Surface Modification of Biochar for Dye Removal from Wastewater
Abstract
:1. Introduction
2. Biochar
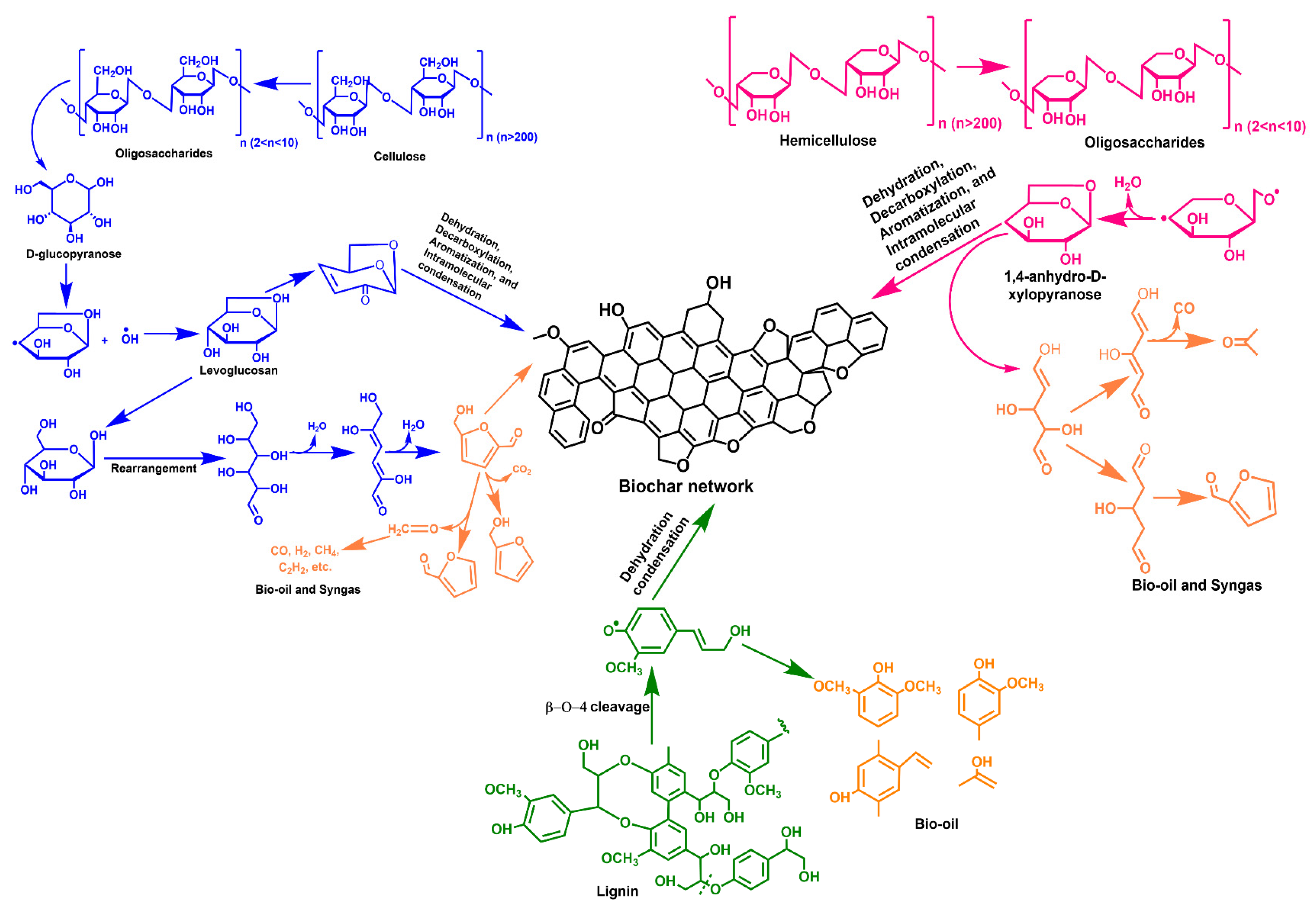
2.1. Production of Biochar
2.2. Characteristics of Biochar
2.2.1. Physical Properties
Nano and Macroporosity
Particle Size Distribution
Density
Mechanical Strength
2.2.2. Chemical Properties
2.2.3. Microchemical Characteristics
2.2.4. Organo-Chemical Characteristics
2.3. Factors Influencing Biochar Sorption Efficiency
2.3.1. Temperature
2.3.2. Solution pH
2.3.3. Adsorbent Dosage
2.3.4. Initial Dye Concentration
2.3.5. Heating Rate
2.3.6. Particle Size
2.3.7. Feedstock Composition
2.4. Mechanisms of Dye Adsorption
2.4.1. Physical Adsorption
2.4.2. Ion Exchange
2.4.3. Electrostatic Attraction
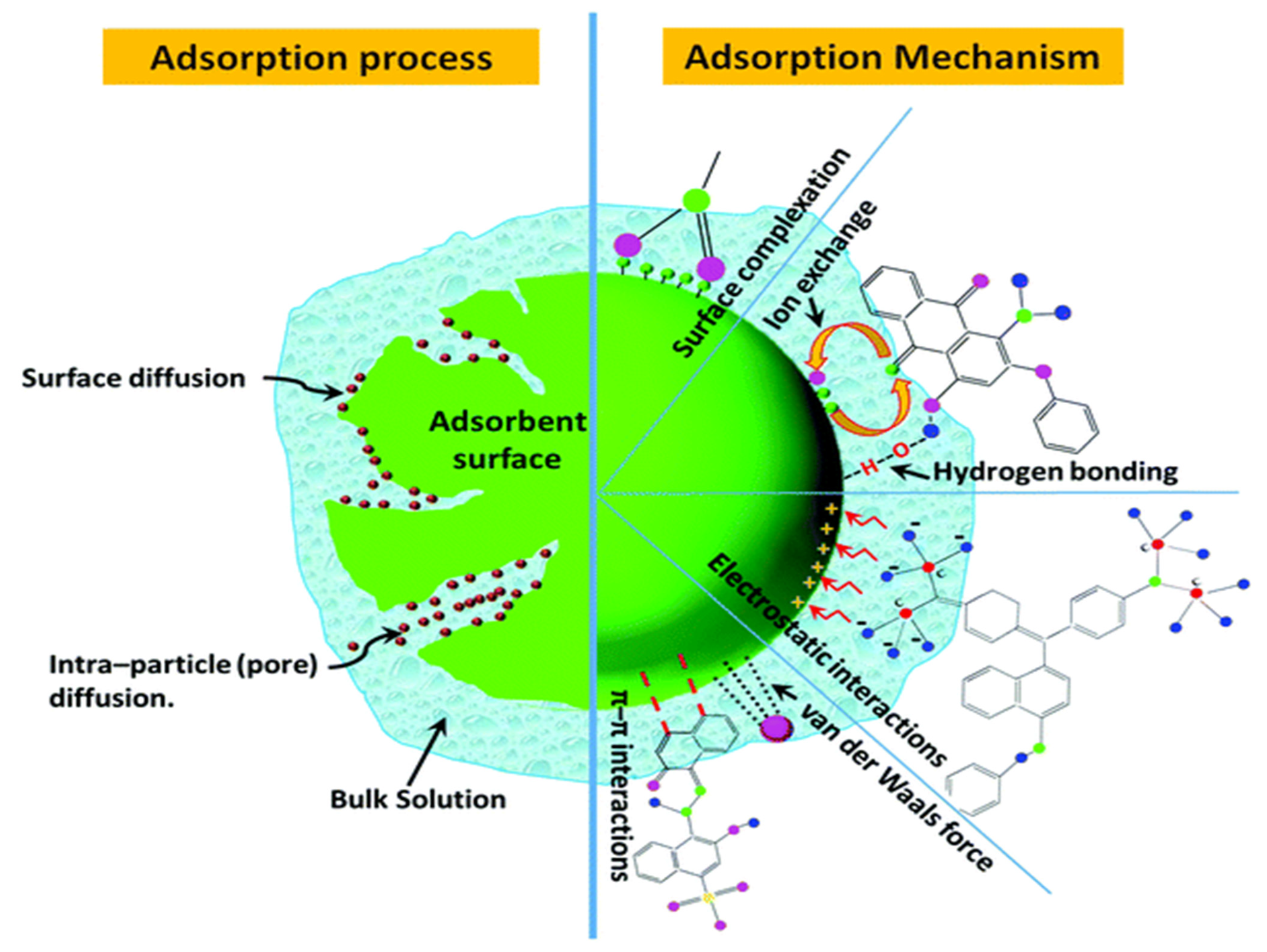
3. Post-Production Modification of Biochar for Dye Removal
| Synthetic Dye | Biochar | Adsorption Capacity | Conditions Adsorption Mechanism | Reference | |
|---|---|---|---|---|---|
| Feedstock | Method of Production | ||||
| Congo red | Litchi peel BC | Hydrothermal carbonization | 404.4 mg g−1 | Pore filling effect π–π interaction Electrostatic interaction Hydrogen bonding | [45] |
| Orange peel waste | Microwave pyrolysis using CO2 and steam activation | 136 mg g−1 | Electrostatic interaction | [82] | |
| Switchgrass | Pyrolysis (900 °C) | 22.6 mg g−1 | Electrostatic interaction π–π interaction | [83] | |
| Acid violet 17 | Pine tree-derived BC | - | 90% | Electrostatic interactions | [84] |
| Malachite green | Cladodes of cactus (Opuntia ficus-indica) | Pyrolysis at 400 °C followed by NaOH impregnation, pyrolysis at 500 °C and rinsing with HCl | 1341 mg g−1 | Π–π EDA interaction Cation-π interaction Hydrogen bonding | [85] |
| Corn straw | HNO3 treatment, followed by washing with distilled water and drying. Then, NaOH activation and drying, followed by pyrolysis at 500 °C and FeCl3 modification by precipitation technique | 515.8 mg g−1 | Electrostatic attraction | [86] | |
| Frass of mealworms (Tenebrio molitor Linnaeus 1758) | Pyrolysis at 800 °C | 1738.6 mg g−1 | Electrostatic interaction π–π interaction Hydrogen bonding | [87] | |
| Litchi peel BC | Hydrothermal carbonization at 850 °C | 2468 mg g−1 | Pore filling effect π–π interaction Electrostatic interaction Hydrogen bonding | [45] | |
| Tapioca peel waste | Pyrolysis of feedstock, then mixing with thiourea and followed by pyrolysis at 800 °C to create sulfur-doped BC | 30.2 mg g−1 | Electrostatic interaction Hydrogen bonding | [67] | |
