Sustainably Recycling and Upcycling of Single-Use Plastic Wastes through Heterogeneous Catalysis
Abstract
:1. Introduction
2. Brief Introduction on Polymer Depolymerization
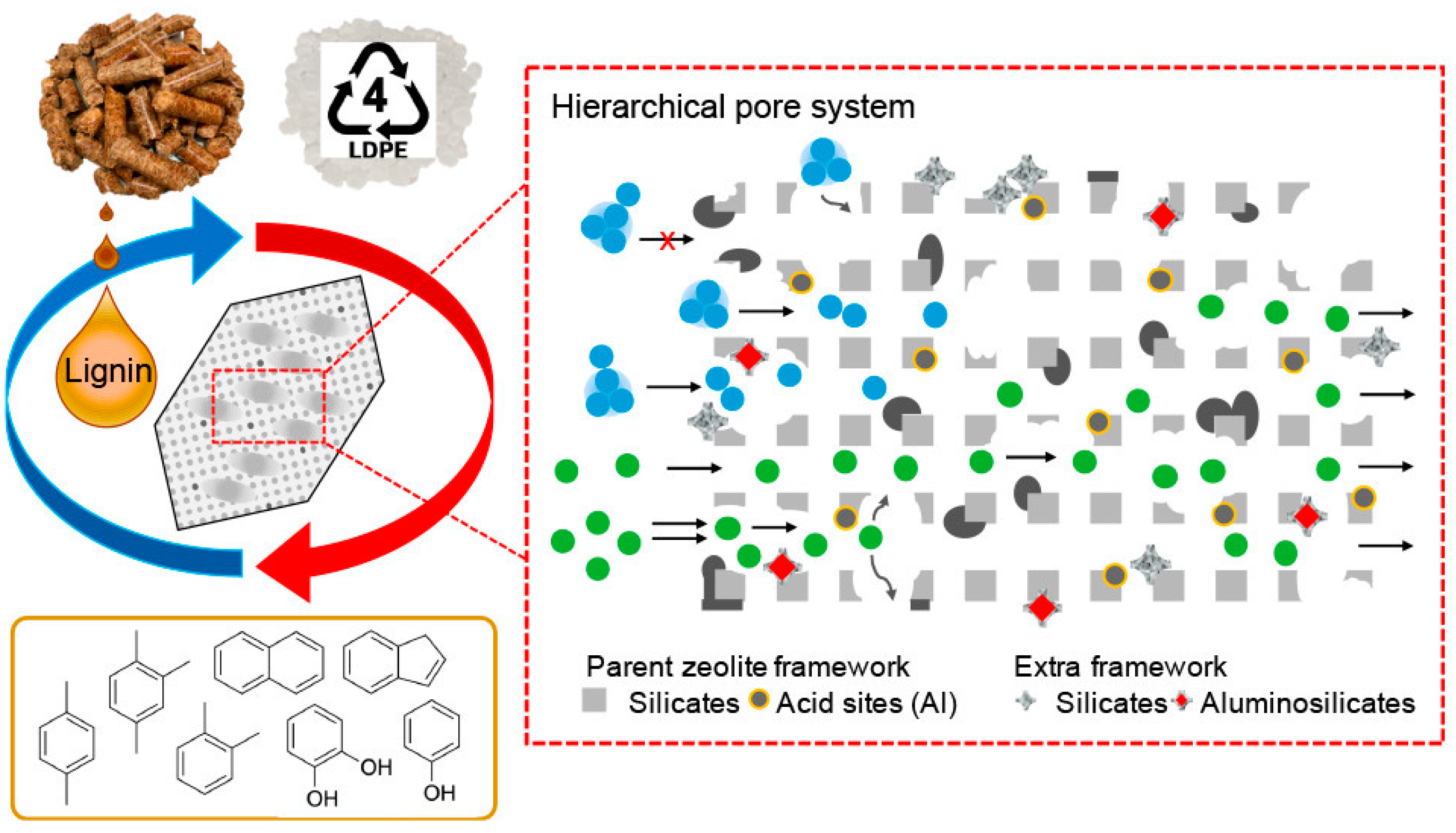
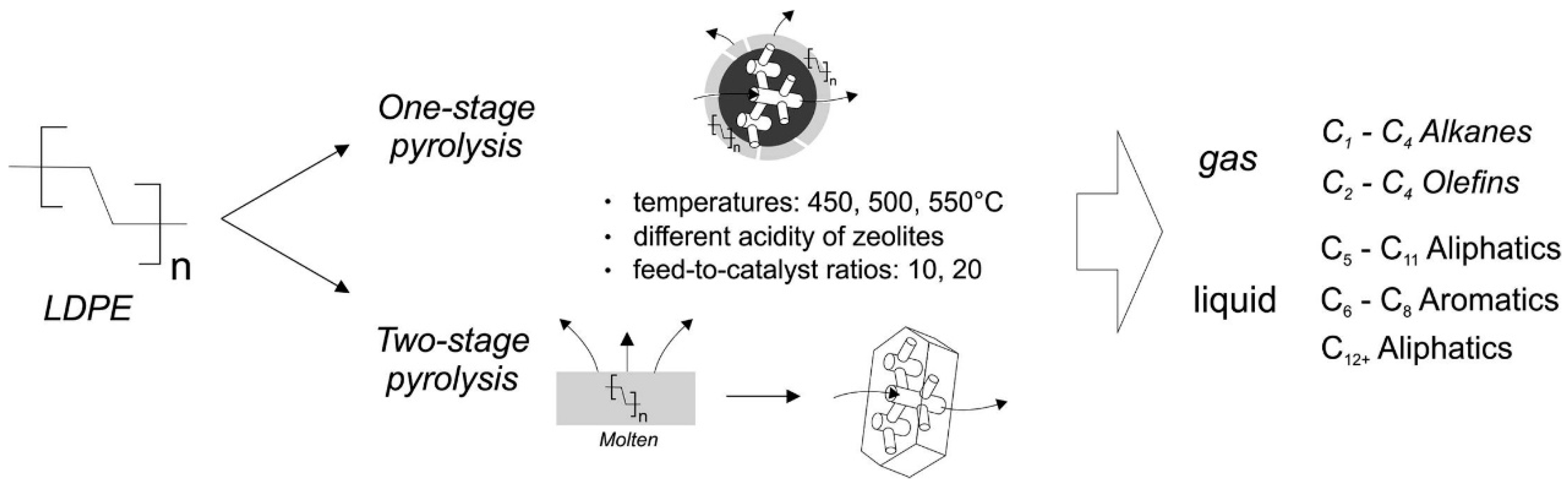
3. Acid-Catalyzed Cracking of Polyolefins without Hydrogen
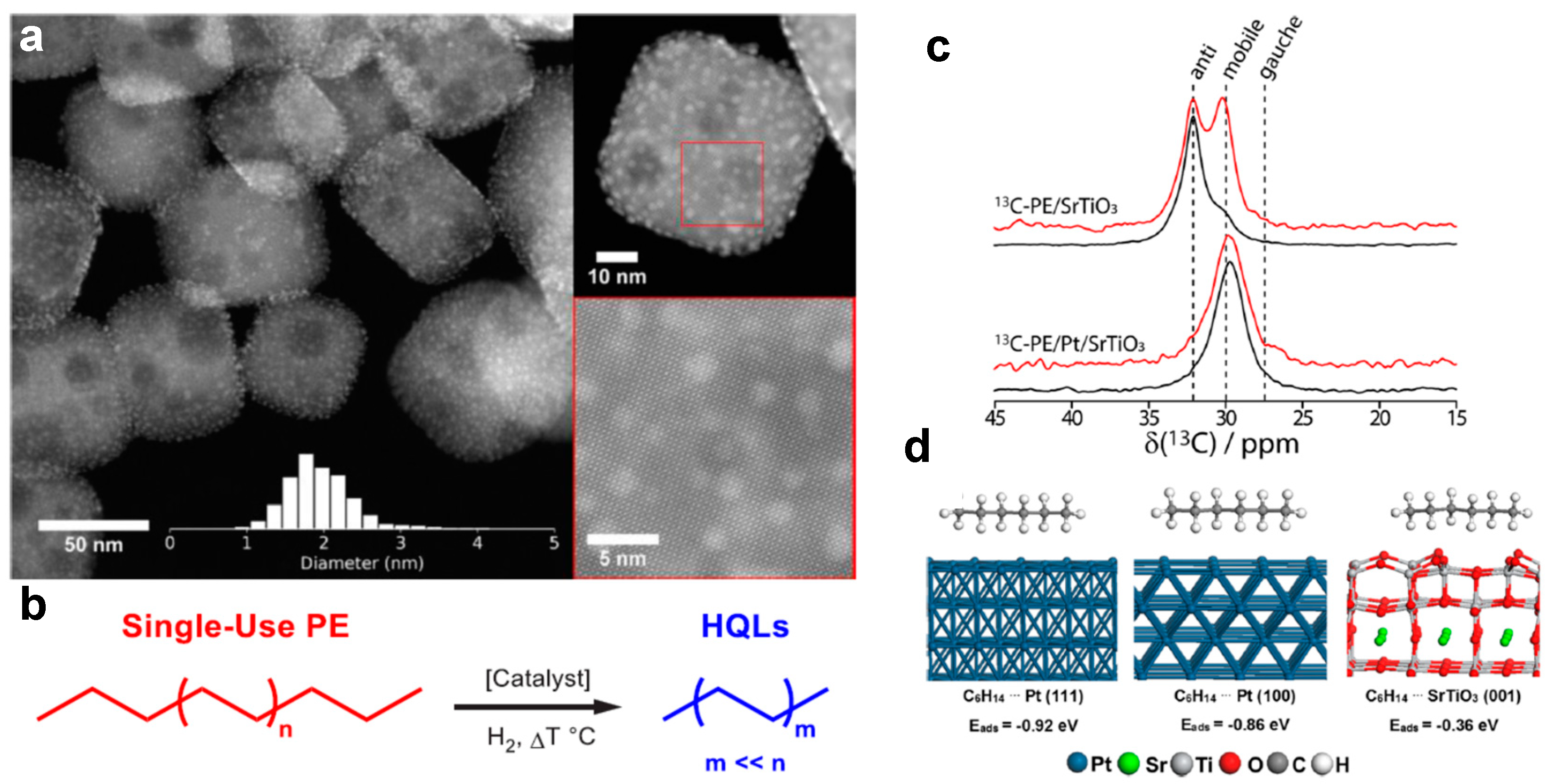
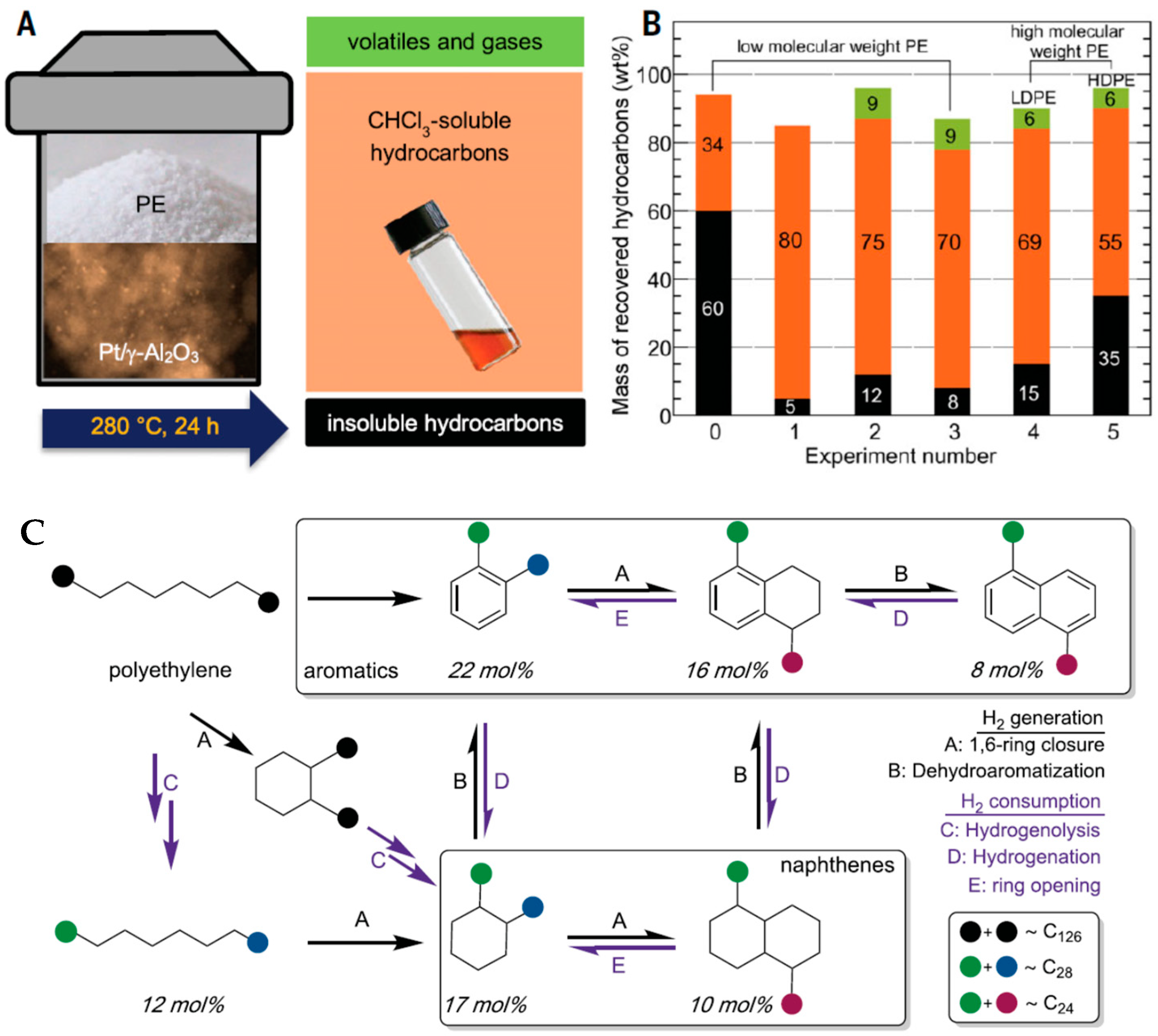
4. Hydrogenolysis of Polyolefins Using Gaseous Hydrogen

5. Hydrogenolysis of Polyesters Using Gaseous Hydrogen
6. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Ellis, L.D.; Rorrer, N.A.; Sullivan, K.P.; Otto, M.; McGeehan, J.E.; Roman-Leshkov, Y.; Wierckx, N.; Beckham, G.T. Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 2021, 4, 539–556. [Google Scholar] [CrossRef]
- Yin, S.; Rajarao, R.; Sahajwalla, V. Thermal transformation of metallized plastic packaging waste into value-added Al/Al3C4/AlN resources. ACS Sustain. Chem. Eng. 2019, 7, 1723–1733. [Google Scholar] [CrossRef]
- Chen, H.; Wan, K.; Zhang, Y.; Wang, Y. Waste to wealth: Chemical recycling and chemical upcycling of waste plastics for a great future. ChemSusChem 2021, 14, 4123–4136. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Darensbourg, D.J. Making plastics from carbon dioxide: Salen metal complexes as catalysts for the production of polycarbonates from epoxides and CO2. Chem. Rev. 2007, 107, 2388–2410. [Google Scholar] [CrossRef] [PubMed]
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Fox, J.A.; Stacey, N.T. Process targeting: An energy based comparison of waste plastic processing technologies. Energy 2019, 170, 273–283. [Google Scholar] [CrossRef]
- Yao, D.; Li, H.; Mohan, B.C.; Prabhakar, A.K.; Dai, Y.; Wang, C.-H. Conversion of waste plastic packings to carbon nanomaterials: Investigation into catalyst material, waste type, and product applications. ACS Sustain. Chem. Eng. 2022, 10, 1125–1136. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Chiba, S.; Saito, H.; Fletcher, R.; Yogi, T.; Kayo, M.; Miyagi, S.; Ogido, M.; Fujikura, K. Human footprint in the abyss: 30 year records of deep-sea plastic debris. Mar. Policy 2018, 96, 204–212. [Google Scholar] [CrossRef]
- Estrin, Y.; Vinogradov, A. Extreme grain refinement by severe plastic deformation: A wealth of challenging science. Acta Mater. 2013, 61, 782–817. [Google Scholar] [CrossRef]
- Nicholson, S.R.; Rorrer, N.A.; Carpenter, A.C.; Beckham, G.T. Manufacturing energy and greenhouse gas emissions associated with plastics consumption. Joule 2021, 5, 673–686. [Google Scholar] [CrossRef]
- Nomura, S.; Kato, K.; Nakagawa, T.; Komaki, I. The effect of plastic addition on coal caking properties during carbonization. Fuel 2003, 82, 1775–1782. [Google Scholar] [CrossRef]
- Garcia, J.M.; Robertson, M.L. The future of plastics recycling Chemical advances are increasing the proportion of polymer waste that can be recycled. Science 2017, 358, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Huber, G.W.; Wang, J.; Guss, A.; O’Neill, H.M.; Lin, C.S.K.; Wang, Y.; Wurm, F.R.; Meng, X. New technologies are needed to improve the recycling and upcycling of waste plastics. ChemSusChem 2021, 14, 3982–3984. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M. Catalyst: Design challenges for the future of plastics recycling. Chem 2016, 1, 813–815. [Google Scholar] [CrossRef] [Green Version]
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 2004, 428, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kwon, E.E.; Lam, S.S.; Chen, W.-H.; Rinklebe, J.; Park, Y.-K. Chemical recycling of plastic waste via thermocatalytic routes. J. Clean. Prod. 2021, 321, 128989. [Google Scholar] [CrossRef]
