High-Performance A-Site Deficient Perovskite Electrocatalyst for Rechargeable Zn–Air Battery
Abstract
:1. Introduction
2. Results and Discussions
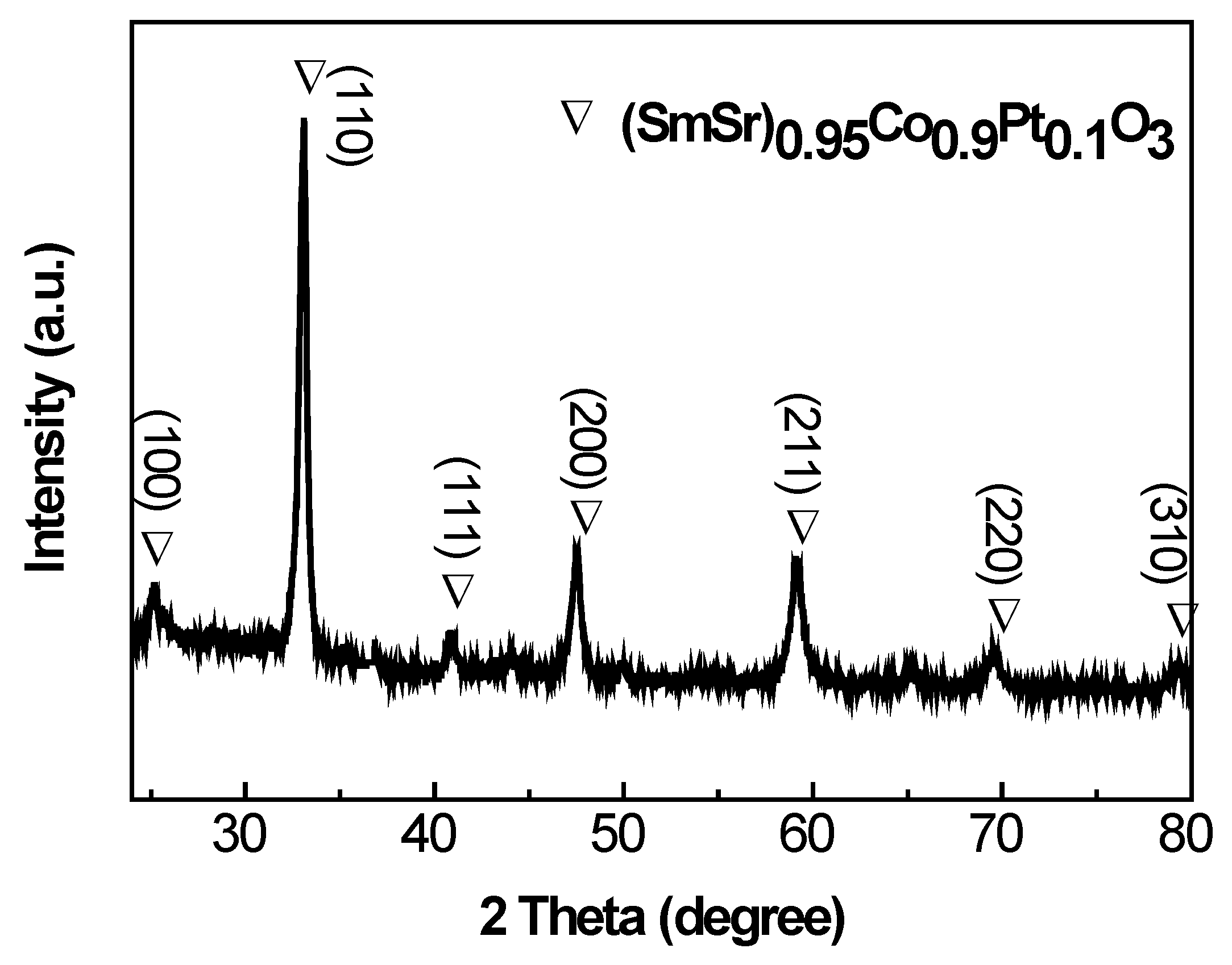
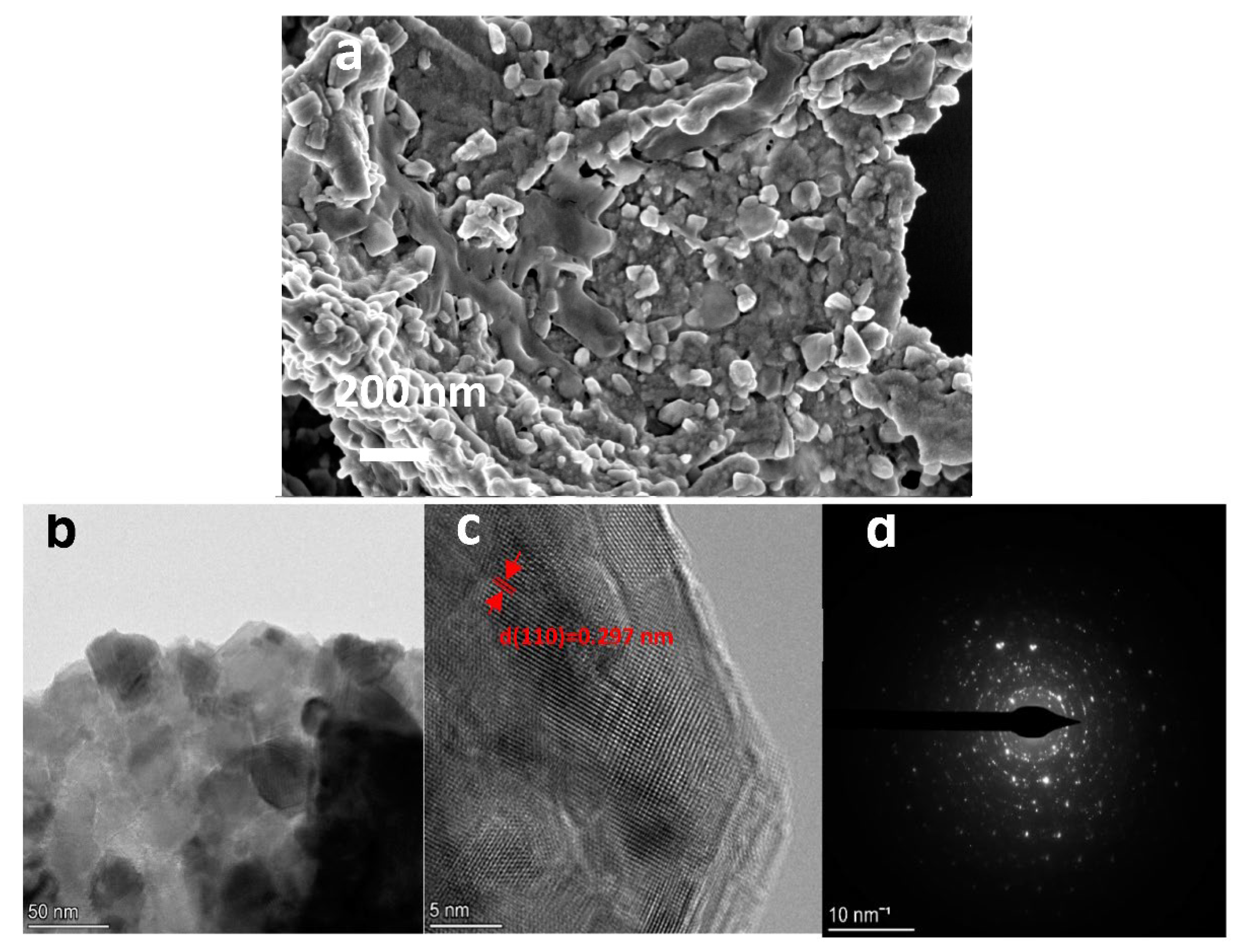
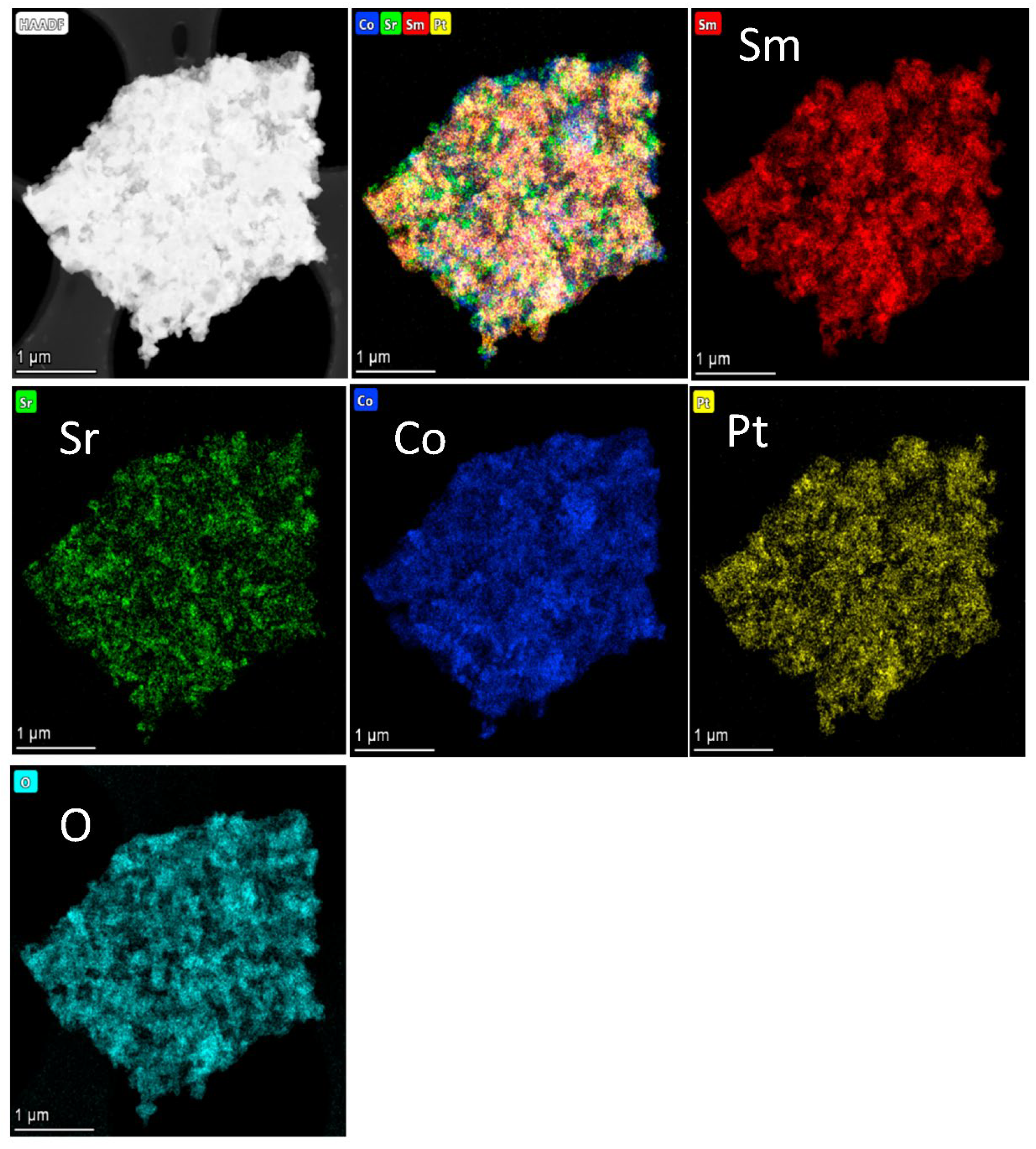
2.1. Phase and Microstructure Characterization
2.2. Electrocatalytic Performance
2.3. Cell Performance
3. Materials and Methods
3.1. Powder Synthesis
3.2. Catalysts Characterization
3.3. Electrochemical Tests
3.4. Battery Assembly and Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, R.; Lee, J.S.; Liu, M.L.; Cho, J. Recent Progress in Non-Precious Catalysts for Metal-Air Batteries. Adv. Energy Mater. 2012, 2, 816–829. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Han, C.; Wang, Z.; Liu, Z.; Tang, Z.; Zhi, C. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Y.Y.; Yang, H.; Dong, Z.; Liu, H.; Xia, B.Y. Advanced Architectures and Relatives of Air Electrodes in Zn-Air Batteries. Adv. Sci. 2018, 5, 1700691–1700721. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, M.; Nam, G.; Lee, J.S.; Cho, J. All-Solid-State Cable-Type Flexible Zinc-Air Battery. Adv. Mater. 2015, 27, 1396. