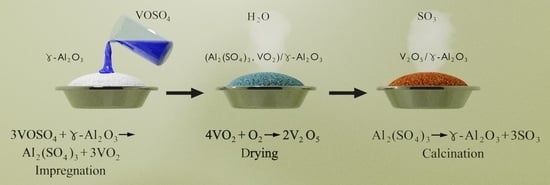
Synthesis of Vanadium-Containing Catalytically Active Phases for Exhaust Gas Neutralizers of Motor Vehicles and Industrial Enterprises
Abstract
:1. Introduction
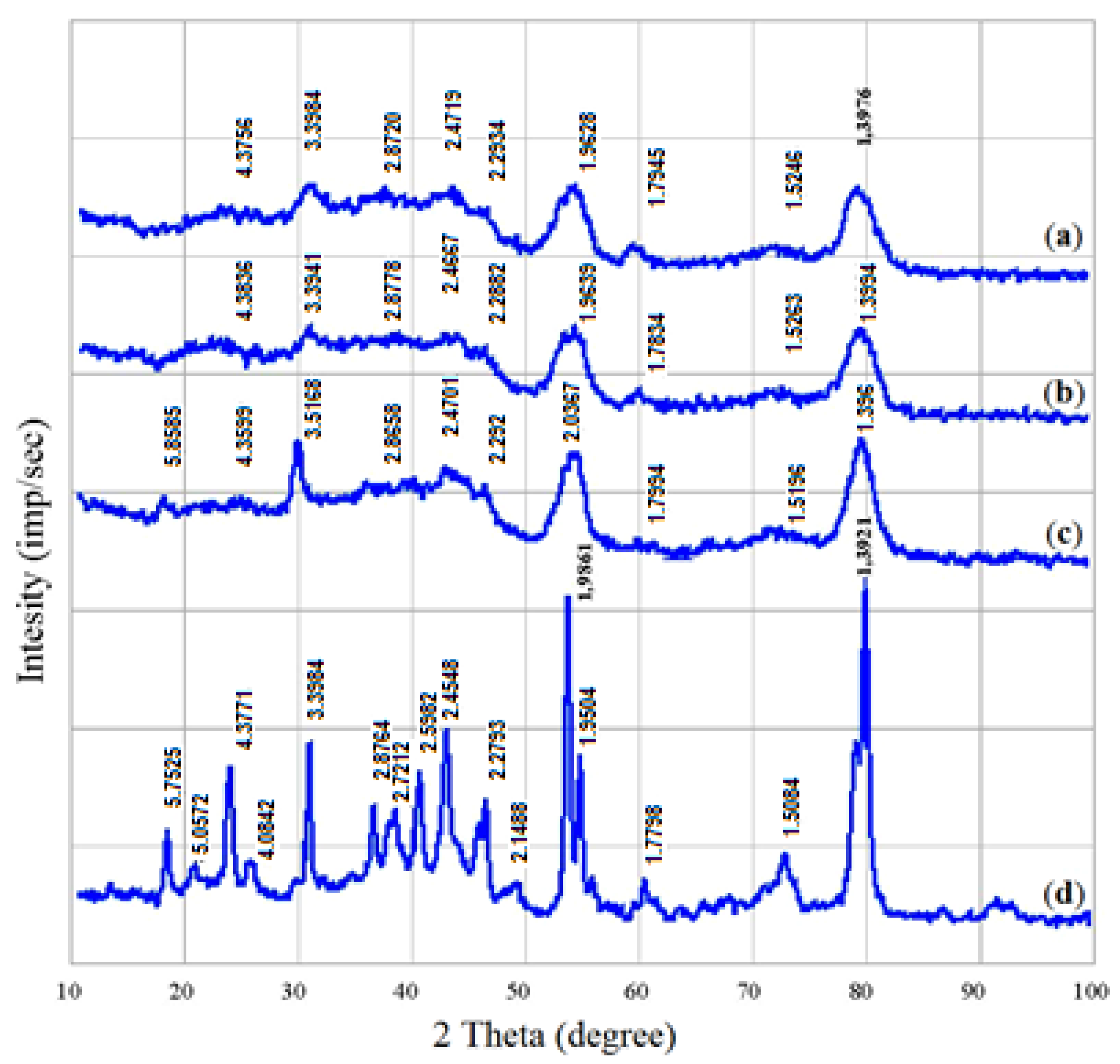
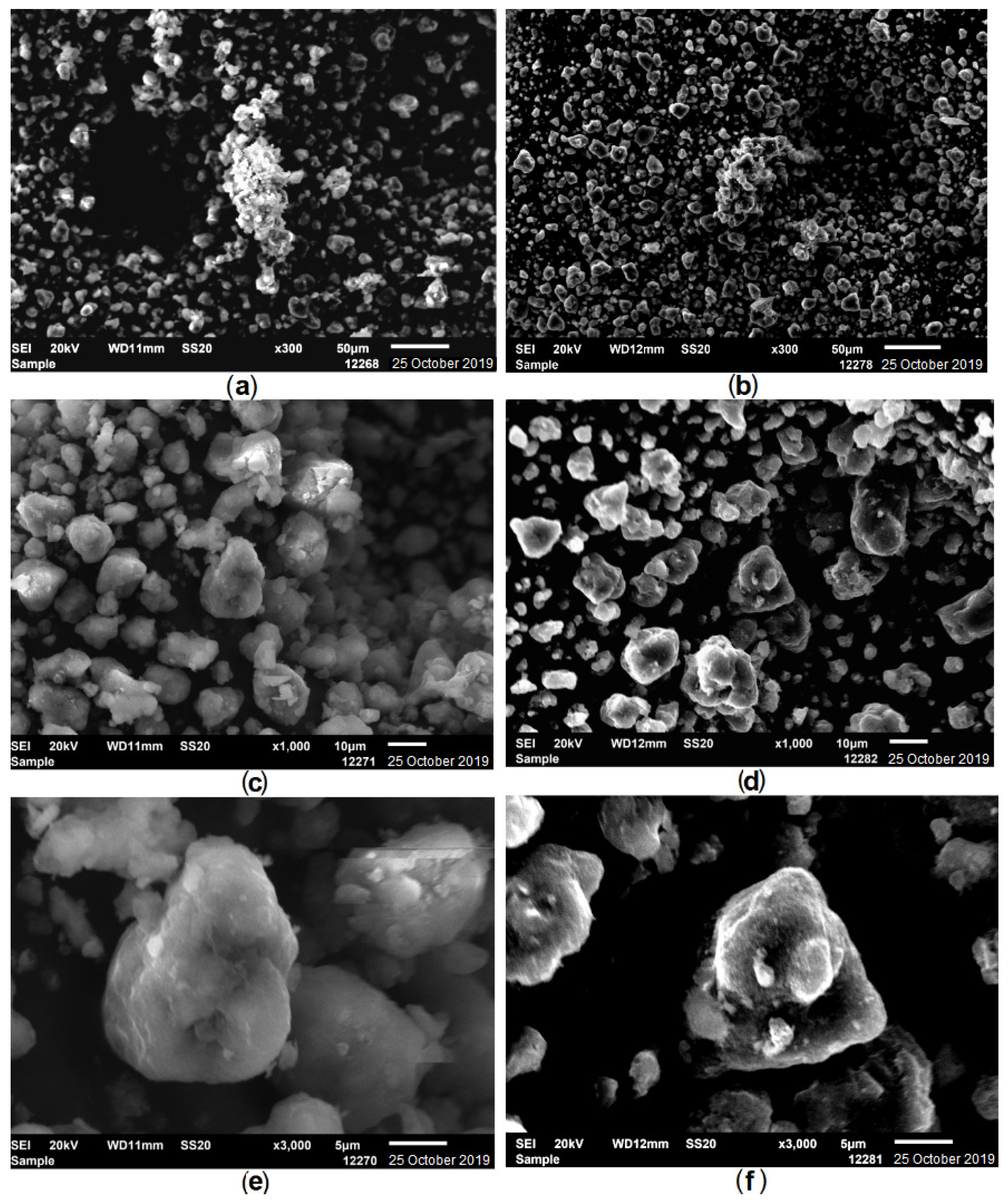
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garbowski, E.; Guenin, M.; Marion, M.; Primet, M. Catalytic properties and surface states of cobalt-containing oxidation catalysts. Appl. Catal. 1990, 64, 209–224. [Google Scholar] [CrossRef]
- Zeng, H.; Ji, L.; Lin, J. Metal−support interactions in Co/Al2O3 catalysts. A comparative study on reactivity of support. Phys. Chem. 2000, 104, 1783–1790. [Google Scholar]
- Thormählen, P.; Skoglundh, M.; Fridell, E.; Andersson, B. Low-temperature CO oxidation over platinum and cobalt oxide catalysts. J. Catal. 1999, 188, 300–310. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T.; Papadopoulou, C.; Batista, J.; Hocevar, S.; Matralis, H.K. A Comparative study of Pt/γ-Al2O3, Au/α-Fe2O3 and CuO–CeO2 catalysts for the selective oxidation of carbon monoxide in excess hydrogen. Catal. Today 2002, 75, 157–167. [Google Scholar] [CrossRef]
- Jansson, J. Low-temperature CO oxidation over Co3O4/Al2O3. J. Catal. 2000, 194, 55–60. [Google Scholar] [CrossRef]
- Ma, W.; Jacobs, G.; Keogh, R.A.; Bukur, D.B.; Davis, B.H. Fischer-Tropsch synthesis: Effect of Pd, Pt, Re, and Ru noble metal promoters on the activity and selectivity of a 25% Co/Al2O3 catalyst. Appl. Catal. A Gen. 2012, 437, 1–9. [Google Scholar] [CrossRef]