- Rahimi, A.; Garcia, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, Y.S.; Kim, S.H. Investigation of thermodynamic parameters in the thermal decomposition of plastic waste-waste lube oil compounds. Environ. Sci. Technol. 2010, 44, 5313–5317. [Google Scholar] [CrossRef]
- Kumar, S.; Panda, A.K.; Singh, R.K. A review on tertiary recycling of high-density polyethylene to fuel. Resour. Conserv. Recycl. 2011, 55, 893–910. [Google Scholar] [CrossRef]
- Roy, P.S.; Garnier, G.; Allais, F.; Saito, K. Strategic approach towards plastic waste valorization: Challenges and promising chemical upcycling possibilities. ChemSusChem 2021, 14, 4007–4027. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Belyakov, A.; Kaibyshev, R.; Miura, H.; Jonas, J.J. Dynamic and post-dynamic recrystallization under hot, cold and severe plastic deformation conditions. Prog. Mater. Sci. 2014, 60, 130–207. [Google Scholar] [CrossRef] [Green Version]
- Easterling, C.P.; Kubo, T.; Orr, Z.M.; Fanucci, G.E.; Sumerlin, B.S. Synthetic upcycling of polyacrylates through organocatalyzed post-polymerization modification. Chem. Sci. 2017, 8, 7705–7709. [Google Scholar] [CrossRef] [Green Version]
- Goto, M.; Sasaki, M.; Hirose, T. Reactions of polymers in supercritical fluids for chemical recycling of waste plastics. J. Mater. Sci. 2006, 41, 1509–1515. [Google Scholar] [CrossRef]
- Himmel, M.E. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 316, 982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inagaki, Y. Converting waste plastic containing acrylonitrile into a water-absorbent polymer. J. Appl. Polym. Sci. 2008, 108, 689–693. [Google Scholar] [CrossRef]
- Maqsood, T.; Dai, J.; Zhang, Y.; Guang, M.; Li, B. Pyrolysis of plastic species: A review of resources and products. J. Anal. Appl. Pyrolysis 2021, 159, 105295. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Nakaji, Y.; Tamura, M.; Miyaoka, S.; Kumagai, S.; Tanji, M.; Nakagawa, Y.; Yoshioka, T.; Tomishige, K. Low-temperature catalytic upgrading of waste polyolefinic plastics into liquid fuels and waxes. Appl. Catal. B 2021, 285, 119805. [Google Scholar] [CrossRef]
- Kyrikou, I.; Briassoulis, D. Biodegradation of agricultural plastic films: A critical review. J. Polym. Environ. 2007, 15, 125–150. [Google Scholar] [CrossRef]
- Selke, S.; Auras, R.; Tuan Anh, N.; Aguirre, E.C.; Cheruvathur, R.; Liu, Y. Evaluation of Biodegradation-Promoting Additives for Plastics. Environ. Sci. Technol. 2015, 49, 3769–3777. [Google Scholar] [CrossRef] [PubMed]
- Veksha, A.; Giannis, A.; Oh, W.-D.; Chang, V.W.C.; Lisak, G. Upgrading of non-condensable pyrolysis gas from mixed plastics through catalytic decomposition and dechlorination. Fuel Process. Technol. 2018, 170, 13–20. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Zhu, L.; Zhu, X.; Qian, M.; Yadavalli, G.; Wu, J.; Chen, S. Thermal behavior and kinetic study for catalytic co-pyrolysis of biomass with plastics. Bioresour. Technol. 2016, 220, 233–238. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Bjorn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [Green Version]
- Purahong, W.; Wahdan, S.F.M.; Heinz, D.; Jariyavidyanont, K.; Sungkapreecha, C.; Tanunchai, B.; Sansupa, C.; Sadubsarn, D.; Alaneed, R.; Heintz-Buschart, A.; et al. Back to the Future: Decomposability of a Biobased and Biodegradable Plastic in Field Soil Environments and Its Microbiome under Ambient and Future Climates. Environ. Sci. Technol. 2021, 55, 12337–12351. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Enein, A.A.; Awadallah, A.E. Production of nanostructured carbon materials using Fe-Mo/MgO catalysts via mild catalytic pyrolysis of polyethylene waste. Chem. Eng. J. 2018, 354, 802–816. [Google Scholar] [CrossRef]
