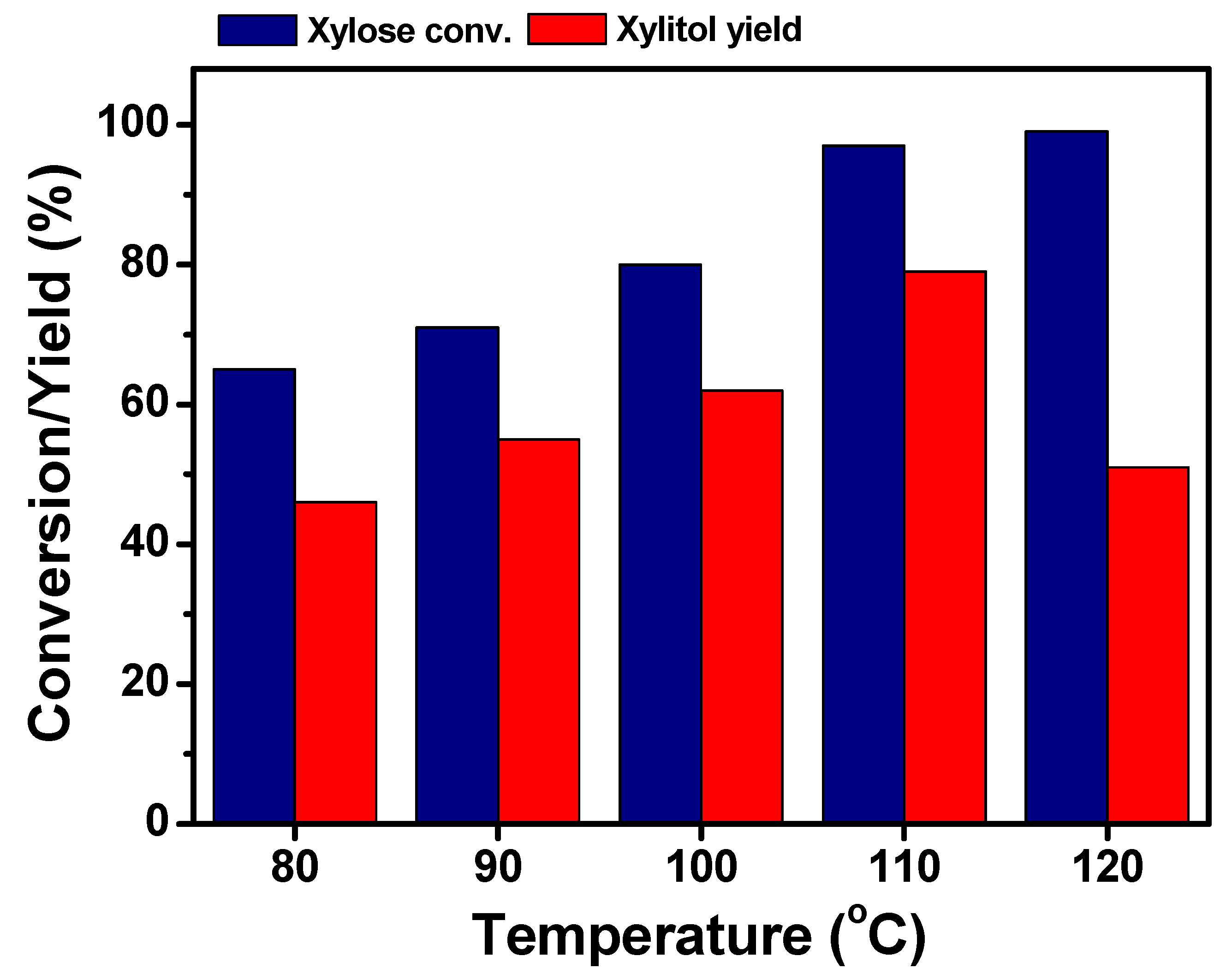
2.3. Effect of the Reaction Temperature
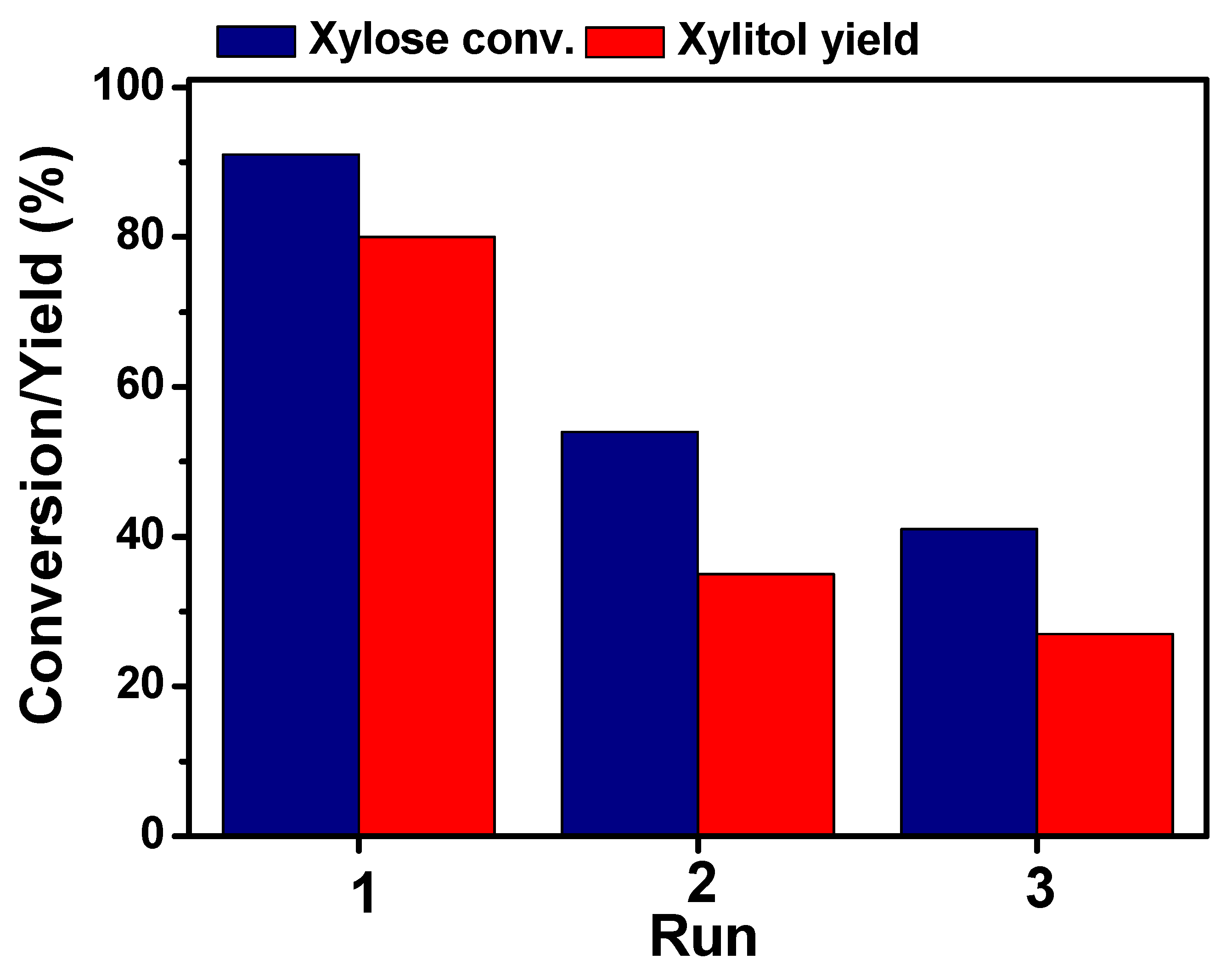
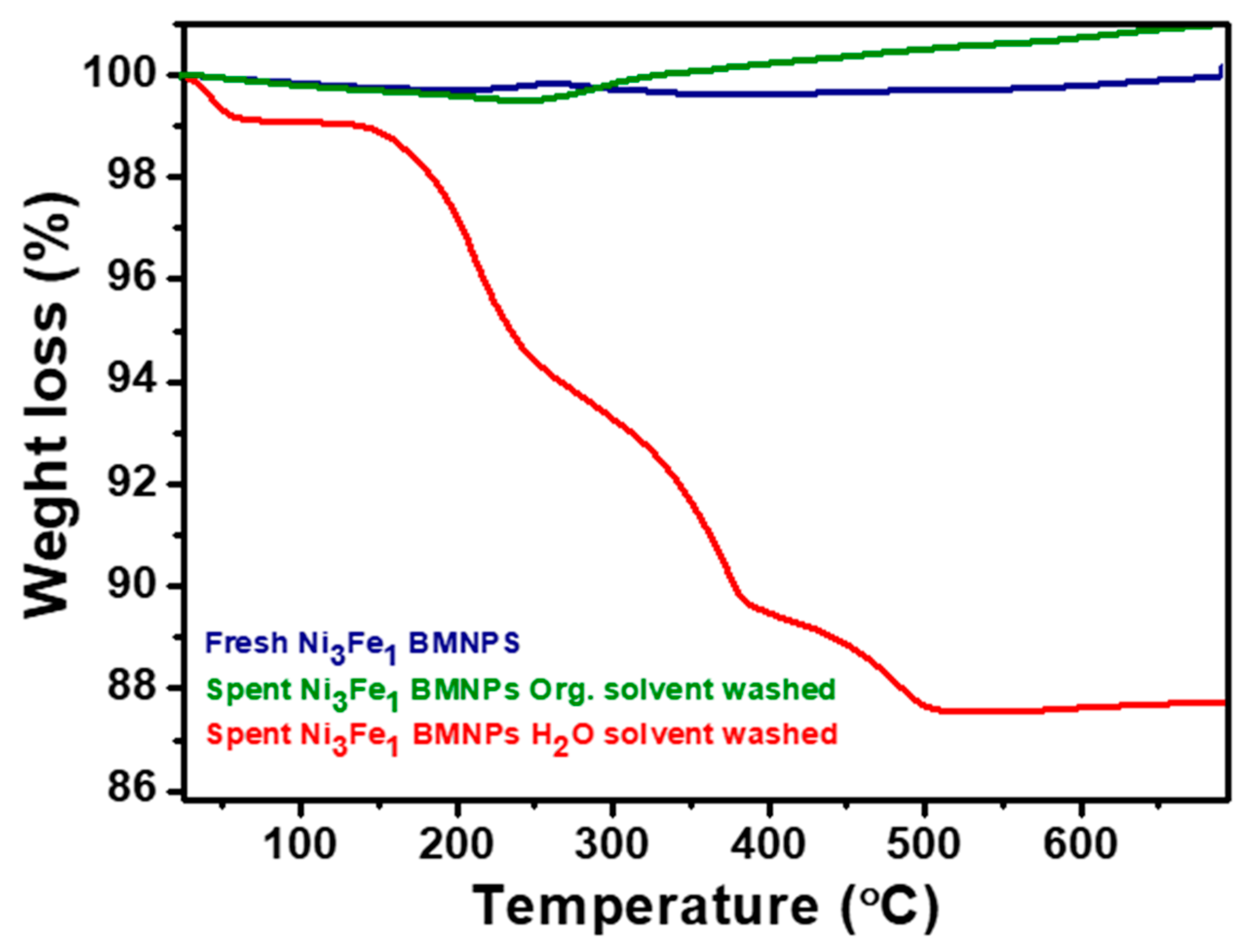
2.4. Stability of the Ni3Fe1 BMNPs
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Ni3Fe1 Bimetallic Nanoparticles (BMNPs)
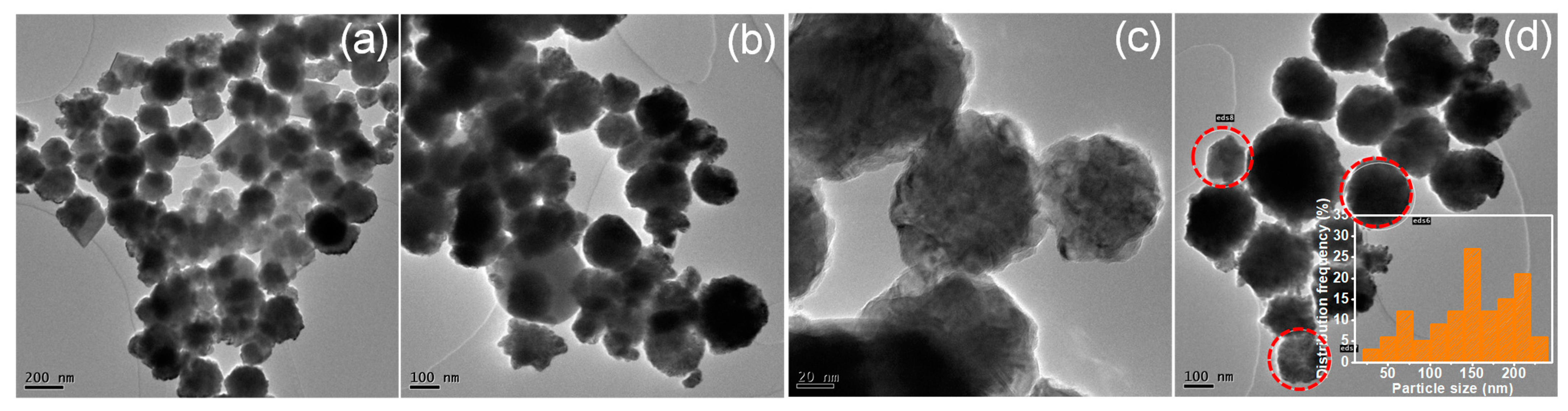
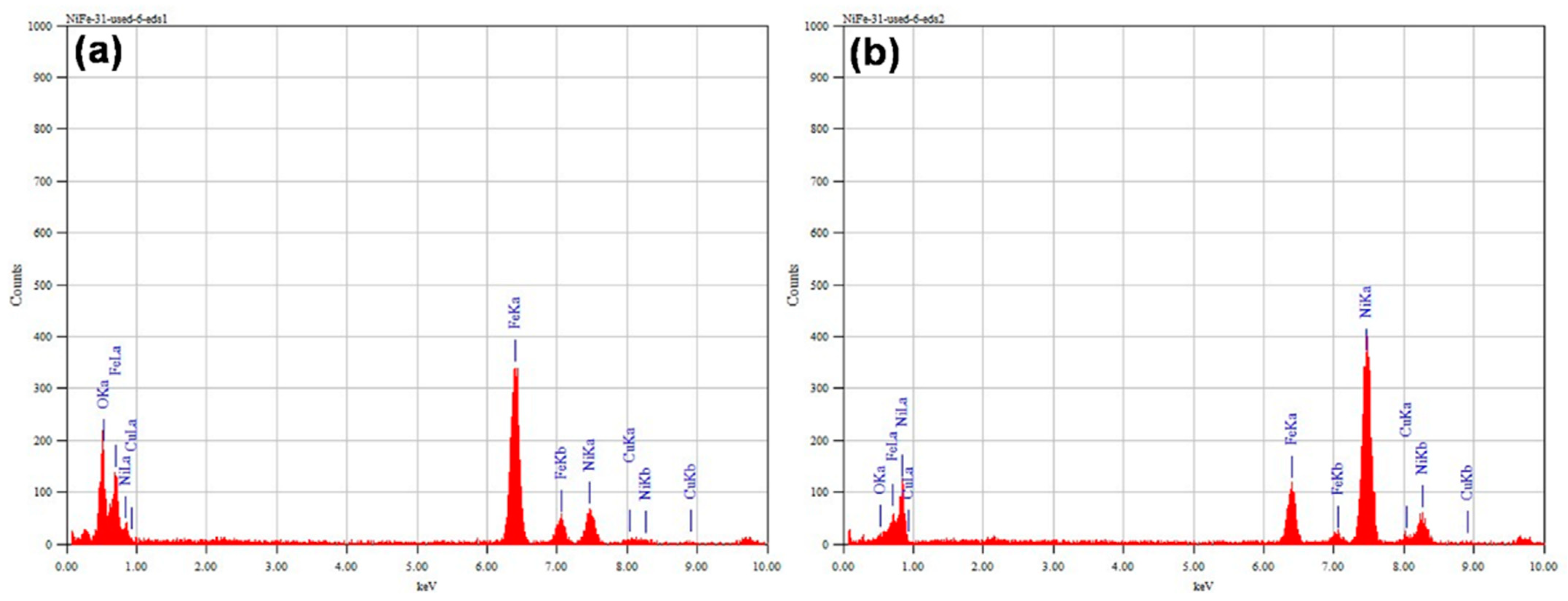
3.3. Catalyst Characterizations
3.4. Hydrogenation of Xylose to Xylitol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Questell-Santiago, Y.M.; Galkin, M.V.; Barta, K.; Luterbacher, J.S. Stabilization strategies in biomass depolymerization using chemical functionalization. Nat. Rev. Chem. 2020, 4, 311–330. [Google Scholar] [CrossRef]
- Stuhr, R.; Bayer, P.; Stark, C.B.W.; von Wangelin, A.J. Light-Driven Waste-To-Value Upcycling: Bio-Based Polyols and Polyurethanes from the Photo-Oxygenation of Cardanols. ChemSusChem 2021, 14, 3325–3332. [Google Scholar] [CrossRef] [PubMed]
- Kazachenko, A.; Akman, F.; Medimagh, M.; Issaoui, N.; Vasilieva, N.; Malyar, Y.N.; Sudakova, I.G.; Karacharov, A.; Miroshnikova, A.; Al-Dossary, O.M. Sulfation of Diethylaminoethyl-Cellulose: QTAIM Topological Analysis and Experimental and DFT Studies of the Properties. ACS Omega 2021, 6, 22603–22615. [Google Scholar] [CrossRef]
