Homogeneous Group IVB Catalysts of New Generations for Synthesis of Ethylene-Propylene-Diene Rubbers: A Mini-Review
Abstract
:1. Introduction
2. Metallocene Catalysts
3. “Constrained” Geometry Complexes
4. Half-Sandwich Titanium Complexes
5. Post-MetalloceneChelate Catalysts
6. Conclusionsand Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Noordermeer, J.W. Ethylene-Propylene Elastomers. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons: New York, NY, USA, 2002; pp. 178–196. [Google Scholar]
- Ravishankar, P.S. Treatise on EPDM. Rubber Chem. Technol. 2012, 85, 327–349. [Google Scholar] [CrossRef]
- Van Duin, M.; van Doremaele, G.; van der Aar, N. Defining EPDM for the past and the next 50 years. KGK 2017, 11–12, 14–23. [Google Scholar]
- Bravaya, N.M.; Faingol’d, E.E.; Badamshina, E.R.; Sanginov, E.A. Advances in synthesis of ethylene–propylene–diene elastomers by ion-coordination polymerization on single-site catalytic systems of new generation. Polym. Sci. Ser. C 2020, 62, 1–16. [Google Scholar] [CrossRef]
- Quirk, R.P.; Hoff, R.E. (Eds.) Transition Metal Catalyzed Polymerizations: Ziegler-Natta and Metathesis Polymerizations; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Karpeles, R.; Grossi, A.V. EPDM rubber technology. In Handbook of Elastomers, 2nd ed.; Bhowmick, A.K., Stephens, H.L., Eds.; Marcel Dekker: New York, NY, USA, 2001; pp. 845–876. [Google Scholar]
- Ma, Y.; Reardon, D.; Gambarotta, S.; Yap, G.; Zahalka, H.; Lemay, C. Vanadium-catalyzed ethylene-propylene copolymerization: the question of the metal oxidation state in Ziegler-Natta polymerization promoted by (β-diketonate)3V. Organometallics 1999, 18, 2773–2781. [Google Scholar] [CrossRef]
- ARLANXEOWebsite/Find Products/Ethylene Propylene Diene Rubber. Available online: https://www.arlanxeo.com/en/products/finder?q=%3aname-asc%3abrandCategory%3akeltan (accessed on 21 June 2022).
- NORDEL™ EPDM Product Selection Guide—265-11001-01-nordel-epdm-product-selection-guide.pdf. Available online: https://www.dow.com/content/dam/dcc/documents/en-us/catalog-selguide/265/265-11001-01-nordel-epdm-product-selection-guide.pdf (accessed on 21 June 2022).
- ExxonMobil/Home/Resources/Technical Data Sheets/EPDM Rubber. Available online: https://www.exxonmobilchemical.com/en/resources/product-data-sheets/epdm-rubber (accessed on 21 June 2022).
- MITSUI EPT™/EPDM Rubber. Available online: https://us.mitsuichemicals.com/service/product/mitsui-ept.htm (accessed on 21 June 2022).
- KUMHO POLYCHEM Website/EPDM/Overview. Available online: https://www.polychem.co.kr/eng/product/epdm?seq=1 (accessed on 21 June 2022).
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in propene polymerization with metallocene catalysts. Chem. Rev. 2000, 100, 1253–1346. [Google Scholar] [CrossRef] [PubMed]