- Rattan, G.; Kumar, M. Carbon monoxide oxidation using cobalt catalysts. Chem. Chem. Technol. 2014, 8, 249–260. [Google Scholar] [CrossRef]
- Zavyalova, U.F.; Tretyakov, V.F.; Burdeinaya, T.N.; Lunin, V.V.; Titkov, A.I.; Salanov, A.N.; Ryzhova, N.D.; Tsyrulnikov, P.G. Oxidation of CO, deep oxidation of CH4 and reduction of NOx by propane on CuCo2O4 and Pd-CeO2 catalysts supported on tape carriers. Pet. Chem. 2005, 45, 281–286. (In Russian) [Google Scholar]
- Zavyalova, U.F.; Petrov, V.F.; Burdeinaya, T.N.; Tsyrulnikov, T.N. Block catalysts for neutralization of exhaust gases synthesized by the combustion method. Chem. Sustain. Dev. 2005, 13, 751–755. (In Russian) [Google Scholar]
- Noskov, A.S.; Pai, Z.P. Technological Methods for Protecting the Atmosphere from Harmful Emissions at Energy Enterprises; SB RAS: Novosibirsk, Russia, 1996; p. 156. (In Russian) [Google Scholar]
- Popova, N.M. Catalysts for the Purification of Gas Emissions from Industrial Production; Nauka: Alma-Ata, Kazakh SSR, 1991; p. 176. (In Russian) [Google Scholar]
- Borshch, V.N.; Pugacheva, E.V.; Zhuk, S.Y.; Andreev, D.E.; Sanin, V.N.; Yukhvid, V.I. Multicomponent metal catalysts for deep oxidation of carbon monoxide and hydrocarbons. Proc. Acad. Sci. 2008, 419, 775–777. (In Russian) [Google Scholar] [CrossRef]
- Vaneman, G.L. Comparison of metal foil and ceramic monolith automotive catalytic converters. Catal. Automot. Pollut. Control II 1991, 71, 537–555. [Google Scholar]