- Fineberg, S.J.; Nandyala, S.V.; Marquez-Lara, A.; Oglesby, M.; Patel, A.A.; Singh, K. Incidence and risk factors for postoperative delirium after lumbar spine surgery (Phila Pa 1976). Spine 2013, 38, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Lari, G.M.; Gröninger, O.G.; Li, Q.; Mondelli, C.; López, N.; Pérez-Ramírez, J. Catalyst and Process Design for the Continuous Manufacture of Rare Sugar Alcohols by Epimerization-Hydrogenation of Aldoses. ChemSusChem 2016, 9, 3407–3418. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Sato, O.; Mimura, N.; Shirai, M. Catalytic production of sugar alcohols from lignocellulosic biomass. Catal. Today 2016, 265, 199–202. [Google Scholar] [CrossRef]
- Audemar, M.; Ramdani, W.; Junhui, T.; Ifrim, A.R.; Ungureanu, A.; Jérôme, F.; Royer, S.; De Oliveira Vigier, K. Selective Hydrogenation of Xylose to Xylitol over Co/SiO 2 Catalysts. ChemCatChem 2020, 12, 1973–1978. [Google Scholar] [CrossRef]
- Sun, J.; Liu, H. Selective hydrogenolysis of biomass-derived xylitol to ethylene glycol and propylene glycol on supported Ru catalysts. Green Chem. 2011, 13, 135–142. [Google Scholar] [CrossRef]