| Wakame (macroalgae) | Chemical activation with KOH followed by pyrolysis at 800 °C | 4066.9 mg g−1 | Electrostatic interaction π–π stacking Hydrogen bonding van der Waals force | [88] | |
| Methylene blue | Macroalgae (Undaria pinnatifida) | Chemical activation with KOH followed by pyrolysis at 800 °C | 841.6 mg g−1 | Electrostatic interaction π–π interaction Hydrogen bonding and van der Waals force | [88] |
| Rice straw and fly ash | Alkali-fusion pre-treatment of fly ash with NaOH, followed by mixing with rice straw and pyrolysis at 700 °C | 143.8 mg g−1 | Electrostatic interaction π–π interaction | [68] | |
| Orange G | Switchgrass | Pyrolysis at 900 °C | 38.2 mg g−1 | Electrostatic interaction π–π interaction | [83] |
| Rhodamine B | Macroalgae (Undaria pinnatifida) | Chemical activation with KOH followed by pyrolysis at 800 °C | 533.8 mg g−1 | Electrostatic interaction π–π interaction Hydrogen bonding van der Waals force | [88] |
| Tapioca peel waste | Pyrolysis of feedstock, then mixing with thiourea and followed by pyrolysis at 800 °C to create sulfur-doped BC | 33.1 mg g−1 | Electrostatic interaction Hydrogen bonding | [67] | |
| Sunset yellow/ Tartrazine | Corncob | Pyrolysis at 400 °C followed by mixing with triethylenetetramine, drying and H2SO4 treatment to achieve a positively charged BC | 77.1 mg g−1 | Amine groups on the surface Electrostatic interaction | [74] |
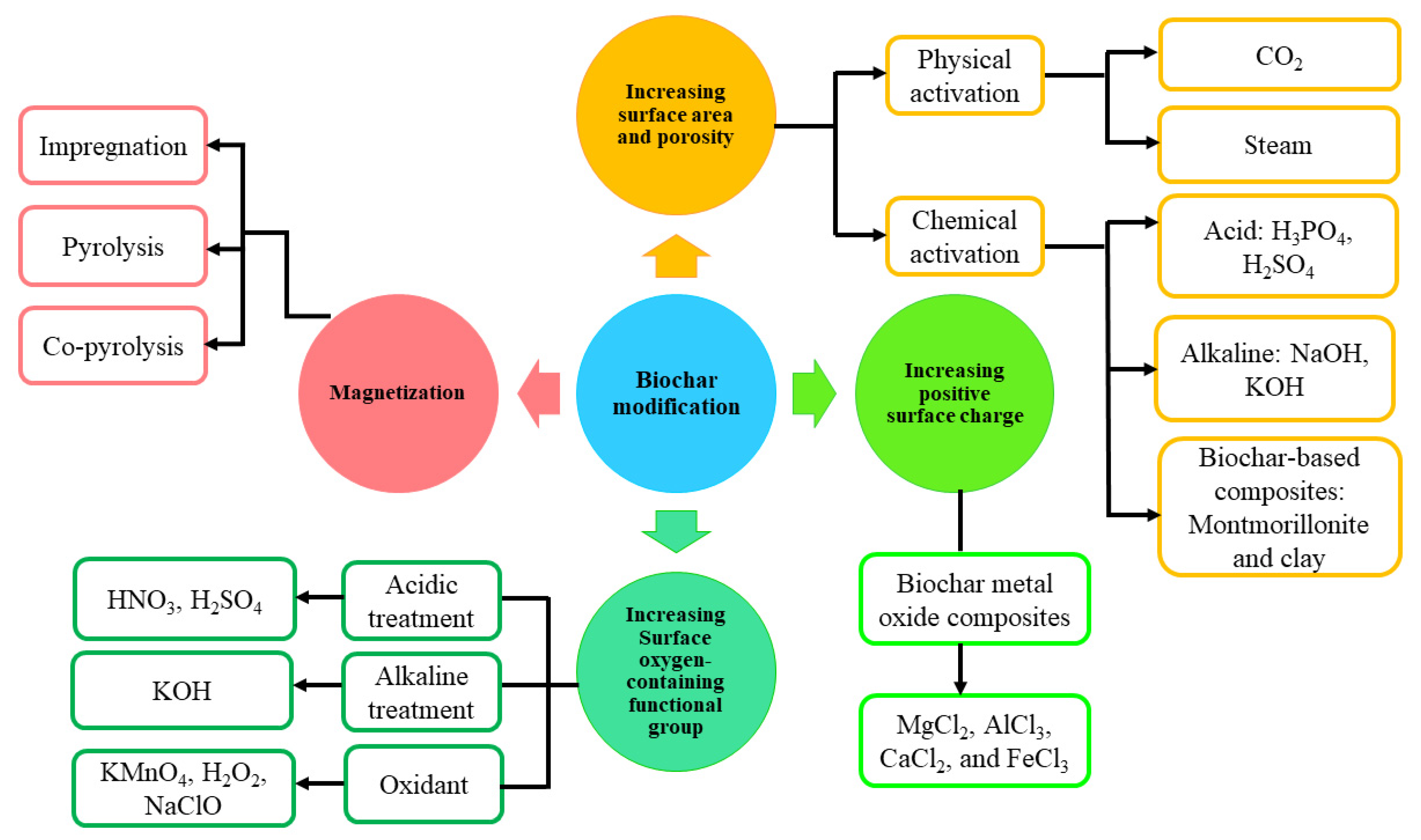
3.1. Acid-Base Activation/Decoration
3.2. Persulfate Activation
3.3. Physical Activation
3.4. Biochar-Based Composites
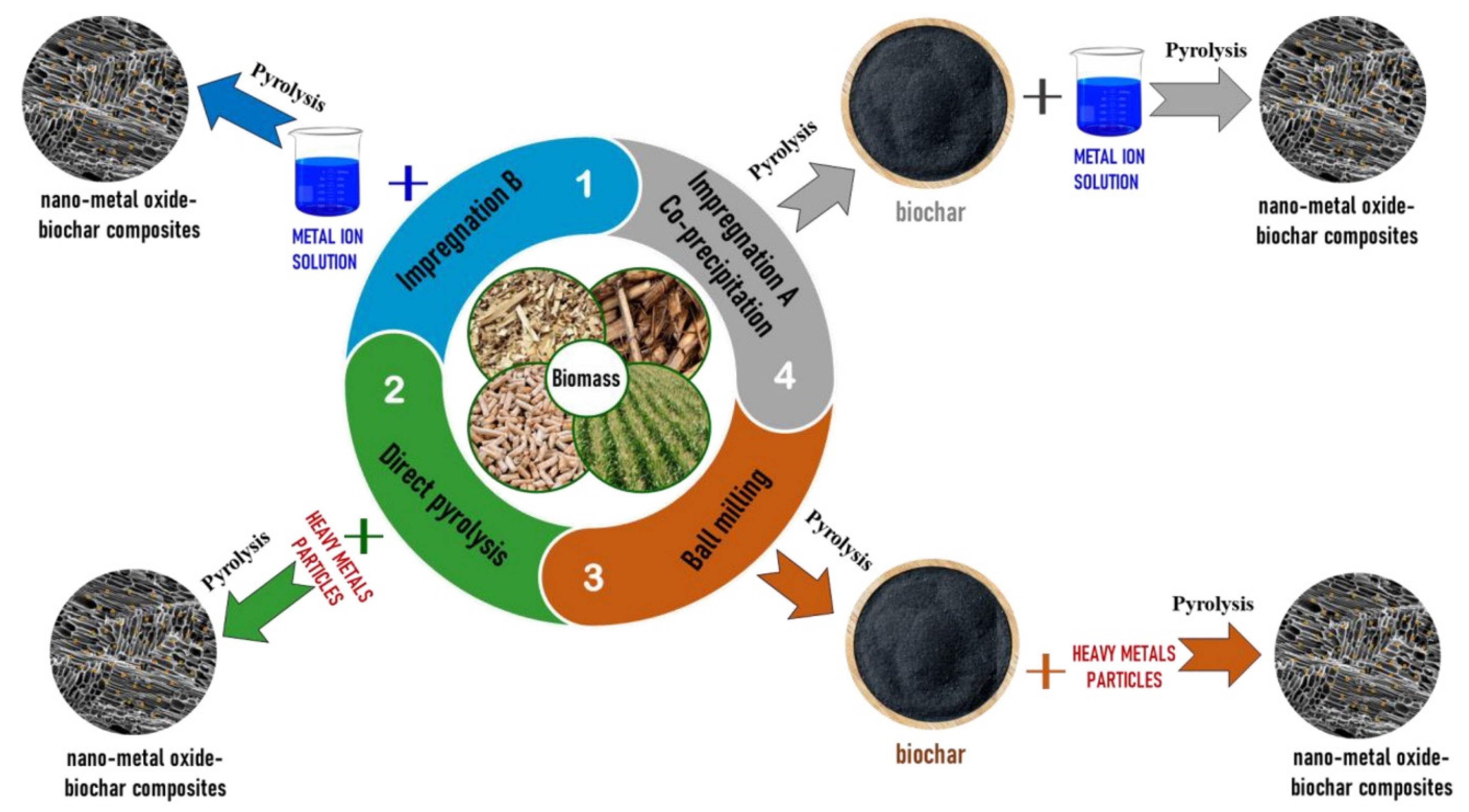
3.4.1. Nano-Metal Oxide/Hydroxide-Biochar Composites
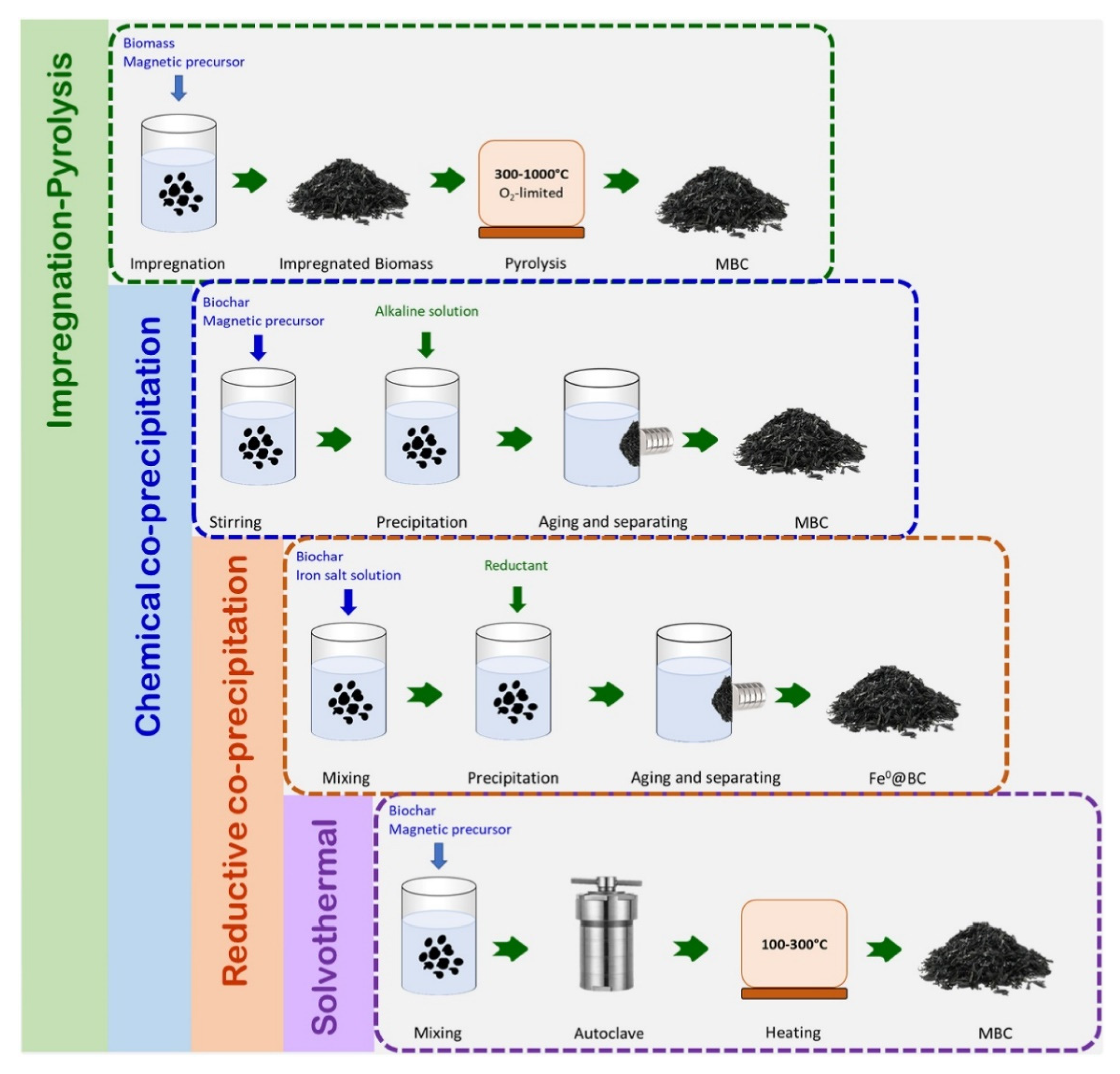
3.4.2. Magnetized Biochar Composites
| S. No. | Surface-Modified Biochar and Its Nanocomposites | Dye | Adsorption Model | Maximum Adsorption Capacity (mg/g) | Mechanism Involved | Reference |
|---|---|---|---|---|---|---|
| 1. | TiO2 supported BC | 3,4-dimethylaniline | Toth adsorption models | 285.71 | - | [151] |
| 2. | Layered double-oxide/BC composites | Congo red | Langmuir | 344.83, | - | [152] |
| 3. | Methyl orange | Langmuir | 588.24 | - | [152] | |
| 4. | CO2 and H2O activator Pine sawdust BC | Methylene blue | Langmuir | 160 | Hydrogen bonding, ion exchange, π–π interaction | [153] |