- Altaf, A.A.; Badshah, A.; Khan, N.; Marwat, S.; Ali, S. Zirconium complexes in homogeneous ethylene polymerization. J. Coord. Chem. 2011, 64, 1815–1836. [Google Scholar] [CrossRef]
- Gillis, D.J.; Karpeles, R. Process for Producing Polyolefin Elastomer Employing a Metallocene Catalyst. Patent US 6225426 B1, 1 May 2001. [Google Scholar]
- Endo, K.; Hiwara, M.; Matsuura, S.; Mizobuchi, Y.; Yamamura, Y.; Noguchi, Y.; Ishii, Y.; Sakai, T.; Shishido, K.; Ichino, K.; et al. Ethylene Alpha-Olefin Non-Conjugated Polyene Copolymer, Use Thereof, and Manufacturing Method Thereof. Patent US 2016/0347894 A1, 1 December 2016. [Google Scholar]
- Ichino, K.; Kikuchi, Y.; Tohi, Y.; Matsugi, T.; Yanagimoto, Y.; Arino, M.; Shishido, K.; Hosoya, M. Ethylene/α-Olefin/Non-Conjugated Polyene Copolymer, and Production Process and Use Thereof. Patent US 10435494 B2, 8 November 2018. [Google Scholar]
- Ichino, K.; Yamaguchi, T.; Aita, Y.; Noguchi, Y. Thermoplastic Elastomer Composition, Use Thereof, Method for Producing Same, Ethylene/α-Olefin/Unconjugated Polyene Copolymer and Use Thereof. Patent US 2018/0072877 A1, 15 March 2018. [Google Scholar]
- Ichino, K.; Kikuchi, Y.; Shishido, K.; Tanaka, J.; Arino, M. Ethylene/Alpha-Olefin/Non-Conjugated Polyene Copolymer, Method for Producing the Same, and Use Thereof. Patent US 2021/0009730 A1, 14 January 2021. [Google Scholar]
- Hong, Y.J.; Shin, E.H.; Jung, S.W.; Woo, H.Y.; Lee, R.H.; Min, J.K.; Chae, B.H. Process for Ethylene/Propylene/Dieneterpolymer Using New Transition Metal Compound. Patent KR 101792956 B1, 11 March 2017. [Google Scholar]
- Chen, E.Y.-X.; Marks, T.J. Cocatalysts for metal-catalyzed olefin polymerization: Activators, activation processes, and structure-activity relationships. Chem. Rev. 2000, 100, 1391–1434. [Google Scholar] [CrossRef]
- Zijlstra, H.S.; Harder, S. Methylalumoxane—History, production, properties, and applications. Eur. J. Inorg. Chem. 2015, 2015, 19–43. [Google Scholar] [CrossRef]
- Bochmann, M. The chemistry of catalyst activation: The case of group 4 polymerization catalysts. Organometallics 2010, 29, 4711–4740. [Google Scholar] [CrossRef]
- Ali, A.; Tufail, M.K.; Jamil, M.I.; Yaseen, W.; Iqbal, N.; Hussain, M.; Ali, A.; Aziz, T.; Fan, Z.; Guo, L. Comparative analysis of ethylene/diene copolymerization and ethylene/propylene/diene terpolymerization using ansa-zirconocene catalyst with alkylaluminum/borate activator: The effect of conjugated and nonconjugated dienes on catalytic behavior and polymer microstructure. Molecules 2021, 26, 2037. [Google Scholar]
- Ali, A.; Akram, M.A.; Guo, Y.; Wu, H.-L.; Liu, W.; Khan, A.; Liu, H.; Fu, Z.; Fan, Z. Ethylene–propylene copolymerization and their terpolymerization with dienes using ansa-zirconocene catalysts activated by borate/alkylaluminum. J. Macromol. Sci. Part A 2020, 57, 156–164. [Google Scholar] [CrossRef]