- Hilpmann, G.; Steudler, S.; Ayubi, M.M.; Pospiech, A.; Walther, T.; Bley, T.; Lange, R. Combining Chemical and Biological Catalysis for the Conversion of Hemicelluloses: Hydrolytic Hydrogenation of Xylan to Xylitol. Catal. Lett. 2019, 149, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Mikkola, J.-P.; Vainio, H.; Salmi, T.; Sjöholm, R.; Ollonqvist, T.; Väyrynen, J. Deactivation kinetics of Mo-supported Raney Ni catalyst in the hydrogenation of xylose to xylitol. Appl. Catal. A Gen. 2000, 196, 143–155. [Google Scholar] [CrossRef]
- Lee, G.D.; Suh, C.S.; Park, J.H.; Park, S.S.; Hong, S.S. Raney Ni catalysts derived from different alloy precursors (I) morphology and characterization. Korean J. Chem. Eng. 2005, 22, 375–381. [Google Scholar] [CrossRef]
- Shi, D.; Wojcieszak, R.; Paul, S.; Marceau, E. Ni Promotion by Fe: What Benefits for Catalytic Hydrogenation? Catalysts 2019, 9, 451. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.; Oh, E.; Lim, C.; Shim, S.E.; Baeck, S.-H. Bimetallic NiFe alloys as highly efficient electrocatalysts for the oxygen evolution reaction. Catal. Today 2020, 352, 27–33. [Google Scholar] [CrossRef]
- Bian, Z.; Das, S.; Wai, M.H.; Hongmanorom, P.; Kawi, S. A Review on Bimetallic Nickel-Based Catalysts for CO2 Reforming of Methane. ChemPhysChem 2017, 18, 3117–3134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Ali, N.; Khan, I.; Zhang, B.; Sadiq, M. Heterogeneous photodegradation of industrial dyes: An insight to different mechanisms and rate affecting parameters. J. Environ. Chem. Eng. 2020, 8, 104364. [Google Scholar] [CrossRef]