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [Green Version]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Lefèvre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.-P. Iron-Based Catalysts with Improved Oxygen Reduction Activity in Polymer Electrolyte Fuel Cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from poly-aniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Liu, S.; Sun, K.; Chen, X.; Wang, B.; Wu, K.; Cao, X.; Cheong, W.C.; Shen, R.; Han, A.; et al. A bimetallic Zn/Fe polyphthalocyanine-derived single-atom FeN4 catalytic site: A superior trifunctional catalyst for overall water splitting and Zn-air batteries. Angew. Chem. Int. Ed. 2018, 130, 8750–8754. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Zhu, Y.; Sun, D.; Liu, X.; Xu, L.; Tang, Y. Atomic Fe dispersed on Ndoped carbon hollow nanospheres for high-effificiency electrocatalytic oxygen reduction. Adv. Mater. 2019, 31, 1806312. [Google Scholar] [CrossRef]
- Jasinski, R. A New Fuel Cell Cathode Catalyst. Nature 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- He, W.; Wang, Y.; Jiang, C.; Lu, L. Structural effects of a carbon matrix in non-precious metal O2-reduction electrocatalysts. Chem. Soc. Rev. 2016, 45, 2396–2409. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Chen, Z.G.; Chen, Y.; Su, C.; Tade, M.O.; Shao, Z. SrNb0.1Co0.7Fe0.2O3-delta perovskite as a next-generation electrocatalyst for oxygen evolution in alkaline solution. Angew. Chem. Int. Ed. Engl. 2015, 54, 3897–3901. [Google Scholar] [CrossRef]
- Su, C.; Wang, W.; Chen, Y.; Yang, G.; Xu, X.; Tade, M.O.; Shao, Z. SrCo0.9Ti0.1O3-delta as a New Electrocatalyst for the Oxygen Evolution Reaction in Alkaline Electrolyte with Stable Performance. ACS Appl. Mater. Interfaces 2015, 7, 17663–17670. [Google Scholar] [CrossRef]
- Xu, X.; Su, C.; Zhou, W.; Zhu, Y.; Chen, Y.; Shao, Z. Co-doping Strategy for Developing Perovskite Oxides as Highly Efficient Electrocatalysts for Oxygen Evolution Reaction. Adv. Sci. 2016, 3, 1500187–1500193. [Google Scholar] [CrossRef]
- Jung, J.-I.; Jeong, H.Y.; Lee, J.-S.; Kim, M.G.; Cho, J. A Bifunctional Perovskite Catalyst for Oxygen Reduction and Evolution. Angew. Chem. Int. Ed. 2014, 53, 4582–4586. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Sunarso, J.; Zhong, Y.; Shao, Z. Phosphorus-Doped Perovskite Oxide as Highly Efficient Water Oxidation Elec-trocatalyst in Alkaline Solution. Adv. Funct. Mater. 2016, 26, 5862–5872. [Google Scholar] [CrossRef]