- Anders, J.J.; Palmqvist, E.C.; Fridell, E.; Skoglundh, M.; Österlund, L.; Thormählen, P.; Langer, V. On the catalytic activity of Co3O4 in low-temperature CO oxidation. J. Catal. 2002, 211, 387–397. [Google Scholar]
- Wang, H.F.; Kavanagh, R.; Guo, Y.L.; Guo, Y.; Hu, P. Synthesis-structure-activity relationships in Co3O4 catalyzed CO oxidation. J. Catal. 2012, 296, 110–119. [Google Scholar] [CrossRef]
- Ozkara, S.; Akin, A.N.; Misirli, Z.; Aksoylu, A.E. The effect of metal loading on structural characteristics and low temperature CO oxidation activity of coprecipitated Co/Al2O3. Turk. J. Chem. 2005, 29, 219–224. [Google Scholar]
- Oliveira, H.A.; Franceschini, D.F.; Passos, F.B. Cobalt catalyst characterization for methane decomposition and carbon nanotube growth. J. Brazil. Chem. Soc. 2014, 25, 53–65. [Google Scholar] [CrossRef]
- Vosoughi, V.; Dalai, A.K.; Abatzoglou, N.; Hu, Y. Performances of promoted cobalt catalysts supported on mesoporous alumina for Fischer-Tropsch synthesis. Appl. Catal. A Gen 2017, 547, 155–163. [Google Scholar] [CrossRef]
- Kurzina, I.A. Deep oxidation of methane on platinum and palladium catalysts supported on silicon nitride. Bull. Tomsk. Polytech. Univ. 2005, 308, 104–109. [Google Scholar]
- Ivanova, N.D.; Ivanov, S.V.; Boldyrev, E.I.; Sokolsky, G.V.; Makeeva, I.S. Highly efficient manganese oxide catalysts for the CO oxidation reaction. J. Appl. Chem. 2002, 75, 1452–1455. [Google Scholar]
- Olsson, L.; Fridell, H.; Skoglundh, M.; Andersson, B. A kinetic study of NO oxidation and NOx storage on Pt/Al2O3 and Pt/BaO/Al2O3. J. Phys. Chem. B 2001, 105, 6895–6906. [Google Scholar] [CrossRef]