- Liu, Y.; Deak, N.; Wang, Z.; Yu, H.; Hameleers, L.; Jurak, E.; Deuss, P.J.; Barta, K. Tunable and functional deep eutectic solvents for lignocellulose valorization. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Larriba, M.; Ayuso, M.; Navarro, P.; Delgado-Mellado, N.; Gonzalez-Miquel, M.; García, J.; Rodríguez, F. Choline Chloride-Based Deep Eutectic Solvents in the Dearomatization of Gasolines. ACS Sustain. Chem. Eng. 2017, 6, 1039–1047. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Quest for Green-Solvent Design: From Hydrophilic to Hydrophobic (Deep) Eutectic Solvents. ChemSusChem 2019, 12, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Verrier, C.; Ahmar, M.; Lai, J.; Ma, C.; Muller, E.; Queneau, Y.; Pera-Titus, M.; Jérôme, F.; Vigier, K.D.O. Unveiling the role of choline chloride in furfural synthesis from highly concentrated feeds of xylose. Green Chem. 2018, 20, 5104–5110. [Google Scholar] [CrossRef]
- Sadier, A.; Shi, D.; Mamede, A.-S.; Paul, S.; Marceau, E.; Wojcieszak, R. Selective aqueous phase hydrogenation of xylose to xylitol over SiO2-supported Ni and Ni-Fe catalysts: Benefits of promotion by Fe. Appl. Catal. B Environ. 2021, 298, 120564. [Google Scholar] [CrossRef]