- Wu, S.-L.; Kuo, J.-H.; Wey, M.-Y. Design of catalysts comprising a nickel core and ceria shell for hydrogen production from plastic waste gasification: An integrated test for anti-coking and catalytic performance. Catal. Sci. Technol. 2020, 10, 3975–3984. [Google Scholar] [CrossRef]
- Lopez-Urionabarrenechea, A.; de Marco, I.; Caballero, B.M.; Adrados, A.; Laresgoiti, M.F. Deactivation and regeneration of ZSM-5 zeolite in catalytic pyrolysis of plastic wastes. Waste Manag. 2011, 31, 1852–1858. [Google Scholar] [CrossRef]
- Sebestyen, Z.; Barta-Rajnai, E.; Bozi, J.; Blazso, M.; Jakab, E.; Miskolczi, N.; Soja, J.; Czegeny, Z. Thermo-catalytic pyrolysis of biomass and plastic mixtures using HZSM-5. Appl. Energy 2017, 207, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Klaimy, S.; Ciotonea, C.; Dhainaut, J.; Royer, S.; Casetta, M.; Duquesne, S.; Tricot, G.; Lamonier, J.-F. Flash Catalytic Pyrolysis of Polyethylene over (Alumino)silicate Materials. ChemCatChem 2020, 12, 1109–1116. [Google Scholar] [CrossRef]
- Islam, M.T.; Dominguez, A.; Turley, R.S.; Kim, H.; Sultana, K.A.; Shuvo, M.; Alvarado-Tenorio, B.; Montes, M.O.; Lin, Y.; Gardea-Torresdey, J.; et al. Development of photocatalytic paint based on TiO2 and photopolymer resin for the degradation of organic pollutants in water. Sci. Total Environ. 2020, 704, 135406. [Google Scholar] [CrossRef]
- Miller, J.H.; Starace, A.K.; Ruddy, D.A. Catalytic activation of polyethylene model compounds over metal-exchanged beta zeolites. ChemSusChem 2022, 15, e202200535. [Google Scholar] [CrossRef] [PubMed]
- Socci, J.; Osatiashtiani, A.; Kyriakou, G.; Bridgwater, T. The catalytic cracking of sterically challenging plastic feedstocks over high acid density Al-SBA-15 catalysts. Appl. Catal. A 2019, 570, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Valanciene, E.; Miknius, L.; Martynaitis, V.; Striugas, N. Influence of equilibrium fluid catalytic cracking catalyst amount on the thermolysis process of various polyolefin plastic wastes in a fixed-bed reactor for gasoline and diesel production. Energy Fuels 2017, 31, 11194–11210. [Google Scholar] [CrossRef]
- Rorrer, J.E.; Troyano-Valls, C.; Beckham, G.T.; Roman-Leshkov, Y. Hydrogenolysis of Polypropylene and Mixed Polyolefin Plastic Waste over Ru/C to Produce Liquid Alkanes. ACS Sustain. Chem. Eng. 2021, 9, 11661–11666. [Google Scholar] [CrossRef]
- Yao, L.; King, J.; Wu, D.; Ma, J.; Li, J.; Xie, R.; Chuang, S.S.C.; Miyoshi, T.; Peng, Z. Non-thermal plasma-assisted rapid hydrogenolysis of polystyrene to high yield ethylene. Nat. Commun. 2022, 13, 885. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.; Samudrala, S.P.; Bhattacharya, S. The route towards sustainable production of ethylene glycol from a renewable resource, biodiesel waste: A review. Catal. Sci. Technol. 2019, 9, 567–577. [Google Scholar] [CrossRef]
- Kratish, Y.; Marks, T.J. Efficient Polyester Hydrogenolytic Deconstruction via Tandem Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202112576. [Google Scholar]
- Lu, S.; Jing, Y.; Feng, B.; Guo, Y.; Liu, X.; Wang, Y. H-2-free Plastic Conversion: Converting PET back to BTX by Unlocking Hidden Hydrogen. ChemSusChem 2021, 14, 4242–4250. [Google Scholar] [CrossRef]
- Lovas, P.; Hudec, P.; Jambor, B.; Hajekova, E.; Hornacek, M. Catalytic cracking of heavy fractions from the pyrolysis of waste HDPE and PP. Fuel 2017, 203, 244–252. [Google Scholar] [CrossRef]