- Ali, A.; Nadeem, M.; Lu, J.; Moradian, J.M.; Rasheed, T.; Aziz, T.; Maouche, C.; Guo, Y.; Awais, M.; Zhiqiang, F.; et al. Rapid kinetic evaluation of homogeneous single-site metallocene catalysts and cyclic diene: How do the catalytic activity, molecular weight, and diene incorporation rate of olefins affect each other? RSC Adv. 2021, 11, 31817–31826. [Google Scholar] [CrossRef]
- Bravaya, N.M.; Panin, A.N. Method of Producing Copolymers of Olefin Monomers with Cyclic or Linear Dienes. Patent RU 2477289 C1, 10 March 2013. [Google Scholar]
- Bravaya, N.M.; Panin, A.N.; Faingol’d, E.E.; Saratovskikh, S.L.; Babkina, O.N.; Zharkov, I.V.; Perepelitsina, E.O. Isobutylalumoxanes as high-performance activators of rac-Et(2-MeInd)2ZrMe2 in copolymerization of ethylene with propylene and ternary copolymerization of ethylene, propylene, and 5-ethylidene-2-norbornene. Polym. Bull. 2016, 73, 473–491. [Google Scholar] [CrossRef]
- Faingol’d, E.E.; Bravaya, N.M.; Panin, A.N.; Saratovskikh, S.L.; Babkina, O.N. Use of Aryl Oxides of Isobutyl Aluminium as Activators Dialkylmetallocene Catalysts for Homopolymerisation Ethylene, Propylene, Copolymerisation of Ethylene with Propylene and Triple Copolymerisation of Ethylene, Propylene and Diene, Homogeneous Metallocene Catalyst Systems for Synthesis of Homo-and Copolymers of Olefins and Dienes, Method of Producing Homo- and Copolymers of Olefins and Dienes, Method for Stabilisation of Homo- and Copolymers of Olefins and Dienes. Patent RU 2588496 C2, 27 June 2016. [Google Scholar]
- Faingol’d, E.E.; Bravaya, N.M.; Panin, A.N.; Babkina, O.N.; Saratovskikh, S.L.; Privalov, V.I. Isobutylaluminum aryloxides as metallocene activators in homo- and copolymerization of olefins. J. Appl. Polym. Sci. 2016, 133, 43276. [Google Scholar] [CrossRef]
- Faingol’d, E.E.; Zharkov, I.V.; Bravaya, N.M.; Panin, A.N.; Saratovskikh, S.L.; Babkina, O.N.; Shilov, G.V. Sterically crowded dimericdiisobutylaluminumaryloxides: Synthesis, characteristics, and application as activators in homo- and copolymerization of olefins. J. Organomet. Chem. 2018, 871, 86–95. [Google Scholar] [CrossRef]
- Faingol’d, E.E.; Saratovskikh, S.L.; Panin, A.N.; Babkina, O.N.; Zharkov, I.V.; Garifullin, N.O.; Shilov, G.V.; Bravaya, N.M. Ethylene/propylene and ethylene/propylene/5-ethylidene-2-norbornene copolymerizations on metallocene/(2,6-tBu2PhO-)AliBu2 catalyst systems. Polymer 2021, 220, 123559. [Google Scholar] [CrossRef]
- Baier, M.C.; Zuideveld, M.A.; Mecking, S. Post-metallocenes in the industrial production of polyolefins. Angew. Chem. Int. Edit. 2014, 53, 9722–9744. [Google Scholar] [CrossRef]