- Massenova, A.; Kalykberdiyev, M.; Ussenov, A.; Sass, A.; Kenzin, N.; Kanatbayev, E.; Baiken, A. Catalytic technologies for the production of eco-friendly gasolines and reducing the toxicity of vehicle exhaust gases. Orient. J. Chem. 2019, 35, 351–357. [Google Scholar] [CrossRef]
- Gruber, M.; Hermann, K. Elementary steps of the catalytic NOx reduction with NH3: Cluster studies on reaction paths and energetics at vanadium oxide substrate. J. Chem. Phys. 2013, 139, 244701–244708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinkin, P.; Kovalenko, O.; Lapina, O.; Khabibulin, D.; Kundo, N. Kinetic peculiarities in the low-temperature oxidation of H2S over vanadium catalysts. J. Mol. Catal. A Chem. 2002, 178, 173–180. [Google Scholar] [CrossRef]
- León, M.; Jiménez-Jiménez, J.; Jiménez-López, A.; Rodríguez-Castellón, E.; Soriano, D.; Nieto, J.M.L. Vanadium oxide-porous phosphate heterostructure catalysts for the selective oxidation of H2S to sulphur. Solid State Sci. 2010, 12, 996–1001. [Google Scholar] [CrossRef]
- Barba, D.; Palma, V.; Ciambelli, P. Screening of catalysts for H2S abatement from biogas to feed molten carbonate fuel cell. Int. J. Hydrogen Energy 2013, 38, 328–335. [Google Scholar] [CrossRef]
- Soriano, M.D.; Nieto, J.M.L.; Ivars, F.; Concepcion, P.; Rodriguez-Castellon, E. Alkali-promoted V2O5 catalysts for the partial oxidation of H2S to sulphur. Catal. Today 2012, 192, 28–35. [Google Scholar] [CrossRef]