- Xue, Y.; Johnston, P.; Bai, X. Effect of catalyst contact mode and gas atmosphere during catalytic pyrolysis of waste plastics. Energy Convers. Manag. 2017, 142, 441–451. [Google Scholar] [CrossRef]
- Yao, D.; Yang, H.; Chen, H.; Williams, P.T. Investigation of nickel-impregnated zeolite catalysts for hydrogen/syngas production from the catalytic reforming of waste polyethylene. Appl. Catal. B 2018, 227, 477–487. [Google Scholar] [CrossRef]
- Zhong, L.; Fang, Z.; Shu, C.; Mo, C.; Chen, X.; Yu, D. Redox Donor-Acceptor Conjugated Microporous Polymers as Ultralong-Lived Organic Anodes for Rechargeable Air Batteries. Angew. Chem. Int. Ed. 2021, 60, 10164–10171. [Google Scholar] [CrossRef]
- Duong Dinh, P.; Cho, J. Low-energy catalytic methanolysis of poly(ethyleneterephthalate). Green Chem. 2021, 23, 511–525. [Google Scholar]
- Haeussler, M.; Eck, M.; Rothauer, D.; Mecking, S. Closed-loop recycling of polyethylene-like materials. Nature 2021, 590, 423–427. [Google Scholar] [CrossRef]
- Raheem, A.B.; Noor, Z.Z.; Hassan, A.; Abd Hamid, M.K.; Samsudin, S.A.; Sabeen, A.H. Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: A review. J. Clean. Prod. 2019, 225, 1052–1064. [Google Scholar] [CrossRef]
- Nguyen, S.T.; McLoughlin, E.A.; Cox, J.H.; Fors, B.P.; Knowles, R.R. Depolymerization of Hydroxylated Polymers via Light-Driven C-C Bond Cleavage. J. Am. Chem. Soc. 2021, 143, 12268–12277. [Google Scholar] [CrossRef]
- Fowley, L.A.; Lee, J.C.; Crabtree, R.H.; Siegbahn, P.E.M. Consequences of the formation organometallic exciplex [Hg(eta 2-arene)] in mercury-photosensitized reactions of arenes: C-C, C-O, and C-N bond cleavage. Organometallics 1996, 15, 1157–1165. [Google Scholar] [CrossRef]
- Moselage, M.; Li, J.; Kramm, F.; Ackermann, L. Ruthenium(II)-Catalyzed C-C Arylations and Alkylations: Decarbamoylative C-C Functionalizations. Angew. Chem. Int. Ed. 2017, 56, 5341–5344. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Gou, T.; Wang, B.-Q.; Shi, Z.-J. Catalytic activations of unstrained C-C bond involving organometallic intermediates. Chem. Soc. Rev. 2018, 47, 7078–7115. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Perea, M.A.; Sarpong, R. Transition-Metal-Mediated Cleavage of C-C Single Bonds: Making the Cut in Total Synthesis. Angew. Chem. Int. Ed. 2020, 59, 18898–18919. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhu, F.; He, W.; Zhang, F.; Wang, W.; Li, H. Periodic Mesoporous Organometallic Silicas with Unary or Binary Organometals inside the Channel Walls as Active and Reusable Catalysts in Aqueous Organic Reactions. J. Am. Chem. Soc. 2010, 132, 1492–1493. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.Q.; Qin, C.; Friedberger, T.; Guan, Z.B.; Huang, Z. Efficient and selective degradation of polyethylenes into liquid fuels and waxes under mild conditions. Sci. Adv. 2016, 2, e1501591. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.-H.; Pu, M.; Chen, B.-H.; Cao, F. Theoretical study on the cracking reaction catalyzed by a solid acid with zeolitic structure: The catalytic cracking of 1-hexene on the surface of H-ZSM-5. Appl. Catal. A 2013, 455, 65–70. [Google Scholar] [CrossRef]
- Kubo, K.; Takahashi, T.; Iida, H.; Namba, S.; Igarashi, A. Reactivities of C6-8 hydrocarbons and effect of coexistence of another hydrocarbon in cracking on H-ZSM-5 catalyst at high temperatures. Appl. Catal. A 2014, 482, 370–376. [Google Scholar] [CrossRef]