- Braunschweig, H.; Breitling, F.M. Constrained geometry complexes—synthesis and applications. Coord. Chem. Rev. 2006, 250, 2691–2720. [Google Scholar] [CrossRef]
- Klosin, J.; Fontaine, P.P.; Figueroa, R. Development of group IV molecular catalysts for high temperature ethylene-α-olefin copolymerization reactions. Account Chem. Res. 2015, 48, 2004–2016. [Google Scholar] [CrossRef]
- Canich, J.A.M. Olefin Polymerization Catalysts. Patent EP 0420436 A1, 3 April 1991. [Google Scholar]
- Thakur, V.; Shan, C.L.P.; Li, G.; Han, T.; Doelder, J.D. Sponge EPDM by design. Plast. Rubber Compos. 2019, 48, 32–41. [Google Scholar] [CrossRef]
- Stevens, J.C.; Timmers, F.J.; Wilson, D.R.; Schmidt, G.F.; Nickias, P.N.; Rosen, R.K.; Knight, G.W.; Lai, S.-Y. Constrained Geometry Addition Polymerization Catalysts, Processes for Their Preparation, Precursors Therefor, Methods of Use, and Novel Polymers Formed Therewith. Patent EP 0416815 A2, 13 March 1991. [Google Scholar]
- Katayama, H.; Nabika, M.; Imai, A.; Miyashita, A.; Watanabe, T.; Johohji, H.; Oda, Y.; Hanaoka, H. Transition Metal Complex, Process for Producing the Same, Olefin Polymerization Catalyst Containing the Transition Metal Complex and Process for Producing Olefin Polymers. Patent US 6329478 B1, 11 December 2001. [Google Scholar]
- Kim, S.-B.; Goo, H.-R.; Hong, Y.-J.; Lee, M.-H.; Do, Y.-G.; Park, M.-H.; Park, J.-H. Dinuclear Metallocene Catalysts and Process for the Manufacture of EP and EPDM Polymers Using Them. Patent KR 101216691 B1, 28 December 2012. [Google Scholar]
- Kim, H.-J.; Kim, H.-S.; Kim, S.K.; Yoon, S.; Koo, H.R.; Son, A. Catalyst Composition for Preparing Elastic Copolymer, and Method for Preparing Elastic Copolymer, Which Comprises Ethylene and Alpha-Olefin or Ethylene, Alpha-Olefin and Unconjugated Diene, by Using Same. Patent US 11220566 B2, 11 January 2022. [Google Scholar]
- Park, S.H.; Yoon, S.C.; Kim, S.K.; Ko, J.S.; Park, S.E.; Choi, S.Y. Elastic Diene Terpolymer and Preparation Method Thereof. Patent US 9428600 B2, 30 June 2016. [Google Scholar]
- Kim, S.K.; Park, S.H.; Yoon, S.C.; Ko, J.S.; Choi, S.Y. Elastic Diene Terpolymer and Preparation Method Thereof. Patent US 9493593 B2, 15 November 2016. [Google Scholar]
- Yoon, S.-C.; Park, S.-H.; Ko, J.-S.; Choi, S.-Y. Elastic Terpolymer Including Diene Group and Preparation Method Thereof. Patent US 9410008 B2, 9 August 2016. [Google Scholar]
- Park, S.H.; Yoon, S.C.; Kim, S.K.; Ko, J.S.; Park, S.E.; Choi, S.Y. Elastic Diene Terpolymer and Preparation Method Thereof. Patent US 9650460 B2, 16 May 2017. [Google Scholar]
- Wang, Q.; Brown, S.J. Process to Prepare Ethylene Propylene Elastomer. Patent EP 1162214 A1, 12 December 2001. [Google Scholar]