- Liu, L.; Gao, F.; Concepción, P.; Corma, A. A new strategy to transform mono and bimetallic non-noble metal nanoparticles into highly active and chemoselective hydrogenation catalysts. J. Catal. 2017, 350, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Laaksonen, A.; Liu, C.; Lu, X.; Ji, X. The peculiar effect of water on ionic liquids and deep eutectic solvents. Chem. Soc. Rev. 2018, 47, 8685–8720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monroe, D.C.; Blumenfeld, R.S.; Keator, D.B.; Solodkin, A.; Small, S.L. One season of head-to-ball impact exposure alters functional connectivity in a central autonomic network. NeuroImage 2020, 223, 117306. [Google Scholar] [CrossRef]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The effect of water upon deep eutectic solvent nanostructure: An unusual transition from ionic mixture to aqueous solution. Angew. Chem. Int. Ed. 2017, 56, 9782–9785. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Hemmateenejad, B.; Safavi, A.; Shojaeifard, Z.; Shahsavar, A.; Mohajeri, A.; Dokoohaki, M.H.; Zolghadr, A.R. Deep eutectic–water binary solvent associations investigated by vibrational spectroscopy and chemometrics. Phys. Chem. Chem. Phys. 2018, 20, 18463–18473. [Google Scholar] [CrossRef]