- Luzgin, M.V.; Stepanov, A.G.; Sassi, A.; Sommer, J. Formation of carboxylic acids from small alkanes in zeolite H-ZSM-5. Chem. Eur. J. 2000, 6, 2368–2376. [Google Scholar] [CrossRef]
- Inayat, A.; Knoke, I.; Spiecker, E.; Schwieger, W. Assemblies of mesoporous FAU-type zeolite nanosheets. Angew. Chem. Int. Ed. 2012, 51, 1962–1965. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, C.; Wu, D.; He, S.; Ren, B. A simple method of preparation of high silica zeolite y and its performance in the catalytic cracking of cumene. J. Nanotechnol. 2016, 2016, 1486107. [Google Scholar] [CrossRef] [Green Version]
- Reiprich, B.; Tarach, K.A.; Pyra, K.; Grzybek, G.; Góra-Marek, K. High-silica layer-like zeolites y from seeding-free synthesis and their catalytic performance in low-density polyethylene cracking. ACS Appl. Mater. Interfaces 2022, 14, 6667–6679. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.H.M.; Muraza, O.; Jamil, A.K.; Shafei, E.N.; Yamani, Z.H.; Choi, K.-H. Steam Catalytic cracking of n-dodecane over Ni and Ni/Co bimetallic catalyst supported on hierarchical BEA zeolite. Energy Fuels 2017, 31, 5482–5490. [Google Scholar] [CrossRef]
- Khalil, U.; Liu, Z.; Peng, C.; Hikichi, N.; Wakihara, T.; Garcia-Martinez, J.; Okubo, T.; Bhattacharya, S. Ultrafast surfactant-templating of *BEA zeolite: An efficient catalyst for the cracking of polyethylene pyrolysis vapours. Chem. Eng. J. 2021, 412, 128566. [Google Scholar] [CrossRef]
- Nakao, R.; Kubota, Y.; Katada, N.; Nishiyama, N.; Kunimori, K.; Tomishige, K. Highly active BEA catalyst for catalytic cracking of n-heptane. Catal. Lett. 2003, 89, 153–157. [Google Scholar] [CrossRef]
- Nakao, R.; Kubota, Y.; Katada, N.; Nishiyama, N.; Kunimori, K.; Tomishige, K. Performance and characterization of BEA catalysts for catalytic cracking. Appl. Catal. A 2004, 273, 63–73. [Google Scholar] [CrossRef]
- Huang, Z.; Shanmugam, M.; Liu, Z.; Brookfield, A.; Bennett, E.L.; Guan, R.; Vega Herrera, D.E.; Lopez-Sanchez, J.A.; Slater, A.G.; McInnes, E.J.L.; et al. Chemical recycling of polystyrene to valuable chemicals via selective acid-catalyzed aerobic oxidation under visible light. J. Am. Chem. Soc. 2022, 144, 6532–6542. [Google Scholar] [CrossRef]
- Jana, S.K.; Takahashi, H.; Nakamura, M.; Kaneko, M.; Nishida, R.; Shimizu, H.; Kugita, T.; Namba, S. Aluminum incorporation in mesoporous MCM-41 molecular sieves and their catalytic performance in acid-catalyzed reactions. Appl. Catal. A 2003, 245, 33–41. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Yang, M.-H. Kinetic and mechanistic modeling of acid-catalyzed degradation of polymers with various cracking catalysts. J. Appl. Polym. Sci. 2009, 114, 2591–2599. [Google Scholar] [CrossRef]
- Sazama, P.; Sobalik, Z.; Dedecek, J.; Jakubec, I.; Parvulescu, V.; Bastl, Z.; Rathousky, J.; Jirglova, H. Enhancement of activity and selectivity in acid-catalyzed reactions by dealuminated hierarchical zeolites. Angew. Chem. Int. Ed. 2013, 52, 2038–2041. [Google Scholar] [CrossRef]
- Christensen, C.H.; Johannsen, K.; Schmidt, I.; Christensen, C.H. Catalytic benzene alkylation over mesoporous zeolite single crystals: Improving activity and selectivity with a new family of porous materials. J. Am. Chem. Soc. 2003, 125, 13370–13371. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Z.; Li, X.; Xie, Z. Mesoporous zeolite materials. Prog. Chem. 2008, 20, 637–643. [Google Scholar]
- Yue, M.B.; Sun, L.B.; Zhuang, T.T.; Dong, X.; Chun, Y.; Zhu, J.H. Directly transforming as-synthesized MCM-41 to mesoporous MFI zeolite. J. Mater. Chem. 2008, 18, 2044–2050. [Google Scholar] [CrossRef]