- Windmuller, P.J.H.; van Doremaele, G.H.J. Process for the Production of a Polymer Comprising Monomeric Units of Ethylene, an α-Olefin and a Vinyl Norbornene. Patent WO 2005/005496 A2, 20 January 2005. [Google Scholar]
- Ijpeij, E.G.; Arts, H.J.; van Doremaele, G.H.J.; Beijer, F.H.; van Der Burgt, F.; Zuideveld, M.A. Process for the Preparation of a Polyolefin. Patent WO 2005/014601 A2, 17 February 2005. [Google Scholar]
- Windmuller, P.; Doremaele, G. Process for the Production of a Polymer Comprising Monomeric Units of Ethylene, an α-Oplefin and a Vinyl Norbornene. Patent US 2006/0205900 A1, 14 September 2006. [Google Scholar]
- Windmuller, P.; Doremaele, G. Process for the Production of a Polymer Comprising Monomeric Units of Ethylene, an α-Oplefin and a Vinyl Norbornene. Patent US 7829645 B2, 9 November 2010. [Google Scholar]
- Van Doremaele, G.; van Duin, M.; Valla, M.; Berthoud, A. On the development of titanium κ1-amidinate complexes, commercialized as Keltan ACE™ technology, enabling the production of an unprecedented large variety of EPDM polymer structures. J. Polym. Sci. Pol. Chem. 2017, 55, 2877–2891. [Google Scholar] [CrossRef] [Green Version]
- Van Doremaele, G.H.J.; Zuideveld, M.A.; Leblanc, A.; Norambuena, V.F.Q. Catalyst Component for the Polymerization of Olefins Having a Guanidinate Ligand. Patent US 8987393 B2, 24 March 2015. [Google Scholar]
- Ijpeij, E.; Arts, H.; van Doremaele, G.; Windmuller, P.; van der Burgt, F.; Zuideveld, M.A. Polymerization Catalyst Comprising Amidine Ligand. Patent US 7956140 B2, 7 June 2011. [Google Scholar]
- Van Doremaele, G.H.J.; Berthoud, A.; Norambuena, V.Q.; Rupnicki, L.; Karbaum, P. Metal Complex with a Cyclic Amidine Ligand. Patent WO 2015113957 A1, 6 August 2015. [Google Scholar]
- Collins, R.A.; Russell, A.F.; Scott, R.T.W.; Bernardo, R.; van Doremaele, G.H.J.; Berthoud, A.; Mountford, P. Monometallic and bimetallic titanium κ1-amidinate complexes as olefin polymerization catalysts. Organometallics 2017, 36, 2167–2181. [Google Scholar] [CrossRef]
- Berthoud, A.; van Doremaele, G.; Norambuena, V.Q.; Scott, R.; Zuideveld, M.A.; Arts, H.J. Bimetallic Complex Comprising Cyclopentadienyl and Amidine Ligands. Patent US 9862736 B2, 9 January 2018. [Google Scholar]
- Van Doremaele, G.H.J.; Zuideveld, M.A.; Mountford, P.; Heath, A.; Scott, R.T.W. Ti Catalyst System Comprising Substituted Cyclopentadienyl, Amidine and Diene Ligand. Patent WO 2011076775 A1, 30 June 2011. [Google Scholar]
- Van Doremaele, G.H.J.; Zuideveld, M.A. Borane Activated ti Catalyst System Comprising Amidine and Diene Ligand. Patent WO 2011076772 A1, 30 June 2011. [Google Scholar]
- Karbaum, P.; Scott, R.T.W.; van De Moosdijk, J. Metal Complex Comprising Amidine and Substituted Cyclopentadienyl Ligands. Patent US 10472431 B2, 12 November 2019. [Google Scholar]