- Bian, J.J.; Li, Z.P.; Li, N.W.; Sun, C.W. Oxygen Deficient LaMn0.75Co0.25O3-Delta Nanofibers as an Efficient Electrocatalyst for Oxygen Evolution Reaction and Zinc-Air Batteries. Inorg. Chem. 2019, 58, 8208–8214. [Google Scholar] [CrossRef]
- Choi, M.J.; Kim, T.L.; Kim, J.K.; Lee, T.H.; Lee, S.A.; Kim, C.; Hong, K.; Bark, C.W.; Ko, K.T.; Jang, H.W. Enhanced Oxygen Evolution Electrocatalysis in Strained a-Site Cation Deficient LaNiO3 Perovskite Thin Films. Nano Lett. 2020, 20, 8040–8045. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Geng, Z.B.; Yuan, L.; Sun, Y.; Cong, Y.G.; Huang, K.K.; Wang, L.; Zhang, W. Nanoscale Architecture of RuO2/La0.9Fe0.92Ru0.08-xO3-Delta Composite Via Manipulating the Exsolution of Low Ru-Substituted a-Site Deficient Perovskite. Acs Sustain. Chem. Eng. 2018, 6, 11999–12005. [Google Scholar] [CrossRef]
- Ma, J.J.; Geng, Z.B.; Jiang, Y.L.; Hou, X.Y.; Ge, X.; Wang, Z.Z.; Huang, K.K.; Zhang, W.; Feng, S.H. Exsolution Manipulated Local Surface Cobalt/Iron Alloying and Dealloying Conversion in La0.95Fe0.8Co0.2O3 Perovskite for Oxygen Evolution Reaction. J. Alloy. Compd. 2021, 854, 157154–157161. [Google Scholar] [CrossRef]