- Amiridis, M.D.; Solar, J.P. Selective catalytic reduction of nitric oxide by ammonia over V2O5/TiO2, V2O5/TiO2/SiO2, and V2O5−WO3 /TiO2 catalysts: Effect of vanadia content on the activation energy. Ind. Eng. Chem. Res. 1996, 35, 978–981. [Google Scholar] [CrossRef]
- Garbarino, G.; Kowalik, P.; Riani, P.; Antoniak-Jurak, K.; Pieta, P.; Lewalska-Graczyk, A.; Lisowski, W.; Nowakowski, R.; Busca, G.; Pieta, I. Improvement of Ni/Al2O3 catalysts for low-temperature CO2 methanation by vanadium and calcium oxide addition. Ind. Eng. Chem. Res. 2021, 60, 6554–6564. [Google Scholar] [CrossRef]
- Wachs, I.E. Catalysis science of supported vanadium oxide catalysts. Dalton Trans. 2013, 42, 11762–11769. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Kleemann, M. Urea-SCR: A promising technique to reduce NOx emissions from automotive diesel engines. Catal. Today 2000, 59, 335–345. [Google Scholar] [CrossRef]
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A Gen 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Borovkov, V.Y.; Mikheeva, E.P.; Zhidomirov, G.M.; Lapina, O.B. Theoretical and experimental studies of the nature of the catalytic activity of VOX/TIO2 systems. Kinet. Catal. 2003, 44, 710–717. [Google Scholar] [CrossRef]
- Wu, M.; Ung, K.C.; Dai, Q.; Wang, X. Catalytic combustion of chlorinated VOCS over VOx/TiO2 catalysts. Catal. Commun. 2012, 18, 72–75. [Google Scholar] [CrossRef]
- Inomata, Y.; Hata, S.; Mino, M.; Kiyonaga, E.; Morita, K.; Hikino, K.; Yoshida, K.; Kubota, H.; Toyao, T.; Shimizu, K.; et al. Bulk vanadium oxide versus conventional V2O5/TiO2: NH3–SCR catalysts working at a low temperature below 150 °C. ACS Catal. 2019, 9, 9327–9331. [Google Scholar] [CrossRef]