- Berthoud, A.; Van Doremaele, G.H.J.; Scott, R.T.W.; Perez, F.; Bernardo, R. Catalyst System. Patent WO 2017/029141 A1, 23 February 2017. [Google Scholar]
- Scott, R.T.W.; Zuideveld, M.A.; Mountford, P. Metal Complex with a Lewis Base Ligand. Patent WO 2014180913 A1, 13 November 2014. [Google Scholar]
- Scott, R.T.W.; Bernardo, R.; van de Moosdijk, J.; Berthoud, A. Metall Complex Comprising Amidine and Substituted Cyclopentadienyl Ligands. Patent EP 3272761 A1, 24 January 2018. [Google Scholar]
- Goryunov, G.P.; Samsonov, O.V.; Uborsky, D.V.; Voskoboynikov, A.Z.; Berthoud, A.; Valla, M. Metal Complex Comprising Amidine and Indole Fused Cyclopentadienyl Ligands. Patent US 20210269465 A1, 2 September 2021. [Google Scholar]
- Bernardo, R.; van Doremaele, G.; van Meerendonk, W.; Windmuller, P. Catalyst Mixture. Patent US 20220056162 A1, 24 February 2022. [Google Scholar]
- DSM Continues to Invest in Innovative Growth in Performance Materials Cluster. Available online: https://www.pressreleasefinder.com/pr/DSMELPR005/en/ (accessed on 19 May 2022).
- History | ARLANXEO Website/Customer Portal. Available online: https://www.arlanxeo.com/en/company/history (accessed on 19 May 2022).
- Arlanxeoto Close Texas Keltan EPDM Plant | Rubber News. Available online: https://www.rubbernews.com/suppliers/arlanxeo-close-texas-keltan-epdm-plant (accessed on 19 May 2022).
- Boussie, T.R.; Bruemmer, O.; Diamond, G.; Goh, C.; Lapointe, A.M.; Leclerc, M.K.; Shoemaker, J.A. Bridged bi-Aromatic Ligands, Complexes, Catalysts and Processes for Polymerizing and Polymers Therefrom. Patent WO 03/091262A1, 6 November 2003. [Google Scholar]
- Boussie, T.; Bruemmer, O.; Diamond, G.; LaPointe, A.; Leclerc, M.; Micklatcher, C.; Sun, P.; Bei, X. Bridged bi-Aromatic Catalysts, Complexes, and Methods of Using the Same. Patent US 20060025548 A1, 2 February 2006. [Google Scholar]
- Boussie, T.; Diamond, G.; LaPointe, A.; Leclerc, M.; Micklatcher, C.; Sun, P.; Bei, X. Bridged bi-Aromatic Catalysts, Complexes, and Methods of Using the Same. Patent US 20060052554 A1, 9 March 2006. [Google Scholar]
- Boussie, T.R.; Diamond, G.M.; Goh, C.; Hall, K.A.; LaPointe, A.M.; Leclerc, M.; Lund, C.; Murphy, V.; Shoemaker, J.A.W.; Tracht, U.; et al. A fully integrated high-throughput screening methodology for the discovery of new polyolefin catalysts: discovery of a new class of high temperature single-site group (IV) copolymerization catalysts. J. Am. Chem. Soc. 2003, 125, 4306–4317. [Google Scholar] [CrossRef] [PubMed]
- Boussie, T.R.; Diamond, G.M.; Goh, C.; Hall, K.A.; LaPointe, A.M.; Leclerc, M.; Murphy, V.; Shoemaker, J.A.W.; Turner, H.; Rosen, R.K.; et al. Nonconventional catalysts for isotactic propene polymerization in solution developed by using high-throughput-screening technologies. Angew. Chem. Int. Ed. 2006, 45, 3278–3283. [Google Scholar] [CrossRef]