- Majee, R.; Islam, Q.A.; Mondal, S.; Bhattacharyya, S. An electrochemically reversible lattice with redox active A-sites of double perovskite oxide nanosheets to reinforce oxygen electrocatalysis. Chem. Sci. 2020, 11, 10180–10189. [Google Scholar] [CrossRef]
- Miao, H.; Wu, X.Y.; Chen, B.; Wang, Q.; Wang, F.; Wang, J.T.; Zhang, C.F.; Zhang, H.C.; Yuan, J.L.; Zhang, Q.J. A-Site De-ficient/Excessive Effects of LaMnO3 Perovskite as Bifunctional Oxygen Catalyst for Zinc-Air Batteries. Electrochim. Acta 2020, 333, 135566–135577. [Google Scholar] [CrossRef]
- Wu, X.; Miao, H.; Hu, R.; Chen, B.; Yin, M.; Zhang, H.; Xia, L.; Zhang, C.; Yuan, J. A-site deficient perovskite nanofibers boost oxygen evolution reaction for zinc-air batteries. Appl. Surf. Sci. 2021, 536, 147806–147813. [Google Scholar] [CrossRef]
- Xu, W.; Yan, L.; Teich, L.; Liaw, S.; Zhou, M.; Luo, H. Polymer-assisted chemical solution synthesis of La0.8Sr0.2MnO3-based perovskite with A-site deficiency and cobalt-doping for bifunctional oxygen catalyst in alkaline media. Electrochim. Acta 2018, 273, 80–87. [Google Scholar] [CrossRef]
- Yao, X.L.; Liu, J.Y.; Wang, W.H.; Lu, F.; Wang, W.C. Origin of Oer Catalytic Activity Difference of Oxygen-Deficient Perovskites A2Mn2O5 (a = Ca, Sr): A Theoretical Study. J. Chem. Phys. 2017, 146, 1404–1427. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Yu, J.; Chen, Y.; Liu, M.; Shao, Z. Enhancing Electrocatalytic Activity of Perovskite Oxides by Tuning Cation Deficiency for Oxygen Reduction and Evolution Reactions. Chem. Mater. 2016, 28, 1691–1697. [Google Scholar] [CrossRef]
- Xia, C.; Rauch, W.; Chen, F.; Liu, M. Sm0.5Sr0.5CoO3 cathodes for low-temperature SOFCs. Solid State Ion. 2002, 148, A795. [Google Scholar] [CrossRef] [Green Version]
- Grimaud, A.; May, K.J.; Carlton, C.E.; Lee, Y.-L.; Risch, M.; Hong, W.T.; Zhou, J.; Shao-Horn, Y. Double perovskites as a family of highly active catalysts for oxygen evolution in alkaline solution. Nat. Commun. 2013, 4, 2439. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, G.; Zhu, Y.; Liu, B.; Zhou, W.; Shao, Z. B-Site Cation Ordered Double Perovskites as Efficient and Stable Electrocatalysts for Oxygen Evolution Reaction. Chem. Eur. J. 2017, 23, 5722–5728. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Huang, Z.; Li, G.; Wang, Q.; He, B.; Zhao, L. Insights into Ni-Fe couple in perovskite electrocatalysts for highly efficient electrochemical oxygen evolution. Electrochim. Acta 2019, 293, 240–246. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Zhao, B.; Huijun, C.; Liu, X.; Zhao, R.; Wang, X.; Liu, J.; Chen, Y.; Liu, M. Oxygen Defect Engineering: Improving the Activity for Oxygen Evolution Reaction by Tailoring Oxygen Defects in Double Perovskite Oxides. Adv. Funct. Mater. 2019, 29, 1970236. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhou, W.; Chen, Y.; Yu, J.; Liu, M.; Shao, Z. ChemInform Abstract: A High-Performance Electrocatalyst for Oxygen Evolution Reaction: LiCo0.8Fe0.2O2. ChemInform 2015, 47, 7150–7155. [Google Scholar] [CrossRef]
- Liu, R.; Liang, F.; Zhou, W.; Yang, Y.; Zhu, Z. Calcium-doped lanthanum nickelate layered perovskite and nickel oxide nanohybrid for highly efficient water oxidation. Nano Energy 2015, 12, 115–122. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Cheng, Y.; Ianni, E.; Lin, B. A highly active and stable La0.5Sr0.5Ni0.4Fe0.6O3-δ perovskite electrocatalyst for oxygen evolution reaction in alkaline media. Electrochim. Acta 2017, 246, 997–1003. [Google Scholar] [CrossRef]

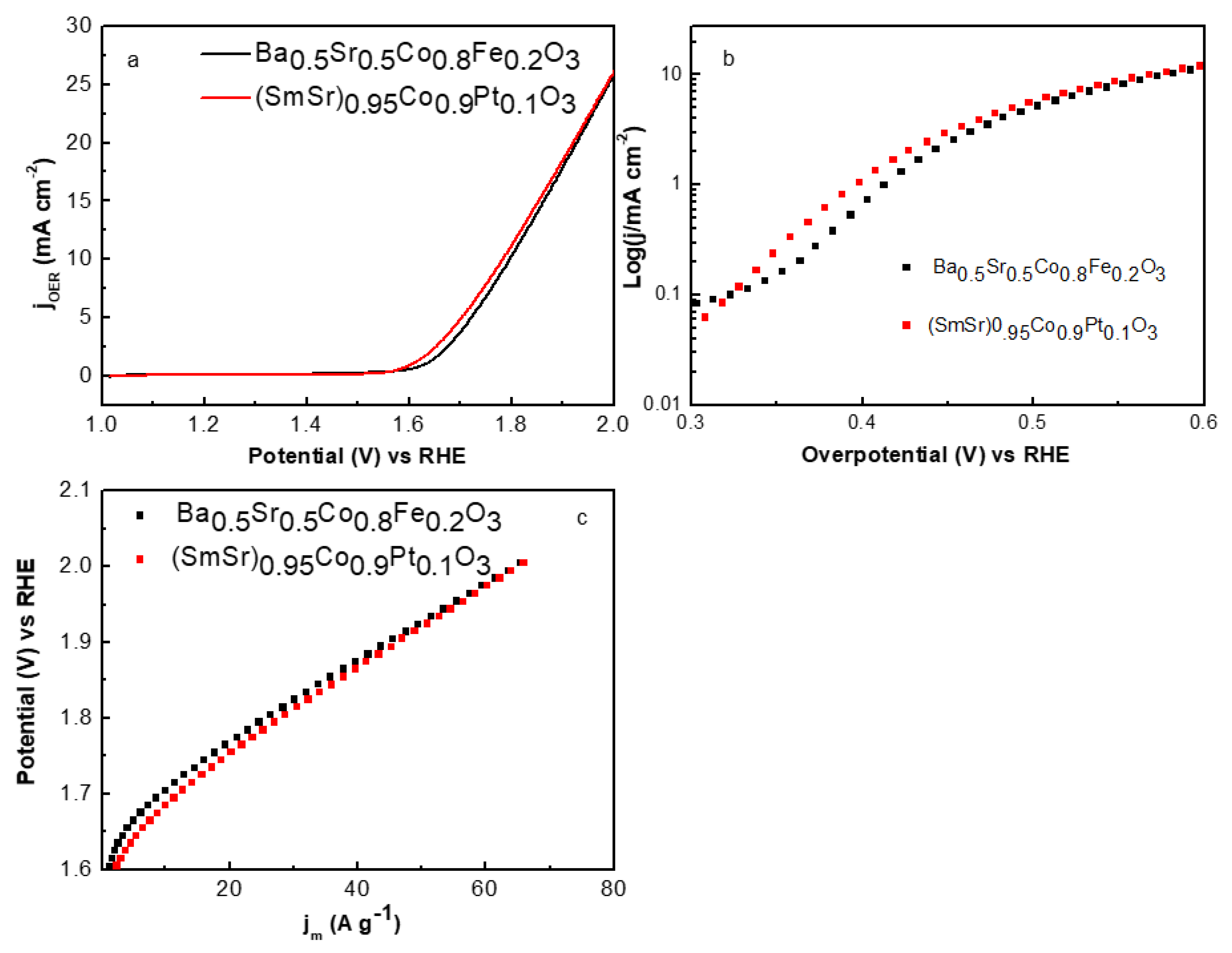
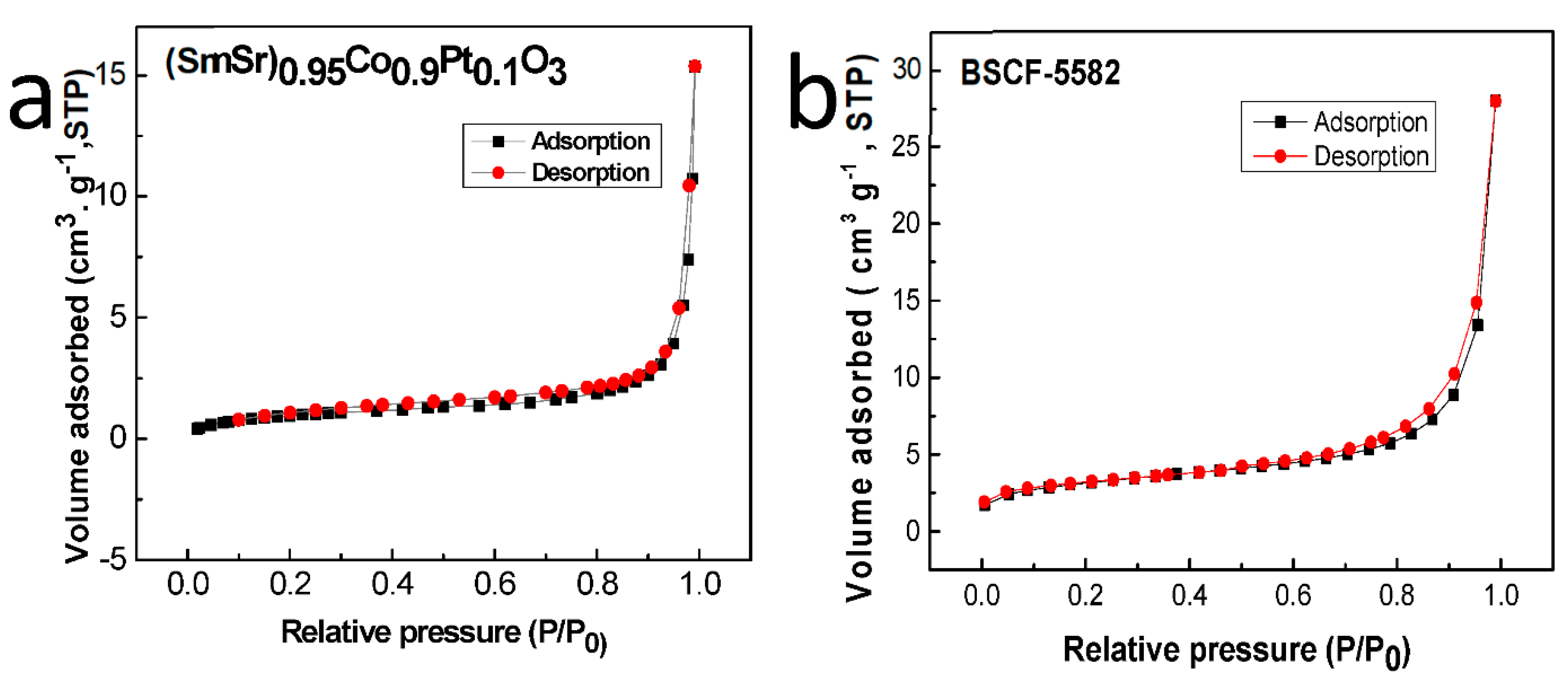
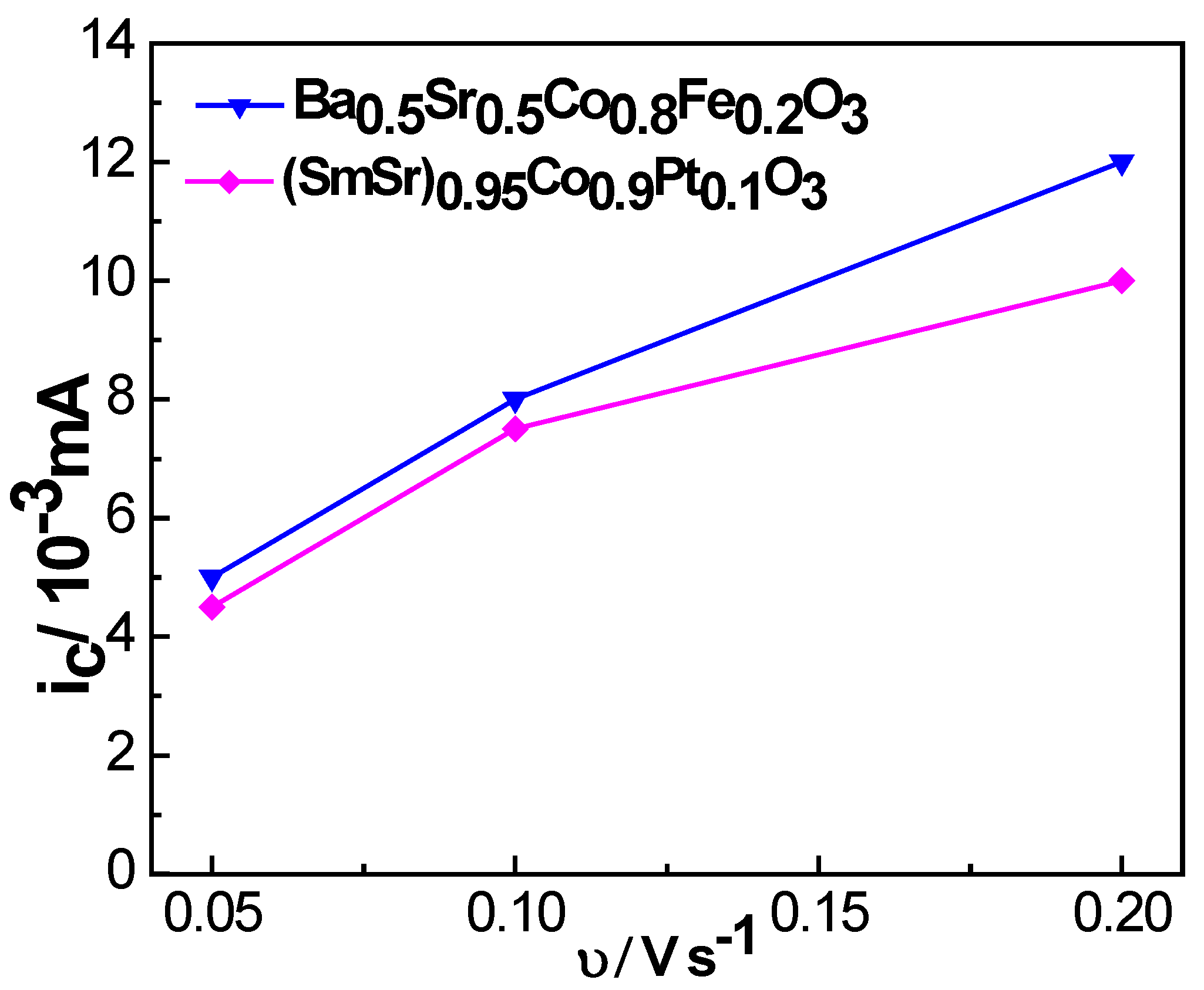

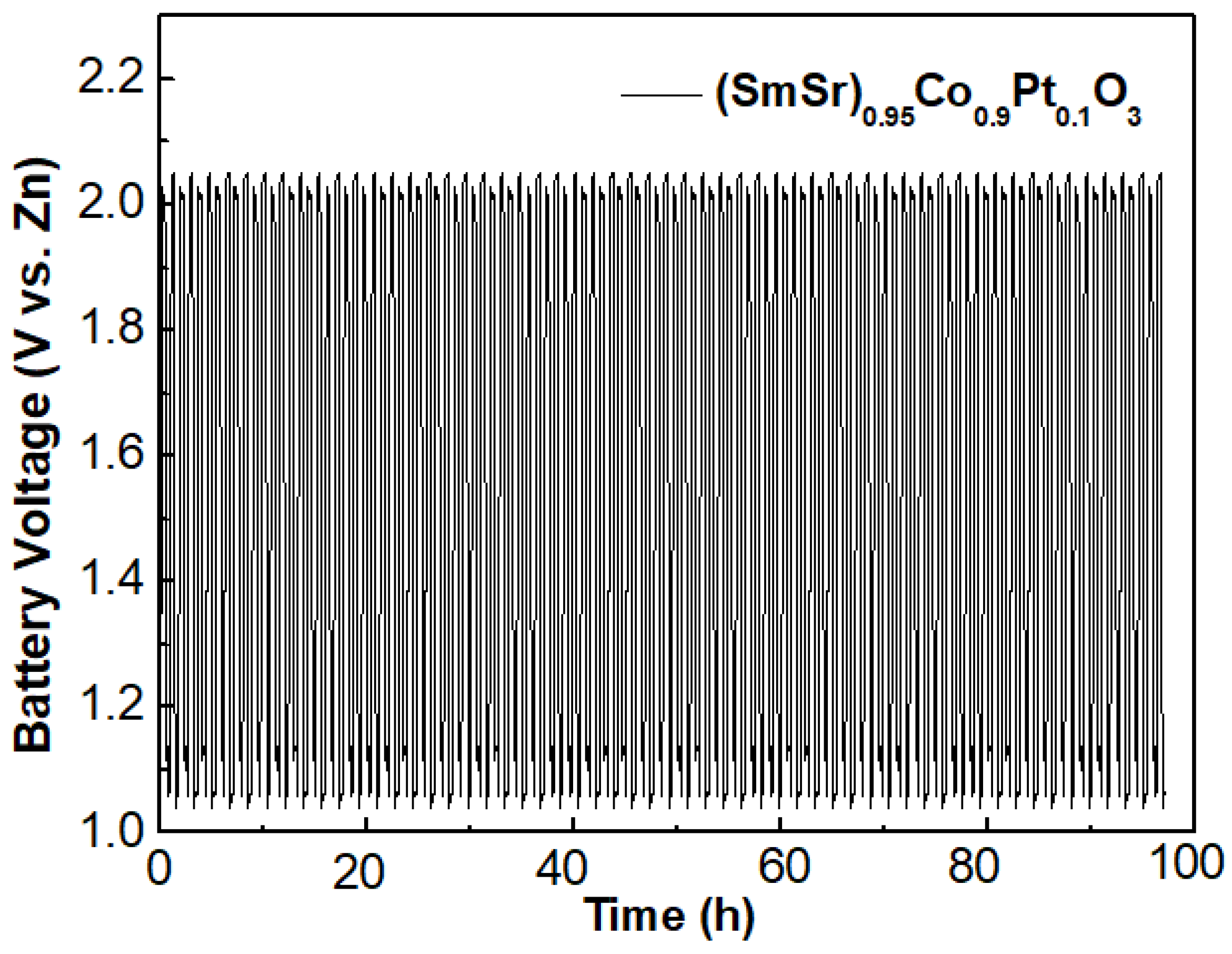
| Sample | Ba0.5Sr0.5Co0.8Fe0.2O3 | (SmSr)0.95Co0.9Pt0.1O3 |
|---|---|---|