- Marberger, A.; Elsener, M.; Ferri, D.; Kröcher, O. VOx surface coverage optimization of V2O5/WO3-TiO2 SCR catalysts by variation of the V loading and by aging. Catalysts 2015, 5, 1704–1720. [Google Scholar] [CrossRef]
- Massenova, A.; Kalykberdiyev, M.; Sass, A.; Kenzin, N.; Ussenov, A.; Baiken, A.; Rakhmetova, K. Catalytic technologies for solving environmental problems in the production of fuels and motor transport in Kazakhstan. Catalysts 2020, 10, 1197. [Google Scholar] [CrossRef]
- Rovenskikh, A.S.; Kariychina, A.E.; Iguminova, V.A. Automobile emission effect on the environment. Investigations of young scientists. In Proceedings of the XV International Scientific Conference, Kazan, Russia, 24 December 2020; pp. 15–18. (In Russian). [Google Scholar]
- Zeidel, A.N.; Prokofiev, V.K.; Raysky, S.M.; Slavny, V.A.; Schrader, E.Y. Tables of Spectral Lines. Directory; Nauka: Moscow, Russia, 1977; p. 800. (In Russian) [Google Scholar]
- Kalinkin, I.P.; Mosichev, V.I. New Handbook of Chemist and Technologist. Analytical Chemistry; Peace and Family: St. Petersburg, Russia, 2003; p. 984. (In Russian) [Google Scholar]
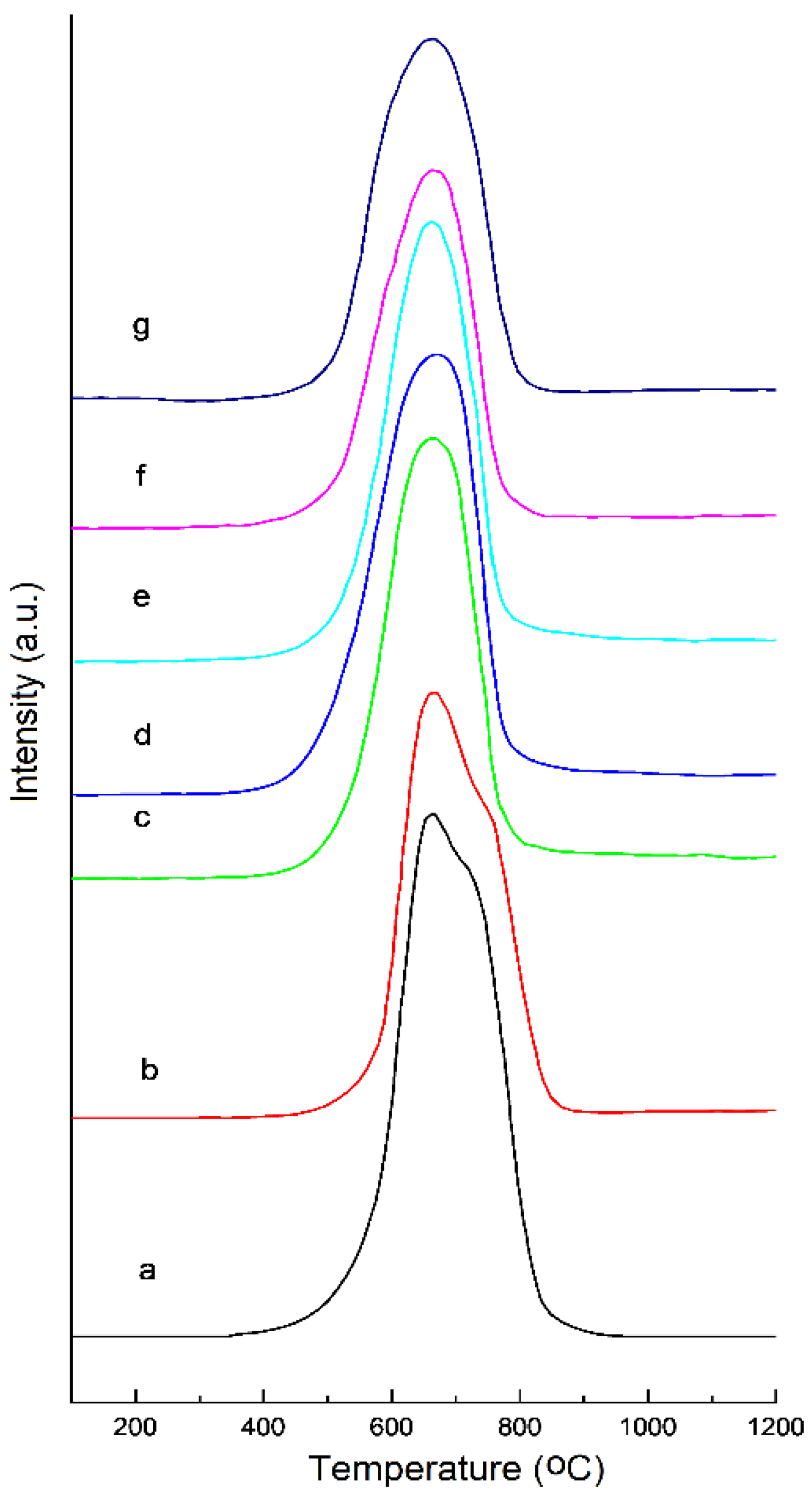
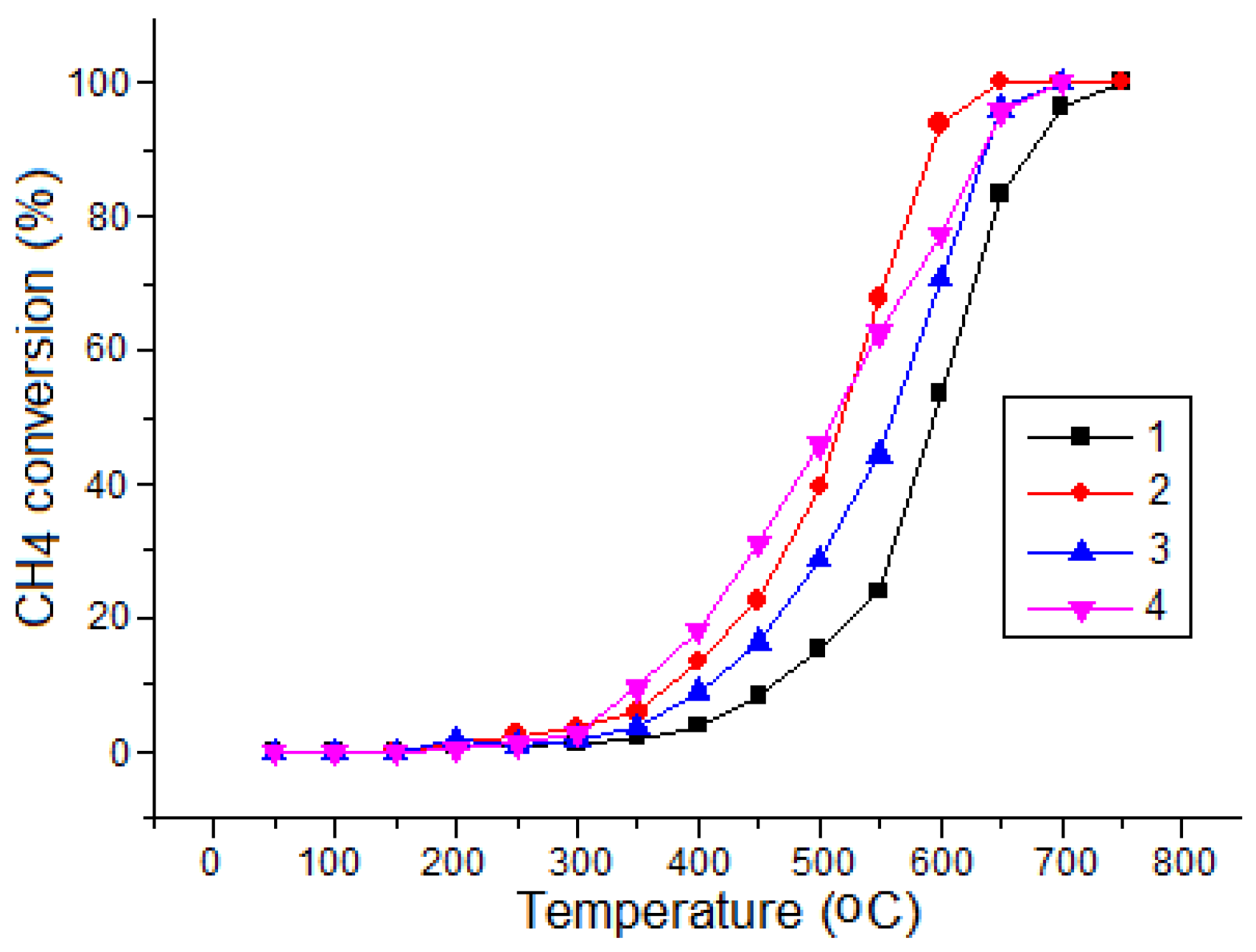
| T (°C) | Elements (wt%) | |||||
|---|---|---|---|---|---|---|
| O | Al | Si | S | V | Total | |
| 25 (initial) | 45.07 | 40.60 | 0.42 | 5.19 | 8.72 | 100 |
| 300 °C | 44.79 | 41.62 | 0.11 | 5.11 | 8.37 | 100 |