- Konze, W.V.; Vanderlende, D.D. Polyolefin Solution Polymerization Process and Polymer. Patent US 8349984 B2, 8 January 2013. [Google Scholar]
- Boone, H.W.; Iverson, C.N.; Konze, W.V.; Vanderlende, D.D. Ethylene/Alpha-Olefin/Diene Solution Polymerization Process and Polymer. Patent US 8299189 B2, 30 October 2012. [Google Scholar]
- Konze, W.V.; Stevens, J.C.; Vanderlende, D.D. High Efficiency Solution Polymerization Process. Patent WO 2007/136495 A3, 17 January 2008. [Google Scholar]
- Klosin, J.; Thomas, P.J.; Iverson, C.N.; Aboelella, N.W.; Frazier, K.A. Process for Polymerizing a Polymerizable Olefin and Catalyst Therefor. Patent US 9029487 B2, 12 May 2015. [Google Scholar]
- LiPiShan, C.; Karjala, T.W.; Smith, M.L.; Spencer, L.P.; Klosin, J. Ethylene/Alpha-Olefin/Nonconjugatedpolyeneinterpolymers and Processes to Form the Same. Patent US 9422383 B2, 23 August 2016. [Google Scholar]
- Spencer, L.P.; Kirschner, J.M. Polymerization Processes for High Molecular Weight Polymers. Patent US 9534070 B2, 3 January 2017. [Google Scholar]
- Fontaine, P.P.; Sun, L.; Shan, C.L.P.; Tuberquia, J.C.; Brown, S.G.; Madenjian, E.O.; Brennan, G.J. Processes for the Production of High Molecular Weight Ethylene/Alpha-Olefin/Non-Conjugated Interpolymers with Low Levels of Long Chain Branching. Patent US 10450394 B2, 22 October 2019. [Google Scholar]
- Wu, X.; Han, T.; Lipishan, C. Ethylene/Propylene/Nonconjugated Diene Interpolymer Composition. Patent WO 2020220244 A1, 5 November 2020. [Google Scholar]
- US DowDuPont to Start Up LDPE, EPDM Plants in Q1|ICIS. Available online: https://www.icis.com/resources/news/2018/02/01/10189241/us-dowdupont-to-start-up-ldpe-epdm-plants-in-q1/ (accessed on 19 May 2022).
- Atienza, C.C.H.; Cano, D.A.; Hagadorn, J.R.; Shah, R.K. Bisphenolate Transition Metal Complexes, Production and Use Thereof. Patent WO 2016153682 A1, 29 September 2016. [Google Scholar]
- Atienza, C.C.H.; Cano, D.A.; Hagadorn, J.R.; Shah, R.K. Bisphenolate Transition Metal Complexes, Production and Use Thereof. Patent US 9745327 B2, 29 August 2017. [Google Scholar]
- Goryunov, G.P.; Sharikov, M.I.; Popov, V.A.; Uborsky, D.V.; Voskoboynikov, A.Z.; Hagadorn, J.R.; Titone, M.E.; Carpenter, A.E.; Faler, C.A.; Canich, J.A.M. Transition Metal Bis(phenolate) Complexes and Their Use as Catalysts for Olefin Polymerization. Patent WO 2020167821 A1, 20 August 2020. [Google Scholar]
- Jiang, P.; Canich, J.A.M.; Hagadorn, J.R.; Xie, R.; Shi, J. Ethylene-Alpha-olefin-diene Monomer Copolymers Obtained Using Transition Metal bis(phenolate) Catalyst Complexes and Homogeneous Process for Production Thereof. Patent WO 2021162747 A1, 19 August 2021. [Google Scholar]