| BET surface area (m2 g−1) | 10.84 ± 0.02 | 14.77 ± 0.03 |
| ECSA(m2 g−1) | 1.79 ± 0.01 | 1.56 ± 0.02 |
| Onset potential (V) | 1.61V | ~1.58 V |
| Jmax (mA cm−2) (η = 0.77 V) | 26.7 | 27 |
| Tafel slope (mV dec−1) | 80 | 82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Hou, B.; Wang, X.; Yu, Z.; Luo, D.; Gholizadeh, M.; Fan, X. High-Performance A-Site Deficient Perovskite Electrocatalyst for Rechargeable Zn–Air Battery. Catalysts 2022, 12, 703. https://doi.org/10.3390/catal12070703
Wang C, Hou B, Wang X, Yu Z, Luo D, Gholizadeh M, Fan X. High-Performance A-Site Deficient Perovskite Electrocatalyst for Rechargeable Zn–Air Battery. Catalysts. 2022; 12(7):703. https://doi.org/10.3390/catal12070703
Chicago/Turabian StyleWang, Chengcheng, Bingxue Hou, Xintao Wang, Zhan Yu, Dawei Luo, Mortaza Gholizadeh, and Xincan Fan. 2022. "High-Performance A-Site Deficient Perovskite Electrocatalyst for Rechargeable Zn–Air Battery" Catalysts 12, no. 7: 703. https://doi.org/10.3390/catal12070703
APA StyleWang, C., Hou, B., Wang, X., Yu, Z., Luo, D., Gholizadeh, M., & Fan, X. (2022). High-Performance A-Site Deficient Perovskite Electrocatalyst for Rechargeable Zn–Air Battery. Catalysts, 12(7), 703. https://doi.org/10.3390/catal12070703