- Zhekenov, T.; Toksanbayev, N.; Kazakbayeva, Z.; Shah, D.; Mjalli, F.S. Formation of type III Deep Eutectic Solvents and effect of water on their intermolecular interactions. Fluid Phase Equilib. 2017, 441, 43–48. [Google Scholar] [CrossRef]
- Gao, Q.; Zhu, Y.; Ji, X.; Zhu, W.; Lu, L.; Lu, X. Effect of water concentration on the microstructures of choline chloride/urea (1:2) /water mixture. Fluid Phase Equilib. 2018, 470, 134–139. [Google Scholar] [CrossRef]
- Mikkola, J.-P.; Salmi, T.; Sjöholm, R.; Mäki-Arvela, P.; Vainio, H. Hydrogenation of xylose to xylitol: Three-phase catalysis by promoted raney nickel, catalyst deactivation and in-situ sonochemical catalyst rejuvenation. In Studies in Surface Science and Catalysis; Elsevier: Granada, Spain, 2000; Volume 130, pp. 2027–2032. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P. Deactivation of metal catalysts in liquid phase organic reactions. Catal. Today 2003, 81, 547–559. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Silva, L.P.; Fernandez, L.; Conceição, J.; Martins, M.A.R.; Sosa, A.; Ortega, J.; Pinho, S.P.; Coutinho, J.A.P. Design and Characterization of Sugar-Based Deep Eutectic Solvents Using Conductor-like Screening Model for Real Solvents. ACS Sustain. Chem. Eng. 2018, 6, 10724–10734. [Google Scholar] [CrossRef] [Green Version]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]