- Christensen, C.H.; Johannsen, K.; Toernqvist, E.; Schmidt, I.; Topsoe, H.; Christensen, C.H. Mesoporous zeolite single crystal catalysts: Diffusion and catalysis in hierarchical zeolites. Catal. Today 2007, 128, 117–122. [Google Scholar] [CrossRef]
- Meng, X.; Nawaz, F.; Xiao, F.-S. Templating route for synthesizing mesoporous zeolites with improved catalytic properties. Nano Today 2009, 4, 292–301. [Google Scholar] [CrossRef]
- Qian, M.; Lei, H.; Zhao, Y.; Villota, E.; Huo, E.; Wang, C.; Zhang, X. Lignin-Mediated Preparation of Hierarchical ZSM-5 Catalysts and Their Effects in the Catalytic Co-pyrolysis of Softwood Biomass and Low-Density Polyethylene Mixtures. ACS Sustain. Chem. Eng. 2021, 9, 12602–12613. [Google Scholar] [CrossRef]
- Inayat, A.; Inayat, A.; Schwieger, W.; Sokolova, B.; Lestinsky, P. Enhancing aromatics and olefins yields in thermo-catalytic pyrolysis of LDPE over zeolites: Role of staged catalysis and acid site density of HZSM-5. Fuel 2022, 314, 123071. [Google Scholar] [CrossRef]
- Jin, X.; Lee, J.H.; Choi, J.W. Catalytic co-pyrolysis of woody biomass with waste plastics: Effects of HZSM-5 and pyrolysis temperature on producing high-value pyrolytic products and reducing wax formation. Energy 2022, 239, 121739. [Google Scholar] [CrossRef]
- Dufaud, V.R.; Basset, J.M. Catalytic hydrogenolysis at low temperature and pressure of polyethylene and polypropylene to diesels or lower alkanes by a zirconium hydride supported on silica-alumina: A step toward polyolefin degradation by the microscopic reverse of Ziegler-Natta polymerization. Angew. Chem. Int. Ed. 1998, 37, 806–810. [Google Scholar]
- Sanchez-Rivera, K.L.; Huber, G.W. Catalytic hydrogenolysis of polyolefins into alkanes. ACS Cent. Sci. 2021, 7, 17–19. [Google Scholar] [CrossRef]
- Wu, X.; Tennakoon, A.; Yappert, R.; Esveld, M.; Ferrandon, M.S.; Hackler, R.A.; LaPointe, A.M.; Heyden, A.; Delferro, M.; Peters, B.; et al. Size-Controlled Nanoparticles Embedded in a Mesoporous Architecture Leading to Efficient and Selective Hydrogenolysis of Polyolefins. J. Am. Chem. Soc. 2022, 144, 5323–5334. [Google Scholar] [CrossRef]
- Nakaji, Y.; Nakagawa, Y.; Tamura, M.; Tomishige, K. Regioselective hydrogenolysis of alga-derived squalane over silica-supported ruthenium-vanadium catalyst. Fuel Process. Technol. 2018, 176, 249–257. [Google Scholar] [CrossRef]
- Oya, S.-i.; Kanno, D.; Watanabe, H.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Catalytic Production of Branched Small Alkanes from Biohydrocarbons. ChemSusChem 2015, 8, 2472–2475. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Fan, Y.; Zhang, X.; Zeng, Y.; Xie, S.; Pei, Y.; Zeng, G.; Qiao, M.; Zong, B. Tungsten-doped siliceous mesocellular foams-supported platinum catalyst for glycerol hydrogenolysis to 1,3-propanediol. Appl. Catal. B 2021, 297, 120428. [Google Scholar] [CrossRef]
- Fan, Y.; Cheng, S.; Wang, H.; Tian, J.; Xie, S.; Pei, Y.; Qiao, M.; Zong, B. Pt-WOx on monoclinic or tetrahedral ZrO2: Crystal phase effect of zirconia on glycerol hydrogenolysis to 1,3-propanediol. Appl. Catal. B 2017, 217, 331–341. [Google Scholar] [CrossRef]
- Mizugaki, T.; Yamakawa, T.; Nagatsu, Y.; Maeno, Z.; Mitsudome, T.; Jitsukawa, K.; Kaneda, K. Direct Transformation of Furfural to 1,2-Pentanediol Using a Hydrotalcite-Supported Platinum Nanoparticle Catalyst. ACS Sustain. Chem. Eng. 2014, 2, 2243–2247. [Google Scholar] [CrossRef]
- Celik, G.; Kennedy, R.M.; Hackler, R.A.; Ferrandon, M.; Tennakoon, A.; Patnaik, S.; LaPointe, A.M.; Ammal, S.C.; Heyden, A.; Perras, F.A.; et al. Upcycling Single-Use Polyethylene into High-Quality Liquid Products. ACS Cent. Sci. 2019, 5, 1795–1803. Available online: https://pubs.acs.org/doi/10.1021/acscentsci.9b00722 (accessed on 18 July 2022). [CrossRef] [Green Version]