| 400 °C | 45.28 | 41.30 | 0.07 | 4.95 | 8.40 | 100 |
| 500 °C | 47.23 | 41.16 | 0.09 | 4.04 | 7.49 | 100 |
| 600 °C | 45.69 | 44.14 | 0.10 | 3.21 | 6.86 | 100 |
| 700 °C | 37.92 | 50.99 | 0.08 | 0.00 | 11.01 | 100 |
| 800 °C | 40.42 | 48.78 | 0.08 | 0.00 | 10.72 | 100 |
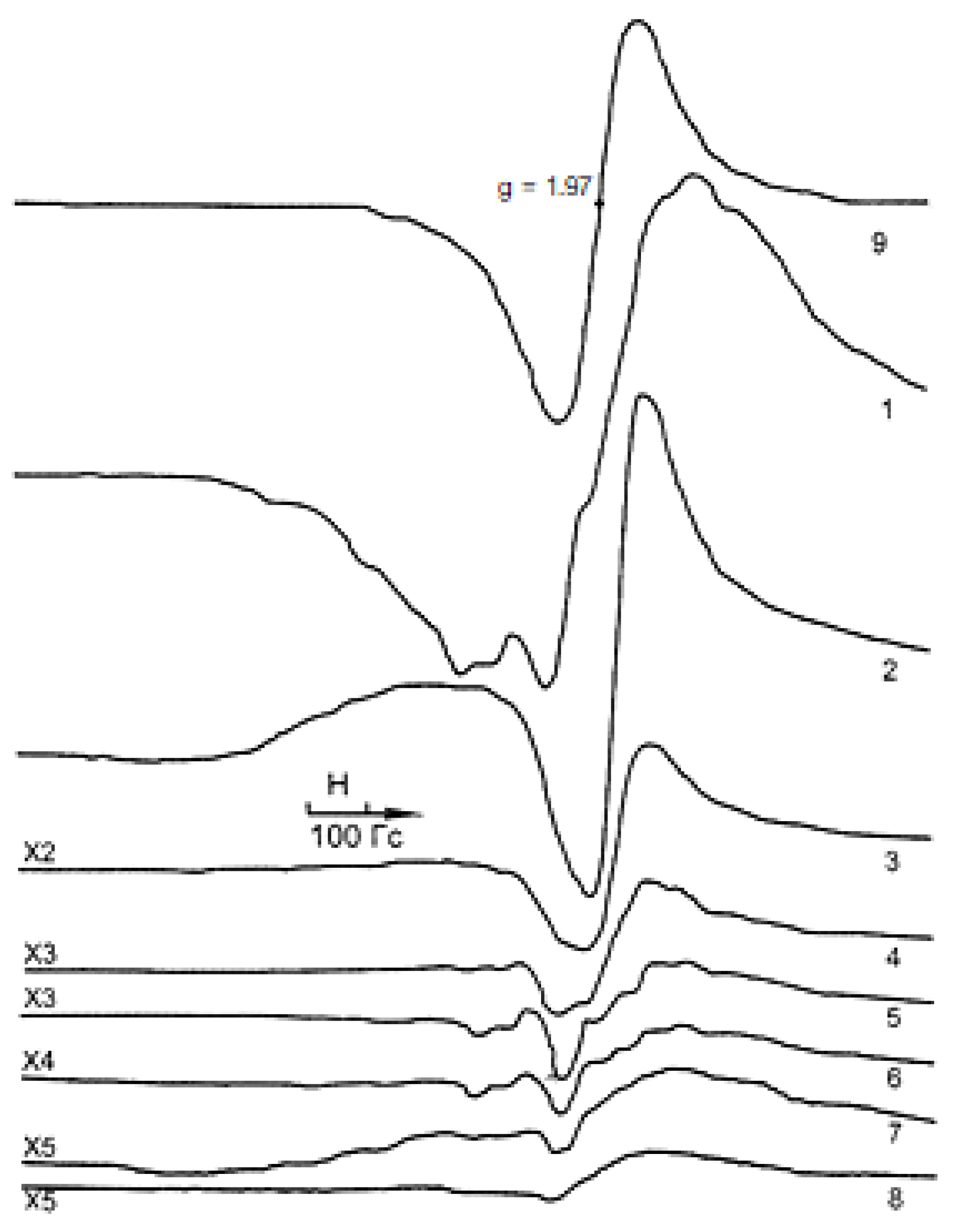
| Sample | Intensity, Amplitude (a.u.) | g-Factor | ∆H (gauss) |
|---|---|---|---|
| Initial 10% V/γ-Al2O3 | 120 | 1.99 | 270 |
| 300 °C | 116 | 1.97 | 261 |
| 400 °C | 24 | 1.97 | 116 |
| 500 °C | 13 | 1.97 | 155 |
| 600 °C | 11 | 1.97 | 193 |
| 700 °C | 6.5 | 1.97 | 213 |
| 800 °C | 1.3 | 1.97 | 213 |
| 900 °C | 1.5 | 1.97 | 174 |
| VOSO4·3H2O | 2250 | 1.97 | 126 |
| Sample | S (m2/g) |
|---|---|
| VOSO4·3H2O vanadyl sulfate | 23 |
| 10% V/γ-Al2O3, initial | 192 |
| 10% V/γ-Al2O3, calcination for 1 h, 300 °C, air | 184 |
| 10% V/γ-Al2O3, calcination for 1 h, 400 °C, air | 195 |
| 10% V/γ-Al2O3, calcination for 1 h, 500 °C, air | 172 |
| 10% V/γ-Al2O3, calcination for 1 h, 600 °C, air | 174 |
| 10% V/γ-Al2O3, calcination for 1 h, 700 °C, air | 27 |
| 10% V/γ-Al2O3, calcination for 1 h, 800 °C, air | 10 |
| γ-Al2O3 after calcination in air at 600 °C, 1 h | 195 |
| γ-Al2O3 after calcination in air at 700 °C, 1 h | 189 |
| Temperature of Heating in Air (°C) | Total Area of Peaks (a.u.) | Temperature of the Peaks (°C) |
|---|---|---|
| 25 | 14,407,832 | 640,670 |
| 300 | 11,858,380 | 640,670 |
| 400 | 11,382,953 | 640 |
| 500 | 11,110,654 | 650 |
| 600 | 11,063,026 | 635 |
| 700 | 7,920,939 | 645 |