| 5. | Phosphomolybdate-modified BC | Methylene blue | Langmuir | 146.23 | Hydrogen bonding, electrostatic interactions, and ion exchange | [154] |
| 6. | Banana peel BC/iron oxide composite | Methylene blue | Langmuir | 862 | - | [54] |
| 7. | Mixed municipal discarded material derived BC | Methylene blue | Langmuir | 7.2 | π–π interactions | [155] |
| 8. | KOH modified lychee seed BC | Methylene blue | Langmuir | 124.5 | - | [156] |
| 9. | Triethylenetetramine corncob BC | Sunset yellow | Langmuir | - | - | [74] |
| 10. | Fe2O3/TiO2 functionalized wasted tea leaves derived BC | Methylene blue | - | - | Reactive radical species | [157] |
| 11. | Rhodamine B | - | - | |||
| 12. | Methyl orange | - | - | |||
| 13. | Manganese-modified lignin BC | Methylene blue | Langmuir | 248.96 | - | [158] |
| 14. | KOH activated pine BC | Methylene blue | Freundlich | 637.5 | Primary polar and π–π interactions | [109] |
| 15. | KMnO4 activated pine BC | Methylene blue | Freundlich | 439.5 | Primary polar and π–π interactions | [109] |
| 16. | Fe3O4-modified Citrus bergamia peel derived BC | Methylene blue | Langmuir | 136.72 | Electrostatic interaction | [26] |
| 17. | Sulfuric acid modified BC from Pumpkin peel | Methylene Blue | Langmuir | 208.3 | - | [159] |
| 18. | Acid activated Pine needle BC | Methylene blue | Langmuir | 153.84 | Hydrogen bonding, electrostatic interaction | [160] |
| 19. | Laccase immobilized pine needle BC | Malachite green | - | - | Enzymatic degradation | [161] |
| 20. | Ozonized saw dust BC | Methylene blue | Langmuir | 200 | Electrostatic interaction and hydrogen bonding | [162] |
| 21. | Sonicated saw dust BC | Methylene blue | Langmuir | 526 | ||
| 22. | Nitric acid-treated Pterospermum acerifolium fruit waste BC | Methylene blue | Langmuir | - | - | [28] |
| 23. | SDS-modified nitric acid-treated Pterospermum acerifolium fruit waste BC | Methylene blue | Langmuir | - | - | [28] |
| 24. | Cetyl trimethyl ammonium bromide modified magnetic BC from pine nut shells | Acid chrome blue K | Langmuir | - | - | [27] |
3.4.3. Modification via Clay Mineral
4. Application of Machine Learning and Artificial Neural Networks into Biochar-Facilitated Wastewater Remediation
5. Conclusions and Future Perspectives
- (1)
- To attain optimum BC efficacy, it is crucial to study the relations amid various parameters, such as production process, modification/functionalization, and handling all in an eco-friendly way.