| Ni3Fe1 BMNPs | Fe (238, 204 nm) mg/L | Ni (216, 555 nm) mg/L |
|---|---|---|
| 1 | 69.9 | 8.7 |
| 2 | 74.8 | 27.0 |
| 3 | 71.3 | 17.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, N.; Jérôme, F.; De Oliveira Vigier, K. Hydrogenation of Xylose to Xylitol in the Presence of Bimetallic Nanoparticles Ni3Fe Catalyst in the Presence of Choline Chloride. Catalysts 2022, 12, 841. https://doi.org/10.3390/catal12080841
Ullah N, Jérôme F, De Oliveira Vigier K. Hydrogenation of Xylose to Xylitol in the Presence of Bimetallic Nanoparticles Ni3Fe Catalyst in the Presence of Choline Chloride. Catalysts. 2022; 12(8):841. https://doi.org/10.3390/catal12080841
Chicago/Turabian StyleUllah, Naseeb, François Jérôme, and Karine De Oliveira Vigier. 2022. "Hydrogenation of Xylose to Xylitol in the Presence of Bimetallic Nanoparticles Ni3Fe Catalyst in the Presence of Choline Chloride" Catalysts 12, no. 8: 841. https://doi.org/10.3390/catal12080841
APA StyleUllah, N., Jérôme, F., & De Oliveira Vigier, K. (2022). Hydrogenation of Xylose to Xylitol in the Presence of Bimetallic Nanoparticles Ni3Fe Catalyst in the Presence of Choline Chloride. Catalysts, 12(8), 841. https://doi.org/10.3390/catal12080841