- Kratish, Y.; Li, J.; Liu, S.; Gao, Y.; Marks, T.J. Polyethylene terephthalate deconstruction catalyzed by a carbon-supported single-site molybdenum-dioxo complex. Angew. Chem. Int. Ed. 2020, 59, 19857–19861. [Google Scholar] [CrossRef]
- Sun, M.; Zhu, L.; Liu, W.; Zhao, X.; Zhang, Y.; Luo, H.; Miao, G.; Li, S.; Yin, S.; Kong, L. Efficient upgrading of polyolefin plastics into C-5-C-12 gasoline alkanes over a Pt/W/Beta catalyst. Sustain. Energy Fuels 2022, 6, 271–275. [Google Scholar] [CrossRef]
- Wu, P.; Lu, G.; Cai, C. Cobalt-molybdenum synergistic catalysis for the hydrogenolysis of terephthalate-based polyesters. Green Chem. 2021, 23, 8666–8672. [Google Scholar] [CrossRef]
- Zaheer, M.; Hermannsdoerfer, J.; Kretschmer, W.P.; Motz, G.; Kempe, R. Robust Heterogeneous Nickel Catalysts with Tailored Porosity for the Selective Hydrogenolysis of Aryl Ethers. ChemCatChem 2014, 6, 91–95. [Google Scholar] [CrossRef]
- Carr, S.H.; Keller, A.; Baer, E. Relationship between self-seeded and epitaxial crystallization from polymer solutions: A potentially new method for molecular weight separation and a new decoration method for alkali halides. J. Polym. Sci. Part B Polym. Phys. 1970, 8, 1467–1490. [Google Scholar] [CrossRef]
- Chen, K.; Hu, Y.; Dong, X.; Sun, Y. Molecular insights into the enhanced performance of ekylated petase toward PET degradation. ACS Catal. 2021, 11, 7358–7370. [Google Scholar] [CrossRef]
- Han, X.; Liu, W.; Huang, J.-W.; Ma, J.; Zheng, Y.; Ko, T.-P.; Xu, L.; Cheng, Y.-S.; Chen, C.-C.; Guo, R.-T. Structural insight into catalytic mechanism of PET hydrolase. Nat. Commun. 2017, 8, 2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, S.; Cho, I.J.; Seo, H.; Son, H.F.; Sagong, H.-Y.; Shin, T.J.; Choi, S.Y.; Lee, S.Y.; Kim, K.-J. Structural insight into molecular mechanism of poly (ethylene terephthalate) degradation. Nat. Commun. 2018, 9, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tennakoon, A.; Wu, X.; Paterson, A.L.; Patnaik, S.; Pei, Y.; LaPointe, A.M.; Ammal, S.C.; Hackler, R.A.; Heyden, A.; Slowing, I.I.; et al. Catalytic upcycling of high-density polyethylene via a processive mechanism. Nat. Catal. 2020, 3, 893–901. [Google Scholar] [CrossRef]
- Jiao, F.; Li, J.; Pan, X.; Xiao, J.; Li, H.; Ma, H.; Wei, M.; Pan, Y.; Zhou, Z.; Li, M.; et al. Selective conversion of syngas to light olefins. Science 2016, 351, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jiao, F.; Pan, X.; Ding, Y.; Feng, J.; Bao, X. Size effects of ZnO nanoparticles in bifunctional catalysts for selective syngas conversion. ACS Catal. 2019, 9, 960–966. [Google Scholar] [CrossRef]
- Pan, X.; Jiao, F.; Miao, D.; Bao, X. Oxide-zeolite-based composite catalyst concept that enables syngas chemistry beyond Fischer-Tropsch synthesis. Chem. Rev. 2021, 121, 6588–6609. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kots, P.A.; Vance, B.C.; Danielson, A.; Vlachos, D.G. Plastic waste to fuels by hydrocracking at mild conditions. Sci. Adv. 2021, 7, eabf8283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zeng, M.; Yappert, R.D.; Sun, J.; Lee, Y.-H.; LaPointe, A.M.; Peters, B.; Abu-Omar, M.M.; Scott, S.L. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 2020, 370, 437–441. [Google Scholar] [CrossRef]
- González-Marcos, M.P.; Fuentes-Ordóñez, E.G.; Salbidegoitia, J.A.; González-Velasco, J.R. Optimization of supports in bifunctional supported Pt catalysts for polystyrene hydrocracking to liquid fuels. Top. Catal. 2021, 64, 224–242. [Google Scholar] [CrossRef]