- (2)
- Moreover, promising sorbents that are effectively suitable for pilot scale must be inexpensive and resources ought to be widely accessible in huge amounts in nature. Recycling of sorbents on a large scale can reduce costs and energy consumption to provide sustainable products.
- (3)
- Involvement of software to optimize factors affecting pollutant removal by BC is an innovative and powerful tool in experimental design and analysis. Applications of ANN and ML can be used to predict and reduce explicit computer programming.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bouckaert, S.; Pales, A.F.; McGlade, C.; Remme, U.; Wanner, B.; Varro, L.; Spencer, T. Net Zero by 2050: A Roadmap for the Global Energy Sector; International Energy Agency: Paris, France, 2021. [Google Scholar]
- Sathe, S.S.; Goswami, L.; Mahanta, C. Arsenic reduction and mobilization cycle via microbial activities prevailing in the Holocene aquifers of Brahmaputra flood plain. Groundw. Sustain. Dev. 2021, 13, 100578. [Google Scholar] [CrossRef]
- Kushwaha, A.; Goswami, S.; Hans, N.; Goswami, L.; Devi, G.; Deshavath, N.N.; Lall, A.M. An insight into biological and chemical technologies for micropollutant removal from wastewater. In Fate and Transport of Subsurface Pollutants; Springer: Singapore, 2021; pp. 199–226. [Google Scholar]
- Mallakpour, S.; Lormahdiabadi, M. Removal of the Anionic Dye Congo Red from an Aqueous Solution Using a Crosslinked Poly (vinyl alcohol)-ZnO-Vitamin M Nanocomposite Film: A Study of the Recent Concerns about Nonlinear and Linear Forms of Isotherms and Kinetics. Langmuir 2022, 38, 4065–4076. [Google Scholar] [CrossRef] [PubMed]
- Hussain, C.M.; Singh, S.; Goswami, L. Emerging Trends to Approaching Zero Waste; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Hussain, C.M.; Singh, S.; Goswami, L. (Eds.) Waste-to-Energy Approaches towards Zero Waste: Interdisciplinary Methods of Controlling Waste; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Choudhary, M.; Kumar, R.; Neogi, S. Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye, Cu+2 and Ni+2 from water. J. Hazard. Mater. 2020, 392, 122441. [Google Scholar] [CrossRef]
- Begum, W.; Goswami, L.; Sharma, B.B.; Kushwaha, A. Assessment of urban river pollution using the water quality index and macro-invertebrate community index. Environ. Dev. Sustain. 2022, 1–26. [Google Scholar] [CrossRef]
- Goswami, S.; Kushwaha, A.; Goswami, L.; Singh, N.; Bhan, U.; Daverey, A.; Hussain, C.M. Biological treatment, recovery, and recycling of metals from waste printed circuit boards. In Environmental Management of Waste Electrical and Electronic Equipment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 163–184. [Google Scholar]
- Lata, K.; Kushwaha, A.; Ramanathan, G. Bacterial enzymatic degradation and remediation of 2,4,6-trinitrotoluene. Microb. Nat. Macromol. 2021, 23, 623–659. [Google Scholar]
- Vadivel, V.K.; Cikurel, H.; Mamane, H. Removal of Indigo Dye by CaCO3/Ca(OH)2 Composites and Resource Recovery. Ind. Eng. Chem. Res. 2021, 60, 10312–10318. [Google Scholar] [CrossRef]
- Kumar, M.; Kushwaha, A.; Goswami, L.; Singh, A.K.; Sikandar, M. A review on advances and mechanism for the phycoremediation of cadmium contaminated wastewater. Clean. Eng. Technol. 2021, 5, 100288. [Google Scholar] [CrossRef]
- Rizvi, S.; Singh, A.; Kushwaha, A.; Gupta, S.K. Recent advances in melanoidin removal from wastewater: Sources, properties, toxicity, and remediation strategies. In Emerging Trends to Approaching Zero Waste; Elsevier: Amsterdam, The Netherlands, 2022; pp. 361–386. [Google Scholar]
- Patra, D.; Patra, B.R.; Pattnaik, F.; Hans, N.; Kushwaha, A. Recent evolution in green technologies for effective valorization of food and agricultural wastes. In Emerging Trends to Approaching Zero Waste; Elsevier: Amsterdam, The Netherlands, 2022; pp. 103–132. [Google Scholar]
- Kushwaha, A.; Goswami, S.; Hans, N.; Singh, A.; Vishwakarma, H.S.; Devi, G.; Hussain, C.M. Sorption of pharmaceutical and personal care products from the wastewater by carbonaceous materials. In Emerging Trends to Approaching Zero Waste; Elsevier: Amsterdam, The Netherlands, 2021; pp. 175–196. [Google Scholar]
- Saha, P.; Goswami, L.; Kim, B.S. Novel Biobased Non-Isocyanate Polyurethanes from Microbially Produced 7,10-Dihydroxy-8 (E)-Octadecenoic Acid for Potential Packaging and Coating Applications. ACS Sustain. Chem. Eng. 2022, 10, 4623–4633. [Google Scholar] [CrossRef]
- Ojaswini, C.; Sathe, S.S.; Mahanta, C.; Kushwaha, A. Evaluation of iron coated natural sand for removal of dissolved arsenic from groundwater and develop sustainable filter media. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100682. [Google Scholar] [CrossRef]
- Gautam, A.; Kushwaha, A.; Rani, R. Microbial remediation of hexavalent chromium: An eco-friendly strategy for the remediation of chromium-contaminated wastewater. In The Future of Effluent Treatment Plants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 361–384. [Google Scholar]
- Gupt, C.B.; Kushwaha, A.; Prakash, A.; Chandra, A.; Goswami, L.; Sekharan, S. Mitigation of groundwater pollution: Heavy metal retention characteristics of fly ash based liner materials. In Fate and Transport of Subsurface Pollutants; Springer: Singapore, 2021; pp. 79–104. [Google Scholar]
- Kushwaha, A.; Rani, R.; Patra, J.K. Adsorption kinetics and molecular interactions of lead [Pb (II)] with natural clay and humic acid. Int. J. Environ. Sci. Technol. 2020, 17, 1325–1336. [Google Scholar] [CrossRef]
- Goswami, L.; Manikandan, N.A.; Taube, J.C.R.; Pakshirajan, K.; Pugazhenthi, G. Novel waste-derived biochar from biomass gasification effluent: Preparation, characterization, cost estimation, and application in polycyclic aromatic hydrocarbon biodegradation and lipid accumulation by Rhodococcus opacus. Environ. Sci. Pollut. Res. 2019, 26, 25154–25166. [Google Scholar] [CrossRef]
- Goswami, L.; Kushwaha, A.; Singh, A.; Saha, P.; Choi, Y.; Maharana, M.; Kim, B.S. Nano-biochar as a sustainable catalyst for anaerobic digestion: A synergetic closed-loop approach. Catalysts 2022, 12, 186. [Google Scholar] [CrossRef]
- Goswami, S.; Kushwaha, A.; Goswami, L.; Gupta, N.R.; Kumar, V.; Bhan, U.; Tripathi, K.M. Nanobiochar-a green catalyst for wastewater remediation. In Bio-Based Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 109–132. [Google Scholar]
- Goswami, L.; Pakshirajan, K.; Pugazhenthi, G. Biological treatment of biomass gasification wastewater using hydrocarbonoclastic bacterium Rhodococcus opacus in an up-flow packed bed bioreactor with a novel waste-derived nano-biochar based bio-support material. J. Clean. Prod. 2020, 256, 120253. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Tripathy, S.; Sahu, S.; Patel, R.K.; Panda, R.B.; Kar, P.K. Novel Fe3O4-Modified Biochar Derived from Citrus Bergamia Peel: A Green Synthesis Approach for Adsorptive Removal of Methylene Blue. ChemistrySelect 2022, 7, e202103595. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Gao, Y. Cetyl trimethyl ammonium bromide modified magnetic biochar from pine nut shells for efficient removal of acid chrome blue K. Bioresour. Technol. 2020, 312, 123564. [Google Scholar] [CrossRef]
- Oraon, A.; Prajapati, A.K.; Ram, M.; Saxena, V.K.; Dutta, S. Synthesis, characterization, and application of microporous biochar prepared from Pterospermum acerifolium plant fruit shell waste for methylene blue dye adsorption: The role of surface modification by SDS surfactant. Biomass Convers. Biorefin. 2022, 1–23. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Zaman, C.Z.; Pal, K.; Yehye, W.A.; Sagadevan, S.; Shah, S.T.; Adebisi, G.A.; Johan, R.B. Pyrolysis: A Sustainable Way to Generate Energy from Waste; IntechOpen: Rijeka, Croatia, 2017; Volume 1, p. 316806. [Google Scholar]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Zeghioud, H.; Fryda, L.; Djelal, H.; Assadi, A.; Kane, A. A comprehensive review of biochar in removal of organic pollutants from wastewater: Characterization, toxicity, activation/functionalization and influencing treatment factors. J. Water Process Eng. 2022, 47, 102801. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Li, Y.; Chen, L.; Jiang, H.; Li, H.; Hou, S. Fabrication, application, and mechanism of metal and heteroatom co-doped biochar composites (MHBCs) for the removal of contaminants in water: A review. J. Hazard. Mater. 2022, 431, 128584. [Google Scholar] [CrossRef]