- Atienza, C.C.H.; Shah, R.K.; Walker, R.; Hagadorn, J.R.; Datta, S. Ethylene-α-Olefin-Diene Elastomers and Methods of Making them. Patent US 20210269567 A1, 2 September 2021. [Google Scholar]
- Hagadorn, J.R.; Canich, J.A.M.; Tsou, A.H.; Dharmarajan, N.; Jiang, P.; Shah, R.K. Branched EPDM Polymers Produced via Use of Vinyl Transfer Agents and Processes for Production Thereof. Patent WO 2018160575 A1, 7 September 2018. [Google Scholar]
- Dharmarajan, N.; Bai, Z.; Tsou, A.H.; Shah, R.K.; Hagadorn, J.R. Elastomeric Formulations Comprising Branched EPDM Polymers. Patent US 20200172717 A1, 4 June 2020. [Google Scholar]
- Tsou, A.H.; Hagadorn, J.R. Bimodal Ethylene, Alpha-Olefin, and Diene Polymers Using Dual Organometallic Catalysts. Patent WO 2019099116 A1, 23 May 2018. [Google Scholar]
- Tsou, A.H.; Hagadorn, J.R. Bimodal Ethylene, Alpha-Olefin, and Diene Polymers Using Dual Organometallic Catalysts. Patent US 11008449, 18 May 2021. [Google Scholar]
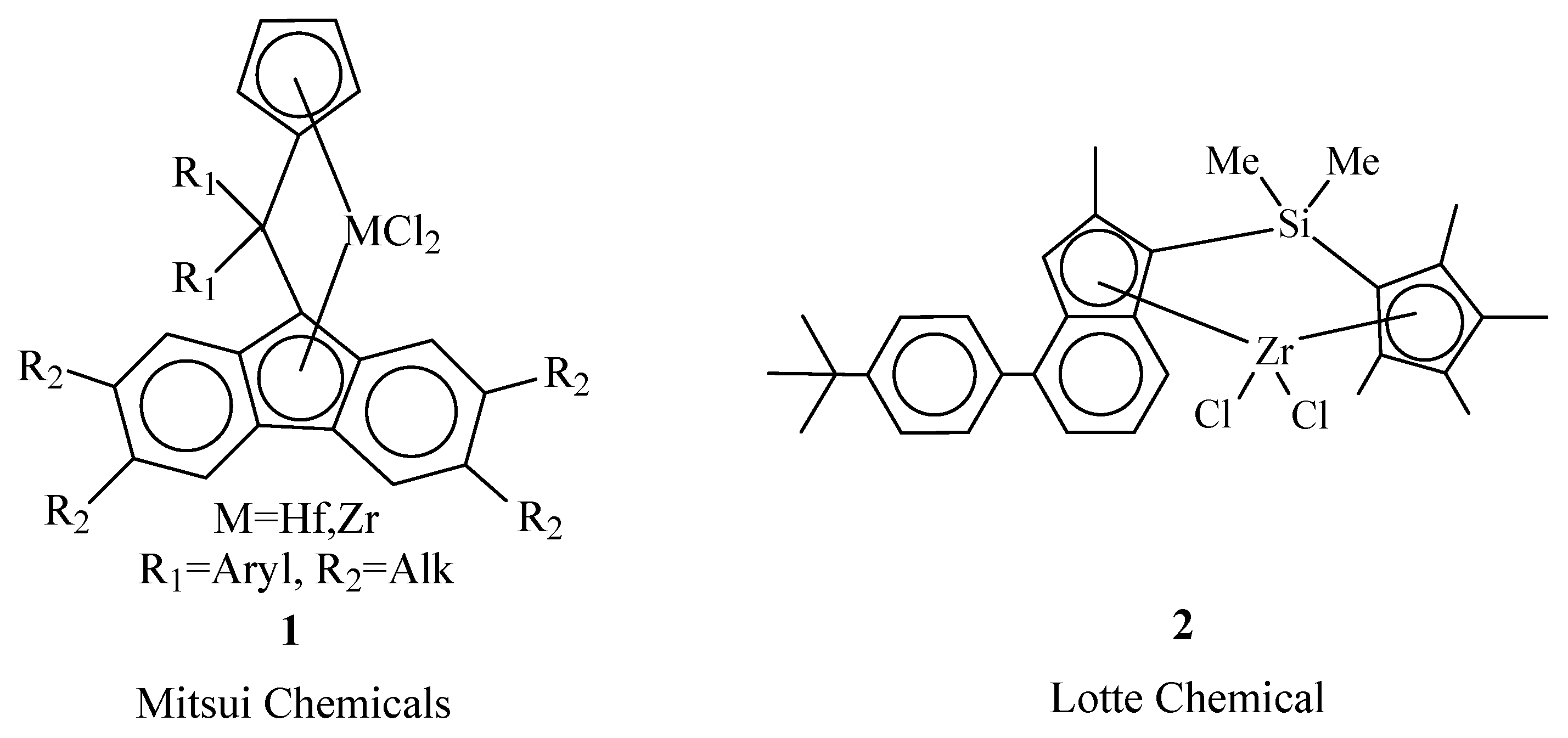
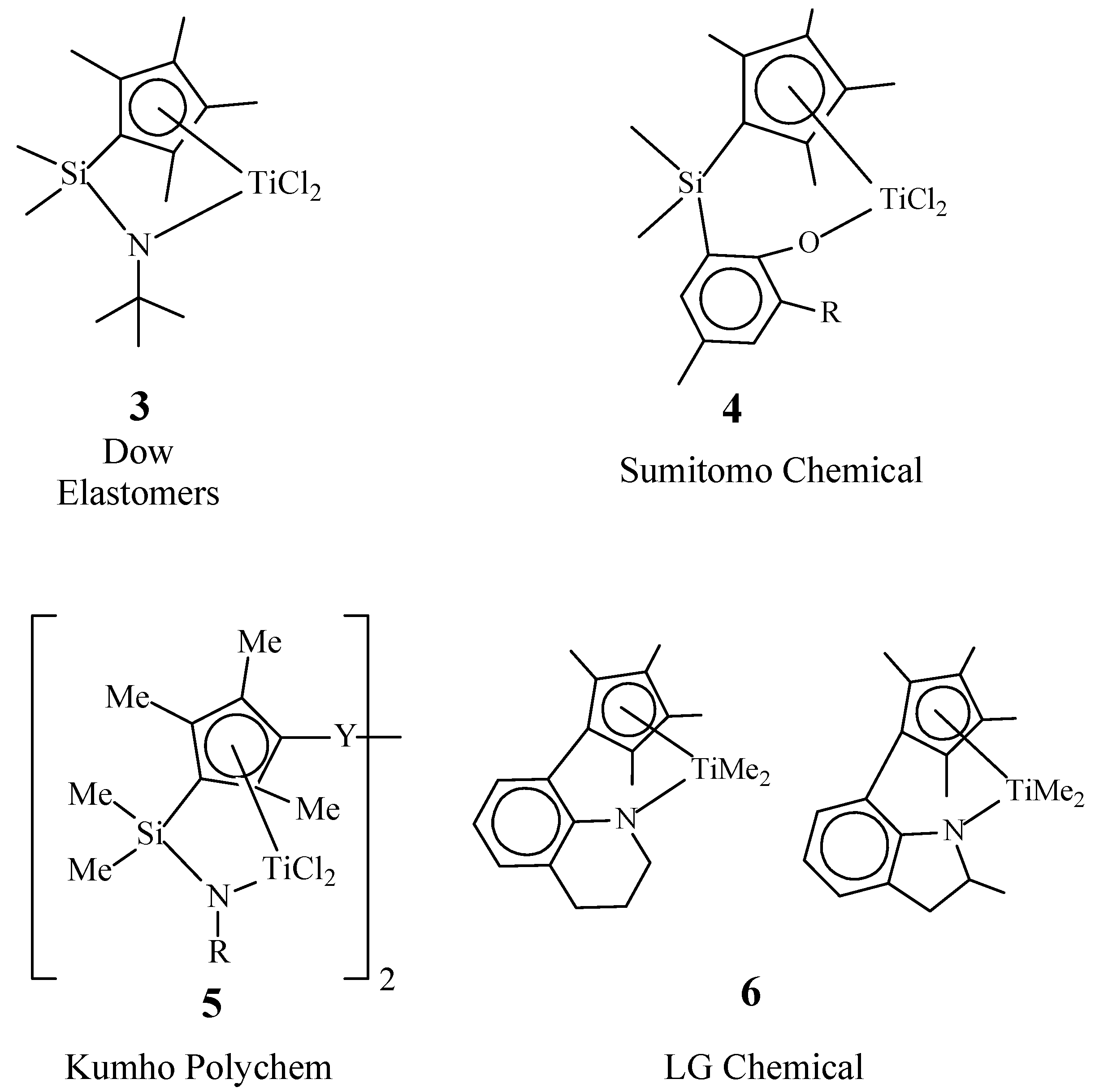
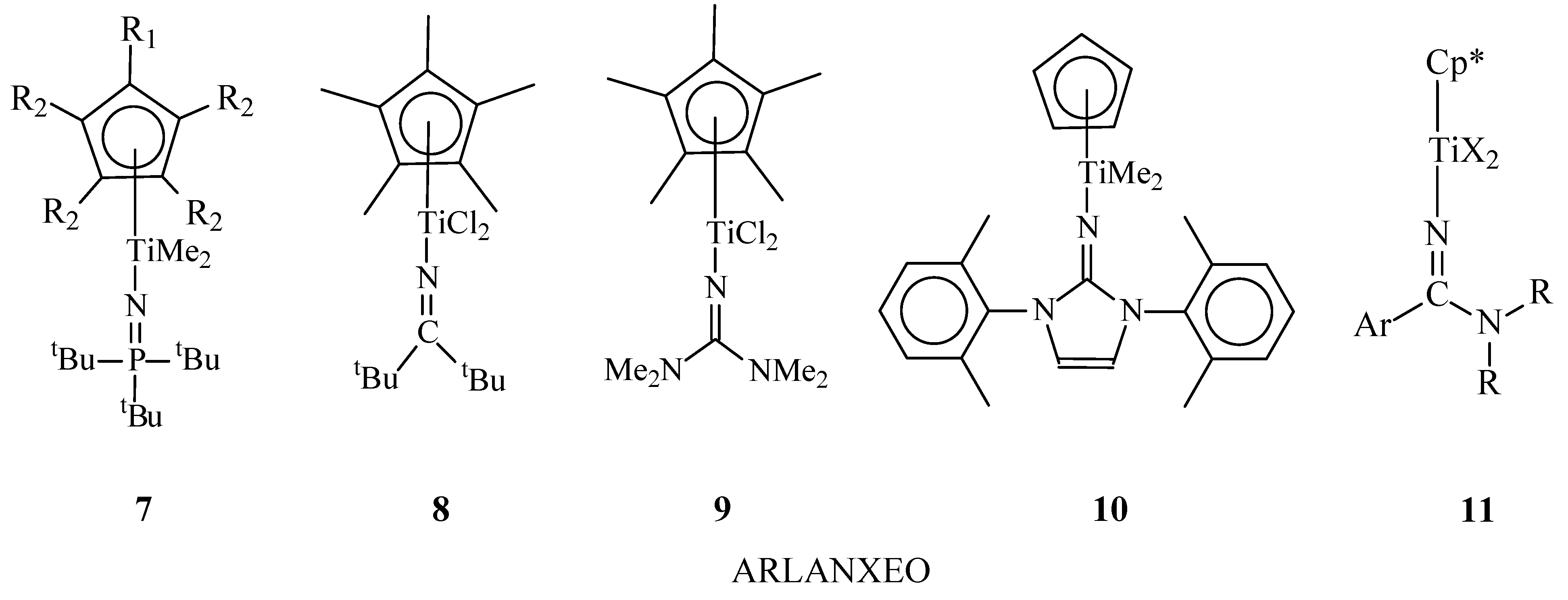
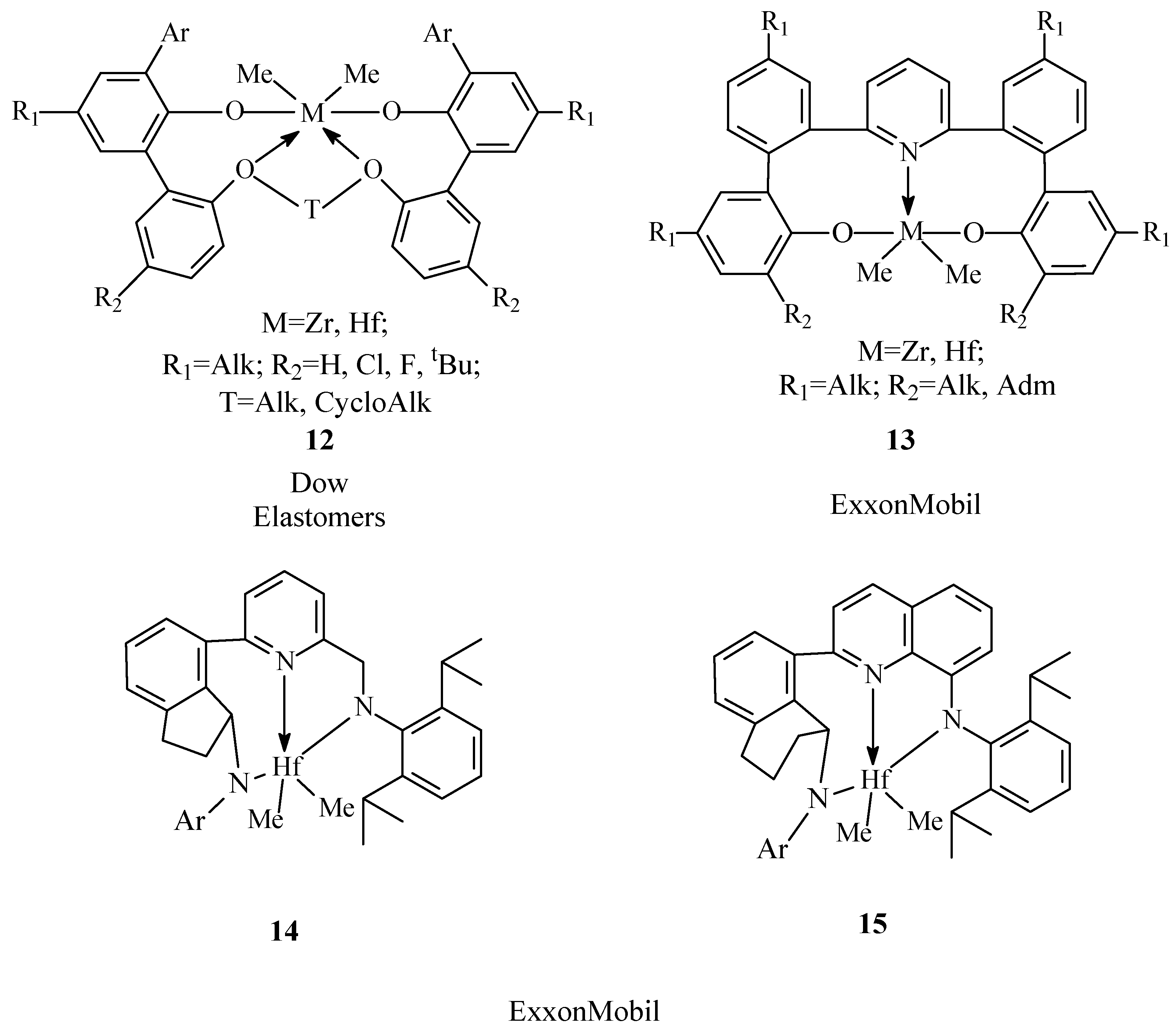
| EPDM Manufacturer | Polymer Grade | Ethylene Content, wt% | Diene (ENB) Content, wt% | Mooney Viscosity ML1+4(125 °C) 1 | Polymer Structures | Ref. |
|---|---|---|---|---|---|---|
| ARLANXEO | Keltan® | 44–71 | 0–11.0 | 22–92 | LCB 2, ND 3, MD 4, BD 5 | [8] |
| DOW Elastomers | NordelTM | 50–85 | 0–8.5 | 18–85 | ND, MD, BD | [9] |
| ExxonMobil | VistalonTM | 54–77 | 0–10.0 | 16–82 | LCB, ND, MD, BD | [10] |
| Mitsui Chemical | Mitsui EPTTM | 41–72 | 0–14.0 | 40–78 | LCB, ND, MD, BD | [11] |
| KumhoPolychem | KEP® | 55–71 | 0–10.0 | 23–95 | No data | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravaya, N.M.; Faingol’d, E.E.; Sanginov, E.A.; Badamshina, E.R. Homogeneous Group IVB Catalysts of New Generations for Synthesis of Ethylene-Propylene-Diene Rubbers: A Mini-Review. Catalysts 2022, 12, 704. https://doi.org/10.3390/catal12070704
Bravaya NM, Faingol’d EE, Sanginov EA, Badamshina ER. Homogeneous Group IVB Catalysts of New Generations for Synthesis of Ethylene-Propylene-Diene Rubbers: A Mini-Review. Catalysts. 2022; 12(7):704. https://doi.org/10.3390/catal12070704
Chicago/Turabian StyleBravaya, Natalia M., Evgeny E. Faingol’d, Evgeny A. Sanginov, and Elmira R. Badamshina. 2022. "Homogeneous Group IVB Catalysts of New Generations for Synthesis of Ethylene-Propylene-Diene Rubbers: A Mini-Review" Catalysts 12, no. 7: 704. https://doi.org/10.3390/catal12070704
APA StyleBravaya, N. M., Faingol’d, E. E., Sanginov, E. A., & Badamshina, E. R. (2022). Homogeneous Group IVB Catalysts of New Generations for Synthesis of Ethylene-Propylene-Diene Rubbers: A Mini-Review. Catalysts, 12(7), 704. https://doi.org/10.3390/catal12070704