- Whiston, K.; Forsyth, S.; Seddon, K.R. Ionic Liquid Solvents and a Process for the Depolymerization of Polyamides. WIPO Patent WO2009016378A3, 10 August 2010. [Google Scholar]
- Liu, F.; Cui, X.; Yu, S.; Li, Z.; Ge, X. Hydrolysis reaction of poly(ethylene terephthalate) using ionic liquids as solvent and catalyst. J. Appl. Polym. Sci. 2009, 114, 3561–3565. [Google Scholar] [CrossRef]
- Song, X.Y.; Liu, F.S.; Li, L.; Yang, X.Q.; Yu, S.T.; Ge, X.P. Hydrolysis of polycarbonate catalyzed by ionic liquid [Bmim] [Ac]. J. Hazard. Mater. 2013, 244, 204–208. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Yehia, F.Z.; Eshaq, G.; Rabie, A.M.; ElMetwally, A.E. Greener routes for recycling of polyethylene terephthalate. Egypt. J. Pet. 2016, 25, 53–64. [Google Scholar] [CrossRef] [Green Version]
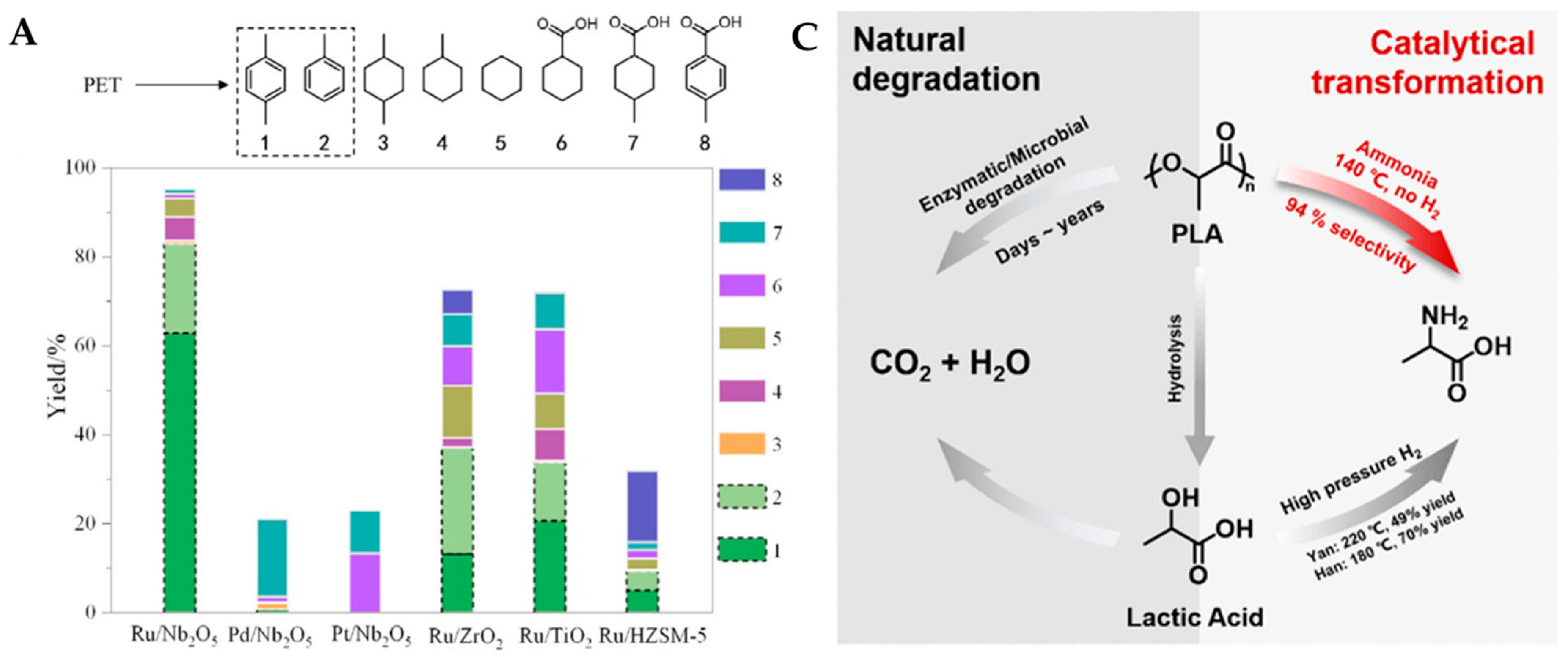
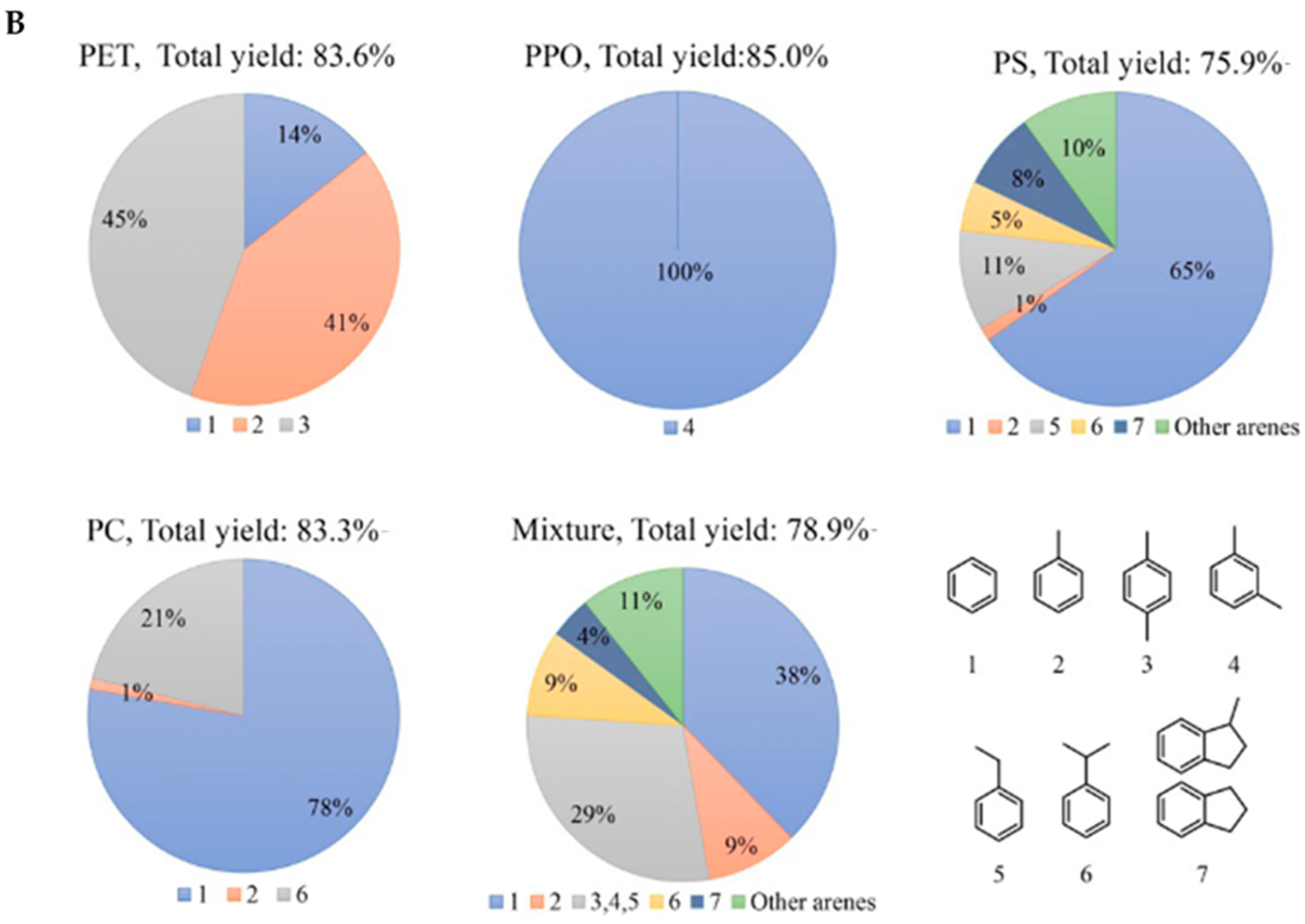
- Jing, Y.X.; Wang, Y.Q.; Furukawa, S.Y.; Xia, J.; Sun, C.Y.; Hulsey, M.J.; Wang, H.F.; Guo, Y.; Liu, X.H.; Yan, N. Towards the circular economy: Converting aromatic plastic waste back to arenes over a Ru/Nb2O5 catalyst. Angew. Chem. Int. Ed. 2021, 60, 5527–5535. [Google Scholar] [CrossRef]
- Li, Y.W.; Wang, M.; Liu, X.W.; Hu, C.Q.; Xiao, D.Q.; Ma, D. Catalytic transformation of PET and CO2 into high-value chemicals. Angew. Chem. Int. Ed. 2022, 61, e202117205. [Google Scholar]
- Tian, S.H.; Jiao, Y.C.; Gao, Z.R.; Xu, Y.; Fu, L.K.; Fu, H.; Zhou, W.; Hu, C.Q.; Liu, G.S.; Wang, M.; et al. Catalytic amination of polylactic acid to alanine. J. Am. Chem. Soc. 2021, 143, 16358–16363. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Bai, B.; Wei, W.; Chen, Y.; Ge, Z.; Shi, J. Hydrothermal liquefaction of polycarbonate (PC) plastics in sub-/supercritical water and reaction pathway exploration. ACS Sustain. Chem. Eng. 2020, 8, 7039–7050. [Google Scholar] [CrossRef]
- Saito, K.; Jehanno, C.; Meabe, L.; Olmedo-Martinez, J.L.; Mecerreyes, D.; Fukushima, K.; Sardon, H. From plastic waste to polymer electrolytes for batteries through chemical upcycling of polycarbonate. J. Mater. Chem. A 2020, 8, 13921–13926. [Google Scholar] [CrossRef]
- Amsden, B. In vivo degradation mechanisms of aliphatic polycarbonates and functionalized aliphatic polycarbonates. Macromol. Biosci. 2021, 21, 2100085. [Google Scholar] [CrossRef] [PubMed]
- Barbarias, I.; Lopez, G.; Artetxe, M.; Arregi, A.; Bilbao, J.; Olazar, M. Valorisation of different waste plastics by pyrolysis and in-line catalytic steam reforming for hydrogen production. Energy Convers. Manag. 2018, 156, 575–584. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xu, S.; Tang, J.; Fu, L.; Karimi-Maleh, H. Sustainably Recycling and Upcycling of Single-Use Plastic Wastes through Heterogeneous Catalysis. Catalysts 2022, 12, 818. https://doi.org/10.3390/catal12080818
Zhang X, Xu S, Tang J, Fu L, Karimi-Maleh H. Sustainably Recycling and Upcycling of Single-Use Plastic Wastes through Heterogeneous Catalysis. Catalysts. 2022; 12(8):818. https://doi.org/10.3390/catal12080818
Chicago/Turabian StyleZhang, Xiaoxia, Shaodan Xu, Junhong Tang, Li Fu, and Hassan Karimi-Maleh. 2022. "Sustainably Recycling and Upcycling of Single-Use Plastic Wastes through Heterogeneous Catalysis" Catalysts 12, no. 8: 818. https://doi.org/10.3390/catal12080818
APA StyleZhang, X., Xu, S., Tang, J., Fu, L., & Karimi-Maleh, H. (2022). Sustainably Recycling and Upcycling of Single-Use Plastic Wastes through Heterogeneous Catalysis. Catalysts, 12(8), 818. https://doi.org/10.3390/catal12080818