- Da Silva Medeiros, D.C.C.; Nzediegwu, C.; Benally, C.; Messele, S.A.; Kwak, J.H.; Naeth, M.A.; El-Din, M.G. Pristine and engineered biochar for the removal of contaminants co-existing in several types of industrial wastewaters: A critical review. Sci. Total Environ. 2021, 809, 151120. [Google Scholar] [CrossRef]
- Das, O.; Bhattacharyya, D.; Hui, D.; Lau, K.T. Mechanical and flammability characterisations of biochar/polypropylene biocomposites. Compos. Part B Eng. 2016, 106, 120–128. [Google Scholar] [CrossRef]
- Zou, H.; Zhao, J.; He, F.; Zhong, Z.; Huang, J.; Zheng, Y.; Gao, B. Ball milling biochar iron oxide composites for the removal of chromium (Cr (VI)) from water: Performance and mechanisms. J. Hazard. Mater. 2021, 413, 125252. [Google Scholar] [CrossRef]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting biochar properties and functions based on feedstock and pyrolysis temperature: A review and data syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Agrafioti, E.; Bouras, G.; Kalderis, D.; Diamadopoulos, E. Biochar production by sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 72–78. [Google Scholar] [CrossRef]
- Amonette, J.E.; Joseph, S. Characteristics of biochar: Microchemical properties. In Biochar for Environmental Management; Routledge: London, UK, 2012; pp. 65–84. [Google Scholar]
- Huang, Q.; Song, S.; Chen, Z.; Hu, B.; Chen, J.; Wang, X. Biochar-based materials and their applications in removal of organic contaminants from wastewater: State-of-the-art review. Biochar 2019, 1, 45–73. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. [Google Scholar] [CrossRef]
- Wu, J.; Yang, J.; Feng, P.; Huang, G.; Xu, C.; Lin, B. High-efficiency removal of dyes from wastewater by fully recycling litchi peel biochar. Chemosphere 2020, 246, 125734. [Google Scholar] [CrossRef] [PubMed]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Kushwaha, A.; Rani, R.; Kumar, S.; Thomas, T.; David, A.A.; Ahmed, M. A new insight to adsorption and accumulation of high lead concentration by exopolymer and whole cells of lead-resistant bacterium Acinetobacter junii L. Pb1 isolated from coal mine dump. Environ. Sci. Pollut. Res. 2017, 24, 10652–10661. [Google Scholar] [CrossRef] [PubMed]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for wastewater treatment-conversion technologies and applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Gautam, R.K.; Goswami, M.; Mishra, R.K.; Chaturvedi, P.; Awashthi, M.K.; Singh, R.S.; Pandey, A. Biochar for remediation of agrochemicals and synthetic organic dyes from environmental samples: A review. Chemosphere 2021, 272, 129917. [Google Scholar] [CrossRef]
- Qiu, Y.; Zheng, Z.; Zhou, Z.; Sheng, G.D. Effectiveness and mechanisms of dye adsorption on a straw-based biochar. Bioresour. Technol. 2009, 100, 5348–5351. [Google Scholar] [CrossRef] [PubMed]
- Kaya, N.; Yıldız, Z.; Ceylan, S. Preparation and characterisation of biochar from hazelnut shell and its adsorption properties for methylene blue dye. Politek. Derg. 2018, 21, 765–776. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C.; Ho, S.H.; Zhou, Y.; Ren, N.Q. Highly efficient adsorption of dyes by biochar derived from pigments-extracted macroalgae pyrolyzed at different temperature. Bioresour. Technol. 2018, 259, 104–110. [Google Scholar]
- Nautiyal, P.; Subramanian, K.A.; Dastidar, M.G. Adsorptive removal of dye using biochar derived from residual algae after in-situ transesterification: Alternate use of waste of biodiesel industry. J. Environ. Manag. 2016, 182, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; O’Connor, D.; Wang, Y.; Jiang, L.; Xia, T.; Wang, L.; Hou, D. A green biochar/iron oxide composite for methylene blue removal. J. Hazard. Mater. 2020, 384, 121286. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Chakraborty, S.; Saha, P. Biosorption of Basic Green 4 from aqueous solution by Ananas comosus (pineapple) leaf powder. Colloids Surf. B Biointerfaces 2011, 84, 520–527. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K.; Phan, C. Adsorption removal of Methylene Blue (MB) dye from aqueous solution by bio-char prepared from Eucalyptus sheathiana bark: Kinetic, equilibrium, mechanism, thermodynamic and process design. Desalination Water Treat. 2016, 57, 28964–28980. [Google Scholar] [CrossRef]
- Biswas, S.; Mohapatra, S.S.; Kumari, U.; Meikap, B.C.; Sen, T.K. Batch and continuous closed circuit semi-fluidized bed operation: Removal of MB dye using sugarcane bagasse biochar and alginate composite adsorbents. J. Environ. Chem. Eng. 2020, 8, 103637. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, B. Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour. Technol. 2013, 146, 485–493. [Google Scholar] [CrossRef]
- Li, C.; Hayashi, J.I.; Sun, Y.; Zhang, L.; Zhang, S.; Wang, S.; Hu, X. Impact of heating rates on the evolution of function groups of the biochar from lignin pyrolysis. J. Anal. Appl. Pyrolysis 2021, 155, 105031. [Google Scholar] [CrossRef]
- Yorgun, S.; Yıldız, D.; Şimşek, Y.E. Activated carbon from paulownia wood: Yields of chemical activation stages. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 2035–2042. [Google Scholar] [CrossRef]
- Harun, N.Y.; Afzal, M.T. Effect of particle size on mechanical properties of pellets made from biomass blends. Procedia Eng. 2016, 148, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Bourke, J.; Manley-Harris, M.; Fushimi, C.; Dowaki, K.; Nunoura, T.; Antal, M.J. Do all carbonized charcoals have the same chemical structure? 2. A model of the chemical structure of carbonized charcoal. Ind. Eng. Chem. Res. 2007, 46, 5954–5967. [Google Scholar] [CrossRef]
- Aliasa, N.; Zaini, M.A.A.; Kamaruddin, M.J. Roles of impregnation ratio of K2CO3 and NaOH in chemical activation of palm kernel shell. J. Appl. Sci. Process Eng. 2017, 4, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, B.; Zhang, H.; Zhang, W. Fe/S modified sludge-based biochar for tetracycline removal from water. Powder Technol. 2020, 364, 889–900. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Y.; Lu, B.; Xiao, B.; Chen, R.; Liu, H. Efficient activation of peroxymonosulfate and degradation of Orange G in iron phosphide prepared by pickling waste liquor. Chemosphere 2021, 269, 129398. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Sirajudheen, P.; Karthikeyan, P.; Meenakshi, S. Fabrication of sulfur-doped biochar derived from tapioca peel waste with superior adsorption performance for the removal of Malachite green and Rhodamine B dyes. Surf. Interfaces 2021, 23, 100920. [Google Scholar] [CrossRef]
- Wang, K.; Peng, N.; Sun, J.; Lu, G.; Chen, M.; Deng, F.; Zhong, Y. Synthesis of silica-composited biochars from alkali-fused fly ash and agricultural wastes for enhanced adsorption of methylene blue. Sci. Total Environ. 2020, 729, 139055. [Google Scholar] [CrossRef]
- Cojocaru, C.; Samoila, P.; Pascariu, P. Chitosan-based magnetic adsorbent for removal of water-soluble anionic dye: Artificial neural network modeling and molecular docking insights. Int. J. Biol. Macromol. 2019, 123, 587–599. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Manzoor, O.; Mohsin, M.; Chaudhry, S.A. Nigella sativa seed-based nanocomposite-MnO2/BC: An antibacterial material for photocatalytic degradation, and adsorptive removal of Methylene blue from water. Environ. Res. 2019, 171, 328–340. [Google Scholar] [CrossRef]