| 800 | 8,052,778 | 645 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khussain, B.; Brodskiy, A.; Sass, A.; Rakhmetova, K.; Yaskevich, V.; Grigor’eva, V.; Ishmukhamedov, A.; Shapovalov, A.; Shlygina, I.; Tungatarova, S.; et al. Synthesis of Vanadium-Containing Catalytically Active Phases for Exhaust Gas Neutralizers of Motor Vehicles and Industrial Enterprises. Catalysts 2022, 12, 842. https://doi.org/10.3390/catal12080842
Khussain B, Brodskiy A, Sass A, Rakhmetova K, Yaskevich V, Grigor’eva V, Ishmukhamedov A, Shapovalov A, Shlygina I, Tungatarova S, et al. Synthesis of Vanadium-Containing Catalytically Active Phases for Exhaust Gas Neutralizers of Motor Vehicles and Industrial Enterprises. Catalysts. 2022; 12(8):842. https://doi.org/10.3390/catal12080842
Chicago/Turabian StyleKhussain, Bolatbek, Alexandr Brodskiy, Alexandr Sass, Kenzhegul Rakhmetova, Vladimir Yaskevich, Valentina Grigor’eva, Altay Ishmukhamedov, Anatoliy Shapovalov, Irina Shlygina, Svetlana Tungatarova, and et al. 2022. "Synthesis of Vanadium-Containing Catalytically Active Phases for Exhaust Gas Neutralizers of Motor Vehicles and Industrial Enterprises" Catalysts 12, no. 8: 842. https://doi.org/10.3390/catal12080842
APA StyleKhussain, B., Brodskiy, A., Sass, A., Rakhmetova, K., Yaskevich, V., Grigor’eva, V., Ishmukhamedov, A., Shapovalov, A., Shlygina, I., Tungatarova, S., & Khussain, A. (2022). Synthesis of Vanadium-Containing Catalytically Active Phases for Exhaust Gas Neutralizers of Motor Vehicles and Industrial Enterprises. Catalysts, 12(8), 842. https://doi.org/10.3390/catal12080842