- Pirbazari, A.E.; Saberikhah, E.; Kozani, S.H. Fe3O4–wheat straw: Preparation, characterization and its application for methylene blue adsorption. Water Resour. Ind. 2014, 7, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Cheng, B.; You, W.; Yu, J.; Ho, W. 3D hierarchical graphene oxide-NiFe LDH composite with enhanced adsorption affinity to Congo red, methyl orange and Cr (VI) ions. J. Hazard. Mater. 2019, 369, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, X.; Zhang, C.; Yu, Q.; Zhao, Z.; Niu, X.; Li, Z. Enhanced adsorptive removal of anionic and cationic dyes from single or mixed dye solutions using MOF PCN-222. RSC Adv. 2017, 7, 16273–16281. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, M.E.; Abdelfattah, A.M.; Tharwat, R.M.; Nabil, G.M. Adsorption of negatively charged food tartrazine and sunset yellow dyes onto positively charged triethylenetetramine biochar: Optimization, kinetics and thermodynamic study. J. Mol. Liq. 2020, 318, 114297. [Google Scholar] [CrossRef]
- Ho, S.H.; Zhu, S.; Chang, J.S. Recent advances in nanoscale-metal assisted biochar derived from waste biomass used for heavy metals removal. Bioresour. Technol. 2017, 246, 123–134. [Google Scholar] [CrossRef]
- Shen, K.; Gondal, M.A. Removal of hazardous Rhodamine dye from water by adsorption onto exhausted coffee ground. J. Saudi Chem. Soc. 2017, 21, S120–S127. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Vijayaraghavan, K. The importance of mineral ingredients in biochar production, properties and applications. Crit. Rev. Environ. Sci. Technol. 2021, 51, 113–139. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, Y.G.; Gu, Y.L.; Xu, Y.; Zeng, G.M.; Hu, X.J.; Li, J. Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef]
- Dai, L.; Lu, Q.; Zhou, H.; Shen, F.; Liu, Z.; Zhu, W.; Huang, H. Tuning oxygenated functional groups on biochar for water pollution control: A critical review. J. Hazard. Mater. 2021, 420, 126547. [Google Scholar] [CrossRef]
- Yek, P.N.Y.; Peng, W.; Wong, C.C.; Liew, R.K.; Ho, Y.L.; Mahari, W.A.W.; Lam, S.S. Engineered biochar via microwave CO2 and steam pyrolysis to treat carcinogenic Congo red dye. J. Hazard. Mater. 2020, 395, 122636. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Wang, J.J.; Meng, Y.; Wei, Z.; DeLaune, R.D.; Seo, D.C. Adsorption/desorption behavior of cationic and anionic dyes by biochars prepared at normal and high pyrolysis temperatures. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 274–282. [Google Scholar] [CrossRef]
- Sterenzon, E.; Vadivel, V.K.; Gerchman, Y.; Luxbacher, T.; Narayanan, R.; Mamane, H. Effective Removal of Acid Dye in Synthetic and Silk Dyeing Effluent: Isotherm and Kinetic Studies. ACS Omega 2021, 7, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.F.; Khandaker, S.; Sarker, F.; Islam, A.; Rahman, M.T.; Awual, M.R. Current treatment technologies and mechanisms for removal of indigo carmine dyes from wastewater: A review. J. Mol. Liq. 2020, 318, 114061. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Mohamed, H.A.; Abd El-Monaem, E.M.; El-Subruiti, G.M. Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye: Characterization, adsorption kinetics, thermodynamics and isotherms. Adv. Powder Technol. 2020, 31, 1253–1263. [Google Scholar] [CrossRef]
- Yang, S.S.; Kang, J.H.; Xie, T.R.; He, L.; Xing, D.F.; Ren, N.Q.; Wu, W.M. Generation of high-efficient biochar for dye adsorption using frass of yellow mealworms (larvae of Tenebrio molitor Linnaeus) fed with wheat straw for insect biomass production. J. Clean. Prod. 2019, 227, 33–47. [Google Scholar] [CrossRef]
- Yao, X.; Ji, L.; Guo, J.; Ge, S.; Lu, W.; Chen, Y.; Song, W. An abundant porous biochar material derived from wakame (Undaria pinnatifida) with high adsorption performance for three organic dyes. Bioresour. Technol. 2020, 318, 124082. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Gaston, L.A.; Zhou, B.; Li, M.; Xiao, R.; Zhang, X. An overview of carbothermal synthesis of metal–biochar composites for the removal of oxyanion contaminants from aqueous solution. Carbon 2018, 129, 674–687. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.J.; Jiang, H.; Chen, J.J.; Li, W.W.; Yu, H.Q. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour. Technol. 2012, 121, 235–240. [Google Scholar] [CrossRef]
- Shen, W.; Li, Z.; Liu, Y. Surface chemical functional groups modification of porous carbon. Recent Pat. Chem. Eng. 2008, 1, 27–40. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Biochar based nanocomposites for photocatalytic degradation of emerging organic pollutants from water and wastewater. Mater. Res. Bull. 2021, 140, 111262. [Google Scholar] [CrossRef]
- Sun, J.; Lin, X.; Xie, J.; Zhang, Y.; Wang, Q.; Ying, Z. Facile synthesis of novel ternary g-C3N4/ferrite/biochar hybrid photocatalyst for efficient degradation of methylene blue under visible-light irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125556. [Google Scholar] [CrossRef]
- Yu, F.; Tian, F.; Zou, H.; Ye, Z.; Peng, C.; Huang, J.; Gao, B. ZnO/biochar nanocomposites via solvent free ball milling for enhanced adsorption and photocatalytic degradation of methylene blue. J. Hazard. Mater. 2021, 415, 125511. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Benis, K.Z.; Damuchali, A.M.; Soltan, J.; McPhedran, K.N. Treatment of aqueous arsenic–A review of biochar modification methods. Sci. Total Environ. 2020, 739, 139750. [Google Scholar] [CrossRef] [PubMed]
- Barquilha, C.E.; Braga, M.C. Adsorption of organic and inorganic pollutants onto biochars: Challenges, operating conditions, and mechanisms. Bioresour. Technol. Rep. 2021, 15, 100728. [Google Scholar] [CrossRef]
- Cho, H.H.; Wepasnick, K.; Smith, B.A.; Bangash, F.K.; Fairbrother, D.H.; Ball, W.P. Sorption of aqueous Zn [II] and Cd [II] by multiwall carbon nanotubes: The relative roles of oxygen-containing functional groups and graphenic carbon. Langmuir 2010, 26, 967–981. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, B.; Shafi, M.; Ma, J.; Guo, J.; Wu, J.; Jin, H. Effects of biochar on growth, and heavy metals accumulation of moso bamboo (Phyllostachy pubescens), soil physical properties, and heavy metals solubility in soil. Chemosphere 2019, 219, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shao, J.; Wang, X.; Deng, Y.; Yang, H.; Chen, H. Characterization of modified biochars derived from bamboo pyrolysis and their utilization for target component (furfural) adsorption. Energy Fuels 2014, 28, 5119–5127. [Google Scholar] [CrossRef]
- Peng, P.; Lang, Y.H.; Wang, X.M. Adsorption behavior and mechanism of pentachlorophenol on reed biochars: pH effect, pyrolysis temperature, hydrochloric acid treatment and isotherms. Ecol. Eng. 2016, 90, 225–233. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, M.; Jin, Z.; Wang, G.; Li, R.; Zhang, X.; Wang, H. Characterization of biochars derived from various spent mushroom substrates and evaluation of their adsorption performance of Cu (II) ions from aqueous solution. Environ. Res. 2021, 196, 110323. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Li, B.; Huang, H.; Luo, L.; Zhang, J.; Yang, Y.; Zhou, Y. Biochar-based functional materials in the purification of agricultural wastewater: Fabrication, application and future research needs. Chemosphere 2018, 197, 165–180. [Google Scholar] [CrossRef]
- Li, B.; Yang, L.; Wang, C.Q.; Zhang, Q.P.; Liu, Q.C.; Li, Y.D.; Xiao, R. Adsorption of Cd (II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere 2017, 175, 332–340. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar modification to enhance sorption of inorganics from water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Cazetta, A.L.; Vargas, A.M.; Nogami, E.M.; Kunita, M.H.; Guilherme, M.R.; Martins, A.C.; Almeida, V.C. NaOH-activated carbon of high surface area produced from coconut shell: Kinetics and equilibrium studies from the methylene blue adsorption. Chem. Eng. J. 2011, 174, 117–125. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, Y.; Fu, Y.; Zhang, N. Activated carbons synthesized from unaltered and pelletized biomass wastes for bio-tar adsorption in different phases. Renew. Energy 2020, 146, 1700–1709. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Li, D.; Liu, C.; Lu, Y.; Lin, X.; Zheng, Z. Insight into the KOH/KMnO4 activation mechanism of oxygen-enriched hierarchical porous biochar derived from biomass waste by in-situ pyrolysis for methylene blue enhanced adsorption. J. Anal. Appl. Pyrolysis 2021, 158, 105269. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, C.; Deng, J.; Jiang, Z.; Zhang, L.; Cheng, Y.; Zeng, G. Factors influencing degradation of trichloroethylene by sulfide-modified nanoscale zero-valent iron in aqueous solution. Water Res. 2018, 135, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, G.; Yu, H.; Zhang, Z. Preparation of ferric-activated sludge-based adsorbent from biological sludge for tetracycline removal. Bioresour. Technol. 2016, 211, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Navarathna, C.M.; Bombuwala Dewage, N.; Keeton, C.; Pennisson, J.; Henderson, R.; Lashley, B.; Mlsna, T. Biochar adsorbents with enhanced hydrophobicity for oil spill removal. ACS Appl. Mater. Interfaces 2020, 12, 9248–9260. [Google Scholar] [CrossRef] [PubMed]
- Zulbadli, N.; Zaini, N.; Soon, L.Y.; Kang, T.Y.; Al-Harf, A.A.A.; Nasri, N.S.; Kamarudin, K.S.N. Acid-modified adsorbents from sustainable green-based materials for crude oil removal. J. Adv. Res. Fluid Mech. Therm. Sci. 2018, 46, 11–20. [Google Scholar]
- Chen, L.; Jiang, X.; Xie, R.; Zhang, Y.; Jin, Y.; Jiang, W. A novel porous biochar-supported Fe-Mn composite as a persulfate activator for the removal of acid red 88. Sep. Purif. Technol. 2020, 250, 117232. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, B.; Theng, B.K.; Wu, P.; Liu, F.; Wang, S.; Zhang, X. Formation and mechanisms of nano-metal oxide-biochar composites for pollutants removal: A review. Sci. Total Environ. 2021, 767, 145305. [Google Scholar] [CrossRef]
- Liu, J.; Huang, S.; Wang, T.; Mei, M.; Chen, S.; Li, J. Peroxydisulfate activation by digestate-derived biochar for azo dye degradation: Mechanism and performance. Sep. Purif. Technol. 2021, 279, 119687. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, C.; Zhao, X.; Bu, X.; Liao, X.; Fan, H.; Huang, Z. Malachite green degradation by persulfate activation with CuFe2O4@ biochar composite: Efficiency, stability and mechanism. J. Environ. Chem. Eng. 2021, 9, 105800. [Google Scholar] [CrossRef]
- Liu, H.; Hu, M.; Zhang, H.; Wei, J. Biosynthesis of Stalk Biochar-nZVI and its Catalytic Reactivity in Degradation of Dyes by Persulfate. Surf. Interfaces 2022, 31, 102098. [Google Scholar] [CrossRef]
- Zhao, Y.; Dai, H.; Ji, J.; Yuan, X.; Li, X.; Jiang, L.; Wang, H. Resource utilization of luffa sponge to produce biochar for effective degradation of organic contaminants through persulfate activation. Sep. Purif. Technol. 2022, 288, 120650. [Google Scholar] [CrossRef]
- Gaspard, S.; Ncibi, M.C. (Eds.) Activated Carbon from Biomass for Water Treatment. Biomass for Sustainable Applications: Pollution Remediation and Energy; Royal Society of Chemistry: London, UK, 2013. [Google Scholar]
- Kim, J.R.; Kan, E. Heterogeneous photocatalytic degradation of sulfamethoxazole in water using a biochar-supported TiO2 photocatalyst. J. Environ. Manag. 2016, 180, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: A review. Biomass Convers. Biorefin. 2020, 12, 925–947. [Google Scholar] [CrossRef]
- Chia, C.H.; Downie, A.; Munroe, P. Characteristics of biochar: Physical and structural properties. In Biochar for Environmental Management; Routledge: London, UK, 2015; pp. 89–109. [Google Scholar]
- Rajapaksha, A.U.; Vithanage, M.; Ahmad, M.; Seo, D.C.; Cho, J.S.; Lee, S.E.; Ok, Y.S. Enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar. J. Hazard. Mater. 2015, 290, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.M.; Santos, A.O.; Sussuchi, E.M.; Nascimento, J.S.; Lima, Á.S.; Freitas, L.S. Pyrolysis of mangaba seed: Production and characterization of bio-oil. Bioresour. Technol. 2015, 196, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Uchimiya, M.; Lima, I.M.; Klasson, K.T.; Wartelle, L.H. Contaminant immobilization and nutrient release by biochar soil amendment: Roles of natural organic matter. Chemosphere 2010, 80, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, N.; Spokas, K.; Schmidt, H.P.; Kägi, R.; Böhler, M.; Bucheli, T. Activated Carbon, Biochar and Charcoal: Linkages and Synergies across Pyrogenic Carbon’s ABCs. Water 2018, 10, 182. [Google Scholar] [CrossRef] [Green Version]
- Jellali, S.; Khiari, B.; Usman, M.; Hamdi, H.; Charabi, Y.; Jeguirim, M. Sludge-derived biochars: A review on the influence of synthesis conditions on pollutants removal efficiency from wastewaters. Renew. Sustain. Energy Rev. 2021, 144, 111068. [Google Scholar] [CrossRef]
- Méndez, A.; Gascó, G.; Freitas, M.M.A.; Siebielec, G.; Stuczynski, T.; Figueiredo, J.L. Preparation of carbon-based adsorbents from pyrolysis and air activation of sewage sludges. Chem. Eng. J. 2005, 108, 169–177. [Google Scholar] [CrossRef]
- Sewu, D.D.; Jung, H.; Kim, S.S.; Lee, D.S.; Woo, S.H. Decolorization of cationic and anionic dye-laden wastewater by steam-activated biochar produced at an industrial-scale from spent mushroom substrate. Bioresour. Technol. 2019, 277, 77–86. [Google Scholar] [CrossRef]
- Jin, H.; Capareda, S.; Chang, Z.; Gao, J.; Xu, Y.; Zhang, J. Biochar pyrolytically produced from municipal solid wastes for aqueous As (V) removal: Adsorption property and its improvement with KOH activation. Bioresour. Technol. 2014, 169, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; Lyu, H.; Zhao, Q.; Jiang, L.; Liu, L. Ball-milled biochar for galaxolide removal: Sorption performance and governing mechanisms. Sci. Total Environ. 2019, 659, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Liu, G.; Yousaf, B.; Ahmed, R.; Irshad, S.; Ashraf, A.; Rashid, M.S. Synthesis, characteristics and mechanistic insight into the clays and clay minerals-biochar surface interactions for contaminants removal—A review. J. Clean. Prod. 2021, 310, 127548. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Li, X.; Jiang, L.; Wang, H. Burgeoning prospects of biochar and its composite in persulfate-advanced oxidation process. J. Hazard. Mater. 2021, 409, 124893. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.C.; Jackson, M.A.; Kim, S.; Palmquist, D.E. Increasing biochar surface area: Optimization of ball milling parameters. Powder Technol. 2012, 228, 115–120. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Huang, H.; Tang, J. Effects of ball milling on the physicochemical and sorptive properties of biochar: Experimental observations and governing mechanisms. Environ. Pollut. 2018, 233, 54–63. [Google Scholar] [CrossRef]
- Zheng, Y.; Wan, Y.; Chen, J.; Chen, H.; Gao, B. MgO modified biochar produced through ball milling: A dual-functional adsorbent for removal of different contaminants. Chemosphere 2020, 243, 125344. [Google Scholar] [CrossRef]
- Sewu, D.D.; Tran, H.N.; Ohemeng-Boahen, G.; Woo, S.H. Facile magnetic biochar production route with new goethite nanoparticle precursor. Sci. Total Environ. 2020, 717, 137091. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, Q.; Wang, Z.; Gao, B.; Fan, Z.; Li, M.; Zhong, M. Degradation of anthraquinone dye reactive blue 19 using persulfate activated with Fe/Mn modified biochar: Radical/non-radical mechanisms and fixed-bed reactor study. Sci. Total Environ. 2021, 758, 143584. [Google Scholar] [CrossRef]
- Zhao, F.; Shan, R.; Li, W.; Zhang, Y.; Yuan, H.; Chen, Y. Synthesis, characterization, and dye removal of ZnCl2-modified biochar derived from pulp and paper sludge. ACS Omega 2021, 6, 34712–34723. [Google Scholar] [CrossRef]
- Feng, Z.; Yuan, R.; Wang, F.; Chen, Z.; Zhou, B.; Chen, H. Preparation of magnetic biochar and its application in catalytic degradation of organic pollutants: A review. Sci. Total Environ. 2021, 765, 142673. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Lee, S.M. Magnetic biochar composite: Facile synthesis, characterization, and application for heavy metal removal. Colloids Surf. A Physicochem. Eng. Asp. 2014, 454, 96–103. [Google Scholar] [CrossRef]
- Qu, S.; Huang, F.; Yu, S.; Chen, G.; Kong, J. Magnetic removal of dyes from aqueous solution using multi-walled carbon nanotubes filled with Fe2O3 particles. J. Hazard. Mater. 2008, 160, 643–647. [Google Scholar] [CrossRef]
- Thines, K.R.; Abdullah, E.C.; Mubarak, N.M.; Ruthiraan, M. Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: A review. Renew. Sustain. Energy Rev. 2017, 67, 257–276. [Google Scholar] [CrossRef]
- Theydan, S.K.; Ahmed, M.J. Adsorption of methylene blue onto biomass-based activated carbon by FeCl3 activation: Equilibrium, kinetics, and thermodynamic studies. J. Anal. Appl. Pyrolysis 2012, 97, 116–122. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef]
- Baig, S.A.; Zhu, J.; Muhammad, N.; Sheng, T.; Xu, X. Effect of synthesis methods on magnetic Kans grass biochar for enhanced As (III, V) adsorption from aqueous solutions. Biomass Bioenergy 2014, 71, 299–310. [Google Scholar] [CrossRef]
- Han, Y.; Cao, X.; Ouyang, X.; Sohi, S.P.; Chen, J. Adsorption kinetics of magnetic biochar derived from peanut hull on removal of Cr (VI) from aqueous solution: Effects of production conditions and particle size. Chemosphere 2016, 145, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhao, L.; Tang, Y.; Zhou, J.; Shi, B. An iron–based biochar for persulfate activation with highly efficient and durable removal of refractory dyes. J. Environ. Chem. Eng. 2022, 10, 106979. [Google Scholar] [CrossRef]
- Abodif, A.M.; Meng, L.; Ma, S.; Ahmed, A.S.; Belvett, N.; Wei, Z.Z.; Ning, D. Mechanisms and models of adsorption: TiO2-supported biochar for removal of 3,4-dimethylaniline. ACS Omega 2020, 5, 13630–13640. [Google Scholar] [CrossRef]
- Deng, H.; Li, A.; Ye, C.; Sheng, L.; Li, Z.; Jiang, Y. Green Removal of Various Pollutants by Microsphere Adsorption: Material Characterization and Adsorption Behavior. Energy Fuels 2020, 34, 16330–16340. [Google Scholar] [CrossRef]
- Liu, S.; Shen, C.; Wang, Y.; Huang, Y.; Hu, X.; Li, B.; Zhang, H. Development of CO2/H2O activated biochar derived from pine pyrolysis: Application in methylene blue adsorption. J. Chem. Technol. Biotechnol. 2022, 97, 885–893. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Xu, S.; Wang, M.; Zhang, Y.; Xue, X. A modified method for enhancing adsorption capability of banana pseudostem biochar towards methylene blue at low temperature. Bioresour. Technol. 2019, 282, 48–55. [Google Scholar] [CrossRef]
- Hoslett, J.; Ghazal, H.; Mohamad, N.; Jouhara, H. Removal of methylene blue from aqueous solutions by biochar prepared from the pyrolysis of mixed municipal discarded material. Sci. Total Environ. 2020, 714, 136832. [Google Scholar] [CrossRef]
- Sahu, S.; Pahi, S.; Tripathy, S.; Singh, S.K.; Behera, A.; Sahu, U.K.; Patel, R.K. Adsorption of methylene blue on chemically modified lychee seed biochar: Dynamic, equilibrium, and thermodynamic study. J. Mol. Liq. 2020, 315, 113743. [Google Scholar] [CrossRef]
- Chen, X.L.; Li, F.; Chen, H.; Wang, H.; Li, G. Fe2O3/TiO2 functionalized biochar as a heterogeneous catalyst for dyes degradation in water under Fenton processes. J. Environ. Chem. Eng. 2020, 8, 103905. [Google Scholar] [CrossRef]
- Liu, X.J.; Li, M.F.; Singh, S.K. Manganese-modified lignin biochar as adsorbent for removal of methylene blue. J. Mater. Res. Technol. 2021, 12, 1434–1445. [Google Scholar] [CrossRef]
- Bal, D.; Özer, Ç.; İmamoğlu, M. Green and ecofriendly biochar preparation from pumpkin peel and its usage as an adsorbent for methylene blue removal from aqueous solutions. Water Air Soil Pollut. 2021, 232, 457. [Google Scholar] [CrossRef]
- Pandey, D.; Daverey, A.; Dutta, K.; Yata, V.K.; Arunachalam, K. Valorization of waste pine needle biomass into biosorbents for the removal of methylene blue dye from water: Kinetics, equilibrium and thermodynamics study. Environ. Technol. Innov. 2022, 25, 102200. [Google Scholar] [CrossRef]
- Pandey, D.; Daverey, A.; Dutta, K.; Arunachalam, K. Bioremoval of toxic malachite green from water through simultaneous decolorization and degradation using laccase immobilized biochar. Chemosphere 2022, 297, 134126. [Google Scholar] [CrossRef]
- Eldeeb, T.M.; Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; El-Nemr, M.A.; Hassaan, M.A.; El Nemr, A. Adsorption of methylene blue (MB) dye on ozone, purified and sonicated sawdust biochars. Biomass Convers. Biorefin. 2022, 1–23. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, W. Insight into interaction between biochar and soil minerals in changing biochar properties and adsorption capacities for sulfamethoxazole. Environ. Pollut. 2019, 245, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yang, Y.; Liu, P.; Li, Y.; Huang, F.; Zeng, L.; Hou, B. Iron-montmorillonite treated corn straw biochar: Interfacial chemical behavior and stability. Sci. Total Environ. 2020, 708, 134773. [Google Scholar] [CrossRef]
- Han, H.; Rafiq, M.K.; Zhou, T.; Xu, R.; Mašek, O.; Li, X. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 2019, 369, 780–796. [Google Scholar] [CrossRef]
- Khandelwal, N.; Tiwari, E.; Singh, N.; Darbha, G.K. Heterogeneously Porous Multiadsorbent Clay–Biochar Surface to Support Redox-Sensitive Nanoparticles: Applications of Novel Clay–Biochar–Nanoscale Zerovalent Iron Nanotrident (C-BC-nZVI) in Continuous Water Filtration. ACS EST Water 2021, 1, 641–652. [Google Scholar] [CrossRef]
- Lakshmi, D.; Akhil, D.; Kartik, A.; Gopinath, K.P.; Arun, J.; Bhatnagar, A.; Muthusamy, G. Artificial intelligence (AI) applications in adsorption of heavy metals using modified biochar. Sci. Total Environ. 2021, 801, 149623. [Google Scholar] [CrossRef]
- Mishra, P.; Gupta, C. Cookies in a Cross-site scripting: Type, Utilization, Detection, Protection and Remediation. In Proceedings of the 2020 8th International Conference on Reliability, Infocom Technologies and Optimization (Trends and Future Directions) (ICRITO), Noida, India, 4–5 June 2020; pp. 1056–1059. [Google Scholar] [CrossRef]
- Babaei, A.A.; Khataee, A.; Ahmadpour, E.; Sheydaei, M.; Kakavandi, B.; Alaee, Z. Optimization of cationic dye adsorption on activated spent tea: Equilibrium, kinetics, thermodynamic and artificial neural network modeling. Korean J. Chem. Eng. 2016, 33, 1352–1361. [Google Scholar] [CrossRef]
- Moosavi, S.; Manta, O.; El-Badry, Y.A.; Hussein, E.E.; El-Bahy, Z.M.; Mohd Fawzi, N.F.B.; Moosavi, S.M.H. A study on machine learning methods’ application for dye adsorption prediction onto agricultural waste activated carbon. Nanomaterials 2021, 11, 2734. [Google Scholar] [CrossRef]
- Alam, G.; Ihsanullah, I.; Naushad, M.; Sillanpää, M. Applications of artificial intelligence in water treatment for optimization and automation of adsorption processes: Recent advances and prospects. Chem. Eng. J. 2022, 427, 130011. [Google Scholar] [CrossRef]
- Taoufik, N.; Boumya, W.; Achak, M.; Chennouk, H.; Dewil, R.; Barka, N. The state of art on the prediction of efficiency and modeling of the processes of pollutants removal based on machine learning. Sci. Total Environ. 2022, 807, 150554. [Google Scholar] [CrossRef]
- Beck, D.A.; Carothers, J.M.; Subramanian, V.R.; Pfaendtner, J. Data science: Accelerating innovation and discovery in chemical engineering. AIChE J. 2016, 62, 1402–1416. [Google Scholar] [CrossRef]
- Ghaedi, A.M.; Vafaei, A. Applications of artificial neural networks for adsorption removal of dyes from aqueous solution: A review. Adv. Colloid Interface Sci. 2017, 245, 20–39. [Google Scholar] [CrossRef]
- Aber, S.; Daneshvar, N.; Soroureddin, S.M.; Chabok, A.; Asadpour-Zeynali, K. Study of acid orange 7 removal from aqueous solutions by powdered activated carbon and modeling of experimental results by artificial neural network. Desalination 2007, 211, 87–95. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goswami, L.; Kushwaha, A.; Kafle, S.R.; Kim, B.-S. Surface Modification of Biochar for Dye Removal from Wastewater. Catalysts 2022, 12, 817. https://doi.org/10.3390/catal12080817
Goswami L, Kushwaha A, Kafle SR, Kim B-S. Surface Modification of Biochar for Dye Removal from Wastewater. Catalysts. 2022; 12(8):817. https://doi.org/10.3390/catal12080817
Chicago/Turabian StyleGoswami, Lalit, Anamika Kushwaha, Saroj Raj Kafle, and Beom-Soo Kim. 2022. "Surface Modification of Biochar for Dye Removal from Wastewater" Catalysts 12, no. 8: 817. https://doi.org/10.3390/catal12080817
APA StyleGoswami, L., Kushwaha, A., Kafle, S. R., & Kim, B.-S. (2022). Surface Modification of Biochar for Dye Removal from Wastewater. Catalysts, 12(8), 817. https://doi.org/10.3390/catal12080817







